- 1Department of Obstetrics and Gynecology, Dokuz Eylul University School of Medicine, İzmir, Türkiye
- 2Department of Medical Biology, Dokuz Eylul University, İzmir, Türkiye
- 3Department of Pathology, Dokuz Eylul University School of Medicine, İzmir, Türkiye
Background: Endometriosis, a prevalent chronic gynecologic disorder, significantly impacts women’s health, with both genetic and environmental factors contributing to its heritability. Within the adaptive immune system, the NOD-like receptors (NLR) pathway plays pivotal roles in various autoinflammatory diseases, regulating interleukins, proinflammatory cytokines, and NF-κB activity. However, the potential association between single nucleotide polymorphisms (SNPs) of the NOD1, NOD2, PYDC1, and PYDC2 genes and the predisposition to endometriosis risk remains unexplored.
Methods: In this cross-sectional study, 54 patients diagnosed with ovarian endometriosis and 54 control subjects were included. The genetic SNPs of NOD1 (rs2075820 and rs2075818) and NOD2 (rs104895461) were assessed using the PCR-RFLP (polymerase chain reaction-restriction fragment length polymorphism) method. Additionally, the polymorphisms of PYDC1 and PYDC2 were evaluated using Sanger sequencing. After conducting polymorphism analysis, the genetic profiles were assessed with the clinical manifestations and the size of ovarian endometriomas, categorized as either small (<4 cm) or large (≥4 cm).
Results: Significant differences in the NOD1 rs2075820 (G: A) genotypes were found. The GG genotype was more prevalent in endometriosis patients (p = 0.04), while the GA genotype was less common (p = 0.029). The AA genotype was associated with higher rates of perimenstrual gastrointestinal symptoms (p = 0.005) and infertility (p = 0.037). The PYDC2 rs293833 variant was detected in 22.2% of patients. Carriers of this variant exhibited higher rates of perimenstrual gastrointestinal symptoms (p = 0.004), infertility (p = 0.001) and larger endometriomas (≥4 cm) (p < 0.001). No significant differences were found in NOD1 rs2075818 genotypes (p = 0.89) and no polymorphisms were detected in NOD2 or PYDC1 genes.
Conclusion: These findings emphasize the influence of genetic polymorphisms on the clinical manifestations of endometriosis. Specifically, gene polymorphisms in NLRs have been found to significantly impact infertility and increase endometrioma size.
Introduction
Endometriosis is characterized by an estrogen-dependent chronic inflammatory pathology that affects reproductive-aged women with pelvic pain and infertility (1). Understanding the mechanisms underlying endometriosis is crucial due to its clinical and therapeutic relevance. While numerous theories have been proposed, none fully explain the disease’s progression and diverse clinical manifestations. Sampson’s retrograde menstruation theory remains the most widely cited explanation (2). However, this theory does not adequately explain why only 10% of women with retrograde menstrual flow develop endometriosis.
A common element in all theories is the dysregulation of hormonal signaling and an inflammatory microenvironment, which, together with genetic and epigenetic factors, drive the disease’s initiation, persistence, and progression (3). Genetic predisposition is significant, as daughters of affected mothers have double the risk of developing endometriosis, and monozygotic twins show a 51% increased risk (4, 5). Ovarian endometriomas are a significant and prominent component of endometriosis. About 17–44% of patients with endometriosis have endometriomas, with bilateral endometriomas occurring in 19–28% of these patients (6). Endometriosis is a chronic pelvic inflammatory condition where local inflammation significantly contributes to pain and infertility. Excessive reactive oxygen species (ROS) production affects gene expression, with NF-κB involvement in the disease. Activated NF-κB in lesions and macrophages drives proinflammatory cytokine production, supporting lesion formation and persistence (7).
The innate immune system detects various danger and pathogen-associated molecular patterns through pattern-recognition receptors (PRRs), such as nod-like receptors (NLRs) (8). The NLR family comprises over 20 members, including nucleotide-binding oligomerization domain-containing proteins 1 and 2 (NOD1 and NOD2) (9). Engagement of NLRs triggers cooperative signaling between mitogen-activated protein kinase (MAPK) and nuclear factor kappa B (NF-κB) pathways, leading to the transcription of pro-inflammatory cytokines, assembly of NLR inflammasomes, and cell death (10). Moreover, pyrin-only protein/pyrin domain (POP/PYDC) domain proteins also disrupt NF-κB signaling by forming an inflammasome complex by certain NLRs and interleukins (11). Studies highlight that mutations and dysregulation in NLRs, such as NOD2 and NLRP3, significantly impact these pathways, altering immune responses and contributing to diseases like Crohn’s disease and cryopyrinopathies. Polymorphisms in the NOD1 and NOD2 genes can disrupt the balance between pro- and anti-inflammatory cytokines, fostering chronic inflammation and increasing the risk of cancer. These findings emphasize the critical role of structure–function relationships in understanding NLR-mediated immune regulation and their relevance to disease pathogenesis (12).
Polymorphisms play a crucial role in understanding the genetic underpinnings of complex diseases, including endometriosis. Given the multifactorial nature of endometriosis, the identification of genetic variants that contribute to disease susceptibility has significant implications for advancing diagnostic and therapeutic strategies. However, there remains a substantial research gap in understanding the precise contribution of genetic polymorphisms to endometriosis, with many studies producing inconsistent results across populations and ethnic groups. This variability underscores the complexity of genetic influence on endometriosis, suggesting that multiple, potentially interacting loci may contribute to its pathology (13, 14).
In this study, we aim to investigate inflammasome regulators PYDC1 and PYDC2 and genetic variations in the NOD1 and NOD2 genes in patients with ovarian endometriosis. Additionally, we will evaluate the genetic profile of these patients with the size of the endometriomas and their clinical symptoms.
Method
Subjects
All subjects provided written informed consent for inclusion before participating in the study. The study was conducted by the Declaration of Helsinki of 1975 (as revised in 2013), and the protocol was reviewed and approved by the Health Research Ethics Committee of Dokuz Eylul University (7511-GOA). Blood samples were collected from a total of 108 patients who had either undergone laparoscopic surgery or exploratory laparotomy between March 2022 and November 2023. The study population comprised 54 patients diagnosed with ovarian endometriosis (endometriosis group) and 54 control subjects without endometriosis (control group).
Endometriosis group
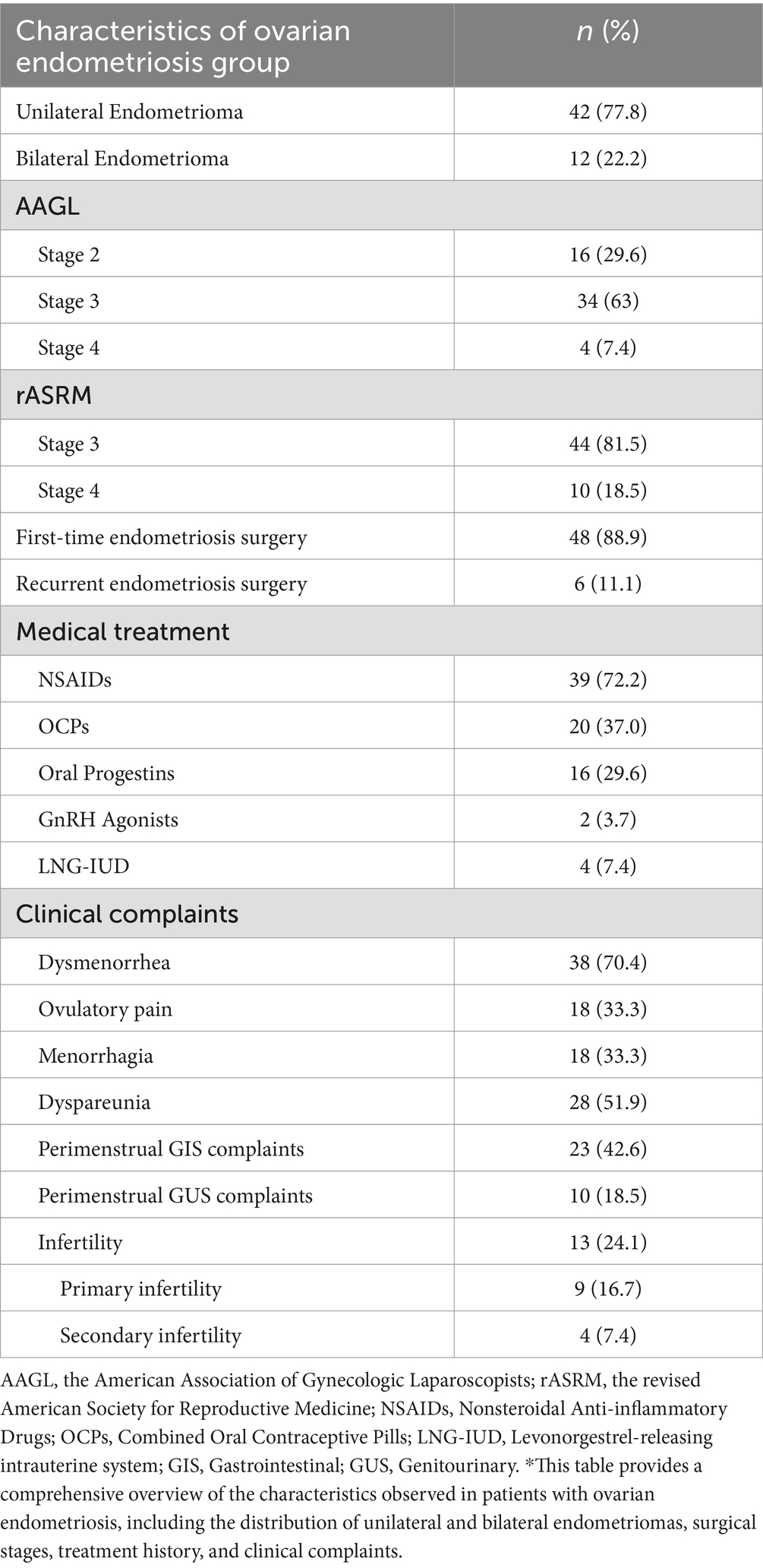
Diagnosis of endometriosis was confirmed and classified based on visual and histopathological examinations according to the American Association of Gynecologic Laparoscopists (AAGL) and the revised American Society for Reproductive Medicine (rASRM) Endometriosis Classification Systems (15, 16). According to AAGL Classification, 16 (29.6%) were in stage II, 34 (63%) in stage III, and 4 (7.4%) in stage IV. When classified with rASRM, 44 patients (81.5%) were classified as stage 3, and 10 patients (18.5%) as stage 4. To explore potential genetic differences related to endometrioma size, a subgroup analysis was performed, categorizing endometriomas as larger (≥4 cm) or smaller (<4 cm).
The control group consisted of patients who underwent surgery for fibroids, menorrhagia, benign adnexal masses, and pelvic organ prolapse. Endometriosis was ruled out in these patients through histopathological evaluation. Patients with additional autoimmune diseases, pelvic inflammatory disease, or gynecological malignancies were excluded from both the endometriosis and control groups.
Genotyping polymorphisms
DNA was collected in 5 mL peripheral blood, followed by ficol separation (Sigma Histopaque-1077, cat no: 10771). DNA isolation was then performed using Trizol (Invitrogen TM, cat no: 15596018). Conventional polymerase chain reaction (PCR) was conducted using Taq DNA Polymerase (A.B.T., cat no: E02-01-50) for the target genes, with the following protocol: 40 cycles of denaturation at 95°C for 10 s, annealing at 60°C for 30 s, and extension at 65°C for 95 min. Primer sequences for the target genes previously created before (17). Genotypes rs2075818 and rs104895461 were determined using the PCR-restriction fragment length polymorphism (PCR-RFLP) method. PCR products were incubated overnight at 37°C with specific restriction enzymes for the restriction enzyme process. Samples were loaded onto a 2% agarose gel to determine allele separation and visualized (Figure 1).
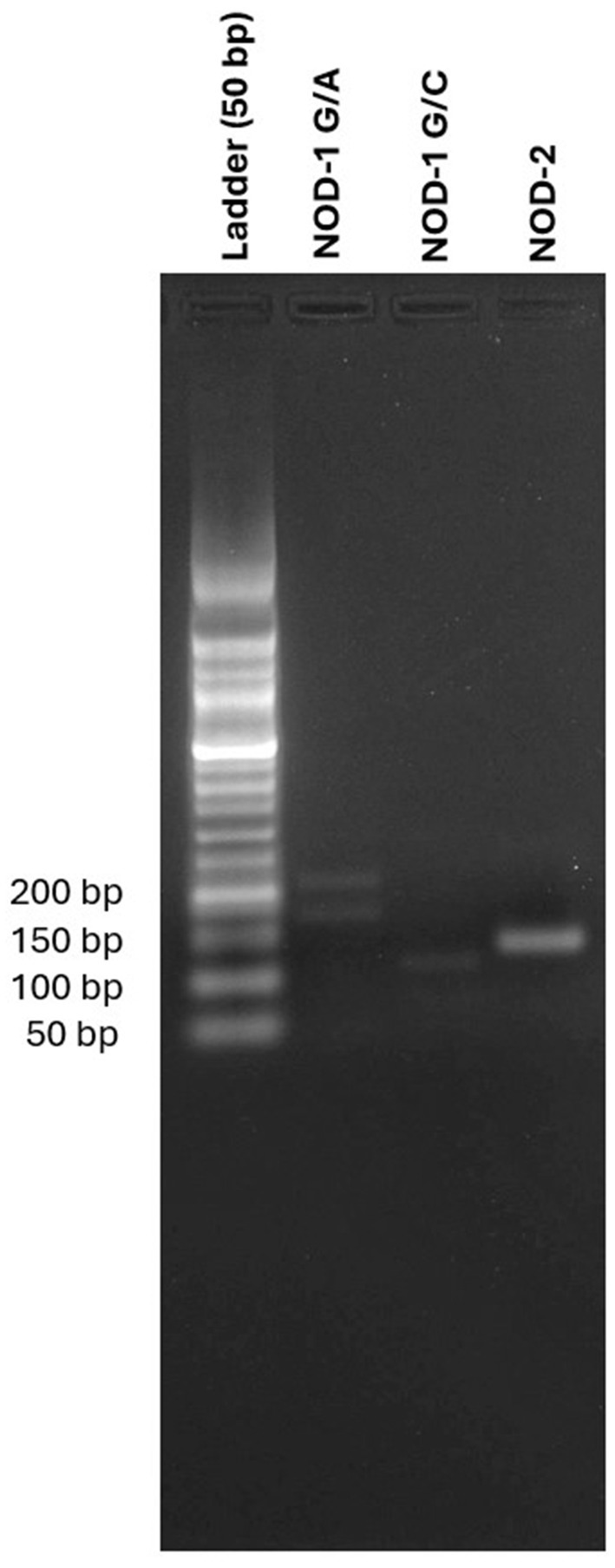
Figure 1. Comparative gel electrophoresis of allele separation with DNA ladder for NOD1 G/A, NOD 1 G/C, and NOD2 variants.
The PCR primers used for Sanger sequencing of the PYDC1 and PYDC2 genes are listed in Supplementary material 1. After PCR amplification, the products were purified, and sequencing reactions were performed (Macrogen Europe). After completing the electrophoresis process, the samples were analyzed using the “Sequence Analysis” program. Sequence comparisons and analyses were conducted using the MutationSurveyor 1.2 program.
Statistical analysis
The sample size was calculated using a power analysis, achieving 95% confidence level. Hardy–Weinberg equilibrium was assessed for each evaluated SNP. The student’s t-test was employed to compare means of continuous variables. The Chi-square test was used to compare mutations and allele frequencies among groups and clinical features within subgroups categorized by endometrioma size. Statistical analyses were conducted using IBM SPSS version 26.0, with a p-value of <0.05 accepted as statistically significant.
Results
There were no significant differences between endometriosis patients and control subjects regarding age (39.28 ± 8.22 vs. 39.31 ± 7.86), BMI (23.1 ± 1.4 vs. 22.8 ± 1.5), and age at menarche (12.4 ± 1.6 vs. 12.1 ± 1.8) (p > 0.05). Out of the patients studied, 54 had ovarian endometriosis. Of these, 42 patients (77.8%) had unilateral ovarian endometriosis, while 12 patients (22.2%) had bilateral involvement. In the endometriosis group, 48 patients (88.9%) underwent first-time surgery, and 6 patients (11.1%) had recurrent endometriomas.
The symptoms reported by patients with endometriosis included dysmenorrhea 38 (70.4%), dyspareunia 28 (51.9%), perimenstrual gastrointestinal system (GIS) complaints 23 (42.6%), ovulatory pain 18 (33.3%), menorrhagia 18 (33.3%), perimenstrual genitourinary system (GUS) complaints 10 (18.5%). Infertility was present in 13 patients (24.1%), with 9 patients (16.7%) experiencing primary infertility and 4 patients (7.4%) experiencing secondary infertility (Table 1).
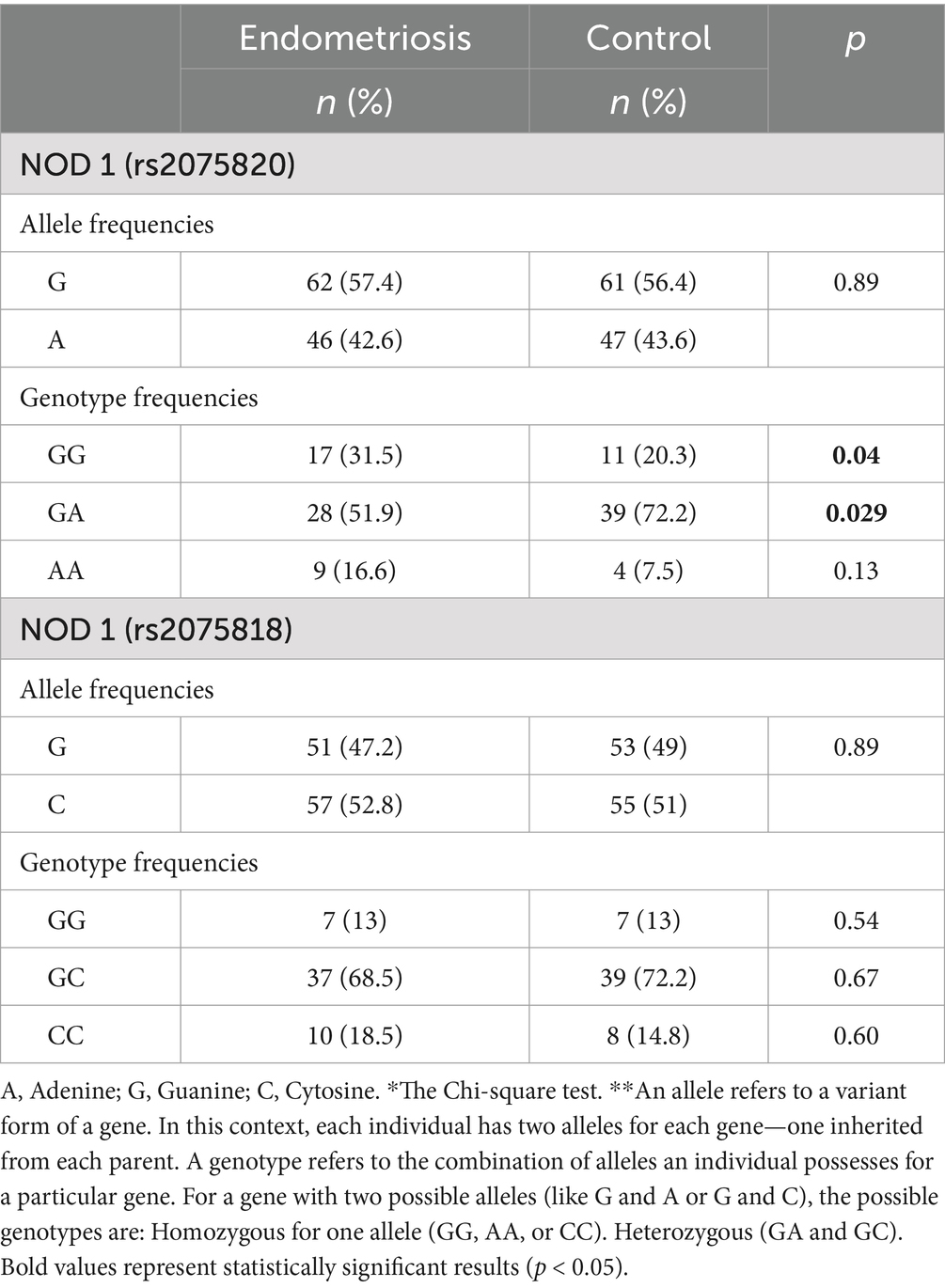
There were no significant differences in allele frequencies between endometriosis and control subjects for NOD1 rs2075820 (G vs. A) (p = 0.89) and rs2075818 (G vs. C) (p = 0.89). A statistically significant difference in the distribution of the rs2075820 (NOD1 G/A) genotypes was observed between endometriosis patients and control subjects. The GG wild-type genotype was found to be significantly more prevalent in the endometriosis group 17 (31.5%) compared to the control group 11 (20.3%) (p = 0.04). Conversely, the GA genotype was significantly less common among endometriosis patients 28 (51.9%) than in controls 39 (72.2%) (p = 0.029). Although the AA genotype was more frequent in endometriosis patients 9 (16.6%) than in control subjects 4 (7.5%), this difference did not reach statistical significance (p = 0.13) (Table 2).
No significant differences were detected when evaluating the NOD1 (rs2075818) genotypes between endometriosis patients and control subjects. The frequencies of the GG genotype were identical in both groups (13% vs. 13%; p = 0.54). Similarly, the distribution of the GC genotype (68.5% in endometriosis patients vs. 72.2% in controls; p = 0.67) and the CC genotype (18.5% in endometriosis patients vs. 14.8% in controls; p = 0.6) showed no significant differences (Table 2). No polymorphisms were detected at the NOD2 (rs104895461) and PYDC1 genes. PYDC2 rs293833 (c.242A>G) variant was detected in 12 endometriosis patients (22.2%).
We also evaluated the association of three polymorphisms in the NOD1, NOD2, and PYDC2 genes with the clinical manifestations of endometriosis. The NOD1 rs2075820 AA genotype was associated with significantly higher rates of perimenstrual GIS symptoms 8 (88.9%) compared to other NOD1 rs2075820 genotypes 17 (37.8%) (p = 0.005). Additionally, infertility was significantly more common in patients with the AA genotype 5 (55.5%) compared to those with other genotypes 8 (17.8%) (p = 0.037) (Table 3).
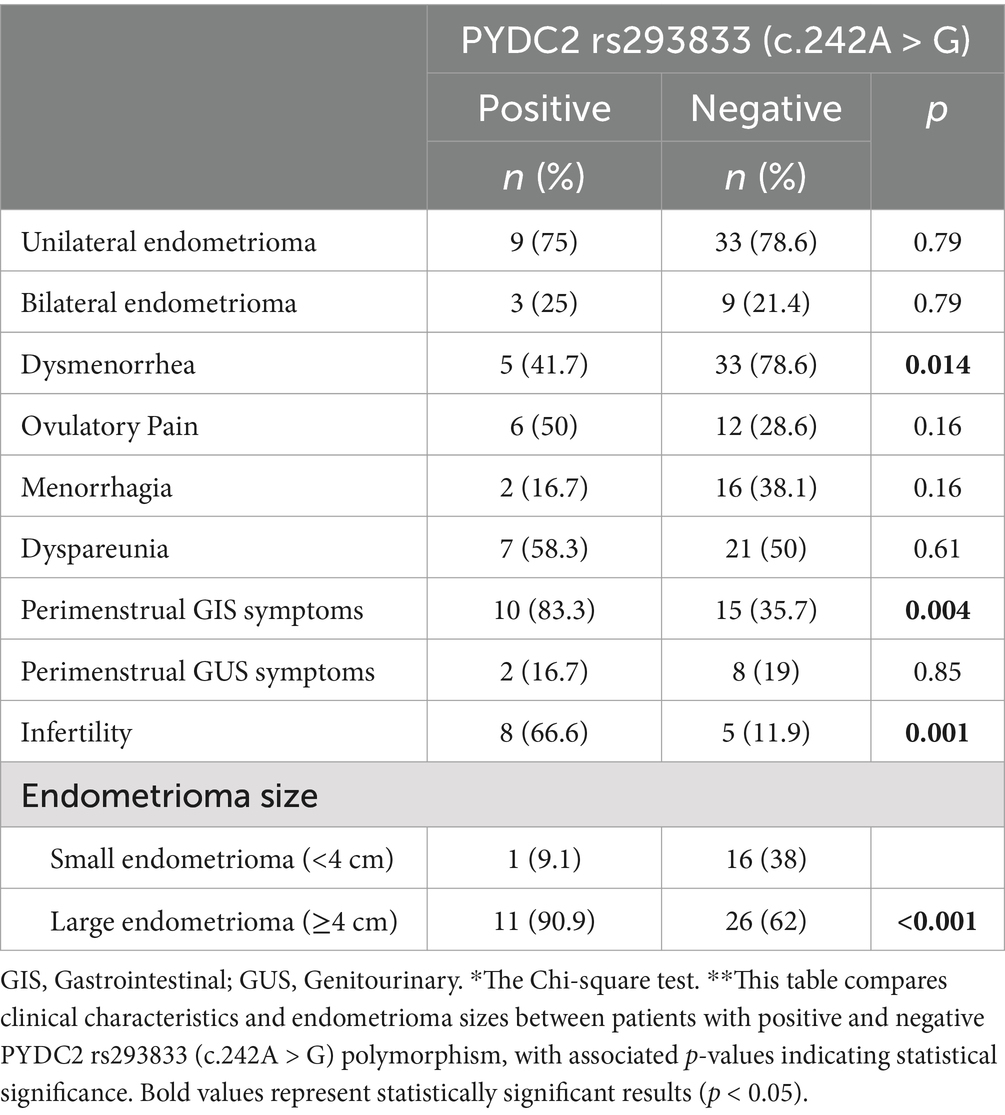
PYDC2 rs293833 (c.242A>G) positive patients exhibited a lower incidence of dysmenorrhea compared to negative patients (41.7% vs. 78.6%; p = 0.014). Moreover, perimenstrual gastrointestinal symptoms were significantly more prevalent in positive patients (83.3% vs. 35.7%; p = 0.004). Additionally, PYDC2-positive patients had significant differences in infertility and the presence of larger endometriomas. Infertility rates were markedly higher in positive patients (66.6% vs. 11.9%; p = 0.001), and large endometriomas were more frequently observed in positive patients (90.9% vs. 62%; p < 0.001) (Table 4).
Discussion
Genome-wide association studies (GWAS) have described that ovarian endometriosis partly contributes to the larger effect sizes observed in ASRM Stage 3-4, indicating a genetic basis distinct from other disease manifestations (18). In this study, we hypothesized that genetic factors may play a role in the pathophysiology of ovarian endometriosis. This study aimed to assess the genetic predisposition to the development and characteristics of this disease, focusing on the presence of four specific inflammasome-related polymorphisms: NOD1 (rs2075820 and rs2075818), NOD2 (rs104895461), PYDC1, and PYDC2 gene polymorphisms. This is the first report to detail the analysis of gene polymorphisms for these genes in endometriosis.
Previously, NOD1 and NOD2 genes were assessed for their potential predisposition to endometrial cancer; however, no associations were observed (19). Our study revealed that the NOD1 rs2075820 had lower (G>A) genotypes in endometriosis patients when compared with the control group. A pro-apoptotic protein NOD1 can trigger apoptosis through interactions with the caspase pathway whereas NF-κB serves to suppress the apoptotic process (20). NOD proteins can initiate signaling pathways involving both NF-κB and caspase in endometriosis. On the other hand, the allele frequencies of G and A in NOD1 rs2075820 did not differ significantly. Other studies revealed that the presence of the A allele of rs2075820 correlated with decreased expression and activation of NF-κB when intracellular Propionibacterium acnes (P. acnes) infection present in the Japanese population (21).
A few studies investigated the expression of NODs in the female reproductive tract. NOD1 and NOD2 are differentially expressed and regulated in the human endometrium, playing roles in the innate immune response and potentially in the inflammatory events associated with menstruation with interleukins (22). In another study, ectopic endometrial stromal cells showed increased levels of NOD1 expression and interleukin-8, while the NOD1 inhibitor ML-130 suppressed proliferation, clonal expansion, invasion, and migration of these cells without impacting apoptosis (23).The pathophysiological mechanism behind diminished ovarian reserve in endometriosis remains unclear. It is debated whether endometriomas reduce functional tissue through mechanical stretching (space-occupying effect) or direct inflammatory impact. Ovarian endometriomas contain immune components like reactive oxygen species (ROS), metalloproteinases, and cytokines, which may progressively damage the ovarian stroma and reduce the primordial follicular reserve over time (24).
Ovarian endometriosis poses a challenge to ovarian reserve, though the extent of its uniform impact on reserve remains debated. A retrospective study on women with ovarian endometriomas (mean diameter 26 ± 8 mm) undergoing multiple ovarian stimulation cycles found consistent oocyte retrieval rates from affected ovaries across cycles, at 44% for both initial and subsequent cycles. Another study reported a statistically significant 26% decrease in anti-müllerian hormone (AMH) levels over six months in 40 women with endometriomas (mean diameter 46 ± 17 mm), indicating a progressive decline in ovarian reserve (25).
Ovarian endometrioma size has been studied in relation to ovarian stimulation, with a 4 cm diameter threshold commonly used to indicate potential impact on ovarian response. Generally, small cysts have minimal effects, while larger cysts can significantly affect ovarian function. Our findings reveal that the NOD1 rs2075820 AA phenotype and PYDC2 rs293833 (c.242A > G) polymorphism are strongly associated with female infertility. Additionally, PYDC2 rs293833 (c.242A > G) correlates with larger endometriomas (≥4 cm). Subgroup analysis supports GWAS recommendations for assessing genetic variations, particularly in cases with larger ovarian cysts and severe endometriosis, to improve reproductive outcomes.
The primary treatments for endometriosis include surgery and pharmacological options like hormone therapy and NSAIDs for pain management. Surgical excision can improve symptoms and fertility; however, recent reviews show recurrence rates of 21.5% at 2 years and 40–50% at 5 years, indicating that recurrences and repeat surgeries may exacerbate pain and further reduce fertility (26).
Therefore, regular and long-term medication use is recommended to prevent postoperative recurrence of endometriosis. However, hormone therapies, due to estrogen’s role in endometriosis development, may suppress follicular development and ovulation, making treatment challenging for women seeking pregnancy. NLRs are hypothesized as promising therapeutic targets for addressing inflammation-associated endometriosis via their pivotal role in innate immunity (10). NLR family pyrin domain containing 3 (NLRP3) and NLR family CARD domain containing 5(NLRC5) have prominent improving effects on endometriosis with altering fibrosis and inflammation in previous studies (27, 28).
The NLRP3/IL-1β pathway plays a role in endometriosis development, and NLRP3 inhibitors may help reduce ovarian endometrioma size and improve ovarian function (29). In a study, increased NOD1 expression and inflammatory cytokines in ectopic endometrial cells in peritoneal fluid, with the NOD1 inhibitor ML130 significantly reducing cell viability and cytokine production (30). Furthermore, mifepristone has been shown to exhibit protective effects against NLRP1 inflammasome activation and to minimize damage to hippocampal neurons caused by dexamethasone (31). Thus, strategies targeting the inflammasome axis may serve as potential therapeutic options for treating endometriosis.
Women with pelvic endometriosis often experience pain due to pelvic visceral hypersensitivity, along with abdominal and pelvic discomfort. Studies show that the inflammatory microenvironment within ectopic lesions activates sensory nerve endings through inflammatory mediators, amplifying pain signal transmission (32). This hypothesis is reinforced by fluctuations in cyclic inflammatory markers during the menstrual cycle, which correlate with heightened gastrointestinal symptoms. The overlap between endometriosis and irritable bowel syndrome (IBS)—more commonly diagnosed in women with pelvic endometriosis—adds complexity to interpreting gastrointestinal symptoms. Additionally, endometriosis patients show lower pain thresholds in response to bowel distension and other gastrointestinal triggers (33, 34). In another study, NOD1 rs2075820 was not associated with inflammatory bowel disease in the Turkish population (35).
Our findings suggest that NOD1 rs2075820 AA phenotype and PYDC2 rs293833 (c.242A>G) polymorphism is strongly associated with increased gastrointestinal complaints in ovarian endometriosis patients. The localization of ovarian endometriosis in areas closely related to the terminal parts of the colon, along with its inflammatory characteristics and local factors such as prostaglandin release, may explain the increased incidence of gastrointestinal complaints in endometriosis patients. However, painful symptoms associated with deep infiltrative endometriosis (DIE) may also cause pain characteristics, often specific to precise anatomical locations or affected organs, such as severe deep dyspareunia or painful defecation.A limitation of the study includes the potential for more robust results if the sample size for subgroup analysis is increased, even though the sample size was previously calculated specifically for ovarian endometriosis. On the other hand, to the best of our knowledge, this is the first study to evaluate endometriomas with their sizes and genetic profiles together. Obtaining significant differences between these groups may provide valuable insights for further studies.
Conclusion
Our study shows a correlation between genetic predispositions, inflammatory pathways, and the clinical manifestations of ovarian endometriosis. By investigating specific inflammasome-related polymorphisms, NOD1, and PYDC2 gene variants, we have uncovered potential associations with infertility and gastrointestinal complaints in affected individuals. These findings imply that the inflammatory microenvironment substantially influences infertility, particularly through pathways associated with the inflammasome complexes. The importance of considering genetic variations is shown in the evaluation and management of endometriosis, especially in subgroups characterized by severe disease phenotypes. Moreover, our results highlight the complex nature of endometriosis pathophysiology, implicating not only mechanical and inflammatory processes but also genetic factors in disease progression and symptomatology.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Health Research Ethics Committee of Dokuz Eylul University (7511-GOA). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
HK: Conceptualization, Data curation, Funding acquisition, Methodology, Visualization, Writing – original draft. BB: Investigation, Methodology, Software, Writing – review & editing. TT: Data curation, Validation, Writing – original draft. PY: Data curation, Writing – original draft. OY: Data curation, Writing – original draft. SK: Methodology, Project administration, Writing – review & editing. EU: Conceptualization, Validation, Writing – original draft. CP: Formal analysis, Funding acquisition, Investigation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Funding of this study supported by the Dokuz Eylul University Scientific Research Program – Project ID: 3013.
Acknowledgments
We also extend special thanks to Nazlı Demirkıran for her recommendations during the funding process.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1495002/full#supplementary-material
References
1. Vercellini, P, Viganò, P, Somigliana, E, and Fedele, L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. (2014) 10:261–75. doi: 10.1038/nrendo.2013.255
2. Sampson, JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. (1927) 14:422–69. doi: 10.1016/S0002-9378(15)30003-X
3. Bonavina, G, and Taylor, HS. Endometriosis-associated infertility: from pathophysiology to tailored treatment. Front Endocrinol. (2022) 13:1020827. doi: 10.3389/fendo.2022.1020827
4. Dalsgaard, T, Hjordt Hansen, MV, Hartwell, D, and Lidegaard, O. Reproductive prognosis in daughters of women with and without endometriosis. Hum Reprod. (2013) 28:2284–8. doi: 10.1093/humrep/det231
5. Hadfield, RM, Mardon, HJ, Barlow, DH, and Kennedy, SH. Endometriosis in monozygotic twins. Fertil Steril. (1997) 68:941–2. doi: 10.1016/S0015-0282(97)00359-2
6. Somigliana, E, Li Piani, L, Paffoni, A, Salmeri, N, Orsi, M, Benaglia, L, et al. Endometriosis and IVF treatment outcomes: unpacking the process. Reprod Biol Endocrinol. (2023) 21:107. doi: 10.1186/s12958-023-01157-8
7. Defrère, S, González-Ramos, R, Lousse, JC, Colette, S, Donnez, O, Donnez, J, et al. Insights into iron and nuclear factor-kappa B (NF-kappaB) involvement in chronic inflammatory processes in peritoneal endometriosis. Histol Histopathol. (2011) 26:1083–92. doi: 10.14670/HH-26.1083
8. Wilmanski, JM, Petnicki-Ocwieja, T, and Kobayashi, KS. NLR proteins: integral members of innate immunity and mediators of inflammatory diseases. J Leukoc Biol. (2008) 83:13–30. doi: 10.1189/jlb.0607402
9. Strober, W, Murray, PJ, Kitani, A, and Watanabe, T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol. (2006) 6:9–20. doi: 10.1038/nri1747
10. Guo, B, Chen, J, Zhang, JH, Fang, Y, Liu, XJ, Zhang, J, et al. Pattern-recognition receptors in endometriosis: a narrative review. Front Immunol. (2023) 14:14. doi: 10.3389/fimmu.2023.1161606
11. Porter, KA, Duffy, EB, Nyland, P, Atianand, MK, Sharifi, H, and Harton, JA. The CLRX.1/NOD24 (NLRP2P) pseudogene codes a functional negative regulator of NF-κB, pyrin-only protein 4. Genes Immun. (2014) 15:392–403. doi: 10.1038/gene.2014.30
12. Zerrad, C, Lkhider, M, Bouqdayr, M, Belkouchi, A, Badre, W, Tahiri, M, et al. NOD1 and NOD2 genetic variants: impact on hepatocellular carcinoma susceptibility and progression in Moroccan population. Gene. (2024) 931:148847. doi: 10.1016/j.gene.2024.148847
13. Falconer, H, D’Hooghe, T, and Fried, G. Endometriosis and genetic polymorphisms. Obstet Gynecol Surv. (2007) 62:616–28. doi: 10.1097/01.ogx.0000279293.60436.60
14. Tempfer, CB, Simoni, M, Destenaves, B, and Fauser, BCJM. Functional genetic polymorphisms and female reproductive disorders: part II--endometriosis. Hum Reprod Update. (2008) 15:97–118. doi: 10.1093/humupd/dmn040
15. American Society. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. (1997) 67:817–21. doi: 10.1016/S0015-0282(97)81391-X
16. Abrao, MS, Andres, MP, Miller, CE, Gingold, JA, Rius, M, Neto, JS, et al. AAGL 2021 endometriosis classification: an anatomy-based surgical complexity score. J Minim Invasive Gynecol. (2021) 28:1941–1950.e1. doi: 10.1016/j.jmig.2021.09.709
17. Kocaaga, A, Cakmak Genc, G, Karakas Celık, S, Koca, R, and Dursun, A. Association of NOD1, NOD2, PYDC1 and PYDC2 genes with Behcet’s disease susceptibility and clinical manifestations. Ophthalmic Genet. (2021) 42:691–7. doi: 10.1080/13816810.2021.1955273
18. Rahmioglu, N, Nyholt, DR, Morris, AP, Missmer, SA, Montgomery, GW, and Zondervan, KT. Genetic variants underlying risk of endometriosis: insights from meta-analysis of eight genome-wide association and replication datasets. Hum Reprod Update. (2014) 20:702–16. doi: 10.1093/humupd/dmu015
19. Ashton, KA, Proietto, A, Otton, G, Symonds, I, McEvoy, M, Attia, J, et al. Toll-like receptor (TLR) and nucleosome-binding oligomerization domain (NOD) gene polymorphisms and endometrial cancer risk. BMC Cancer. (2010) 10:382. doi: 10.1186/1471-2407-10-382
20. Kasimsetty, SG, Hawkes, A, Barekatain, K, Soo, E, Welch, AK, and McKay, DB. TLR2 and NODs1 and 2 cooperate in inflammatory responses associated with renal ischemia reperfusion injury. Transpl Immunol. (2020) 58:101260. doi: 10.1016/j.trim.2019.101260
21. Tanabe, T, Ishige, I, Suzuki, Y, Aita, Y, Furukawa, A, Ishige, Y, et al. Sarcoidosis and NOD1 variation with impaired recognition of intracellular Propionibacterium acnes. Biochim Biophys Acta. (2006) 1762:794–801. doi: 10.1016/j.bbadis.2006.07.006
22. King, AE, Horne, AW, Hombach-Klonisch, S, Mason, JI, and Critchley, HOD. Differential expression and regulation of nuclear oligomerization domain proteins NOD1 and NOD2 in human endometrium: a potential role in innate immune protection and menstruation. Mol Hum Reprod. (2009) 15:311–9. doi: 10.1093/molehr/gap020
23. Li, C, Luo, S, Guo, A, Su, Y, Zhang, Y, Song, Y, et al. Human endometrium derived mesenchymal stem cells with aberrant NOD1 expression are associated with ectopic endometrial lesion formation. Int. J Stem Cells. (2024) 17:309–18. doi: 10.15283/ijsc22200
24. Sanchez, AM, Viganò, P, Somigliana, E, Panina-Bordignon, P, Vercellini, P, and Candiani, M. The distinguishing cellular and molecular features of the endometriotic ovarian cyst: from pathophysiology to the potential endometrioma-mediated damage to the ovary. Hum Reprod Update. (2014) 20:217–30. doi: 10.1093/humupd/dmt053
25. Kasapoglu, I, Ata, B, Uyaniklar, O, Seyhan, A, Orhan, A, Yildiz Oguz, S, et al. Endometrioma-related reduction in ovarian reserve (ERROR): a prospective longitudinal study. Fertil Steril. (2018) 110:122–7. doi: 10.1016/j.fertnstert.2018.03.015
26. Koga, K, Takamura, M, Fujii, T, and Osuga, Y. Prevention of the recurrence of symptom and lesions after conservative surgery for endometriosis. Fertil Steril. (2015) 104:793–801. doi: 10.1016/j.fertnstert.2015.08.026
27. Guo, C, Fu, R, Wang, S, Huang, Y, Li, X, Zhou, M, et al. NLRP3 inflammasome activation contributes to the pathogenesis of rheumatoid arthritis. Clin Exp Immunol. (2018) 194:231–43. doi: 10.1111/cei.13167
28. Wang, Y, Huang, C, Bian, E, Lei, T, Lv, X, and Li, J. NLRC5 negatively regulates inflammatory responses in LPS-induced acute lung injury through NF-κB and p38 MAPK signal pathways. Toxicol Appl Pharmacol. (2020) 403:115150. doi: 10.1016/j.taap.2020.115150
29. Murakami, M, Osuka, S, Muraoka, A, Hayashi, S, Bayasula, X, Kasahara, Y, et al. Effectiveness of NLRP3 inhibitor as a non-hormonal treatment for ovarian endometriosis. Reprod Biol Endocrinol. (2022) 20:924. doi: 10.1186/s12958-022-00924-3
30. Wei, X, Liu, Y, Li, W, and Shao, X. Nucleotide-binding oligomerization domain-containing protein 1 regulates inflammatory response in endometriosis. Curr Protein Pept Sci. (2022) 23:31. doi: 10.2174/1389203723666220322125031
31. Zhang, B, Zhang, Y, Xu, T, Yin, Y, Huang, R, Wang, Y, et al. Chronic dexamethasone treatment results in hippocampal neurons injury due to activate NLRP1 inflammasome in vitro. Int Immunopharmacol. (2017) 49:222–30. doi: 10.1016/j.intimp.2017.05.039
32. Patel, BG, Lenk, EE, Lebovic, DI, Shu, Y, Yu, J, and Taylor, RN. Pathogenesis of endometriosis: interaction between endocrine and inflammatory pathways. Best Pract Res Clin Obstet Gynaecol. (2018) 50:50–60. doi: 10.1016/j.bpobgyn.2018.01.006
33. Seaman, HE, Ballard, KD, Wright, JT, and de Vries, CS. Endometriosis and its coexistence with irritable bowel syndrome and pelvic inflammatory disease: findings from a national case–control study—part 2. BJOG. (2008) 115:1392–6. doi: 10.1111/j.1471-0528.2008.01879.x
34. Issa, B, Onon, TS, Agrawal, A, Shekhar, C, Morris, J, Hamdy, S, et al. Visceral hypersensitivity in endometriosis: a new target for treatment? Gut. (2012) 61:367–72. doi: 10.1136/gutjnl-2011-300306
Keywords: endometriosis, infertility, pain, gene polymorphism, NOD, PYDC
Citation: Kula H, Balbal B, Timur T, Yalcın P, Yavuz O, Kızıldag S, Ulukus EC and Posaci C (2025) NOD1, NOD2, PYDC1, and PYDC2 gene polymorphisms in ovarian endometriosis. Front. Med. 11:1495002. doi: 10.3389/fmed.2024.1495002
Edited by:
Lan Zheng, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Mohd Faizal Ahmad, National University of Malaysia, MalaysiaRahul Basu, University of Texas at San Antonio, United States
Copyright © 2025 Kula, Balbal, Timur, Yalcın, Yavuz, Kızıldag, Ulukus and Posaci. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hakan Kula, YWxpaGFrYW4ua3VsYUBkZXUuZGV1LnRy; Cemal Posaci, Y2VtYWwucG9zYWNpQGRldS5lZHUudHI=
†ORCID: Hakan Kula, orcid.org/0000-0003-1443-5796
Beste Balbal, orcid.org/0000-0003-1407-2497
Tunc Timur, orcid.org/0000-0002-1250-8579
Pelin Yalcın, orcid.org/0000-0001-9743-9930
Onur Yavuz, orcid.org/0000-0003-3716-2145
Sefa Kızıldag, orcid.org/0000-0001-7939-5153
Cemal Posaci, orcid.org/0000-0001-5010-5204
 Hakan Kula
Hakan Kula Beste Balbal2†
Beste Balbal2† Pelin Yalcın
Pelin Yalcın Onur Yavuz
Onur Yavuz