
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med. , 04 March 2025
Sec. Infectious Diseases: Pathogenesis and Therapy
Volume 12 - 2025 | https://doi.org/10.3389/fmed.2025.1539661
Introduction: Blastocystis hominis (B. hominis), a protozoan parasite often found in the human intestinal tract, is typically identified through fecal examination. Its presence in ascitic fluid is quite uncommon, making such a detection significant in the context of medical diagnosis.
Case presentation: In this case report, we describe a 53-year-old female patient who presented with a 6-month history of recurrent diarrhea and fecal incontinence subsequent to the diagnosis of rectal signet ring cell carcinoma. The patient was discovered to have a severe abdominal infection, with B. hominis identified in both the abdominal cavity and the intestinal tract. Marked improvement in diarrheal symptoms was achieved following peritoneal lavage with metronidazole.
Conclusion: This case underscores the significance of detecting B. hominis infection in the ascitic fluid of a patient afflicted with rectal signet ring cell carcinoma. B. hominis, a prevalent opportunistic pathogen, often exploits the compromised immune states and nutritional deficiencies prevalent in cancer patients, rendering them more susceptible to such infections. It is imperative to enhance diagnostic accuracy and mitigate the risk of misdiagnosis to subsequently improve the clinical outcomes and overall quality of life for individuals battling cancer.
Blastocystis hominis is a ubiquitous protozoan that inhabits the human large intestine. Its prevalence ranges approximately 10% in developed countries, yet this figure escalates to between 50 and 60% in developing nations (1). The pathogenicity of B. hominis remains a subject of debate, as the majority of individuals carrying this bacterium exhibit no discernible clinical symptoms. Nevertheless, there are exceptions, particularly among patients who have concurrent infections or compromised immune systems. These individuals may experience a range of symptoms including abdominal pain, diarrhea, nausea, and vomiting, with severe cases potentially leading to fatal outcomes (2–5). In this report, we delve into the uncommon detection of B. hominis in the abdominal fluid of a patient diagnosed with intestinal adenocarcinoma. Our findings shed light on the complexities of managing B. hominis infections in the context of patients grappling with malignant tumors, offering valuable insights for clinical practice.
A 53-year-old female patient was admitted to the Gastroenterology Department at The Third People’s Hospital of Chengdu, presenting with a six-month history of recurrent diarrhea and a one-month history of abdominal bloating. She had no prior medical record of diabetes, hypertension, coronary artery disease, hepatitis B infection, or tuberculosis. Additionally, the patient denied any history of drug or food allergies, blood transfusions, surgical procedures, or injuries.
The patient presented with abdominal distention and a positive shifting dullness sign upon physical examination. A computed tomography (CT) scan of the abdomen and pelvis revealed massive ascites, enlarged lymph nodes in the retroperitoneum, abdomen, and pelvis, and a suspicious nodule on the right hemidiaphragm, indicative of potential metastasis. Cytological analysis of the ascitic fluid showed a high concentration of nucleated cells, bacteria, and ruptured cells (Table 1). Wright’s stain identified round, lightly stained vacuole protozoa of varying sizes with large transparent bodies, consistent with B. hominis (as indicated by arrows in Figures 1, 2). We then conducted a routine and microscopic examination of the patient’s stool samples, which also uncovered the presence of Blastocystis (Table 2). Ascitic fluid culture further revealed an infection with Escherichia coli (E. coli), and confirmed that the isolate E. coli was positive for Extended-Spectrum Beta-Lactamases (ESBLs). Susceptibility testing further showed that the ESBL-producing E. coli was sensitive to meropenem. The enteroscope revealed lesions encircling the intestine, accompanied by erosive, congestive, edematous, and hardened mucosa. A subsequent biopsy of the mass identified it as a signet ring cell carcinoma (Figures 3–5). Genetic testing of the rectal biopsy specimen ruled out K-ras gene mutation. Based on these findings, the patient was diagnosed with severe abdominal infection (B. hominis andE. coli), rectal signet ring cell carcinoma with metastasis to the retroperitoneal lymph nodes, peritoneum, and diaphragm, severe abdominal infection, B. hominis infection affecting the abdomen and intestine, severe malnutrition, acute enteritis, and hypoalbuminemia, carcinomatous ascites.
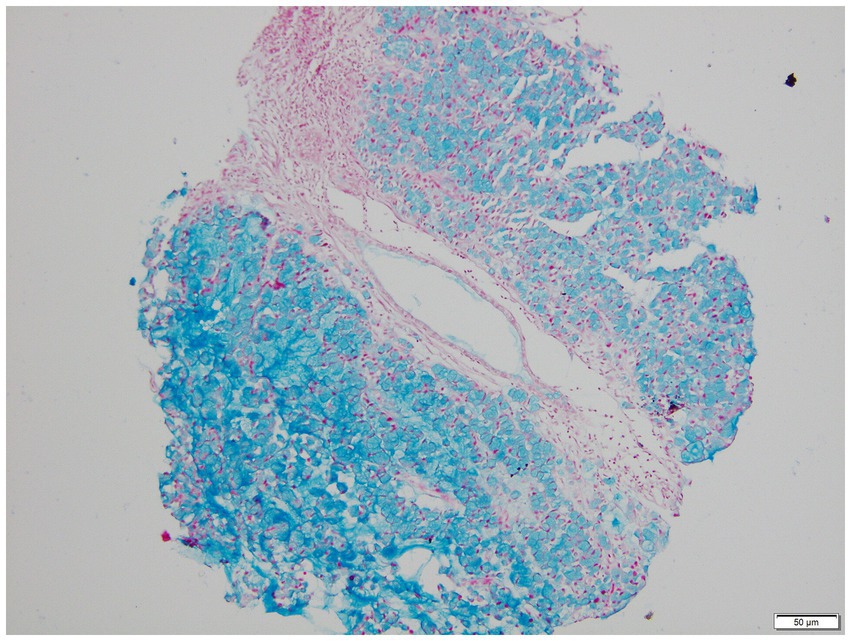
Figure 5. AB-PAS stained microscopic view of signet ring cell carcinoma biopsy (100x Magnification).
The patient’s ascites was effectively managed through paracentesis and subsequent drainage. A comprehensive treatment regimen was implemented, consisting of a combined chemotherapy approach with raltitrexed and tegafur gimeraciloteracil, alongside radiation therapy that incorporated planning target volume techniques. Additionally, cetuximab was administered as part of the targeted therapy. Following peritoneal lavage with metronidazole and levofloxacin, along with the intravenous administration of meropenem, there was a significant reduction in drainage volume, and the ascites odor was considerably diminished. The patient experienced notable improvements in symptoms such as abdominal bloating, pain, and diarrhea. Regrettably, the patient, after a period of hospitalization, was discharged with a palliative care plan and the patient passed away 9 days following their discharge.
Blastocystis hominis is a prevalent intestinal protozoan with a global distribution. Despite its widespread presence, the impact of B. hominis on human health and its role in disease pathology remain largely enigmatic (2, 6). Several studies have shed light on factors that may contribute to the pathogenicity of B. hominis (7–11) with a particular emphasis on the association between the parasite and immunodeficiency or immunosuppression. Notably, B. hominis infection has been identified as a significant cause of diarrhea in individuals living with HIV/AIDS, indicating its potential as a pathogenic agent in this vulnerable population (12, 13). Our case report highlights a severe ascites infection caused by B. hominis in a patient with signet ring cell carcinoma. This observation suggests a close link between cancer-related immunosuppression and B. hominis infection. Consistent with this hypothesis, B. hominis infections have been documented in other cancer patients experiencing immune compromise (14). This is further supported by the identification of B. hominis in patients with various cancers, including colorectal cancer, haematological malignancies, bladder, breast, lung, pancreas, basal cell carcinoma, laryngeal, renal cell carcinoma, and prostate cancer (15–19).
The detection of B. hominis in ascitic fluid, as observed in our patient, could potentially be traced back to the compromised intestinal mucosal barrier resulting from signet ring cell carcinoma and potential microperforations induced by tumor invasion. This compromise enables B. hominis to migrate from the intestinal lumen into the abdominal cavity. The observed erosion, congestion, edema, and induration of the intestinal mucosa during enteroscopy further suggest a weakened barrier, facilitating this migration. The pervasive occurrence of B. hominis among many cancer types indicates its possible function as an opportunistic pathogen, taking advantage of the impaired immune systems and overall deteriorated health of cancer patients. These findings underscore the propensity of B. hominis to cause opportunistic infections. It is worth noting that the infection rates of B. hominis are significantly higher in patients with digestive system tumors, especially colorectal cancer, as compared to those with tumors in other bodily systems (18, 20). Furthermore, according to in vitro studies (21), B. hominis has the ability to stimulate the growth of colorectal cancer cell lines by suppressing apoptosis. Additional studies (22, 23) have revealed that B. hominis can also enhance cancer cell proliferation by downregulating the host’s immune response, indicating a potential involvement of B. hominis in the development of colorectal cancer. The rapid proliferation of B. hominis in immunocompromised hosts can lead to a spectrum of intestinal pathologies, ranging from mild to severe. It is imperative for medical professionals to recognize that anti-tumor treatments in cancer patients may further suppress the immune system, potentially triggering the reactivation of dormant B. hominis or new infections (8). Our study serves as a stark reminder of the critical importance of timely diagnosis of B. hominis infections for the appropriate management and treatment of cancer patients.
In the majority of clinical laboratories, microscopic examination of stained slides remains the gold standard for diagnosing B. hominis infections. Drawing from personal experience, several strategies can enhance the detection rate of this protozoan. Firstly, conducting multiple stool examinations can significantly boost the diagnostic yield, particularly for patients presenting with chronic diarrhea. Our laboratory protocol involves the use of normal saline to prepare direct smears of stool samples. These smears are initially scanned at low magnification for objects resembling fat globules, starch granules, or white blood cells. Upon identifying suspicious structures, a coverslip is applied, and the slide is then examined at high magnification. B. hominis typically appears colorless or light yellow, with a round or oval shape, varying in size. It features a large, transparent body encircled by a thin rim of cytoplasm, within which a few refractile bodies may be observed. The images presented in this article depict vacuolar protozoa with thick walls after staining, showcasing a centrally located, darkly stained area that pushes the nucleus (appearing purple) to the periphery of the organism. In addition to direct smears with normal saline, various staining techniques can be employed to improve detection, including iodine staining, Giemsa staining, iron hematoxylin staining, and direct culture methods. Lastly, for ascites samples, a routine procedure involves centrifugation at 1500 revolutions per minute for 5 min. The sediment at the bottom is then used to create a thin, uniform film. After drying, Wright’s staining is applied, which has been shown to markedly enhance the detection rate of B. hominis. Through above described methods, including direct fecal smear examination and staining techniques, this study achieved successful identification of B. hominis. Although these methods are simple and rapid, they possess certain limitations, such as insufficient sensitivity. This limitation primarily stems from the polymorphic characteristics, size variations, and varying cell counts of the parasite, which complicate microscopic detection. In particular, a low cell count within the species may have resulted in a higher incidence of false negative results. Compared to these methods, molecular biological diagnostic techniques, represented by PCR technology, offer a sensitive, specific, and reliable method for detecting B. hominis. Additionally, these techniques allow for the identification of genetic subtypes of B. hominis, providing a valuable foundation for potential correlational research between the epidemiology of B. hominis, its genotype, and disease patterns.
Our study underscores the detrimental impact of B. hominis infection in cancer patients by identifying its presence in ascites samples. The current research elucidates that significant strides remain to be made in understanding the pathogenicity of B. hominis, developing effective treatment strategies, and implementing robust control measures to mitigate its harmful effects.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Third People’s Hospital of Chengdu/Affiliated Hospital of Southwest Jiaotong University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
QL: Data curation, Methodology, Writing – original draft. JH: Data curation, Writing – original draft. YC: Data curation, Methodology, Writing – review & editing. XW: Data curation, Writing – original draft. YM: Data curation, Writing – original draft. QY: Conceptualization, Writing – original draft. PL: Methodology, Writing – original draft, Writing – review & editing. XC: Conceptualization, Funding acquisition, Investigation, Resources, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Chengdu Third People’s Hospital Independent Research Project [No. CSY-YN-01-2023-017], Chengdu Medical Research Project [No. 202313052863] and Southwest Jiaotong University Medical and Industrial Training Project [No. 2682024ZTPY001].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Duda, A, Kosik-Bogacka, D, Lanocha, N, and Szymański, S. Blastocystis hominis- parasites or commensals? Ann Acad Med Stetin. (2014) 60:23–8. doi: 10.21164/POMJLIFESCI.7
2. Tan, KS. New insights on classification, identification, and clinical relevance of Blastocystis spp. Clin Microbiol Rev. (2008) 21:639–65. doi: 10.1128/CMR.00022-08
3. Patino, WD, Cavuoti, D, Banerjee, SK, Swartz, K, Ashfaq, R, and Gokaslan, T. Cytologic diagnosis of Blastocystis hominis in peritoneal fluid. Acta Cytol. (2008) 52:718–20. doi: 10.1159/000325628
4. Taşova, Y, Sahin, B, Koltaş, S, and Paydaş, S. Clinical significance and frequency of Blastocystis hominis in Turkish patients with hematological malignancy. Acta Med Okayama. (2000) 54:133–6.
5. Toychiev, A, Abdujapparov, S, Imamov, A, Navruzov, B, Davis, N, Badalova, N, et al. Intestinal helminths and protozoan infections in patients with colorectal cancer: prevalence and possible association with cancer pathogenesis. Parasitol Res. (2018) 117:3715–23. doi: 10.1007/s00436-018-6070-9
6. Stensvold, CR, and Clark, C. Parasitology international. Current status of Blastocystis: A personal view. Parasitol Int. (2016) 65:763–71. doi: 10.1016/j.parint.2016.05.015
7. Andiran, N, Acikgoz, ZC, Turkay, S, and Andiran, F. Blastocystis hominis--an emerging and imitating cause of acute abdomen in children. J Pediatr Surg. (2006) 41:1489–91. doi: 10.1016/j.jpedsurg.2006.04.037
8. Rondón, L, Vargas, CM, Velarde, CN, At, I, and Tello, R. Human blastocystosis: prospective study symptomatology and associated epidemiological factors. Revista De Gastroenterologia Del Peru Organo Oficial De La Sociedad De Gastroenterologia Del Peru. (2003) 23:29–35.
9. El-Shazly, AM, Abdel-Magied, AA, El-Beshbishi, SN, El-Nahas, HA, and Monib, M. Blastocystis hominis among symptomatic and asymptomatic individuals in talkha center, dakahlia governorate, Egypt. J Egypt Soc Parasitol. (2005) 35:653–66.
10. Tai, WP, Hu, PJ, Jing, W, and Lin, XC. Six ulcerative colitis patients with refractory symptoms co-infective with Blastocystis hominis in China. Parasitol Res. (2011) 108:1207–10. doi: 10.1007/s00436-010-2164-8
11. Stark, D, Fotedar, R, Hal, SV, Beebe, N, and Marriott, D. Prevalence of enteric protozoa in human immunodeficiency virus (HIV)-positive and HIV-negative men who have sex with men from Sydney. Austral Am J Tropical Med Hygiene. 76:549–552.
12. Gassama, A, Sow, PS, Fall, F, Camara, P, and Aïdara-Kane, A. Ordinary and opportunistic enteropathogens associated with diarrhea in senegalese adults in relation to human immunodeficiency virus serostatus. Int J Infectious Dis. (2001) 5:192–8. doi: 10.1016/S1201-9712(01)90069-4
13. Hailemariam, G, Kassu, A, Abebe, G, Abate, E, Damte, D, Mekonnen, E, et al. Intestinal parasitic infections in HIV/AIDS and HIV seronegative individuals in a teaching hospital. Ethiopia Japanese J Infectious Diseases. (2004) 57:41–3.
14. Chandramathi, S, Suresh, K, Anita, ZB, and Kuppusamy, UR. Infections of Blastocystis hominis and microsporidia in cancer patients: are they opportunistic? Trans R Soc Trop Med Hyg. (2012) 106:267–9. doi: 10.1016/j.trstmh.2011.12.008
15. Rauff-Adedotun, AA, Meor Termizi, FH, Shaari, N, and Lee, IL. The coexistence of Blastocystis spp. in humans, animals and environmental sources from 2010-2021 in Asia. Biology. (2021) 10:990. doi: 10.3390/biology10100990
16. Öncü Öner, T, Karabey, M, Can, H, Değirmenci Döşkaya, A, Karakavuk, M, Gül, A, et al. Molecular investigation of Blastocystis sp. and its subtypes in cancer patients under chemotherapy in Aegean region, Turkey. Acta Trop. (2022) 233:106577. doi: 10.1016/j.actatropica.2022.106577
17. Yersal, O, Malatyali, E, Ertabaklar, H, Oktay, E, Barutca, S, and Ertug, S. Blastocystis subtypes in cancer patients: analysis of possible risk factors and clinical characteristics. Parasitol Int. (2016) 65:792–6. doi: 10.1016/j.parint.2016.02.010
18. Labania, L, Zoughbor, S, Ajab, S, Olanda, M, Shantour, SNM, and Al, RZ. The associated risk of Blastocystis infection in cancer: a case control study. Front Oncol. (2023) 13:1115835. doi: 10.3389/fonc.2023.1115835
19. Łanocha, A, Łanocha-Arendarczyk, N, Wilczyńska, D, Zdziarska, B, and Kosik-Bogacka, D. Protozoan intestinal parasitic infection in patients with hematological malignancies. J Clin Med. (2022) 11:2847. doi: 10.3390/jcm11102847
20. Mohamed, AM, Ahmed, MA, Ahmed, SA, Al-Semany, SA, Alghamdi, SS, and Zaglool, DA. Predominance and association risk of Blastocystis hominis subtype I in colorectal cancer: a case control study. Infect Agent Cancer. (2017) 12:21. doi: 10.1186/s13027-017-0131-z
21. Chandramathi, S, Suresh, K, and Kuppusamy, UR. Solubilized antigen of Blastocystis hominis facilitates the growth of human colorectal cancer cells, HCT116. Parasitol Res. (2010) 106:941–5. doi: 10.1007/s00436-010-1764-7
22. Chan, KH, Chandramathi, S, Suresh, K, Chua, KH, and Kuppusamy, UR. Effects of symptomatic and asymptomatic isolates of Blastocystis hominis on colorectal cancer cell line, HCT116. Parasitol Res. (2012) 110:2475–80. doi: 10.1007/s00436-011-2788-3
Keywords: Blastocystis hominis, cytology, intestine perforation, colorectal carcinoma, case report
Citation: Lin Q, Huang J, Chen Y, Wu X, Ma Y, Yang Q, Long P and Chen X (2025) Cytological identification of Blastocystis hominis in the ascites of a patient with rectal carcinoma: a case report. Front. Med. 12:1539661. doi: 10.3389/fmed.2025.1539661
Received: 04 December 2024; Accepted: 04 February 2025;
Published: 04 March 2025.
Edited by:
Vivek P. Chavda, L. M. College of Pharmacy, IndiaReviewed by:
Stefania Tocci, University of Massachusetts Lowell, United StatesCopyright © 2025 Lin, Huang, Chen, Wu, Ma, Yang, Long and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pan Long, bG9uZ3BhbjEwMDVAcXEuY29t; Xin Chen, eGluY2hlbmNkQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.