
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 22 January 2025
Sec. Pulmonary Medicine
Volume 12 - 2025 | https://doi.org/10.3389/fmed.2025.1445809
This article is part of the Research TopicTargeting Cellular Signalling Pathways for Disease Therapy: The Potential of Cellular Reprogramming and Protein Kinase InhibitorsView all 7 articles
 Yanxiong Mao1†
Yanxiong Mao1† Anyi Guo1†
Anyi Guo1† Ying Zhang2†
Ying Zhang2† Jianxing Lai1†
Jianxing Lai1† Dian Yuan1
Dian Yuan1 Hao Zhang1
Hao Zhang1 Wenqi Diao1
Wenqi Diao1 Weisong Chen3
Weisong Chen3 Fugui Yan1*
Fugui Yan1*Introduction: Baricitinib is a selective inhibitor of Janus kinase (JAK)1 and JAK2, which is associated with clinical improvement in non-severe COVID-19 patients. But in severe COVID-19 patients, the effectiveness of baricitinib is still controversial.
Methods: A propensity score-matched and retrospective study was conducted to evaluate the effectiveness of baricitinib in severe COVID-19 patients requiring invasive mechanical ventilation (IMV).
Results: A total number of 48 patients treated with baricitinib were included, and 48 patients were assigned to control group by propensity score matching. The mean ages were high in both group (baricitinib group vs. control group: 78.80 ± 9.04 vs. 82.57 ± 9.27), and most were unvaccinated (62.5% vs. 66.7%. Baricitinib group had a higher proportion of patients with hypertension (73.9% vs. 45.5%, p = 0.006). Control group had higher level of creatine kinase-myocardial band (247.50 vs. 104.50, p = 0.021). Patients in the baricitinib group were more likely to receive nirmatrelvir/ritonavir (39.6% vs. 16.7%, p = 0.017) and intravenous immunoglobin (14.6% vs. 0, p = 0.007). Baricitinib group had significantly lower all-cause 28-days mortality than control group (72.9% vs. 89.6%, p = 0.004).
Conclusion: The present study revealed baricitinib reduced 28-days mortality in severe COVID-19 patients on IMV. The effectiveness of baricitinib in treating patients with severe COVID-19 on IMV needs to be further investigated through future studies.
Since 2019, the COVID-19 pandemic has represented a major cause of mortality worldwide. People of all ages are susceptible to COVID-19 (1). However, elderly patients are more likely to develop severe COVID-19 infection that requires invasive mechanical ventilation (IMV) (2, 3). There are various therapeutic options for COVID-19 including antiviral drugs, glucocorticoids, cytokine antagonists, Janus kinase (JAK) inhibitors and so on (4). Antiviral drugs target viral proteins to block the virus life cycle, but most of them need the administration in early stage of symptom onset (4, 5). Glucocorticoids, which are known to reduce the mortality among severe COVID-19 patients, are recommended mostly for inpatients with COVID-19 who are receiving oxygen support (6). Tocilizumab, an interleukin (IL)-6 receptor antagonist, in combination with glucocorticoids, reduces mortality in patients with severe or critical COVID-19 (7). Before COVID-19 pandemic, JAK inhibitors were used alone or with other medications to treat rheumatoid arthritis (8). The use of JAK inhibitors in COVID-19 were tested by various studies, and baricitinib stood out for its effectiveness and received attention from researchers and physicians (7, 9).
Baricitinib was a selective inhibitor of JAK1 and JAK2. In early 2020, artificial intelligence identified baricitinib as a potential intervention for COVID-19 due to its known anti-cytokine properties and potential for targeting host proteins for its antiviral mechanism (10, 11). Following the identification, numerous studies investigated the potential use of baricitinib in COVID-19 and provided substantial evidence of clinical improvement associated with baricitinib in non-severely hospitalized patients (12–14). The phase 3 COV-BARRIER study found that baricitinib, in addition to standard of care, was associated with reduced mortality in hospitalized patients with COVID-19, and had a similar safety profile to that of standard of care alone (10). The RECOVERY study revealed that baricitinib significantly reduced the risk of death in patients hospitalized with COVID-19 (15). With established safety profiles, baricitinib offers potential benefits for inpatients with COVID-19, especially the elderly (4). Based on substantial evidence, the use of baricitinib was recommend in hospitalized patients with COVID-19 (13).
But in severe hospitalized patients with COVID-19, the effectiveness of baricitinib was still controversial. On one hand, some studies found that baricitinib reduced mortality in severely ill patients. A small sample size exploratory trial showed that baricitinib reduced mortality in critically ill hospitalized patients with COVID-19 on IMV or extracorporeal membrane oxygenation (ECMO) (16). On the other hand, some studies showed that baricitinib brought no benefit on the mortality in severely ill patients. In the Bari-SolidAct study, no statistically significant difference was observed on 60-day mortality in hospitalized patients with severe/critical COVID-19 receiving either baricitinib or placebo (17). In subgroup analysis of RECOVERY study, baricitinib didn’t reduce mortality among patients on IMV (15). So the effect of baricitinib on severe COVID-19 was still controversial, more research was needed to draw a definite conclusion. To answer the question, further phase 3 randomized controlled trials (RCT) with large sample size are warranted. However along with massive vaccination across the globe, the incidence of severe COVID-19 is in decline, which makes the phase 3 RCT with large sample size less feasible. So the real-world studies, which have a mutually supplementary relationship with RCT, might be a good alternative. Real-world studies, which reflects the actual clinical aspects, could use data from collected from diversified areas including Electric Medical Record System (EMRS) used in hospitals. EMRS data, which are recognized as definitive data with the highest reliability among real-world data, are increasingly used for medical research (18). It is reasonable to assume that retrospective real-world studies by using EMRS data may provide further evidence of use of baricitinib in severe hospitalized patients with COVID-19. So we aimed to evaluate the effectiveness of baricitinib in severe COVID-19 patients requiring IMV in a propensity score-matched and retrospective study.
All patients admitted to the study hospital between December 1st 2022 to January 31st 2023 with discharge diagnosis of COVID-19 infection were retrieved from EMRS. During this time period, China had a policy shift in COVID-19 control, and our tiered medical system had dealt with a rise in infections in the country.
Two pulmonologists (AYG and HZ) reviewed the cases one by one independently and identified severe COVID-19 patients on IMV. Where differences arose, the third pulmonologist (JXL) validated the assessment. The standard of the reviewing was based on the following inclusion criteria and exclusion criteria. The inclusion criteria were as follows: (1) aged 18 years or older; (2) laboratory-confirmed COVID-19 infection; (3) use of IMV; (4) evidence of pneumonia or clinical symptoms of COVID-19. The exclusion criteria were as follows: (1) unknown vaccination status of COVID-19; (2) hospital-acquired COVID-19 infections; (3) intubated prior to admission.
Treatment strategies were based on institutional protocols that were updated weekly by a multidisciplinary panel, based on national COVID-19 guidelines published by National Health Commission of the People’s Republic of China. All severe COVID-19 patients on IMV received standard of care therapies, which included corticosteroids and prone position ventilation. The use of antiviral drugs, baricitinib, tocilizumab, intravenous immunoglobin (IVIG) or ECMO were left at the discretion of the physicians. During the study period, the Omicron variant of COVID-19 was the dominant variant in our province.
The primary outcome of current study was 28-days all-cause mortality after admission. Data were extracted from EMRS by a combination of drug utilization reports and manual chart reviews. Demographics, vaccination status, lab test results on admission, disease comorbidities, and pharmacotherapy were collected.
Patients who were treated with baricitinib during their hospital stay were defined as patients of baricitinib group. Per hospital’s treatment strategy, baricitinib 4 mg daily was the standard dose. To ensure a valid comparison of similar patients, we then used propensity score matching to 1:1 match patients treated with baricitinib and control. The following covariates were used for the calculation of the propensity score: age, sex, vaccination status, heart failure, renal insufficiency, diabetes mellitus, rheumatoid disease, interstitial lung disease, chronic obstructive pulmonary disease (COPD) and history of malignancy.
The results were analyzed using IBM SPSS Statistics 20. Continuous data was presented as the mean with stand deviation (SD) or median with interquartile range (IQR), depending on the distribution of data. For continuous data before PSM, if the data followed the normal distribution, variables were compared using Student’s t-test for independent samples. If the data didn’t follow the normal distribution, Mann-Whitney U test was used.
For continuous data after PSM, if the data followed the normal distribution, variables were compared using Student’s t-test for paired samples. If the data didn’t follow the normal distribution, Wilicoxon signed-rank for paired samples test was used. Categorical data were presented as absolute value and percentage. For categorical data before PSM, Chi-square test was used. For categorical data after PSM, McNemar test for paired data was used. Kaplan-Meier curves were used for survival analysis, and a dependent samples log-rank test was used to compare the curves. Statistical significance was set at p < 0.05.
Between December 1st 2022 to January 31st 2023, a total number of 2809 patients with discharge diagnosis of COVID-19 infection were identified from EMRS. After review, 424 patients met the inclusion criteria. Of those 424 patients, 104 patients were excluded. A final number of 320 patients were eligible for further analysis, which consisting of 48 patients treated with baricitinib. After PSM, 48 patients were assigned to control group (Figure 1). Covariates used for the calculation of the propensity score were compared before and after PSM (Table 1). The mean participant age were high in both group (baricitinib group vs. control group: 78.80 ± 9.04 vs. 82.57 ± 9.27). Most patients were unvaccinated in both groups (baricitinib group vs. control group: 62.5% vs. 66.7%). Both groups had a large proportion of patients with heart failure (baricitinib group vs. control group: 39.6% vs. 47.9%) and renal insufficiency (39.6% vs. 41.7%). Baseline demographic and comorbidity data were balanced between two groups, except for hypertension. Compared to control group, baricitinib group had a higher proportion of patients with hypertension (70.8% vs. 41.7%, p = 0.029) (Table 2).
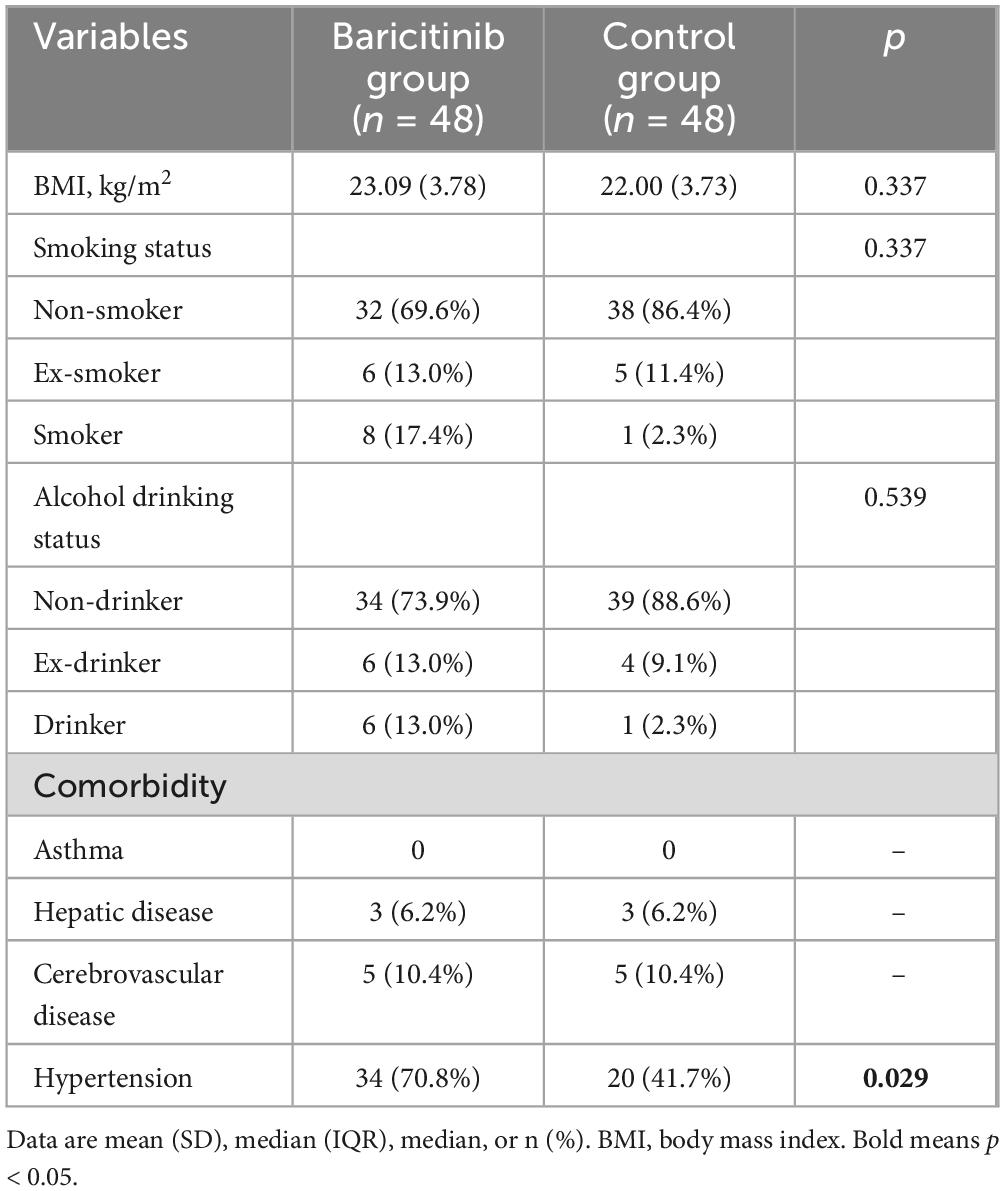
Table 2. Comparison of baseline demographic and comorbidity data between baricitinib group and control group.
The time from admission to intubation was similar between groups (Table 3). Regarding biomarkers of infection, both groups had similar levels of C-reactive protein (CRP), IL-6 and procalcitonin, which suggested that the severity of COVID-19 infection were similar between groups. Control group had higher level of creatine kinase-myocardial band (CK-MB) than baricitinib group (247.50 vs. 104.50, p = 0.021). But both groups had similar level of creatine kinase (CK). There was no significant difference in arterial pressure of oxygen and arterial pressure of carbon dioxide between two groups.
All patients in both groups received systemic corticosteroids, and the daily dose of systemic corticosteroids was similar between groups (Table 4). The majority in both groups received at least one of the three antiviral drugs including nirmatrelvir/ritonavir, azvudine and molnupiravir (baricitinib group vs. control group: 81.2% vs. 72.9%). Baricitinib group had a significant higher proportion of patients received nirmatrelvir/ritonavir than control group (39.6% vs. 16.7%, p = 0.017), and higher proportion of patients who switched antiviral drugs during treatment (31.2% vs. 12.5%, p = 0.033). There was no significant difference in use of antibiotics, low molecular weight heparin, tocilizumab and ECMO between the two groups. But patients in the baricitinib group were more likely to receive IVIG (14.6% vs. 0, p = 0.007).
Both groups reported high all-cause 28-days mortality (baricitinib group: 72.9% vs. control group: 89.6%). The Kaplan-Meier analysis identified significant difference between two groups in all-cause 28-days mortality after admission (log rank, p = 0.004) (Figure 2).
Our observational study compared real-world outcomes with baricitinib versus control for treatment in patients hospitalized with severe COVID-19 on IMV in a propensity score-matched and retrospective cohort. The study found high mortality among patients hospitalized with severe COVID-19 on IMV. The result suggested that baricitinib reduce 28-days mortality in severe COVID-19 patients on IMV.
One of the major strengths of our study was the PSM design. There are a few known risk factors for severe COVID-19, which could have impact on prognosis in a retrospective study. So far, age and comorbidities such as diabetes, COPD, chronic kidney disease, have been reported to be independent predictors of mortality for COVID-19 patients (3, 19–24). Vaccine has been shown to bring survival benefit in COVID-19, which can also influence the outcomes (25, 26). With the expanding coverage of COVID-19 vaccination, its influence on reducing mortality could not been overlooked. So the current study excluded patients with unknown vaccination status. Given these potentially confounding variables and the retrospective, observational nature of current study, PSM was applied to minimize bias. Therefore, the outcomes were assessed between two comparable groups, which increased validity of our study. Moreover in our study, the infection biomarkers such CRP, IL-6 and procalcitonin, were balanced between both groups, which suggested that the severity of infection was similar. So the balanced baseline demographics, comorbidity profile and severity of infection lend credibility to our findings.
Our study showed a significant difference in the 28-days all-cause mortality between severe COVID-19 patients on IMV treated with baricitinib versus control. In less severe hospitalized COVID-19 patients, the benefit of baricitinib on reducing mortality was reported by numerous studies. It was roughly estimated that baricitinib or another JAK inhibitor was associated with a 20% proportional reduction in mortality (15). However, in severe COVID-19, there were discrepant results about effectiveness of baricitinib among studies. On one hand, some studies found that baricitinib reduced mortality in severely ill patients. In an exploratory trial conducted in severe patients on IMV or ECMO, treatment with baricitinib compared with placebo reduced 28-days all-cause mortality by 46% and 60-day all-cause mortality by 44% (16). But this trial had a relatively small sample size. On the other hand, some studies showed that baricitinib brought no benefit on the mortality in severely ill patients. In the Bari-SolidAct study, no statistically significant difference was observed on 60-days mortality in hospitalized patients with severe/critical COVID-19 receiving either baricitinib or placebo (17). Of note, the trial was stopped before reaching planned sample size, so the results should be treated with caution. In subgroup analysis of RECOVERY study, baricitinib didn’t reduce mortality among patients on IMV (15). Our findings provided a new support of the use of baricitinib in severe COVID-19 patients on IMV. But the effectiveness of baricitinib in severe COVID-19 was still needed more research to draw a definite conclusion.
Despite the use of standard care, our study reported high mortality of 72.9% for baricitinib group and 89.6% for control group, which were much higher than reported in previous studies. In an exploratory trial conducted in severe patients, the overall 28-days all-cause mortality for those on IMV or ECMO at baseline was 39% in participants who received baricitinib and 58% in those who received placebo (16). The open-label RECOVERY study reported that 28-days mortality of 49% in participants who received tocilizumab while on IMV versus 51% in those who received standard of care (15). In one large meta-analysis, the case fatality rate of 45% was reported for patients with severe COVID-19 requiring IMV (16). This discrepancy in mortality may be explained by the following reasons. First, our study included older patients than previous studies. In our study, the mean age was 78.80 ± 9.04 years for baricitinib group and 82.57 ± 9.27 years for control group. The mean participant age was 58.6 ± 13.8 years in the above-mentioned exploratory trial, and 58.1 ± 15.5 years in the RECOVERY study. Studies have shown that older age is a risk factor for fatality related to COVID-19 (3, 27). Second, our study had a large proportion of patients with comorbidities, especially heart failure (39.6–47.9%) and renal insufficiency (39.6–41.7%). In the RECOVERY study, only about 18–19% of patients had heart disease and about 2% had severe kidney impairment (15). The discrepancy indicated that the patients in our study were in poorer health state, which may contribute to a worse prognosis and high mortality. Third, delayed use of antiviral drugs may also contribute to high morbidity. Most of our patients received antiviral drugs after intubation instead of in early phase of COVID-19, so maybe it was too late. It was well-known that early use of antiviral drugs lead to better prognosis (4, 5).
Except for the standard of care therapies, the additional treatments such as antiviral drugs, tocilizumab, or ECMO were left at the discretion of the physicians in the study. During the surge there was a national drug shortage, which made the use of additional treatments variable among patients. Our study found that patients in the baricitinib group were more likely to receive nirmatrelvir/ritonavir and IVIG. Nirmatrelvir/ritonavir could reduce risk of progression to severe COVID-19 and mortality for non-hospitalized adults, and its effectiveness on severe COVID-19 was unknown (5, 28). In the current study, most of patients received nirmatrelvir/ritonavir after intubated and admitted to ICU instead of within five days of the onset of symptoms, which was not supported by evidence. The effectiveness of delayed use of nirmatrelvir/ritonavir complicated matters even further. Regarding IVIG, available data do not support its use in severe COVID-19. In a multicenter, double-blind, placebo-controlled, phase 3 trial of patients with moderate-to-severe COVID-19-associated acute respiratory distress syndrome, IVIG did not improve clinical outcomes at day 28 and tended to be associated with an increased frequency of serious adverse events (29). Two retrospective studies reported similar results and found IVIG was not associated with significant changes in mortality in severe COVID-19 patients (30, 31). In conclusion, although published studies showed that neither nirmatrelvir/ritonavir nor IVIG reduce mortality in severe COVID-19, their effect on outcome were difficult to account for in a retrospective study design.
Due to PSM, most comorbidities were balanced between groups except hypertension. Our study reported that baricitinib group had a higher proportion of patients with hypertension. Hypertension has been identified as the most prevalent cardiovascular comorbidity in patients infected with COVID-19 (21). So far, the evidence had been mixed about the association between hypertension and COVID-19 prognosis (32–34). In a study conducted in Italy, hypertension was not an independent predictor of mortality in the multivariate analysis (24). Another European registry study, which included more than 9,000 patients, reported that hypertension was not independently related with in-hospital mortality (35). On the other hand, a large-scale analysis in China showed that hypertension was a significant risk factor for poor outcomes including admission to an intensive care unit, invasive ventilation, or death (21). A meta-analysis found that hypertension acted as an independent risk factor for deterioration of COVID-19 (36). So inconsistent results about association between hypertension and COVID-19 prognosis make the interpretation of our findings troublesome. The retrospective design of our study further made it difficult to clarify the impact of hypertension on the outcome.
The current study also found there were disagreement about CK and CK-MB in patients. Compared to baricitinib group, the control group had higher level of CK-MB and similar levels of CK. Previous results showed that the cardiovascular system of the COVID-19 patients had been notably damaged, and the degree of damage could be evaluated by cardiac biomarkers such as CK and CK-MB. Most studies showed that the elevated levels of CK and CK- MB were significantly associated with an increased risk of the mortality in COVID-19 infected patients (37). For example, in a retrospective analysis including 2954 COVID-19 patients, those with higher CK-MB had a significantly higher mortality rate compared to patients with normal levels (38). Two meta-analysis revealed that higher CK and CK-MB were associated with the mortality and severe disease in COVID-19 patients (39, 40). But there were some studies reporting conflicting results as well. A study found CK-MB and CK had no significant difference in the prediction effect of the mortality in COVID-19 (41). The disagreement of CK and CK-MB found in our study made it difficult to clarify whether patients in the control group had more severe cardiac damage than patients in the baricitinib group. As a result, the impact of cardiac damage on the outcome could be evaluated neither.
Some limitations of the study merit consideration. First, the present study was a retrospective study, so there were inherent problems related to this design. Second, we did not have data about the specific variant or sub-variant of each patient in our cohort. Third, some disparities in baseline characteristics and concomitant treatment were present which may have influenced the outcome parameters.
The present study revealed baricitinib reduced 28-days mortality in severe COVID-19 patients on IMV. The effectiveness of baricitinib in treating patients with severe COVID-19 on IMV needs to be further investigated through future studies.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Ethics Committee of Second Affiliated Hospital of Zhejiang University School of Medicine (Approval Number: 2023-0135). The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by- product of routine care or industry. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
YM: Conceptualization, Data curation, Investigation, Writing – original draft, Writing – review & editing. AG: Data curation, Investigation, Writing – review & editing. YZ: Data curation, Investigation, Writing – review & editing. JL: Data curation, Investigation, Writing – review & editing. DY: Data curation, Writing – review & editing. HZ: Data curation, Investigation, Writing – review & editing. WD: Data curation, Writing – review & editing. WC: Data curation, Writing – review & editing. FY: Conceptualization, Investigation, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Zhejiang University Education Foundation Project (No. 100000-11533/002), Zhejiang Provincial Medical and Health Science and Technology Project (No. 2019KY742), Bethune Charitable Foundation (No. J202201E026), and Beijing Health Alliance Charitable Foundation (Health Development Promotion Project).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Tahaghoghi-Hajghorbani S, Zafari P, Masoumi E, Rajabinejad M, Jafari-Shakib R, Hasani B, et al. The role of dysregulated immune responses in COVID-19 pathogenesis. Virus Res. (2020) 290:198197.
2. Lee JS, Park S, Jeong HW, Ahn JY, Choi SJ, Lee H, et al. Immunophenotyping of COVID-19 and influenza highlights the role of type I interferons in development of severe COVID-19. Sci Immunol. (2020) 5:eabd1554. doi: 10.1126/sciimmunol.abd1554
3. Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. (2020) 180:934–43.
4. Li G, Hilgenfeld R, Whitley R, De Clercq E. Therapeutic strategies for COVID-19: Progress and lessons learned. Nat Rev Drug Discov. (2023) 22:449–75.
5. Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N Engl J Med. (2022) 386:1397–408.
6. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. (2021) 384:693–704.
7. Peterson JH, Paranjape NS, Grundlingh N, Priestley JL. Outcomes and adverse effects of baricitinib versus tocilizumab in the management of severe COVID-19. Crit Care Med. (2023) 51:337–46.
8. Shi JG, Chen X, Lee F, Emm T, Scherle PA, Lo Y, et al. The pharmacokinetics, pharmacodynamics, and safety of baricitinib, an oral JAK 1/2 inhibitor, in healthy volunteers. J Clin Pharmacol. (2014) 54:1354–61. doi: 10.1002/jcph.354
9. Karampitsakos T, Papaioannou O, Tsiri P, Katsaras M, Katsimpris A, Kalogeropoulos AP, et al. Tocilizumab versus baricitinib in hospitalized patients with severe COVID-19: An open label, randomized controlled trial. Clin Microbiol Infect. (2023) 29:372–8.
10. Marconi VC, Ramanan AV, de Bono S, Kartman CE, Krishnan V, Liao R, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): A randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med. (2021) 9:1407–18. doi: 10.1016/S2213-2600(21)00331-3
11. Stebbing J, Phelan A, Griffin I, Tucker C, Oechsle O, Smith D, et al. COVID-19: Combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. (2020) 20:400–2.
12. Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. New England Journal of Medicine. (2020) 384:795–807.
13. Selvaraj V, Finn A, Lal A, Khan MS, Dapaah-Afriyie K, Carino GP. Baricitinib in hospitalised patients with COVID-19: A meta-analysis of randomised controlled trials. Eclinicalmedicine. (2022) 49:101489.
14. Wolfe CR, Tomashek KM, Patterson TF, Gomez CA, Marconi VC, Jain MK, et al. Baricitinib versus dexamethasone for adults hospitalised with COVID-19 (ACTT-4): A randomised, double-blind, double placebo-controlled trial. Lancet Respir Med. (2022) 10:888–99.
15. Group RC. Baricitinib in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial and updated meta-analysis. Lancet. (2022) 400:359–68. doi: 10.1016/S0140-6736(22)01109-6
16. Ely EW, Ramanan AV, Kartman CE, de Bono S, Liao R, Piruzeli MLB, et al. Efficacy and safety of baricitinib plus standard of care for the treatment of critically ill hospitalised adults with COVID-19 on invasive mechanical ventilation or extracorporeal membrane oxygenation: An exploratory, randomised, placebo-controlled trial. Lancet Respir Med. (2022) 10:327–36.
17. Trøseid M, Arribas JR, Assoumou L, Holten AR, Poissy J, Terzić V, et al. Efficacy and safety of baricitinib in hospitalized adults with severe or critical COVID-19 (Bari-SolidAct): A randomised, double-blind, placebo-controlled phase 3 trial. Crit Care (London, England). (2023) 27:9.
18. Kim HS, Lee S, Kim JH. Real-world evidence versus randomized controlled trial: Clinical research based on electronic medical records. J Korean Med Sci. (2018) 33:e213.
19. Javid FA, Waheed FA, Zainab N, Khan H, Amin I, Bham A, et al. COVID-19 and diabetes in 2020: A systematic review. J Pharm Policy Pract. (2023) 16:42.
20. Higham A, Mathioudakis A, Vestbo J, Singh D. COVID-19 and COPD: A narrative review of the basic science and clinical outcomes. Eur Respir Rev. (2020) 29:200199. doi: 10.1183/16000617.0199-2020
21. Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur Respir J. (2020) 55:2000547. doi: 10.1183/13993003.00547-2020
22. Janice ML, Masahiro N, Cheng Wei Tony Y, Don DS. COVID-19 and COPD. Eur Respir J. (2020) 56:2002108.
23. Schiffl H, Lang SM. Long-term interplay between COVID-19 and chronic kidney disease. Int Urol Nephrol. (2023) 55:1977–84.
24. Iaccarino G, Grassi G, Borghi C, Ferri C, Salvetti M, Volpe M. Age and multimorbidity predict death among COVID-19 patients: Results of the SARS-RAS study of the Italian society of hypertension. Hypertension. (2020) 76:366–72. doi: 10.1161/HYPERTENSIONAHA.120.15324
25. Pooley N, Abdool Karim SS, Combadière B, Ooi EE, Harris RC, El Guerche Seblain C, et al. Durability of vaccine-induced and natural immunity against COVID-19: A narrative review. Infect Dis Ther. (2023) 12:367–87.
26. Shah A, Coiado OC. COVID-19 vaccine and booster hesitation around the world: A literature review. Front Med (Lausanne). (2022) 9:1054557. doi: 10.3389/fmed.2022.1054557
27. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
28. Arbel R, Wolff Sagy Y, Hoshen M, Battat E, Lavie G, Sergienko R, et al. Nirmatrelvir use and severe COVID-19 outcomes during the omicron surge. N Engl J Med. (2022) 387:790–8.
29. Mazeraud A, Jamme M, Mancusi RL, Latroche C, Megarbane B, Siami S, et al. Intravenous immunoglobulins in patients with COVID-19-associated moderate-to-severe acute respiratory distress syndrome (ICAR): Multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. (2022) 10:158–66. doi: 10.1016/S2213-2600(21)00440-9
30. Liu J, Chen Y, Li R, Wu Z, Xu Q, Li Z, et al. Intravenous immunoglobulin treatment for patients with severe COVID-19: A retrospective multicentre study. Clin Microbiol Infect. (2021) 27:1488–93.
31. Chen Y, Xie J, Wu W, Li S, Hu Y, Hu M, et al. Intravenous immunoglobulin therapy for critically ill COVID-19 patients with different inflammatory phenotypes: A multicenter, retrospective study. Front Immunol. (2021) 12:738532. doi: 10.3389/fimmu.2021.738532
32. Peng M, He J, Xue Y, Yang X, Liu S, Gong Z. Role of hypertension on the severity of COVID-19: A review. J Cardiovasc Pharmacol. (2021) 78:e648–55.
33. Gallo G, Calvez V, Savoia C. Hypertension and COVID-19: Current evidence and perspectives. High Blood Pressure Cardiovasc Prev. (2022) 29:115–23.
34. Shibata S, Kobayashi K, Tanaka M, Asayama K, Yamamoto E, Nakagami H, et al. COVID-19 pandemic and hypertension: An updated report from the Japanese Society of Hypertension project team on COVID-19. Hypertens Res. (2023) 46:589–600. doi: 10.1038/s41440-022-01134-5
35. McFarlane E, Linschoten M, Asselbergs FW, Lacy PS, Jedrzejewski D, Williams B. The impact of pre-existing hypertension and its treatment on outcomes in patients admitted to hospital with COVID-19. Hypertens Res. (2022) 45:834–45.
36. Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: Evidence from meta-analysis. Aging (Albany NY). (2020) 12:6049–57.
37. Cersosimo A, Cimino G, Amore L, Calvi E, Pascariello G, Inciardi RM, et al. Cardiac biomarkers and mortality in COVID-19 infection: A review. Monaldi Arch Chest Dis. (2022) 93:2276.
38. Li P, Wu W, Zhang T, Wang Z, Li J, Zhu M, et al. Implications of cardiac markers in risk-stratification and management for COVID-19 patients. Crit Care (London, England). (2021) 25:158.
39. Zinellu A, Sotgia S, Fois AG, Mangoni AA, Serum CK-MB. COVID-19 severity and mortality: An updated systematic review and meta-analysis with meta-regression. Adv Med Sci. (2021) 66:304–14.
40. Cao B, Jing X, Liu Y, Wen R, Wang C. Comparison of laboratory parameters in mild vs. severe cases and died vs. survived patients with COVID-19: Systematic review and meta-analysis. J Thorac Dis. (2022) 14:1478–87. doi: 10.21037/jtd-22-345
Keywords: COVID-19, baricitinib, mortality, propensity score matching, invasive mechanical ventilation
Citation: Mao Y, Guo A, Zhang Y, Lai J, Yuan D, Zhang H, Diao W, Chen W and Yan F (2025) Baricitinib treatment for hospitalized patients with severe COVID-19 on invasive mechanical ventilation: a propensity score-matched and retrospective analysis. Front. Med. 12:1445809. doi: 10.3389/fmed.2025.1445809
Received: 08 June 2024; Accepted: 07 January 2025;
Published: 22 January 2025.
Edited by:
Ariane Zamoner, Federal University of Santa Catarina, BrazilReviewed by:
Ourania Papaioannou, General University Hospital of Patras, GreeceCopyright © 2025 Mao, Guo, Zhang, Lai, Yuan, Zhang, Diao, Chen and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fugui Yan, eWZnMDcyNkB6anUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.