
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 22 January 2025
Sec. Geriatric Medicine
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1488536
 Zenghui Niu1†
Zenghui Niu1† Meiyu Cui1†
Meiyu Cui1† Yingkun Fu2
Yingkun Fu2 Lingfeng Zhou1
Lingfeng Zhou1 Jiali Wang1,3
Jiali Wang1,3 Yan Lei1
Yan Lei1 Xinrong Fan1
Xinrong Fan1 Qiang Wang4*
Qiang Wang4* Jing Yang1*
Jing Yang1*Background: Aging is the primary factor contributing to the development of aging-related diseases. As research on exosomes continues to advance, its relationship with aging and aging-related diseases has become a hot topic This article analyzes the research hotspots of exosomes in aging and aging-related diseases, aiming to fill the gap in bibliometric research in this field and help researchers better understand the current status and future trends of both fundamental and clinical research in this field.
Methods: The articles were retrieved and exported from WoSCC on December 18, 2023. The visual analysis of countries and regions, institutions, authors, references, and keywords in exosomes of aging was conducted using VOSviewer 1.6.18, CiteSpace 6.2.R7, and Bibliometrix.
Results: The bibliometric analysis included 1628 articles. China and the United States emerged as the top two leading countries in this field. A total of 2,321 research institutions from 78 countries and regions were primarily led by China and the United States. Both Kapogiannis D and Goetzl E were active authors in this field. Thery C, Valadi H, and Raposo G were the important promoters in this field. Thery C proposed the method of differential centrifugation and density gradient centrifugation to extract exosomes. Valadi H discovered cells could send RNA-messages to each other by loading them into exosome-vesicles. The journal with the highest number of articles was International Journal of Molecular Sciences, while PLoS One was the most frequently cited journal. The keyword analysis revealed that future research on exosomes in aging will possibly focus on “inflammation, cellular senescence, angiogenesis, insulin resistance, and Alzheimer's disease.”
Conclusion: We identified the research trends of exosomes in the field of aging through this bibliometric analysis. The present study provides valuable new perspectives on the history and current status of exosomes in the field of aging and aging-related diseases, and also offering guidance for future research directions.
Aging is an inevitable process that organisms spontaneously undergo over time, manifesting structural and functional degeneration, loss of adaptability and resistance. Concurrently, aging is an inevitable and complex phenomenon characterized by a variety of factors, including genomic instability, telomere shortening, epigenetic modifications, mitochondrial dysfunction, cellular senescence, alterations in intercellular communication, and inflammatory responses (1). Despite outstanding progress in the medical research of aging risk factors, aging-related diseases remain the leading cause affecting health issues in humans.
Exosomes are cell-secreted vesicles of a lipid bilayer membrane with a diameter about 30~150 nm (the average diameter is about 100 nm), and they are one of the important carriers for regulating intercellular communication. Furthermore, exosomes influence the senescence-associated secretory phenotype (SASP) in aging and aging-related diseases. Juan Antonio Fafian-Labora demonstrated that exosomes isolated from young fibroblasts reduce tissue damage by decreasing oxidative stress and lipid peroxidation (2). Another study showed that exosomes derived from bone marrow mesenchymal stem cells (MSCs) play a role in regulating nucleus pulposus cell senescence (3) and aging-related insulin resistance (IR) (4). Besides that, exosomes can also regulate the microenvironment and induce cellular senescence through autocrine or paracrine pathways. Hadi Valadi discovered that exosomes deliver mRNAs and miRNAs to new cells and play a functional role within these cells (5). Another study showed that exosomes spread to the surrounding environment and induce aging in young cells (6). What's more, miRNAs have been recognized as the biomarkers of certain diseases and pathological states, such as exosomal miR-24-3p in the saliva of elderly individuals (7). Therefore, it is necessary to explore the mechanisms of aging and prevention and treatment of diseases to analyze the relationship between exosomes and aging.
The bibliometrics emerged as an independent discipline in 1969 (8), which is a quantitative approach to the existing studies in a particular field and time period (9). CiteSpace describes the evolution of research fields and the historical development of clustering from a temporal perspective (10). VOSviewer provides network, overlay, and density, visualizations for keywords, and other parameters to build visual network maps (11–14). Bibliometrix allows for rapid integration and upgrading with other statistical and graphical tools in bibliometric analysis (15). Through bibliometric analysis, we can not only deeply explore the relationship between a thesis and its information, but also predict the development of a field. However, bibliometrics has not been used to investigate exosomes in aging. Therefore, in this study, we used bibliometric methods, searched the Web of Science Core Collection (WoSCC), and explored the hotspots and development trends of exosomes in the field of aging and drew a map of scientific knowledge in order to support intercellular communication-based aging research (Figure 1).
The literature was retrieved from WoSCC, which is regarded as the most appropriate database for high-quality digital bibliometric resources (16). All literature was downloaded in plain text format within 1 day, on December 18, 2023. The search themes were as follows: “TS = (aging OR senescence),” AND “TS = (exosomes),” AND “Reference Type: Article AND Review, AND Language: English,” and the retrieval date range was from January 1, 2007, to December 1, 2023. A bibliometric analysis was conducted on a total of 1,628 selected works of literature.
All WoSCC standards-compliant data was imported into VOSviewer 1.6.18, CiteSpace 6.2.R7 and Bibliometrix for literature visualization analysis. Bibliometrix shows the evaluation results for metadate in our data (Supplementary Figure 1). CiteSpace, VOSviewer and Bibliometrix were used to analyze the visual distribution of countries and regions, authors and co-cited authors, journals and co-cited journals, co-cited references, keywords cluster analysis, and timelines.
This research involved a total of 6698 keywords. In Table 1, miRNAs were the most frequently used keyword in exosome-related aging research, followed by biomarkers MSCs, mechanism, and microvesicles. MiRNAs and biomarkers had appeared more than 250 times among these keywords, indicating that these research fields were hotspots and might have substantial research potential. In Supplementary Figure 2, extracellular vesicles and miRNAs were closer to red, representing their higher density and indicating that the keyword was a research focus and hotspot in the field, while the other parts closer to blue indicated that the field was not currently receiving extensive attention. Figure 2A and Supplementary Table 1 depicted five different clusters representing five different directions. Figure 2B presented a timezone map categorizing keywords. The first stage was from 2007 to 2012, the primary keywords were cellular senescence, expression, apoptosis, biomarkers, and so on. The field of aging was beginning to look at exosome-related research, focusing on apoptosis, gene expression, RNA, and related diseases such as Alzheimer's disease (AD) and cancer. Most of the research themes generated during this period continue to be researched and advanced to this day. From 2013 to 2017, the second phase focused on MSCs, oxidative stress, inflammation, therapy. The regenerative ability of MSCs had received widespread attention, and the research on the mechanism of exosomes interfering with aging and aging-related diseases was continuously deepened. The third stage was from 2018 to 2023, mainly focusing on stromal cells, proliferation, Parkinson's disease, communication, transplantation, regenerative medicine. This stage was characterized by the intensification of research fields and the expansion of research topics. In recent years, several prominent research areas have emerged, notably osteoporosis, skeletal muscle, knee osteoarthritis, obstructive sleep apnea, and cell-free therapy.

Figure 2. (A) VOSviewer visualization map of keywords clustering analysis on exosomes in aging. (B) CiteSpace visualization map of timezone viewer related to exosomes in aging.
A total of 1,628 publications were obtained from the WoSCC, comprising 1,222 articles and 406 reviews. As depicted in Figure 3A, the fewest published articles were recorded in 2007, with only 1 article, whereas the highest count was 301 articles in 2022. From 2007 to 2012, research on exosomes in aging was in its early stages, with 30 articles. The second phase spanned from 2013 to 2017, during which 217 articles were published. The final stage spanned from 2018 to 2023, during which the annual number of articles consistently exceeded 100. From 2020 to 2023, the annual number of publications exceeded 200, indicating a substantial increase compared to that in 2019. In the past 6 years, 1,381 articles were published, accounting for 84.83% of the overall publications. This trend underscores a growing momentum in research activities related to the correlation between exosomes and the aging process.
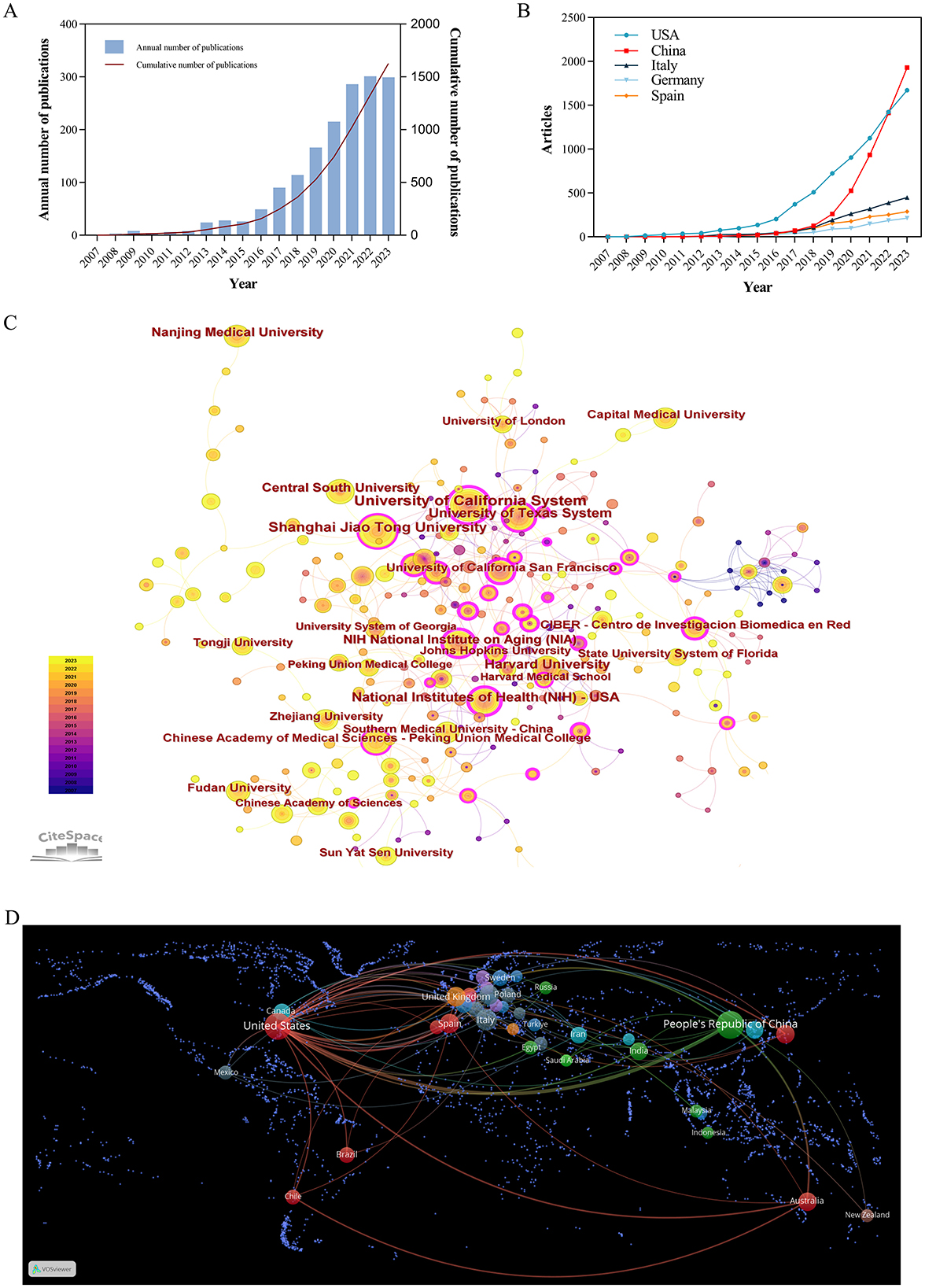
Figure 3. (A) Time trend of the publications on exosomes in aging from 2007 to 2023. (B) Distribution of publications from different countries. (C) CiteSpace visualization map of institutions involved in exosomes in aging. (D) Map of cooperation between countries.
This research encompassed a total of 2,321 institutions across 78 countries and regions. As shown in Figure 3B and Table 2 most articles came from China (524, 32.19%), and the US (490, 30.1%). The University of California System from the United States had emerged as the leading research institution with the highest number of published articles (54 articles, 3.32%), closely followed by Shanghai Jiao Tong University from China (49 articles, 3.01%). Among the top ten institutions based on the quantity of published articles, there are five institutions in the United States and China.
Betweenness centrality (BC) values above 0.1 indicate pivotal nodes. In Table 2, several countries exhibited a higher degree of centrality compared to others such as the United States (0.58), Italy (0.18), and France (0.18). The line between the circles indicated the cooperative relationship between countries and institutions. In Figures 3C, D, the United States had developed extensive cooperative relationship with the United Kingdom, Netherlands, Spain, Australia, Brazil, China, Japan, and Korea. Additionally, the University of California System maintained collaborative partnership with the US Department of Veterans Affairs, the University System of Georgia, the University of Miami, Veterans Health Administration (VHA), the University of Texas System.
However, most countries/regions and research institutions were not sufficiently focused and lacked stable and in-depth communication and collaboration. For example, in Figure 3B and Table 2, China had published 524 articles on exosomes in aging, but its Centrality is only 0.12. The United States had the second highest number of publications (490, 30.1%), but it had a high centrality (0.58). As shown in Figure 3D, Supplementary Figures 3, 4, the number of international cooperation nodes in the United States was significantly higher than that of China. Although China had initially formed a national cooperation network, there was still a need to continue to expand the scope and depth of international cooperation. Meanwhile, in Figure 4A, the number of MCP publications in the countries of the corresponding authors in the United States was more than that in China. Therefore, it was also important to emphasize international cooperation and shared research results to promote scientific progress while we focused on research.
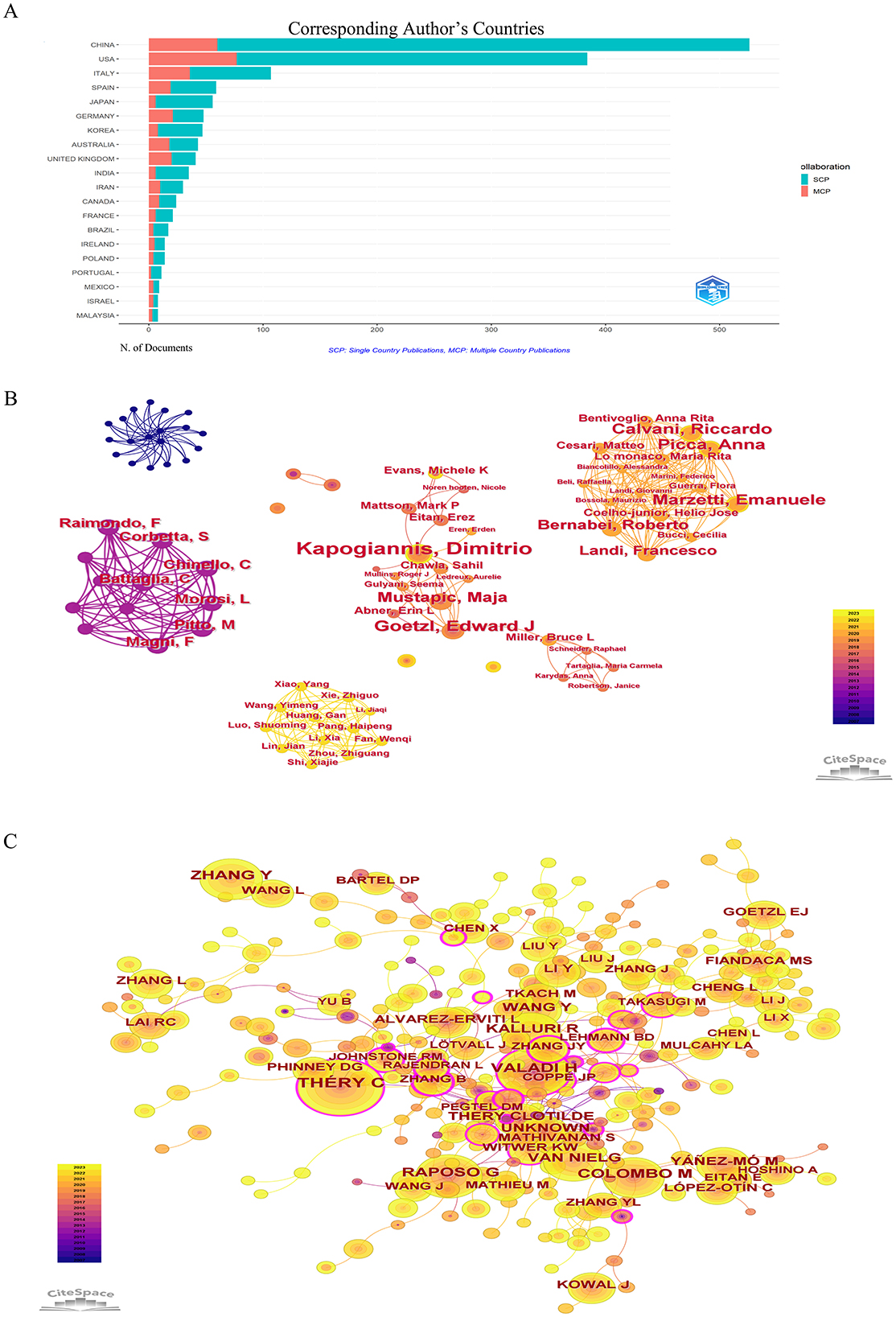
Figure 4. (A) Cooperation in the country where the corresponding author is located. (B) CiteSpace visualization map of authors involved in exosomes in aging. (C) Co-cited authors involved in exosomes in aging.
A total of 10,659 authors had contributed to articles on exosomes in aging. As shown in Table 3, Kapogiannis D was the most published author (17 articles, 1.04%), the second position was occupied by Goetzl E (11 articles, 0.68%). Each node represented an author, and the larger the node, the greater the number of articles published by that author. Thicker lines represented greater collaboration between authors. Differently colored connection nodes represented cooperative clusters among different authors. According to Figure 4B, authors established a network of communication and collaboration. Co-cited authors were two or more authors who were cited together in the same paper or in multiple articles (Figure 4C). As shown in Supplementary Table 2, Thery C (339, 0.18) was the most cited author, followed by Valadi H (230, 0.06), Raposo G (200, 0.05), and Zhang Y (179, 0).
A total of 697 academic journals had published articles on exosomes in aging. As shown in Table 4, International Journal of Molecular Sciences had published the highest number of articles (71 articles), followed by Cells (36 articles), Scientific Reports (31 articles), and Stem Cell Research & Therapy (29 articles). The journal with the highest impact factor among the top 10 was Journal of Extracellular Vesicles (IF: 15.5). In the co-cited journal of 6,738, 32 reference number more than 500 times, and 11 journals had been cited over 1,000 times. Table 4 revealed that the top 10 journals had been cited more than 1,000 times. Nature had the highest impact factor among the top 10 literature, followed by Cell (IF: 45.5), Nature Communications (IF: 14.7), and Journal of Extracellular Vesicles (IF: 16).
Through co-citation analysis, this study identified research hotspots that demonstrated the progression of a particular academic discipline. Figure 5 presented the top 50 references with the strongest citation bursts. It could be seen that the first reference to the literature began in 2009. The short duration of the citation literature suggested that this research field was poised for expansion and diversification in the coming years. Supplementary Table 3 showed the top 10 co-cited references. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells are the most frequently cited articles (233 times).
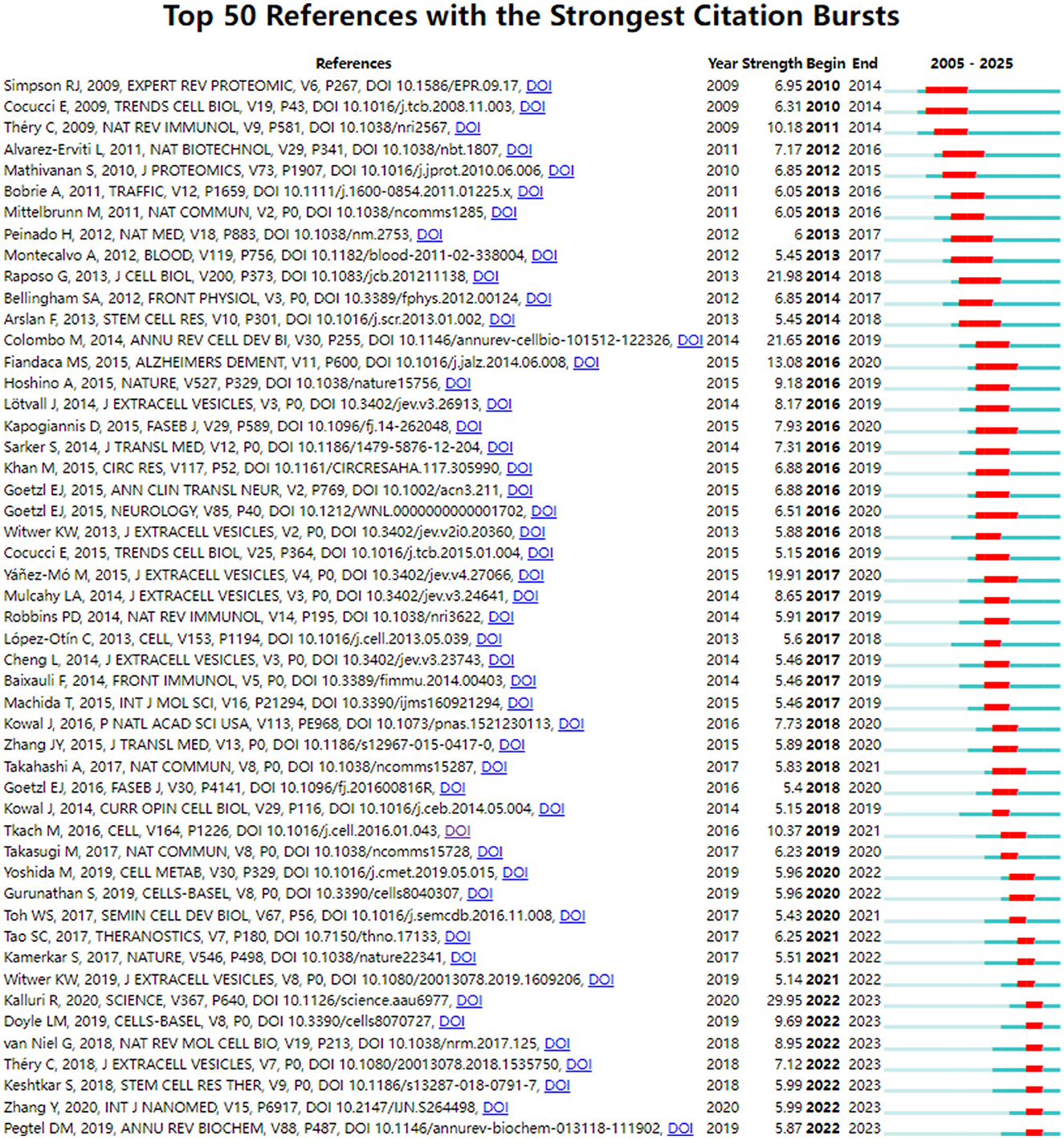
Figure 5. CiteSpace visualization map of top 50 references with the strongest citation bursts involved in exosomes in aging.
Bibliometrics is fundamentally based on empirical statistical principles, including but not limited to Bradford's Law (17), Lotka's Law (18), Zipf's Law (19), and Price's Law (20). Bibliometric analysis software, which adheres to established scientific principles, offers an efficient and dependable method that provide a foundation for the analysis of this study. Our study strictly rigorously adhered to the principles of scientific statistics and provided new insights into the research of exosomes in aging, examining trends, emerging areas, research focal points, and collaborations among countries, institutions, and authors.
As shown in Figure 5 and Supplementary Table 3, during the period from 2007 to 2012, the role of exosomes as a medium for intercellular communication was established, which was distinguished from shedding microvesicles and apoptotic blebs (21). mRNA and miRNA contained in exosomes are increasingly recognized as potential biomarkers for diseases (22). Théry C indicated that the combination of exosomes from different sources with immunotherapies in clinical diseases is an important direction in the future research (23). Some studies had found the therapeutic effects of exosomes on neurological disorders (24, 25) and tumor (26). Meanwhile, the microvesicles had been shown to be distinct from exosomes, and the expression and role in immune regulation, inflammatory diseases, and cancer were increasingly being studied (27). From 2013 to 2017, one of the most significant achievements was the formulation of an experimental standard for extracellular vesicles by the International Society for Extracellular Vesicles (28). This development established a recognized standard for the research related to extracellular vesicles, enabling greater experiment standardization and ensuring research quality in the rapidly advancing field of extracellular vesicles. In the study of AD, various neurogenic plasma-derived exosomes could be assessed not only to predict progression of disease before the onset of AD (29), but also to potentially evaluate the effectiveness of clinical drugs (30). The focus on tumor cells was also a research hotspot. Masaki Takasugi found that exosomes released from senescent cells promote tumor proliferation through EphA2 (31). At the same time, an increasing number of studies had emphasized the importance of focusing on the microenvironment where the subject was situated and observing intercellular communication mediated by exosomes (32). From 2018 to 2023, a large number of reviews focused on the classification of exosomes and techniques of isolation (33, 34), including ultracentrifugation, immuno-affinity purification, microfluidics-based isolation techniques, size-based filtration, size-exclusion chromatography, and polymer precipitation, among others. Several studies had also indicated that exosomes from young individuals had the potential to delay aging. At the same time, the therapeutic role of MSC-derived exosomes in disease was emphasized. However, the application of MSC-derived exosomes encountered similar challenges related to isolation, quality control, and the reproducibility of efficacy that needed to be addressed (35).
In Figure 2A and Table 1, miRNAs were the most frequently appearing keywords besides exosomes and senescence. These results suggested that the study of exosomal miRNAs was very important in aging. In fact, exosomal miRNAs were involved in many processes leading to aging (Table 5). Autophagy played a crucial role in the degradation of abnormal proteins and was essential for the preservation of protein homeostasis. In the upstream, exosomal miRNAs were involved not only in autophagy but also in loss of proteostasis. Endothelial colony-forming cell-derived exosomal miR-21-5p regulated autophagic flux to promote vascular endothelial repair in atherosclerosis (36), but the effect of exosomes-derived miRNAs on autophagy was controversial. Another study showed that exosomal miR-4645-5p derived from BMSCs enhanced cellular autophagy, proliferation, and migration in diabetic murine models (37). It had been established that exosomal miRNAs played a significant role in the process of autophagy in aging. The influence of exosomal miRNAs on cellular aging was contingent upon the origin of the exosomes. Exosomal miR-3200-3p, originating from VEGFR suppressed tumor cells, had the capacity to target DDB1, thereby promoting T cell senescence (38). However, exosomal miR-214-3p derived from senescent osteoblasts promoted the acceleration of endothelial cell senescence to aggravate the osteoporosis (39). Nevertheless, the involvement of exosomal miRNAs in the aging process might be influenced by a range of factors, including the tissue source, cell type, physiological and pathological conditions. Consequently, additional research was required to elucidate these complexities.
The phenomenon of elevated inflammatory markers in organisms and cells leads to the emergence of low levels of chronic pro-inflammatory states. Aging-related diseases are also exacerbated in inflammatory state, such as type 2 diabetes, cardiovascular diseases, and neurodegenerative diseases (50). Exosomes play a bi-directional role in regulating inflammation through intercellular communication, accelerating or delaying the process of aging and aging-associated diseases.
Exosomes regulate the initiation and progression of inflammation, particularly through exosomal miRNAs. For example, miR-19b, miR-21, miR-138, miR-146b, and miR-155 in endothelial cell-derived exosomes were closely related to vascular inflammation (51–54). It showed that IL-1β and TNF-α were increased in exosomes and contribute to the generation of inflammatory cascades (55). Based on these studies, it can be postulated that exosomes and exosomal miRNAs exacerbate cellular senescence and disease by influencing SASP-related inflammatory factors. In the same disease, exosomes from different sources exhibit varying effects on inflammation. Proinflammatory miRNAs of exosomes released by M1-type macrophages exacerbate myocardial injury in myocardial infarction mice (56). In contrast, exosomes from adipose-derived MSCs exhibit reduced levels of inflammatory markers as IL-1β, IL-6, TNF-α, and IFN-γ in patients with myocardial infarction (57). The beneficial anti-inflammatory effects in nervous system were obtained in exosomes and exosomal miRNAs, and these findings have significant implications for the management of neuroinflammatory diseases. Exosomes originating from human adipose-derived MSCs had been shown to suppress NF-κB and p38 mitogen-activated protein kinase, in addition to inhibiting the activation of microglia and macrophages (58). This mechanism is significant in mitigating neuroinflammation and facilitating functional recovery after brain injury. Moreover, exosomes derived from human adipose MSCs also alleviated microglia-mediated neuroinflammation through IRAK1/TRAF6 (59). Therefore, an effective way to delay aging and aging-related diseases is to fully utilize the anti-inflammatory effects of exosomes.
Cellular senescence is distinct from aging, characterized by the stable arrest of the cell cycle and concomitant alterations in morphology, structure, and function (60). The characteristics of cellular senescence can be observed at the early stage of embryonic development. Simultaneously, as the aging process progresses or diseases, senescent cells continue to accumulate within the body. Exosomes widely exist in almost all kinds of cells and affect the microenvironment through released miRNAs (61). It showed that exosomes and exosome-derived miRNAs exist a close relationship with the occurrence and progress of aging and aging-related diseases (62). It is important to mention that some of the exosome-derived miRNAs also have the potential to become the biomarkers of cellular senescence.
The study by Lehmann was significant because they found that aging of prostate cancer cells is closely linked to the role of P53 on exosomes in the extracellular environment (63). Numerous studies had shown that some exosomes and exosome-derived miRNAs accelerate cellular senescence. Exosomal miR-139-5p and senescent osteoblast-derived exosome-mediated miR-139-5p contributed to aging and cell apoptosis (64). In addition, inflammatory exosomes derived from senescent dendritic cells caused a large number of surrounding dendritic cells to aging through paracrine secretion (65). On the other hand, exosomes exhibit a delaying and inhibitory effects on cellular senescence. The mechanism of action was at least related to anti-inflammatory and anti-oxidative stress pathway. Dan Xu found that miR-22 was able to inhibit the development of breast cancer in mice, and this was attributed to miR-22 reactivating the cellular senescence program in cancer cells (66). MSCs-derived exosomes increased antioxidant capacity in senescent granulosa cells, thereby delaying cellular decline (67). Exosome-mediated adipose-derived MSCs enhanced the anti-inflammatory capacity of senescent cells in arthritis (68). Interestingly, exosomal miRNAs not only have the capacity to influence cellular senescence and aging-related diseases but also hold promises as biomarkers for SASP and aging-related diseases. For example, levels of exosomal miR-34a-5p and miR-183-5p increased with age (69, 70). Additionally, exosomal miR-24-3p in saliva from elderly patients showed the potential to serve as a biomarker of aging (7). Exosome-released miRNAs might serve as biomarkers to evaluate the quality of oocytes in the reproductive aging process (71).
Regenerative medicine aims to find effective biological therapeutic methods to promote self-repair and regeneration of the organism. However, the efficiency of cell and tissue transplantation limits the development of regenerative medicine. The emergence of the paracrine hypothesis provides an opportunity to address this issue and attract much attention in regenerative medicine, especially regarding exosomes. According to the results of the bibliometric analysis, we focused on the therapeutic value and application of exosomes in angiogenesis.
Exosomes play a role in angiogenesis in different types of cancers. Mao revealed that exosomal miR-141 induces angiogenesis, playing a pro-cancer role in small cell lung cancer (72). Exosomal miR-210 not only exhibited elevated expression but also induced angiogenesis in lung cancer, breast cancer, and hepatocellular carcinoma (73–75). Several research advances had made on exosomes and exosomal miRNAs in the treatment of tumors. The direct transfection of exosomal miRNA-340 derived from young bone marrow stromal cells restored the anti-angiogenic effect of exosomes from old bone marrow stromal cells in bone tumors (76). Exosomal miR-944 derived from glioma stem cells delayed glioma progression by exerting angiogenesis inhibition (77). However, the utilization of exosomes is insufficient. The effective approach may involve regulating angiogenesis by targeting exosomes and exosomal miRNAs using drugs, biomaterials, and other methods.
IR is a decrease in the body's efficiency in promoting glucose uptake and utilization by insulin, which is an important feature of diabetes mellitus. Immune aging is a manifestation of aging, and the process of immune aging affecting insulin metabolism is highly correlated with various SASP inflammatory factors (78).
Mitochondrial dysfunction represents a significant factor in the aging process; however, exosomes derived from macrophages had been shown to adversely affect mitochondrial function in murine models of type 2 diabetes (79). Li showed that miR-27-3p from M2-derived exosomes exacerbated the development of IR and diabetes through mitophagy (80). The different effects on IR may be related to the source of exosomes and the function of different macrophages. The exosomes from Natural killer cells improved IR, and the exosomal miR-1249-3p improved cellular insulin sensitivity (81). Su showed that the level of exosomal miR-29b-3p derived from bone marrow MSCs of aging mice was closely related to Sirt1, and inhibition the level of exosomal miR-29b-3p improved the IR associated with aging (4). Jalabert found that the changes in exosomal levels in mice might be partially closely related to PPARγ, resulting in modifications to IR (82). The AMP-activated protein kinase (AMPK) pathway enhanced insulin sensitivity by inhibiting antagonistic insulin signaling (83), and mammalian target of rapamycin (mTOR) was involved in glucose metabolism and angiogenesis (84). Therefore, exosomes derived from different cells play a crucial role in regulating IR by facilitating the transfer of nuclear materials or transmitting intercellular signals. This process ultimately alleviates IR-induced aging and aging-related diseases.
AD is one of the most common neurodegenerative diseases, and its incidence is increasing every year as the world's population ages. The nervous system is capable of producing and releasing exosomes, which contributes to synaptic plasticity, myelination, neurogenesis, and the regulation of neuroinflammation (85). Exosomes transmit information between neurons and neuronal-glial cells and play a role in the development of AD, leading to the accumulation of proteins such as amyloid beta (Aβ) and tau.
Extensive studies have been conducted on the treatment of AD with favorable results, such as stem cell-derived exosomes and exosomal miRNAs. MSC-secreted exosomes effectively reduced the expression of the inflammatory mediators TNF-α, IL-1β, and IL-6 in AD mice (86). Another study showed that MSCs-derived exsomes enhanced resistance to soluble oligomers of the Aβ-induced oxidative stress and synaptic damage in AD rats (87). The ability of MSCs to internalize soluble Aβ oligomers provides significant research directions and value to MSC-derived exosomes for the treatment of AD. Besides MSCs-derived exosomes, exosomes from other types of stem cells exhibit similar capabilities. Exosomal miRNAs derived from neuronal stem cells have been shown to enhance synaptic resistance to amyloid oligomers (88). The research on using exosomes as carriers for drug delivery is also increasing. For example, Ashok Iyaswamy ameliorated cognitive decline in AD mice using exosomes derived from hippocampal neuronal cells overexpressing Fe65 as a carrier for the targeted delivery of Corynoxine-B (89). Saliva-derived exosomes from AD patients had the potential to serve as new biomarkers for AD. The expression of neurogenic exosomes was elevated in the saliva of AD patients (90). Several clinical research has demonstrated changes in the expression of miRNAs in salivary-derived exosomes in AD patients, such as exosomal miR-342-3p and miRNA-485-3p (91, 92). Although exosomes in the cerebrospinal fluid of AD patients appear to be more sensitive, exosomes and exosomal miRNAs derived from saliva are more easily accessible. It is important to emphasize the role of exosomes from different sources for AD treatment, particularly from stem cells.
1. Currently, the most important research direction in exosomes is to find easier and more effective methods for exosome isolation and purification.
2. Exosomes have emerged as potential biomarkers for detecting and diagnosing certain aging-related diseases. Nonetheless, current research lacks systematicity and breakthrough findings. Future research should focus on enhancing the exploration of exosomes as biomarkers of aging, which can hold promise for effectively evaluating the aging process and the risk of aging-related diseases.
3. The current hot direction is the role of exosomes in the pathogenesis of aging-related diseases, such as neurodegenerative diseases, cardiovascular diseases, and tumors. It is indispensable to study the molecular mechanisms of exosomes production and release in aging and aging-related diseases. At the same time, researchers should also focus on the specific mechanisms of exosomes in intercellular communication and tissue microenvironment.
4. The utilization of exosomes as therapeutic drug delivery vehicles for the treatment of aging-related diseases represents a promising avenue for future research. However, the drug delivery efficiency of exosomes is poor at present. The combination of biomaterials and exosomes is one of the viable options to enhance the efficiency of exosomes therapy. In future research, we should pay more attention to the development and application of biomaterials combined with exosomes.
5. SASP plays a significant role in aging and aging-related diseases. However, it is uncertain whether exosomes play a therapeutic role in aging and aging-related diseases by intervening SASP. Therefore, it is necessary to strengthen the research on the synergistic effect of human exosomes and SASP to change the cellular microenvironment and affect the proliferation and differentiation of adjacent cells.
6. There is no definitive conclusion about the effects of exosomes on cellular senescence programs. In the future, the relationship between exosomes and aging processes such as telomere shortening, DNA damage, decreased mitochondrial function, and lipid metabolism should be further investigated.
7. Stem cell research is a significant focus in the field of regenerative medicine, and extracellular vesicles also possess regenerative capabilities similar to stem cells. To delay aging and treat aging-related diseases, it is necessary to optimize the regenerative capacity of extracellular vesicles from different sources, and to apply extracellular vesicle therapy in clinical settings.
This is the first study to perform a visual analysis of exosomes in aging. CiteSpace, VOSviewer and Bibliometrix were used for clustering in order to make the derived analysis data more intuitive. Through the bibliometric retrieval and data analysis of this study, researchers can efficiently comprehend the fundamental concepts, research basis, processes, hotspots, and trends on “exosomes in aging.” In addition, it can also provide a reference for scholars to search for literature, research topics, and journal submissions.
However, there are also limitations to our investigation. CiteSpace, VOSviewer, and Bibliometrix are only bibliometric analysis software and cannot completely replace systematic retrieval systems. Documents were retrieved from January 1, 2007, to December 18, 2023, and the article was written in English. Articles published in this area before 2007 and after 2024 were excluded from our analysis. Despite these limitations, we don't believe that they significantly impact the overall context and trend of “exosomes in aging.” Therefore, this study still holds reference value for subsequent research.
The research on exosomes in aging demonstrates a steadily rising trend, with increasing number of scholars, institutions, and countries joining the effort. They have consistently published high-quality research results in internationally influential journals and will continue to advance the development of this field. However, the research among different countries, institutions, and scholars is relatively isolated and lacks stable exchange and collaboration. The exchange and cooperation among countries, institutions, and scholars should be continuously strengthened. The exploration of this area should focus more on the fundamental and clinical transformations of the research. The current and future research is centered on investigating the role of exosomes in the process of aging and aging-related diseases, as well as exploring how interventions involving exosomes can potentially mitigate the progression of aging and associated conditions.
ZN: Writing – original draft, Conceptualization, Investigation. MC: Writing – original draft, Investigation. YF: Writing – original draft, Data curation, Investigation. LZ: Writing – original draft, Software, Formal analysis, Visualization. JW: Writing – original draft, Methodology, Supervision. YL: Writing – review & editing, Methodology. XF: Writing – review & editing, Visualization, Supervision. QW: Writing – review & editing, Formal analysis, Supervision, Visualization. JY: Writing – review & editing, Conceptualization, Supervision.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research is funded by the National Natural Science Foundation of China (Nos. 82074260 and 82104673) and the Fundamental Research Funds for the Central Public Welfare Research Institutes (Nos. CI2021A05021 and FZ2022001).
The authors are grateful to the Experimental Research Center, China Academy of Chinese Medical Sciences, the Beijing Key Laboratory of Research of Chinese Medicine on Prevention and Treatment for Major Diseases for their support of this work, and to the reviewers for allowing us to make improvements to the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1488536/full#supplementary-material
1. Aunan JR, Watson MM, Hagland HR, Søreide K. Molecular and biological hallmarks of ageing. Br J Surg. (2016) 103:e29–46. doi: 10.1002/bjs.10053
2. Fafián-Labora JA, Rodríguez-Navarro JA, O'Loghlen A. Small extracellular vesicles have GST activity and ameliorate senescence-related tissue damage. Cell Metab. (2020) 32:71–86.e5. doi: 10.1016/j.cmet.2020.06.004
3. Guan M, Liu C, Zheng Q, Chu G, Wang H, Jin J, et al. Exosome-laden injectable self-healing hydrogel based on quaternized chitosan and oxidized starch attenuates disc degeneration by suppressing nucleus pulposus senescence. Int J Biol Macromol. (2023) 232:123479. doi: 10.1016/j.ijbiomac.2023.123479
4. Su T, Xiao Y, Xiao Y, Guo Q, Li C, Huang Y, et al. Bone marrow mesenchymal stem cells-derived exosomal MiR-29b-3p regulates aging-associated insulin resistance. ACS Nano. (2019) 13:2450–62. doi: 10.1021/acsnano.8b09375
5. Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. (2007) 9:654–9. doi: 10.1038/ncb1596
6. Hamdan Y, Mazini L, Malka G. Exosomes and micro-RNAs in aging process. Biomedicines. (2021) 9:968. doi: 10.3390/biomedicines9080968
7. Machida T, Tomofuji T, Ekuni D, Maruyama T, Yoneda T, Kawabata Y, et al. MicroRNAs in salivary exosome as potential biomarkers of aging. Int J Mol Sci. (2015) 16:21294–309. doi: 10.3390/ijms160921294
9. Mayr P, Scharnhorst A. Scientometrics and information retrieval: weak-links revitalized [Editorial Material]. Scientometrics. (2015) 102:2193–9. doi: 10.1007/s11192-014-1484-3
10. Chen CM. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inform Sci Technol. (2006) 57:359–77. doi: 10.1002/asi.20317
11. Waltman L, van Eck NJ. A smart local moving algorithm for large-scale modularity-based community detection. Eur Phys J B. (2013) 86:1–14. doi: 10.1140/epjb/e2013-40829-0
12. van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. (2010) 84:523–38. doi: 10.1007/s11192-009-0146-3
13. Van Eck NJ, Waltman L. Text mining and visualization using VOSviewer. arXiv preprint arXiv:11092058 (2011).
14. Eck NJv, Waltman L. Visualizing bibliometric networks. In: Measuring scholarly impact: Methods and practice. Cham: Springer International Publishing (2014). p. 285–320. doi: 10.1007/978-3-319-10377-8_13
15. Aria M, Cuccurullo C. bibliometrix: an R-tool for comprehensive science mapping analysis. J Informetr. (2017) 11:959–75. doi: 10.1016/j.joi.2017.08.007
16. Ding X, Yang Z. Knowledge mapping of platform research: a visual analysis using VOSviewer and CiteSpace. Electronic Commer Res. (2022) 22:787–809. doi: 10.1007/s10660-020-09410-7
17. Bradford SC. Sources of information on specific subjects. J Inf Sci. (1985) 10:173–80. doi: 10.1177/016555158501000406
18. Lotka AJ. The frequency distribution of scientific productivity. J Washington Acad Sci. (1926) 16:317–23.
19. Zipf GK. Selected Studies of the Principle of Relative Frequency in Language. Cambridge, MA and London, England: Harvard University Press (1932).
20. Price DJDS. Little Science, Big Science. New York Chichester, West Sussex: Columbia University Press (1963). doi: 10.7312/pric91844
21. Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. (2010) 73:1907–20. doi: 10.1016/j.jprot.2010.06.006
22. Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics. (2009) 6:267–83. doi: 10.1586/epr.09.17
23. Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. (2009) 9:581–93. doi: 10.1038/nri2567
24. Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. (2011) 29:341–5. doi: 10.1038/nbt.1807
25. Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JM, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. (2012) 119:756–66. doi: 10.1182/blood-2011-02-338004
26. Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. (2012) 18:883–91. doi: 10.1038/nm.2753
27. Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. (2009) 19:43–51. doi: 10.1016/j.tcb.2008.11.003
28. Lötvall J, Hill AF, Hochberg F, Buzás EI, Di Vizio D, Gardiner C, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. (2014) 3:26913. doi: 10.3402/jev.v3.26913
29. Fiandaca MS, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz JB, et al. Identification of preclinical Alzheimer's disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case-control study. Alzheimers Dement. (2015) 11:600–7.e1. doi: 10.1016/j.jalz.2014.06.008
30. Kapogiannis D, Boxer A, Schwartz JB, Abner EL, Biragyn A, Masharani U, et al. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer's disease. FASEB J. (2015) 29:589–96. doi: 10.1096/fj.14-262048
31. Takasugi M, Okada R, Takahashi A, Virya Chen D, Watanabe S, Hara E. Small extracellular vesicles secreted from senescent cells promote cancer cell proliferation through EphA2. Nat Commun. (2017) 8:15729. doi: 10.1038/ncomms15728
32. Tkach M, Théry C. Communication by extracellular vesicles: where we are and where we need to go. Cell. (2016) 164:1226–1232. doi: 10.1016/j.cell.2016.01.043
33. Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomedicine. (2020) 15:6917–34. doi: 10.2147/IJN.S264498
34. Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. (2019) 8:307. doi: 10.3390/cells8040307
35. Witwer KW, Van Balkom BWM, Bruno S, Choo A, Dominici M, Gimona M, et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J Extracell Vesicles. (2019) 8:1609206. doi: 10.1080/20013078.2019.1609206
36. Ke X, Liao Z, Luo X, Chen JQ, Deng M, Huang Y, et al. Endothelial colony-forming cell-derived exosomal miR-21-5p regulates autophagic flux to promote vascular endothelial repair by inhibiting SIPL1A2 in atherosclerosis. Cell Commun Signal. (2022) 20:30. doi: 10.1186/s12964-022-00828-0
37. Shi Y, Wang S, Liu D, Wang Z, Zhu Y, Li J, et al. Exosomal miR-4645-5p from hypoxic bone marrow mesenchymal stem cells facilitates diabetic wound healing by restoring keratinocyte autophagy. Burns Trauma. (2024) 12:tkad058. doi: 10.1093/burnst/tkad058
38. Hui K, Dong C, Hu C, Li J, Yan D, Jiang X, et al. affects miR-3200-3p-mediated regulatory T cell senescence in tumour-derived exosomes in non-small cell lung cancer. Funct Integr Genomics. (2024) 24:31. doi: 10.1007/s10142-024-01305-2
39. Guo Z, Li J, Tan J, Sun S, Yan Q, Qin H. Exosomal miR-214-3p from senescent osteoblasts accelerates endothelial cell senescence. J Orthop Surg Res. (2023) 18:391. doi: 10.1186/s13018-023-03859-6
40. Zhou Y, Wang X, Song M, He Z, Cui G, Peng G, et al. secreted microRNA disrupts autophagy in distinct tissues of Caenorhabditis elegans upon ageing. Nat Commun. (2019) 10:4827. doi: 10.1038/s41467-019-12821-2
41. Li J, Tan J, Song Q, Yang X, Zhang X, Qin H, et al. Exosomal miR-767 from senescent endothelial-derived accelerating skin fibroblasts aging via inhibiting TAB1. J Mol Histol. (2023) 54:13–24. doi: 10.1007/s10735-022-10107-4
42. Yang S, Li A, Lv L, Zheng Z, Liu P, Min J, et al. Exosomal miRNA-146a-5p derived from senescent hepatocellular carcinoma cells promotes aging and inhibits aerobic glycolysis in liver cells via targeting IRF7. J Cancer. (2024) 15:4448–66. doi: 10.7150/jca.96500
43. Mensà E, Guescini M, Giuliani A, Bacalini MG, Ramini D, Corleone G, et al. Small extracellular vesicles deliver miR-21 and miR-217 as pro-senescence effectors to endothelial cells. J Extracell Vesicles. (2020) 9:1725285. doi: 10.1080/20013078.2020.1725285
44. Yin Q, Tang TT, Lu XY Ni WJ, Yin D, Zhang YL, Liu BC. Macrophage-derived exosomes promote telomere fragility and senescence in tubular epithelial cells by delivering miR-155. Cell Commun Signal. (2024) 22:357. doi: 10.1186/s12964-024-01708-5
45. Okada M, Kim HW, Matsu-ura K, Wang YG, Xu M, Ashraf M. Abrogation of age-induced MicroRNA-195 rejuvenates the senescent mesenchymal stem cells by reactivating telomerase stem. Cells. (2016) 34:148–59. doi: 10.1002/stem.2211
46. Bao Z, Li J, Cai J, Yao S, Yang N, Yang J, et al. Plasma-derived exosome miR-10a-5p promotes premature ovarian failure by target BDNF via the TrkB/Akt/mTOR signaling pathway. Int J Biol Macromol. (2024) 277:134195. doi: 10.1016/j.ijbiomac.2024.134195
47. Zhang Y, Huang X, Sun T, Shi L, Liu B, Hong Y, et al. MicroRNA-19b-3p dysfunction of mesenchymal stem cell-derived exosomes from patients with abdominal aortic aneurysm impairs therapeutic efficacy. J Nanobiotechnol. (2023) 21:135. doi: 10.1186/s12951-023-01894-3
48. Luo P, Chen X, Gao F, Xiang AP, Deng C, Xia K, et al. Human umbilical cord mesenchymal stem cell-derived exosomes rescue testicular aging. Biomedicines. (2024) 12:98. doi: 10.3390/biomedicines12010098
49. Dai J, Hu Z, Zeng F, Gong X, Tang H, Deng J, et al. Osteoclast-derived exosomal miR-212-3p suppressed the anabolism and accelerated the catabolism of chondrocytes in osteoarthritis by targeting TGF-β1/Smad2 signaling. Arch Biochem Biophys. (2024) 751:109827. doi: 10.1016/j.abb.2023.109827
50. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. (2014) 69:S4–9. doi: 10.1093/gerona/glu057
51. Gou L, Liu G, Ma R, Regmi A, Zeng T, Zheng J, et al. High fat-induced inflammation in vascular endothelium can be improved by Abelmoschus esculentus and metformin via increasing the expressions of miR-146a and miR-155. Nutr Metab. (2020) 17:35. doi: 10.1186/s12986-020-00459-7
52. Li C, Li S, Zhang F, Wu M, Liang H, Song J, et al. Endothelial microparticles-mediated transfer of microRNA-19b promotes atherosclerosis via activating perivascular adipose tissue inflammation in apoE(-/-) mice. Biochem Biophys Res Commun. (2018) 495:1922–9. doi: 10.1016/j.bbrc.2017.11.195
53. Zhang A, Wang G, Jia L, Su T, Zhang L. Exosome-mediated microRNA-138 and vascular endothelial growth factor in endometriosis through inflammation and apoptosis via the nuclear factor-κB signaling pathway. Int J Mol Med. (2019) 43:358–70. doi: 10.3892/ijmm.2018.3980
54. Zhou J, Wang KC, Wu W, Subramaniam S, Shyy JY, Chiu JJ Li JY, et al. MicroRNA-21 targets peroxisome proliferators-activated receptor-alpha in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proc Natl Acad Sci U S A. (2011) 108:10355–60. doi: 10.1073/pnas.1107052108
55. Zhao Y, Fu Y, Zou M, Sun Y, Yin X, Niu L, et al. Analysis of deep sequencing exosome-microRNA expression profile derived from CP-II reveals potential role of gga-miRNA-451 in inflammation. J Cell Mol Med. (2020) 24:6178–90. doi: 10.1111/jcmm.15244
56. Liu S, Chen J, Shi J, Zhou W, Wang L, Fang W, et al. M1-like macrophage-derived exosomes suppress angiogenesis and exacerbate cardiac dysfunction in a myocardial infarction microenvironment. Basic Res Cardiol. (2020) 115:22. doi: 10.1007/s00395-020-0781-7
57. Deng S, Zhou X, Ge Z, Song Y, Wang H, Liu X, et al. Exosomes from adipose-derived mesenchymal stem cells ameliorate cardiac damage after myocardial infarction by activating S1P/SK1/S1PR1 signaling and promoting macrophage M2 polarization. Int J Biochem Cell Biol. (2019) 114:105564. doi: 10.1016/j.biocel.2019.105564
58. Chen Y, Li J, Ma B, Li N, Wang S, Sun Z, et al. MSC-derived exosomes promote recovery from traumatic brain injury via microglia/macrophages in rat. Aging. (2020) 12:18274–96. doi: 10.18632/aging.103692
59. Zhang Z, Zou X, Zhang R, Xie Y, Feng Z, Li F, et al. Human umbilical cord mesenchymal stem cell-derived exosomal miR-146a-5p reduces microglial-mediated neuroinflammation via suppression of the IRAK1/TRAF6 signaling pathway after ischemic stroke. Aging. (2021) 13:3060–79. doi: 10.18632/aging.202466
60. Borghesan M, Hoogaars WMH, Varela-Eirin M, Talma N, Demaria M, A. senescence-centric view of aging: implications for longevity and disease. Trends Cell Biol. (2020) 30:777–91. doi: 10.1016/j.tcb.2020.07.002
61. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. (2020) 367:eaau6977. doi: 10.1126/science.aau6977
62. Mahbubfam S, Rezaie J, Nejati V. Crosstalk between exosomes signaling pathway and autophagy flux in senescent human endothelial cells. Tissue Cell. (2022) 76:101803. doi: 10.1016/j.tice.2022.101803
63. Beer L, Zimmermann M, Mitterbauer A, Ellinger A, Gruber F, Narzt MS, et al. Analysis of the secretome of apoptotic peripheral blood mononuclear cells: impact of released proteins and exosomes for tissue regeneration. Sci Rep. (2015) 5:16662. doi: 10.1038/srep16662
64. Lu Q, Qin H, Tan H, Wei C, Yang X, He J, et al. Senescence osteoblast-derived exosome-mediated mir-139-5p regulates endothelial cell functions. Biomed Res Int. (2021) 2021:5576023. doi: 10.1155/2021/5576023
65. Elsayed R, Elashiry M, Liu Y, El-Awady A, Hamrick M, Cutler CW. Porphyromonas gingivalis provokes exosome secretion and paracrine immune senescence in bystander dendritic cells. Front Cell Infect Microbiol. (2021) 11:669989. doi: 10.3389/fcimb.2021.669989
66. Xu D, Takeshita F, Hino Y, Fukunaga S, Kudo Y, Tamaki A, et al. miR-22 represses cancer progression by inducing cellular senescence. J Cell Biol. (2011) 193:409–24. doi: 10.1083/jcb.201010100
67. Xing J, Zhang M, Zhao S, Lu M, Lin L, Chen L, et al. EIF4A3-Induced Exosomal circLRRC8A alleviates granulosa cells senescence via the miR-125a-3p/NFE2L1 axis. Stem Cell Rev Rep. (2023) 19:1994–2012. doi: 10.1007/s12015-023-10564-8
68. Tofiño-Vian M, Guillén MI, Pérez Del Caz MD, Castejón MA, Alcaraz MJ. Extracellular vesicles from adipose-derived mesenchymal stem cells downregulate senescence features in osteoarthritic osteoblasts. Oxid Med Cell Longev. (2017) 2017:7197598. doi: 10.1155/2017/7197598
69. Davis C, Dukes A, Drewry M, Helwa I, Johnson MH, Isales CM, et al. MicroRNA-183-5p increases with age in bone-derived extracellular vesicles, suppresses bone marrow stromal (Stem) cell proliferation, and induces stem cell senescence. Tissue Eng Part A. (2017) 23:1231–40. doi: 10.1089/ten.tea.2016.0525
70. Fulzele S, Mendhe B, Khayrullin A, Johnson M, Kaiser H, Liu Y, et al. Muscle-derived miR-34a increases with age in circulating extracellular vesicles and induces senescence of bone marrow stem cells. Aging. (2019) 11:1791–803. doi: 10.18632/aging.101874
71. Zhang D, Lv J, Tang R, Feng Y, Zhao Y, Fei X, et al. Association of exosomal microRNAs in human ovarian follicular fluid with oocyte quality. Biochem Biophys Res Commun. (2021) 534:468–73. doi: 10.1016/j.bbrc.2020.11.058
72. Mao S, Lu Z, Zheng S, Zhang H, Zhang G, Wang F, et al. Exosomal miR-141 promotes tumor angiogenesis via KLF12 in small cell lung cancer. J Exper Clin Cancer Res. (2020) 39:193. doi: 10.1186/s13046-020-01680-1
73. Lin XJ, Fang JH, Yang XJ, Zhang C, Yuan Y, Zheng L, et al. Hepatocellular carcinoma cell-secreted exosomal MicroRNA-210 promotes angiogenesis in vitro and in vivo. Molec Ther Nucleic Acids. (2018) 11:243–52. doi: 10.1016/j.omtn.2018.02.014
74. Fan J, Xu G, Chang Z, Zhu L, Yao J. miR-210 transferred by lung cancer cell-derived exosomes may act as proangiogenic factor in cancer-associated fibroblasts by modulating JAK2/STAT3 pathway. Clin Sci. (2020) 134:807–25. doi: 10.1042/CS20200039
75. Kosaka N, Iguchi H, Hagiwara K, Yoshioka Y, Takeshita F, Ochiya T. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. J Biol Chem. (2013) 288:10849–59. doi: 10.1074/jbc.M112.446831
76. Umezu T, Imanishi S, Azuma K, Kobayashi C, Yoshizawa S, Ohyashiki K, et al. Replenishing exosomes from older bone marrow stromal cells with miR-340 inhibits myeloma-related angiogenesis. Blood Adv. (2017) 1:812–23. doi: 10.1182/bloodadvances.2016003251
77. Jiang J, Lu J, Wang X, Sun B, Liu X, Ding Y, et al. Glioma stem cell-derived exosomal miR-944 reduces glioma growth and angiogenesis by inhibiting AKT/ERK signaling. Aging. (2021) 13:19243–59. doi: 10.18632/aging.203243
78. Shimi G, Sohouli MH, Ghorbani A, Shakery A, Zand H. The interplay between obesity, immunosenescence, and insulin resistance. Immun Ageing. (2024) 21:13. doi: 10.1186/s12979-024-00414-7
79. Tian F, Tang P, Sun Z, Zhang R, Zhu D, He J, et al. miR-210 in exosomes derived from macrophages under high glucose promotes mouse diabetic obesity pathogenesis by suppressing NDUFA4 expression. J Diabetes Res. (2020) 2020:6894684. doi: 10.1155/2020/6894684
80. Li JM Li X, Chan LWC, Hu R, Zheng T, Li H, Yang S. Lipotoxicity-polarised macrophage-derived exosomes regulate mitochondrial fitness through Miro1-mediated mitophagy inhibition and contribute to type 2 diabetes development in mice. Diabetologia. (2023) 66:2368–86. doi: 10.1007/s00125-023-05992-7
81. Wang Y, Li M, Chen L, Bian H, Chen X, Zheng H, et al. Natural killer cell-derived exosomal miR-1249-3p attenuates insulin resistance and inflammation in mouse models of type 2 diabetes. Signal Transduct Target Ther. (2021) 6:409. doi: 10.1038/s41392-021-00805-y
82. Jalabert A, Reininger L, Berger E, Coute Y, Meugnier E, Forterre A, et al. Profiling of ob/ob mice skeletal muscle exosome-like vesicles demonstrates combined action of miRNAs, proteins and lipids to modulate lipid homeostasis in recipient cells. Sci Rep. (2021) 11:21626. doi: 10.1038/s41598-021-00983-3
83. Wang DH, He X, He Q. Combining use of Phillyrin and autophagy blocker alleviates laryngeal squamous cell carcinoma via AMPK/mTOR/p70S6K signaling. Biosci Rep. (2019) 39:BSR20190459. doi: 10.1042/BSR20190459
84. Wu WY, Yan H, Wang XB, Gui YZ, Gao F, Tang XL, et al. Sodium tanshinone IIA silate inhibits high glucose-induced vascular smooth muscle cell proliferation and migration through activation of AMP-activated protein kinase. PLoS ONE. (2014) 9:e94957. doi: 10.1371/journal.pone.0094957
85. Lizarraga-Valderrama LR, Sheridan GK. Extracellular vesicles and intercellular communication in the central nervous system. FEBS Lett. (2021) 595:1391–410. doi: 10.1002/1873-3468.14074
86. Zhang X, Hou X, Te L, Zhongsheng Z, Jiang J, Wu X. Mesenchymal stem cells and exosomes improve cognitive function in the aging brain by promoting neurogenesis. Front Aging Neurosci. (2022) 14:1010562. doi: 10.3389/fnagi.2022.1010562
87. de Godoy MA, Saraiva LM, de Carvalho LRP, Vasconcelos-Dos-Santos A, Beiral HJV, Ramos AB, et al. Mesenchymal stem cells and cell-derived extracellular vesicles protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-β oligomers. J Biol Chem. (2018) 293:1957–75. doi: 10.1074/jbc.M117.807180
88. Micci MA, Krishnan B, Bishop E, Zhang WR, Guptarak J, Grant A, et al. Hippocampal stem cells promotes synaptic resistance to the dysfunctional impact of amyloid beta oligomers via secreted exosomes. Mol Neurodegener. (2019) 14:25. doi: 10.1186/s13024-019-0322-8
89. Iyaswamy A, Thakur A, Guan XJ, Krishnamoorthi S, Fung TY, Lu K, et al. Fe65-engineered neuronal exosomes encapsulating corynoxine-B ameliorate cognition and pathology of Alzheimer's disease. Signal Transduct Target Ther. (2023) 8:404. doi: 10.1038/s41392-023-01657-4
90. Kurian TK, Banik S, Gopal D, Chakrabarti S, Mazumder N. Elucidating methods for isolation and quantification of exosomes: a review. Mol Biotechnol. (2021) 63:249–66. doi: 10.1007/s12033-021-00300-3
91. Lugli G, Cohen AM, Bennett DA, Shah RC, Fields CJ, Hernandez AG, et al. Plasma exosomal miRNAs in persons with and without Alzheimer disease: altered expression and prospects for biomarkers. PLoS ONE. (2015) 10:e0139233. doi: 10.1371/journal.pone.0139233
92. Ryu IS, Kim DH, Ro JY, Park BG, Kim SH, Im JY, et al. The microRNA-485-3p concentration in salivary exosome-enriched extracellular vesicles is related to amyloid β deposition in the brain of patients with Alzheimer's disease. Clin Biochem. (2023) 118:110603. doi: 10.1016/j.clinbiochem.2023.110603
Keywords: bibliometric, exosomes, aging, senescence, aging-related diseases
Citation: Niu Z, Cui M, Fu Y, Zhou L, Wang J, Lei Y, Fan X, Wang Q and Yang J (2025) A bibliometric analysis of exosomes in aging from 2007 to 2023. Front. Med. 11:1488536. doi: 10.3389/fmed.2024.1488536
Received: 30 August 2024; Accepted: 04 November 2024;
Published: 22 January 2025.
Edited by:
Rabeea Siddique, Second Affiliated Hospital of Zhengzhou University, ChinaReviewed by:
Changqing Deng, Hunan University of Chinese Medicine, ChinaCopyright © 2025 Niu, Cui, Fu, Zhou, Wang, Lei, Fan, Wang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Wang, d3F3aW4yMUAxNjMuY29t; Jing Yang, eWFuZ2ppbmdkckAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.