- 1Department of Pediatrics, Nanfang Hospital, Southern Medical University, Guangzhou, China
- 2Department of Pharmacy, Nanfang Hospital, Southern Medical University, Guangzhou, China
- 3Department of Nephrology, Peking University Third Hospital, Beijing, China
Background: Gabapentinoids, such as gabapentin and pregabalin, are opioid substitutes commonly included in perioperative multimodal analgesia regimens. We investigated whether the initiation of gabapentin and pregabalin during the perioperative period have varying effects on the adverse renal outcomes.
Methods: This study included adult participants who received surgery in the INSPIRE database. The exposure of interest was the initiation of pregabalin or gabapentin during the perioperative period. The primary outcome was renal function decline. Secondary outcomes included incident chronic kidney disease (CKD), hospital-acquired acute kidney injury (AKI), and in-hospital mortality. We conducted a propensity score to balance the baseline characteristics. Cox proportional hazard regression was used to estimate the hazard ratio (HR) of the initiation of gabapentin compared with pregabalin.
Results: Among 640 pairs of pregabalin and gabapentin initiators in the matched cohort, the initiation of gabapentin was associated with a higher risk of kidney function decline (HR, 1.40; 95% confidence interval [CI], 1.04–1.89) as compared with pregabalin. After excluding participants who were diagnosed with CKD at the baseline, the initiation of gabapentin was associated with a higher risk of incident CKD (HR, 1.46; 95% CI, 1.03–2.05) as compared with pregabalin. For the in-hospital outcomes, the proportion of AKI and mortality were similar between participants initiating gabapentin and pregabalin. In addition, the risk of kidney function decline did not vary across each subgroup.
Conclusion: The initiation of gabapentin during the perioperative period was associated with a higher risk of kidney function decline and incident CKD as compared with pregabalin.
1 Introduction
Adequate postoperative pain control is an important component of the Enhanced Postoperative Recovery (ERAS) pathway, which has been related to better outcomes, shorter hospital stays, and lower costs (1–3). In recent years, multimodal analgesia targeting various pain pathways has been an increasingly adopted strategy in the ERAS pathway, which aims to improve postoperative pain relief with minimal or no opioid consumption, and thus reduce opioid-related adverse events (4). Gabapentinoids, such as pregabalin and its predecessor, gabapentin, are now commonly included in multimodal analgesia regimens to reduce postoperative pain and opioid requirements, which were also found to reduce the incidence of postoperative nausea, vomiting, and pruritus (5, 6). However, despite these benefits, gabapentinoids have been reported to increase the risk of adverse effects such as sedation, dizziness, visual disturbances, ataxia, cognitive impairment, and respiratory depression, particularly when used concurrently with opioids (6–8).
Gabapentinoids were originally designed as analogs of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA), but they have no significant antagonistic effect on GABAA or GABAB receptors (9). Instead, their mechanism of action involves targeting the α-2-δ subunit of presynaptic voltage-dependent calcium channels in the spinal cord and peripheral nerves, inhibiting calcium influx, and thus decreasing the release of excitatory neurotransmitters and reducing spinal sensitization (10). Gabapentinoids are not metabolized by the liver; they are primarily eliminated by the kidneys in an unchanged form, with clearance proportional to the creatinine clearance. Accumulation of gabapentinoids can lead to kidney failure and other adverse effects (11). Therefore, in patients with chronic kidney disease (CKD), appropriate dosing of gabapentinoids is crucial to minimize the risk of adverse events (12). A single center retrospective cohort study reported that patients with decreased creatinine clearance (<60 mL/min) often take inappropriate high-dose gabapentin, which may exacerbate adverse effects (13). Even in patients whose kidney function was previously normal, several case reports indicated that gabapentin can directly induce rhabdomyolysis and cause acute kidney injury, suggesting potential renal adverse reactions of gabapentin (14–19).
Although gabapentin and pregabalin share similar chemical structures and mechanisms of action but differ considerably in pharmacodynamic and pharmacokinetic profiles (11). Pregabalin, which developed after gabapentin, is more potent and has the benefits of more rapid peak blood concentration and better bioavailability than gabapentin (20). Previous studies have reported that substituting gabapentin with pregabalin may result in improved pain relief and fewer adverse events, such as sedation, dizziness, and peripheral edema (21). However, to our knowledge, it is uncertain whether the risk of adverse kidney outcomes varies between gabapentin and pregabalin, as there are no direct comparisons.
Therefore, this study aims to compare the risk of adverse kidney outcomes in participants undergoing perioperative initiating gabapentin or pregabalin, hoping to provide some reference for the selection of Gabapentinoids during the perioperative period.
2 Methods
2.1 Target trial emulation
We emulated a target trial with new-user, active comparator design to compare the risk of kidney outcomes in participants underwent surgery and initiated pregabalin or gabapentin during the perioperative period. Supplementary Table S1 summarizes the key design elements of this trial.
2.2 Data source
Participants were identified from a publicly available research dataset in perioperative medicine, which includes appropriately 130,000 cases who underwent anesthesia for surgery at an academic institution in South Korea between January 2011 and December 2020. This comprehensive dataset includes patient characteristics such as age, sex, American Society of Anesthesiologists physical status classification, diagnosis, surgical procedure code, department, and type of anesthesia. It also includes vital signs in the operating theater, general wards, and intensive care units (ICUs), laboratory results from 6 months before admission to 6 months after discharge, and medication during hospitalization. Complications include total hospital and ICU length of stay and in-hospital death. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
2.3 Study population
This study included participants aged 18–90 years who underwent surgery with general, neuraxial, regional, or monitored anesthesia care, as recorded in the INSPIRE database (22, 23). We included participants who received any prescription of pregabalin or gabapentin during the perioperative period. Participants using both pregabalin and gabapentin concurrently were excluded. Additionally, to avoid prevalent user bias, we excluded participants with prior use of the study drugs. The index date was defined as the date of the first prescription of either pregabalin or gabapentin during the perioperative period. Participants with a baseline estimated glomerular filtration rate (eGFR) <15 mL/min/1.73 m2 or those lacking laboratory measurements after discharge were also excluded. The flowchart of participants selection was shown in Figure 1.
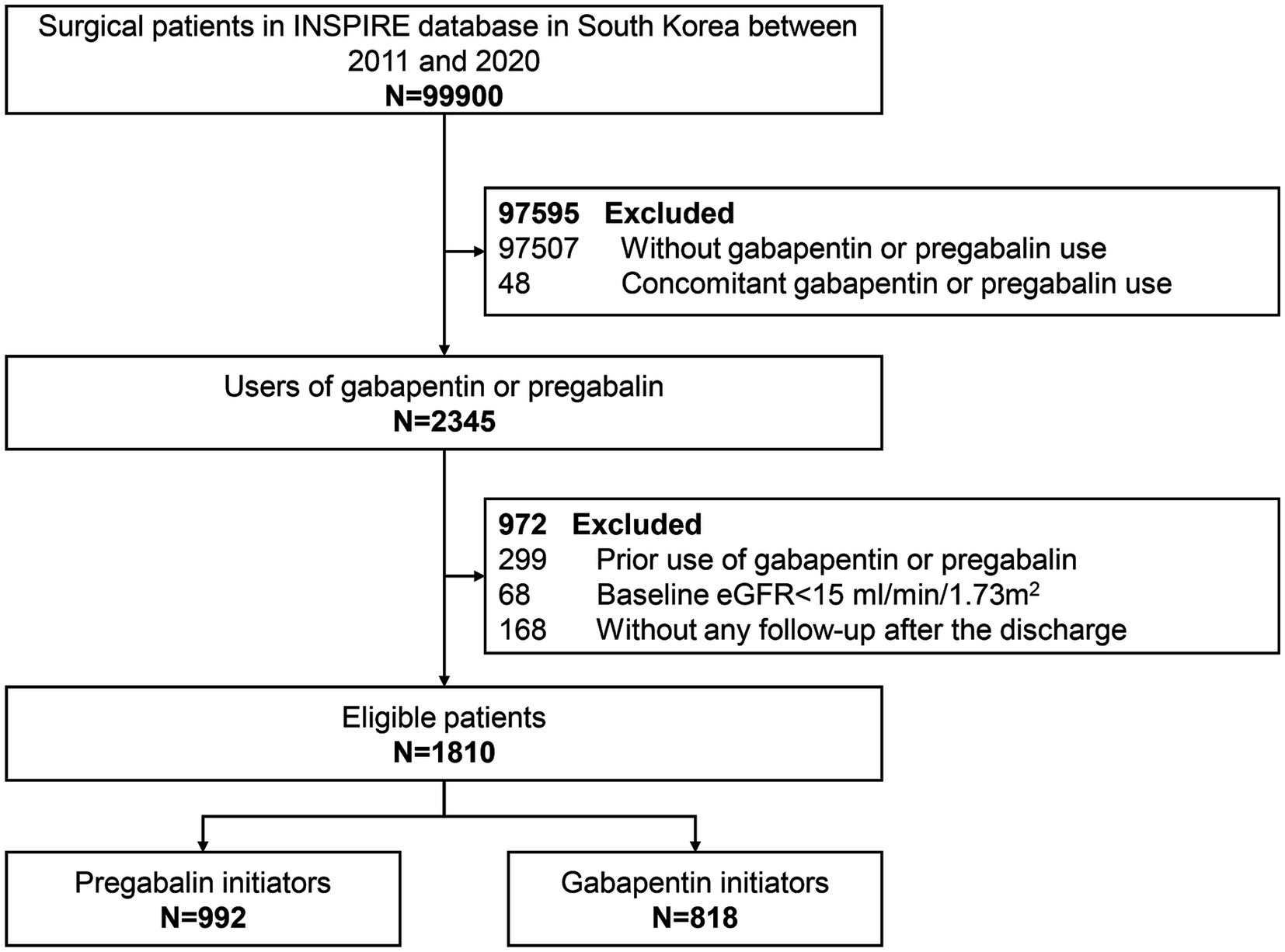
Figure 1. Study flow diagram of participants initiating pregabalin or gabapentin in the INSPIRE database between 2011 and 2020.
2.4 Exposure
The exposure of interest was the initiation of pregabalin or gabapentin during the perioperative period, with the start date of treatment for each participant defined as the index date. To mimic the intention-to-treat approach of a randomized clinical trial, participants were considered to remain on the study drug for the entire duration of the analysis.
2.5 Outcomes
The primary outcome was the kidney function decline, defined as the >40% decline in eGFR from the baseline within 6 months. Secondary outcomes included the incident CKD within 6 months, hospital-acquired acute kidney injury (HA-AKI), and in-hospital mortality. The incident CKD was defined as the new-onset eGFR < 60 mL/min/1.73 m2 and was assessed among participants with baseline eGFR > 60 mL/min/1.73 m2. HA-AKI was defined as an increase in serum creatinine (SCr) by 0.3 mg/dL within 48 h or a 50% increase in SCr from the baseline within 7 days according to the KDIGO criteria (24). Methods for determining HA-AKI have been reported in our previous studies (25). Follow-up began at the date of the initiation of study drugs until the occurrence of the outcome of interest, death, or the end of the study period (31 December 2020), whichever came first.
2.6 Covariates
The baseline characteristics included the demographic characteristics, body mass index (BMI), calendar year of the drugs initiation, operation and anesthesia-related parameters, intraoperative factors (plasma solution infused and sustained hypotension), diagnosis, vital signs, laboratory results, or prescription and administration of the medications were extracted from the clinical data warehouse of the Seoul National University Hospital (SUPREME version 1.0 and 2.0). Laboratory measurements were recorded from 6 months before the operation to 6 months after the last discharge. Potential confounding factors in our study included age, sex, blood pressure, type of surgery (orthopedic, gastroenterological, cardiac, neurological, and other surgeries), laboratory measurements (eGFR, hemoglobin, and serum albumin), comorbidities (hypertension, diabetes, cancer, heart failure, and coronary heart disease, sepsis), and co-medications (nonsteroidal anti-inflammatory drugs [NASIDs], opioids, renin-angiotensin system inhibitors [RASi], statins, proton pump inhibitors [PPI], aminoglycosides, loop diuretics, and antibiotics).
2.7 Statistical analysis
Baseline characteristics for the overall cohort and across the two initiation groups were presented as median (interquartile range, IQR) for continuous variables and frequencies with proportions for categorical variables. Standardized mean differences (SMDs) were computed and presented, with values less than 0.1 considered indicative of balance (26).
To balance baseline characteristics between the two initiation groups, we conducted propensity score matching (PSM) using logistic regression to model the probability of initiating gabapentin, adjusting for baseline covariates described in Table 1. Patients receiving gabapentin were matched with those initiating pregabalin in a 1:1 ratio (nearest-neighbor) based on a maximum caliper width of 0.1 of the standard deviation of the logit of the propensity score. Cumulative incidence curves for kidney function decline and incident CKD were plotted in the matched sample. Incidence rates per 100 person-years with 95% confidence intervals (CIs) were calculated using 1,000 nonparametric bootstrap samples. Cox proportional hazard regression was performed to estimate the hazard ratio of gabapentin initiation compared with pregabalin initiation after PSM, without further adjustment. Our primary analyses adhered to the intention-to-treat principle; thus, participants who initiated pregabalin and subsequently initiated gabapentin were retained in the pregabalin group, and vice versa.
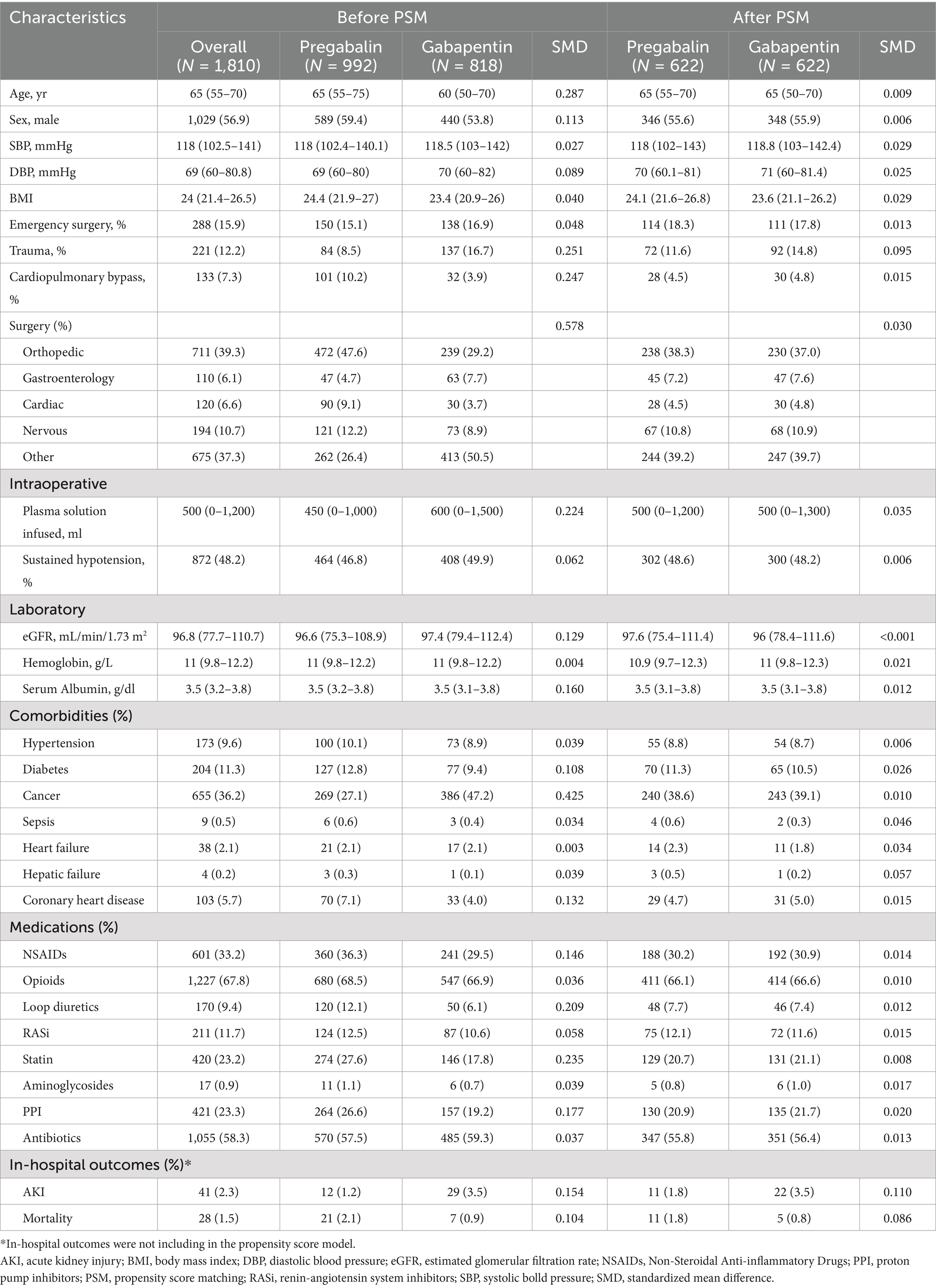
Table 1. Baseline characteristics between participants with pregabalin or gabapentin initiation before and after PSM.
2.8 Sensitivity and subgroup analyses
Four sensitivity analyses were conducted. First, we used propensity score overlap weighting instead of matching and repeated our analysis. Second, participants with less than 90 days of follow-up were excluded to minimize the potential for reverse causality. Third, participants who AKI or died during hospitalization were excluded to mitigate the impact of severe conditions on prognosis. Fourth, in addition to the intention-to-treat analysis, we performed a sensitivity analysis using a per-protocol approach. In the per-protocol analysis, participants were censored at the date they switched study drugs (deviated from the initially initiated drug). Fifth, we re-define the kidney outcome as a sustained decrease in eGFR >40% from baseline, confirmed by two consecutive SCR measurements. Sixth, we performed the same analysis under the assumption of Missing Non at Random (MNAR) mechanism and compared these results with the primary analysis. Subgroup analyses were conducted to explore potential effect modifications among participants stratified by age (≥60 and <60 years), sex, hypertension, diabetes, cancer, and use of statins and PPI. Missing values were imputed using multiple imputation (using the ‘mice’ package in R).
3 Results
3.1 Baseline characteristics
Data from 1,810 eligible participants were included in this analysis (median (IQR) age, 65 [55–70] years; 1,029 [56.9%] male; and 711 [39.3%] underwent orthopedics surgery; with median [IQR] eGFR, 96.8 [77.7–110.7]). Participants initiating pregabalin and gabapentin differed in pertinent baseline characteristics (defined as SMDs >10%). For instance, gabapentin initiators were younger, had a lower proportion undergoing orthopedic surgery, were more likely to have a diagnosis of cancer, and were less likely to use statins and PPI compared to those initiating pregabalin. After PSM, 640 pregabalin initiators were matched to 640 gabapentin initiators. All variables included in the propensity score model were balance between the two groups (no SMDs exceeded 0.1). Regarding in-hospital outcomes, the proportions of AKI and mortality were similar between participants initiating gabapentin and pregabalin. During the 10-year study, there was no notable trend in the initiation rates of gabapentin versus pregabalin (Supplementary Figure S1). Baseline characteristics between participants with pregabalin or gabapentin initiation before and after PSM were shown in Table 1. The proportion of missingness of covariates were shown at Supplementary Table S2.
3.2 Risk of kidney function decline and incident CKD
In the propensity score-matched sample participants (Table 2), the frequency of the SCR measurements after the study drugs initiation was consistent between the two groups (Supplementary Figure S2). The incidence rate of kidney function decline per 100 person-year of follow-up was 8.20 (6.63–10.19) among pregabalin initiators and 9.70 (8.16–11.49) among gabapentin initiators. Gabapentin initiation was associated with a higher risk of kidney function decline (HR 1.40; 95% CI 1.04–1.89) compared to pregabalin (Figure 2 and Table 2). Subgroup analysis indicated no significant variation in the risk of kidney function decline across different subgroups (Figure 3, p for interaction >0.05).

Table 2. The association of kidney outcomes with the initiation of gabapentin versus pregabalin after PSM.
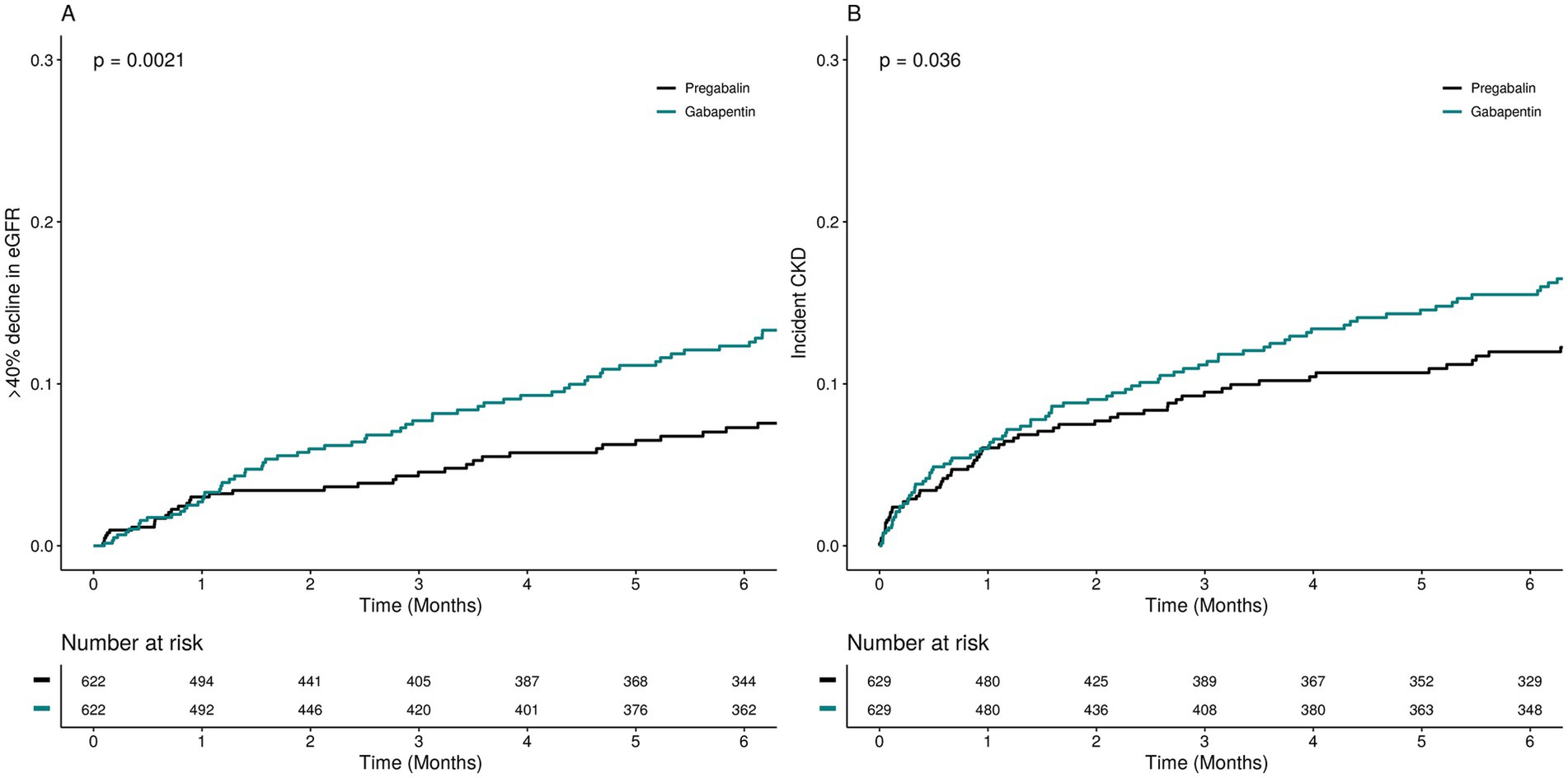
Figure 2. Cumulative incidence of the kidney outcomes among pregabalin initiators and gabapentin initiators.
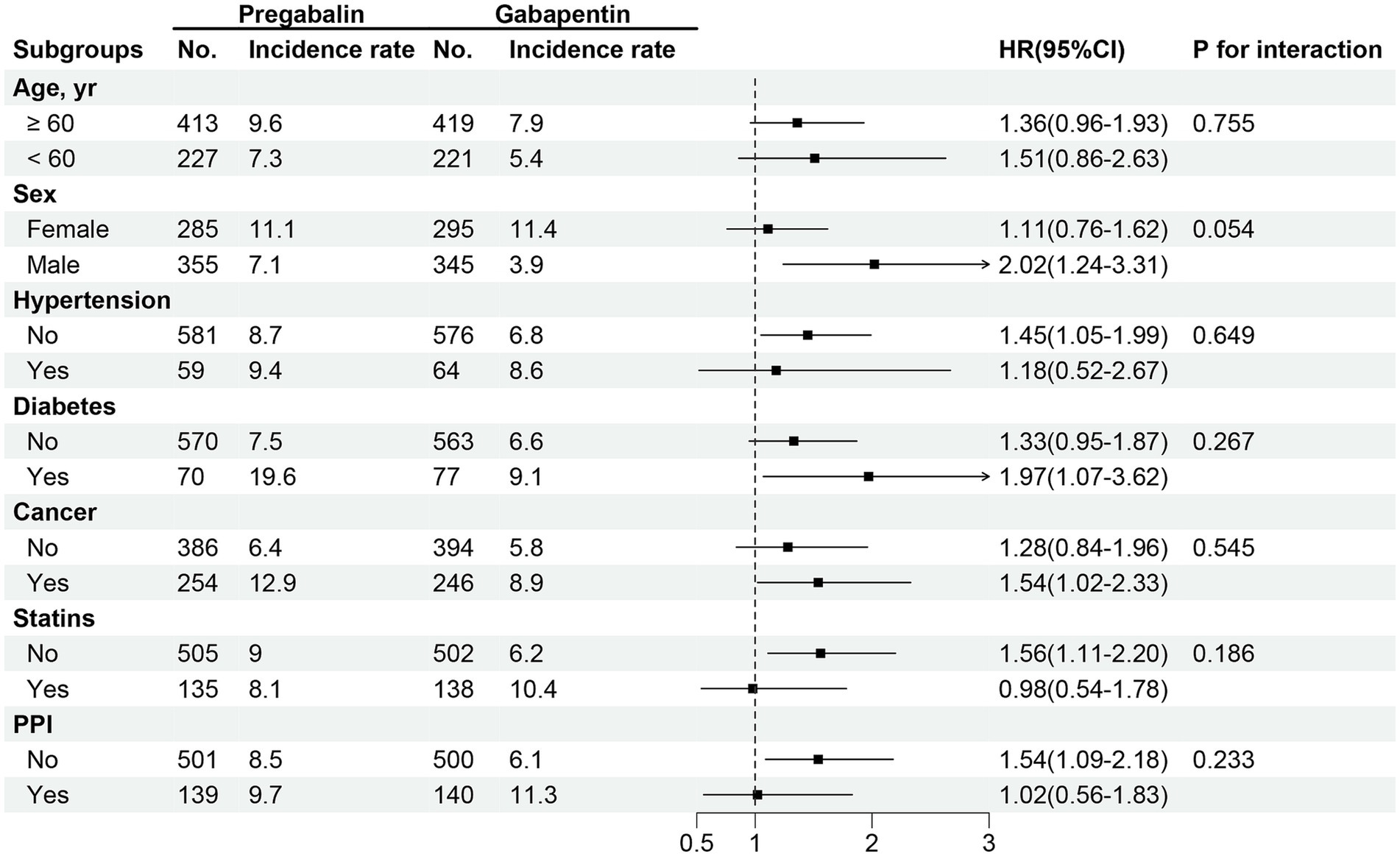
Figure 3. HRs for the association between pregabalin initiators versus gabapentin initiators and risk of kidney function decline among different subgroups.
To assess the association between gabapentin and incident chronic kidney disease (CKD) (Table 2), we excluded 644 participants diagnosed with CKD at baseline and performed separate PSM in the incident CKD cohort. The incidence rate of CKD was 7.88 per 100 person-years among pregabalin initiators and 10.21 per 100 person-years among gabapentin initiators, indicating a significantly higher risk of incident CKD associated with gabapentin initiation (HR 1.46; 95% CI 1.03–2.05).
3.3 Sensitivity analyses
Similar findings were observed in sensitivity analyses using propensity score overlap weighting (Supplementary Table S3). After excluding participants who experienced outcomes or were lost to follow-up within 90 days (Supplementary Table S4), initiation of gabapentin was significantly associated with an increased risk of kidney function decline (HR 1.47; 95% CI 1.06–2.02) and incident chronic kidney disease (HR 1.47; 95% CI 1.03–2.10) compared to pregabalin. Consistent results were found in sensitivity analyses after excluding participants who developed acute kidney injury or died during hospitalization (Supplementary Table S5). In the per-protocol analysis, increased risks of kidney function decline and incident chronic kidney disease were observed among participants initiating gabapentin compared to those initiating pregabalin (Supplementary Table S6). Consistent results were obtained when re-defined the kidney outcome to require confirmation by two consecutive eGFR measurements (Supplementary Table S7) and assuming a MNAR mechanism (Supplementary Table S8).
4 Discussion
Using clinical data from participants who underwent surgery under general, neuraxial, regional, and monitored anesthesia care in the INSPIRE database, we conducted a target trial with a new-user, active comparator design to compare the risk of adverse kidney outcomes in participants initiating pregabalin or gabapentin during the perioperative period. We found that initiation of gabapentin was associated with a higher risk of kidney function decline (HR 1.40; 95% CI 1.04–1.89) and incident chronic kidney disease (HR 1.46; 95% CI 1.03–2.05) compared to pregabalin. The results of the sensitivity analyses and subgroup analyses remained consistent. Our study contributes to filling this gap and offers insights that may guide the rational selection of gabapentinoids during the perioperative period.
Gabapentinoids, including gabapentin and pregabalin, are increasingly used in multimodal analgesia regimens to minimize opioid consumption during the perioperative period (5). Administered orally, gabapentinoids are primarily excreted unchanged via the kidneys. Their half-life ranges from 5 to 7 h, which increases with declining kidney function (27). Patients with CKD are particularly susceptible to gabapentin toxicity (28). Therefore, cautious selection of initial doses and dose adjustments are crucial in this patient population (29). A previous population-based cohort study involving 74,084 older adults with CKD examined the 30-day risk of severe adverse events associated with different starting doses of gabapentinoids. The study found that initiating gabapentinoids at higher doses correlated with an increased risk of hospital visits due to encephalopathy, falls, fractures, or hospitalizations for respiratory depression (30). A recent retrospective cohort study focused on older patients during the perioperative period and assessed gabapentin-related adverse effects. PSM revealed that compared to non-users, gabapentin users had a heightened risk of delirium, particularly pronounced in patients with CKD (31). The risk of toxicity is further elevated in patients undergoing dialysis. Research involving hemodialysis patients indicated that higher doses of gabapentin or pregabalin were associated with increased risks of altered mental status, falls, and fractures (32). These findings underscore the importance of judicious use of gabapentinoids based on kidney function, highlighting the need for future research to establish optimal dosing strategies.
Renal functional impairment has been reported as a delayed adverse effect of gabapentin (33). However, the mechanisms by which gabapentin cause renal dysfunction remains poorly understood. Several case reports indicated that gabapentin can directly induce rhabdomyolysis and cause acute kidney injury, even in patients whose kidney function was previously normal (14–19). In addition, in experimental animal models, gabapentin was reported to induce apoptosis and lead to structural alterations in the kidney, including renal tubular epithelial degeneration, hemorrhage, and glomerular atrophy (34). Although pregabalin and gabapentin share similar chemical structures and mechanisms of action, pregabalin is known to be more potent and faster-acting than gabapentin (11). Gabapentin is almost 100% excreted in its original form through the kidneys, which may increase the risk of drug accumulation and toxicity in the kidneys, thereby increasing the risk of kidney damage. In contrast, the proportion of pregabalin excreted through the kidneys is relatively small, which has a relatively small burden on the kidneys (11). Previous research has also suggested that switching from gabapentin to pregabalin could potentially enhance pain relief and reduce adverse events such as sedation, dizziness, and peripheral edema (21). However, the comparative risk of adverse kidney outcomes between gabapentin and pregabalin remains unclear due to the absence of direct comparisons in previous studies. In our study, utilizing a new-user, active comparator design, we found that initiating gabapentin was associated with a higher risk of kidney function decline and incident CKD compared to pregabalin. Importantly, this risk of kidney function decline was consistent across different subgroups analyzed. To our knowledge, our study represents the first direct comparison of kidney adverse outcomes between gabapentin and pregabalin, suggesting that pregabalin may carry a lower risk of kidney adverse events than gabapentin.
The strengths of the current study include its real-world-based dataset, new-user design, and use of hard kidney outcomes. Furthermore, sophisticated statistical methods were employed to mitigate confounding and indication biases. However, the study also has several limitations. Firstly, despite PSM to balance baseline characteristics between gabapentin and pregabalin initiators, residual confounding from unmeasured factors may still impact outcomes. Sensitivity analyses were conducted to address this concern and reinforce result robustness. Secondly, the study did not explore the potential differential impact of varying initial doses of gabapentin or pregabalin on kidney outcomes, warranting future investigations in this area. Thirdly, the study population exclusively comprised patients from South Korea, necessitating validation of findings across diverse populations and geographic regions. Fourthly, due to the scattered distribution of surgical types, we were unable to perform subgroup analyses to access the impact of the heterogeneity in surgical procedures and the unknown intraoperative events. However, we have attempted to adjust for some intraoperative events such as plasma solution infused and sustained hypotension. Lastly, this study is hypothesis-generating and requires further validation through randomized controlled trials.
In conclusion, the initiation of gabapentin during the perioperative period was associated with a higher risk of kidney function decline and incident CKD as compared with pregabalin. These findings suggest that perioperative use of pregabalin might pose a lower risk of adverse kidney outcomes than gabapentin.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://physionet.org/content/inspire/1.3/.
Ethics statement
The studies involving humans were approved by the Institutional Review Board (IRB) of Seoul National University Hospital (SNUH, IRB No. H-2210-078-1368). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because of the retrospective nature of the study design. No potentially identifiable images or data are presented in this study.
Author contributions
YH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Writing – original draft. LM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Writing – review & editing. JL: Funding acquisition, Data curation, Formal analysis, Writing – original draft. DL: Conceptualization, Investigation, Methodology, Project administration, Writing – original draft. JN: Writing – original draft. YL: Data curation, Formal analysis, Writing – original draft. QZ: Software, Validation, Writing – original draft. YG: Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from Beijing Natural Science Foundation (7244432) and Wu Jieping Medical Foundation (320.6750.2020-04-1).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1488773/full#supplementary-material
References
1. Joshi, GP, and Kehlet, H. Enhanced recovery pathways: looking into the future. Anesth Analg. (2019) 128:5–7. doi: 10.1213/ane.0000000000003746
2. Gerbershagen, HJ, Pogatzki-Zahn, E, Aduckathil, S, Peelen, LM, Kappen, TH, van Wijck, AJM, et al. Procedure-specific risk factor analysis for the development of severe postoperative pain. Anesthesiology. (2014) 120:1237–45. doi: 10.1097/aln.0000000000000108
3. Hah, JM, Cramer, E, Hilmoe, H, Schmidt, P, McCue, R, Trafton, J, et al. Factors associated with acute pain estimation, postoperative pain resolution, opioid cessation, and recovery: secondary analysis of a randomized clinical trial. JAMA Netw Open. (2019) 2:e190168. doi: 10.1001/jamanetworkopen.2019.0168
4. Beverly, A, Kaye, AD, Ljungqvist, O, and Urman, RD. Essential elements of multimodal analgesia in enhanced recovery after surgery (ERAS) guidelines. Anesthesiol Clin. (2017) 35:e115–43. doi: 10.1016/j.anclin.2017.01.018
5. Patel, AS, Abrecht, CR, and Urman, RD. Gabapentinoid use in perioperative care and current controversies. Curr Pain Headache Rep. (2022) 26:139–44. doi: 10.1007/s11916-022-01012-2
6. Mishriky, BM, Waldron, NH, and Habib, AS. Impact of pregabalin on acute and persistent postoperative pain: a systematic review and meta-analysis. Br J Anaesth. (2015) 114:10–31. doi: 10.1093/bja/aeu293
7. Kharasch, ED, Clark, JD, and Kheterpal, S. Perioperative Gabapentinoids: deflating the bubble. Anesthesiology. (2020) 133:251–4. doi: 10.1097/aln.0000000000003394
8. Cavalcante, AN, Sprung, J, Schroeder, DR, and Weingarten, TN. Multimodal analgesic therapy with gabapentin and its association with postoperative respiratory depression. Anesth Analg. (2017) 125:141–6. doi: 10.1213/ane.0000000000001719
9. Patel, R, and Dickenson, AH. Mechanisms of the gabapentinoids and α 2 δ-1 calcium channel subunit in neuropathic pain. Pharmacol Res Perspect. (2016) 4:e00205. doi: 10.1002/prp2.205
10. Kremer, M, Salvat, E, Muller, A, Yalcin, I, and Barrot, M. Antidepressants and gabapentinoids in neuropathic pain: mechanistic insights. Neuroscience. (2016) 338:183–206. doi: 10.1016/j.neuroscience.2016.06.057
11. Chincholkar, M. Gabapentinoids: pharmacokinetics, pharmacodynamics and considerations for clinical practice. Br J Pain. (2020) 14:104–14. doi: 10.1177/2049463720912496
12. Wells, DA, Washington, K, Cave, B, Zhu, R, and Hudson, JQ. Gabapentinoid dosing and adverse events in patients with chronic kidney disease. Clin Nephrol. (2022) 98:147–54. doi: 10.5414/cn110815
13. Knowles, GM, LaFleur, GE, and Churchwell, MD. Evaluation of gabapentin and Pregabalin use in hospitalized patients with decreased kidney function. J Pharm Technol. (2024) 40:30–6. doi: 10.1177/87551225231217906
14. Tuccori, M, Lombardo, G, Lapi, F, Vannacci, A, Blandizzi, C, and Del Tacca, M. Gabapentin-induced severe myopathy. Ann Pharmacother. (2007) 41:1301–5. doi: 10.1345/aph.1K077
15. Bilgir, O, Calan, M, Bilgir, F, et al. Gabapentin-induced rhabdomyolysis in a patient with diabetic neuropathy. Internal Med. (2009) 48:1085–7. doi: 10.2169/internalmedicine.48.1766
16. Torregrosa-de Juan, E, Olagüe-Díaz, P, Royo-Maicas, P, Fernández-Nájera, E, and García-Maset, R. Acute renal failure due to gabapentin. a case report and literature. Nefrologia. (2012) 32:130–1. doi: 10.3265/Nefrologia.pre2011.Nov.11087
17. Falconi, D, Tattoli, F, Brunetti, C, De Prisco, O, Gherzi, M, Marazzi, F, et al. Rhabdomyolysis from gabapentin: a case report. G Ital Nefrol. (2015) 32:gin/32.2.37.
18. Choi, MS, Jeon, H, Kim, HS, Jang, BH, Lee, YH, Park, HS, et al. A case of gabapentin-induced rhabdomyolysis requiring renal replacement therapy. Hemodial Int. (2017) 21:E4–e8. doi: 10.1111/hdi.12458
19. Qiu, X, Tackett, E, and Khitan, Z. A case of gabapentin overdose induced rhabdomyolysis requiring renal replacement therapy. Clin Case Reports. (2019) 7:1596–9. doi: 10.1002/ccr3.2302
20. Schmidt, PC, Ruchelli, G, Mackey, SC, and Carroll, IR. Perioperative gabapentinoids: choice of agent, dose, timing, and effects on chronic postsurgical pain. Anesthesiology. (2013) 119:1215–21. doi: 10.1097/ALN.0b013e3182a9a896
21. Toth, C. Substitution of gabapentin therapy with pregabalin therapy in neuropathic pain due to peripheral neuropathy. Pain Med. (2010) 11:456–65. doi: 10.1111/j.1526-4637.2009.00796.x
22. Lim, L, Lee, H, Jung, C-W, Sim, D, Borrat, X, Pollard, TJ, et al. INSPIRE, a publicly available research dataset for perioperative medicine. Sci Data. (2024) 11:655. doi: 10.1038/s41597-024-03517-4
23. Lee, H-C, and Jung, C-W. VitalDB, a high-fidelity multi-parameter vital signs database in surgical patients. (version 1.0.0). PhysioNet. (2022)
24. KDIGO. 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. (2021) 100:S1–s276. doi: 10.1016/j.kint.2021.05.021
25. Su, L, Li, Y, Chen, R, Zhang, X, Cao, Y, Luo, F, et al. Epidemiology and outcomes of post-AKI proteinuria. Clin Kidney J. (2023) 16:2262–70. doi: 10.1093/ckj/sfad129
26. Austin, PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. (2009) 28:3083–107. doi: 10.1002/sim.3697
27. Athavale, A, and Murnion, B. Gabapentinoids: a therapeutic review. Aust Prescr. (2023) 46:80–5. doi: 10.18773/austprescr.2023.025
28. Zand, L, McKian, KP, and Qian, Q. Gabapentin toxicity in patients with chronic kidney disease: a preventable cause of morbidity. Am J Med. (2010) 123:367–73. doi: 10.1016/j.amjmed.2009.09.030
29. Raouf, M, Atkinson, TJ, Crumb, MW, and Fudin, J. Rational dosing of gabapentin and pregabalin in chronic kidney disease. J Pain Res. (2017) 10:275–8. doi: 10.2147/jpr.S130942
30. Muanda, FT, Weir, MA, Ahmadi, F, Sontrop, JM, Cowan, A, Fleet, JL, et al. Higher-dose Gabapentinoids and the risk of adverse events in older adults with CKD: a population-based cohort study. Am J Kidney Dis. (2022) 80:98–107.e1. doi: 10.1053/j.ajkd.2021.11.007
31. Park, CM, Inouye, SK, Marcantonio, ER, Metzger, E, Bateman, BT, Lie, JJ, et al. Perioperative gabapentin use and in-hospital adverse clinical events among older adults after major surgery. JAMA Intern Med. (2022) 182:1117–27. doi: 10.1001/jamainternmed.2022.3680
32. Ishida, JH, McCulloch, CE, Steinman, MA, Grimes, BA, and Johansen, KL. Gabapentin and Pregabalin use and association with adverse outcomes among hemodialysis patients. J Am Soc Nephrol. (2018) 29:1970–8. doi: 10.1681/asn.2018010096
33. Grunze, H, Dittert, S, Bungert, M, and Erfurth, A. Renal impairment as a possible side effect of gabapentin. A single case report. Neuropsychobiology. (1998) 38:198–9. doi: 10.1159/000026537
Keywords: gabapentin, pregabalin, perioperative analgesia, kidney function, acute kidney injury
Citation: He Y, Mo L, Li J, Lu D, Niu J, Li Y, Zeng Q and Gao Y (2024) Association of perioperative initiation of gabapentin versus pregabalin with kidney function: a target trial emulation study. Front. Med. 11:1488773. doi: 10.3389/fmed.2024.1488773
Edited by:
Tetsu Ohnuma, Duke University, United StatesReviewed by:
Yoshihisa Miyamoto, Oak Ridge Institute for Science and Education (ORISE), United StatesJulien Cobert, University of California, San Francisco, United States
Copyright © 2024 He, Mo, Li, Lu, Niu, Li, Zeng and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yueming Gao, MTMyNTY3MzM2NTBAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Yanfang He1†
Yanfang He1† Dongying Lu
Dongying Lu Yueming Gao
Yueming Gao