
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 23 January 2025
Sec. Hepatobiliary Diseases
Volume 12 - 2025 | https://doi.org/10.3389/fmed.2025.1528733
This article is part of the Research Topic Chronic Hepatitis B Management: Current Status and Future Directions View all 14 articles
Objective: Inflammatory reactions and dyslipidemia are associated with the pathogenesis and prognosis of hepatitis B virus-related cirrhosis. We aimed to assess the predictive ability of these parameters in patients with hepatitis B virus-related cirrhosis and overt hepatic encephalopathy (HBV-related OHE).
Design: We conducted an analysis of 1,404 participants diagnosed with HBV-related OHE between January 2008 and July 2023. The prognostic significance of the neutrophil-to-high-density lipoprotein cholesterol (HDL-C) ratio (NHR), lymphocyte-to-HDL-C ratio (LHR), and monocyte-to-HDL-C ratio (MHR) was evaluated using the area under the receiver operating characteristic curve (AUC). Restrictive cubic splines (RCS) were employed to explore the relationship between NHR and 12-month transplant-free (TF) mortality. This study included a prospective test cohort of 328 patients.
Results: NHR was identified as an independent risk factor for 12-month TF mortality. The AUC for NHR (0.776) was similar to that of the model end-stage liver disease (MELD) score (AUC: 0.777). In the test cohort, NHR demonstrated AUC values comparable to MELD, with significantly higher AUCs than LHR and MHR (both p < 0.05). Based on cutoff values for NHR and MELD, patients were classified into four risk subgroups: very-low (NHR < 10 and MELD <18), low (NHR ≥ 10 and MELD <18), moderate (NHR < 10 and MELD ≥18), and high (NHR ≥ 10 and MELD ≥18). The 12-month TF mortality rates in the training cohort were 7.2, 23.5, 30.8, and 51.4%, respectively, for these subgroups, while in the test cohort, the rates were 8.7, 20.5, 30.7, and 46.0%.
Conclusion: NHR is a valuable and accessible prognostic indicator for 12-month TF mortality in patients with HBV-related OHE. Patients with both NHR ≥ 10 and MELD ≥18 are at the highest risk of mortality.
Chronic hepatitis B virus (HBV) infection is the leading cause of cirrhosis in China, often progressing to severe liver failure and life-threatening complications (1). Hepatic encephalopathy (HE), a complex neurological manifestation of cirrhosis, is characterized by high recurrence and mortality rates (2). This condition significantly diminishes patients’ quality of life and imposes a substantial burden on healthcare systems (3). Clinical studies have identified overt HE (OHE) as a major driver of hospital admissions and readmissions among cirrhotic patients (4, 5). Given its considerable impact on morbidity and mortality, it is crucial to identify high-risk patients using reliable biomarkers to enable closer monitoring and timely intervention, thereby preventing the progression of HBV-related OHE.
Decompensated cirrhosis is frequently complicated by infections and carries a high risk of mortality due to dysregulated innate immune responses, which result in both inflammation and immune paralysis (6). Research has highlighted the role of systemic inflammation and lipid abnormalities in the pathogenesis and progression of HE (7, 8). Neutrophils, as key mediators of inflammation, release cytotoxic substances that contribute to the body’s defense against pathogens (9). High-density lipoprotein cholesterol (HDL-C), on the other hand, has protective roles in anti-infection, anti-oxidation, and antithrombotic processes (10). HDL-C also functions as an endogenous inhibitor of inflammation, showing inverse correlations with inflammatory markers (11, 12). Reduced HDL-C levels are strongly linked to poor prognoses in various diseases (13, 14). However, relying on inflammatory or lipid markers alone may not provide sufficient prognostic accuracy. Recent studies have focused on the interplay between inflammatory and lipid markers and their combined predictive value in chronic liver diseases. Ratios such as neutrophil-to-HDL-C (NHR) (14, 15), lymphocyte-to-HDL-C (LHR) (16), and monocyte-to-HDL-C (MHR) (17, 18) have been explored as predictors for conditions including stroke, Parkinson’s disease, cardiovascular disease, and metabolic syndrome. Nevertheless, limited evidence exists regarding the prognostic significance of these biomarkers in predicting 12-month transplant-free (TF) mortality among patients with HBV-related OHE.
This study aims to evaluate the predictive capabilities of NHR, LHR, and MHR for 12-month TF mortality and develop a risk stratification strategy for patients with HBV-related OHE.
From January 2008 to July 2023, a total of 2,534 hospitalized patients with OHE aged 18–75 years were screened at Beijing Ditan Hospital. Chronic hepatitis B was diagnosed by the presence of hepatitis B surface antigen for more than six months (19). OHE diagnosis was confirmed based on the West Haven Criteria (20). Cirrhosis was identified through liver biopsy, endoscopic or radiological evidence of portal hypertension (e.g., ultrasound, computed tomography, or magnetic resonance imaging), and/or complications of cirrhosis, including ascites, HE, or upper gastrointestinal bleeding (21).
Exclusion criteria included co-infections (e.g., HIV, hepatitis A, C, D, or E), malignant tumors, liver transplantation, neuropsychiatric disorders, use of psychotropic drugs, minimal HE, and other liver diseases such as alcoholic liver disease, autoimmune liver disease, metabolic-associated fatty liver disease (MASLD), drug-induced liver injury, cholestatic liver disorders, or inherited conditions. Patients with incomplete clinical data were also excluded. For patients with comorbidities such as hypertension and diabetes, these conditions were not exclusion criteria. However, MASLD was excluded based on clinical and imaging findings (e.g., liver ultrasound or MRI), and histopathological evaluation was performed when necessary to ensure the liver disease was primarily attributable to HBV-related cirrhosis.
Ultimately, 1,076 patients met the inclusion criteria and formed the training cohort. An additional 328 patients treated between August 2018 and July 2023 were included as the test cohort (Figure 1). The index date was the time of OHE diagnosis, and outcomes were assessed as TF mortality or survival at the end of the 12-month follow-up period. The study complied with the Declaration of Helsinki Ethical Guidelines and was approved by the Ethics Committee of Beijing Ditan Hospital.
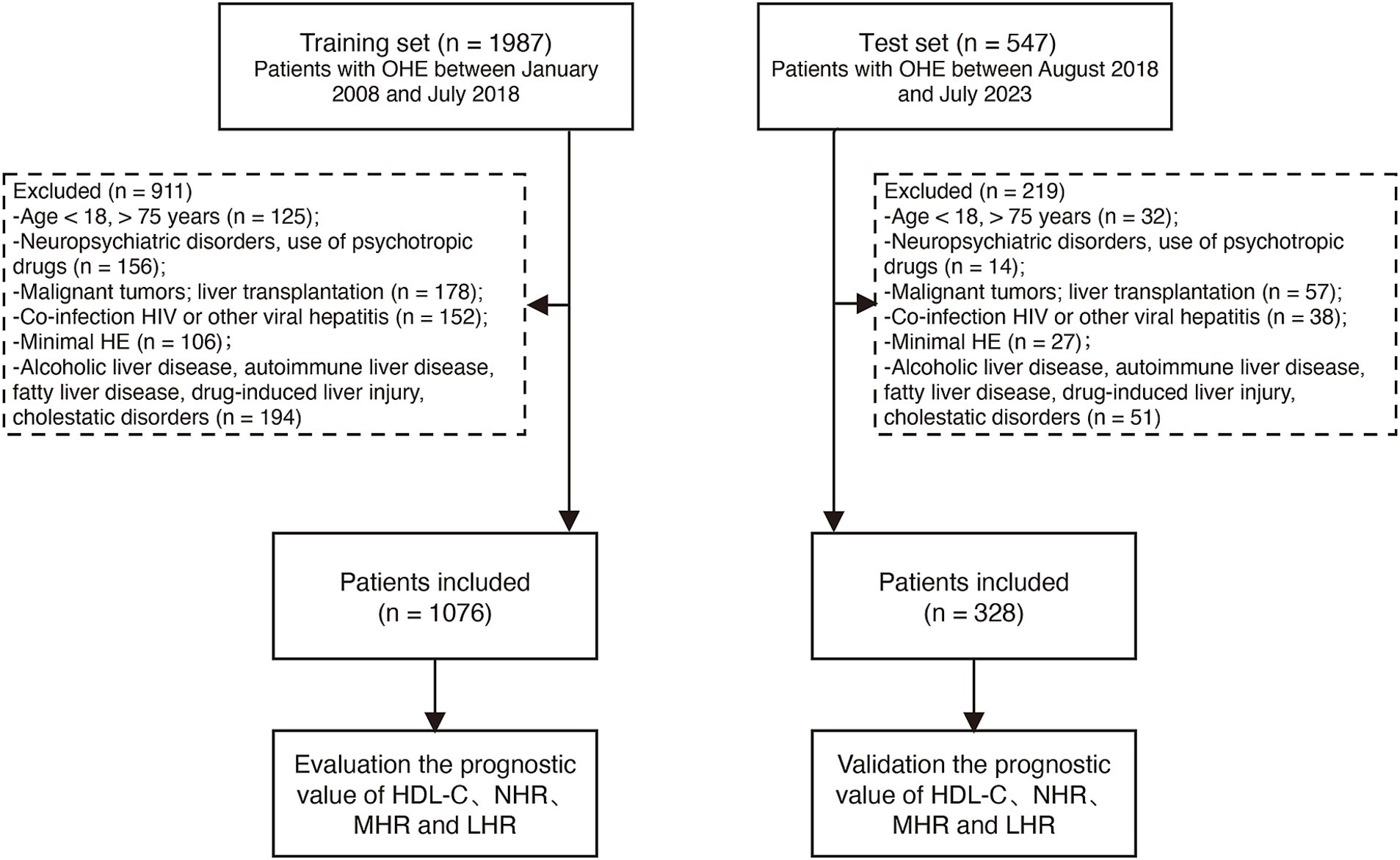
Figure 1. Participant flow in the study. HBV, hepatitis B virus; OHE, overt hepatic encephalopathy; HDL-C, high-density lipoprotein cholesterol; NHR, neutrophil-to-high-density lipoprotein cholesterol ratio; LHR, lymphocyte-to-high-density lipoprotein cholesterol ratio; MHR, monocyte-to-high-density lipoprotein cholesterol ratio.
Management of OHE was tailored according to the clinical classification of HE, the severity of symptoms, and the underlying liver disease status. Standard treatment included a combination of L-ornithine L-aspartate, lactulose, and/or rifaximin. L-ornithine L-aspartate was administered to enhance ammonia metabolism, lactulose was used to reduce ammonia levels by promoting its excretion, and rifaximin provided antimicrobial effects by decreasing ammonia production from gut bacteria. The selection and combination of therapies were guided by the severity of encephalopathy, the presence of comorbidities, and the patient’s response to treatment. This individualized approach aimed to optimize outcomes by addressing the multifactorial nature of OHE.
Baseline characteristics and biochemical data were obtained from the medical record system. These included patient demographics (age, sex), complications, and laboratory parameters such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), albumin (ALB), platelet count, international normalized ratio (INR), creatinine (Cr), neutrophils, lymphocytes, monocytes, total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). Ratios such as NHR, LHR, and MHR were calculated by dividing neutrophil, lymphocyte, and monocyte counts by HDL-C levels. The severity of liver disease was assessed using the Model for End-Stage Liver Disease (MELD) score.
Continuous variables were summarized as mean ± standard deviation (SD) or median with interquartile range (IQR), and comparisons between two groups were performed using the T-test or Mann–Whitney U test. Categorical variables were presented as frequencies and percentages, with group differences evaluated using chi-square or Fisher’s exact tests. Multivariate Cox regression analysis included variables with clinical relevance or statistical significance in univariate analysis (p < 0.05). A stepwise backward elimination approach was used to finalize the model, retaining only significant predictors. Adjusted hazard ratios (aHR) and 95% confidence intervals (CIs) were calculated, with significance defined as p < 0.05 (two-tailed). Statistical analyses were conducted using SPSS (version 25.0; SPSS Inc., Chicago, IL, USA).
The discriminatory power of NHR, LHR, MHR, and MELD scores was evaluated using the area under the receiver operating characteristic curve (AUC). Comparative analyses at 1, 3, and 12 months were performed using the DeLong test (22). A restricted cubic spline (RCS) with four knots (10th, 50th, 70th, and 90th percentiles of NHR) was employed to assess potential nonlinear relationships between NHR and prognosis (23). The importance of predictive indicators was analyzed using random forest models with tenfold cross-validation (24). Optimal cutoff values for NHR and MELD scores were determined using X-tile software. Survival rates were estimated with Kaplan–Meier curves using R software (version 4.2.2; The R Foundation, Vienna, Austria).
Among 1,404 patients, 1,132 deaths (80.6%) were attributed to liver-related causes, while 84 deaths (6.0%) were non-liver-related, with the remainder unclassified. Liver-related mortality included upper gastrointestinal bleeding and hemorrhagic shock (416 cases, 29.6%), multiple organ failure (351 cases, 25%), hepatic encephalopathy (116 cases, 8.3%), toxic shock from infection (212 cases, 15.1%), and liver failure (225 cases, 16%). Non-liver-related causes of death included heart failure (56 cases, 4%) and respiratory failure (28 cases, 2%).
Baseline clinical data for the training and test cohorts are shown in Table 1. The median age of patients was 55 years (IQR: 48–64), with 71.2% being male (1,000/1,404), and 25.2% having diabetes (358/1,404). Median NHR, LHR, and MHR were 7.5, 1.7, and 0.7, respectively. The 12-month TF mortality rates were 25.6% (276/1,076) in the training cohort and 24.3% (80/328) in the test cohort. Deceased patients were older, had a higher prevalence of variceal bleeding, infection, and ascites, and demonstrated elevated MELD scores, ALT, AST, TBIL, INR, Cr, NHR, LHR, and MHR levels, with significantly lower TC, ALB, LDL-C, and HDL-C levels (all p < 0.001, Table 2). Figures 2A–C illustrate differences in NHR, LHR, and MHR between survivors and deceased patients (all p < 0.001).
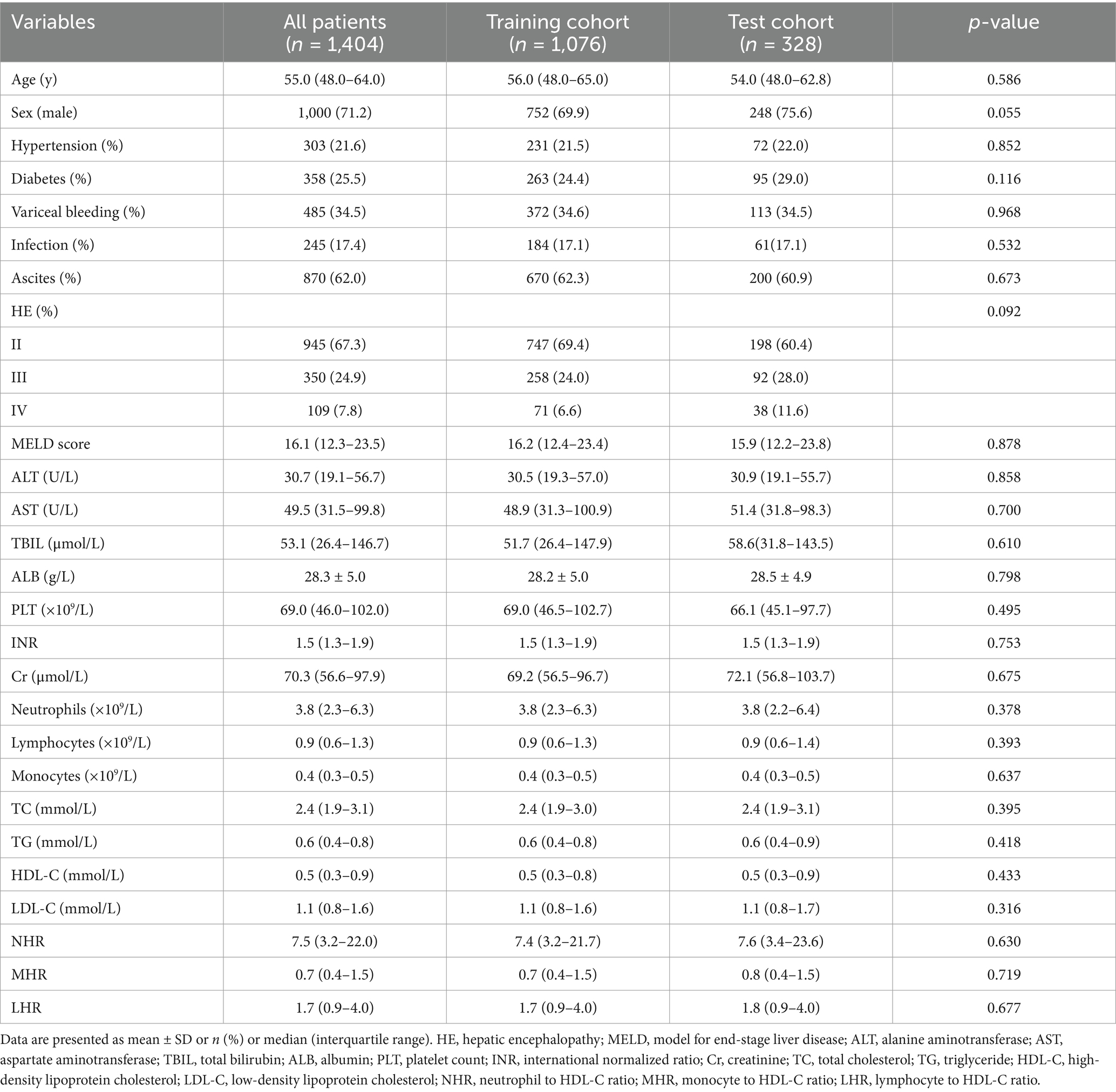
Table 1. Baseline demographics and clinical characteristics of patients with hepatitis B virus-related cirrhosis and overt hepatic encephalopathy in the training and test cohorts.
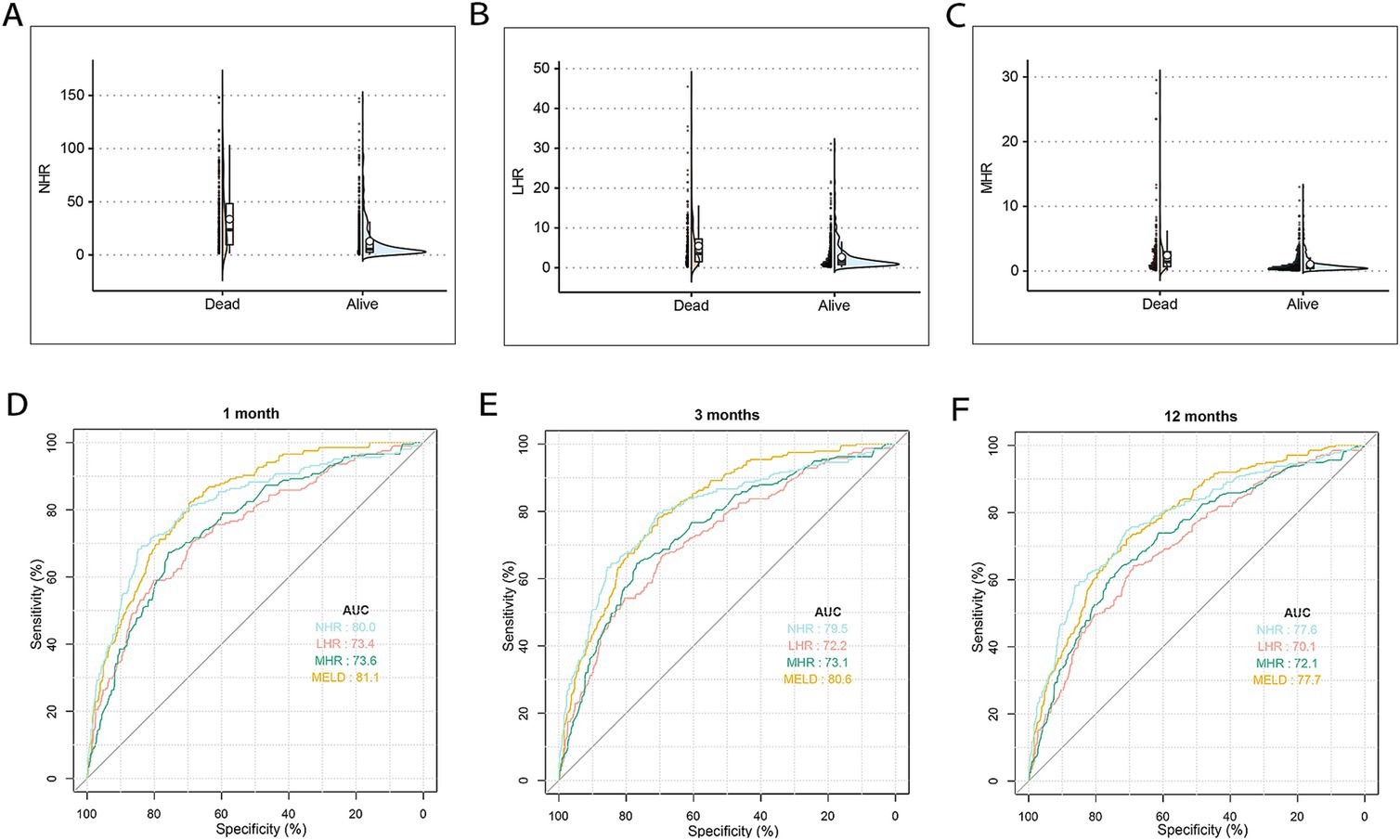
Figure 2. The differences and discrimination ability in NHR, LHR, and MHR between the surviving and deceased patients in the training cohort. (A) The difference in NHR between surviving and deceased patients. (B) The difference in LHR between surviving and deceased patients. (C) The difference in MHR between surviving and deceased patients. (D–F) The discrimination ability in NHR, MHR, LHR, and MELD score for TF mortality. ROC curves of NHR, MHR, LHR, and MELD score predicting 1 (D), 3 (E), and 12 months (F) TF mortality. MELD, model for end-stage liver disease; HDL-C, high-density lipoprotein cholesterol; NHR, neutrophil to HDL-C ratio; MHR, monocyte to HDL-C ratio; LHR, lymphocyte to HDL-C ratio; TF, transplant-free; ROC, receiver operating characteristic.
Univariate analysis identified significant associations between 12-month TF mortality and variables such as age, sex, variceal bleeding, infection, ascites, MELD score, NHR, LHR, MHR, ALT, AST, ALB, and TC (all p < 0.05). Multivariate Cox regression analysis revealed that age (HR = 1.015, 95% CI: 1.005–1.026; p = 0.009), MELD score (HR = 1.092, 95% CI: 1.077–1.107; p < 0.001), NHR (HR = 1.006, 95% CI: 1.002–1.011; p = 0.003), LHR (HR = 1.013, 95% CI: 1.001–1.025; p = 0.033), and ALB (HR = 0.970, 95% CI: 0.945–0.996; p = 0.024) were independent predictors of 12-month TF mortality (Table 3).
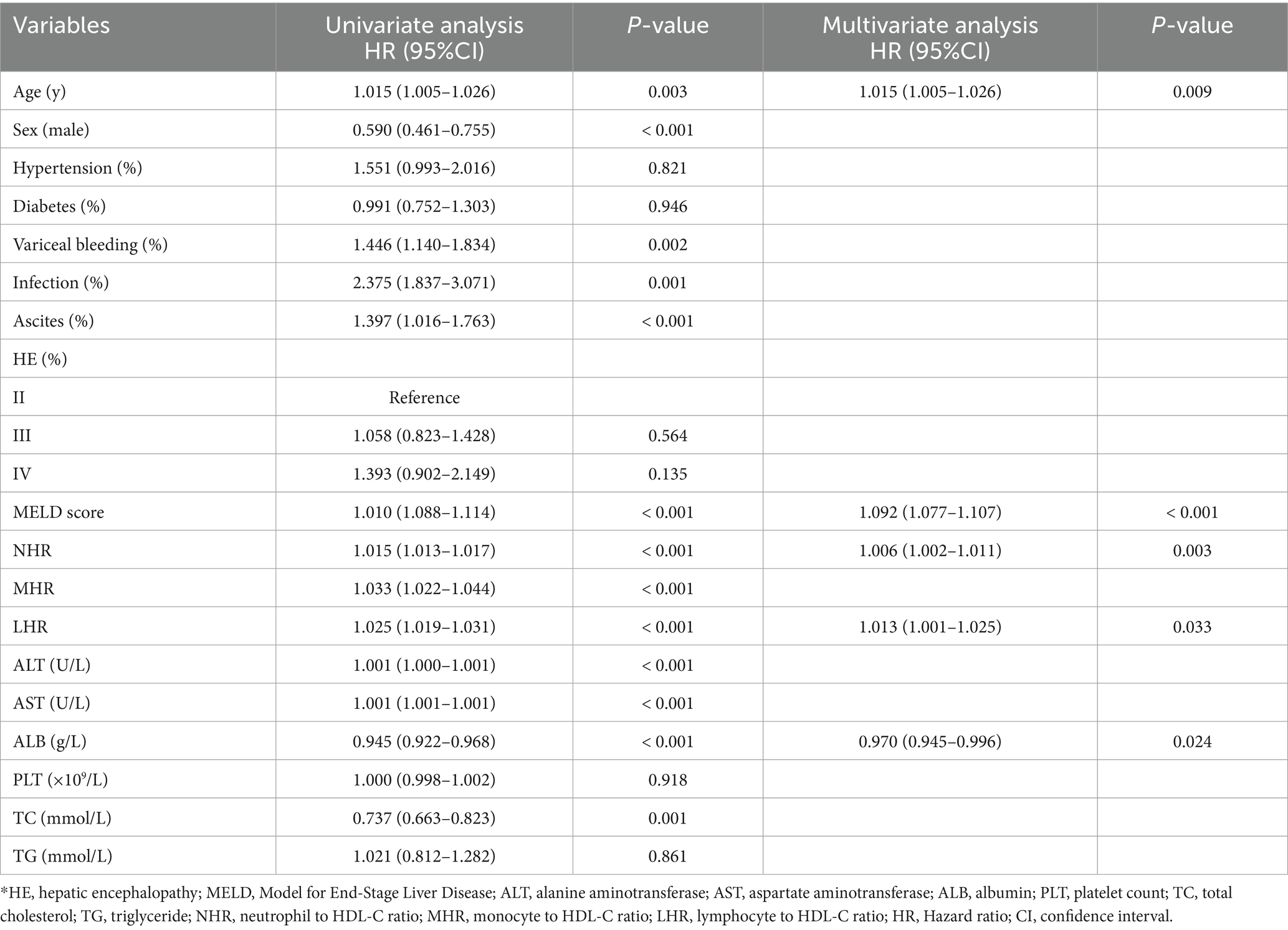
Table 3. Univariate and multivariate Cox hazards analysis for 12 months transplant-free mortality among patients with hepatitis B virus-related cirrhosis and overt hepatic encephalopathy.
The predictive performance of HDL-C levels, NHR, LHR, MHR, and MELD scores was evaluated using receiver operating characteristic (ROC) curves (Figures 2D–F). At 1, 3, and 12 months in the training set, NHR demonstrated predictive accuracy comparable to the MELD score, with AUC values of 0.800, 0.795, and 0.776 for NHR and 0.811, 0.806, and 0.777 for MELD, respectively. NHR outperformed LHR (AUC: 0.734, 0.722, and 0.701) and MHR (AUC: 0.736, 0.731, and 0.721) across the same time intervals (all p < 0.05). Random forest analysis further identified NHR as a key predictive factor, ranking second only to MELD and TBIL levels (Figure 3). These findings underscore NHR’s significance as a reliable prognostic marker.
Using X-tile software, the optimal cutoff values for 12-month TF mortality were determined to be 10 for NHR and 18 for MELD scores. Scatter plots illustrated that patients with NHR ≥ 10 and MELD ≥18 had the poorest prognosis (Figure 4A). Patients with MELD scores ≥18 exhibited higher rates of infection and ascites, as well as elevated ALT, AST, TBIL, INR, Cr, TC, NHR, and LHR levels compared to those with MELD <18 (all p < 0.001; Table 4). RCS analysis revealed a nonlinear relationship between NHR and 12-month TF mortality risk (unadjusted and adjusted, both p < 0.001; Figures 4B,C). Mortality risk was relatively low when NHR was <10 but increased significantly when NHR exceeded 10.
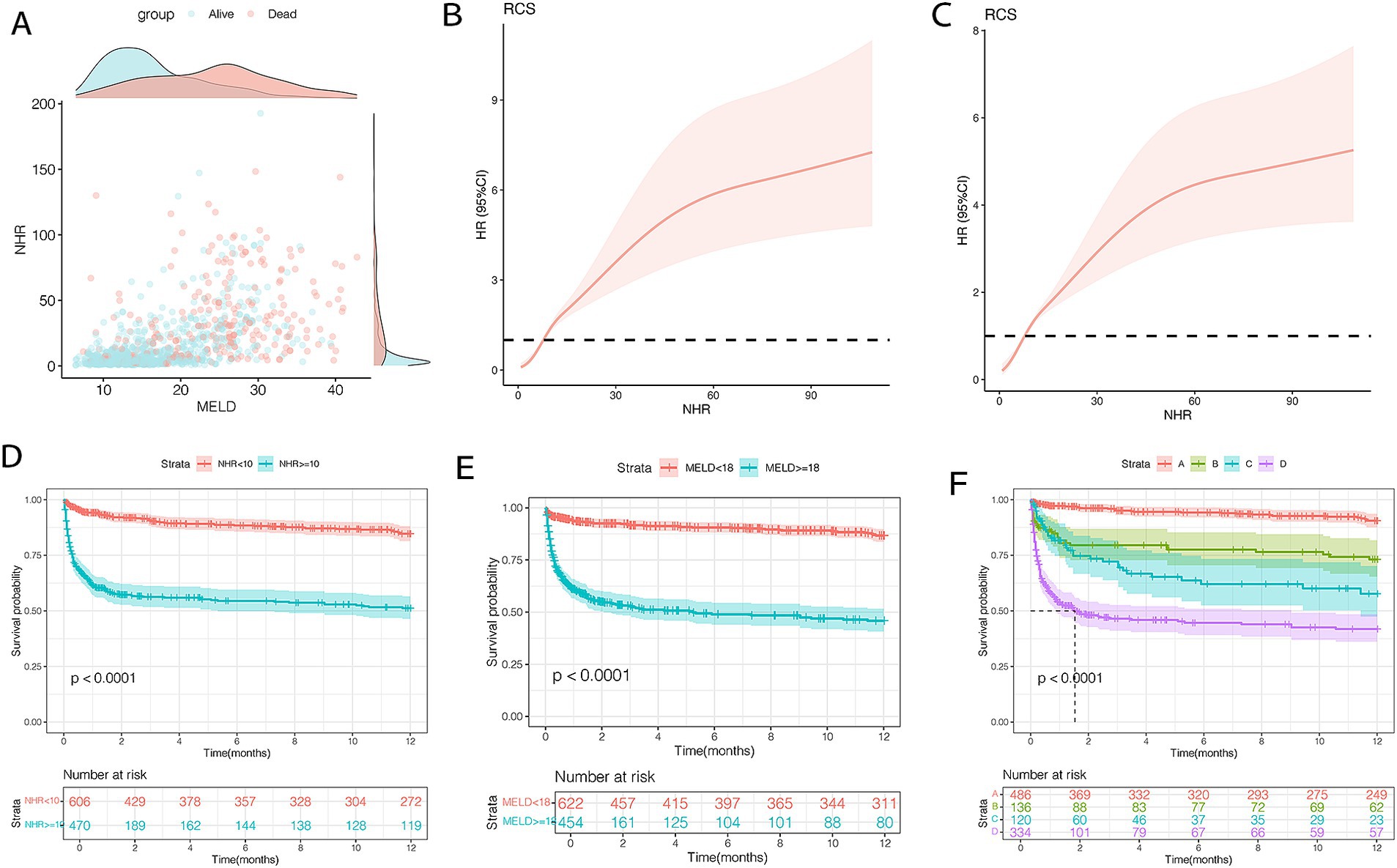
Figure 4. Association between NHR levels and mortality (A–C) and survival curves (D–F) of patients with HBV-related cirrhosis and OHE in the training cohort (n = 1,076). (A) The distribution of survival and death patients. (B)The association between NHR and 12-month TF mortality (unadjusted). (C) The association between NHR and 12-month TF mortality (adjusted). (D) Survival probability in patients with NHR < 10 (n = 609) and ≥ 10 (n = 467). (E) Survival probability in patients with MELD < 18 (n = 622) and ≥ 18 (n = 454). (F) Survival probability in the very low- (NHR < 10 and MELD <18, n = 486), low- (NHR ≥ 10 and MELD <18, n = 136), moderate- (NHR < 10 and MELD ≥18, n = 120), and high-risk (NHR ≥ 10 and MELD ≥18, n = 334) groups. HBV, hepatitis B virus; NHR, neutrophil to high-density lipoprotein cholesterol ratio; OHE, overt hepatic encephalopathy; MELD, model for end-stage liver disease; TF, transplant-free.
Kaplan–Meier analysis showed that patients with NHR < 10 had a 12-month mortality rate of 12.1%, compared to 43.2% for those with NHR ≥ 10 (p < 0.0001; Figure 4D). Similarly, mortality was 10.7% in patients with MELD scores <18 and 46.0% in those with MELD ≥18 (p < 0.0001; Figure 4E). Based on these thresholds, patients were classified into four risk groups: very low (NHR < 10, MELD <18), low (NHR ≥ 10, MELD <18), moderate (NHR < 10, MELD ≥18), and high (NHR ≥ 10, MELD ≥18). Mortality rates for the four groups were 7.2, 23.5, 30.8, and 51.4%, respectively (p < 0.0001; Figure 4F).
To assess mortality risk in subgroups, patients were stratified by age (< 55 or ≥ 55 years), sex, and OHE grades (II, III, or IV). Across all subgroups, patients with NHR ≥ 10 and MELD ≥18 (high risk) consistently had significantly higher 12-month mortality compared to those in the very low-risk group (NHR < 10, MELD <18), regardless of age (both p < 0.0001; Figures 5A,B). Among male patients, the 12-month mortality rates in the very low-, low-, moderate-, and high-risk groups were 5.3, 20.1, 22.5, and 47.8%, respectively (p < 0.0001; Figure 5C). Similar trends were observed among female patients (p < 0.0001; Figure 5D) and in patients with different OHE grades (p < 0.0001; Figures 5E,F).
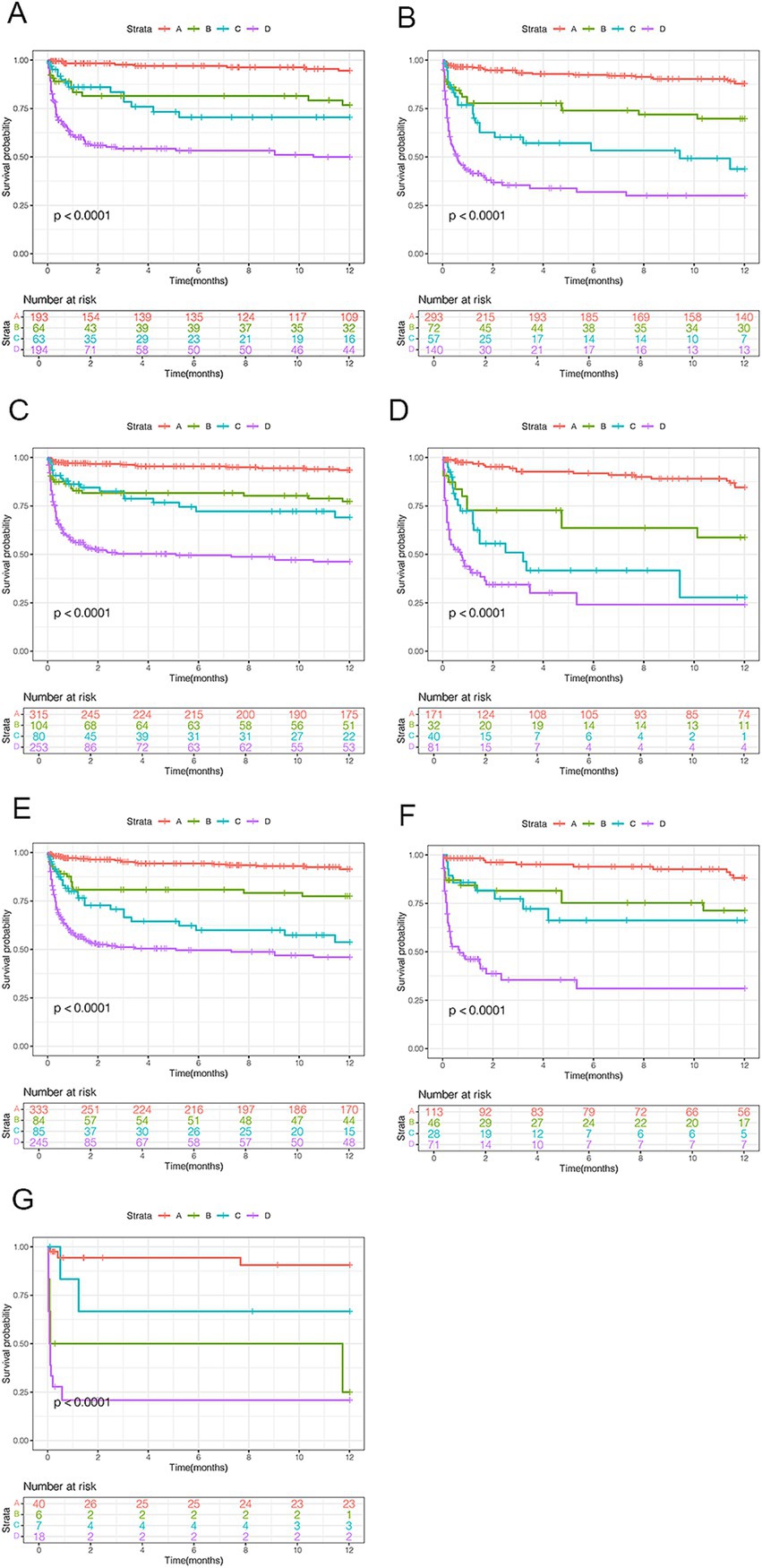
Figure 5. Survival analyses by clinical characteristics for patients with HBV-related cirrhosis and OHE. (A) Age < 55 years. (B) Age ≥ 55 years. (C) Male patients. (D) Female patients. (E–G) OHE grades II, III, and IV. HBV, hepatitis B virus; OHE, overt hepatic encephalopathy.
The AUCs of the MELD score and NHR were calculated at 1, 3, and 12 months (Figures 6A–C). MELD scores achieved the highest predictive accuracy at all time points (AUC: 0.824, 0.790, and 0.772), followed by NHR (AUC: 0.800, 0.771, and 0.751). Both metrics significantly outperformed LHR (AUC: 0.725, 0.694, and 0.668) and MHR (AUC: 0.729, 0.703, and 0.678) (all p < 0.05). Patients with NHR < 10 had a 12-month TF mortality rate of 15.1%, compared to 37.2% for those with NHR ≥ 10 (p < 0.0001; Figure 6D). Similarly, patients with MELD scores <18 exhibited lower mortality (10.7%) than those with MELD ≥18 (42.2%; p < 0.0001; Figure 6E). Among the 328 patients in the test cohort, the distribution across risk groups was as follows: 45.1% in very low risk, 11.9% in low risk, 11.9% in moderate risk, and 31.1% in high risk. Corresponding 12-month mortality rates were 8.7, 20.5, 30.7, and 46.0%, respectively (p < 0.001; Figure 6F).
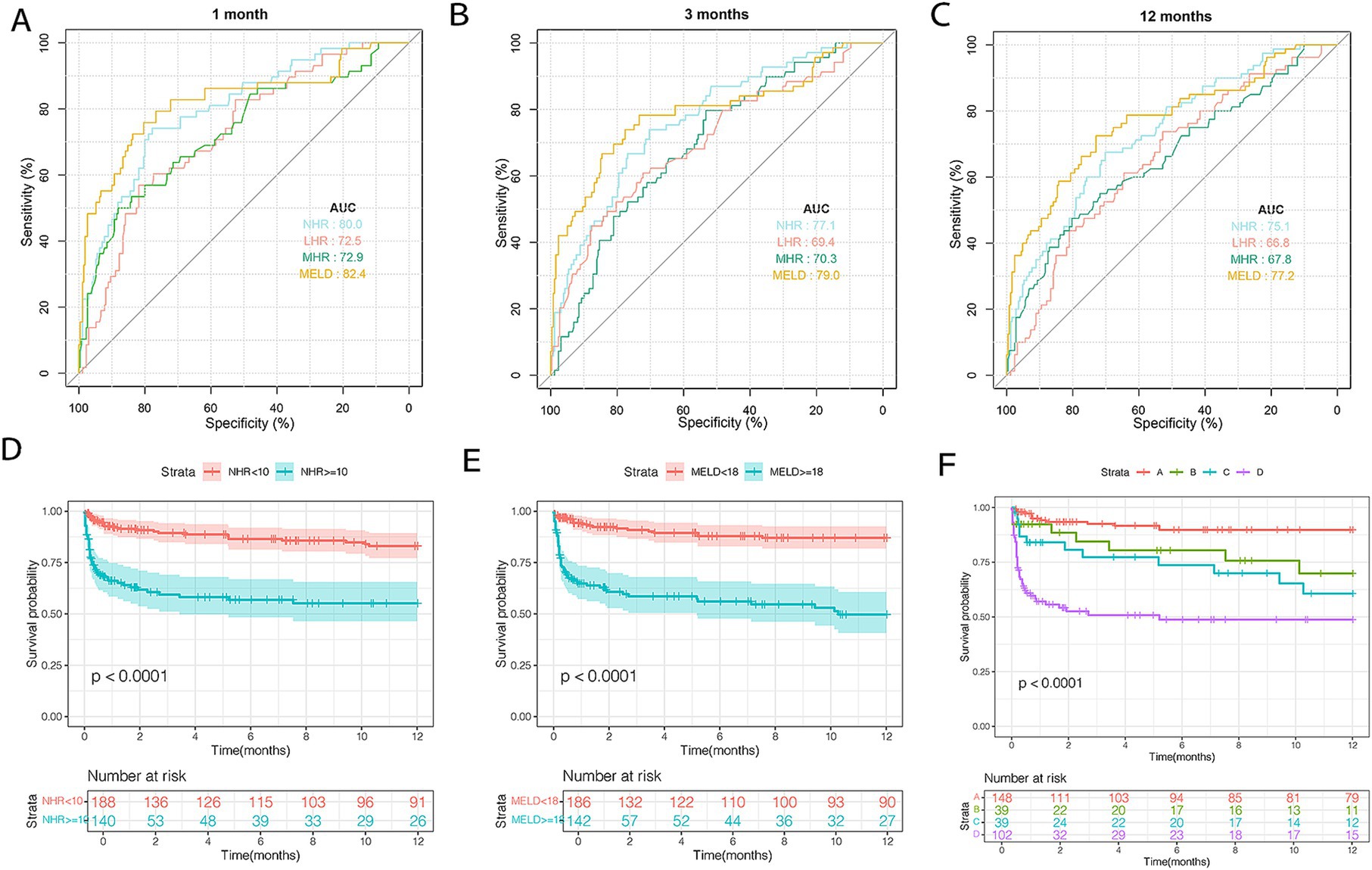
Figure 6. The discrimination ability of these indicators and risk stratification in the test cohort. ROC curves of NHR, MHR, LHR, and MELD score predicting 1 (A), 3 (B), and 12 months (C) TF mortality. (D) Survival probability in patients with NHR < 10 (n = 191) and ≥ 10 (n = 137). (E) Survival probability in patients with MELD < 18 (n = 186) and ≥ 18 (n = 142). (F) Survival probability in the very low- (NHR < 10 and MELD <18, n = 148), low- (NHR ≥ 10 and MELD <18, n = 39), moderate- (NHR < 10 and MELD ≥18, n = 39), and high-risk (NHR ≥ 10 and MELD ≥18, n = 102) groups. HDL-C, high-density lipoprotein cholesterol; NHR, neutrophil to HDL-C ratio; MHR, monocyte to HDL-C ratio; LHR, lymphocyte to HDL-C ratio; TF, transplant-free; MELD, model for end-stage liver disease.
OHE, a severe complication of cirrhosis, is associated with significant liver dysfunction and poor prognosis (25). Identifying reliable predictive biomarkers is crucial for informing patients and guiding timely interventions. In this study, we demonstrated that NHR is a practical, inexpensive, and effective lipid-related inflammatory biomarker. Compared to LHR and MHR, NHR exhibited superior predictive value for 12-month TF mortality in patients with HBV-related OHE, highlighting its potential role in clinical risk stratification.
The liver plays a central role in lipid metabolism, and liver dysfunction often leads to impaired lipid synthesis and clearance. In advanced liver diseases such as cirrhosis or liver failure, the liver’s ability to regulate lipids, particularly HDL cholesterol, is significantly reduced. Consequently, lower circulating HDL-C levels are commonly observed and are strongly associated with disease severity (11, 26). Disruption of lipid metabolism can also activate inflammatory responses (27). Clinical and lipidomic studies have linked HDL-C-related indicators and lipid mediators to inflammatory markers (11, 28). Inflammation and dyslipidemia play crucial roles in promoting liver dysfunction and the progression of cirrhosis (8). Ratios such as NHR, MHR, and LHR reflect the interplay between inflammatory responses and lipid changes (29). In this study, we found that NHR was independently associated with 12-month transplant-free (TF) mortality. NHR, which can be easily calculated from routine blood tests, demonstrated superior predictive accuracy compared to LHR and MHR at 1-, 3-, and 12-month intervals (all p < 0.05). Neutrophils, as the first immune cells to infiltrate the liver, play a key role in clearing dead and infected cells while producing cytokines that modulate monocytes and lymphocytes (30). This may explain why NHR outperforms MHR and LHR as a prognostic marker. These findings were validated in an independent dataset, highlighting the potential of NHR as a valuable tool for predicting mortality in patients with HBV-related OHE.
HBV-related OHE is associated with high hospitalization and mortality rates. Identifying high-risk individuals is critical for initiating timely treatment and reducing OHE-related mortality. The MELD score is a well-established prognostic tool that reflects liver function and predicts short-term mortality in advanced liver disease (31). Similarly, NHR, derived from routine blood tests, is closely linked to inflammation and poor prognosis in cirrhotic patients. Our findings showed that NHR demonstrated predictive ability comparable to the MELD score. This combination of heightened inflammation and impaired liver function suggests a unique pathophysiological mechanism requiring attention in pretransplant mortality risk assessment. Notably, patients with elevated MELD scores (≥18) and NHR (≥10) had the worst prognoses, as shown by scatter plot distributions. In the training cohort, four risk subgroups were identified based on NHR and MELD thresholds: very low-risk (NHR < 10, MELD <18), low-risk (NHR ≥ 10, MELD <18), moderate-risk (NHR < 10, MELD ≥18), and high-risk (NHR ≥ 10, MELD ≥18). Patients in the very low-risk group, accounting for approximately 45% of the cohort, had a mortality rate of 7.2%, indicating relatively stable liver function. Clinical management for this group may focus on regular follow-up and lifestyle modifications. In the low-risk group, elevated NHR indicated active inflammation and potential liver deterioration, even with lower MELD scores, suggesting the need for early anti-inflammatory interventions. The moderate-risk group exhibited significant liver impairment but lower inflammation levels, potentially reflecting immune suppression or inflammatory exhaustion in late-stage compensated cirrhosis. Close monitoring of liver function and complications is essential for these patients. Finally, the high-risk group, characterized by severe inflammation, lipid dysregulation, and liver dysfunction, requires comprehensive management targeting inflammation, lipid metabolism, and liver support. This stratification provides a precise prognostic assessment and informs individualized treatment strategies.
This study sheds light on the complex interactions among inflammation, lipid metabolism, and TF mortality in patients with HBV-related OHE. Bacterial infections and inflammation exacerbate immune dysfunction, accelerating liver deterioration and increasing mortality risk (32, 33). Conversely, HDL-C suppresses monocyte activation and transformation, thereby attenuating inflammatory responses. Inflammation alters HDL-C composition and functionality, while dysfunctional HDL-C further exacerbates cirrhosis progression and complications (26). NHR reflects the dynamic interplay between neutrophil activity and HDL-C levels. The liver regulates lipoprotein synthesis and degradation, while HDL inhibits neutrophil activation, proliferation, and migration (34). Abnormal neutrophil activation disrupts HDL-C composition and functionality, leading to increased neutrophil production and heightened mortality risk (35).
Despite its novel findings, this study has several limitations. First, the prediction of 1-year mortality based solely on baseline data may be biased by emerging clinical events or additional decompensation during follow-up. While our analysis focused on baseline characteristics, the dynamic nature of the disease must be acknowledged. Second, although this single-center study demonstrated and validated NHR as a prognostic marker, multicenter studies are needed to confirm its predictive value. Third, due to the retrospective design, patients received various treatments and combination therapies, and subgroup analyses based on treatment modalities were not performed. Future prospective studies should explore the impact of these indicators in different treatment settings to provide more comprehensive prognostic guidance. Finally, although confounding variables were adjusted to the greatest extent possible, some unmeasured factors may have influenced the results.
This cohort study supports NHR as a robust predictor of 12-month TF mortality in patients with HBV-related OHE. NHR ≥ 10 and MELD ≥18 effectively identify high-risk patients and provide valuable insights for optimizing clinical management. Further research is warranted to validate the prognostic utility of NHR in larger, multicenter studies.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by this study was approved by the Ethical Review Committee of the Beijing Ditan Hospital (Beijing, China). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
KS: Data curation, Methodology, Software, Writing – original draft. XOW: Data curation, Investigation, Methodology, Writing – original draft. ZY: Data curation, Methodology, Software, Validation, Writing – original draft. YL: Data curation, Formal analysis, Investigation, Supervision, Validation, Visualization, Writing – original draft. YF: Funding acquisition, Methodology, Resources, Software, Writing – review & editing. XNW: Conceptualization, Funding acquisition, Methodology, Resources, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the high-level Chinese Medicine Key Discipline Construction Project, No. zyyzdxk-2023005; Capital’s Funds for Health improvement and Research, no. 2024–1-2173; the Beijing Municipal Natural Science Foundation, no. 7232272; National Natural Science Foundation of China, No. 82474419 and 82474426; the Beijing Traditional Chinese Medicine Technology Development Fund Project, No. BJZYYB-2023-06.
We are grateful to Ting Zhao for her constructive comments in improving the grammar and readability of the paper.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1528733/full#supplementary-material
1. Zhou, RR, Song, YH, Xu, CY, Zhang, YY, Wu, XW, Zhang, L, et al. Altered counts and mitochondrial mass of peripheral blood leucocytes in patients with chronic hepatitis B virus infection. J Cell Mol Med. (2024) 28:e18440. doi: 10.1111/jcmm.18440
2. Rose, CF, Amodio, P, Bajaj, JS, Dhiman, RK, Montagnese, S, Taylor-Robinson, SD, et al. Hepatic encephalopathy: novel insights into classification, pathophysiology and therapy. J Hepatol. (2020) 73:1526–47. doi: 10.1016/j.jhep.2020.07.013
3. Sanyal, AJ, Kowdley, KV, Reau, NS, Pyrsopoulos, NT, Allen, C, Heimanson, Z, et al. Rifaximin plus lactulose versus lactulose alone for reducing the risk of HE recurrence. Hepatol Commun. (2024) 8:e0436. doi: 10.1097/HC9.0000000000000436
4. Guimarães, L, Piedade, J, Duarte, J, Baldin, C, Victor, L, Costa, B, et al. Hepatic encephalopathy in cirrhotic patients with bacterial infections: frequency, clinical characteristics, and prognostic relevance. J Clin Exp Hepatol. (2023) 13:559–67. doi: 10.1016/j.jceh.2023.01.004
5. Nardelli, S, Riggio, O, Marra, F, Gioia, S, Saltini, D, Bellafante, D, et al. Episodic overt hepatic encephalopathy after transjugular intrahepatic portosystemic shunt does not increase mortality in patients with cirrhosis. J Hepatol. (2024) 80:596–602. doi: 10.1016/j.jhep.2023.11.033
6. Weiss, E, de la Peña-Ramirez, C, Aguilar, F, Lozano, JJ, Sánchez-Garrido, C, Sierra, P, et al. Sympathetic nervous activation, mitochondrial dysfunction and outcome in acutely decompensated cirrhosis: the metabolomic prognostic models (CLIF-C MET). Gut. (2024) 72:1581–91. doi: 10.1136/gutjnl-2022-328708
7. Lin, YC, Swendeman, S, Moreira, IS, Ghosh, A, Kuo, A, Rosário-Ferreira, N, et al. Designer high-density lipoprotein particles enhance endothelial barrier function and suppress inflammation. Sci Signal. (2024) 17:eadg9256. doi: 10.1126/scisignal.adg9256
8. Trieb, M, Horvath, A, Birner-Gruenberger, R, Spindelboeck, W, Stadlbauer, V, Taschler, U, et al. Liver disease alters high-density lipoprotein composition, metabolism and function. Biochim Biophys Acta. (2016) 1861:630. doi: 10.1016/j.bbalip.2016.04.013
9. Engelmann, C, Clària, J, Szabo, G, Bosch, J, and Bernardi, M. Pathophysiology of decompensated cirrhosis: portal hypertension, circulatory dysfunction, inflammation, metabolism and mitochondrial dysfunction. J Hepatol. (2021) 75:S49–66. doi: 10.1016/j.jhep.2021.01.002
10. Tanaka, S, Couret, D, Tran-Dinh, A, Duranteau, J, Montravers, P, Schwendeman, A, et al. High-density lipoproteins during sepsis: from bench to bedside. Crit Care. (2020) 24:134. doi: 10.1186/s13054-020-02860-3
11. Trieb, M, Rainer, F, Stadlbauer, V, Douschan, P, Horvath, A, Binder, L, et al. HDL-related biomarkers are robust predictors of survival in patients with chronic liver failure. J Hepatol. (2020) 73:113–20. doi: 10.1016/j.jhep.2020.01.026
12. Lodge, S, Litton, E, Gray, N, Ryan, M, Millet, O, Fear, M, et al. Stratification of Sepsis patients on admission into the intensive care unit according to differential plasma metabolic phenotypes. J Proteome Res. (2024) 23:1328–40. doi: 10.1021/acs.jproteome.3c00803
13. Wang, Y, Shen, W, Huang, F, Yu, C, Xi, L, Gao, J, et al. HDL-C levels added to the MELD score improves 30-day mortality prediction in Asian patients with cirrhosis. J Int Med Res. (2022) 50:3000605221109385. doi: 10.1177/03000605221109385
14. Chen, G, Yang, N, Ren, J, He, Y, Huang, H, Hu, X, et al. Neutrophil counts to high-density lipoprotein cholesterol ratio: a potential predictor of prognosis in acute ischemic stroke patients after intravenous thrombolysis. Neurotox Res. (2020) 38:1001–9. doi: 10.1007/s12640-020-00274-1
15. Jiang, M, Sun, J, Zou, H, Li, M, Su, Z, Sun, W, et al. Prognostic role of neutrophil to high-density lipoprotein cholesterol ratio for all-cause and cardiovascular mortality in the general population. Front Cardiovasc Med. (2022) 9:807339. doi: 10.3389/fcvm.2022.807339
16. Guo, X, Shen, R, Su, Y, and Ma, L. High-density lipoprotein-related inflammatory indices predict repeat revascularization in coronary drug-eluting stenting. Biomark Med. (2023) 17:959–69. doi: 10.2217/bmm-2023-0399
17. Usta, A, Avci, E, Bulbul, CB, Kadi, H, and Adali, E. The monocyte counts to HDL cholesterol ratio in obese and lean patients with polycystic ovary syndrome. Reprod Biol Endocrinol. (2018) 16:34. doi: 10.1186/s12958-018-0351-0
18. Jiang, L, Zhong, Z, Huang, J, Bian, H, and Huang, W. Monocytohigh-density lipoprotein ratio has a high predictive value for the diagnosis of multiple system atrophy and the differentiation from Parkinson's disease, Monocytohigh-density lipoprotein ratio has a high predictive value for the diagnosis of multiple system atrophy and the differentiation from Parkinson's disease. Front Aging Neurosci. (2022) 14:1035437. doi: 10.3389/fnagi.2022.1035437
19. Shiha, G, Sarin, SK, Ibrahim, AE, Omata, M, Kumar, A, Lesmana, LA, et al. Liver fibrosis: consensus recommendations of the Asian Pacific Association for the Study of the liver (APASL). Hepatol Int. (2009) 3:323–33. doi: 10.1007/s12072-008-9114-x
20. Shi, K, Huang, Y, Zhang, Q, Ran, C, Hou, J, Zhang, Y, et al. A dynamic nomogram to predict transplant-free mortality in patients with hepatitis B-related cirrhosis and overt hepatic encephalopathy. Int Immunopharmacol. (2022) 108:108879. doi: 10.1016/j.intimp.2022.108879
21. Xu, XY, Ding, HG, Li, WG, Xu, JH, Han, Y, Jia, JD, et al. Chinese guidelines on the management of liver cirrhosis. World J Gastroenterol. (2020) 26:7088–103. doi: 10.3748/wjg.v26.i45.7088
22. DeLong, ER, DeLong, DM, and Clarke-Pearson, DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. (1988) 44:837–45. doi: 10.2307/2531595
23. Lee, DH, Keum, N, Hu, FB, Orav, EJ, Rimm, EB, Willett, WC, et al. Predicted lean body mass, fat mass, and all cause and cause specific mortality in men: prospective US cohort study. BMJ. (2018) 362:k2575. doi: 10.1136/bmj.k2575
24. Wang, W, Li, L, Gu, H, Chen, Y, Zhen, Y, and Dong, Z. Random Forest-based prediction of acute respiratory distress syndrome in patients undergoing cardiac surgery. Heart Surg Forum. (2022) 25:E854–9. doi: 10.1532/hsf.5113
25. Reed, V. Hepatic encephalopathy: diagnosis and treatment in advanced liver disease. Crit Care Nurs Clin North Am. (2022) 34:331–9. doi: 10.1016/j.cnc.2022.04.011
26. Zhang, Y, Chen, P, Zhang, Y, Nie, Y, and Zhu, X. Low high-density lipoprotein cholesterol levels predicting poor outcomes in patients with hepatitis B virus-related acute-on-chronic liver failure. Front Med. (2022) 9:1001411. doi: 10.3389/fmed.2022.1001411
27. Trovato, FM, Zia, R, Artru, F, Mujib, S, Jerome, E, Cavazza, A, et al. Lysophosphatidylcholines modulate immunoregulatory checkpoints in peripheral monocytes and are associated with mortality in people with acute liver failure. J Hepatol. (2023) 78:558–73. doi: 10.1016/j.jhep.2022.10.031
28. López-Vicario, C, Checa, A, Urdangarin, A, Aguilar, F, Alcaraz-Quiles, J, Caraceni, P, et al. Targeted lipidomics reveals extensive changes in circulating lipid mediators in patients with acutely decompensated cirrhosis. J Hepatol. (2020) 73:817–28. doi: 10.1016/j.jhep.2020.03.046
29. Huang, JB, Chen, YS, Ji, HY, Xie, WM, Jiang, J, Ran, LS, et al. Neutrophil to high-density lipoprotein ratio has a superior prognostic value in elderly patients with acute myocardial infarction: a comparison study. Lipids Health Dis. (2020) 19:59. doi: 10.1186/s12944-020-01238-2
30. Rosales, C. Neutrophils at the crossroads of innate and adaptive immunity. J Leukoc Biol. (2020) 108:377–96. doi: 10.1002/JLB.4MIR0220-574RR
31. Ruf, A, Dirchwolf, M, and Freeman, RB. From child-Pugh to MELD score and beyond: taking a walk down memory lane. Ann Hepatol. (2022) 27:100535. doi: 10.1016/j.aohep.2021.100535
32. Shawcross, DL, Sharifi, Y, Canavan, JB, Yeoman, AD, Abeles, RD, Taylor, NJ, et al. Infection and systemic inflammation, not ammonia, are associated with grade 3/4 hepatic encephalopathy, but not mortality in cirrhosis. J Hepatol. (2011) 54:640–9. doi: 10.1016/j.jhep.2010.07.045
33. Helil, AS, Haile, SA, Birhanu, Y, Desalegn, H, Desalegn, DM, Geremew, RA, et al. Bacterial profile, drug resistance pattern, clinical and laboratory predictors of ascites infection in cirrhosis patients. BMC Infect Dis. (2024) 24:528. doi: 10.1186/s12879-024-09418-6
34. Jia, C, Anderson, JLC, Gruppen, EG, Lei, Y, Bakker, SJL, Dullaart, RPF, et al. High-density lipoprotein anti-inflammatory capacity and incident cardiovascular events. Circulation. (2021) 143:1935–45. doi: 10.1161/CIRCULATIONAHA.120.050808
Keywords: hepatitis B virus, hepatic encephalopathy, model end-stage liver disease score, inflammatory, high density lipoprotein cholesterol
Citation: Shi K, Wang X, Yi Z, Li Y, Feng Y and Wang X (2025) Inflammatory lipid biomarkers and transplant-free mortality risk in hepatitis B-related cirrhosis and hepatic encephalopathy. Front. Med. 12:1528733. doi: 10.3389/fmed.2025.1528733
Received: 15 November 2024; Accepted: 08 January 2025;
Published: 23 January 2025.
Edited by:
Krzysztof Tomasiewicz, Medical University of Lublin, PolandReviewed by:
Simona Parisse, Sapienza University of Rome, ItalyCopyright © 2025 Shi, Wang, Yi, Li, Feng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianbo Wang, d2FuZ3hiQGNjbXUuZWR1LmNu; Ying Feng, ZmVuZ3lpbmdAY2NtdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.