
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 14 February 2025
Sec. Intensive Care Medicine and Anesthesiology
Volume 12 - 2025 | https://doi.org/10.3389/fmed.2025.1466346
This article is part of the Research Topic Safety of Corticosteroids in Respiratory Medicine View all 4 articles
 Vikas Bansal1†
Vikas Bansal1† Nitesh K. Jain2,3†
Nitesh K. Jain2,3† Amos Lal4
Amos Lal4 Anwar Khedr2
Anwar Khedr2 Aysun Tekin1
Aysun Tekin1 Abbas B. Jama2
Abbas B. Jama2 Noura Attallah2
Noura Attallah2 Esraa Hassan2
Esraa Hassan2 Hisham Ahmed Mushtaq2
Hisham Ahmed Mushtaq2 Sara Robinson5
Sara Robinson5 Marjan Jahani Kondori5
Marjan Jahani Kondori5 Thoyaja Koritala2
Thoyaja Koritala2 Donna Lee Armaignac6
Donna Lee Armaignac6 Amy B. Christie7
Amy B. Christie7 Umamaheswara Raju8
Umamaheswara Raju8 Ashish Khanna9
Ashish Khanna9 Rodrigo Cartin-Ceba10
Rodrigo Cartin-Ceba10 Devang K. Sanghavi11
Devang K. Sanghavi11 Abigail La Nou12
Abigail La Nou12 Karen Boman13
Karen Boman13 Vishakha Kumar13
Vishakha Kumar13 Allan J. Walkey14
Allan J. Walkey14 Juan Pablo Domecq1,2
Juan Pablo Domecq1,2 Rahul Kashyap15,16
Rahul Kashyap15,16 Syed Anjum Khan2* and the Society of Critical Care Medicine (SCCM) Discovery Viral Infection and Respiratory Illness Universal Study (VIRUS): COVID-19 Registry Investigator Group
Syed Anjum Khan2* and the Society of Critical Care Medicine (SCCM) Discovery Viral Infection and Respiratory Illness Universal Study (VIRUS): COVID-19 Registry Investigator GroupBackground: Corticosteroids improve survival in hospitalized COVID-19 patients needing supplemental oxygen. However, concern exists about increased risk of secondary infections. This study investigated the impact of early corticosteroids use on these infections.
Methods: Data from the Society of Critical Care Medicine Discovery Viral Infection and Respiratory Illness Universal Study (VIRUS): COVID-19 registry were analyzed for adult patients, stratified by early corticosteroid use (within 48 h of admission). The primary outcome was documented secondary infections, including bacteremia, bacterial pneumonia, empyema, meningitis/encephalitis, septic shock, and ventilator-associated pneumonia. Univariate and multivariable logistic regression models were used to assess the association between early corticosteroids and these outcomes.
Results: Among 17,092 eligible patients, with 13.5% developed at least one secondary bacterial infection during hospitalization. Patients receiving early corticosteroids were older (median 63 years) compared to those who did not (median 60 years), with a similar gender distribution (42.5% vs. 44.2% female). Unadjusted analysis revealed a higher risk for any secondary infection (OR 1.93, 95% CI 1.76–2.12). This association persisted for specific infections including bacteremia (OR 2.0, 95% CI 1.58–2.54), bacterial pneumonia (OR 1.5, 95% CI 1.27–1.77), and septic shock (OR 1.67, 95% CI 1.44–1.93). However, the effect on meningitis/encephalitis (OR 0.62, 95% CI 0.24–1.57) and ventilator-associated pneumonia (VAP; OR 1.08, 95% CI 0.75–1.57) was non-significant. Adjusted analysis maintained significance for any secondary infection (OR 1.15, 95% CI 1.02–1.29), bacteremia (OR 1.43, 95% CI 1.09–1.88), and infections with unknown sources (OR 1.63, 95% CI 1.31–2.02). Notably, the association weakened and became non-significant for bacterial pneumonia (OR 0.98, 95% CI 0.81–1.20) and septic shock (OR 0.94, 95% CI 0.79–1.11), while it became significant for meningitis/encephalitis (OR 0.26, 95% CI 0.08–0.82). VAP remained non-significant (OR 0.87, 95% CI 0.56–1.34).
Conclusion: Early use of corticosteroids increased overall secondary infection risk in hospitalized COVID-19 patients, but the impact varied. Risk of bacteremia was notably increased, while the association with bacterial pneumonia and septic shock weakened after adjustment becoming non-significant and surprisingly reduced meningitis/encephalitis risk was noted suggesting the complexity of corticosteroid effects. Further research is needed to understand how corticosteroids influence specific secondary infections, and thereby optimize the treatment strategies.
COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has had far reaching social, political, and economic impact since it was first reported in November 2019. It has led to significant morbidity and mortality with a high rate of hospitalization. Multiple risk factors are involved in the disease prognosis, and patients infected with SARS-CoV-2 display a spectrum of clinical presentations that range from asymptomatic to life-threatening manifestation (1–7). For this multi-system disorder (8–12) different therapeutic strategies have been tested since the disease’s inception (13–18). The Mayo Clinic and Society of Critical Care (SCCM) Discovery Network created a global Viral Infection and Respiratory Illness Universal Study (VIRUS): COVID-19 registry for the collection of high-quality data in real-time (19) and for the study of COVID-19 treatments and related outcomes (20–22).
Historically pandemics such as influenza have had a high rate of mortality attributed to co-infections (23). Co-infections occur when a patient is infected with two or more pathogens (viruses, bacteria) at the same time (24). In contrast, the co-infection rate in COVID-19 patients was also noted, but much lower (around 3–8%) compared to historical influenza outbreaks (25, 26). However, occurrence of secondary infections, which develop after the initial COVID-19 infection (24), was noted to be above 40%, a rate much higher than the non-COVID-19 patients (27–33). A number of factors contributed to this secondary infection burden such as prolonged hospitalization, high device utilization rates such as central line and urinary catheter use, invasive mechanical ventilation, pandemic surge overwhelming healthcare capacity, high burn out rate, high comorbidity rate, and immunocompromised state of patients (33–36).
Corticosteroids have been used as therapeutic agents in viral pneumonia and Adult respiratory distress syndrome (ARDS) for many decades (37–39). However, their therapeutic benefit or harm was not well defined with contradictory results in ARDS studies and lack of benefit or even harm in Influenza pneumonia and Middle East Respiratory Syndrome (MERS) (40–42). Given this uncertainty and amidst the chaos of the COVID-19 pandemic, corticosteroids were shown to improve survival in patients with severe COVID-19 requiring supplemental oxygen and respiratory assist devices (40, 43–45). Indeed, the survival effect was more pronounced in critically ill patients needing invasive mechanical ventilation (40, 44, 46). However, the possibility of corticosteroids causing harm when used in patients with ARDS secondary to prolonged viral shedding, hyperglycemia secondary to use of corticosteroids, immunosuppression of the host and therefore increasing the risk of secondary infection remained unsettled (34, 36, 40, 47).
After the “Dexamethasone in Hospitalized Patients with COVID-19” (The RECOVERY collaborative group) (44), corticosteroids became a mainstay of treatment for treating hospitalized patients with severe COVID-19 requiring supplementation oxygen and respiratory assist devices. However, this landmark trial was not powered to detect the differences between the groups with regards to the secondary infection rate, a side effect profile of corticosteroid uses in COVID-19 patients (44). Many other randomized control trials which suspended recruitment post “Dexamethasone in Hospitalized Patients with COVID-19” reported a very small number of patients with secondary infection, which left this question unanswered (48, 49). Observational studies with their inherent confounding limitations reported conflicting results of corticosteroids being a risk for of secondary infections (27, 34–36, 42). There are several mechanisms on how corticosteroids might cause harm when used in patients with severe COVID-19. Prolonged viral shedding, steroid induced hyperglycemia, and immunosuppression are among the most accepted mechanism on how steroids might increase the risk of secondary infections, but still this important question remains unsettled (34, 36, 40, 47).
Considering the existing evidence of corticosteroid-induced immunosuppression and the high prevalence of secondary infections in hospitalized COVID-19 patients, this study aims to investigate the potential association between early corticosteroid use and secondary infection rates within a large, multicenter. Multinational SCCM Discovery VIRUS COVID-19 registry, a rich repository of demographics, treatment details, healthcare processes, hospital outcomes, and documented complications for hospitalized COVID-19 patients from 183 hospitals across 24 countries.
Our analysis utilized data from the SCCM Discovery VIRUS COVID-19 registry, a comprehensive collection of clinical information for hospitalized patients of all ages confirmed to have SARS-CoV-2 infection (by PCR or similar methods) across 183 hospitals in 24 countries from March 1, 2020. The registry was established through the Society of Critical Care Medicine (SCCM) Discovery Network. Established in early 2020, the registry served as a consolidated international repository for ongoing COVID-19 related clinical research. Details of registry design, data management, process of resolution of qualitative and quantitative data related issues have been described elsewhere (20–22), and initial findings from the registry have been previously reported (2, 50).
The registry included patients from both intensive care units and general medical wards, encompassing a diverse spectrum of respiratory support needs. These needs ranged from supplemental oxygen to more advanced interventions like high-flow oxygen, noninvasive ventilation, invasive mechanical ventilation, proning, neuromuscular paralysis, and extracorporeal membrane oxygenation (ECMO). Patient were followed up until discharge or death, whichever occurred first. This registry received exempt status from human participant research review by the Mayo Clinic Institutional Review Board (IRB#: 20-002610) and is registered on Clinicaltrials.gov (NCT04323787). All participating investigative sites obtained local ethical approval and a data use agreement before data collection commenced. As stipulated in the approved protocol, informed consent was waived under Common Rule 45 CFR 46.116, and individual study sites signed a data use agreement to acquire permission for de-identified data extraction and entry into registry case report forms (CRFs). The case report forms were adapted from the World Health Organization templates (51) and modified for an ICU-specific context through rapid, iterative editing to balance feasibility, efficiency, and comprehensiveness, with input from multiple clinical specialties and adding pertinent data for clinical research.
This ancillary study utilizes data from the “SCCM Viral Infection and Respiratory Illness Universal Study (VIRUS) – COVID-19 registry.” We focused on adult patients aged 18 years and older, adhering to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline (52). Our primary exposure of interest was early administration of systemic corticosteroids within 48 h of hospital admission. Patients were categorized into two groups: those receiving early corticosteroids and those who did not. While complete corticosteroid treatment details (duration and type of corticosteroid formulation) were not captured, relevant elements like COVID-19 diagnosis confirmation, patient demographics, comorbid conditions, COVID-19 disease severity, early corticosteroid use, pre-hospital and in-hospital medications, hospital complications and pertinent clinical outcomes were collected from the VIRUS CRF.
Patients were excluded if:
(1) Hospitalization was less than 48 h
(2) They had a secondary or co-infection diagnosed within the first 48 h of hospitalization
(3) Data was missing on medication use on days 0–2 and
(4) Microbiology results or complications during hospitalization were missing.
Admission diagnoses of bacteremia, bacterial pneumonia, meningitis/encephalitis, or positive microbiological findings within 48 h were classified as community-acquired infections and considered co-infections. Complications of bacteremia, empyema, endocarditis, lung abscess, septic shock, ventilator-associated pneumonia, and positive microbiological findings after 48 h were classified as hospital-acquired infections and considered as secondary infections.
The study’s primary focus was the occurrence of any documented secondary infection. This included infections like bacteremia, bacterial pneumonia, empyema, meningitis/encephalitis, septic shock, ventilator-associated pneumonia (VAP) and those with an unknown source.
Continuous variables with normal distributions were summarized using means and standard deviations (SD). For non-normal continuous variables, medians, and interquartile ranges (IQRs) were reported, indicating the 25th and 75th percentiles. Categorical variables were presented as frequencies and percentages. Differences between groups for categorical variables were assessed using Pearson’s Chi-squared tests. To compare non-parametric continuous variables, a Kruskal-Wallis ANOVA was employed with subsequent Mann–Whitney U tests for pairwise comparisons. All analyses were conducted on available data, with the number of observations reported for each variable. Missing data was not imputed. We investigated the association between early in-hospital administration of systemic corticosteroids and these outcomes using both univariate and multivariable logistic regression models. We used existing literature and our univariate analysis to find certain patient characteristics and comorbidities associated with our outcomes of interest. These predictor variables were included into the multivariable model, after evaluating for collinearity. The multivariable logistic regression model adjusted for potential confounding factors, including age, gender, race, ethnicity, body mass index (BMI), ICU admission during index hospitalization, various comorbidities such as coronary artery disease (CAD), hypertension, heart failure, chronic obstructive pulmonary disease (COPD), asthma, chronic kidney disease, diabetes (DM), stroke/other neurological disorders, and dyslipidemia, the highest documented COVID-19 severity as per the WHO Ordinal scale during hospitalization, length of hospital stay, concurrent medication use (including tocilizumab, baricitinib), and antibacterials exposure within the first 48 h of admission. Finally, we compared the frequency of these outcomes between those who received early corticosteroids and those who did not. Diagnoses of these infections were based on the VIRUS registry records and were evaluated and documented as “yes” or “no” in the case report forms. All analyses were conducted using JMP® Software, version Pro 14 (SAS Institute Inc., Cary, NC, USA).
A total of 17,092 patients met eligibility criteria between March 1, 2020, and March 14, 2023, and were included in the analysis (Figure 1). Of these, 9,641 received early inpatient systemic corticosteroids (Figure 1). Table 1 presents a comparison of baseline demographics between the early corticosteroid and control groups. Notably, the median age of patients receiving early corticosteroids was 63 years (IQR 51–75) with 42.5% female, compared to 60 years (IQR 45–74) with 44.2% female in the control group. Table 2 further details the patient characteristics of the index hospitalization based on early steroid use within 48 h of admission.
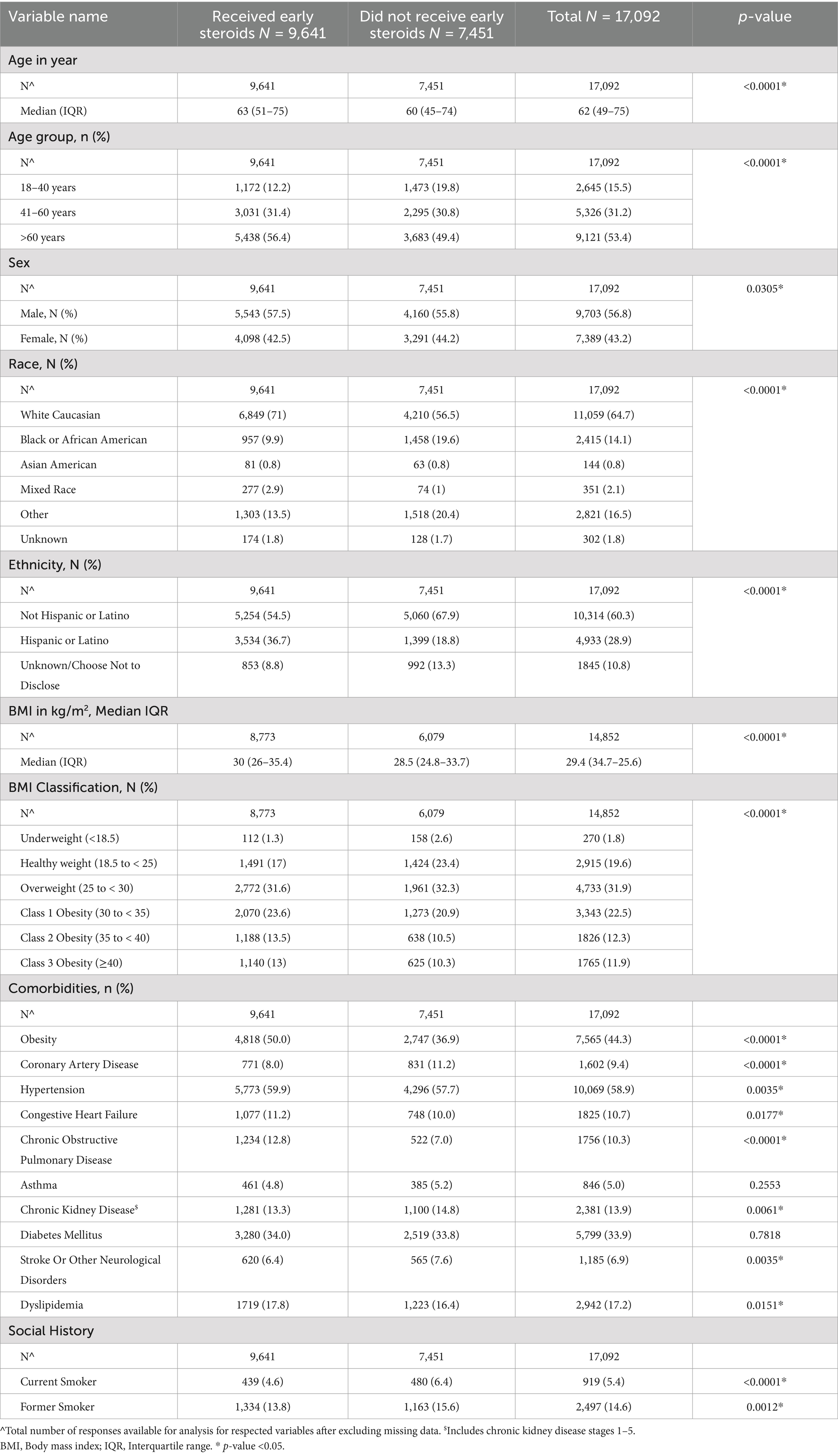
Table 1. Baseline demographics of patients in VIRUS COVID-19 registry by early steroid use within 48 hours of hospital admission.
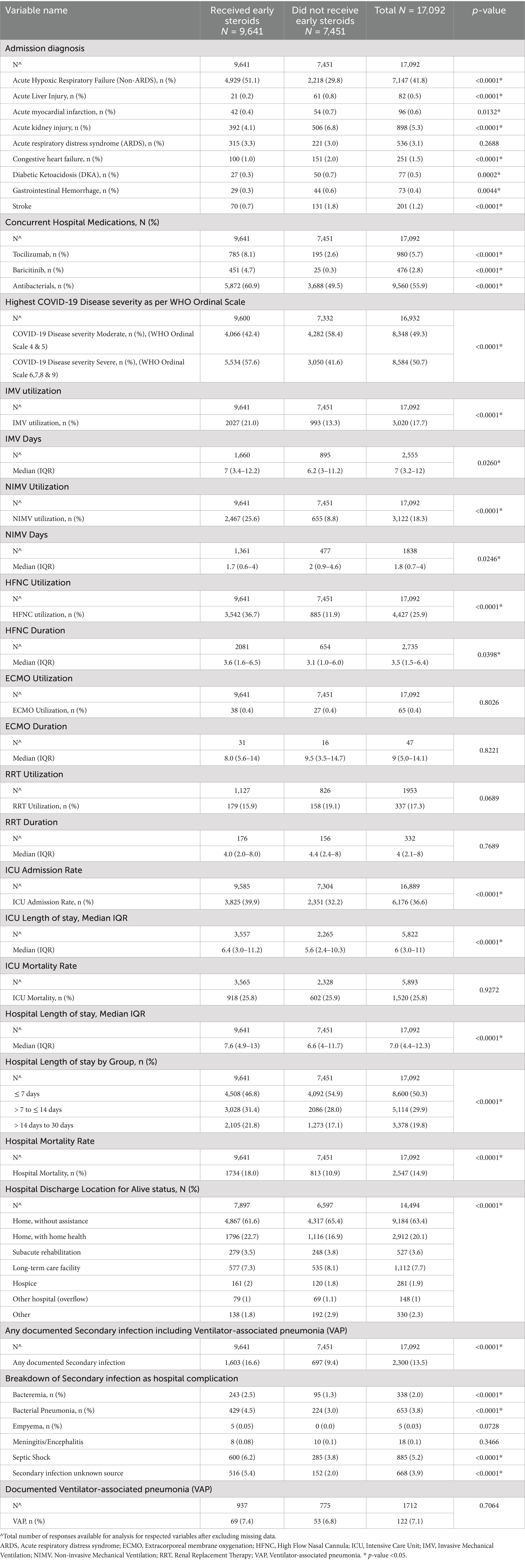
Table 2. Patient characteristics of the index hospitalization in VIRUS COVID-19 registry by early steroid use within 48 hours of hospital admission.
Overall, 13.5% of patients developed at least one of the studied secondary bacterial infections during their hospitalization. Unadjusted analysis (Table 3) revealed that early corticosteroid administration significantly increased the odds of any secondary infection (OR 1.93, 95% CI 1.76–2.12; p-value <0.001). This association remained significant for specific infections like bacteremia (OR 2.0, 95% CI 1.58–2.54; p-value <0.001), bacterial pneumonia (OR 1.5, 95% CI 1.27–1.77; p-value <0.001), septic shock (OR 1.67, 95% CI 1.44–1.93; p-value <0.001), and secondary infection from unknown source (OR 2.72, 95% CI 2.26–3.26, p-value <0.001).

Table 3. Unadjusted and adjusted risk of secondary infections with early steroid use within 48 hours of hospital admission in hospitalized COVID-19 patients.
After adjusting for potential confounding factors, the association between early corticosteroid use and secondary infections persisted for some but not all outcomes. The adjusted odds of developing any secondary infection (OR 1.15, 95% CI 1.02–1.29; p-value = 0.023), bacteremia (OR 1.43, 95% CI 1.09–1.88; p-value = 0.010), meningitis/encephalitis (OR 0.26, 95% CI 0.08–0.82; p-value = 0.021), and secondary infection from unknown source (OR 1.63, 95% CI 1.31–2.02, p-value <0.001) remained statistically significant. However, the association with bacterial pneumonia and septic shock did not reach statistical significance in the adjusted model (OR 0.94, 95% CI 0.79–1.11, p-value = 0.468).
The administration of corticosteroids early in the course of hospitalization for COVID-19 significantly increased the overall risk of secondary infections. However, the effects on specific infection types were heterogeneous. Bacteremia risk was substantially elevated, while the associations with bacterial pneumonia and septic shock became less evident after controlling for potential confounding factors. Notably, meningitis/encephalitis exhibited a surprising trend toward decreased risk.
The overall rate of secondary infection in our study was 13.5% with the early corticosteroid group having a rate of 16.6% and non-corticosteroid group a rate of 9.4%. This secondary rate of infection in our study was consistent with other reported studies in literature elsewhere (53, 54). The rate of secondary infections in COVID-19 has been found to be higher than in the pre COVID-19 time period (34, 53, 54). As noted previously, this has been attributed to a number of causes unique to the pandemic such as excessive use of antibiotics, lax infection control measures during pandemic, prolonged hospitalization especially in critically ill patients associated with increased device utilization rates, and ventilator associated pneumonia amongst others (34, 53, 54).
Our study demonstrated increased secondary infection rate, bacteremia, and secondary infection from an after unknown source after adjustment, whereas no difference was noted in bacterial pneumonia, VAP, and septic shock. This increased rate of infection is remarkably similar to other reported retrospective large series such as the ESICM UNITE-COVID study (34). Furthermore, a study on mechanically ventilated patients also reported an increased risk of bacterial pneumonia and fungal infections with dexamethasone, emphasizing superinfections in critically ill patients (55). Similarly, another study focused on ICU patients found that prolonged corticosteroid use significantly raised the risk of bloodstream infections, consistent with our observation of increased bacteremia risk (56). It is noteworthy that the early steroid group had an increased incidence of acute hypoxic respiratory failure at admission and a higher COVID-19 disease severity scale. Interestingly, the early steroid group had a higher incidence of severe illness at admission, indicated by increased acute hypoxic respiratory failure scores and higher COVID-19 disease severity scales. This likely explains the association with a greater use and duration of respiratory support devices, including invasive mechanical ventilation (IMV) in the early steroid group. Additionally, the early steroid group had a higher ICU admission rate, longer ICU and hospital lengths of stay, and increased hospital mortality, but not ICU mortality. It is important to note that our study covered a period of nearly 3 years, encompassing both the pre and post-RECOVERY trial period. The RECOVERY trial (44) established the survival benefits of corticosteroids in COVID-19, likely leading to the widespread adoption of dexamethasone during the latter part of the study timeframe. This shift in standard care practices might explain why most patients requiring respiratory support received dexamethasone in the later stages of our study. However, the widespread adoption of corticosteroids as standard care after this trial makes it difficult to disentangle the independent effect of corticosteroids on infection rates. Similar to the ESICM UNITE-COVID study (34), we did not observe an overall ICU mortality benefit, but a trend toward increased mortality in the entire hospitalized population receiving early steroids. While our study was not designed to assess mortality definitively, this could be due to the higher acuity of illness in the group receiving early corticosteroids.
However, our results differ from other studies, such as the Mount Sinai COVID Informatics Center (MSCIC) analysis, which reported a lower coinfection rate in patients receiving corticosteroids (57). The MSCIC data consisted of more than 4,000 patients but also was reported early in the pandemic (54). Similarly, a study of critically ill patients found no significant association between corticosteroid use and secondary infections, contrasting with our findings of increased overall risk (58). This study focused on critically ill ICU patients with severe COVID-19, who were receiving intensive care and mechanical ventilation. In contrast to both studies our study consists of patients both from the general medical ward and ICU, spread out over a three-year period, during which time care for COVID-19 patients gradually became more standardized with hospitals only experiencing periods of intermittent surge. Thus, our results are more generalizable, more reflective of and applicable to real world with less bias.
Our study explores the link between corticosteroids and secondary infections in COVID-19 patients. While our data hints at fewer cases of meningitis/encephalitis with steroid use, the small group (18 people) limits firm conclusions. COVID-19 itself can cause this complication, as shown by a study of 32 cases by Huo et al. (59). The same study mentions individual cases where meningitis/encephalitis patients improved after getting steroids. This suggests that the benefit we observed might not be entirely due to steroids alone. It highlights the need to explore how these complications arise and how steroids influence their development. In hospitalized COVID-19 patients, meningitis and encephalitis are less often linked to secondary bacterial infections. Instead, they are usually caused by immune-driven inflammation in the brain or the virus directly invading the nervous system (59). Corticosteroids like dexamethasone help reduce cytokine storms and brain inflammation, which might explain the lower risk of these complications with steroid use (60). However, our preliminary finding of decreased incidence needs further research with larger groups and stronger methods to definitively assess this, confirm or refute our findings, uncover the underlying mechanisms, and thereby guide optimal clinical management.
Our study investigates the complex relationship between early corticosteroid use and secondary infections in COVID-19, offering several key strengths. This large-scale, international registry study (20–22) encompasses data from diverse hospitals across the globe, providing a robust and generalizable perspective on this critical relationship. Focusing on real-world data from actual clinical settings directly translates our findings to everyday patient care, enhancing their relevance and applicability. Furthermore, the comprehensive analysis of a wide range of secondary infections provides a clear picture of the potential risks associated with early corticosteroids. Importantly, we adjusted for various potential confounders, strengthening the validity and reliability of our observed associations. Finally, the consistency of our results with other large studies further reinforces the credibility and reproducibility of this association (53).
However, it is crucial to acknowledge the study’s limitations, which necessitate further research. While this observational study offers valuable real-world insights, it cannot definitively prove causality, and the retrospective design may limit the accuracy and completeness of data, especially when it comes to secondary infections like VAP and bacteremia. Additionally, variations in treatment regimes, patient populations across hospitals, and viral strains during different surge periods could have influenced our findings, which could not be adjusted in our study. Notably, different corticosteroid formulations, doses, and durations were employed based on local practices, and although this is unlikely to significantly impact our secondary infection results given the consistent effect observed in previous studies (43, 61), it warrants further investigation. Similarly, the determination of secondary infections relied on investigator judgment and reporting across multiple sites, introducing potential bias due to non-standardization. This is unavoidable given the geographic spread of hospitals and the multitude of investigators involved. The analysis of microbiological data also faces limitations. Firstly, data collection depends on what researchers recorded, potentially introducing bias, particularly for free-text information like antimicrobial use. Secondly, we cannot directly link microbiological findings to clinical outcomes. Thirdly, excluding patients with missing data may overestimate infection rates, as missing data is more likely for negative cultures. Additionally, for bloodstream and urinary tract infections, it is difficult to distinguish catheter-related infections. Finally, evaluating multi-bacterial culture positivity and microorganism susceptibility presents challenges. Despite these limitations, the consistency of our findings with other large studies is reassuring (34).
While our study has provided valuable information on how early corticosteroid use, COVID-19 itself, and secondary infections are linked, there are still unanswered questions. We need to understand better how exactly early steroids affect the risk of secondary infections; especially why different types of infections are impacted differently. Additionally, figuring out the exact role of COVID-19 versus corticosteroids in causing secondary infections requires studies that look closely at the types of pathogens involved and potentially treatments aimed at specific parts of the immune system. Investigating specific pathogens and their susceptibility to corticosteroids could provide more refined understanding of the risk profile. To improve patient outcomes, future research should explore the possibility of personalized corticosteroid plans based on individual risk factors for secondary infections and tailored to different types of COVID-19. Additionally, investigating additional treatments that could reduce the increased risk of secondary infections associated with early corticosteroids could be helpful in clinical practice. By addressing these remaining questions thorough research, we can get a clear picture of the complex relationship between COVID-19, corticosteroids, and secondary infections, ultimately leading to better treatment options and improved patient outcomes.
Our study suggests that early in-hospital corticosteroids use significantly increased the risk of any secondary infection in patients with COVID-19, but their effect on specific infections varied significantly. While bacteremia risk substantially increased, associations with bacterial pneumonia and septic shock was weakened upon adjusting for confounding factors. Notably, meningitis/encephalitis showed a surprising decreased incidence, highlighting a critical knowledge gap in the existing data. This is particularly concerning considering that existing data from trials like the RECOVERY collaboration group and others did not report on secondary infections. This emphasizes the urgent need for careful consideration of both potential benefits and risks when using corticosteroids in this setting. Future randomized controlled trials should explicitly address the potential risk of secondary infections as an outcome to fully assess the risk–benefit profile of corticosteroid therapy so as to guide optimal clinical management.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Mayo Clinic Institutional Review Board (IRB#: 20-002610). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this was a prospective observational registry. No intervention was tested in the registry.
SCCM Discovery VIRUS investigators collaborative co-author list is in the Acknowledgements section. The journal is requested to PubMed index the list of VIRUS: COVID-19 Registry Investigator Group as collaborative co-authors submitted along with this manuscript.
VB: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. NJ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AL: Writing – original draft, Writing – review & editing. AnK: Writing – original draft, Writing – review & editing, Data curation. AT: Writing – original draft, Writing – review & editing, Data curation. AJ: Writing – original draft, Writing – review & editing, Data curation. NA: Writing – original draft, Writing – review & editing, Data curation. EH: Writing – original draft, Writing – review & editing, Data curation. HM: Writing – original draft, Writing – review & editing, Data curation. SR: Writing – original draft, Writing – review & editing, Data curation. MK: Writing – original draft, Writing – review & editing, Data curation. TK: Writing – original draft, Writing – review & editing, Data curation. DA: Writing – original draft, Writing – review & editing. AC: Writing – original draft, Writing – review & editing. UR: Writing – original draft, Writing – review & editing. AsK: Writing – original draft, Writing – review & editing. RC-C: Writing – original draft, Writing – review & editing. DS: Writing – original draft, Writing – review & editing. AN: Writing – original draft, Writing – review & editing. KB: Writing – original draft, Writing – review & editing. VK: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization. AW: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization. JD: Writing – original draft, Writing – review & editing. RK: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. SK: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The VIRUS: COVID-19 Registry was supported, in part, by the Gordon and Betty Moore Foundation, and Janssen Research & Development, LLC. They have no role in data gathering, analysis, interpretation, and writing.
Data from this study was submitted and presented as an abstract format for the CHEST 2023 Annual Meeting in Honolulu, Hawaii, USA. We acknowledge Grammarly for assistance with the English Language review of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1466346/full#supplementary-material
1. Shah, A, Kashyap, R, Tosh, P, Sampathkumar, P, and O'Horo, JC. Guide to understanding the 2019 novel coronavirus. Mayo Clin Proc. (2020) 95:646–52. doi: 10.1016/j.mayocp.2020.02.003
2. Domecq, JP, Lal, A, Sheldrick, CR, Kumar, VK, Boman, K, Bolesta, S, et al. Outcomes of patients with coronavirus disease 2019 receiving organ support therapies: the international viral infection and respiratory illness universal study registry. Crit Care Med. (2021) 49:437–48. doi: 10.1097/CCM.0000000000004879
3. Bhalala, US, Gist, KM, Tripathi, S, Boman, K, Kumar, VK, Retford, L, et al. Characterization and outcomes of hospitalized children with coronavirus disease 2019: a report from a multicenter, viral infection and respiratory illness universal study (coronavirus disease 2019) registry. Crit Care Med. (2022) 50:e40–51. doi: 10.1097/CCM.0000000000005232
4. Deo, N, Tekin, A, Bansal, V, Koritala, T, Mullen, B, Armaignac, DL, et al. Cutaneous manifestations of hospitalized COVID-19 patients in the VIRUS COVID-19 registry. Int J Dermatol. (2022) 61:623–5. doi: 10.1111/ijd.16134
5. Tripathi, S, Gist, KM, Bjornstad, EC, Kashyap, R, Boman, K, Chiotos, K, et al. Coronavirus disease 2019-associated PICU admissions: a report from the society of critical care medicine discovery network viral infection and respiratory illness universal study registry. Pediatr Crit Care Med. (2021) 22:603–15. doi: 10.1097/PCC.0000000000002760
6. Tripathi, S, Sayed, IA, Dapul, H, McGarvey, JS, Bandy, JA, Boman, K, et al. Risk factors for critical coronavirus disease 2019 and mortality in hospitalized young adults: an analysis of the Society of Critical Care Medicine discovery viral infection and respiratory illness universal study (VIRUS) coronavirus disease 2019 registry. Crit Care Explor. (2021) 3:e 0514. doi: 10.1097/CCE.0000000000000514
7. Singh, R, Rathore, SS, Khan, H, Karale, S, Chawla, Y, Iqbal, K, et al. Association of obesity with COVID-19 severity and mortality: an updated systemic review, meta-analysis, and meta-regression. Front Endocrinol. (2022) 13:780872. doi: 10.3389/fendo.2022.780872
8. Razonable, RR, Pennington, KM, Meehan, AM, Wilson, JW, Froemming, AT, Bennett, CE, et al. A collaborative multidisciplinary approach to the management of coronavirus disease 2019 in the hospital setting. Mayo Clin Proc. (2020) 95:1467–81. doi: 10.1016/j.mayocp.2020.05.010
9. Sheraton, M, Deo, N, Kashyap, R, and Surani, S. A review of neurological complications of COVID-19. Cureus. (2020) 12:e8192. doi: 10.7759/cureus.8192
10. Khan, H, Sabzposh, H, Deshpande, S, and Kashyap, R. Pregnancy during COVID-19 pandemic - maternal and neonatal outcomes: a concise review. Int J Acad Med. (2020) 6:287–93. doi: 10.4103/Ijam.Ijam_94_20
11. Shah, K, Mann, S, Singh, R, Bangar, R, and Kulkarni, R. Impact of COVID-19 on the mental health of children and adolescents. Cureus. (2020) 12:e10051. doi: 10.7759/cureus.10051
12. Sheraton, M, Deo, N, Dutt, T, Surani, S, Hall-Flavin, D, and Kashyap, R. Psychological effects of the COVID 19 pandemic on healthcare workers globally: a systematic review. Psychiatry Res. (2020) 292:113360. doi: 10.1016/j.psychres.2020.113360
13. Md Insiat Islam, R. Current drugs with potential for treatment of COVID-19: a literature review. J Pharm Pharm Sci. (2020) 23:58–64. doi: 10.18433/jpps31002
14. Gilzad-Kohan, H, and Jamali, F. Anti-inflammatory properties of drugs used to control COVID-19 and their effects on the renin-angiotensin system and angiotensin-converting Enzyme-2. J Pharm Pharm Sci. (2020) 23:259–77. doi: 10.18433/jpps31346
15. Bansal, V, Mahapure, KS, Bhurwal, A, Gupta, I, Hassanain, S, Makadia, J, et al. Mortality benefit of Remdesivir in COVID-19: a systematic review and Meta-analysis. Front Med. (2020) 7:606429. doi: 10.3389/fmed.2020.606429
16. Bansal, V, Mahapure, KS, Mehra, I, Bhurwal, A, Tekin, A, Singh, R, et al. Mortality benefit of convalescent plasma in COVID-19: a systematic review and Meta-analysis. Front Med. (2021) 8:624924. doi: 10.3389/fmed.2021.624924
17. Singh, R, Rathore, SS, Khan, H, Bhurwal, A, Sheraton, M, Ghosh, P, et al. Mortality and severity in COVID-19 patients on ACEIs and ARBs-A systematic review, Meta-analysis, and Meta-regression analysis. Front Med. (2021) 8:703661. doi: 10.3389/fmed.2021.703661
18. Garcia, MA, Johnson, SW, Bosch, NA, et al. Variation in use of repurposed medications among patients with coronavirus disease 2019. From the Society of Critical Care Medicine discovery viral infection and respiratory illness universal study: coronavirus disease 2019 registry Investigator Group. Criti Care Explorat. (2021) 3:p e0566. doi: 10.1097/CCE.0000000000000566
19. The Society of Critical Care Medicine, Lyntek Medical Technologies Inc. VIRUS COVID-19 registry dashboard: a COVID-19 registry of current ICU and hospital care patterns. Available at: https://sccmcovid19.org/
20. Walkey, AJ, Kumar, VK, Harhay, MO, Bolesta, S, Bansal, V, Gajic, O, et al. The viral infection and respiratory illness universal study (VIRUS): an international registry of coronavirus 2019-related critical illness. Crit Care Explor. (2020) 2:e0113. doi: 10.1097/CCE.0000000000000113
21. Walkey, AJ, Sheldrick, RC, Kashyap, R, Kumar, VK, Boman, K, Bolesta, S, et al. Guiding principles for the conduct of observational critical care research for coronavirus disease 2019 pandemics and beyond: the Society of Critical Care Medicine discovery viral infection and respiratory illness universal study registry. Crit Care Med. (2020) 48:e1038–44. doi: 10.1097/CCM.0000000000004572
22. Turek, JR, Bansal, V, Tekin, A, Singh, S, Deo, N, Sharma, M, et al. Lessons from a rapid project management exercise in the time of pandemic: methodology for a global covid-19 virus registry database. JMIR Res Protoc. (2022) 11:e27921. doi: 10.2196/27921
23. Brundage, JF, and Shanks, GD. Deaths from bacterial pneumonia during 1918-19 influenza pandemic. Emerg Infect Dis. (2008) 14:1193–9. doi: 10.3201/eid1408.071313
24. Feldman, C, and Anderson, R. The role of co-infections and secondary infections in patients with COVID-19. Pneumonia. (2021) 13:5. doi: 10.1186/s41479-021-00083-w
25. Centers for Disease C, Prevention. Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1) - United States, May-August 2009. MMWR Morb Mortal Wkly Rep. (2009) 58:1071–4.
26. Echenique, IA, Chan, PA, Chapin, KC, Andrea, SB, Fava, JL, and Mermel, LA. Clinical characteristics and outcomes in hospitalized patients with respiratory viral co-infection during the 2009 H1N1 influenza pandemic. PLoS One. (2013) 8:e60845. doi: 10.1371/journal.pone.0060845
27. Bardi, T, Pintado, V, Gomez-Rojo, M, Escudero-Sanchez, R, Azzam Lopez, A, Diez-Remesal, Y, et al. Nosocomial infections associated to COVID-19 in the intensive care unit: clinical characteristics and outcome. Eur J Clin Microbiol Infect Dis. (2021) 40:495–502. doi: 10.1007/s10096-020-04142-w
28. Bassetti, M, Kollef, MH, and Timsit, JF. Bacterial and fungal superinfections in critically ill patients with COVID-19. Intensive Care Med. (2020) 46:2071–4. doi: 10.1007/s00134-020-06219-8
29. Maes, M, Higginson, E, Pereira-Dias, J, Curran, MD, Parmar, S, Khokhar, F, et al. Ventilator-associated pneumonia in critically ill patients with COVID-19. Crit Care. (2021) 25:25. doi: 10.1186/s13054-021-03460-5
30. Pickens, CO, Gao, CA, Cuttica, MJ, Smith, SB, Pesce, LL, Grant, RA, et al. Bacterial superinfection pneumonia in patients mechanically ventilated for COVID-19 pneumonia. Am J Respir Crit Care Med. (2021) 204:921–32. doi: 10.1164/rccm.202106-1354OC
31. Rouze, A, Martin-Loeches, I, Povoa, P, Makris, D, Artigas, A, Bouchereau, M, et al. Relationship between SARS-CoV-2 infection and the incidence of ventilator-associated lower respiratory tract infections: a European multicenter cohort study. Intensive Care Med. (2021) 47:188–98. doi: 10.1007/s00134-020-06323-9
32. Wang, D, Hu, B, Hu, C, Zhu, F, Liu, X, Zhang, J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. (2020) 323:1061–9. doi: 10.1001/jama.2020.1585
33. Weiner-Lastinger, LM, Pattabiraman, V, Konnor, RY, Patel, PR, Wong, E, Xu, SY, et al. The impact of coronavirus disease 2019 (COVID-19) on healthcare-associated infections in 2020: a summary of data reported to the National Healthcare Safety Network. Infect Control Hosp Epidemiol. (2022) 43:12–25. doi: 10.1017/ice.2021.362
34. Conway Morris, A, Kohler, K, De Corte, T, Ercole, A, De Grooth, HJ, De Elbers, PWG, et al. Co-infection and ICU-acquired infection in COIVD-19 ICU patients: a secondary analysis of the UNITE-COVID data set. Crit Care. (2022) 26:236. doi: 10.1186/s13054-022-04108-8
35. Kubin, CJ, McConville, TH, Dietz, D, Zucker, J, May, M, Nelson, B, et al. Characterization of bacterial and fungal infections in hospitalized patients with coronavirus disease 2019 and factors associated with health care-associated infections. Open Forum Infect Dis. (2021) 8:ofab201. doi: 10.1093/ofid/ofab201
36. Ritter, LA, Britton, N, Heil, EL, Teeter, WA, Murthi, SB, Chow, JH, et al. The impact of corticosteroids on secondary infection and mortality in critically ill COVID-19 patients. J Intensive Care Med. (2021) 36:1201–8. doi: 10.1177/08850666211032175
37. Landolf, KM, Lemieux, SM, Rose, C, Johnston, JP, Adams, CD, Altshuler, J, et al. Corticosteroid use in ARDS and its application to evolving therapeutics for coronavirus disease 2019 (COVID-19): a systematic review. Pharmacotherapy. (2022) 42:71–90. doi: 10.1002/phar.2637
38. Khilnani, GC, and Hadda, V. Corticosteroids and ARDS: a review of treatment and prevention evidence. Lung India. (2011) 28:114–9. doi: 10.4103/0970-2113.80324
39. Briel, M, Spoorenberg, SMC, Snijders, D, Torres, A, Fernandez-Serrano, S, Meduri, GU, et al. Corticosteroids in patients hospitalized with community-acquired pneumonia: systematic review and individual patient data meta-analysis. Clin Infect Dis. (2018) 66:346–54. doi: 10.1093/cid/cix801
40. Prescott, HC, and Rice, TW. Corticosteroids in COVID-19 ARDS: evidence and hope during the pandemic. JAMA. (2020) 324:1292–5. doi: 10.1001/jama.2020.16747
41. Tsai, MJ, Yang, KY, Chan, MC, Kao, KC, Wang, HC, Perng, WC, et al. Impact of corticosteroid treatment on clinical outcomes of influenza-associated ARDS: a nationwide multicenter study. Ann Intensive Care. (2020) 10:26. doi: 10.1186/s13613-020-0642-4
42. Ni, YN, Chen, G, Sun, J, Liang, BM, and Liang, ZA. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit Care. (2019) 23:99. doi: 10.1186/s13054-019-2395-8
43. WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working GroupSterne, JAC, Murthy, S, Diaz, JV, Slutsky, AS, Villar, J, et al. Association between Administration of Systemic Corticosteroids and Mortality among Critically ill Patients with COVID-19: a Meta-analysis. JAMA. (2020) 324:1330–41. doi: 10.1001/jama.2020.17023
44. Recovery Collaborative GroupHorby, P, Lim, WS, Emberson, JR, Mafham, M, Bell, JL, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. (2021) 384:693–704. doi: 10.1056/NEJMoa2021436
45. Mehra, I, Mahapure, K, Armaly, P, Madas, N, Shah, V, Gupta, I, et al. 146: controversial role of corticosteroids on mortality in COVID-19: systematic review and Meta-analysis. Crit Care Med. (2021) 49:58. doi: 10.1097/01.ccm.0000726472.05866.e3
46. Fang, F, Zhang, Y, Tang, J, Lunsford, LD, Li, T, Tang, R, et al. Association of Corticosteroid Treatment with Outcomes in adult patients with Sepsis: a systematic review and Meta-analysis. JAMA Intern Med. (2019) 179:213–23. doi: 10.1001/jamainternmed.2018.5849
47. Steinberg, KP, Hudson, LD, Goodman, RB, Hough, CL, Lanken, PN, Hyzy, R, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. (2006) 354:1671–84. doi: 10.1056/NEJMoa051693
48. Angus, DC, Derde, L, Al-Beidh, F, Annane, D, Arabi, Y, Beane, A, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. (2020) 324:1317–29. doi: 10.1001/jama.2020.17022
49. Dequin, PF, Heming, N, Meziani, F, Plantefève, G, Voiriot, G, Badié, J, et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. JAMA. (2020) 324:1298–306. doi: 10.1001/jama.2020.16761
50. Lal, A, Garces, JPD, Bansal, V, Tekin, A, Zec, S, Khanna, AK, et al. Pre-hospital aspirin use and patient outcomes in COVID-19: results from the international viral infection and respiratory illness universal study (VIRUS). Arch Bronconeumol. (2022) 58:746–53. doi: 10.1016/j.arbres.2022.07.017
51. WHO ISARIC COVID-19 Clinical Research Resources. (2020). Available at: https://isaric.tghn.org/covid-19-clinical-research-resources/ (Accessed March 31, 2020).
52. Vandenbroucke, JP, von Elm, E, Altman, DG, Gøtzsche, PC, Mulrow, CD, Pocock, SJ, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int J Surg. (2014) 12:1500–24. doi: 10.1016/j.ijsu.2014.07.014
53. Langford, BJ, So, M, Raybardhan, S, Leung, V, Westwood, D, MacFadden, DR, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. (2020) 26:1622–9. doi: 10.1016/j.cmi.2020.07.016
54. Markovskaya, Y, Gavioli, EM, Cusumano, JA, and Glatt, AE. Coronavirus disease 2019 (COVID-19): secondary bacterial infections and the impact on antimicrobial resistance during the COVID-19 pandemic. Antimicrob Steward Healthc Epidemiol. (2022) 2:e114. doi: 10.1017/ash.2022.253
55. Sovik, S, Barrat-Due, A, Kasine, T, Olasveengen, T, Strand, MW, Tveita, AA, et al. Corticosteroids and superinfections in COVID-19 patients on invasive mechanical ventilation. J Infect. (2022) 85:57–63. doi: 10.1016/j.jinf.2022.05.015
56. Dupper, AC, Malik, Y, Cusumano, JA, Nadkarni, D, Banga, J, Berbel Caban, A, et al. Longer steroid treatment increases secondary bloodstream infection risk among patients with COVID-19 requiring intensive care. Infect Dis Clin Pract. (2022) 30. doi: 10.1097/IPC.0000000000001188
57. Ho, KS, Narasimhan, B, Difabrizio, L, Rogers, L, Bose, S, Li, L, et al. Impact of corticosteroids in hospitalised COVID-19 patients. BMJ Open Respir Res. (2021) 8:e000766. doi: 10.1136/bmjresp-2020-000766
58. Pearce, AK, Zawaydeh, Q, McGuire, WC, Husain, A, Ayoub, C, Sweeney, DA, et al. Secondary infections in critically ill patients with COVID-19 receiving steroid therapy. Sci Prog. (2023) 106:368504231207209. doi: 10.1177/00368504231207209
59. Huo, L, Xu, KL, and Wang, H. Clinical features of SARS-CoV-2-associated encephalitis and meningitis amid COVID-19 pandemic. World J Clin Cases. (2021) 9:1058–78. doi: 10.12998/wjcc.v9.i5.1058
60. Hodzic, E, Hasbun, R, Granillo, A, Tröscher, AR, Wagner, H, von Oertzen, TJ, et al. Steroids for the treatment of viral encephalitis: a systematic literature review and meta-analysis. J Neurol. (2023) 270:3603–15. doi: 10.1007/s00415-023-11715-0
61. Tomazini, BM, Maia, IS, Cavalcanti, AB, Berwanger, O, Rosa, RG, Veiga, VC, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. (2020) 324:1307–16. doi: 10.1001/jama.2020.17021
Keywords: SARS-CoV-2, COVID-19, corticosteroids, secondary infections, hospitalized patients, risk factors, early steroid treatment outcomes, VIRUS COVID-19 registry
Citation: Bansal V, Jain NK, Lal A, Khedr A, Tekin A, Jama AB, Attallah N, Hassan E, Mushtaq HA, Robinson S, Kondori MJ, Koritala T, Armaignac DL, Christie AB, Raju U, Khanna A, Cartin-Ceba R, Sanghavi DK, La Nou A, Boman K, Kumar V, Walkey AJ, Domecq JP, Kashyap R, Khan SA and the Society of Critical Care Medicine (SCCM) Discovery Viral Infection and Respiratory Illness Universal Study (VIRUS): COVID-19 Registry Investigator Group (2025) The association between early corticosteroid use and the risk of secondary infections in hospitalized patients with COVID-19: a double-edged sword. Results from the international SCCM discovery viral infection and respiratory illness universal study (VIRUS) COVID-19 registry. Front. Med. 12:1466346. doi: 10.3389/fmed.2025.1466346
Received: 17 July 2024; Accepted: 15 January 2025;
Published: 14 February 2025.
Edited by:
Semra Bilaceroglu, University of Health Sciences, TürkiyeReviewed by:
Filiz Koşar, Yedikule Teaching Hospital, TürkiyeCopyright © 2025 Bansal, Jain, Lal, Khedr, Tekin, Jama, Attallah, Hassan, Mushtaq, Robinson, Kondori, Koritala, Armaignac, Christie, Raju, Khanna, Cartin-Ceba, Sanghavi, La Nou, Boman, Kumar, Walkey, Domecq, Kashyap, Khan and the Society of Critical Care Medicine (SCCM) Discovery Viral Infection and Respiratory Illness Universal Study (VIRUS): COVID-19 Registry Investigator Group. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Syed Anjum Khan, S2hhbi5TeWVkQG1heW8uZWR1
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.