
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 03 March 2025
Sec. Pulmonary Medicine
Volume 12 - 2025 | https://doi.org/10.3389/fmed.2025.1440121
 Kaihan Su1,2†
Kaihan Su1,2† Xiaoyu Wang1,2†
Xiaoyu Wang1,2† ShiYin Zhang1,2
ShiYin Zhang1,2 Jiantong Wu1,2
Jiantong Wu1,2 Yuqi Chen3
Yuqi Chen3 Lianjun Yin4
Lianjun Yin4 Haunan Li1,2
Haunan Li1,2 Jingui Wang1,2*
Jingui Wang1,2*Objectives: This study aims to systematically evaluate the efficacy of acupuncture on stroke-associated pneumonia (SAP).
Methods: English and Chinese databases were searched from their inception until 15 March 2024 to collect randomized controlled trials (RCTs). The risk of bias was assessed using Cochrane collaboration tools. RevMan 5.4.0 software was used to analyze the included studies, and the Grades of Recommendations, Assessment, Development, and Evaluation (GRADE) assessment was used to evaluate the quality of the study outcomes.
Results: 16 studies involving 1,125 patients were included in this meta-analysis. Compared with the control group, the results showed that acupuncture significantly improved the effective rate [RR = 1.20, 95% CI (1.13, 1.27), P < 0.00001] and reduced the level of white blood cells (WBC) [MD = −6.52, 95% CI (−8.31, −4.73), P < 0.00001], C reactive protein (CRP) [MD = −6.50, 95% CI (−9.97, −3.03), P = 0.0002], neutrophil percentage (Neu%) [MD = −6.66, 95% CI (−8.96, −4.36), P < 0.00001], and procalcitonin (PCT) [MD = −0.81, 95% CI (−1.21, −0.40), P < 0.0001]. Additionally, acupuncture therapy shortened the duration of coughing [MD = −3.22, 95% CI (−4.73, −1.72), P < 0.0001], duration until disappearance of rales [MD = −3.99, 95% CI (−6.44, −1.54), P = 0.001], and duration of antibiotic use [MD = −4.51, 95% CI (−5.46, −3.57), P < 0.00001]. It also reduced the clinical pulmonary infection score (CPIS) [MD = −1.71, 95% CI (−2.71, −0.71), P = 0.0008] and National Institute of Health Stroke Scale (NIHSS) [MD = −3.93, 95% CI (−5.78, −2.09), P < 0.00001]. Moreover, acupuncture therapy increased the forced vital capacity (FVC) [MD = 0.46, 95% CI (0.02, 0.89), P = 0.04] and Forced Expiratory Volume in One Second (FEV1) [MD = 0.49, 95% CI (0.14, 0.84), P = 0.006].
Conclusion: This study found that acupuncture has a positive effect in treating SAP. However, owing to the low-quality evidence, more rigorous studies are needed in the coming years to confirm these findings.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023462846, identifier CRD42023462846.
According to the Global Burden of Disease Study 2021 (1), stroke is the third leading cause of death worldwide (1). As revealed by the Global Burden of Disease Study 2016, stroke not only causes death but also disability, profoundly affecting the health and quality of life of millions of people (2). Infection is both a risk factor for stroke and a determinant of post-stroke outcomes (3). The patient’s immune response is suppressed following a stroke, adversely influencing survival and recovery (4). Pneumonia is the most common type of infection and has a greater impact on clinical outcomes (5). Additionally, approximately 42% of stroke patients experience post-stroke dysphagia (PSD), which heightens the risk of silent aspiration and penetration, potentially resulting in aspiration pneumonia (6). Stroke-associated pneumonia (SAP) is a frequent complication that arises from lower respiratory tract infections within the first week following a stroke (7), affecting approximately 14.3% of stroke patients (8). Moreover, it is closely associated with a variety of complications such as gastrointestinal bleeding (8.35%), bedsores (5.31%), deep vein thrombosis (4.27%), epileptic seizures (3.96%), urinary tract infections (3.34%), atrial fibrillation/flutter (3.17%), and recurrent stroke (2.65%) (9). It elevates mortality rates for up to one year, extends the duration of hospital stays, and deteriorates functional outcomes at discharge (10, 11).
SAP plays a vital role in the development of various complications after stroke, emphasizing the need for integrative therapy to help patients get better outcomes. Antibiotics remain the primary medication for treating pneumonia (12). However, their use failed to improve the prognosis for stroke patients (13). In addition, the current evidence does not support using antibiotics or immunomodulatory approaches as a preventative measure to prevent pneumonia in stroke patients with dysphagia, even if they are at a high risk of aspiration (13, 14). Even though Angiotensin Converting Enzyme (ACE) inhibitors were associated with lower pneumonia-related mortality, the data is not convincing (15). Similarly, while metoclopramide could reduce the incidence of pneumonia after stroke, it did not decrease mortality rates and lack of robust evidence (16). Consequently, given the limitations of conventional treatment (CT) in improving the prognosis of SAP, exploring effective alternative therapies for SAP is essential. Research has proven that dysphagia screening and rehabilitation, feeding modification, oral care, airway management, position management, and Traditional Chinese Medicine (TCM) nursing techniques have significant effects on the prevention of SAP (17).
Acupuncture has been widely used in the recovery after stroke and has shown favorable results by regulating various mechanisms within the central nervous system (CNS) (18). Moreover, acupuncture therapy has been shown to reduce the inflammatory response by affecting the regulation of multiple cytokines, making it an effective potential treatment for respiratory disease (19). In recent years, several small-sample randomized controlled trials (RCTs) on acupuncture for SAP have been conducted domestically and internationally (20–35). While these studies have confirmed the positive effects of acupuncture on SAP, due to the varying outcome measures among these studies, there is still lacking robust and high-quality evidence to support these findings.
According to our knowledge, no high-quality systematic review or meta-analysis currently provides a comprehensive summary of this topic. Therefore, further research is needed to fully understand this subject. This meta-analysis significantly enhances our understanding of the role of acupuncture in SAP. We hope that the findings of this study will capture the interest of healthcare professionals, researchers, and patients alike.
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines (36). The study protocol was registered in PROSPERO (number CRD42023462846).
We searched PubMed, Embase, Sinomed, the Cochrane Library, Web of Science, China National Knowledge Infrastructure (CNKI), Chinese Scientific Journal Database (VIP), and Wanfang Data database. The search covered the period from the inception of these databases to 15 March, 2024. The keywords and search terms were imposed: “stroke,” “pneumonia,” “stroke associated pneumonia,” “acupuncture,” and “RCT.” More search strategies are available in Supplementary File 1.
Inclusion criteria:
1. Types of participants: Individuals meeting the diagnostic criteria for SAP were diagnosed by the “Chinese expert consensus on the diagnosis and treatment of stroke-associated pneumonia” (37) or “Diagnosis of stroke-associated pneumonia: recommendations from pneumonia in stroke consensus group” (7), with no restrictions on nationality, ethnicity, age, gender, or other demographic factors.
2. Types of intervention: The treatment group receives acupuncture and CT.
3. Types of comparison: The control group receives CT without acupuncture therapy.
4. Types of outcomes: The primary outcome measure was the effective rate. The secondary outcomes were the inflammatory markers [C-reactive protein (CRP), procalcitonin (PCT), white blood cell count (WBC), neutrophil percentage (Neu%)], pulmonary symptoms forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), cough duration, duration until disappearance of rales, and clinical pulmonary infection score (CPIS), national institute of health stroke scale (NIHSS), duration of antibiotic use and incidence of adverse reactions.
5. Types of study: RCTs.
Exclusion Criteria:
1. Reviews, basic experiments, and reports.
2. Unpublished or duplicate publications.
3. Studies with missing data.
4. The control group receives acupuncture therapy.
Two reviewers (KHS and XYW) independently reviewed the titles and abstracts of identified studies to exclude unrelated studies that did not fit the subject of the study, and disagreements were resolved through discussion with the third reviewer (JTW). Subsequently, a researcher (KHS) extracted the information required for this study from the selected articles into Excel 2016, including the first author, publication year, average age, randomization method, sample size, intervention method, adverse reactions, allocation concealment, outcome measures, and their data integrity information. After data extraction, all data were checked by another investigator (XYW), and discrepancies were discussed and resolved by the third reviewer (JTW).
Review Manager 5.4.0 and Stata 14.0 were used for systematic evaluation and meta-analysis in this study. Continuous data was represented by mean difference (MD) with a corresponding 95% confidence interval (CI), while binary data was represented by risk ratio (RR) with a corresponding 95% confidence interval. Heterogeneity between studies was assessed using the chi-square test and I-squared (I2) index. A fixed effects model was employed if the P ≥ 0.05 and I2 ≤ 50%; otherwise, a random effects model was chosen. Statistical significance was denoted by a significance level of P ≤ 0.05. If the number of studies was > 10 for comparison, we assessed the possibility of publication bias using funnel plots and assessed funnel plot asymmetry using Egger’s regression test (P < 0.1).
The Cochrane bias risk assessment tool (38) was used by two researchers (KHS and XYW) to evaluate the risk of bias in the included literature, and disagreements were resolved through discussion with the third reviewer (JTW). This evaluation covered aspects such as randomisation processes, allocation concealment, blinding techniques, completeness of data, reporting selectivity, and other bias risks. The outcomes of this evaluation were classified into three levels: “high risk,” “unclear,” or “low risk.”
The Grades of Recommendations, Assessment, Development, and Evaluation (GRADE) assessment (39) was used to evaluate the quality of the study outcomes. The quality of the evidence was categorized as “high,” “medium,” “low,” or “very low” depending on the study’s findings Grade. Factors affecting the grade of evidence include risk of bias, inconsistency, indirectness, precision, and publication bias. Details of the GRADE evidence quality assessment are presented in Table 1.
Initially, a total of 1,798 studies were obtained from the database. Subsequently, 1,085 duplicate records were removed, and 1,014 irrelevant records were excluded based on titles and abstracts. After screening the complete text, 55 trials were further excluded. Ultimately, 16 trials (20–35) were included. Details of the selection process are presented in Figure 1.
The studies were conducted by Chinese researchers and published from 2014 to 2023 in China. A total of 16 trials (20–35) involving 1,125 patients were included in the study. There were 613 patients in the experimental group and 612 patients in the control group. In the control group, CT was used alone in 8 trials (23, 26, 29, 31–35), combined with swallowing rehabilitation in 2 trials (25, 30), with extracorporeal diaphragm pacing in 1 trial (27), with breathing training in 4 trials (21, 22, 27, 28), and with both breathing training and ultrashort wave electrotherapy in 1 trial (24). The detailed intervention methods for the control group are provided in Supplementary File 2. In the experimental groups, patients were treated with manual acupuncture (MA) in 13 trials (20–24, 27–32, 34, 35); Electroacupuncture (EA) in 2 trials (25, 26); and acupuncture point embedding (APE) in one trial (33). The most frequent retention time of MA and EA was 30 min (21, 23–32, 34), and 1 MA was 15 min (20), 1 MA was 20 min (22), and 1 MA only aimed to achieve “De qi” without retaining the needle (35). Additionally, 1 trial required the APE to be done once a week without mention of retention time (33). The treatment period varied from 10 to 28 days, and the most frequent treatment period was 14 days (20–22, 26, 28–35). All trials reported acupoints, and the top 5 acupoints with the highest frequency were BL13 (eight times) (21, 22, 25, 26, 29, 30, 33, 34), LI4 (seven times) (20, 21, 24, 27, 28, 31, 34), GV20 (six times) (20, 24, 25, 28, 31, 34), CV23 (six times) (20, 23, 24, 28, 34, 35), and GB20 (five times) (20, 24, 25, 28, 29), as shown in Figure 2. Adverse events were not mentioned. Detailed information regarding study design, participant demographics, and measured outcomes can be found in Table 2.
We assessed the risk of bias for all 16 trials (20–35), and the detailed assessment data are presented in Figure 3. For randomized sequence generation, 10 trials (20, 22, 24–26, 28, 30, 31, 33) were at low risk of bias, 2 trials (27, 29) were at high risk, and 4 trials (21, 23, 34, 35) were at unclear risk of bias. For allocation concealment, 15 trials (20–24, 26–35) were at unclear risk of bias in this area, and 1 trial (25) was at low risk of bias. 1 trial (29) reported blinding of participants and researchers, whereas other trials (20–28, 30–35) did mention this aspect, thereby leaving unclear risk of bias. The risk of bias in the blinding of outcome assessments was unclear in all studies. Regarding incomplete outcome data, 15 trials (20–25, 27–35) had a low risk of bias, and 1 trial (26) had a high risk of bias. In terms of selective reporting, all 16 trials (20–35) were at low risk of bias. For other biases, the risk of bias for all 16 trials (20–35) was unclear.
11 trials using a fixed effect model reported the effective rate between the intervention and control groups. The results demonstrated that the intervention group showed superior effectiveness than the control group [RR = 1.20, 95% Cl (1.13, 1.27), P < 0.00001], with no significant heterogeneity detected (I2 = 0%, P = 0.79) (Figure 4).
8 trials using a random effects model reported the CRP levels between the intervention and control groups. The results demonstrated that the intervention group had a significantly lower CRP level than the control group [MD = −6.50, 95% CI (−9.97, −3.03), P = 0.0002]. However, it is essential to note the considerable heterogeneity (I2 = 93%, P < 0.00001) (Figure 5A).
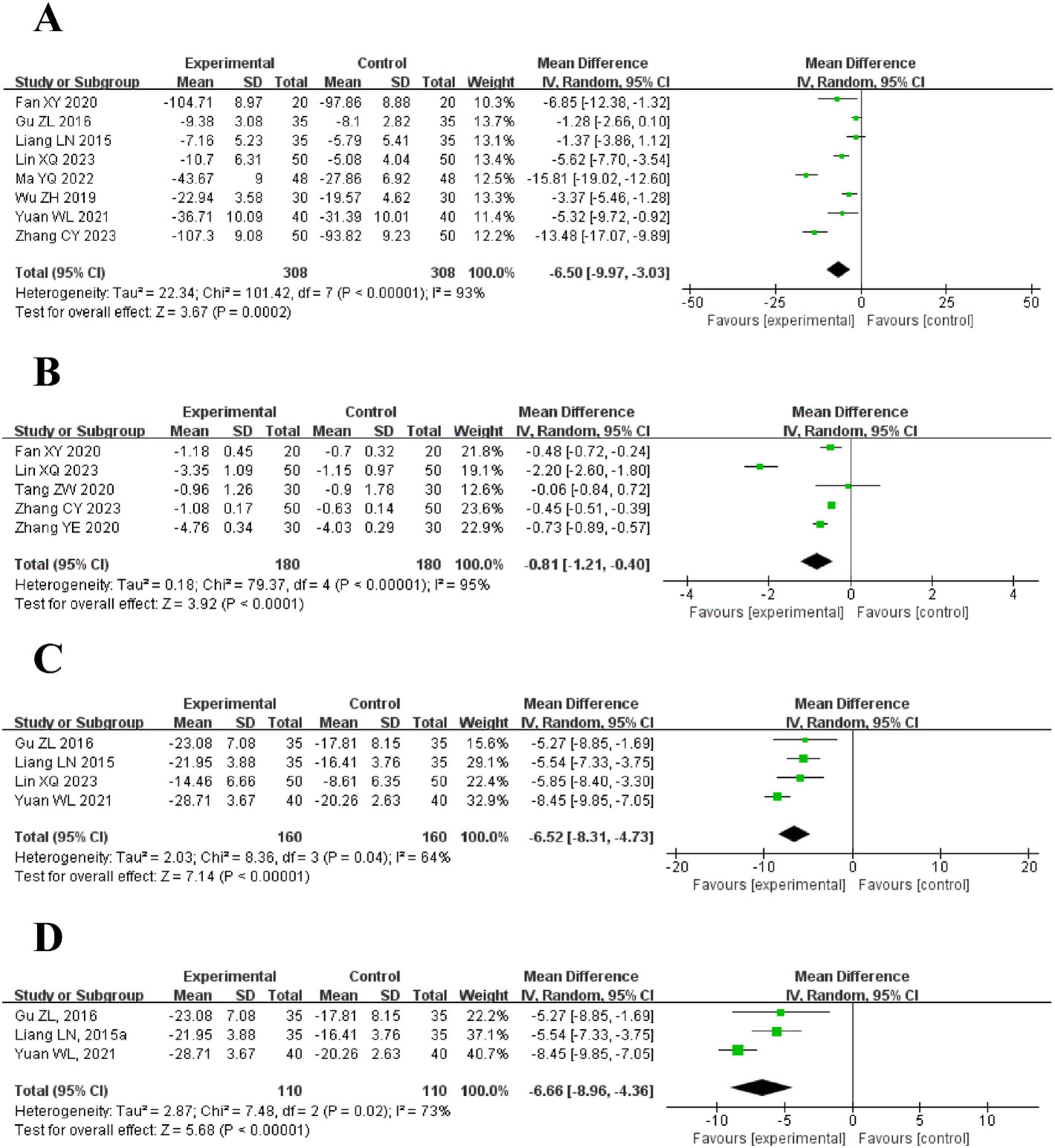
Figure 5. The forest plot of inflammatory markers in blood routine compared to acupuncture plus CT vs. CT. (A) C-Reactive Protein (CRP) Levels. (B) Procalcitonin (PCT) Levels. (C) White Blood Cell Count (WBC) Levels. (D) Neutrophil Percentage (Neu%) Levels.
5 trials used a random effects model to compare the PCT levels between the intervention and control groups. The results demonstrated that the intervention group had a significantly lower PCT level than the control group [MD = −0.81, 95% CI (−1.21, −0.40), P < 0.0001]. However, substantial heterogeneity was observed (I2 = 95%, P < 0.00001) (Figure 5B).
4 trials reported the WBC levels between the intervention and control groups using a random effects model. The results demonstrated that the intervention group had a significantly lower WBC level than the control group [MD = −6.52, 95% CI (−8.31, −4.73), P < 0.00001], The included trials exhibited moderate heterogeneity (I2 = 64%, P = 0.04) (Figure 5C).
3 trials reported the Neu% levels between the intervention and control groups using a random effects model. The results demonstrated that the intervention group had a significantly lower WBC level than the control group [MD = −6.66, 95% CI (−8.96, −4.36), P < 0.00001], Moderate heterogeneity was observed among the trials (I2 = 73%, P = 0.02) (Figure 5D).
5 trials reported cough duration showed that the intervention group had a significantly shorter cough duration than the control group [MD = −3.22, 95% CI (−4.73, −1.72), P < 0.0001] with high heterogeneity (I2 = 94%, P < 0.00001). As shown in Figure 6A.
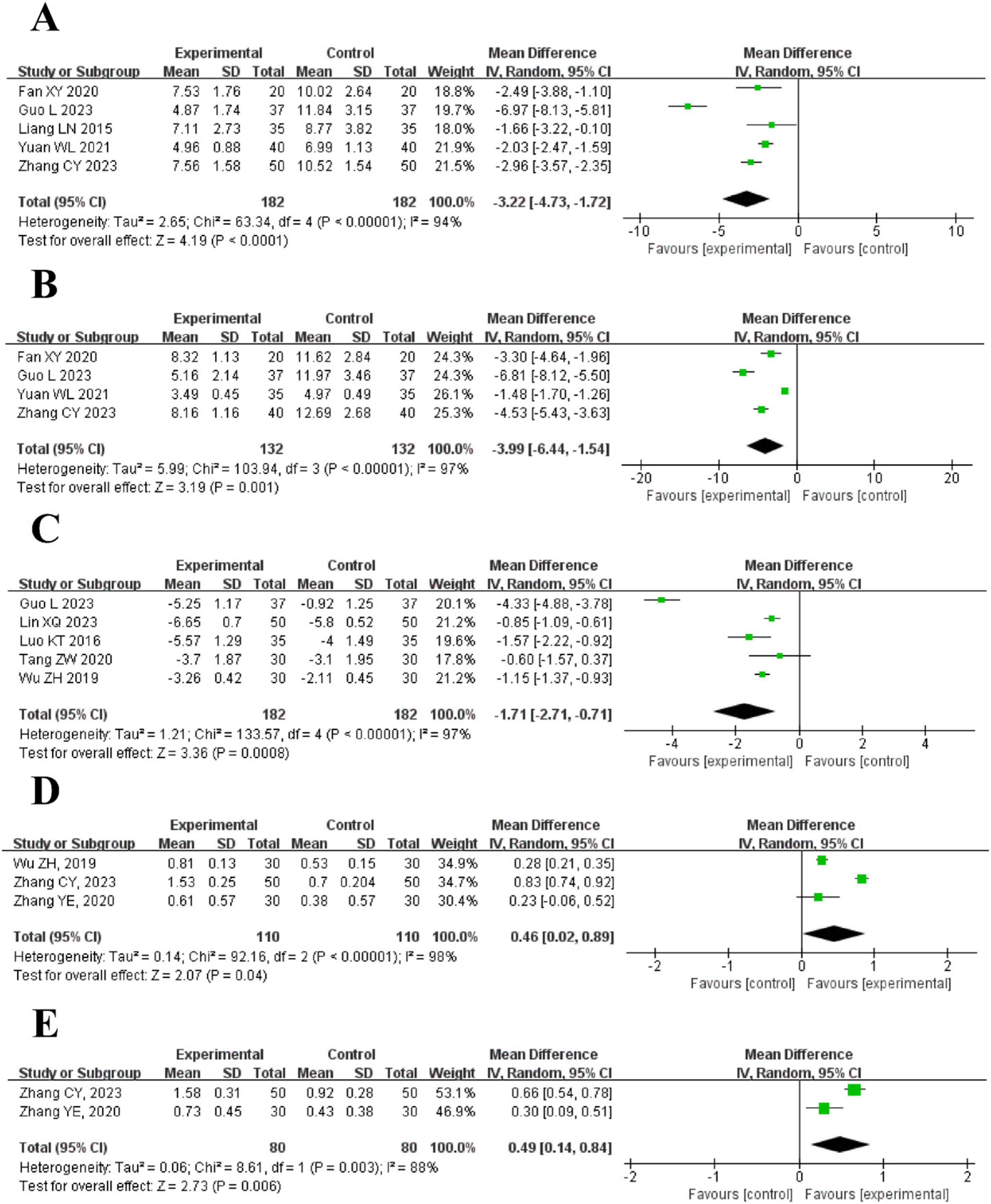
Figure 6. The forest plot of pulmonary symptoms compared to acupuncture plus CT vs. CT. (A) Cough Duration. (B) Duration Until Disappearance of Rales. (C) Clinical pulmonary infection score (CPIS). (D) Forced vital capacity (FVC). (E) Forced expiratory volume in 1 s (FEV1).
4 trials indicated that the duration until the disappearance of rales was shorter in the intervention group [MD = −3.99, 95% CI (−6.44, −1.54), P = 0.001]. High heterogeneity was found (I2 = 97%, P < 0.00001). As shown in Figure 6B.
5 trials reported CPIS showed that the intervention group had a lower score than the control group [MD = −1.71, 95% CI (−2.71, −0.71), P = 0.0008], with high heterogeneity (I2 = 97%, P < 0.00001). As shown in Figure 6C.
3 trials showed that FVC was higher in the intervention group [MD = 0.46, 95% CI (0.02, 0.89), P = 0.04], with high heterogeneity (I2 = 98%, P < 0.00001). As shown in Figure 6D.
2 trials found that FEV1 was higher in the intervention group [MD = 0.49, 95% CI (0.14, 0.84), P = 0.006], with high heterogeneity (I2 = 88%, P = 0.003). As shown in Figure 6E.
4 trials reported the NIHSS between the intervention and control groups using a random-effects model. The results demonstrated that the intervention group had a significantly lower NIHSS than the control group [MD = −3.93, 95% CI (−5.78, −2.09), P < 0.00001], with high heterogeneity (I2 = 97%, P < 0.00001). As shown in Figure 7.
2 trials compared the duration of antibiotic use between the intervention group and the control group. The results of the meta-analysis showed that the intervention group had a significantly shorter duration of antibiotic use than the control group [MD = −4.51, 95% CI (−5.46, −3.57), P < 0.00001]. The studies demonstrated low heterogeneity and no statistically significant difference (I2 = 22%, P = 0.26). As shown in Figure 8.
As shown in Figure 9A, the funnel plot shows that the research results are biased toward one side of the funnel plot, which may suggest publication bias. We used Egger’s tests to evaluate publication bias in the included studies. As shown in Figure 9B, Egger’s tests indicated that there was no evidence of publication bias regarding the effective rate (P = 0.489).
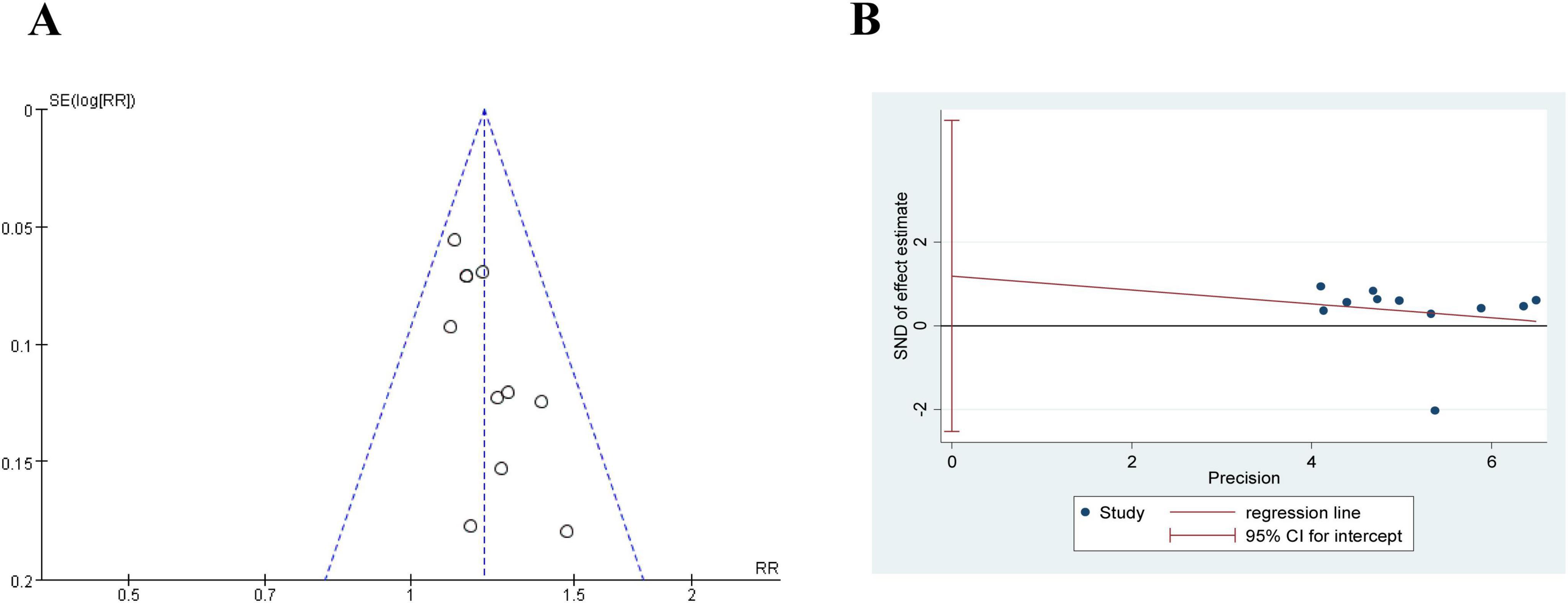
Figure 9. The publication bias of effective rate in comparing acupuncture plus CT vs. CT. (A) Funnel plots. (B) Egger’s tests.
Although no adverse effects associated with acupuncture treatment were reported in the selected literature, potential adverse effects cannot be excluded entirely.
This meta-analysis included 16 trials involving 1,125 patients, comparing the effects of acupuncture therapy to CT. Our findings indicate that patients who underwent acupuncture treatment had a higher efficacy rate, lower levels of inflammation (WBC, CRP, Neu%, and PCT), and a reduced duration of antibiotic use. Additionally, acupuncture improved pulmonary conditions such as FVC and FEV1, shortened the duration of coughing, quicker the disappearance of rales, and resulted in a lower CPIS and NIHSS than those who did not receive acupuncture treatment. Additionally, although no adverse events related to acupuncture were reported, the possibility of adverse reactions cannot be excluded entirely due to the lack of definitive reporting in the included trials. Finally, due to the low-quality evidence, more rigorous studies are needed in the coming years to confirm these findings.
Our study findings indicate that the majority of patients with SAP included in the study were elderly individuals aged 60 years and older, a demographic characterized by advanced age, which is a major risk factor for SAP (40). Advanced-age stroke patients often present with multiple comorbidities and require prolonged bed rest, posing various challenges in clinical management. The misuse of antibiotics has led to the emergence of antimicrobial resistance, while post-stroke immunosuppression further complicates the clinical treatment of SAP (41, 42). Moreover, there is a lack of consensus regarding the definition and diagnostic criteria for SAP, resulting in inadequacies and delays in its prevention, diagnosis, and treatment in clinical practice (8). This may contribute to decreased treatment efficacy and poor prognosis for patients. In contrast to the limitations of antibiotic therapy, acupuncture offers the advantage of minimal side effects (43). In our study, the effective rate in the treatment group was significantly higher than that in the control group, with a statistically meaningful difference (RR = 1.20, 95% CI [1.13, 1.27], P < 0.00001). Furthermore, there was no significant heterogeneity between the two groups (P = 0.79, I2 = 0%), indicating that acupuncture interventions positively impact the clinical treatment efficacy of SAP.
Recent studies have indicated a close relationship between SAP and systemic immune dysregulation induced by post-stroke brain injury. Stroke can trigger the release of various immune mediators, including IL-1β, TNF-α, calcitonin gene-related peptides, neuropeptides, and vasoactive intestinal peptides. These immune mediators activate specific signaling pathways that lead to systemic immune suppression, exacerbating neurological deficits post-stroke, diminishing host immune function, and increasing susceptibility to infections as well as issues related to antibiotic resistance (44, 45). CRP and PCT have been identified as independent risk factors influencing pulmonary infections in stroke patients, and elevated levels of CRP are considered a significant risk factor for mortality in elderly patients with SAP (46, 47). During infection or trauma, CRP levels can rise rapidly, demonstrating high sensitivity in the early stages of infection (48). Furthermore, increased serum PCT levels correlate positively with the severity of neuroinflammation and injury, as well as with adverse outcomes in patients with intracerebral hemorrhage (49). WBC and Neu% are commonly used clinical inflammatory markers that participate in the progression of inflammatory lesions. In patients with pneumonia, these markers are typically elevated, aiding in the assessment of the condition and infection severity in SAP patients (50). Results from included studies demonstrate that, compared to conventional Western Medical treatment alone, acupuncture combined with conventional therapy significantly reduces the levels of CRP, WBC, Neu%, and PCT, suggesting that acupuncture may improve patient conditions by lowering inflammatory factor levels. This phenomenon may be attributed to acupuncture’s ability to regulate systemic Qi and blood circulation by stimulating specific acupoints, harmonizing organ function, thereby alleviating inflammatory responses and reducing pneumonia symptoms (51, 52). Acupuncture stimulation can affect nerve endings, activate the nervous system, and modulate immune function through neuro-endocrine-immune network mechanisms, inhibiting the release of inflammatory factors. Additionally, acupuncture can stimulate the body to release endogenous anti-inflammatory substances, such as β-endorphins, thereby suppressing the inflammatory process and alleviating pulmonary inflammation (53).
Neurological damage resulting from stroke can significantly impair respiratory muscle function, consequently affecting lung capacity and airway clearance (54). FVC and FEV1 are critical indicators for assessing pulmonary function. Stroke patients often exhibit marked declines in these parameters, leading to inadequate pulmonary ventilation and an increased risk of infection (55). Furthermore, the cough reflex in stroke patients may be suppressed, resulting in a shortened cough duration that hampers the ability to clear airway secretions, thereby exacerbating the risk of pulmonary infections. CPIS integrates clinical, microbiological, and radiological indicators, effectively reflecting the severity of pulmonary infections; higher scores indicate more severe infections (56). The duration of the disappearance of rales is also a crucial indicator for assessing the progression of pulmonary infections, with prolonged duration potentially signaling worsening conditions. Therefore, timely assessment of pulmonary function and infection risk in stroke patients, along with proactive preventive and therapeutic measures, is essential for reducing the incidence of SAP and improving patient outcomes. Our study demonstrates that the acupuncture combined treatment group shows superior efficacy in alleviating pulmonary symptoms compared to the control group, suggesting that acupuncture may be beneficial in managing SAP by improving symptoms and signs of pulmonary infection.
The NIHSS score greater than 15 is recognized as an independent risk factor for the development of SAP (57). Higher NIHSS scores indicate more severe neurological impairment, which correlates with an increased risk of pulmonary infections (58). Previous studies have demonstrated that acupuncture can enhance immune function and promote neurological recovery (59, 60). Our meta-analysis reveals that the acupuncture treatment group significantly reduces NIHSS scores, indicating improved neurological outcomes. Furthermore, research indicates prolonged antibiotic therapy may disrupt the gut microbiota, thereby increasing the risk of infections (61, 62). Our findings suggest that acupuncture as an adjunct therapy can significantly reduce the duration of antibiotic use by an average of 4.51 days, which may help mitigate antibiotic-related side effects and the risk of antibiotic resistance.
After a stroke, inflammatory responses aid tissue healing and removing necrotic cells. However, when inflammation becomes excessive, it can cause additional harm (4). Research indicates that the BL13 acupoint modulates pneumonia by targeting multiple genes, such as FCER2, IL4R, FASLG, and others, to modulate cytokine signaling in the immune system (63). Additionally, it has been shown that electroacupuncture at BL13 down-regulates the lung index and serum TNF-α levels and up-regulates serum IL-10 levels in mice with viral pneumonia (64). Additionally, electroacupuncture at GV20 and ST36 demonstrates anti-inflammatory effects by inhibiting the release of cytokines, such as localized TNFα, and suppressing the expression of heat shock protein 70 (HSP70) (65). Moreover, electroacupuncture at GB20 activates the cerebral cortex regions associated with swallowing, enhancing functional connectivity and brain remodeling and improving swallowing function (66). Besides, acupuncture at GV20, ST36, and LI4 has been shown to benefit the rehabilitation of the central nervous system in patients recovering from ischemic stroke (18). Finally, electroacupuncture at CV23 improves swallowing function in mouse models of post-stroke dysphagia by facilitating motor cortex inputs to the nucleus tractus solitaries through the parabrachial nuclei (67).
In summary, acupuncture on specific acupoints, such as BL13, GV20, ST36, LI11, GB20, and CV23, exerts anti-inflammatory effects through multiple mechanisms, which supports the effectiveness of acupuncture for SAP. These findings provide a solid theoretical basis for further clinical applications and research.
As secondary to stroke, the effective management of the primary condition (stroke) is crucial in the treatment of SAP. Standard medical management should be regarded as the primary therapeutic option in both experimental and control groups, with detailed implementation protocols comprehensively documented within the study.
The specifics of acupuncture treatment protocols vary across studies; however, many of the included trials inadequately reported essential information, failing to meet all Standards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA) criteria (68). This lack of detail limits the reader’s ability to assess the quality of acupuncture interventions. Syndrome differentiation, a common practice in clinical acupuncture, poses challenges in replication and evaluation due to its individualized diagnostic approach. Therefore, standardized acupuncture treatment protocols should remain the preferred choice in clinical trials. Nevertheless, numerous studies have demonstrated the efficacy of syndrome-based treatments, highlighting the importance of balancing standardized interventions with flexible, personalized approaches.
In summary, future research should focus on increasing sample sizes and employing more rigorous and effective experimental designs to evaluate the efficacy and safety of acupuncture for SAP. Sample size calculations should be based on standardized formulas and previous research findings. To enhance methodological quality, future trials should: (1) utilize more standardized approaches for syndrome differentiation and acupoint selection; (2) classify recruited patients more meticulously according to the severity and duration of SAP; (3) adhere to STRICTA guidelines and Consolidated Standards of Reporting Trials (CONSORT) statements (69); (4) implement appropriate blinding methods; and (5) prioritize follow-up assessments as a critical component of future studies.
This systematic review and meta-analysis is subject to several limitations. Firstly, the included studies exhibited considerable variability in quality. Secondly, there was a lack of standardized experimental designs and interventions across the studies, with notable discrepancies in acupoint selection, needle retention time, treatment frequency, and session duration. Thirdly, the incomplete reporting of essential aspects of the experimental design, such as the specific acupuncture techniques employed and the researchers’ backgrounds, significantly hampers our ability to assess the overall quality of the literature. Lastly, potential publication bias necessitates caution in the interpretation of the results of this study.
The research found that acupuncture has a positive effect in treating SAP. However, owing to the low-quality evidence, additional studies are warranted to corroborate these findings in the coming years.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in this article/Supplementary material.
KS: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Writing – original draft, Writing – review and editing. XW: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Writing – original draft, Writing – review and editing. SZ: Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft. JiaW: Data curation, Investigation, Methodology, Writing – original draft. YC: Data curation, Methodology, Writing – original draft. LY: Data curation, Investigation, Methodology, Writing – original draft. HL: Conceptualization, Formal Analysis, Project administration, Supervision, Visualization, Writing – original draft, Writing – review and editing. JinW: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review and editing.
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This study was financially supported by Tianjin Tuina Research Institute, Regional Chinese Medicine (Tuina Specialist) Diagnosis and Treatment Center (963042), State Administration of Traditional Chinese Medicine High-level Key Subject of Chinese Medicine – Tuina (2024ZDK002), and National Science Foundation of China (No. 82274674).
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1440121/full#supplementary-material
1. GBD (2021) Causes of Death Collaborators. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990-2021: A systematic analysis for the Global burden of disease study 2021. Lancet. (2024) 403:2100–32. doi: 10.1016/s0140-6736(24)00367-2
2. GBD (2016) Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. (2019) 18:459–80. doi: 10.1016/s1474-4422(18)30499-x
3. Elkind M, Boehme A, Smith C, Meisel A, Buckwalter M. Infection as a stroke risk factor and determinant of outcome after stroke. Stroke. (2020) 51:3156–68. doi: 10.1161/strokeaha.120.030429
4. Liu D, Chu S, Chen C, Yang P, Chen N, He X. Research progress in stroke-induced immunodepression syndrome (SIDS) and stroke-associated pneumonia (SAP). Neurochem Int. (2018) 114:42–54. doi: 10.1016/j.neuint.2018.01.002
5. Westendorp W, Nederkoorn P, Vermeij J, Dijkgraaf M, van de Beek D. Post-stroke infection: A systematic review and meta-analysis. BMC Neurol. (2011) 11:110. doi: 10.1186/1471-2377-11-110
6. Banda K, Chu H, Kang X, Liu D, Pien L, Jen H, et al. Prevalence of dysphagia and risk of pneumonia and mortality in acute stroke patients: A meta-analysis. BMC Geriatr. (2022) 22:420. doi: 10.1186/s12877-022-02960-5
7. Smith C, Kishore A, Vail A, Chamorro A, Garau J, Hopkins S, et al. Diagnosis of stroke-associated pneumonia: Recommendations from the pneumonia in stroke consensus group. Stroke. (2015) 46:2335–40. doi: 10.1161/strokeaha.115.009617
8. Kishore A, Vail A, Chamorro A, Garau J, Hopkins S, Di Napoli M, et al. How is pneumonia diagnosed in clinical stroke research? A systematic review and meta-analysis. Stroke. (2015) 46:1202–9. doi: 10.1161/strokeaha.114.007843
9. Ji R, Wang D, Shen H, Pan Y, Liu G, Wang P, et al. Interrelationship among common medical complications after acute stroke: Pneumonia plays an important role. Stroke. (2013) 44:3436–44. doi: 10.1161/strokeaha.113.001931
10. Finlayson O, Kapral M, Hall R, Asllani E, Selchen D, Saposnik G. Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurology. (2011) 77:1338–45. doi: 10.1212/WNL.0b013e31823152b1
11. Teh W, Smith C, Barlas R, Wood A, Bettencourt-Silva J, Clark A, et al. Impact of stroke-associated pneumonia on mortality, length of hospitalization, and functional outcome. Acta Neurol Scand. (2018) 138:293–300. doi: 10.1111/ane.12956
12. De Pascale G, Bello G, Tumbarello M, Antonelli M. Severe pneumonia in intensive care: Cause, diagnosis, treatment and management: A review of the literature. Curr Opin Pulm Med. (2012) 18:213–21. doi: 10.1097/MCP.0b013e328351f9bd
13. Westendorp W, Dames C, Nederkoorn P, Meisel A. Immunodepression, infections, and functional outcome in ischemic stroke. Stroke. (2022) 53:1438–48. doi: 10.1161/strokeaha.122.038867
14. Kalra L, Irshad S, Hodsoll J, Simpson M, Gulliford M, Smithard D, et al. Prophylactic antibiotics after acute stroke for reducing pneumonia in patients with dysphagia (STROKE-INF): A prospective, cluster-randomised, open-label, masked endpoint, controlled clinical trial. Lancet. (2015) 386:1835–44. doi: 10.1016/s0140-6736(15)00126-9
15. Caldeira D, Alarcão J, Vaz-Carneiro A, Costa J. Risk of pneumonia associated with use of angiotensin converting enzyme inhibitors and angiotensin receptor blockers: Systematic review and meta-analysis. BMJ. (2012) 345:e4260. doi: 10.1136/bmj.e4260
16. Warusevitane A, Karunatilake D, Sim J, Lally F, Roffe C. Safety and effect of metoclopramide to prevent pneumonia in patients with stroke fed via nasogastric tubes trial. Stroke. (2015) 46:454–60. doi: 10.1161/strokeaha.114.006639
17. Gandolfi M, Smania N, Bisoffi G, Squaquara T, Zuccher P, Mazzucco S. Improving post-stroke dysphagia outcomes through a standardized and multidisciplinary protocol: An exploratory cohort study. Dysphagia. (2014) 29:704–12. doi: 10.1007/s00455-014-9565-2
18. Chavez L, Huang S, MacDonald I, Lin J, Lee Y, Chen Y. Mechanisms of acupuncture therapy in ischemic stroke rehabilitation: A literature review of basic studies. Int J Mol Sci. (2017) 18:2270. doi: 10.3390/ijms18112270
19. Lee S, Kim S. The effect of acupuncture on modulating inflammatory cytokines in rodent animal models of respiratory disease: A systematic review and meta-analysis. Front Immunol. (2022) 13:878463. doi: 10.3389/fimmu.2022.878463
20. Zhang C, Zhang G, Yang C. Effects of acupuncture and moxibustion and lung rehabilitation training on pulmonary function and peripheral blood inflammatory factors in patients with stroke-associated pneumonia. Hainan Yi Xue. (2023) 34:1836–40. doi: 10.3969/i.issn.1003-6350.2023.13.004
21. Lin X, Shi M, Tang L. Acupuncture combined with pulmonary rehabilitation training in the treatment of stroke-related pneumonia. Zhongguo Zhong Yi Yao Xian Dai Yuan Cheng Jiao Yu. (2023) 21:114–7. doi: 10.3969/j.issn.1672-2779.2023.22.036
22. Guo Y, He C, Feng X. Clinical study on three-point and five-needling method combined with pulmonary rehabilitation training for cerebral apoplexy complicated with pulmonary infection. Xin Zhong Yi. (2023) 55:174–9. doi: 10.13457/j.cnki.jncm.2023.23.033
23. Guo L, Zhou Y, Liu J, Mou X. Clinical observation on the treatment of aspiration pneumonia in the acute stage of cerebral infarction with acupuncture tongue three needles. Zhongguo Min Jian Liao Fa. (2023) 31:55–8. doi: 10.19621/j.cnki.11-3555/r.2023.0918
24. Ma Y, Li J, Zhu H, Dong H. Role of acupuncture combined with comprehensive rehabilitation therapy in the treatment of pulmonary infection in older patients with stroke. Guo Ji Lao Nian Yi Xue Za Zhi. (2022) 43:571–5. doi: 10.3969/j.issn.1674-7593.2022.05.013
25. Yuan W, Shao S, Li Z. Observation on Shao’s five-needle therapy as adjuvant treatment for stroke-associated pneumonia. Zhongguo Zhen Jiu. (2021) 41:3–7. doi: 10.13703/j.0255-2930.20191231-k0002
26. Tang Z, Cui Y. Clinical observation on acupuncture with reinforcement before purgation in treating stroke-associated pneumonia of Phlegm-Damp and Qi deficiency type. Guangzhou Zhong Yi Yao Da Xue Xue Bao. (2021) 38:734–8. doi: 10.13359/j.cnki.gzxbtcm.2021.04.015
27. Zhang Y, Zhen Y, Li S, Xia L, Xu Z. Effect of acupuncture combined with extracorporeal diaphragm pacing on the immune function and inflammatory factors in stroke-associated pneumonia. Wu Jing Hou Qin Xue Yuan Xue Bao (Yi Xue Ban). (2020) 29:36–9. doi: 10.16548/j.2095-3720.2020.04.009
28. Fan X. Effect of acupuncture combined with respiratory training on pulmonary symptoms and serum inflammatory factors in patients with stroke complicated with pulmonary infection. Zhongguo Min Jian Liao Fa. (2020) 28:24–6. doi: 10.19621/j.cnki.11-3555/r.2020.1613
29. Xia J, Zhu J, Ye Z. Acupuncture Therapy combined with comprehensive pulmonary rehabilitation management to reduce lung infection rate in post-stroke swallowing disorders. Shanxi Yi Yao Za Zhi. (2019) 48:2766–9. doi: 10.3969/j.issn.0253-9926.2019.22.013
30. Wu Z, Zou G, Huang F. Effect of acupuncture at Back-Shu points combined with respiratory training on pulmonary infection in post-stroke patients after tracheotomy. Zhongguo Kang Fu. (2019) 34:175–8. doi: 10.3870/zgkf.2019.04.002
31. Luo K, Yang F, Bian X, Lou Z, Ge J. Clinical study of acupuncture treatment for pulmonary infection after acute cerebral infarction. Shanghai Zhen Jiu Za Zhi. (2016) 35:1070–2. doi: 10.13460/j.issn.1005-0957.2016.09.1070
32. Liu Y, Nie Y, Zheng Y, Li X, Li X. The efficacy of Liuchuan acupoint decongestive stabbing method in the treatment of post-stroke dysphagia with concomitant lung infection. Zhong Yi Yao Xue Bao. (2016) 44:108–10. doi: 10.19664/j.cnki.1002-2392.2016.06.033
33. Gu Z, Huang F, Chen T, Xiao T, Yuan Z, Liang P. Therapeutic observation of thread embedding at Back-Shu points for stroke-associated pneumonia. Shanghai Zhen Jiu Za Zhi. (2016) 35:513–6. doi: 10.13460/j.issn.1005-0957.2016.05.0513
34. Liang L. The application effect analysis of acupuncture and rehabilitation therapy in stroke associated pneumonia. Zhongguo Dang Dai Yi Yao. (2015) 22:129–31.
35. Gao Y, Su M. Effect of acupuncture treatment of post-stroke dysphagia on stroke-associated pneumonia in 30 cases of stroke. Zhongguo Min Zu Min Jian Yi Yao. (2014) 23:59–60.
36. Page M, McKenzie J, Bossuyt P, Boutron I, Hoffmann T, Mulrow C, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
37. Wang Y, Chen Y, Lv C, Zhao X, Guo W, Bi Q, et al. Stroke-associated pneumonia: Diagnosis and treatment chinese expert consensus (2019 updated version). Chin J Stroke. (2019) 14:1251–62.
38. Higgins J, Altman D, Gøtzsche P, Jüni P, Moher D, Oxman A, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj. (2011) 343:d5928. doi: 10.1136/bmj.d5928
39. Guyatt G, Oxman A, Kunz R, Falck-Ytter Y, Vist G, Liberati A, et al. Going from evidence to recommendations. Bmj. (2008) 336:1049–51. doi: 10.1136/bmj.39493.646875.AE
40. Li D, Yuan L, Wang T, Rong Y, Li C, You M, et al. Risk factors for stroke-related pneumonia in patients with ischaemic stroke: A systematic evaluation and meta-analysis. Clin Neurol Neurosurg. (2024) 246:108593. doi: 10.1016/j.clineuro.2024.108593
41. Hu J, Zheng X, Shi G, Guo L. Associations of multiple chronic disease and depressive symptoms with incident stroke among Chinese middle-aged and elderly adults: A nationwide population-based cohort study. BMC Geriatr. (2022) 22:660. doi: 10.1186/s12877-022-03329-4
42. Meisel C, Meisel A. Suppressing immunosuppression after stroke. N Engl J Med. (2011) 365:2134–6. doi: 10.1056/NEJMcibr1112454
43. Ernst G, Strzyz H, Hagmeister H. Incidence of adverse effects during acupuncture therapy-a multicentre survey. Complement Ther Med. (2003) 11:93–7. doi: 10.1016/s0965-2299(03)00004-9
44. Planas A, Gorina R, Chamorro A. Signalling pathways mediating inflammatory responses in brain ischaemia. Biochem Soc Trans. (2006) 34:1267–70. doi: 10.1042/bst0341267
45. Faura J, Bustamante A, Miró-Mur F, Montaner J. Stroke-induced immunosuppression: Implications for the prevention and prediction of post-stroke infections. J Neuroinflammation. (2021) 18:127. doi: 10.1186/s12974-021-02177-0
46. Liu Q, Sun G, Huang L. Association of the NLR, BNP, PCT, CRP, and D-D with the severity of community-acquired pneumonia in older adults. Clin Lab. (2023) 69: doi: 10.7754/Clin.Lab.2023.220330
47. Arinzon Z, Peisakh A, Schrire S, Berner YC-. reactive protein (CRP): An important diagnostic and prognostic tool in nursing-home-associated pneumonia. Arch Gerontol Geriatr. (2011) 53:364–9. doi: 10.1016/j.archger.2011.01.006
48. Sproston N, Ashworth J. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. (2018) 9:754. doi: 10.3389/fimmu.2018.00754
49. Shi G, Li M, Zhou R, Wang X, Xu W, Yang F, et al. Procalcitonin related to stroke-associated pneumonia and clinical outcomes of acute ischemic stroke after IV rt-PA treatment. Cell Mol Neurobiol. (2022) 42:1419–27. doi: 10.1007/s10571-020-01031-w
50. Vaughn V, Dickson R, Horowitz J, Flanders S. Community-acquired pneumonia: A review. JAMA. (2024) 332:1282–95. doi: 10.1001/jama.2024.14796
51. Katsuya E, de Castro M, Carneiro C, Yamamura Y, Silveira V. Acupuncture reduces immune-mediated pulmonary inflammatory lesions induced in rats. Forsch Komplementmed. (2009) 16:413–6. doi: 10.1159/000262326
52. Kim S, Bae H. Acupuncture and immune modulation. Auton Neurosci. (2010) 157:38–41. doi: 10.1016/j.autneu.2010.03.010
53. Ding S, Hong S, Wang C, Guo Y, Wang Z, Xu Y. Acupuncture modulates the neuro-endocrine-immune network. Qjm. (2014) 107:341–5. doi: 10.1093/qjmed/hct196
54. Zhang Y, Zhang K, Huang L, Wei J, Bi Z, Xiao J, et al. The effects of respiratory muscle training on respiratory function and functional capacity in patients with early stroke: A meta-analysis. Eur Rev Aging Phys Act. (2024) 21:4. doi: 10.1186/s11556-024-00338-7
55. Wu M, Mo M, Huang X, Wei J. Implications for respiratory muscle training in patients with stroke-associated pneumonia: A meta-analysis. Disabil Rehabil. (2024) 46:5791–7. doi: 10.1080/09638288.2024.2314159
56. Gunalan A, Sistla S, Sastry A, Venkateswaran R. Concordance between the national healthcare safety network (NHSN) surveillance criteria and clinical pulmonary infection score (CPIS) criteria for diagnosis of ventilator-associated pneumonia (VAP). Indian J Crit Care Med. (2021) 25:296–8. doi: 10.5005/jp-journals-10071-23753
57. Dang P, Nguyen M, Mai X, Pham D, Dang M, Nguyen D, et al. A comparison of the national institutes of health stroke scale and the gugging swallowing screen in predicting stroke-associated pneumonia. Ther Clin Risk Manag. (2020) 16:445–50. doi: 10.2147/tcrm.S251658
58. Phan T, Kooblal T, Matley C, Singhal S, Clissold B, Ly J, et al. Stroke severity versus dysphagia screen as driver for post-stroke pneumonia. Front Neurol. (2019) 10:16. doi: 10.3389/fneur.2019.00016
59. Liu F, Wang Y, Lyu K, Du X, Zhou M, Shi J, et al. Acupuncture and its ability to restore and maintain immune homeostasis. Qjm. (2024) 117:167–76. doi: 10.1093/qjmed/hcad134
60. Qin S, Zhang Z, Zhao Y, Liu J, Qiu J, Gong Y, et al. The impact of acupuncture on neuroplasticity after ischemic stroke: A literature review and perspectives. Front Cell Neurosci. (2022) 16:817732. doi: 10.3389/fncel.2022.817732
61. Ramirez J, Guarner F, Bustos Fernandez L, Maruy A, Sdepanian V, Cohen H. Antibiotics as major disruptors of gut microbiota. Front Cell Infect Microbiol. (2020) 10:572912. doi: 10.3389/fcimb.2020.572912
62. Budden K, Gellatly S, Wood D, Cooper M, Morrison M, Hugenholtz P, et al. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol. (2017) 15:55–63. doi: 10.1038/nrmicro.2016.142
63. Xu Y, Cai J, Li W, Miao J, Mei Y, Wang X, et al. Examining the effector mechanisms of the feishu Acupoint (BL13) in the treatment of pneumonia based on systematic acupuncture and moxibustion research. Evid Based Complement Alternat Med. (2021) 2021:5578104. doi: 10.1155/2021/5578104
64. Luo W, Wang J, Liu C, Huang C. Effect of electroacupuncture stimulation of “Feishu” (BL 13) on lung index, serum and lung IL-10 and TNF-alpha levels in mice with viral pneumonia. Zhen Ci Yan Jiu. (2014) 39:293–7.
65. Xu H, Sun H, Chen S, Zhang Y, Piao Y, Gao Y. Effects of acupuncture at Baihui (DU20) and Zusanli (ST36) on the expression of heat shock protein 70 and tumor necrosis factor α in the peripheral serum of cerebral ischemia-reperfusion-injured rats. Chin J Integr Med. (2014) 20:369–74. doi: 10.1007/s11655-014-1800-z
66. Fu X, Li H, Yang W, Li X, Lu L, Guo H, et al. Electroacupuncture at HT5 + GB20 promotes brain remodeling and significantly improves swallowing function in patients with stroke. Front Neurosci. (2023) 17:1274419. doi: 10.3389/fnins.2023.1274419
67. Yao L, Ye Q, Liu Y, Yao S, Yuan S, Xu Q, et al. Electroacupuncture improves swallowing function in a post-stroke dysphagia mouse model by activating the motor cortex inputs to the nucleus tractus solitarii through the parabrachial nuclei. Nat Commun. (2023) 14:810. doi: 10.1038/s41467-023-36448-6
68. MacPherson H, Altman D, Hammerschlag R, Youping L, Taixiang W, White A, et al. Revised standards for reporting interventions in clinical trials of acupuncture (STRICTA): Extending the CONSORT statement. J Altern Complement Med. (2010) 16:St1–14. doi: 10.1089/acm.2010.1610
Keywords: acupuncture, stroke-associated pneumonia, meta-analysis, systematic review, SAP
Citation: Su K, Wang X, Zhang S, Wu J, Chen Y, Yin L, Li H and Wang J (2025) Efficacy of acupuncture for stroke-associated pneumonia: a systematic review and meta-analysis. Front. Med. 12:1440121. doi: 10.3389/fmed.2025.1440121
Received: 29 May 2024; Accepted: 28 January 2025;
Published: 03 March 2025.
Edited by:
Narayanaswamy Venketasubramanian, Raffles Hospital, SingaporeReviewed by:
Nannan Yu, Air Force Medical University, ChinaCopyright © 2025 Su, Wang, Zhang, Wu, Chen, Yin, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingui Wang, d2pnNjV0akAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.