- 1Department of Nephrology, Ruian Hospital of Traditional Chinese Medicine, Wenzhou, Zhejiang, China
- 2Department of Clinical Laboratory, Ruian Hospital of Traditional Chinese Medicine, Wenzhou, Zhejiang, China
- 3Department of Nephrology, Yueyang Hospital of Integrated Traditional Chinese Medicine and Western Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China
Background: Nocardia species are rare opportunistic pathogens in the clinic, with strong invasiveness and dissemination, that can cause serious pulmonary infection, especially in immunocompromised patients, chronic lung diseases and hormone use, and is easy to be missed and misdiagnosed, preventing patients from obtaining timely and effective treatment, resulting in a high mortality rate.
Case presentation: Here, we present a rare case of a patient with chronic bronchiectasis and IgA nephropathy who developed Nocardia otitidiscaviarum pneumonia shortly after hormone therapy. The patient presented with tongue and lip ulcers, chest distress, cough, expectoration, and fever as the initial symptoms, which were extremely similar to common bacterial pulmonary infections. The laboratory examination and pulmonary computer tomography results indicated pulmonary infection, but the blood and multiple sputum cultures failed to identify the pathogen. Empirical treatment with piperacillin/tazobactam sodium and ceftriaxone was ineffective, and the patient’s condition worsened and progressed to respiratory failure. Subsequently, a bronchoscopy examination was performed, and the bronchoalveolar lavage fluid was collected for bacterial culture, which indicated Nocardia infection, however the treatment used of trimethoprim-sulfamethoxazole combined with imipenem was not effective. Finally, the patient was confirmed to have Nocardia otitidiscaviarum infection by mass spectrometry. According to the antibiotic sensitivity test and minimum inhibitory concentration (MIC) value results, Nocardia otitidiscaviarum was resistant to imipenem, so the treatment was changed to trimethoprim-sulfamethoxazole combined with linzolid. The patient’s condition improved rapidly and he was discharged after his condition was stable.
Conclusion: This case reminded us that for patients with a history of chronic lung disease, when pulmonary infection occurs during hormone or immunosuppressive therapy for kidney disease, the possibility of Nocardia infection should be fully considered, and high-quality specimens should be collected as early as possible. Appropriate bacterial culture methods and efficient identification techniques should be adopted to promptly identify pathogens, and personalized treatment plans should be developed based on antibiotic sensitivity tests to save patients’ lives.
Introduction
Nocardia species are widely present in natural environments, especially in soils rich in organic matter, decaying vegetation, and stagnant water, Gram staining and weak acid-fast staining are both positive (1, 2). They are conditional pathogenic bacteria, first discovered by Edmond Nocard in 1888. Currently, 119 species have been identified, of which 54 can cause human infections (2). Nocardia related infections occur sporadically all over the world. The annual incidence rate in North America, Europe and Australia is about 0.375 cases/100000, which is related to work and environmental exposure, and about 60% of them have immune dysfunction. In addition, hormones and immune preparations are also considered risk factors for Nocardia infection. Despite the increasing number of reports related to Nocardia in recent years, there is still no large-scale epidemiological data or prospective studies, and there is still a lack of consensus on the best empirical treatment. The clinical symptoms of Nocardia pneumonia are non-specific, and some types of Nocardia can easily lead to disseminated infections. Moreover, Nocardia ususally grows slowly and is prone to missed diagnosis, resulting in a high mortality rate for patients who do not receive timely and effective treatment. Compared with other Nocardia, infections caused by Nocardia otitidiscaviarum (N. otitidiscaviarum) are relatively rare, accounting for only 3% to 5% of all reported Nocardia infections (3, 4). Herein, we report a case of a patient with chronic bronchiectasis and IgA nephropathy who developed N. otitidiscaviarum pneumonia shortly after hormone therapy.
Case presentation
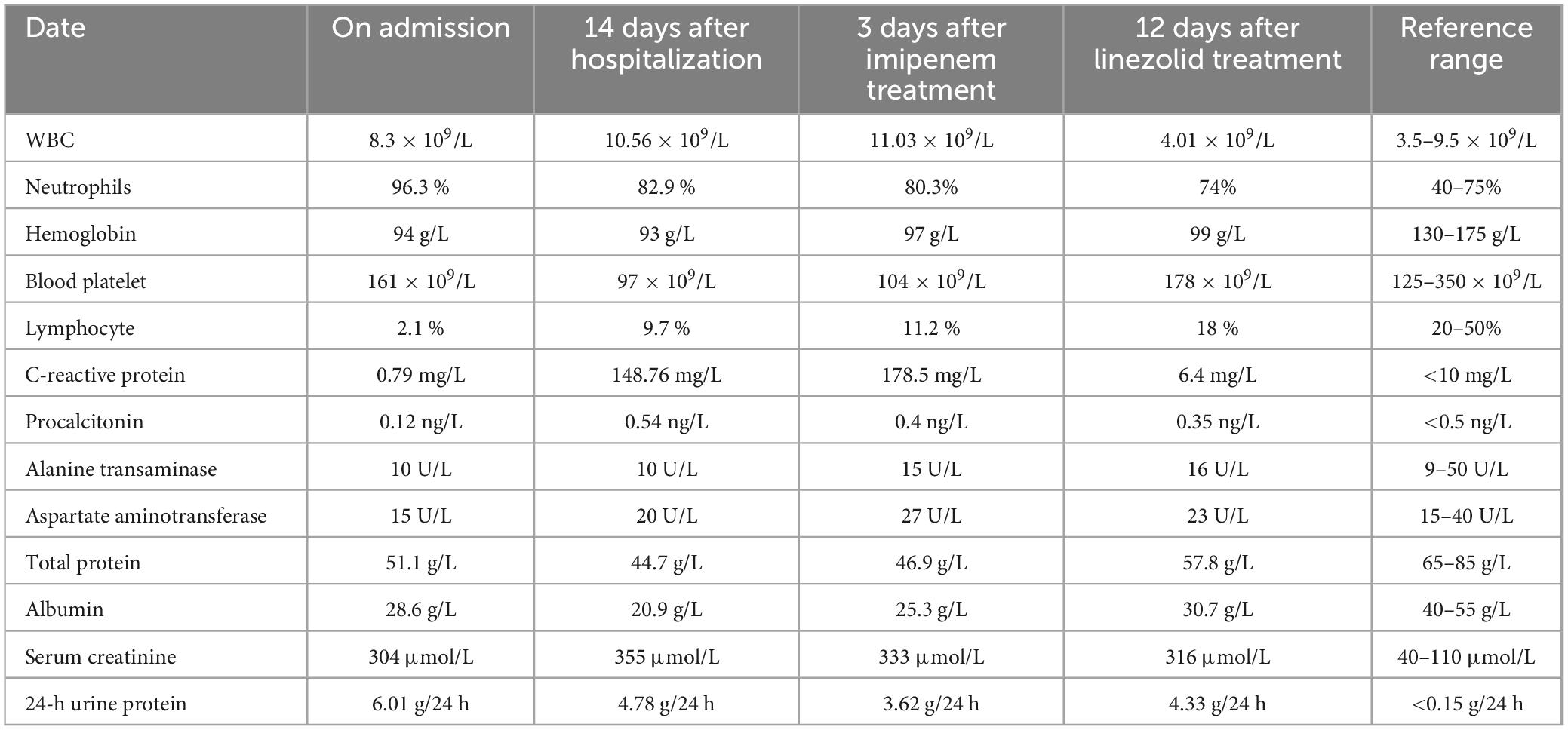
A 69 year-old man was admitted to the hospital due to “cough, expectoration, and fever for 2 days”. He had a history of hypertension for 10 years, chronic bronchiectasis for 10 years, and underwent surgical treatment for bladder and prostate tumors 2 years previously. 4 years ago, he was found to have an increase in serum creatinine (Scr) with a maximum of 120 μmol/L. Since then, he had been irregularly followed up with Scr fluctuating between 110 and 130 μmol/L. 2 months before admission, due to unexplained proteinuria and rapid increase in Scr (up to 294 μmol/L), a renal tissue biopsy was performed. Pathological results showed that immunoglobulin IgA and complement C3 were deposited in clumps and granules in the mesangial area and capillary loops of the glomerulus (Figures 1A–D). The patient was then diagnosed with “IgA nephropathy,” but he refused treatment. 12 days before admission, after pulmonary computer tomography (CT) examination to rule out pulmonary infection and other potential infections (Figure 2A), the patient was treated with prednisone 30 mg/day orally due to increased proteinuria and progressive lower limb edema. At the same time, trimethoprim-sulfamethoxazole (TMP-SMZ) was given 0.24 g/day to prevent potential infection. 4 days before admission, the patient developed tongue and lip ulcers for unknown reasons. 2 days before admission, the patient began to experience mild chest distress, occasional cough and purulent sputum, without chest pain or fever. The patient’s laboratory results are displayed in Table 1.
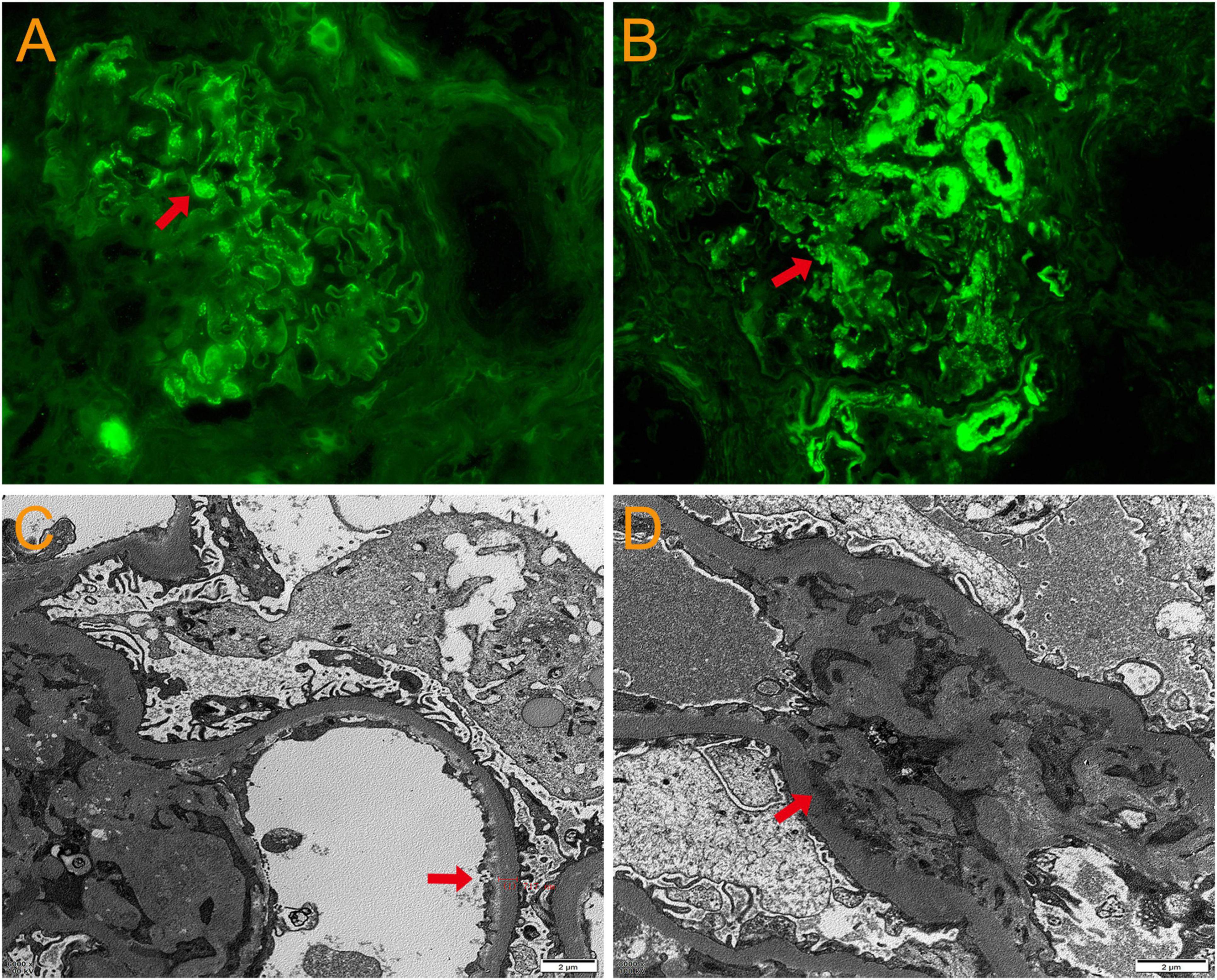
Figure 1. Pathological results of renal tissue biopsy. Immunofluorescence: immunoglobulin IgA (A) and complement C3 (B) deposited in clumps and granules in the mesangial area and capillary loops of the glomerulus. Original magnification, × 400. (C) Electron microscopy: thickening of the basement membrane of glomerular capillary loops. Original magnification, × 3000. (D) Electron microscopy: electron dense deposition in the glomerular mesangial area. Original magnification, × 3000.
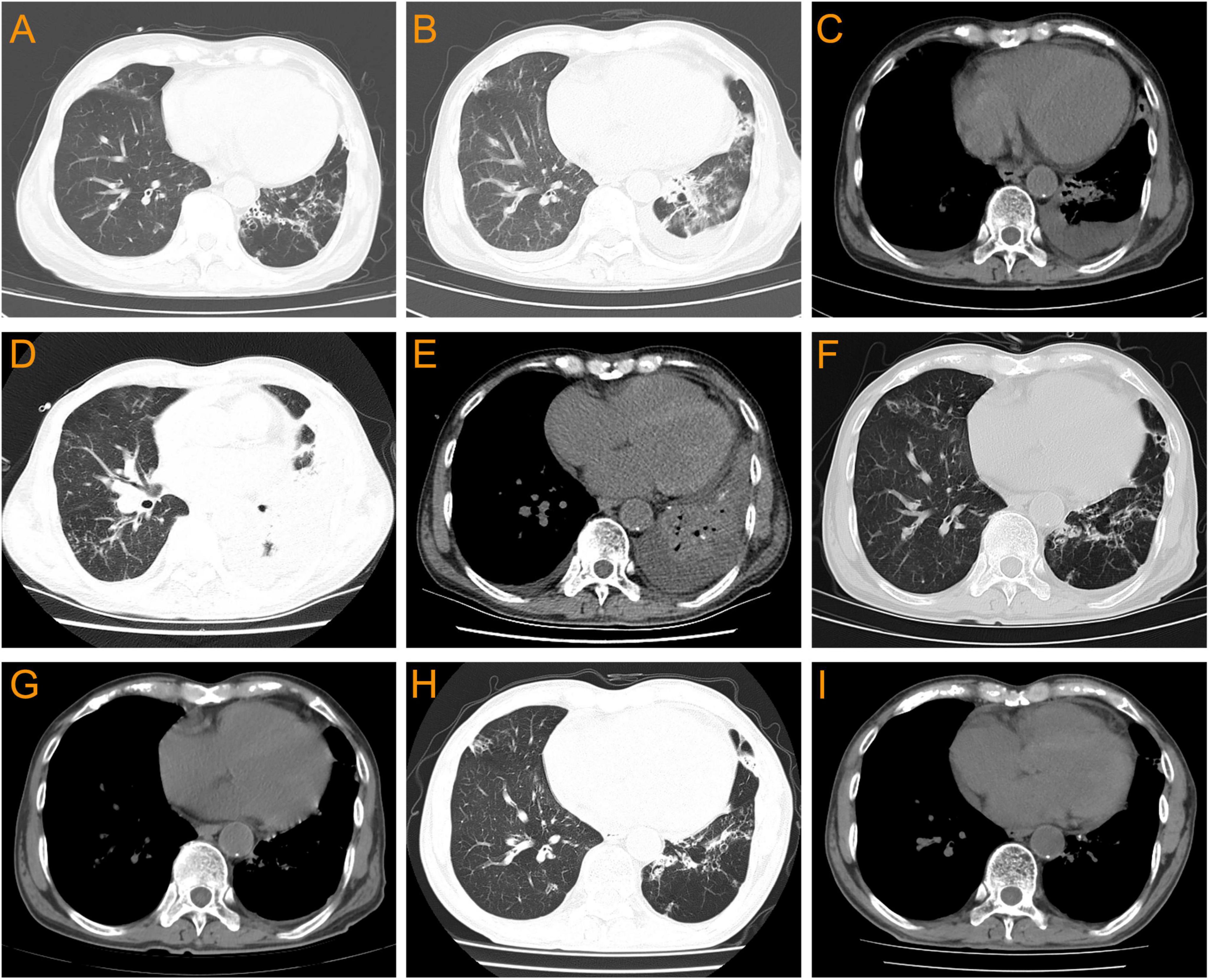
Figure 2. Pulmonary CT changes of this patient before and after Nocardia infection. (A) 12 days before admission (with no Nocardia infection): bronchiectasis without obvious pulmonary infection or pleural effusion. (B,C) On admission: bilateral bronchiectasis with pulmonary infection and bilateral pleural effusion. (D,E) On the 14th day of admission: worsening of bilateral pulmonary infections and bilateral pleural effusion, accompanied by left atelectasis. (F,G) 12 days after linezolid combined with TMP-SMZ treatment: bilateral pulmonary infection improved significantly, with a small amount of left pleural effusion. (H,I) 2 months after discharge: bronchiectasis with no obvious pulmonary infection and pleural effusion.
On the day of admission, the patient went to the hospital due to worsening chest distress and cough, expectoration, and fever. The laboratory examination results showed that WBC 8.3 × 109/L, neutrophils 96.3%, lymphocyte 2.1%,C-reactive protein (CRP) 0.79 mg/L, and procalcitonin 0.12 ng/L. The body temperature was 38.70C, and the arterial oxygen saturation was normal. Pulmonary CT results showed bronchiectasis with scattered infections, emphysema, and a small amount of bilateral pleural effusion (Figures 2B, C). The patient was then diagnosed with pneumonia. After collecting blood and sputum samples for bacterial culture, empirical treatment with piperacillin/tazobactam sodium (9.0 g/day) was administered. However, the patient’s clinical symptoms did not show significant improvement and there was still recurrent fever. During the treatment period, a total of one blood culture, four sputum cultures, as well as fungal (1, 3)-β-D glucan detection, fungal galactomannan antigen detection, and tuberculosis infection T cell spot test were performed, but the results were all negative.
On the 14th day of admission, the patient experienced a progressive decrease in blood oxygen saturation, with a minimum of 85%. Pulmonary CT results showed worsening of bilateral pulmonary infections and pleural effusion, accompanied by left atelectasis (Figures 2D, E). The laboratory examination results showed that WBC 10.56 × 109/L, neutrophils 82.9%, lymphocyte 9.7%, CRP 148.76 mg/L, and procalcitonin 0.54 ng/L. Subsequently, bronchoscopy was performed to collect bronchoalveolar lavage fluid (BALF) for laboratory examination and bacterial culture. The BALF was grayish-white, with a nucleated cell count of 2120/μL, while a red blood cell count of 3840/μL, neutrophils 89%, lymphocytes 4%, and alveolar phagocytic cells 7%. The BALF smear examination only detected oral and pharyngeal microbiota, with no fungi or Mycobacterium tuberculosis, while fluorescence staining also did not detect acid-fast bacilli. Besides, Aspergillus antigen was negative and fungal (1, 3)-β-D glucan <37.5 pg/mL. Then piperacillin/sulbactam sodium was discontinued and the antibiotic was changed to TMP-SMZ (5.76 g/day) combined with ceftriaxone (2.0 g/day).
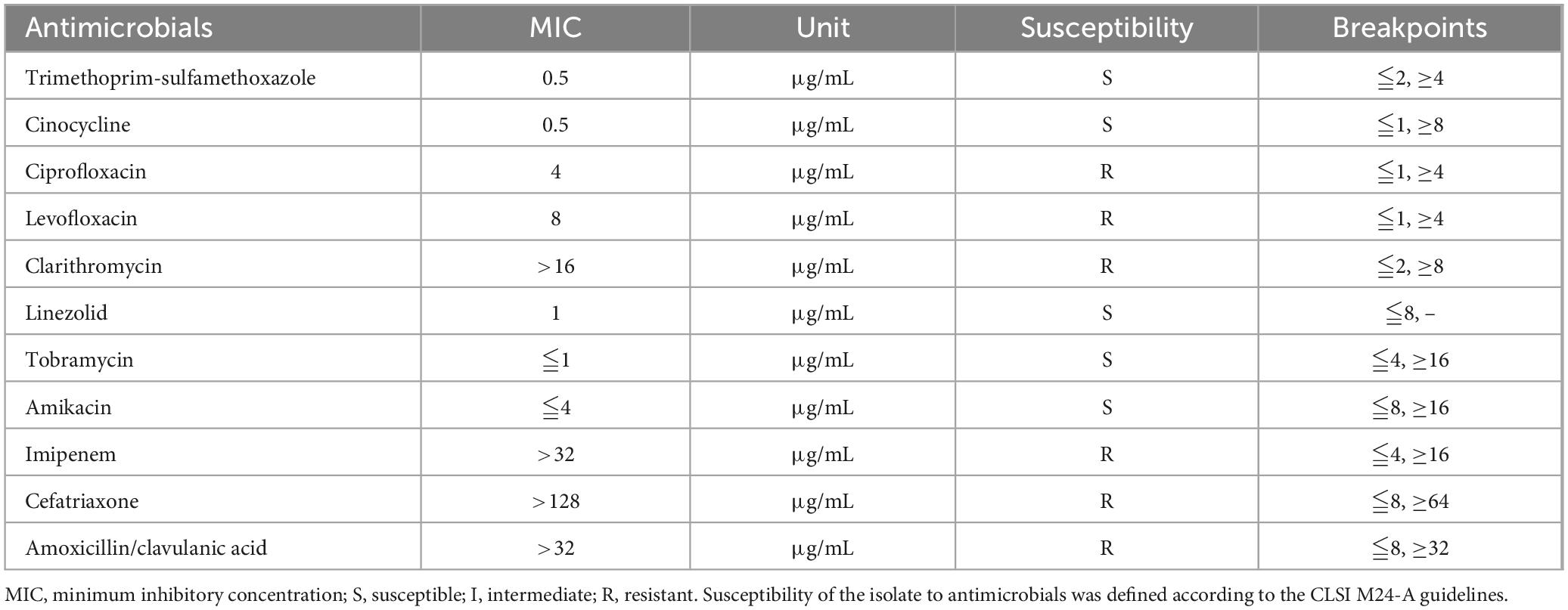
In addition, The BALF culture results showed that pin-sized white colonies could be seen after 2 days, but it was difficult to identify the bacteria species (Figure 3A). Large, dry, white colonies were observed after 5 days (Figures 3B, C), which were Gram staining positive and weak acid-fast staining positive (Figures 3D, E), indicating Nocardia infection, a bacterium widely present in soil and dust. Upon further inquiry, it was found that the patient enjoyed planting flowers, indicating a risk of close contact with Nocardia in the soil, so the patient was considered to be infected with Nocardia. According to the US guidelines (5), imipenem was the preferred empirical treatment for Nocardia, so ceftriaxone was discontinued on day 19 of admission and imipenem (2.0 g/d) was used instead. However, the patient’s condition did not improve after 3 days of imipenem combined with TMP-SMZ treatment, and chest tightness, fever, and respiratory failure were still not relieved. Besides, the patient experienced mild headaches, listlessness, and slow reaction that lasted for several days. A head magnetic resonance imaging (MRI) examination was performed, and the results showed white matter lesions (Fezekas grade 2) without any signs of brain abscess. Finally, the cultured bacteria was confirmed to be rare N. otitidiscaviarum by VITEK MS (bioMérieux, France) with a confidence value of 99.9%, and as shown in Table 2, the antibiotic sensitivity test results and minimum inhibitory concentration (MIC) value indicated that it was resistant to imipenem, but sensitive to TMP-SMZ and linezolid. Linezolid was also one of the recommended drugs for the treatment of Nocardia infection (5). Due to the lack of improvement in the patient’s condition after receiving imipenem treatment, and based on the results of drug sensitivity tests, imipenem was discontinued and replaced with linezolid (1.2 g/day). After 3 days of treatment with linezolid and TMP-SMZ, the patient’s symptoms of chest distress, cough, expectoration and fever were significantly improved, the tongue tip ulcer disappeared, and the lip skin ulcer gradually improved (Figure 3F). The patient’s arterial oxygen saturation increased to over 98%, and the above-mentioned central nervous system symptoms disappeared. Afterwards, the patient’s condition continued to improve. After 12 days of linezolid combined with TMP-SMZ treatment, pulmonary CT showed a significant improvement in pulmonary infection compared to before, only with a small amount of pleural effusion on the left side (Figures 2F, G), and the laboratory examination results showed WBC 4.01 × 109/L, neutrophils 74%,lymphocyte 18%,CRP 6.4 mg/L, and procalcitonin 0.35 ng/L. After 20 days of treatment with linezolid, the patient’s respiratory symptoms and lip ulcers disappeared. In the course of treatment, considering the patient’s renal dysfunction and the toxic side effects of TMP-SMZ on the kidneys, we gradually reduced the dosage of TMP-SMZ until discontinuation. Besides, the dose of prednisone was also gradually reduced from 30 mg/day to 5 mg/day during hospitalization.
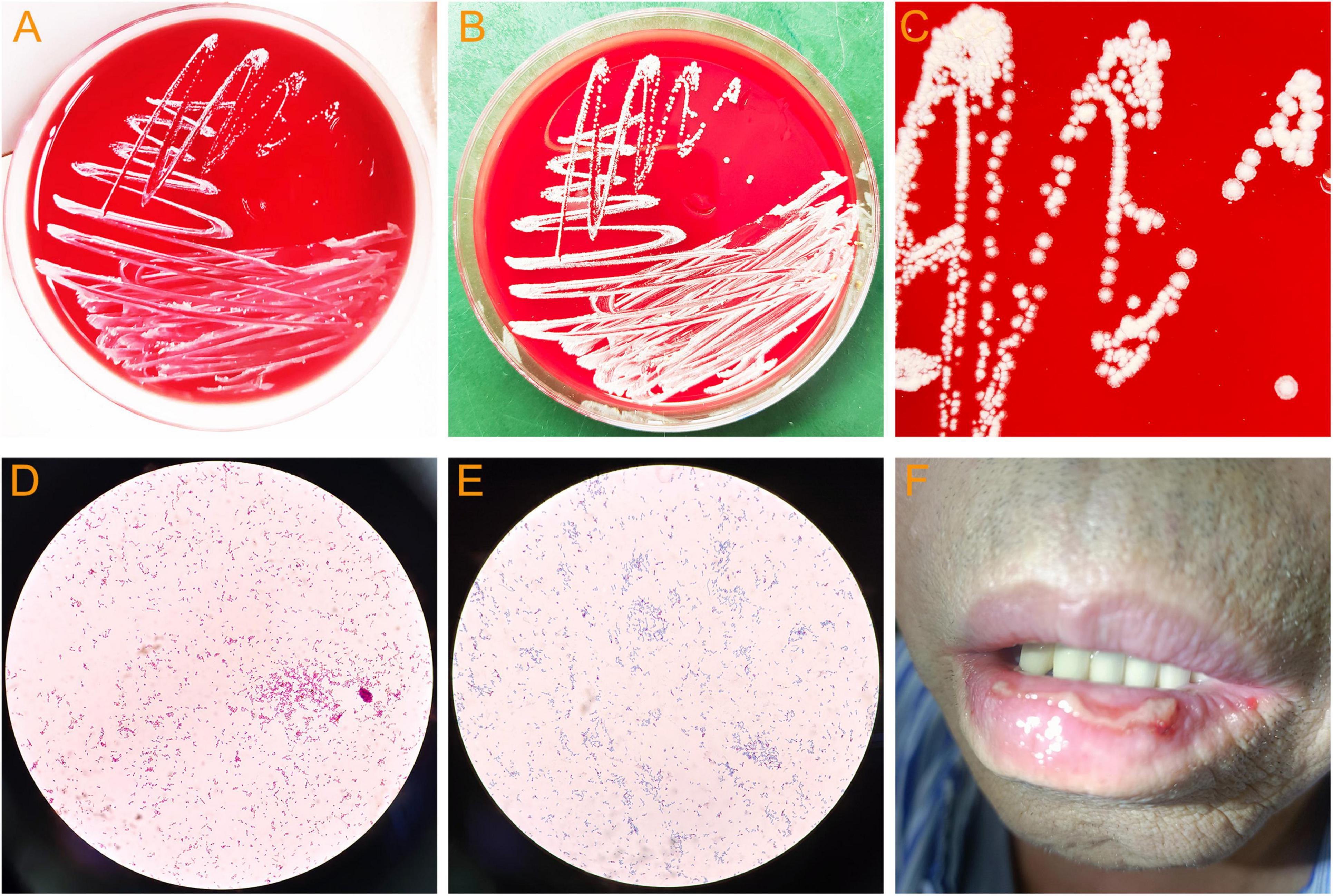
Figure 3. Bacterial culture of bronchoalveolar lavage fluid and lip ulcers in patient. (A) Colonies of N. otitidiscaviarum cultured for 2 days. (B) Colonies of N. otitidiscaviarum cultured for 5 days. (C) Colony morphology of N. otitidiscaviarum. (D) N. otitidiscaviarum by Gram staining. (E) N. otitidiscaviarum by weak acid-fast staining. (F) Lip ulcers in patient (This image was obtained with the patient’s informed consent).
After 42 days of hospitalization, the patient was discharged and continued to receive long-term oral treatment with linezolid at a dose of 0.6 g/day (The timeline of key events is shown in Figure 4). After a 2-month follow-up after discharge, except for occasional cough, the patient had no chest tightness, sputum or fever, and pulmonary CT showed no obvious infection or pleural effusion (Figures 2H, I).

Figure 4. Timeline of key events. IgAN, IgA nephropathy; TMP-SMZ, trimethoprim-sulfamethoxazole; BALF, bronchoalveolar lavage fluid; MIC, minimum inhibitory concentration.
Discussion
Nocardia infection tends to occur in patients with weakened immune function, altered lung structure, and chronic lung disease. Nocardia can cause local or transmitted infections, with the lungs being the most common site of primary infection (4, 6). This type of bacteria can invade broken skin causing a suppurative skin infection, which can lead to a pulmonary infection when it enters the respiratory tract (2). In addition, it can cause disseminated infection of the kidneys or other organs through hematogenous dissemination, leading to acute or chronic suppurative granulomatous disease (7). According to statistics, the mortality rate of pulmonary Nocardia infection is about 18% to 30%, which is higher in disseminated infections, and can reach 50% after involvement of the central nervous system (8). Nocardia is easily misdiagnosed, leading to ineffective treatment. The main reasons include slow growth of Nocardia, requiring longer cultrue time, and some strains forming colonies only after 2 weeks; High technical requirements for rapid detection of Nocardia and its sensitivity to interference from other microorganisms; Non-specific clinical and imaging features that easily be confused with other bacterial diseases such as pulmonary tuberculosis and cutaneous tuberculosis. These factors may be the important causes of high mortality rates in patients (1, 4, 9). In recent years, due to the rapid development of detection technologies such as mass spectrometry and metagenome next-generation sequencing (mNGS), which have been increasingly used to detect N. otitidiscaviarum and other rare taxa, the mortality rate of Nocardia infections has decreased (10, 11).
The reason why Nocardia is more common in immunocompromised populations may be related to its pathogenic characteristics, as it has strong pathogenicity and can invade the human body through damaged skin, the respiratory tract, the digestive tract (12, 13). In addition, immunocompromised patients have decreased immune system function and weakened immune response ability, making it difficult to effectively resist the invasion and reproduction of Nocardia, which significantly increases the risk of infection (14). Studies have shown that most patients with pulmonary or disseminated Nocardia disease have immune dysfunction mainly characterized by cellular immune deficiency, and such patients have a high rate of macrophage dysregulation under the long-term action of various inflammatory factors, which partly leads to Nocardia susceptibility (15). Therefore, immunosuppression-related diseases such as HIV infection, cancer, chemotherapy, solid organ transplantation (especially lung transplantation), allogeneic hematopoietic stem cell transplantation patients, diabetes, autoimmune diseases, glucocorticoid use, and other diseases that lead to cellular immune deficiency provide a potential basis for Nocardia infection (16).
Despite the increasing recognition of Nocardia, infections caused by N. otitidiscaviarum are relatively rare compared to other Nocardia species, accounting for only 3% to 5% of all reported Nocardia infections (3, 4). In a study conducted in the United States, only 2.9% (10/347) of all Nocardia infections were caused by N. otitidiscaviarum (17), and a similar result was obtained in a study conducted in China, which was 5.9% (26/441) (18). The low infection rate of N. otitidiscaviarum may be related to its low pathogenicity, limited distribution in soil, or underreporting of cases (19). In addition, there are some differences in pathogenicity and epidemiology between N. otitidiscaviarum and other Nocardia species. The former usually infects people with specific underlying diseases or immune impairments, while the latter, such as N. asteroides and N. brasiliensis, are relatively more common in healthy people but can also occur in patients with underlying diseases or immune impairments. In terms of pathogenicity, N. otitidiscaviarum can usually cause pulmonary infections, skin and central nervous system infections, while other Nocardia mainly enter the body through the respiratory tract, causing primary purulent pulmonary infections, and in some cases, the infection may also spread to other parts, such as the brain, forming brain abscesses (20, 21).
This study reports a case of N. otitdiscaviarum pulmonary infection. The patient had a history of bladder and prostate tumors and chronic bronchiectasis. After receiving hormone therapy for newly diagnosed IgA nephropathy, he was infected with N. otitdiscaviarum within a short period of time. Previous studies had shown that corticosteroids and immune agents were considered risk factors for Nocardia infection. Steinbrink et al. (22) analyzed clinical data of 112 patients with Nocardia infection and found that Nocardia infection in immunosuppressed patients was associated with the use of high-dose glucocorticoid therapy and hematopoietic stem cell transplantation therapy. A multicenter case-control study in Europe showed that long-term use of high-dose corticosteroids (>20 mg/day prednisone for at least 1 month) was an independent risk factor for Nocardia infection (23). Another retrospective study in Israel confirmed that the use of systemic corticosteroids could increase the risk of Nocardia infection, especially in patients with chronic lung disease (24). In addition, research reports on kidney disease patients showed that the median dose of steroids for patients with Nocardia infection was 20 mg/day, and the median course of treatment was 4–6 months (25). In this case, the reasons for rapid infection with N. otitidiscaviarum after hormone therapy potentially included a history of cancer, chronic bronchiectasis, high initial hormone dose (30 mg/day), and the patient’s interest in planting flowers, which increased the possibility of close contact with Nocardia in soil. Under the combined effect of these factors, the risk of N. otitidiscaviarum infection in this patient was significantly increased.
Similar to the symptoms of pulmonary infections caused by common bacteria, Nocardia pneumonia initially present with fever, cough, expectoration, and chest distress (2). As the condition progresses, lung consolidation, increased pleural effusion, dyspnea, and even respiratory failure may occur. In this case, the patient experienced a similar disease progression. As shown in Figure 2, the pulmonary CT showed that the patient’s pulmonary infection was progressively worsening, with increased lung consolidation and pleural effusion, as well as symptoms of respiratory failure. In some previous studies, more than half of pulmonary Nocardia patients had extrapulmonary dissemination, with the brain being the most common site of dissemination (7, 8). Therefore, it is recommended that all patients with Nocardia pneumonia or disseminated infection undergo cranial imaging examination to rule out intracranial infection. Although the patient in this case experienced mild headaches, listlessness, and slow reaction for several days during the illness, no imaging evidence of intracranial infection was found in the brain MRI examination. However, as shown in Figure 3F, the patient developed severe tongue and lip ulcers in the early stages of the disease. As mentioned earlier, Nocardia can cause skin infections, with N. brasiliensis being the most common, and the pathogenic factors of Nocardia skin infections are mostly horticultural work and trauma (2). Skin Nocardia disease needs to distinguish between primary skin Nocardia infection and other organ Nocardia infections affecting the skin. The former can manifest as skin lymphadenopathy, foot mycosis, and cellulitis, with a few reports of Nocardia causing keratitis, bone and joint infections (26). The latter is a manifestation of Nocardia infection in other organs accompanied by skin damage. In this case, the patient’s lip and tongue ulcers appeared in the early stages of N. otitidiscaviarum infection and gradually improved after the pneumonia was controlled, and no other skin lesions were found in other parts. Therefore, it could be considered that the mucosal lesions of the patient might be related to pulmonary infection caused by N. otitidiscaviarum. This phenomenon suggested that skin and mucosal damage may occur earlier than respiratory symptoms and pulmonary imaging changes after N. otitidiscaviarum infection, which had not been previously reported.
In this case, the patient was ultimately diagnosed with an N. otitidiscaviarum infection, but it was not promptly identified in the early stages of the disease. The reasons for delayed diagnosis might mainly included atypical early clinical symptoms, difficulties in Nocardia culture, and drug interference. The gold standard for diagnosing Nocardia infection is to isolate and culture Nocardia (4). However, we did not find it in the four sputum cultures where samples were taken separately in the early stages of the disease. As mentioned above, some strains of Nocardia grow slowly in culture medium, many microbiology laboratories culture sputum at regular times and discard the culture medium too early, resulting in belated diagnosis for some Nocardia patients, which might be one of the reasons for early misdiagnosis in this case. In addition, drug interference also needs to be taken into account in this case. The patient was treated with hormone therapy for IgA nephropathy while a low-dose of TMP-SMZ was used to prevent potential infections. Theoretically, a low-dose of TMP-SMZ can reduce the probability of infection in high-risk patients, but some previous clinical studies indicated that a low-dose of TMP-SMZ could not prevent potential infections and might increase the risk of drug resistance (27). According to some treatment guidelines, TMP-SMZ is also one of the preferred medication for treating Nocardia infections (5, 28). Therefore, when this patient was administered a low-dose of TMP-SMZ to prevent potential infection, it might also inhibit the growth of Nocardia, thereby interfering with the detection of Nocardia in sputum. Fortunately, Nocardia was found in higher quality BALF specimens and further confirmed as N. otitidiscaviarum by mass spectrometry, providing favorable evidence for the diagnosis of the case.
Nocardia varies in regional distribution and drug resistance, and treatment recommendations for Nocardia may vary from region to region. In the treatment guidelines of the American Society of Transplantation for Nocardia infection, TMP-SMZ is recommended as the first choice for transplant patients with mild to moderate pulmonary infection, followed by imipenem combined with amikacin, ceftriaxone, minocycline, or linezolid, with a course of treatment of 6–12 months. For patients with severe pulmonary infection, brain abscesses and disseminated infections, the guidelines recommend imipenem combined with amikacin or TMP-SMZ as the first choice, with a course of treatment of 6–12 months (5). The treatment guidelines for Nocardia released by Australia recommend the use of TMP-SMZ for patients with mild to moderate infections, for patients with severe infections, it is recommended to use TMP-SMZ in combination with linezolid, acaricin, imipenem, or meropenem (28). Comparing the guidelines of the United States and Australia, it can be found that the first recommended combination therapy for pulmonary or disseminated infections in the United States is TMP-SMZ combined with imipenem. Although some reports indicated that the resistance rate of Nocardia to TMP-SMZ reached 42% and to imipenem ranged from 30 to 55% (28, 29), there were also studies suggesting that early and sufficient drug treatment could still achieve good curative effect (18, 30). In this case, when Nocardia was identified in the BALF, TMP-SMZ combined with imipenem were administered immediately according to the guidelines, but it was ineffective and the patient’s condition did not improve. Finally, the pathogen was identified as N. otitidiscaviarum by mass spectrometry, and according to the results of drug sensitivity tests, the antibiotic was changed to TMP-SMZ combined with linezolid, after which the patient’s condition improved rapidly. After discharge, the patient received oral maintenance treatment with linezolid and his condition remained stable. Although imipenem is one of the recommended drugs for the treatment of Nocardia, the resistance rate in Nocardia is increasing, while the resistance rate to linezolid is relatively low. This case further confirmed this, which was consistent with some recent reports (31–33). This case reminded us that early identification of pathogens and personalized treatment based on drug sensitivity results are key to curing patients with Nocardia disease. In addition, previous studies suggested that reducing hormone dosage in the short term might help with the treatment of Nocardia infection (34). In this case, while receiving antibiotic treatment, the dosage of hormone was gradually decreased, and the patient’s condition gradually improved, indicating that the adjustment of the dosage of hormone might also be related to the rapid improvement of N. otitidiscaviarum pneumonia.
Conclusion
In conclusion, this case reports a patient with a history of cancer and chronic bronchiectasis who was infected with the rare N. otitidiscaviarum during hormone therapy for IgA nephropathy. The atypical clinical manifestations of Nocardia pneumonia and the characteristics of Nocardia itself pose a serious challenge to clinical physicians. Currently, there is still a lack of consistent and effective empirical treatment plans for Nocardia infection, and its high severity and mortality rates require full attention from clinical physicians. We recommend that for patients with a history of chronic lung disease, when pulmonary infection occurs during hormone or immunosuppressive therapy for kidney disease, the possibility of Nocardia infection should be fully considered, and high-quality specimens should be collected as early as possible. Appropriate bacterial culture methods and efficient identification techniques, such as mass spectrometry and mNGS, should be adopted to promptly identify pathogens, and personalized treatment should be developed based on antibiotic sensitivity tests to save patients’ lives.
Data availability statement
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YL: Conceptualization, Formal analysis, Investigation, Writing – original draft. Z-ZJ: Conceptualization, Formal analysis, Investigation, Writing – original draft. X-QC: Data curation, Investigation, Writing – review and editing. J-SC: Data curation, Investigation, Writing – review and editing. CW: Investigation, Software, Writing – original draft. CZ: Investigation, Writing – original draft. Y-YW: Data curation, Resources, Writing – original draft. G-LX: Methodology, Project administration, Resources, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Zhejiang Province “The thirteenth-five” Key Specialized Construction Fund for Traditional Chinese Medicine and Wenzhou Medical and Health Research Project (No. Z2022012).
Acknowledgments
We would like to thank the patient and their family for their great help in this report.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Traxler R, Bell M, Lasker B, Headd B, Shieh W, McQuiston J. Updated review on Nocardia species: 2006-2021. Clin Microbiol Rev. (2022) 35:e0002721. doi: 10.1128/cmr.00027-21
2. Lynch J III, Reid G, Clark N. Nocardia spp.: A rare cause of Pneumonia globally. Semin Respir Crit Care Med. (2020) 41:538–54. doi: 10.1055/s-0040-1708816
3. Jiang Y, Huang A, Fang Q. Disseminated nocardiosis caused by Nocardia otitidiscaviarum in an immunocompetent host: A case report and literature review. Exp Ther Med. (2016) 12:3339–46. doi: 10.3892/etm.2016.3755
4. Fatahi-Bafghi M. Nocardiosis from 1888 to 2017. Microb Pathog. (2018) 114:369–84. doi: 10.1016/j.micpath.2017.11.012
5. Restrepo A, Clark N. Infectious diseases community of practice of the American society of transplantation. Nocardia infections in solid organ transplantation: Guidelines from the infectious diseases community of practice of the American society of transplantation. Clin Transplant. (2019) 33:e13509. doi: 10.1111/ctr.13509
6. Coussement J, Lebeaux D, Rouzaud C, Lortholary O. Nocardia infections in solid organ and hematopoietic stem cell transplant recipients. Curr Opin Infect Dis. (2017) 30:545–51. doi: 10.1097/QCO.0000000000000404
7. Huang L, Chen X, Xu H, Sun L, Li C, Guo W, et al. Clinical features, identification, antimicrobial resistance patterns of Nocardia species in China: 2009-2017. Diagn Microbiol Infect Dis. (2019) 94:165–72. doi: 10.1016/j.diagmicrobio.2018.12.007
8. Margalit I, Lebeaux D, Tishler O, Goldberg E, Bishara J, Yahav D, et al. How do I manage nocardiosis? Clin Microbiol Infect. (2021) 27:550–8. doi: 10.1016/j.cmi.2020.12.019
9. Yuan D, Shen L, Qin B, Xu X, Su Z, Liu J, et al. Central nervous system nocardiosis diagnosed by metagenomic next-generation sequencing: A case series and literature review. Adv Clin Exp Med. (2023) 32:1453–63. doi: 10.17219/acem/175818
10. Srivastava S, Samaddar A, Khan S, Tak V, Bohra G, Sharma D, et al. Nocardia otitidiscaviarum causing pulmonary nocardiosis: A case report and its review of the literature. Access Microbiol. (2024) 6:000530.v5. doi: 10.1099/acmi.0000530.v5
11. Fan N, Fang H, Huang F, Zhou J, Liu P, Li M, et al. Metagenome next-generation sequencing plays a key role in the diagnosis and selection of effective antibiotics on the treatment of Nocardia pneumonia: A case report. Front Med (Lausanne). (2024) 11:1373319. doi: 10.3389/fmed.2024.1373319
12. Engelbrecht A, Saad H, Gross H, Kaysser L. Natural products from Nocardia and their role in pathogenicity. Microb Physiol. (2021) 31:217–32. doi: 10.1159/000516864
13. Doyle C, Costa Blasco M, MacEneaney O, Ryan C, Ní Raghallaigh S. Disseminated cutaneous nocardia. Int J Dermatol. (2023) 62:e29–31. doi: 10.1111/ijd.16402
14. Lafont E, Marciano B, Mahlaoui N, Neven B, Bustamante J, Rodriguez-Nava V, et al. Nocardiosis associated with primary immunodeficiencies (Nocar-DIP): An international retrospective study and literature review. J Clin Immunol. (2020) 40:1144–55. doi: 10.1007/s10875-020-00866-8
15. Belchamber K, Donnelly L. Macrophage dysfunction in respiratory disease. Results Probl Cell Differ. (2017) 62:299–313. doi: 10.1007/978-3-319-54090-0_12
16. Nieves Perez C, Sánchez Pérez M, Vargas A, Franco M, Molina Obana M. Cerebral abscess due to Nocardia beijingensis associated With HIV: Case report and mini review. Cureus. (2023) 15:e47571. doi: 10.7759/cureus.47571
17. Brown-Elliott B, Brown J, Conville P, Wallace R Jr. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. (2006) 19:259–82. doi: 10.1128/CMR.19.2.259-282.2006
18. Wang H, Zhu Y, Cui Q, Wu W, Li G, Chen D, et al. Epidemiology and antimicrobial resistance profiles of the Nocardia Species in China, 2009 to 2021. Microbiol Spectr. (2022) 10:e0156021. doi: 10.1128/spectrum.01560-21
19. Pelaez A, Garcia-Suarez Mdel M, Manteca A, Melon O, Aranaz C, Cimadevilla R, et al. A fatal case of Nocardia otitidiscaviarum pulmonary infection and brain abscess: Taxonomic characterization by molecular techniques. Ann Clin Microbiol Antimicrob. (2009) 8:11. doi: 10.1186/1476-0711-8-11
20. Gupta S, Grant L, Powers H, Kimes K, Hamdi A, Butterfield R, et al. Invasive Nocardia infections across distinct geographic regions, United States. Emerg Infect Dis. (2023) 29:2417–25. doi: 10.3201/eid2912.230673
21. Martínez-Barricarte R. Isolated nocardiosis, an unrecognized primary immunodeficiency? Front Immunol. (2020) 11:590239. doi: 10.3389/fimmu.2020.590239
22. Steinbrink J, Leavens J, Kauffman C, Miceli M. Manifestations and outcomes of nocardia infections: Comparison of immunocompromised and nonimmunocompromised adult patients. Medicine (Baltimore). (2018) 97:e12436. doi: 10.1097/MD.0000000000012436
23. Coussement J, Lebeaux D, van Delden C, Guillot H, Freund R, Marbus S, et al. Nocardia infection in solid organ transplant recipients: A multicenter european case-control study. Clin Infect Dis. (2016) 63:338–45. doi: 10.1093/cid/ciw241
24. Margalit I, Goldberg E, Ben Ari Y, Ben-Zvi H, Shostak Y, Krause I, et al. Clinical correlates of nocardiosis. Sci Rep. (2020) 10:14272. doi: 10.1038/s41598-020-71214-4
25. Han Y, Huang Z, Zhang H, He L, Sun L, Liu Y, et al. Nocardiosis in glomerular disease patients with immunosuppressive therapy. BMC Nephrol. (2020) 21:516. doi: 10.1186/s12882-020-02179-9
26. Dodiuk-Gad R, Cohen E, Ziv M, Goldstein L, Chazan B, Shafer J, et al. Cutaneous nocardiosis: Report of two cases and review of the literature. Int J Dermatol. (2010) 49:1380–5. doi: 10.1111/j.1365-4632.2010.04554.x
27. Takiguchi Y, Ishizaki S, Kobayashi T, Sato S, Hashimoto Y, Suruga Y, et al. Pulmonary nocardiosis: A clinical analysis of 30 cases. Intern Med. (2017) 56:1485–90. doi: 10.2169/internalmedicine.56.8163
28. McGuinness S, Whiting S, Baird R, Currie B, Ralph A, Anstey N, et al. Nocardiosis in the tropical northern territory of Australia, 1997-2014. Open Forum Infect Dis. (2016) 3:ofw208. doi: 10.1093/ofid/ofw208
29. Davidson N, Grigg M, Mcguinness S, Baird R, Anstey N. Safety and outcomes of linezolid use for nocardiosis. Open Forum Infect Dis. (2020) 7:ofaa090. doi: 10.1093/ofid/ofaa090
30. Passerini M, Nayfeh T, Yetmar Z, Coussement J, Goodlet K, Lebeaux D, et al. Trimethoprim-sulfamethoxazole significantly reduces the risk of nocardiosis in solid organ transplant recipients: Systematic review and individual patient data meta-analysis. Clin Microbiol Infect. (2024) 30:170–7. doi: 10.1016/j.cmi.2023.10.008
31. Han Y, Cheng M, Li Z, Chen H, Xia S, Zhao Y, et al. Clinical characteristics and drug resistance of Nocardia in Henan, China, 2017-2023. Ann Clin Microbiol Antimicrob. (2024) 23:23. doi: 10.1186/s12941-024-00677-4
32. Hershko Y, Levytskyi K, Rannon E, Assous M, Ken-Dror S, Amit S, et al. Phenotypic and genotypic analysis of antimicrobial resistance in Nocardia species. J Antimicrob Chemother. (2023) 78:2306–14. doi: 10.1093/jac/dkad236
33. Cordioli G, Di Pietra G, Asa’ad S, Meneghello S, Del Vecchio C, De Canale E, et al. Nocardia infections in Italy: From a local to a national view. New Microbiol. (2023) 46:68–74.
Keywords: pneumonia, Nocardia otitidiscaviarum, bronchiectasis, case report, linezolid, trimethoprim-sulfamethoxazole, hormone, IgA nephropathy
Citation: Lin Y, Jiang Z-Z, Chi X-Q, Chen J-S, Wen C, Zhang C, Wang Y-Y and Xie G-L (2025) Severe pneumonia caused by Nocardia otitidiscaviarum in a patient with bronchiectasis and IgA nephropathy: a case report. Front. Med. 12:1496814. doi: 10.3389/fmed.2025.1496814
Received: 15 September 2024; Accepted: 13 January 2025;
Published: 04 February 2025.
Edited by:
Yutaka Yoshii, The Jikei University School of Medicine, JapanReviewed by:
Michael John Calcutt, University of Missouri, United StatesHe Zhou, Fourth Military Medical University, China
Copyright © 2025 Lin, Jiang, Chi, Chen, Wen, Zhang, Wang and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guang-Liang Xie, eGd1YW5nbGlhbmdAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Yi Lin
Yi Lin Zhao-Zhao Jiang1†
Zhao-Zhao Jiang1† Guang-Liang Xie
Guang-Liang Xie