
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 26 February 2025
Sec. Gastroenterology
Volume 12 - 2025 | https://doi.org/10.3389/fmed.2025.1487892
This article is part of the Research Topic Diverticulitis - A Neglected Disease Despite its Clinical Burden View all 4 articles
 Antonio Tursi1,2*‡
Antonio Tursi1,2*‡ Giorgia Procaccianti3†
Giorgia Procaccianti3† Rudi De Bastiani4
Rudi De Bastiani4 Silvia Turroni3†
Silvia Turroni3† Federica D’Amico3
Federica D’Amico3 Leonardo Allegretta5
Leonardo Allegretta5 Natale Antonino6
Natale Antonino6 Elisabetta Baldi4
Elisabetta Baldi4 Carlo Casamassima7
Carlo Casamassima7 Giovanni Casella4
Giovanni Casella4 Mario Ciuffi4
Mario Ciuffi4 Marco De Bastiani4
Marco De Bastiani4 Lorenzo Lazzarotto4
Lorenzo Lazzarotto4 Claudio Licci8
Claudio Licci8 Maurizio Mancuso4
Maurizio Mancuso4 Antonio Penna9
Antonio Penna9 Giuseppe Pranzo10
Giuseppe Pranzo10 Guido Sanna4
Guido Sanna4 Cesare Tosetti4
Cesare Tosetti4 Maria Zamparella4
Maria Zamparella4 Marcello Picchio11
Marcello Picchio11The role of gut microbiota (GM) in the pathogenesis of Symptomatic Uncomplicated Diverticular Disease (SUDD) remains controversial. Here, we assessed the efficacy of a butyrate formulation in modulating GM and abdominal pain in patients with SUDD. A retrospective study was conducted in patients with SUDD who were treated with a delayed- and colonic-release formulation of butyrate (two capsules bid, for a total dose of 400 mg butyrate) for 3 months. GM was profiled before (T0) and after 90 days of treatment (T2) using 16S rRNA amplicon sequencing. The primary endpoint was change in GM at T2; secondary endpoints were reduction in abdominal pain severity according to VAS (Visual Analog Scale, 0: absence; 10: maximum severity) at T1 (45 days) and T2, stool characteristics according to the Bristol stool form scale at T0, T1 and T2, and safety of treatment. Fifty-nine patients with SUDD (59.3% male; median age 65.5 years, interquartile range 55–71 years) completed treatment. The butyrate formulation increased GM diversity and resulted in several compositional changes that were closely related to baseline abdominal pain severity. Regarding secondary endpoints, abdominal pain decreased significantly over time, while the Bristol stool form scale did not. Mild adverse events were recorded in 3 (5.08%) patients. This study showed that a microencapsulated and colonic-release formulation of butyrate favorably modulates GM and reduces abdominal pain in patients with SUDD.
Symptomatic Uncomplicated Diverticular Disease (SUDD) is the most common clinical presentation in patients with colonic diverticulosis, with a prevalence of up to 20% (1). Although the pathogenesis of SUDD is not clearly understood, heredity, inflammation, motility alterations and gut microbiota (GM) imbalance (i.e., dysbiosis) are the main pathogenetic factors hypothesized. In particular, the GM of patients with SUDD has been found to be depleted in taxa with anti-inflammatory properties, such as Clostridium cluster IV, Clostridium cluster IX, Fusobacterium, and Lactobacillaceae (2), while enriched in mucin-degrading bacteria, such as Akkermansia muciniphila (3). More recently, our work using next-generation sequencing has confirmed that specific taxa may be related to SUDD, but the associations vary depending on the severity of abdominal pain (4).
Regarding treatment options, dietary fiber appears to be useful in preventing the onset of colonic diverticulosis, but there is no clear evidence of its benefit in SUDD (5–8). Mesalazine, an intestinal anti-inflammatory drug, may be effective in the symptoms of SUDD and in the prevention of diverticulitis (9), but not in the prevention of recurrence of diverticulitis (10). Probiotics, particularly bifidobacteria, appear to be effective in the prevention of SUDD (3, 11), even in primary care settings (12). Recent studies in general practice have confirmed the high efficacy, safety and excellent tolerability of rifaximin, a non-absorbable enteric antibiotic (12–15). Very often, however, treatment to prevent the symptoms of SUDD is given in a pulsatile manner (7–10 days per month) without scientific evidence (16). Among foods for special purposes, butyric acid may be effective in SUDD by improving gut health in a variety of ways (16, 17). In fact, butyrate is a short-chain fatty acid (SCFA) that serves as an important source of energy for colonocytes, regulates motility, pH and blood flow, improves mucosal barriers, and exerts antioxidant, anti-inflammatory and antimicrobial properties (18). It has been successfully used in the form of sodium butyrate in the treatment of several pathologies affecting the colon, ranging from irritable bowel syndrome (IBS) (19) to inflammatory bowel disease (IBD) (20), but no data are available for SUDD.
Here, we aimed to evaluate the efficacy and safety of a delayed- and colonic-release butyrate formulation in modulating GM and abdominal pain in patients with SUDD treated in primary care. As general practitioners (GPs) often diagnose the disease and request a GM assessment for patients complaining of gastrointestinal symptoms (5, 21), such a study could be helpful for primary care, which is increasingly involved in the management of patients with SUDD.
We retrospectively assessed the impact of a delayed- and colonic-release butyrate formulation (Butyrose®, SILA Spa) in a population of patients with SUDD managed in primary care by GPs and territorial gastroenterologists. We analyzed stool samples collected by fecal swab for microbiological studies and stored at the Unit of Microbiome Science and Biotechnology, Department of Pharmacy and Biotechnology, University of Bologna (Bologna, Italy). Among them, we identified SUDD patients whose fecal samples were collected before and after treatment with Butyrose® between 1 March 2022 and 1 March 2023. All fecal swabs were collected using the eNAT® System (Copan, Brescia, Italy), and shipped to the Unit of Microbiome Science and Biotechnology, Department of Pharmacy and Biotechnology, University of Bologna (Bologna, Italy), where they were stored at −80°C until processing.
A common database was created to collect the following demographic and clinical data at baseline: gender; age at diagnosis; smoking habit; disease duration; comorbidities; concomitant medications; body mass index; method used to pose the diagnosis of SUDD [colonoscopy, computed tomography (CT), ultrasonography] (1); type of diet followed: Mediterranean diet, predominantly meat-based diet (i.e., more than 5 meals per week based on meat), predominantly fish-based diet (i.e., more than 5 meals per week based on fish), predominantly plant-based diet (i.e., more than 5 meals per week based on fruit and vegetables), vegetarian diet [i.e., a diet that excludes meat (fresh or processed, including cured meats) and fish, but includes the consumption of animal products such as dairy products, eggs and honey] (22, 23), vegan diet, i.e., a diet that excludes all foods of animal origin, such as meat and fish, but also dairy products, eggs and honey (22, 24). The severity of abdominal pain was measured using a 10-point visual analog scale (VAS) before treatment (T0), after 45 days of treatment and at the end of treatment (T2).
The study was conducted according to clinical practice guidelines and following the principles of the Declaration of Helsinki. All patients gave written informed consent before undergoing endoscopy and/or CT scan and/or fecal sampling. Ethic committee approval for this retrospective study was obtained from Azienda Ospedaliero-Universitaria “Ospedali Riuniti,” Foggia, Italy (PROT. 164/CE/2023, October 23, 2023).
Inclusion criteria were: males and females aged >18 years; colonic diverticulosis diagnosed by colonoscopy or imaging (abdominal CT scan and/or ultrasonography); diagnosis of SUDD (defined as left-lower and long-lasting quadrant pain in patients with diverticulosis) (25) during the 6 months prior to enrolment; possibility of retrospectively reconstructing the symptoms and clinical history of patients with SUDD; availability of a fecal sample before (T0) and after (T2) 90 days of Butyrose® supplementation for GM assessment (see also “Primary endpoint”).
Exclusion criteria were: current or previous diagnosis (by abdominal CT and/or ultrasonography) of acute diverticulitis (defined as inflammation of the colonic wall harboring diverticula with fat stranding, with or without complications such as abscesses, stenosis or fistulas, namely uncomplicated or complicated diverticulitis) (1); IBD; ischemic colitis; prior colonic resection; patients with severe liver failure (Child-Pugh C); patients with severe kidney failure; pregnant women; women of childbearing potential not using a highly effective method of contraception; patients currently using or who have received any laxative agents <4 weeks prior to enrolment; patients currently using or who have received any mesalamine compounds <4 weeks prior to enrolment; patients currently using or who have received any probiotic agents <4 weeks prior to enrolment; use of non-steroidal anti-inflammatory drugs (NSAIDs; except for acetyl-salicylic acid ≤100 mg/day) <4 weeks prior to enrolment; patients treated with antibiotics (including those not absorbed) <4 weeks prior to enrolment; patients with a history of cancer, of any origin, at the time of SUDD diagnosis and/or under treatment with chemotherapy and/or radiotherapy; a history of alcohol, drug, or chemical abuse; patients with a current or recent (≤3 months) episode of COVID-19 (26).
As noted above, only patients taking Butyrose® (two tablets per day, 1 after lunch and 1 after dinner, for a total of 1,100 mg of sodium butyrate per day) as mono-therapy for 3 months were enrolled. Butyrose® is a micro-encapsulated sodium butyrate-based supplement manufactured with a modified-release LSC MicroCaps® delivery mechanism (EU patent 2,352,386) for exclusive release in the colon. This formulation was studied because sodium butyrate is a very active molecule but has a high degree of dissociation (pKa 4.82) and would not reach the colon/rectum without an adequate delayed-release mechanism. Butyrose® is manufactured and marketed by SILA s.p.a. (Noale, VE, Italy) and its marketing was notified to the Italian Regulatory Authorities in October 2021.
Fecal samples were processed as described by Tursi et al. (4). Briefly, swabs were vortexed and centrifuged at 13,000 rpm for 10 min at 4°C. The pellets were resuspended in 1 mL of lysis buffer (500 mM NaCl, 50 mM Tris–HCl pH 8, 50 mM EDTA, and 4% SDS) and subjected to three rounds of bead-beating in a FastPrep instrument (MP Biomedicals, Irvine, CA, United States) at 5.5 movements/s for 1 min, in the presence of four 3-mm glass beads and 0.5 g of 0.1-mm zirconia beads (BioSpec Products, Bartlesville, OK, United States). After incubation at 95°C for 15 min, the samples were centrifuged at 13,000 rpm for 5 min, and the supernatants were added with 260 μL of 10 M ammonium acetate for protein precipitation. After centrifugation at 13,000 rpm for 10 min, nucleic acids were precipitated with isopropanol, and the pellets were washed with 70% ethanol before being resuspended in 100 μL of TE buffer (10 mM Tris–HCl, 1 mM EDTA, pH 8.0). After treatment with 2 μL of 10 mg/mL DNase-free RNase at 37°C for 15 min, DNA was purified using the DNeasy Blood and Tissue Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. DNA quantity and quality were assessed using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, United States).
The V3-V4 hypervariable regions of the 16S rRNA gene were amplified using the 341F and 785R primers with Illumina adapter overhangs as previously described (27). After amplicon purification using a magnetic bead-based clean-up system (Agencourt AMPure XP, Beckman Coulter, Brea, CA, United States), indexed libraries were prepared by limited-cycle PCR using Nextera technology, further purified and pooled at an equimolar concentration of 4 nM. The pool was denatured and diluted to 5 pM prior to sequencing on an Illumina MiSeq platform (Illumina, San Diego, CA, United States) using a 2 × 250 bp paired-end protocol. Raw sequencing data were deposited in the National Center for Biotechnology Information Sequence Read Archive (BioProject ID: PRJNA1216941).
Raw sequences were processed using PANDASeq (28) and QIIME 2 (29) and filtered for length and quality. Amplicon sequence variants (ASVs) were identified using DADA2 (30) and taxonomically classified using the VSEARCH algorithm (31) against the SILVA database (August 2020 release) (32). Alpha diversity was calculated using various metrics, such as the number of observed ASVs, the Shannon index and Faith’s phylogenetic diversity. Beta diversity was calculated using UniFrac distances, which were used to construct Principal Coordinates Analysis (PCoA) plots.
We retrospectively evaluated the impact of a butyrate formulation (Butyrose®) on the GM of a cohort of patients with SUDD. GM was assessed using next-generation sequencing of fecal samples collected before (T0) and after (T2) 90 days of treatment.
The following secondary endpoints were evaluated:
• Efficacy of the butyrate formulation (Butyrose®) in reducing abdominal pain in patients with SUDD, defined as at least a 50% reduction in abdominal pain, assessed at T0, T1 (after 45 days of treatment) and T2 using VAS (0: absence; 10: maximum severity);
• Characteristics of evacuation according to the Bristol stool form scale (33) at T0, T1 and T2;
• Safety of therapy, assessed as the number and type of adverse events observed during the 3 months of treatment.
All statistical analyses were performed using R software and the vegan1 and Made4 (34) packages. GM data separation in PCoA was tested using a permutation test with pseudo-F ratio (PERMANOVA). Pre-post differences in alpha diversity, relative taxon abundance and abdominal pain were assessed using the Wilcoxon signed-rank test. The chi-square test was used to compare pre-post differences in Bristol stool form scale. p-values were adjusted for multiple comparisons using the Benjamin-Hochberg method, with a false discovery rate (FDR) ≤0.05 considered statistically significant.
According to the inclusion and exclusion criteria, 72 patients with SUDD treated with Butyrose® were identified. Of these, 59 patients completed the 3 months of treatment, while 13 did not and were excluded from the final evaluation: 4 patients were lost at follow-up, 1 developed a urinary tract infection, 1 had a recurrence of SUDD, 1 developed acute uncomplicated diverticulitis, and 6 received adjunctive treatment for symptom control (2 with mesalazine, 3 with rifaximin, and 1 with mesalazine plus rifaximin). Demographic and clinical characteristics of the patients are reported in Table 1.
Fecal samples were collected from 59 SUDD patients before and after 90 days of taking Butyrose® and analyzed for GM changes.
A significant increase in alpha diversity was observed after treatment (Wilcoxon test, p < 0.001; Figure 1A). Similarly, PCoA based on weighted UniFrac distances showed a significant separation before and after treatment (PERMANOVA, p = 0.001; Figure 1B). From the taxonomic point of view, many differences emerged. In particular, the phylum Desulfobacterota, the families Eggerthellaceae, Rikenellaceae (and its genus Alistipes), Barnesiellaceae (and Barnesiella) and Desulfovibrionaceae (and Desulfovibrio), and the genera Lachnospiraceae_NK4A136_group and Oscillibacter were enriched after treatment (Wilcoxon test, p < 0.05; Figures 1C–E).
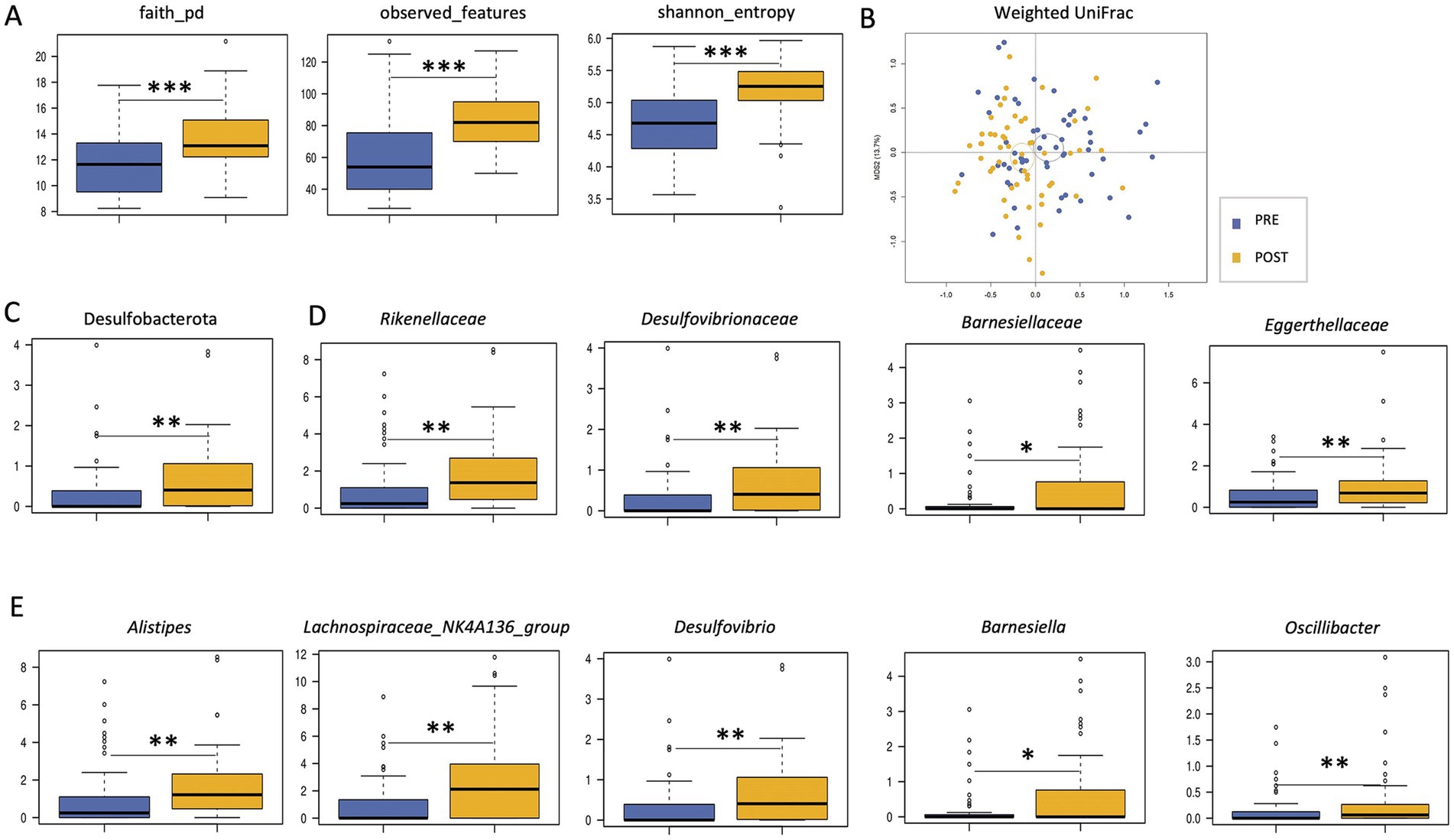
Figure 1. Impact of Butyrose® on the gut microbiota of SUDD patients. (A) Boxplots showing the distribution of alpha diversity, computed according to Faith’s phylogenetic diversity, number of observed ASVs and Shannon entropy, in the gut microbiota of SUDD patients before (PRE) and after (POST) 90 days of Butyrose® supplementation. (B) Principal Coordinates Analysis (PCoA) based on weighted UniFrac distances between groups. Ellipses include 95% confidence area based on the standard error of the weighted average of sample coordinates. A significant separation was found (PERMANOVA, p = 0.001). Boxplots showing the relative abundance distribution of phyla (C), families (D) and genera (E) differentially represented between groups. Wilcoxon test, *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001.
SUDD patients were stratified by basal abdominal pain severity [mild (VAS score 1–3) vs. moderate (VAS score 4–7) vs. severe (VAS score 8–10)] and all analyses were repeated. The treatment-related increase in alpha diversity was confirmed in all severity groups (Wilcoxon test, p < 0.01; Figure 2A). For beta diversity, a significant segregation among groups was observed in the weighted UniFrac-based PCoA (PERMANOVA, p = 0.019; Figure 2B). Taxonomically, none of the differences observed in the overall cohort were replicated in all groups, suggesting that the impact of Butyrose® was dependent on basal abdominal pain severity. In particular, the increase in the family Rikenellaceae (and its genus Alistipes) was significant only in the severe group, the increase in Barnesiellaceae (and Barnesiella) only in the mild group, and the increase in Lachnospiraceae_NK4A136_group only in the moderate group (Wilcoxon test, p < 0.05; Figures 2C,D). Furthermore, the mild group showed enrichment in Coriobacteriaceae (and Collinsella) and Oscillospirales_UCG-010 (p < 0.05). The moderate group showed enrichment in [Eubacterium] coprostanoligenes_group and depletion in Blautia (p < 0.05). Finally, the severe group showed enrichment in Lachnospiraceae, Bacteroidaceae (and Bacteroides), Erysipelotrichaceae_UCG-003 and Phascolarctobacterium, and depletion in Veillonellaceae (and Dialister; p < 0.05).
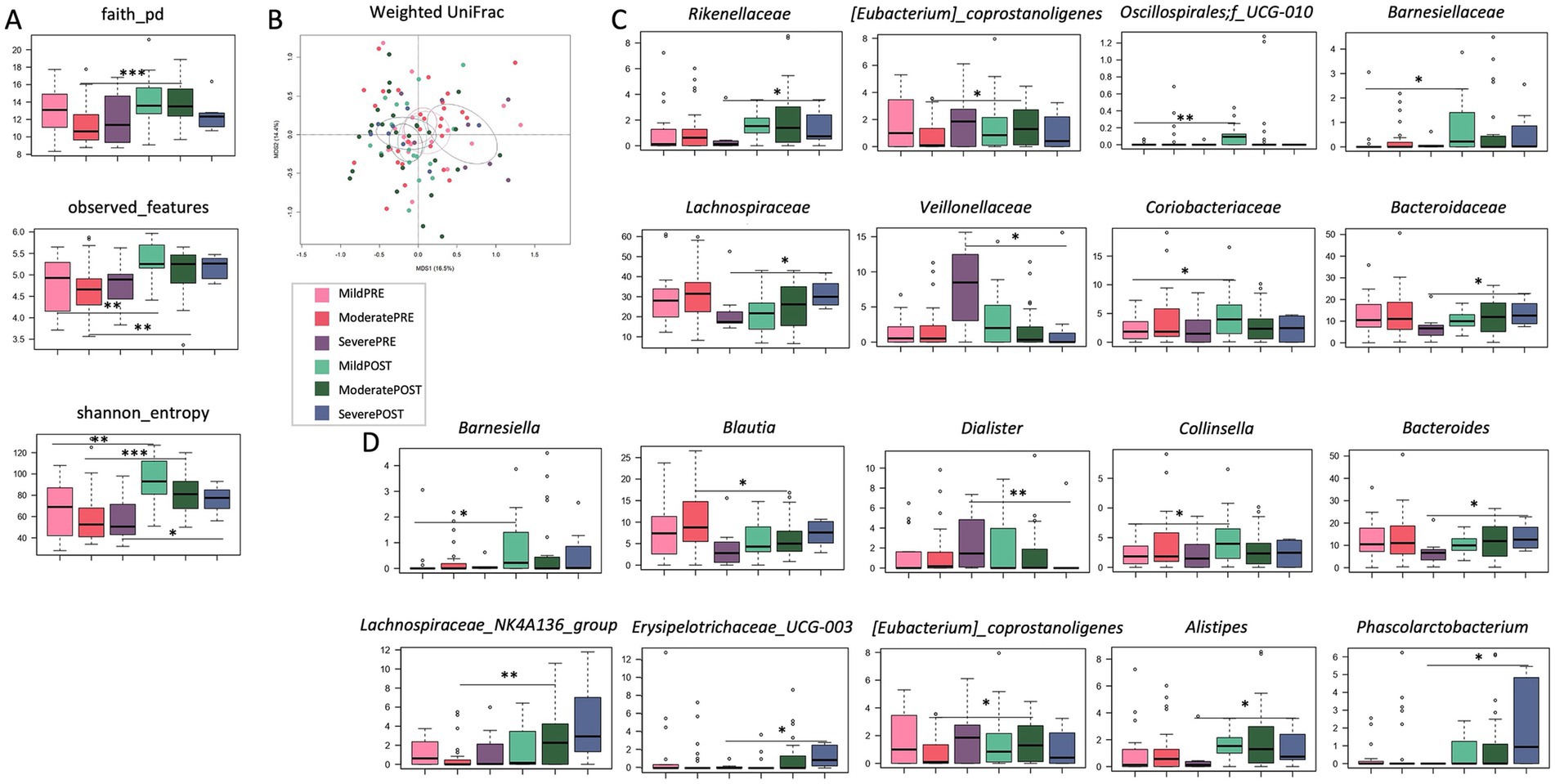
Figure 2. Effects of Butyrose® according to basal abdominal pain severity. (A) Boxplots showing the distribution of alpha diversity, computed according to Faith’s phylogenetic diversity, number of observed ASVs and Shannon entropy, in the gut microbiota of SUDD patients stratified by abdominal pain severity [estimated by visual analog scale, VAS: mild (VAS score 1–3) vs. moderate (VAS score 4–7) vs. severe (VAS score 8–10)] before (PRE) and after (POST) 90 days of Butyrose® supplementation. (B) Principal Coordinates Analysis (PCoA) based on weighted UniFrac distances between groups. A significant separation was found (PERMANOVA, p = 0.019). Boxplots showing the relative abundance distribution of families (C) and genera (D) differentially represented between groups. Wilcoxon test, *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001.
Abdominal pain decreased significantly over time [median (interquartile range, IQR) VAS score: 5 (3–10) at T0, 2 (1–3.7) at T1 and 1 (0–2) at T2; Wilcoxon test, p < 0.000; Figure 3]. The Bristol stool form scale did not change significantly over time (p = 0.996; Table 2). Adverse events were recorded in 3 patients (5.08%): they were mild (1 patient complained of nausea and 2 of diarrhea), and did not require discontinuation. Notably, all these events occurred during a concurrent epidemic of viral gastroenteritis.
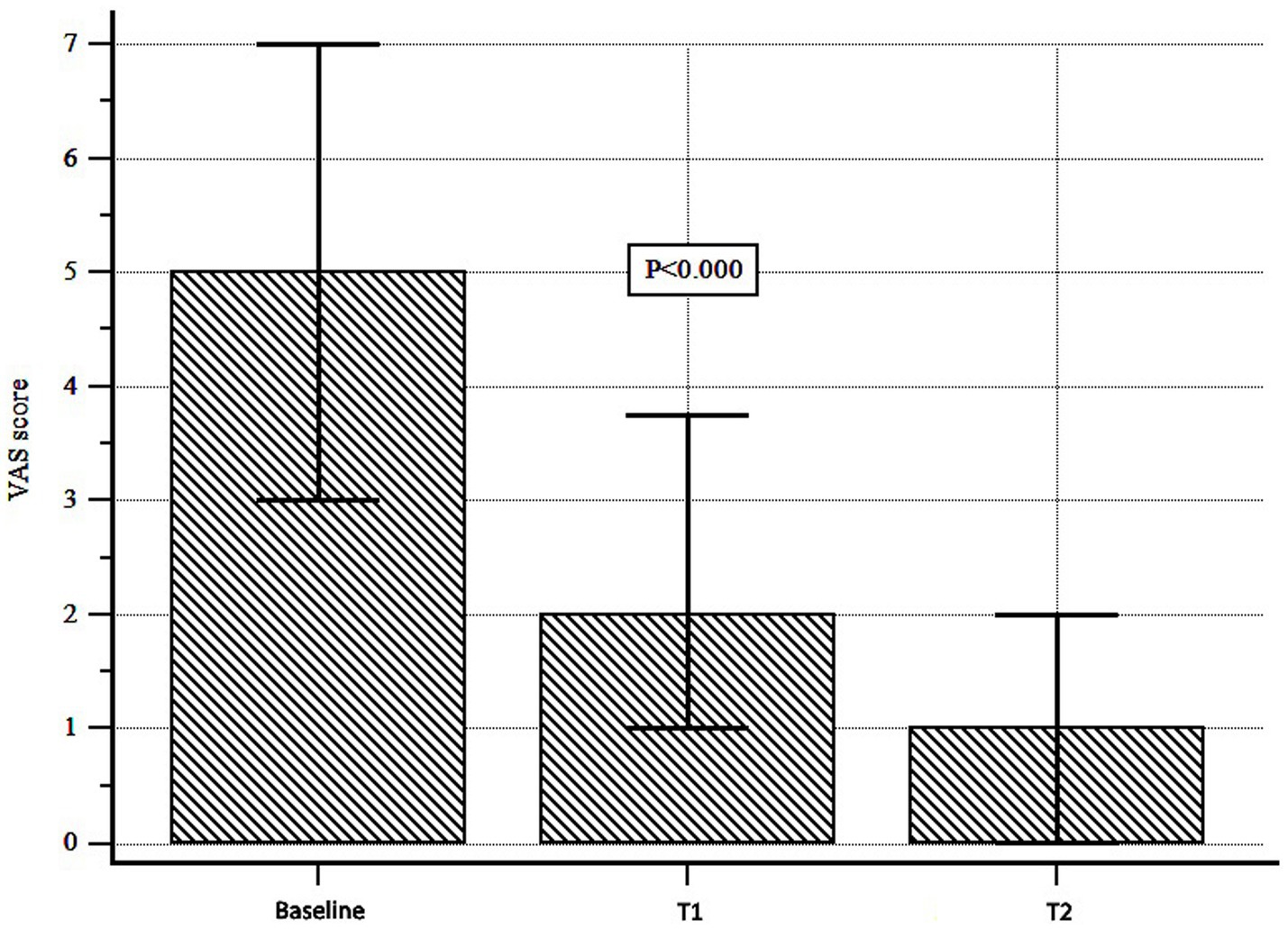
Figure 3. Abdominal pain assessment during Butyrose® treatment. Abdominal pain was assessed by visual analog scale (VAS). A significant reduction was observed over time (Wilcoxon test, p < 0.000).
Here, we investigated the effects of a butyrate formulation (Butyrose®) on GM in patients with SUDD. Moreover, we investigated the effect of this formulation on abdominal pain and its association with GM.
With regard to the primary endpoint, namely the impact of Butyrose® on GM, we found that treatment resulted in an increase in alpha diversity, the reduction of which is a typical hallmark of dysbiosis in a variety of diseases, both local and systemic (35). At the compositional level, the patients’ GM was found to be enriched in several taxa after treatment, including Desulfovibrio (and its family Desulfovibrionaceae and its phylum Desulfobacterota), Alistipes (and its family Rikenellaceae), Barnesiella (and Barnesiellaceae), Eggerthellaceae, Lachnospiraceae_NK4A136_group and Oscillibacter. In particular, Desulfovibrio belongs to sulfate-reducing bacteria, which are considered pathobionts that may contribute to intestinal and extra-intestinal diseases, probably through the production of hydrogen sulfide, lipopolysaccharide and mucolytic enzymes, and the secretion of outer membrane vesicles (36). Although apparently unfavorable, the increase in sulfate-reducing bacteria may be related to their ability to use various organic compounds, including butyric acid, as electron donors for sulfate reduction (37–39). It should be noted that some Desulfovibrio species/strains appear to have more pathogenic potential than others, particularly in unhealthy individuals (36), highlighting the need for high-resolution taxonomic profiling and in-depth assessment of context-dependent features, including interaction networks within the microbial community and with the host. Another potentially harmful genus enriched after treatment was Alistipes, a bile-tolerant microorganism that has been shown to contribute to some diseases but also to protect against others (40), again emphasizing the need for further studies, including animal models, to better understand its role in health and disease. On the other hand, the increases in the SCFA producers Oscillibacter and Lachnospiraceae_NK4A136_group can certainly be considered positive, in addition to being consistent with the available literature on the effects of Butyrose® on the GM of patients with IBD (41). In particular, Lachnospiraceae_NK4A136_group has been shown to have protective and anti-inflammatory effects, including improvement of gut barrier function (42–44). Oscillibacter, together with Alistipes species, has recently been found to be enriched in subjects with lower plasma triglycerides and glucose and higher plasma HDL in ethnically distinct cohorts (45), suggesting a role in cardiovascular health, the impairment of which may be related to SUDD (46).
Based on our previous work showing that the GM of SUDD patients stratified by severity of abdominal pain (as assessed by VAS) (4), we next investigated the effects of Butyrose® in patients with mild vs. moderate vs. severe SUDD. While the increase in alpha diversity was common to all severity groups, the compositional variations were closely related to baseline severity (of abdominal pain and dysbiosis), with the severe group showing an overall greater rearrangement compared to the other groups. In particular, the severe group showed an enrichment in Rikenellaceae (and Alistipes), Lachnospiraceae, Bacteroidaceae (and Bacteroides), Erysipelotrichaceae_UCG-003 and Phascolarctobacterium, and a depletion in Veillonellaceae (and Dialister) after treatment. Again, some changes could be beneficial, such as those in the SCFA producers Lachnospiraceae and Phascolarctobacterium (47), while others could not, notably that in Erysipelotrichaceae_UCG-003, which has previously been linked to intestinal dysfunction, inflammation and metabolic disorders (48). Conversely, the other severity groups showed fewer changes, in line with the lower extent of their dysbiosis, namely an enrichment in Lachnospiraceae_NK4A136_group and [Eubacterium] coprostanoligenes_group and a depletion in Blautia in the moderate group, and an enrichment in Barnesiellaceae (and Barnesiella), Coriobacteriaceae (and Collinsella) and Oscillospirales_UCG-010 in the mild group. Among these, it is worth noting that Blautia has previously been positively associated with gastrointestinal symptoms (particularly diarrhea) and IBS (49), making its reduction potentially desirable also in the context of SUDD. On the other hand, the increase in Collinsella is again questionable, as this genus has been negatively associated with IBS severity (50), but is also known as a pathobiont capable of increasing gut permeability and triggering pro-inflammatory cytokines (51, 52).
With regard to secondary endpoints, we found that Butyrose® was effective in controlling abdominal pain, the main symptom characterizing SUDD patients. This was expected as butyrate is a SCFA that, among other effects, improves gut permeability, increases the rate of cell regeneration, reduces oxidative stress and mucosal inflammation (18), and has previously been shown to be reduced in stool samples from patients with SUDD (4, 5). In contrast, no changes in stool appearance were recorded under treatment with Butyrose®. This may mean that other factors, such as colonic motility, are involved in determining stool form in these patients and/or that the type of fecal output did not influence the clinical response to butyrate. Importantly, however, this means that Butyrose® does not cause constipation, an adverse event that has often been hypothesized but never confirmed with older formulations of butyrate (53). As for adverse events, their incidence was quite low in our population (~5%). However, it is not easy to explain this occurrence, in particular for patients experiencing diarrhea. In fact, sodium butyrate is recommended for the treatment of diarrheal disorders such as traveler’s diarrhea (54). However, we are not sure that these adverse events could really be related to butyrate, as they all occurred during a concurrent epidemic of viral gastroenteritis.
The main limitations of the study include: (i) the use of 16S rRNA amplicon sequencing, which is still the gold standard for microbiota profiling, but does not allow high-resolution taxonomic profiling down to species level and functional insights; (ii) the lack of mechanistic information; and (iii) the retrospective design. In particular, the retrospective design may have led to the loss of information that could have influenced the final results (e.g., recall of symptom severity may not have been reliable, and GM-associated confounding factors, such as proton pump inhibitor use, may not have been captured).
In conclusion, Butyrose® supplementation for 90 days significantly modulated the GM of patients with SUDD. Specifically, it led to increased diversity and some compositional changes that were closely related to baseline abdominal pain severity. Some changes may be beneficial, such as the increase in SCFA producers, while others may not be, such as the increase in taxa with dubious pathogenic potential. Regardless of the significance of the increase in these taxa, these changes in GM were associated with a significant improvement in abdominal pain. Further studies in larger cohorts with longer observation periods and using other omics, such as metagenomics and metabolomics, are needed to validate our findings and to gain deeper insights into the impact of Butyrose® on SUDD, including the composition (down to species level, including interaction networks) and function of the GM. Such studies could pave the way for personalized intervention strategies based on disease severity for faster and more effective resolution of symptoms.
The raw sequencing data of this study were deposited in the National Center for Biotechnology Information Sequence Read Archive (BioProject ID: PRJNA1216941) https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA1216941.
The study was conducted according to clinical practice guidelines and following the principles of the Declaration of Helsinki. All patients gave written informed consent before undergoing endoscopy and/or CT scan and/or fecal sampling. Ethic committee approval for this retrospective study was obtained from Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Foggia, Italy (PROT. 164/CE/2023, October 23, 2023).
AT: Conceptualization, Data curation, Formal analysis, Investigation, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, Methodology. GoP: Data curation, Formal analysis, Methodology, Software, Writing – review & editing. RB: Conceptualization, Investigation, Resources, Writing – review & editing. ST: Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. FD'A: Writing – review & editing, Data curation, Formal analysis, Methodology, Software. LA: Investigation, Resources, Writing – review & editing. NA: Writing – review & editing, Investigation, Resources. EB: Investigation, Resources, Writing – review & editing. CC: Investigation, Resources, Writing – review & editing. GC: Investigation, Resources, Writing – review & editing. MC: Investigation, Resources, Writing – review & editing. MB: Investigation, Resources, Writing – review & editing. LL: Investigation, Resources, Writing – review & editing. CL: Investigation, Resources, Writing – review & editing. MM: Investigation, Resources, Writing – review & editing. AP: Investigation, Resources, Writing – review & editing. GuP: Investigation, Resources, Writing – review & editing. GS: Investigation, Resources, Writing – review & editing. CT: Investigation, Resources, Writing – review & editing. MZ: Investigation, Resources, Writing – review & editing. MP: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer BS declared a shared affiliation with the authors GoP, ST, and FD’A to the handling editor at time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Tursi, A, Scarpignato, C, Strate, LL, Lanas, A, Kruis, W, Lahat, A, et al. Colonic diverticular disease. Nat Rev Dis Prim. (2020) 6:20. doi: 10.1038/s41572-020-0153-5
2. Barbara, G, Scaioli, E, Barbaro, MR, Biagi, E, Laghi, L, Cremon, C, et al. Gut microbiota, metabolome and immune signatures in patients with uncomplicated diverticular disease. Gut. (2017) 66:1252–61. doi: 10.1136/gutjnl-2016-312377
3. Tursi, A, Mastromarino, P, Capobianco, D, Elisei, W, Miccheli, A, Capuani, G, et al. Assessment of fecal microbiota and fecal metabolome in symptomatic uncomplicated diverticular disease of the Colon. J Clin Gastroenterol. (2016) 50:S9–S12. doi: 10.1097/MCG.0000000000000626
4. Tursi, A, De Bastiani, R, Turroni, S, Procaccianti, S, D’Amico, F, Allegretta, L, et al. Gut microbiota in symptomatic uncomplicated diverticular disease stratifies by severity of abdominal pain. Eur J Gastroenterol Hepatol. (2025) 37:147–53. doi: 10.1097/MEG.0000000000002884
5. Crowe, FL, Balkwill, A, Cairns, BJ, Appleby, PN, Green, JN, and Greeves, GK. Et al; million women study collaborators; million women study collaborators. Source of dietary fibre and diverticular disease incidence: a prospective study of UK women. Gut. (2014) 63:1450–6. doi: 10.1136/gutjnl-2013-304644
6. Aldoori, WH, Giovannucci, EL, Rockett, HR, Sampson, L, Rimm, EB, and Willett, WC. A prospective study of dietary fiber types and symptomatic diverticular disease in men. J Nutr. (1998) 128:714–9. doi: 10.1093/jn/128.4.714
7. Crowe, FL, Appleby, PN, Allen, NE, and Key, TJ. Diet and risk of diverticular disease in Oxford cohort of European prospective investigation into Cancer and nutrition (EPIC): prospective study of British vegetarians and non-vegetarians. Br Med J. (2011) 343:d4131. doi: 10.1136/bmj.d4131
8. Ünlü, C, Daniels, L, Vrouenraets, BC, and Boermeester, MA. A systematic review of high-fibre dietary therapy in diverticular disease. Int J Color Dis. (2012) 27:419–27. doi: 10.1007/s00384-011-1308-3
9. Picchio, M, Elisei, W, and Tursi, A. Mesalazine to treat symptomatic uncomplicated diverticular disease and to prevent acute diverticulitis occurrence. A systematic review with meta-analysis of randomized, placebo-controlled trials. J Gastrointestin Liver Dis. (2018) 27:291–7. doi: 10.15403/jgld.2014.1121.273.pic
10. Carter, F, Alsayb, M, Marshall, JK, and Yuan, Y. Mesalamine (5-ASA) for the prevention of recurrent diverticulitis. Cochrane Database Syst Rev. (2017) 2017:CD009839. doi: 10.1002/14651858.CD009839.pub2
11. Scarpignato, C, Bertelé, A, and Tursi, A. Probiotics for the treatment of symptomatic uncomplicated diverticular disease: rationale and current evidence. J Clin Gastroenterol. (2016) 50:S70–3. doi: 10.1097/MCG.0000000000000641
12. Campanini, A, De Conto, U, Cavasin, F, Bastiani, F, Camarotto, A, Gardini, L, et al. A primary-care interventional model on the diverticular disease: searching for the optimal therapeutic schedule. J Clin Gastroenterol. (2016) 50:S93–6. doi: 10.1097/MCG.0000000000000670
13. di, F, Miraglia, C, Cambiè, G, Violi, A, Nouvenne, A, Franceschi, M, et al. Long-term efficacy of rifaximin to manage the symptomatic uncomplicated diverticular disease of the colon. J Investig Med. (2019) 67:767–70. doi: 10.1136/jim-2018-000901
14. Moniuszko, A, and Rydzewska, G. The effect of cyclic rifaximin therapy on symptoms of diverticular disease from the perspective of the gastroenterology outpatient clinic: a “real-life” study. Gastroenterol Rev. (2017) 2:145–51. doi: 10.5114/pg.2017.68167
15. Stallinger, S, Eller, N, and Högenauer, C. Non-interventional study evaluating efficacy and tolerability of rifaximin for treatment of uncomplicated diverticular disease. Wien Klin Wochenschr. (2014) 126:9–14. doi: 10.1007/s00508-013-0447-7
16. de, R, Sanna, G, Bertolusso, L, Casella, G, de, M, Zamparella, M, et al. General practitioners' management of symptomatic uncomplicated diverticular disease of the colon by using rifaximin, a non-adsorbable antibiotic. Eur Rev Med Pharmacol Sci. (2021) 25:423–30. doi: 10.26355/eurrev_202101_24410
17. Krokowicz, L, Stojcev, Z, Kaczmarek, BF, Kociemba, W, Kaczmarek, E, Walkowiak, J, et al. Microencapsulated sodium butyrate administered to patients with diverticulosis decreases incidence of diverticulitis--a prospective randomized study. Int J Color Dis. (2014) 29:387–93. doi: 10.1007/s00384-013-1807-5
18. Koh, A, De Vadder, F, Kovatcheva-Datchary, P, and Bäckhed, F. From dietary Fiber to host physiology: short-chain fatty acids as Key bacterial metabolites. Cell. (2016) 165:1332–45. doi: 10.1016/j.cell.2016.05.041
19. Vernero, M, de, F, Ribaldone, DG, Bugianesi, E, Pellicano, R, Saracco, GM, et al. The usefulness of microencapsulated sodium butyrate add-on therapy in maintaining remission in patients with ulcerative colitis: a prospective observational study. J Clin Med. (2020) 9:3941. doi: 10.3390/jcm9123941
20. Firoozi, D, Masoumi, SJ, Mohammad-Kazem Hosseini Asl, S, Labbe, A, Razeghian-Jahromi, I, Fararouei, M, et al. Effects of short-chain fatty acid-butyrate supplementation on expression of circadian-clock genes, sleep quality, and inflammation in patients with active ulcerative colitis: a double-blind randomized controlled trial. Lipids Health Dis. (2024) 23:216. doi: 10.1186/s12944-024-02203-z
21. Wensaas, KA, and Hungin, AP. Diverticular disease in the Primay care setting. J Clin Gastroenterol. (2016) 50:S86–8. doi: 10.1097/MCG.0000000000000596
22. Craig, WJ, and Mangels, AR. Position of the American dietetic association: vegetarian diets. J Am Diet Assoc. (2009) 109:1266–82. doi: 10.1016/j.jada.2009.05.027
23. NHS Choices . The vegetarian diet (Inglese). (2022). Available at: https://www.nhs.uk/live-well/eat-well/how-to-eat-a-balanced-diet/the-vegetarian-diet/ (accessed on 11th March, 2024)
24. NHS Choices . The vegan diet (Inglese). (2022). Available at: https://www.nhs.uk/live-well/eat-well/how-to-eat-a-balanced-diet/the-vegan-diet/ (accessed on 11th March, 2024)
25. Tursi, A, Elisei, W, Picchio, M, Giorgetti, GM, and Brandimarte, G. Moderate to severe and prolonged left-lower abdominal pain is the best symptom characterizing symptomatic uncomplicated diverticular disease of the colon: a comparison with fecal calprotectin in clinical setting. J Clin Gastroenterol. (2015) 49:218–21. doi: 10.1097/MCG.0000000000000094
26. Freedberg, DE, and Chang, L. Gastrointestinal symptoms in COVID-19: the long and the short of it. Curr Opin Gastroenterol. (2022) 38:555–61. doi: 10.1097/MOG.0000000000000876
27. D'Amico, F, Rinaldi, M, Pascale, R, Fabbrini, M, Morelli, MC, Siniscalchi, A, et al. Gut microbiome dynamics and Enterobacterales infection in liver transplant recipients: a prospective observational study. JHEP Reports. (2024) 6:101039. doi: 10.1016/j.jhepr.2024.101039
28. Masella, AP, Bartram, AK, Truszkowski, JM, Brown, DG, and Neufeld, JD. PANDAseq: paired-end assembler for illumina sequences. BMC Bioinformatics. (2012) 13:31. doi: 10.1186/1471-2105-13-31
29. Bolyen, E, Rideout, JR, Dillon, MR, Bokulich, NA, Abnet, CC, al-Ghalith, G, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. (2019) 37:852–7. doi: 10.1038/s41587-019-0209-9
30. Callahan, BJ, McMurdie, PJ, Rosen, MJ, Han, AW, Johnson, AJ, and Holmes, SP. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. (2016) 13:581–3. doi: 10.1038/nmeth.3869
31. Rognes, T, Flouri, T, Nichols, B, Quince, C, and Mahé, F. VSEARCH: a versatile open source tool for metagenomics. Peer J. (2016) 4:e2584. doi: 10.7717/peerj.2584
32. Quast, C, Pruesse, E, Yilmaz, P, Gerken, J, Schweer, T, Yarza, P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. (2013) 41:D590–6. doi: 10.1093/nar/gks1219
33. Heaton, KW, Radvan, J, Cripps, H, Mountford, RA, Braddon, FE, and Hughes, AO. Defecation frequency and timing, and stool form in the general population: a prospective study. Gut. (1992) 33:818–24. doi: 10.1136/gut.33.6.818
34. Culhane, AC, Thioulouse, J, Perrière, G, and Higgins, DG. MADE4: an R package for multivariate analysis of gene expression data. Bioinformatics. (2005) 21:2789–90. doi: 10.1093/bioinformatics/bti394
35. Hou, K, Wu, ZX, Chen, XY, Wang, JQ, Zhang, D, Xiao, C, et al. Microbiota in health and diseases. Signal Transduct Target Ther. (2022) 7:135. doi: 10.1038/s41392-022-00974-4
36. Singh, SB, Carroll-Portillo, A, and Lin, HC. Desulfovibrio in the gut: the enemy within? Microorganisms. (2023) 11:1772. doi: 10.3390/microorganisms11071772
37. Xiulan, S, and Zhang, Z. Notice of retraction. Sulfate reducing rate of SRB with acetic, propionic, n-butyric acids as carbon sources (2011). doi: 10.1109/ICBBE.2011.5781016
38. Balk, M, Altinbas, M, Rijpstra, WIC, Damsté, JSS, and Stams, AJM. Desulfatirhabdium butyrativorans gen. Nov., sp. nov., a butyrate-oxidizing, sulfate-reducing bacterium isolated from an anaerobic bioreactor. Int J Syst Evol Microbiol. (2008) 58:110–5. doi: 10.1099/ijs.0.65396-0
39. Suzuki, D, Ueki, A, Shizuku, T, Ohtaki, Y, and Ueki, K. Desulfovibrio butyratiphilus sp. nov., a gram-negative, butyrate-oxidizing, sulfate-reducing bacterium isolated from an anaerobic municipal sewage sludge digester. Int J Syst Evol Microbiol. (2010) 60:595–602. doi: 10.1099/ijs.0.013771-0
40. Parker, BJ, Wearsch, PA, Veloo, ACM, and Rodriguez-Palacios, A. The genus Alistipes: gut Bacteria with emerging implications to inflammation, Cancer, and mental health. Front Immunol. (2020) 11:906. doi: 10.3389/fimmu.2020.00906
41. Facchin, S, Vitulo, N, Calgaro, M, Buda, A, Romualdi, C, Pohl, D, et al. Microbiota changes induced by microencapsulated sodium butyrate in patients with inflammatory bowel disease. Neurogastroenterol Motil. (2020) 32:e13914. doi: 10.1111/nmo.13914
42. Xia, T, Duan, W, Zhang, Z, Li, S, Zhao, Y, Geng, B, et al. Polyphenol-rich vinegar extract regulates intestinal microbiota and immunity and prevents alcohol-induced inflammation in mice. Food Res Int. (2021) 140:110064. doi: 10.1016/j.foodres.2020.110064
43. Ma, L, Ni, Y, Wang, Z, Tu, W, Ni, L, Zhuge, F, et al. Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut Microbes. (2020) 12:1832857–19. doi: 10.1080/19490976.2020.1832857
44. Wu, M-R, Chou, T-S, Huang, C-Y, and Hsiao, J-K. A potential probiotic- Lachnospiraceae NK4A136 group: evidence from the restoration of the dietary pattern from a high-fat diet (2020). doi: 10.21203/RS.3.RS-48913/V1,
45. Li, C, Stražar, M, Mohamed, AMT, Pacheco, JA, Walker, RL, Lebar, T, et al. Gut microbiome and metabolome profiling in Framingham heart study reveals cholesterol-metabolizing bacteria. Cell. (2024) 187:1834–1852.e19. doi: 10.1016/j.cell.2024.03.014
46. Yamamichi, N, Shimamoto, T, Takahashi, Y, Sakaguchi, Y, Kakimoto, H, Matsuda, R, et al. Trend and risk factors of diverticulosis in Japan: age, gender, and lifestyle/metabolic-related factors may cooperatively affect on the colorectal diverticula formation. PLoS One. (2015) 10:e0123688. doi: 10.1371/journal.pone.0123688
47. Chen, Y-R, Zheng, H-M, Zhang, G, Chen, F-L, Chen, L-D, and Yang, Z-C. High Oscillospira abundance indicates constipation and low BMI in the Guangdong gut microbiome project. Sci Rep. (2020) 10:9364. doi: 10.1038/s41598-020-66369-z
48. Kaakoush, NO . Insights into the role of Erysipelotrichaceae in the human host. Front Cell Infect Microbiol. (2015) 5:84. doi: 10.3389/fcimb.2015.00084
49. Zhan, S, Liu, C, Meng, J, Mao, R, Tu, T, Lin, J, et al. Mucosa-associated Oscillospira sp. is related to intestinal stricture and post-operative disease course in Crohn’s disease. Microorganisms. (2023) 11:794. doi: 10.3390/microorganisms11030794
50. Malinen, E, Krogius-Kurikka, L, Lyra, A, Nikkilä, J, Jääskeläinen, A, Rinttilä, T, et al. Association of symptoms with gastrointestinal microbiota in irritable bowel syndrome. World J Gastroenterol. (2010) 16:4532–40. doi: 10.3748/wjg.v16.i36.4532
51. Gargari, G, Mantegazza, G, Cremon, C, Taverniti, V, Valenza, A, Barbaro, MR, et al. Collinsella aerofaciens as a predictive marker of response to probiotic treatment in non-constipated irritable bowel syndrome. Gut Microbes. (2024) 16:2298246. doi: 10.1080/19490976.2023.2298246
52. Chen, J, Wright, K, Davis, JM, Jeraldo, P, Marietta, EV, Murray, J, et al. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. (2016) 8:43. doi: 10.1186/s13073-016-0299-7
53. Pituch, A, Walkowiak, J, and Banaszkiewicz, A. Butyric acid in functional constipation. Przeglad Gastroenterologiczny. (2013) 5:295–8. doi: 10.5114/pg.2013.38731
Keywords: diverticulosis, symptomatic uncomplicated diverticular disease, gut microbiota, primary care, abdominal pain
Citation: Tursi A, Procaccianti G, De Bastiani R, Turroni S, D’Amico F, Allegretta L, Antonino N, Baldi E, Casamassima C, Casella G, Ciuffi M, De Bastiani M, Lazzarotto L, Licci C, Mancuso M, Penna A, Pranzo G, Sanna G, Tosetti C, Zamparella M and Picchio M (2025) Micro-encapsulated and colonic-release sodium butyrate modulates gut microbiota and improves abdominal pain in patients with symptomatic uncomplicated diverticular disease. Front. Med. 12:1487892. doi: 10.3389/fmed.2025.1487892
Received: 28 August 2024; Accepted: 28 January 2025;
Published: 26 February 2025.
Edited by:
Ciro Celsa, University of Palermo, ItalyReviewed by:
Giacomo Emanuele Maria Rizzo, Mediterranean Institute for Transplantation and Highly Specialized Therapies (ISMETT), ItalyCopyright © 2025 Tursi, Procaccianti, De Bastiani, Turroni, D’Amico, Allegretta, Antonino, Baldi, Casamassima, Casella, Ciuffi, De Bastiani, Lazzarotto, Licci, Mancuso, Penna, Pranzo, Sanna, Tosetti, Zamparella and Picchio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonio Tursi, YW50b3R1cnNpQHRpc2NhbGkuaXQ=
†These authors have contributed equally to this work
‡ORCID: Antonio Tursi, https://orcid.org/0000-0001-5767-5541
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.