
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 06 March 2025
Sec. Nephrology
Volume 12 - 2025 | https://doi.org/10.3389/fmed.2025.1517019
This article is part of the Research TopicEarly Diagnosis of Kidney Disease in Young AdulthoodView all 4 articles
Objectives: This study aimed to analyze the potential of the estimated protein excretion rate (ePER) as a substitute for the spot urinary protein-creatinine ratio (uPCR) in clinical reports for accurately assessing urinary protein excretion in China.
Methods: We included 1721 patients in the study and compared the differences in levels, correlation, bias, methodological evaluation between uPCR, ePER, and 24-h urinary protein.
Results: Significant differences (Z = −17.568, p < 0.001) were found between uPCR and 24-h urine protein levels in all cases. However, no statistically significant difference (Z = −0.652, p = 0.514) was found between ePER and 24-h urine protein. The bias analysis revealed that the negative bias rate between ePER and 24-h urine protein was −4.33%, significantly lower compared to uPCR (−30.88%). Incorporating ePER significantly boosted its sensitivity to 91.3% in this cohort. Furthermore, ePER demonstrated a higher correlation (r = 0.74, p < 0.001) and kappa consistency (κ = 0.802, p = 0.015) with 24-h urinary protein compared to uPCR (r = 0.71, p < 0.001; κ = 0.737, p = 0.016). However, in the >65 age group, those with estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73m2 group and spot urinary creatinine <500 mg/L exhibited a higher ePER bias compared to uPCR.
Conclusion: These findings highlight the potential of ePER as a valuable tool for accurately assessing urinary protein excretion. Nonetheless, its limitations should be considered, especially in specific patient populations.
Proteinuria is a primary symptom of kidney disease. Evaluating proteinuria aids in diagnosis, disease monitoring, and treatment effectiveness assessment (1, 2). The “gold standard” method for the assessment of proteinuria involves quantitative determination of protein concentration in a 24-h urine collection, termed “timed urine collection.” Protein fluctuation during the day has only a slight impact on the results. However, the conventional method of quantifying protein concentration in a 24-h urine collection is time-consuming, troublesome for patients, and prone to errors such as accidental contamination, which can significantly affect its accuracy (3–5). An alternative, quick, and simple method for quantitatively evaluating urinary protein excretion may be the assessment of spot (random) urine protein or the albumin-to-creatinine ratio (uPCR/uACR). This method uses creatinine concentration in a spot urine sample as an internal control and offers a faster and simpler alternative to timed urine collection.
Literature confirms that evaluating uPCR/uACR in a single urine sample is a viable alternative to 24-h urine collection for assessing proteinuria, especially in patients with renal insufficiency (6–8). However, uPCR/uACR has limitations as it may vary according to age, sex, weight, and muscle mass (9, 10). Ix et al. developed a new equation to estimate creatinine excretion rate (eCER) based on age, sex, weight, and race (11). This equation utilizes commonly available variables and exhibits minimal bias and moderate precision, making it useful for assessing the accuracy of timed urine collection. However, weight as a predictor variable cannot be automatically applied to laboratory reports. In a separate study, Fotheringham et al. derived and validated a creatinine excretion rate (CER) estimation equation relying solely on age, sex, and race (12). The formula not only confirmed the validity of the equation but also demonstrated its superiority over uPCR and uACR in measuring protein and albumin excretion rates from timed 24-h urine collection. The formula was more convenient for clinical application, but regrettably, there is no Chinese population in the selected objects of this study. Racial and ethnic disparities represent a substantial factor influencing the evaluation of urinary protein. Research has shown that variations in urinary creatinine excretion are linked to race and ethnicity. More specifically, the uPCR may systematically underestimate 24-h proteinuria in Black and Hispanic individuals, while overestimating it in White individuals (13). Due to the large population in China and the significant number of complex cases among kidney disease patients, so it is important to verify the validity of this formula in Chinese population for clinical application.
In our study, our objective was to investigate whether ePER offers advantages compared to uPCR and to explore the potential application of ePER in clinical reports, resulting in a more accurate evaluation of urinary protein excretion in patients with kidney disease in China.
This is a prospective study utilizing historical data. This clinical study was conducted at the First Affiliated Hospital of Zhengzhou University between April 2020 and September 2020, encompassing a total of 1721 patients diagnosed with various kidney diseases. All patients during this period utilized the same batch of uPCR reagents and 24-h urine protein assay reagents, in conjunction with consistent internal quality control procedures, thereby minimizing potential systematic errors across the datasets. The inclusion criteria required morning spot urine samples collected on the same day that patients completed the 24-h urine collection while patients without kidney disease were excluded. The Ethics Review Committee of the First Affiliated Hospital of Zhengzhou University approved this study (approval ID: 2023-KY-0810) according to institutional guidelines and waived the requirement to obtain informed consent due to its non-interventional nature.
We reviewed baseline demographics, clinical, and laboratory data, extracting information on age, sex, eGFR, and body mass index (BMI) from medical records. eGFR (mL/min/1.73m2) for males = 141 × [SCr (mg/dL)/0.9]−0.411 × 0.993age if SCr < 0.9 mg/dL, 141 × [SCr (mg/dL)/0.9]−1.209 × 0.993age if SCr > 0.9 mg/dL; eGFR (mL/min/1.73m2) for females = 144 × [SCr (mg/dL)/0.7]−0.329 × 0.993age if SCr < 0.7 mg/dL, 144 × [SCr (mg/dL)/0.7]−1.209 × 0.993age if SCr > 0.7 mg/dL (14).
The ePER and eAER were calculated by multiplying the uPCR and uACR by the eCER. The eCER was determined using the following formula: eCER (mg/24 h) = 1307.3 + (23.1 × age) − (0.3 × age^2) for males and 1051.1 + (5.3 × age) − (0.1 × age^2) for females (12).
Basic information including sex, age, diseases, BMI, eGFR, spot urine creatinine, and proteinuria between groups was compared, with continuous variables tested for normality. Categorical data were expressed as percentages, while continuous variables were described as mean ± standard deviation (SD) or median (interquartile range) depending on normality. The Chi-square test was employed for categorical data analysis, while variables with non-normal distribution were assessed using the Wilcoxon signed-rank or Mann–Whitney U test. Bias was assessed as the median difference between 24-h urine protein and uPCR or ePER. Linear regression analysis, along with Spearman’s correlation coefficient (r) (15), was conducted to explore the correlation among uPCR, ePER, uACR, eAER, and 24-h urine protein or albumin concentration. In general, the following regression coefficients were utilized to determine the degree of correlation: 0.7–1.0 denotes a strong correlation, 0.4–0.7 indicates a slight correlation, 0.2–0.4 signifies a weak correlation, and 0–0.2 suggests almost no correlation. Cohen’s kappa coefficient (κ) (16) was calculated to evaluate the consistency between uPCR, ePER, uACR, eAER, and 24-h urine protein or albumin. The κ coefficient was employed to define the level of agreement: < 0 suggests poor agreement, 0–0.20 denotes minimal agreement, 0.21–0.40 represents fair agreement, 0.41–0.60 indicates moderate agreement, 0.61–0.80 signifies substantial agreement, and 0.81–1.0 implies almost perfect or perfect agreement. Bias was calculated as the median difference between uPCR, ePER, uACR, eAER, and 24-h urine protein or albumin levels. The sensitivity and specificity for detecting severely increased proteinuria and albuminuria (17) were evaluated using cut-off points of 1,000 mg/24 h and 300 mg/24 h, respectively. A proteinuria threshold of 1,000 mg/24 h previously has been recommended as an indication for lower blood pressure targets (18).
All statistical analyses were performed using SPSS version 26 software (IBM Corp., Armonk, NY, United States), with statistical significance defined as p < 0.05.
A population sample comprising 1721 individuals aged 9 to 93 years was included in this study. Among them, 539 were Membranous nephropathy (MN), 110 were Lupus nephritis (LN), 132 were Minimal change disease (MCD), 107 were Diabetic nephropathy (DN), 252 were IgA nephropathy (IgAN), 311 were chronic kidney disease (CKD), and 270 were classified as others. The median age of participants was 47 years. Descriptive statistics for the study cohort are presented in Table 1.
The levels of uPCR and 24-h urine protein showed significant differences (Z = −17.568, p < 0.001) across all cases in terms of urinary protein levels. However, there was no significant difference (Z = −0.652, p = 0.514) between ePER and 24-h urine protein levels. We evaluated the bias, sensitivity, specificity, consistency, and correlation of uPCR, ePER, uACR, and eAER against 24-h urine protein as the reference standard in the datasets (Table 2). Bias analysis revealed a negative bias rate of −30.88% between uPCR and 24-h urine protein, while the bias rate reduced to −4.33% between ePER and 24-h urine protein. In the methodological evaluation, although ePER (89.1%) marginally compromised the specificity of urine protein assessment compared with uPCR (92.5%), ePER (91.3%) significantly enhanced the sensitivity relative to uPCR (83.1%) in this cohort. Additionally, the κ coefficient of ePER (κ = 0.802, p = 0.015) was higher than uPCR (κ = 0.737, p = 0.016) in kappa consistency analysis. Similar results were found in ACR versus eAER.
To assess how effectively ePER perform compared to uPCR in evaluating urinary protein excretion and their influencing factors, we divided the results into two groups: improved and non-improved (Table 3). We defined the improved group as cases where |24-h urine protein – ePER| < |24-h urine protein – uPCR|. Conversely, the non-improved group included cases where |24-h urine protein – ePER| > |24-h urine protein – uPCR|. We then compared these two groups (there were no data with the same results between uPCR and ePER in this study cohort). As indicated in Table 3, we found no significant differences in gender (χ2 = 0.120, p < 0.729) and 24-h protein values (Z = −0.914, p = 0.361) between the two groups. However, significant differences emerged in age (Z = −5.436, p < 0.001), BMI (Z = −3.198, p = 0.001), eGFR (Z = −12.192, p < 0.001), and spot urine creatinine levels (Z = −16.712, p < 0.001) between them. Similar results were observed for urinary albumin excretion (Supplementary Table 1).
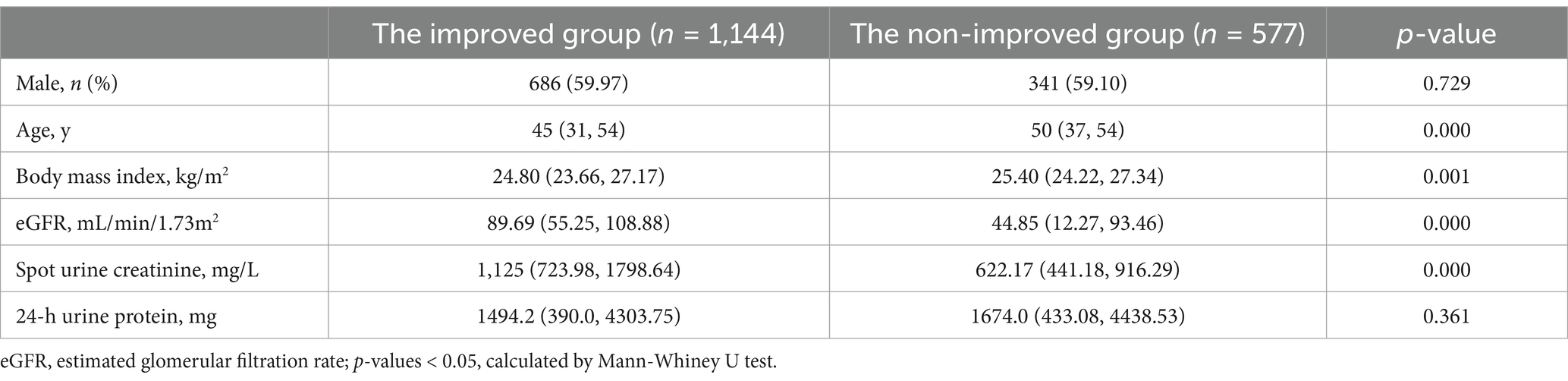
Table 3. The comparison between the improved group and the non-improved group in the urinary protein excretion.
We compared uPCR and ePER with 24-h urinary protein levels across various groups, as presented in Table 4. The results showed that in males (Z = −18.875, p < 0.001) and females (Z = −3.392, p = 0.001), < 18 years old (Z = −4.505, p < 0.001), BMI < 24 kg/m2 (Z = −11.636, p < 0.001) and 24–28 kg/m2 (Z = −10.495, p < 0.001), eGFR 30–59 mL/min/1.73 m2 (Z = −5.669, p < 0.001) and 60–89 mL/min/1.73 m2 (Z = −9.646, p < 0.001) groups, uPCR and 24-h urine protein were statistically significant. While no statistically significant differences were found between ePER and 24-h urinary protein levels in these groups. Interestingly, there were statistically significant difference between ePER and 24-h urinary protein in age > 65 years (Z = −6.534, p < 0.001), BMI > 28 kg/m2 (Z = −2.397, p = 0.017), eGFR <30 mL/min/1.73 m2 (Z = −11.130, p < 0.001) groups. But there was no significant difference between uPCR and 24-h urinary protein levels. The correlation among uPCR, ePER, and 24-h urine protein levels was meticulously analyzed in each group (Table 4). In the male group, uPCR exhibited a slight correlation with 24-h urinary protein (r = 0.694, p < 0.001), as did the eGFR 60-89 mL/min/1.73 m2 group (r = 0.699, p < 0.001). However, ePER demonstrated a strong correlation with 24-h urinary protein levels in both groups, with correlation coefficients of 0.709 (p < 0.001) and 0.763 (p < 0.001), respectively. Furthermore, the correlation between both uPCR and ePER with 24-h urinary protein was strong, except for the age > 65 years and eGFR <30 mL/min/1.73 m2 group, where only a slight correlation was observed. Additionally, the magnitude of the correlation coefficient between ePER and 24-h urinary protein consistently exceeded that of uPCR in almost all groups, except for the >65 years group. We performed the same analysis for urinary albumin excretion (Supplementary Table 2).
We conducted a bias analysis and methodological evaluation of outcomes obtained from uPCR and ePER compared with 24-h urine protein measurements across various study cohorts (Supplementary Table 3). Based on bias analysis, ePER demonstrated a lower bias rate than uPCR in most groups. For instance, the uPCR exhibited a bias rate of −40.14% for males and −14.02% for females, while the ePER showed a reduced bias rate of merely −0.47% and − 9.35%, respectively. However, the bias rates of ePER compared to uPCR were increased in age > 65 years (26.50% vs. 5.63%), BMI > 28 kg/m2 (6.72% vs. 1.32%), eGFR <30 mL/min/1.73 m2 (32.34% vs. 0.90%), and spot urine creatinine <500 mg/L (45.92% vs. −15.91%) groups.
Regarding methodological evaluation, the utilization of ePER led to a slight reduction in specificity but significantly enhanced sensitivity in urine protein assessment across nearly all cohorts. The κ coefficients of ePER showed higher values across most groups compared to uPCR, indicating substantial or nearly perfect consistency with 24-h urinary protein measurements. However, when considering individuals with an eGFR <30 mL/min/1.73 m2, the κ coefficient indicated only moderate agreement between uPCR (κ = 0.587, p = 0.056) or ePER (κ = 0.580, p = 0.059) and the gold standard for assessing urinary protein levels. We performed the same analysis for urinary albumin excretion (Supplementary Table 4).
Quantifying proteinuria is crucial for clinically assessing patients with kidney diseases due to its strong correlation with renal prognosis (19–22). While the gold standard remains 24-h urine protein measurement, random uPCR is commonly used due to the challenges associated with collecting complete 24-h urine samples. The existing literature has consistently demonstrated a strong correlation between uPCR and 24-h urinary protein levels (23, 24). However, some studies have shown that the two are only moderately correlated and consistent (25). Studies have also highlighted clinically unacceptable deviations between 24-h urinary protein levels and uPCR values (26). Therefore, a new method using spot urine protein and creatinine values that is able to minimize under or over estimation is still warranted. In our study, we demonstrated the potential of ePER as a valuable tool for accurately assessing urinary protein excretion. This research validates the effectiveness of ePER in optimizing uPCR for evaluating urinary protein excretion in a Chinese population. Despite certain limitations, ePER effectively mitigated discrepancies between uPCR and 24-h urine protein levels.
In this study, we compared the uPCR using the newly calculated ePER based on a formula (12). Our results confirm that this formula can also optimize uPCR results in most cases in the Chinese population. The application of ePER not only significantly reduced the discrepancy between uPCR and 24-h urine protein levels but also improved relevance and consistency. Methodological evaluation showed that the utilization of ePER led to a slight decrease in specificity while significantly enhancing the sensitivity of urine protein assessment across nearly all cohorts. These findings align with those of a previous study (12). Based on the data from this study, the ePER derived from an age- and sex-based formula appears to mitigate the confounding effect of sex, allowing both males and females to effectively minimize the disparity between uPCR and 24-h urinary protein levels. Additionally, no statistically significant difference was found in the 24-h protein content between the improved and non-improved groups, suggesting that changes in urinary protein levels did not affect the optimization effect of ePER on uPCR.
In this study, the use of ePER greatly improved the uPCR results in the group under 65 years of age, and the difference between uPCR and 24-h urinary protein was greatly reduced, especially in adolescent patients under 18 years of age. However, despite using a formula derived from age and sex optimization to calculate ePER, the impact of age remains partially unaccounted for in the findings of this study. In this study, it is evident that ePER does not completely mitigate the influence of age and is not applicable to patients aged >65 years, in contrast to the findings of Fotheringham et al. (12). These contradictory results on the one hand may arise from disparities in population selection, with the equation derivation and validation cohorts of previous study (12) containing only a small number of participants older than 70 years and none older than 80 years, and on the other hand may be related to disease characteristics, treatment approaches, and sample size.
For BMI group comparison, uPCR results were more affected at low BMI, and the bias rate from 24-h urine protein was the largest, up to −46.12%. However, the effect of this factor was well reduced by the use of ePER, and the bias rate was less than −10%. Although ePER did not play an optimization role in BMI > 28 kg/m2, the bias rate was only 6.72%. Unfortunately, a previous study (12) did not consider grouping BMI, nor did it provide analytical discussions on these subgroups.
In a comparative study of urinary protein excretion in the eGFR group, uPCR had the best correlation with 24-h urinary protein in the eGFR 30–59 mL/min/1.73m2 group and a moderate correlation in the eGFR <30 mL/min/1.73m2 group. This is consistent with the findings of Ahmed et al.[21], moderate correlation (r = 0.535) was seen in patients with advanced renal failure (eGFR <15 mL/min/1.73m2). However, it is worth noting that the bias rates of uPCR and 24-h urine protein were positively correlated with eGFR values. The bias rate of the eGFR <30 mL/min/1.73m2 group was only 0.9%, which was much lower than that of other eGFR groups. The use of ePER not only increased the correlation with 24-h urine protein, but also significantly reduced the bias rate in these groups, especially for patients with eGFR 30–89 mL/min/1.73m2. But unfortunately, when eGFR was <30 mL/min/1.73 m2, the application of ePER did not improve uPCR results, which is consistent with results previously reported by other researchers (12).
Additionally, when spot urine creatinine levels <500 mg/L, ePER performs less effectively compared to uPCR. However, when spot urine creatinine >500 mg/L, ePER played a different degree of optimization, especially when spot urine creatinine was 500–1,000 mg/L. In this study, the reasons why ePER was not optimized were related to old age, obesity, low eGFR, and low urinary creatinine point. However, the main factor may be related to urinary creatinine excretion rate, because more than half of the patients in the no-improvement group had spot urine creatinine level < 500 mg/L. And other indicators also more or less affected the urinary creatinine excretion rate. Previous investigations have shown a correlation between age and urinary creatinine excretion rate, with a decline observed as age increases (27, 28). Taylor et al. found that urinary creatinine excretion rate increased with BMI (29), and Negri et al. reached the same conclusion for both men and women (30). Moreover, previous studies have suggested that individuals with lower eGFR tend to have lower urinary creatinine excretion rates (8, 30, 31). To validate our study, we recruited 244 additional patients with positive urine protein and tested their urine protein and creatinine in morning urine and 24-h urine (As some studies are still ongoing, complete data are not yet available). We conducted a preliminary analysis of the data from this batch of patients and found that ePER (6.46%) had the lowest bias rate and was more favorable than the 24-h uPCR (−11.49%). Through this data analysis, we were able to validate the research findings presented in this study.
In this study, the implementation of ePER effectively reduced the disparity between uPCR and 24-h urinary protein levels. However, certain limitations were identified. The current study demonstrated that ePER performed better than uPCR in assessing urinary protein excretion in most cases, laying the groundwork for its future practical application of in China. However, uPCR showed certain advantages in specific subgroups, with limited optimization by ePER, displaying a superior bias rate, correlation, and consistency. This indicates that a combined approach using both ePER and uPCR may be more suitable in clinical practice at present.
Unfortunately, 24-h urinary creatinine data were unavailable for this analysis due to its non-routine use in renal function assessment, a limitation of the study. In future practice, we will prioritize incorporating 24-h urine creatinine into our protocols and compare uPCR, ePER, and 24-h urine protein excretion using an adequate database to enhance renal function evaluations. This effort is designed to identify a simple and reliable protein excretion test that can potentially eliminate the need for 24-h urine collection. We believe ePER would become a powerful tool for kidney diseases screening.
In conclusion, this study validates the effectiveness of ePER in optimizing uPCR for evaluating urinary protein excretion in a Chinese population. Despite some limitations, ePER effectively reduced disparities between uPCR and 24-h urine protein levels. Advanced age, obesity, reduced eGFR, and abnormal urinary creatinine excretion were identified as significant determinants impacting the efficacy of ePER improvement. This study suggests that the combination of ePER and uPCR holds promise for practical applications, pending further validation using clinical data.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the First Affiliated Hospital of Zhengzhou University Ethics Review Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because waived the requirement to obtain informed consent due to its non-interventional nature.
YJ: Writing – original draft, Writing – review & editing, Data curation, Investigation, Methodology, Formal analysis. LZ: Writing – original draft, Writing – review & editing, Formal analysis, Software. FW: Formal analysis, Software, Writing – review & editing. JS: Funding acquisition, Project administration, Writing – review & editing, Conceptualization. ZZ: Conceptualization, Supervision, Writing – review & editing, Resources, Funding acquisition.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Henan Province Young and middle-aged health science and technology innovation Talents Project, Henan Provincial Natural Science Foundation Outstanding Youth Fund, the National Natural Science Foundation of China (Grant No. 82170738), the Funding for Scientific Research and Innovation Team of The First Affiliated Hospital of Zhengzhou University (ZYCXTD2023009, QNCXTD2023009). Zhengzhou University teaching reform project (No. 2022ZZUSX022) and Medical Education Research Project of Henan Province (WJLX2023044).
We thank all the participants who contributed to the article and the patients who provided the data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1517019/full#supplementary-material
1. Keane, WF, and Eknoyan, G. Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): a position paper of the National Kidney Foundation. Am J Kidney Dis. (1999) 33:1004–10. doi: 10.1016/s0272-6386(99)70442-7
2. Aitekenov, S, Gaipov, A, and Bukasov, R. Review: detection and quantification of proteins in human urine. Talanta. (2021) 223:121718. doi: 10.1016/j.talanta.2020.121718
3. Ruggenenti, P, Gaspari, F, Perna, A, and Remuzzi, G. Cross sectional longitudinal study of spot morning urine protein:creatinine ratio, 24 hour urine protein excretion rate, glomerular filtration rate, and end stage renal failure in chronic renal disease in patients without diabetes. BMJ. (1998) 316:504–9. doi: 10.1136/bmj.316.7130.504
4. Cote, AM, Firoz, T, Mattman, A, Lam, EM, von Dadelszen, P, and Magee, LA. The 24-hour urine collection: gold standard or historical practice? Am J Obstet Gynecol. (2008) 199:625.e1–6. doi: 10.1016/j.ajog.2008.06.009
5. Patil, P, Shah, V, and Shah, B. Comparison of spot urine protein creatinine ratio with 24 hour urine protein for estimation of proteinuria. J Assoc Physicians India. (2014) 62:406–10.
6. Chitalia, VC, Kothari, J, Wells, EJ, Livesey, JH, Robson, RA, Searle, M, et al. Cost-benefit analysis and prediction of 24-hour proteinuria from the spot urine protein-creatinine ratio. Clin Nephrol. (2001) 55:436–47.
7. Montero, N, Soler, MJ, Pascual, MJ, Barrios, C, Marquez, E, Rodriguez, E, et al. Correlation between the protein/creatinine ratio in spot urine and 24-hour urine protein. Nefrologia. (2012) 32:494–501. doi: 10.3265/Nefrologia.pre2012.11300
8. Kimura, Y, Azuma, Y, Notsu, S, Bessho, S, Kobori, A, Kubota, T, et al. A formula for the estimation of 24-hour urinary creatinine excretion: a derivation and validation study. J Ren Nutr. (2022) 32:214–23. doi: 10.1053/j.jrn.2021.05.002
9. Ginsberg, JM, Chang, BS, Matarese, RA, and Garella, S. Use of single voided urine samples to estimate quantitative proteinuria. N Engl J Med. (1983) 309:1543–6. doi: 10.1056/NEJM198312223092503
10. Naresh, CN, Hayen, A, Craig, JC, and Chadban, SJ. Day-to-day variability in spot urine protein-creatinine ratio measurements. Am J Kidney Dis. (2012) 60:561–6. doi: 10.1053/j.ajkd.2012.04.010
11. Ix, JH, Wassel, CL, Stevens, LA, Beck, GJ, Froissart, M, Navis, G, et al. Equations to estimate creatinine excretion rate: the CKD epidemiology collaboration. Clin J Am Soc Nephrol. (2011) 6:184–91. doi: 10.2215/CJN.05030610
12. Fotheringham, J, Campbell, MJ, Fogarty, DG, El Nahas, M, and Ellam, T. Estimated albumin excretion rate versus urine albumin-creatinine ratio for the estimation of measured albumin excretion rate: derivation and validation of an estimated albumin excretion rate equation. Am J Kidney Dis. (2014) 63:405–14. doi: 10.1053/j.ajkd.2013.08.009
13. Mattix, HJ, Hsu, C-Y, Shaykevich, S, and Curhan, G. Use of the albumin/creatinine ratio to detect microalbuminuria: implications of sex and race. J Am Soc Nephrol. (2002) 13:1034–9. doi: 10.1681/ASN.V1341034
14. Levey, AS, Stevens, LA, Schpmid, CH, Zhang, YL, Castro, AF, Feldman, HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
15. Mukaka, MM. Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J. (2012) 24:69–71.
16. Cohen, J. Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull. (1968) 70:213–20. doi: 10.1037/h0026256
17. Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 clinical practice guideline for the Management of Glomerular Diseases. Kidney Int. (2021) 100, 100:S1–S276. doi: 10.1016/j.kint.2021.05.021
18. Peterson, JC, Adler, S, Burkart, JM, Greene, T, Hebert, LA, Hunsicker, LG, et al. Blood pressure control, proteinuria, and the progression of renal disease. The modification of diet in renal disease study. Ann Intern Med. (1995) 123:754–62. doi: 10.7326/0003-4819-123-10-199511150-00003
19. Reich, HN, Troyanov, S, Scholey, JW, and Cattran, DCToronto Glomerulonephritis Registry Group. Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol. (2007) 18:3177–83. doi: 10.1681/ASN.2007050526
20. Barbour, SJ, and Reich, HN. Risk stratification of patients with IgA nephropathy. Am J Kidney Dis. (2012) 59:865–73. doi: 10.1053/j.ajkd.2012.02.326
21. Troyanov, S, Wall, CA, Miller, JA, Scholey, JW, and Cattran, DCToronto Glomerulonephritis Registry Group. Idiopathic membranous nephropathy: definition and relevance of a partial remission. Kidney Int. (2004) 66:1199–205. doi: 10.1111/j.1523-1755.2004.00873.x
22. Troyanov, S, Wall, CA, Miller, JA, Scholey, JW, and Cattran, DCToronto Glomerulonephritis Registry Group. Focal and segmental glomerulosclerosis: definition and relevance of a partial remission. J Am Soc Nephrol. (2005) 16:1061–8. doi: 10.1681/ASN.2004070593
23. Methven, S, MacGregor, MS, Traynor, JP, O'Reilly, DS, and Deighan, CJ. Assessing proteinuria in chronic kidney disease: protein-creatinine ratio versus albumin-creatinine ratio. Nephrol Dial Transplant. (2010) 25:2991–6. doi: 10.1093/ndt/gfq140
24. Zhao, YF, Zhu, L, Liu, LJ, Shi, SF, Lv, JC, and Zhang, H. Measures of urinary protein and albumin in the prediction of progression of IgA nephropathy. Clin J Am Soc Nephrol. (2016) 11:947–55. doi: 10.2215/CJN.10150915
25. Lochner, J, Mohr, B, Garcia-Gutierrez, I, Schoppelrey, HP, Gummer, M, and Breit, R. Congenital pachyonychia type II (Jackson-Lawler syndrome). Hautarzt. (2000) 51:192–5. doi: 10.1007/s001050051103
26. Wiholm, BE, Mortimer, O, Boethius, G, and Haggstrom, JE. Tardive dyskinesia associated with metoclopramide. Br Med J (Clin Res Ed). (1984) 288:545–7. doi: 10.1136/bmj.288.6416.545
27. Walser, M. Creatinine excretion as a measure of protein nutrition in adults of varying age. JPEN J Parenter Enteral Nutr. (1987) 11:73S–8S. doi: 10.1177/014860718701100510
28. James, GD, Sealey, JE, Alderman, M, Ljungman, S, Mueller, FB, Pecker, MS, et al. A longitudinal study of urinary creatinine and creatinine clearance in normal subjects. Race, sex, and age differences. Am J Hypertens. (1988) 1:124–31. doi: 10.1093/ajh/1.2.124
29. Taylor, EN, and Curhan, GC. Body size and 24-hour urine composition. Am J Kidney Dis. (2006) 48:905–15. doi: 10.1053/j.ajkd.2006.09.004
30. Negri, AL, Spivacow, FR, Del Valle, EE, Forrester, M, Rosende, G, and Pinduli, I. Role of overweight and obesity on the urinary excretion of promoters and inhibitors of stone formation in stone formers. Urol Res. (2008) 36:303–7. doi: 10.1007/s00240-008-0161-5
Keywords: kidney disease, urinary protein excretion, ePER, uPCR, 24-h urinary protein excretion
Citation: Jia Y, Zhao L, Wang F, Shang J and Zhao Z (2025) Comparing estimated protein excretion rate and spot urinary protein-creatinine ratio in assessing urinary protein excretion in patients with kidney disease in China: a single center study. Front. Med. 12:1517019. doi: 10.3389/fmed.2025.1517019
Received: 25 October 2024; Accepted: 21 February 2025;
Published: 06 March 2025.
Edited by:
Antonino Sidoti, Azienda USL Toscana Sud Est, ItalyReviewed by:
Karl Martin Wissing, University Hospital Brussels, BelgiumCopyright © 2025 Jia, Zhao, Wang, Shang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhanzheng Zhao, emhhbnpoZW5nemhhb0B6enUuZWR1LmNu; Jin Shang, ZmNjc2hhbmdqMkB6enUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.