- 1Department of Hepatobiliary Surgery, Mianyang Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Mianyang, China
- 2NHC Key Laboratory of Nuclear Technology Medical Transformation, Mianyang Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Mianyang, China
- 3The Central Health Center of Longchi Town, Dujiangyan, China
- 4Department of Urology, Mianyang Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Mianyang, China
Background: The aim of this study is to evaluate the association between triglyceride glucose-waist height ratio (TyG-WHtR) index and the prevalence of gallstone disease (GSD), alongside the age at first gallstone surgery among adult populations within the United States.
Methods: We screened participants using the National Health and Nutrition Examination Survey (NHANES). Logistic regression analysis, generalized additive modeling, smoothed curve fitting, and subgroup analysis were employed to assess the association between the TyG-WHtR index, prevalence of GSD, and the age at initial gallstone surgical intervention.
Results: In this study, 3,728 participants were enrolled, among whom 395 individuals reported a prior history of GSD. The association between the TyG-WHtR index and the prevalence of GSD demonstrated a non-linear, positive association. After adjusting for all potential confounders, for each incremental unit rise in the TyG-WHtR index, there was a 47% escalation in the prevalence of GSD (OR = 1.47, 95% CI: 1.29, 1.68). Subgroup analyses indicated a more pronounced association between the TyG-WHtR index and the prevalence of GSD among individuals aged 20–80 years, females, non-Hispanic white population, non-Hispanic black population, other racial groups, and non-diabetic cohorts. Additionally, this study identified that the TyG-WHtR index may be negatively correlated with age at first surgical treatment of gallstones.
Conclusion: An elevated TyG-WHtR index demonstrates a positive association with the prevalence of GSD. However, more prospective studies are needed to validate our findings.
1 Introduction
Gallstone disease (GSD) stands as a prevalent affliction within the global spectrum of digestive system disorders and constitutes a recognized risk element for gallbladder cancer (1). Epidemiological surveys have revealed that the incidence of GSD varies considerably between regions and ethnicities. For instance, the prevalence of GSD is higher in the United States and Europe than in Asia (2). Within the United States, the occurrence rate of GSD ranges from approximately 10–15% among Caucasians to as high as 70% among individuals of Indian descent (3). About 75% of patients with GSD have no obvious clinical symptoms during the initial phases of the condition and are often diagnosed during physical health examinations (4). Typical clinical symptoms of GSD include epigastric pain, nausea, vomiting, and anorexia triggered by fatty foods. A small number of patients develop acute cholangitis, acute pancreatitis, or infectious shock due to stones entering the common bile duct (5).
Although significant progress has been made in the early detection and management, the precise etiology of GSD remains incompletely understood. Factors such as age, sex, nutrition, environment, genetics, cholesterol metabolism disorders, gallbladder dysfunction, and gut microorganisms are involved in the pathogenesis of GSD (4, 6, 7). Notably, studies have increasingly highlighted the role of metabolic disorders and obesity in the pathogenesis of GSD (8–10). Within this array of factors, insulin resistance (IR) has surfaced as a pivotal element in the complex interplay between metabolic dysregulation and associated pathologies. The triglyceride-glucose (TyG) index serves as a reliable surrogate indicator of IR and has been validated as a useful tool for evaluating conditions such as diabetes mellitus, non-alcoholic fatty liver disease, and cardiovascular disease (11–13). However, the TyG index fails to account for changes in adipose tissue distribution, especially in central obesity, which is strongly associated with metabolic abnormalities. The waist-to-height ratio (WHtR) is widely used as a simple visual measure to evaluate adipose tissue distribution and central obesity (14). By integrating the TyG index and the WHtR, the TyG-WHtR index can provide a more comprehensive assessment of IR, metabolic disorders, and adipose tissue distribution. Xuan et al. demonstrated that the TyG-WHtR index outperforms the TyG index and other markers in identifying individuals at risk for type 2 diabetes mellitus (15).
However, despite evidence that metabolic disorders and obesity are inextricably linked to the development of GSD, reliable indicators for predicting and assessing the risk of GSD are still lacking. Therefore, the aim of this study was to assess the significance of the TyG-WHtR index in the development of GSD.
2 Methods
2.1 Source of data and study population
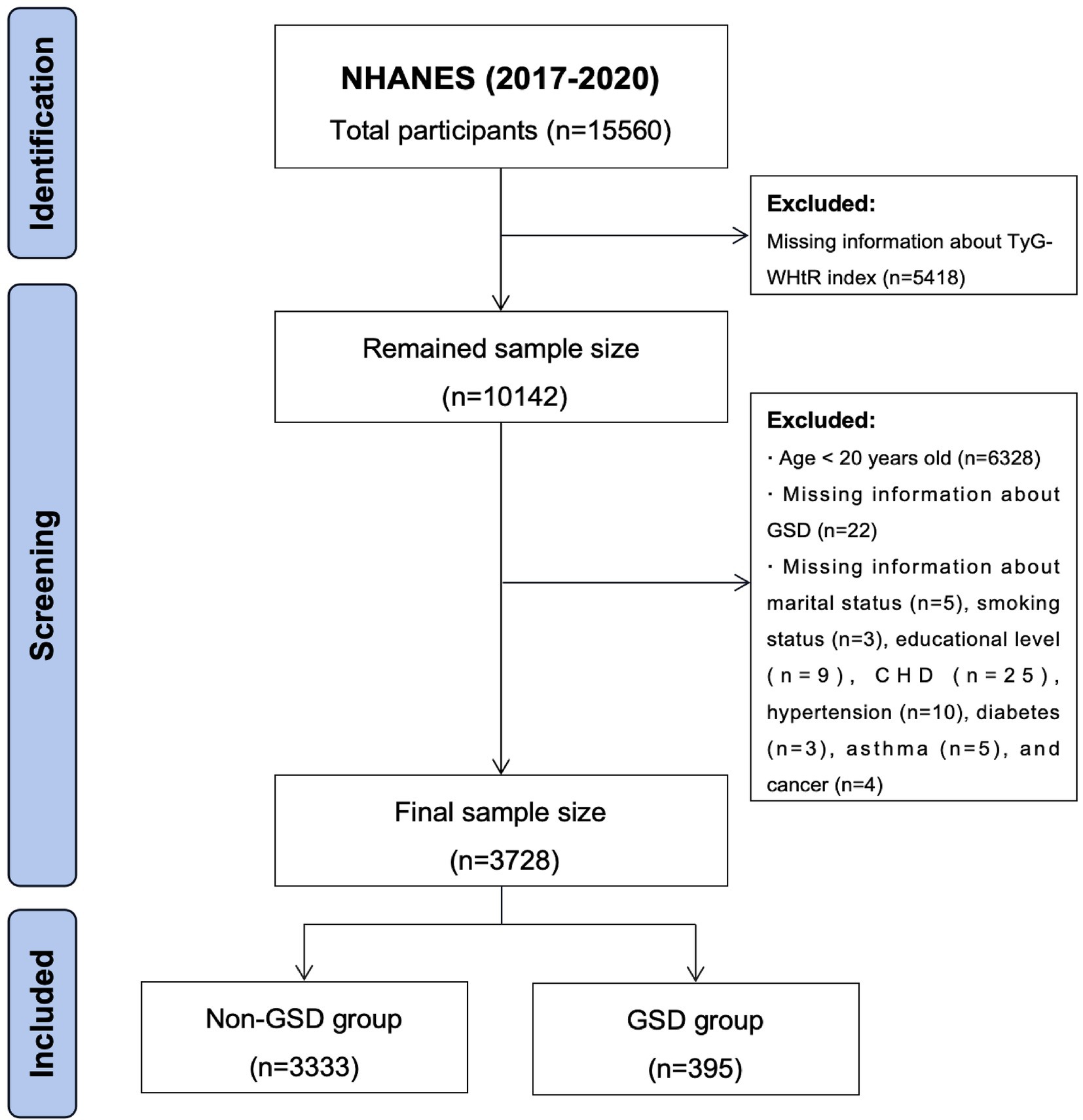
All data utilized in this study were obtained from the National Health and Nutrition Examination Survey (NHANES), an open-source database officially maintained by the Centers for Disease Control and Prevention (CDC). The NHANES undergoes biennial updates and typically encompasses a population of around 10,000 individuals per cycle. Due to the prevalence of COVID-19, the NHANES program was suspended in March 2020. Information on GSD was available only from 2017 to March 2020, so data from this period were used for the study. Since the questionnaires on GSD were only available for adults aged 20 years and older, participants younger than 20 years were excluded from the study. The detailed exclusion criteria were established based on the study objectives (Figure 1). Ultimately, 3,728 individuals were included in this study, of whom 395 had a history of GSD.
2.2 Data collection and parameter definition
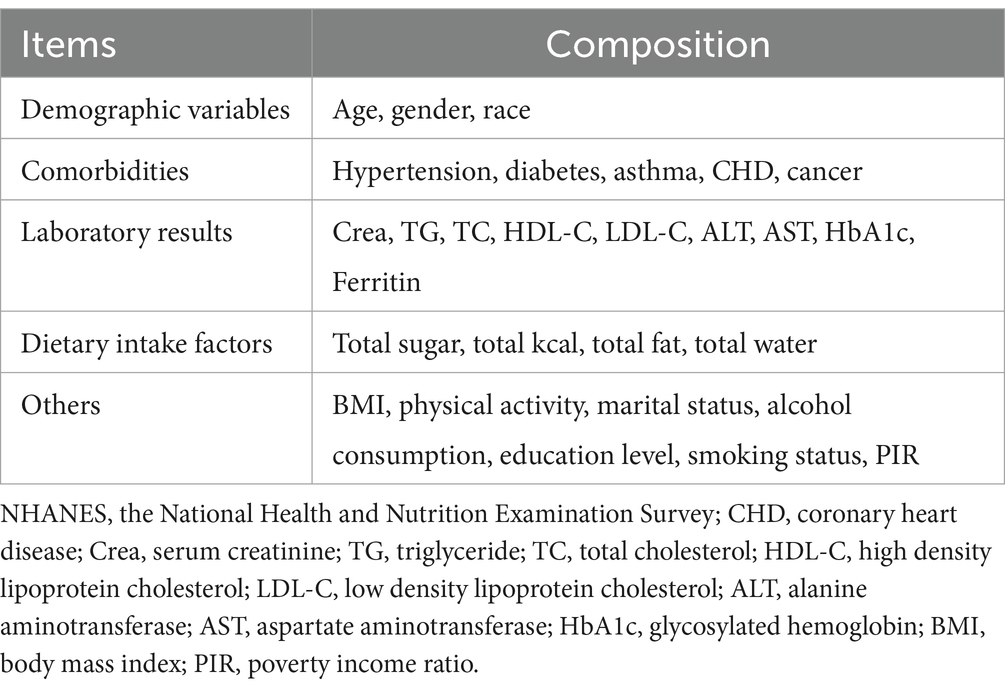
Questionnaires were employed to ascertain the presence of GSD among participants and to document their age at the initial gallbladder surgery for GSD. The covariates included in this study comprised five main components: demographic variables, comorbidities, dietary intake factors, laboratory results, and others (Table 1). TyG-WHtR index served as the independent variable of interest. The TyG index = Ln (fasting triglycerides [mg/dL] × fasting glucose [mg/dL]/2) (16). The WHtR was defined as waist circumference (WC) divided by height (17). The TyG-WHtR index was computed using the formula: TyG-WHtR index = TyG index × WHtR (18).
All subjects enrolled in this study underwent a 24-h dietary recall process; thus, the mean intake derived from the two recalls was utilized for the analytical procedures. Detailed measurement procedures for all variables are available on the official NHANES website at www.cdc.gov/nchs/nhanes/. All procedures in compliance with the U.S. Department of Health and Human Services (HHS) Policy for the Protection of Human Research Subjects were followed throughout the NHANES protocols. These protocols are subject to annual review and standardization by the NCHS Research Ethics Review Board. All individuals participating in the NHANES study provided written informed consent; therefore, no further permissions or ethical reviews were necessary.
2.3 Outcome indicators
In this study, the prevalence of GSD and the age of patients at the time of their initial gallbladder surgery for GSD were considered as the outcome variables.
2.4 Statistical analysis
For normally distributed data, continuous variables were presented as mean ± standard deviation (SD), while for skewed data, continuous variables were described as median (interquartile range [IQR]). Categorical variables were represented as counts and percentages. Comparisons of continuous variables were conducted using t-tests or one-way ANOVA, whereas Pearson’s χ2 test and Fisher’s exact test were employed for comparisons of categorical variables. In accordance with established protocols, multivariate logistic regression models were employed to examine the association among the TyG-WHtR index, various TyG-WHtR tertiles, and the prevalence of GSD across diverse models. Model 1 served as the baseline model without any covariate adjustments. Model 2 was adjusted for age, sex, race, and marital status. Model 3 included adjustments for all potential confounders. The relationship between the TyG-WHtR index and the prevalence of GSD, as well as the age at initial gallstone surgery, was additionally assessed through generalized additive model (GAM) regression and smoothed curve fitting employing the penalized spline method. Upon identification of a non-linear relationship, a natural spline test was utilized to determine the values of inflection points. Subgroup analyses were conducted considering age, sex, race, body mass index (BMI), hypertension, and diabetes. Statistical significance was defined as p < 0.05 in this study. All analyses were performed using R version 4.0.2 (http://www.R-project.org; R Foundation for Statistical Computing) and Empower (www.empowerstats.com; X&Y Solutions Inc., Boston, MA, United States).
3 Results
3.1 Baseline characteristics of the study population
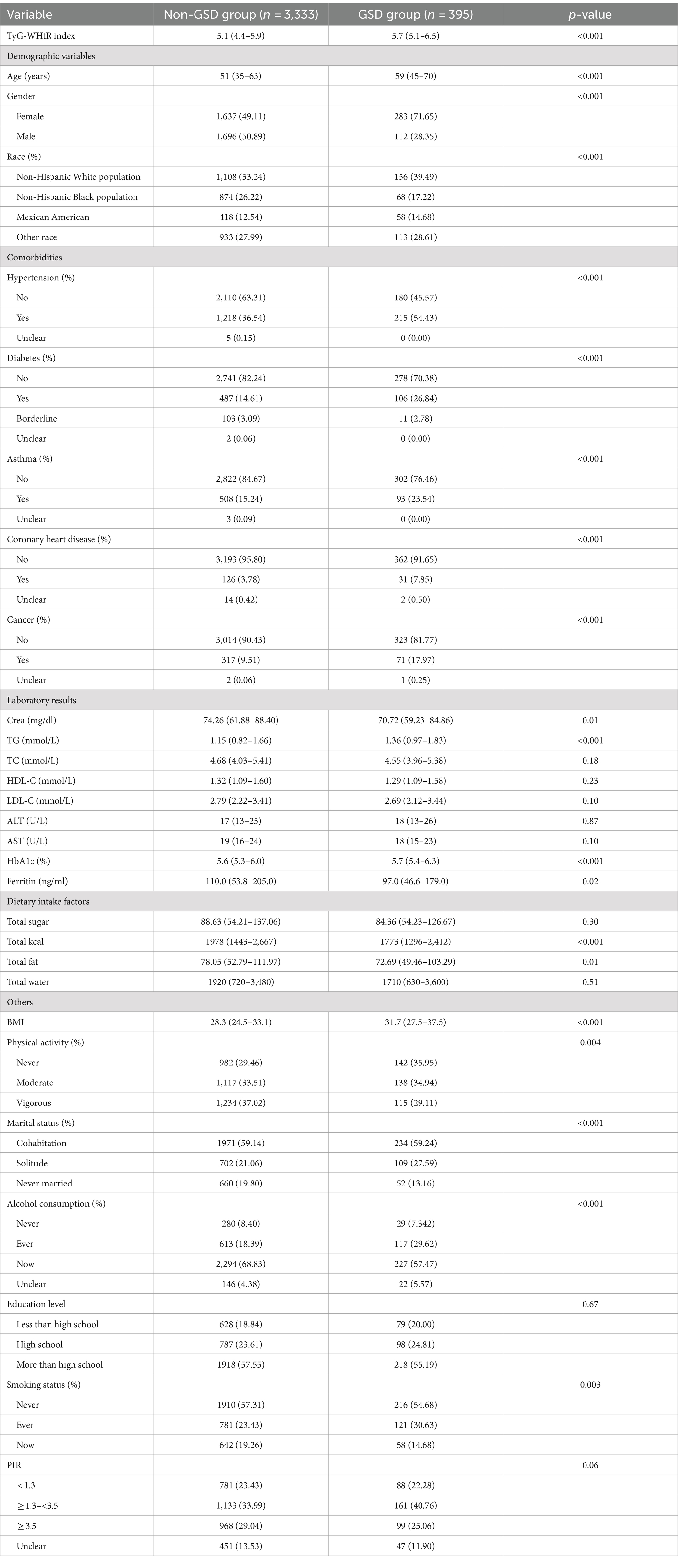
A total of 3,728 individuals were enrolled in this study, among whom 395 were identified as individuals with GSD based on self-reporting. Table 2 presents the baseline demographics of the enrolled participants in the study. The TyG-WHtR index was 5.1 (4.4–5.9) in non-GSD participants and 5.7 (5.1–6.5) in participants with GSD, and the disparity between the two cohorts was found to be statistically significant ( p < 0.001). Moreover, notable distinctions were observed between the two cohorts concerning age, sex, race, comorbidities, serum creatinine, triglycerides, glycosylated hemoglobin, BMI, physical activity, marital status, alcohol consumption, and smoking status (all p < 0.05).
3.2 Logistic regression results between the TyG-WHtR index and GSD
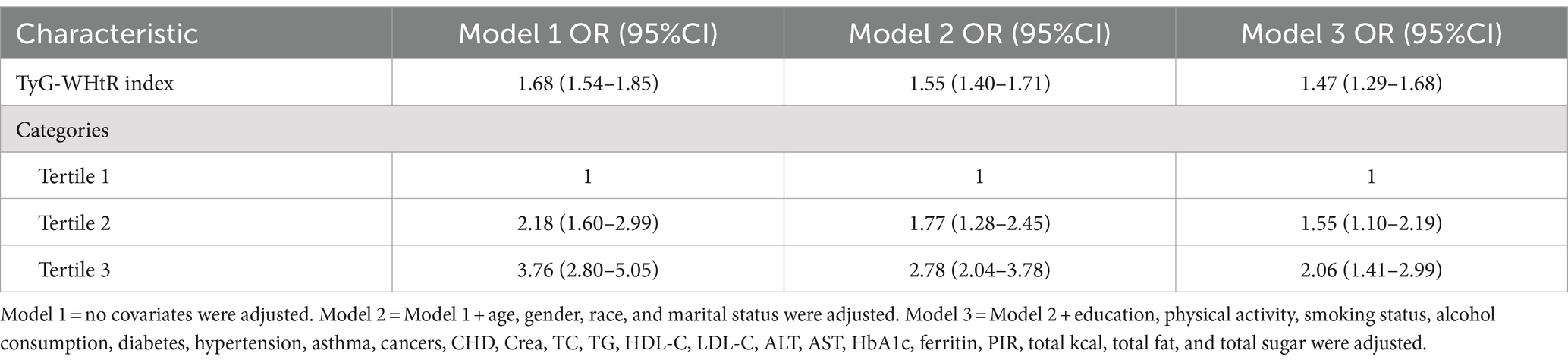
The logistic regression analysis unveiled a direct relationship between the TyG-WHtR index and the prevalence of GSD (Table 3). This positive association persisted in the fully adjusted model (Model 3) (OR = 1.47, 95% CI: 1.29, 1.68). These results suggest that for every 1-unit increase in the TyG-WHtR index, the prevalence of GSD increases by 47%. Furthermore, when the TyG-WHtR index was converted from a continuous variable to a categorical variable (tertiles) for sensitivity analysis, there was a 1.06-fold rise in the prevalence of GSD in Tertile 3 (OR = 2.06, 95% CI: 1.41, 2.99) compared to Tertile 1, the lowest TyG-WHtR index tertile.
3.3 Dose–response and threshold effects of the TyG-WHtR index on GSD prevalence
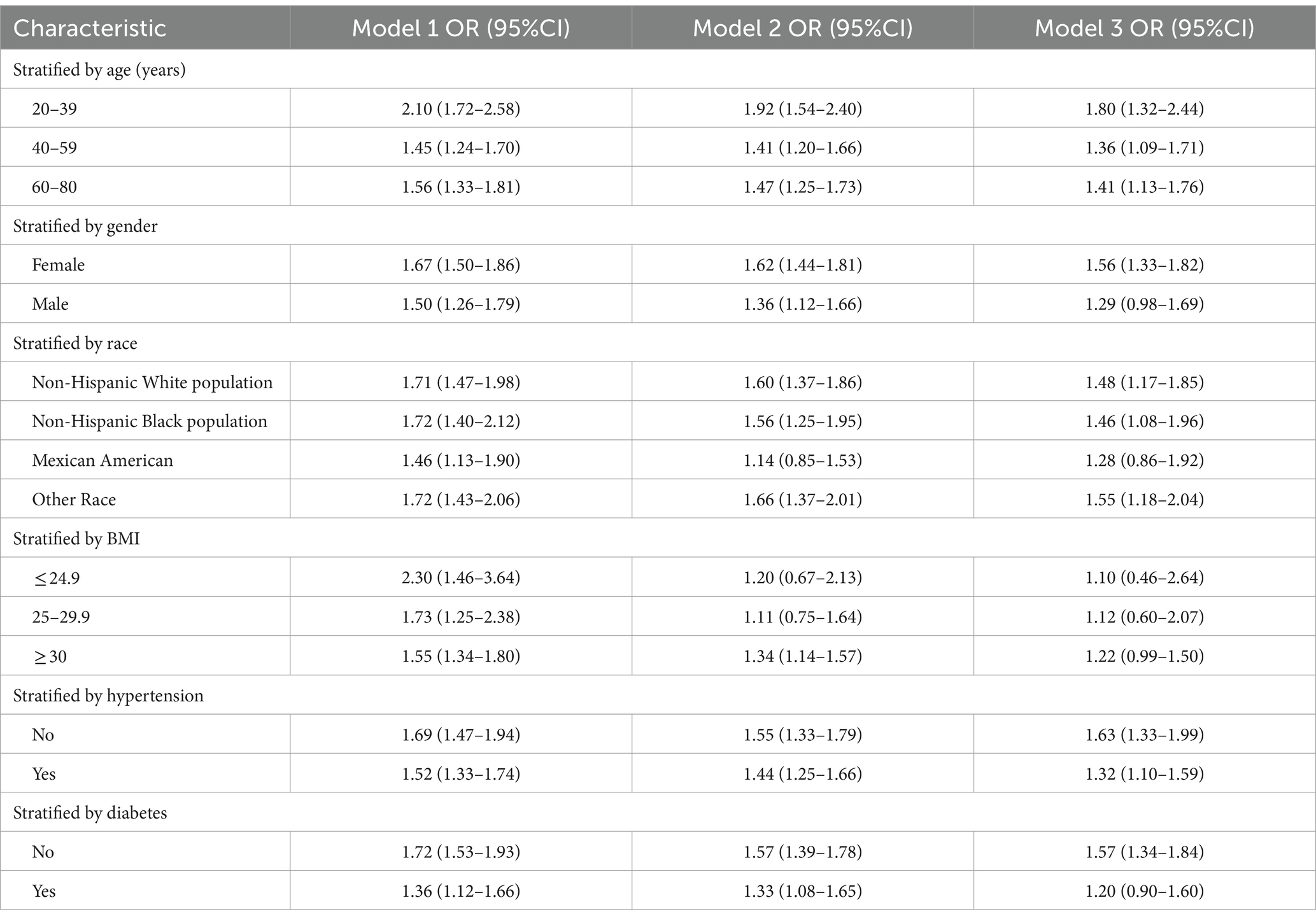
Generalized additive modeling and smooth curve fitting were employed to further investigate the association between the TyG-WHtR index and the prevalence of GSD. Figure 2 illustrates the non-linear association (positive) between the TyG-WHtR index and the prevalence of GSD. Taking into account the saturation threshold effect, the likelihood ratio test indicated that the optimal threshold for the TyG-WHtR was 4.15 (Table 4).
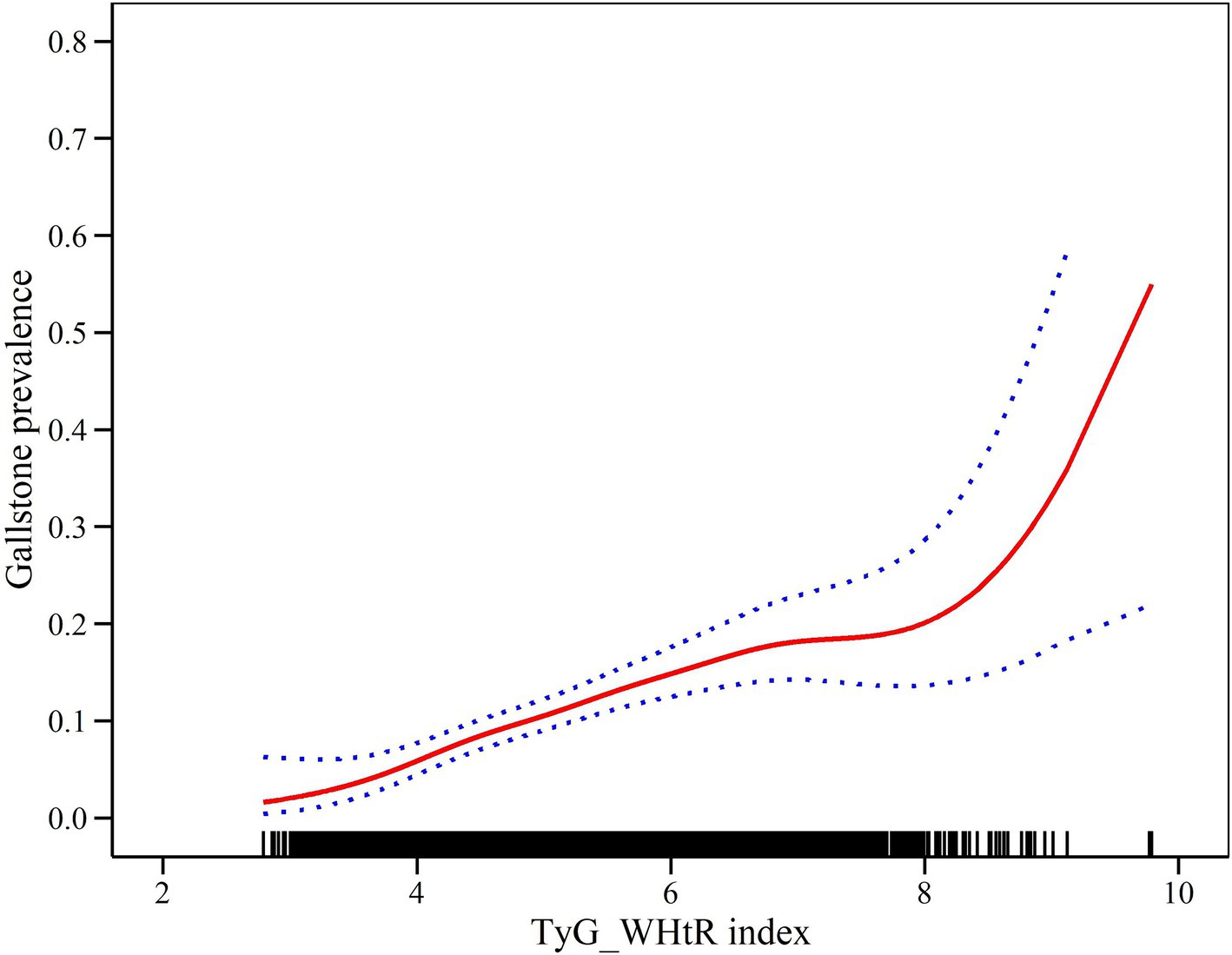
Figure 2. Density dose–response relationship between TyG-WHtR index and the prevalence of GSD. The area between the upper and lower dashed lines represents the 95% CI.
Table 4. Two-piecewise linear regression and logarithmic likelihood ratio test explained the threshold effect analysis of TyG-WHtR index with GSD prevalence.
3.4 Subgroup analysis
To reinforce the validity of the findings, a subgroup analysis was undertaken. When adjusted for all potential confounders, we found that the association between TyG-WHtR index and GSD prevalence was more significant in these populations: Age group 20–39 years (OR = 1.80, 95% CI: 1.32, 2.44); Age group 40–59 years (OR = 1. 36, 95% CI: 1.09, 1.71); Age group 60–80 years (OR = 1.41, 95% CI: 1.13, 1.76); Female group (OR = 1.56, 95% CI: 1.33, 1.82); Non-Hispanic white population (OR = 1.48, 95% CI: 1.17, 1.85); Non-Hispanic black population (OR = 1.46, 95% CI: 1.08, 1.96); Other race group (OR = 1.55, 95% CI: 1.18, 2.04); Non-hypertensive population group (OR = 1.63, 95% CI: 1.33, 1.99); Hypertensive population group (OR = 1.32, 95% CI: 1.10, 1.59); Non-diabetic population group (OR = 1.57, 95% CI: 1.34, 1.84). More details of the results are presented in Table 5.
3.5 Density dose–response relationship between TyG-WHtR index and age at first gallstone surgery
In order to delve deeper into the association between the TyG-WHtR index and the age of initial gallstone surgery, we employed generalized additive modeling along with smoothed curve fitting techniques. Figure 3 shows that the TyG-WHtR index may be negatively correlated with age at first gallstone surgery.
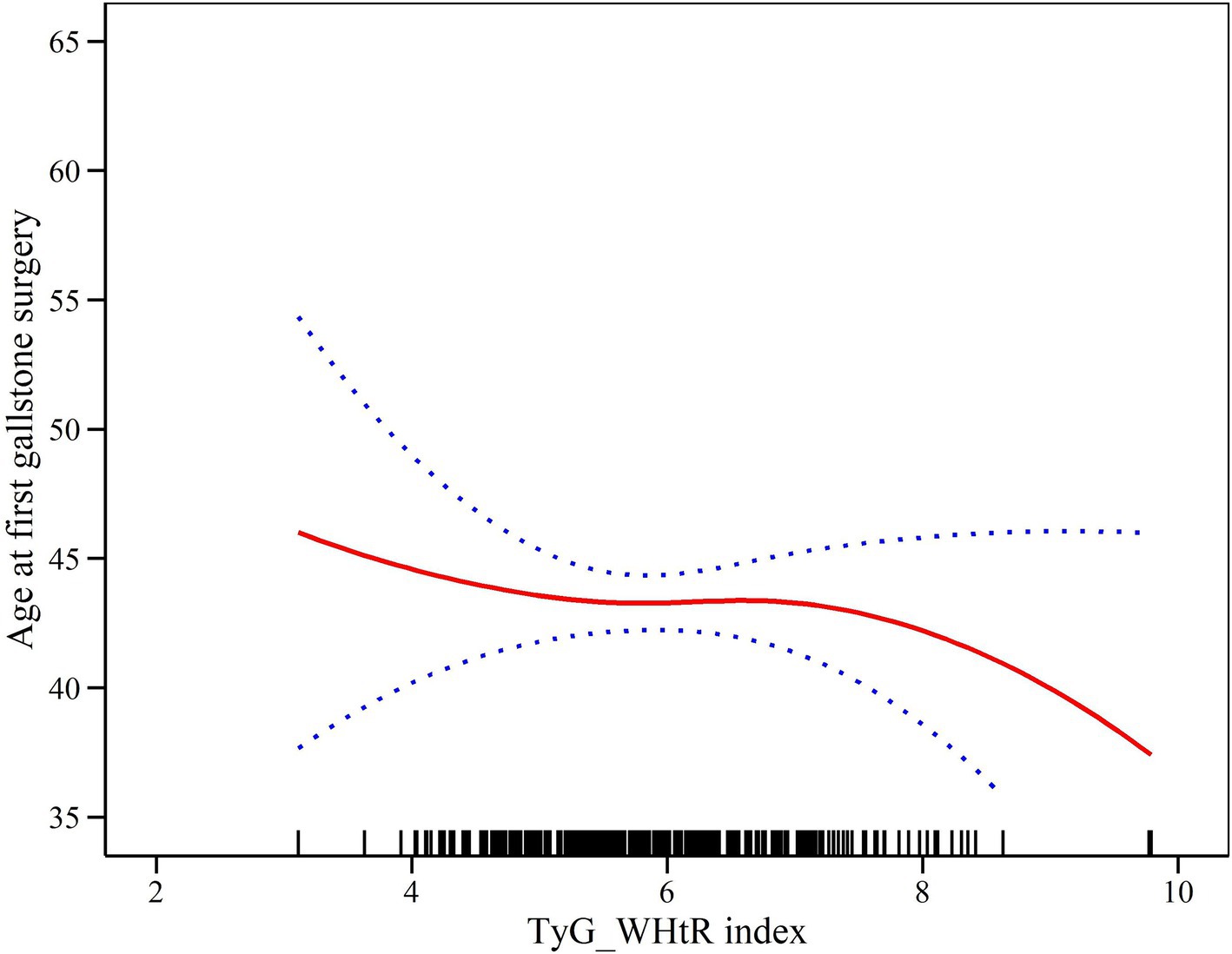
Figure 3. Density dose–response relationship between TyG-WHtR index and age at first gallstone surgery. The area between the upper and lower dashed lines represents the 95% CI.
4 Discussion
This study represents the inaugural comprehensive analysis exploring the interplay among the TyG-WHtR index, the incidence of GSD, and the age of initial gallstone surgical intervention. We observed a non-linear positive association between the TyG-WHtR index and the incidence of GSD, suggesting that for each 1-unit rise in the TyG-WHtR index, the prevalence of GSD escalated by 47%. Moreover, upon categorizing the TyG-WHtR index from a continuous to a categorical format (tertiles), we observed that individuals in the highest TyG-WHtR index tertile exhibited a 1.06-fold higher likelihood of GSD incidence compared to those in the lowest tertile. Subgroup analyses indicated that the association between the TyG-WHtR index and the prevalence of GSD exhibited greater significance among individuals aged 20–80 years, females, non-Hispanic white population, non-Hispanic black population, other racial groups, and non-diabetic populations. Additionally, we found that an increased TyG-WHtR index was associated with an earlier age at first gallstone surgery.
The elevated prevalence of GSD presents a substantial public health concern on a global scale, with 1.5 million individuals in the United States alone seeking medical assistance for GSD in 2015 (19). As mentioned in the introduction, GSD pathogenesis is influenced by various factors. The incidence of GSD differs by sex, with females generally exhibiting a higher prevalence than males (20). This disparity is primarily attributed to hormone levels, particularly the biological effects of estrogen, which plays a pivotal role (21, 22). Estrogen can increase cholesterol deposition in bile, thereby promoting gallstone crystallization (22). Additionally, estrogen can affect gallbladder contractility, leading to prolonged bile retention, thereby increasing the risk of stone formation (21). Moreover, estrogen can alter the composition of bile constituents, further increasing the risk of stone development (21). Our results also confirm a higher prevalence of GSD in females compared to males. Most existing studies identify advanced age as a contributing risk factor for the onset of GSD (23). Nonetheless, a growing body of literature indicates ongoing debate regarding the classification of age as a significant risk factor for GSD. According to a recent study, influences such as obesity and disorders in cholesterol metabolism exert a more pronounced influence on the prevalence of GSD, particularly among younger populations (24). Genetic factors also influence GSD pathogenesis, with Caucasians generally exhibiting a higher prevalence of GSD compared to non-Hispanic black population (3). For example, in the United States, the prevalence among non-Hispanic white women is 16.6% compared to 8.6% in men, and among non-Hispanic black women, it is 13.9% compared to 5.3% in men (9). These differences may be attributed to a wide range of factors such as genetics, nutrition, lifestyle, and environment among different ethnic groups. For instance, Caucasians have greater access to higher-quality diets compared to most non-Hispanic black population, which may contribute to their higher GSD incidence, along with higher calorie intake and lifestyles (25, 26).
The contribution of metabolic abnormalities (such as IR, diabetes, and obesity) to the development of GSD is increasingly recognized in medical research. Studies have demonstrated a strong association between metabolic syndrome and the incidence of GSD (5, 27, 28). Obesity, which constitutes a core aspect of metabolic disorders, emerges as notably impactful in predisposing individuals to GSD. Several studies have revealed a significant association between obesity and GSD (10, 29). Obesity promotes stone formation mainly through several mechanisms. Firstly, obese patients are usually associated with abnormal cholesterol metabolism, and when cholesterol levels in the bile are too high, cholesterol precipitates and forms stones (8, 9, 30). Secondly, obese patients are often associated with metabolic syndrome and IR, which can also lead to weakened gallbladder motility, bile siltation in the gallbladder to form cholesterol crystals, and ultimately the formation of gallstones (30–32). In addition, obese patients are usually accompanied by hypertriglyceridemia, high levels of triglycerides will further affect cholesterol metabolism, exacerbate the oversaturation of bile, and increase the risk of gallstone formation (5, 8, 9, 31). Moreover, obese people often tend to consume high-calorie, low-fibre food, this diet structure will not only increase the intake of cholesterol, but also affect the normal contraction and emptying function of the gallbladder, which further promotes the formation of gallstones (10, 20, 22, 33). Numerous recent epidemiological studies have additionally proposed that individuals with metabolic conditions such as diabetes mellitus face a notably heightened susceptibility to GSD development (31, 33). Effective early management of blood glucose levels, including regular metformin administration, can contribute to reducing the development of GSD in patients with diabetes (34). Butyrylcholinesterase (BChE) is a non-specific cholinesterase that is associated with abnormal liver function (35, 36). Notably, elevated BChE activity may reflect the extent of lipid metabolism disorders and IR in the body, which may also exacerbate the risk of cholesterol stones (35, 36). In addition, BChE activity may be associated with systemic inflammation and oxidative stress, which also play a role in gallstone development (35, 36). However, the detailed relationship between BChE and GSD deserves further study.
IR is one of the core mechanisms by which metabolic disorders and obesity affect the prevalence of GSD. The TyG index has demonstrated its validity as a suitable substitute for IR (11–13). However, the TyG index does not account for variations in adipose tissue distribution. While WC can reflect central obesity, it does not clearly differentiate between subcutaneous and visceral adipose tissues. Research indicates that visceral adipose tissue contributes more significantly to IR development than subcutaneous adipose tissue (37). Previous research has emphasized that height is a crucial factor in adipose tissue distribution, making the WHtR a superior predictor compared to WC alone (14, 38). For instance, Paajanen et al. found that patients with short stature are more likely to develop coronary heart disease (39). Therefore, combining WC with height enhances the accuracy of assessing central obesity and metabolism. The TyG-WHtR index represents a straightforward and intuitive measure incorporating fasting plasma glucose, triglyceride levels, WC, and height (40). With the integration of the TyG index and WHtR, the TyG-WHtR index can provide a more comprehensive assessment of IR and adipose tissue distribution. Our findings further corroborated the strong association between the TyG-WHtR index and both the prevalence of GSD and the age at initial gallstone surgery.
To our knowledge, this study represents the initial study into the association between the TyG-WHtR index and the prevalence of GSD. In summary, our study identified a non-linear, positive association between the TyG-WHtR index and the prevalence of GSD, demonstrating a 47% escalation in incidence for every 1-unit increment in the TyG-WHtR index. Our study possesses several strengths. First, this study represents the pioneering effort to delineate the influence of the TyG-WHtR index on the prevalence of GSD. Second, the NHANES participants constituted a representative sample from the United States, adhering closely to a meticulously designed study protocol and undergoing rigorous quality control measures, thus ensuring the reliability of our conclusions. Third, the NHANES offered comprehensive demographic and metabolic information, along with subsequent follow-up assessments, facilitating the adjustment for potential confounding variables in our multivariate analyses. Last, we conducted subgroup analyses to ensure the generalizability of our results across different populations, underscoring the thoroughness and robustness of our study. TyG-WHtR index may have many potential applications in the future. For example, the positive association between the TyG-WHtR index and the prevalence of GSD suggests that it could be used as a valuable biomarker for predicting an individual’s risk of developing GSD, particularly in women, non-diabetics, and ethnically diverse groups. By monitoring this index, clinicians can identify high-risk populations and thus develop early intervention and prevention measures. In addition, the TyG-WHtR index is strongly associated with metabolic syndrome and could be used in the future as part of the criteria in health screening, especially to identify individuals with metabolic abnormalities associated with GSD. In public health policy, the TyG-WHtR index may be used to guide prevention strategies for GSD, especially in obese, metabolically abnormal, and high-risk populations.
However, our study also has some limitations that should be acknowledged. First, given its cross-sectional design, we cannot establish a causal relationship between the TyG-WHtR index and the prevalence of GSD. Second, the diagnosis of GSD relied on a questionnaire, potentially introducing recall (recollection) bias. Third, due to the design of the NHANES, we could only measure fasting glucose and triglyceride levels at a single time point during baseline assessment; however, these laboratory parameters may change during follow-up. Last, despite adjusting for numerous possible confounders, we could not completely eliminate the effects of other unmeasured confounders.
Despite these limitations, this study is clinically important. The TyG-WHtR index represents a novel biomarker that integrates both IR and adipose tissue distribution, offering a more comprehensive assessment of metabolic health. Our study also revealed, for the first time, an association between the TyG-WHtR index and the prevalence of GSD, offering compelling evidence for the TyG-WHtR index’s predictive value in GSD development. In the future, conducting a multicenter prospective cohort study will be essential to explore the capacity of the TyG-WHtR index as an independent predictor of GSD incidence.
5 Conclusion
A higher TyG-WHtR index is positively associated with the prevalence of GSD and may be related to an earlier age at first gallstone surgery. A multicenter prospective cohort study is needed to investigate the potential of the TyG-WHtR index as an independent predictor of GSD prevalence.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
JW: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing. SC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Supervision, Visualization, Writing – original draft, Writing – review & editing. XC: Conceptualization, Formal analysis, Validation, Visualization, Writing – original draft, Writing – review & editing. CQ: Formal analysis, Methodology, Project administration, Writing – original draft, Writing – review & editing. JH: Data curation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. XZ: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. HuaL: Formal analysis, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. PY: Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. HuiL: Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing, Data curation, Formal analysis. CY: Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. RS: Conceptualization, Data curation, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. DW: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by NHC Key Laboratory of Nuclear Technology Medical Transformation (Mianyang Central Hospital) (grant no. 2023HYX032), National Natural Science Foundation of China (NSFC) (grant no. 82400758), and House-level Project of Mianyang Central Hospital (grant no. 2021YJ011).
Acknowledgments
We are grateful to the NHANES participants and staff. We appreciate all the reviewers who participated in the review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BMI, body mass index; GSD, gallstone disease; TyG, triglyceride-glucose index; IR, insulin resistance; NHANES, National Health and Nutrition Examination Survey; OR, odds ratio; WC, waist circumference; WHtR, waist-to-height ratio; CI, confidence interval; GAM, generalized additive model; IQR, interquartile range; SD, standard deviation.
References
1. Wang, X, Yu, W, Jiang, G, Li, H, Li, S, Xie, L, et al. Global epidemiology of gallstones in the 21st century: a systematic review and Meta-analysis. Clin Gastroenterol Hepatol. (2024) 22:1586–95. doi: 10.1016/j.cgh.2024.01.051
2. Fujita, N, Yasuda, I, Endo, I, Isayama, H, Iwashita, T, Ueki, T, et al. Evidence-based clinical practice guidelines for cholelithiasis 2021. J Gastroenterol. (2023) 58:801–33. doi: 10.1007/s00535-023-02014-6
3. Shaffer, EA . Gallstone disease: epidemiology of gallbladder stone disease. Best Pract Res Clin Gastroenterol. (2006) 20:981–96. doi: 10.1016/j.bpg.2006.05.004
4. Sun, H, Warren, J, Yip, J, Ji, Y, Hao, S, Han, W, et al. Factors influencing gallstone formation: a review of the literature. Biomol Ther. (2022) 12:550. doi: 10.3390/biom12040550
5. Magnano San Lio, R, Barchitta, M, Maugeri, A, Quartarone, S, Basile, G, and Agodi, A. Preoperative risk factors for conversion from laparoscopic to open cholecystectomy: a systematic review and Meta-analysis. Int J Environ Res Public Health. (2022) 20:408. doi: 10.3390/ijerph20010408
6. Chen, J, Liu, ZT, Lyu, JT, and Jiang, GP. Impact of metabolic disorders on gallstone disease and perioperative recovery after laparoscopic cholecystectomy. Hepatobiliary Pancreat Dis Int. (2024) S1499-3872:00106–1. doi: 10.1016/j.hbpd.2024.08.001
7. Luo, M, Chen, P, Tian, Y, Rigzin, N, Sonam, J, Shang, F, et al. Hif-1α expression targets the TMA/Fmo 3/TMAO axis to participate in gallbladder cholesterol stone formation in individuals living in plateau regions. Biochim Biophys Acta Mol basis Dis. (2024) 1870:167188. doi: 10.1016/j.bbadis.2024.167188
8. Ke, B, Sun, Y, Dai, X, Gui, Y, and Chen, S. Relationship between weight-adjusted waist circumference index and prevalence of gallstones in U.S. adults: a study based on the NHANES 2017-2020. Front Endocrinol (Lausanne). (2023) 14:1276465. doi: 10.3389/fendo.2023.1276465
9. Zhang, G, Ding, Z, Yang, J, Wang, T, Tong, L, Cheng, J, et al. Higher visceral adiposity index was associated with an elevated prevalence of gallstones and an earlier age at first gallstone surgery in US adults: the results are based on a cross-sectional study. Front Endocrinol (Lausanne). (2023) 14:1189553. doi: 10.3389/fendo.2023.1189553
10. Wang, J, Yang, J, Chen, Y, Rui, J, Xu, M, and Chen, M. Association of METS-IR index with prevalence of gallbladder stones and the age at the first gallbladder stone surgery in US adults: a cross-sectional study. Front Endocrinol (Lausanne). (2022) 13:1025854. doi: 10.3389/fendo.2022.1025854
11. Zhuang, Y, Qiu, L, Han, D, Qiao, Z, Wang, F, Jiang, Q, et al. The association between triglyceride-glucose index and related parameters and risk of cardiovascular disease in American adults under different glucose metabolic states. Diabetol Metab Syndr. (2024) 16:102. doi: 10.1186/s13098-024-01340-w
12. Zhang, X, Wang, Y, Li, Y, Gui, J, Mei, Y, Yang, X, et al. Optimal obesity-and lipid-related indices for predicting type 2 diabetes in middle-aged and elderly Chinese. Sci Rep. (2024) 14:10901. doi: 10.1038/s41598-024-61592-4
13. Khamseh, ME, Malek, M, Jahangiri, S, Nobarani, S, Hekmatdoost, A, Salavatizadeh, M, et al. Insulin resistance/sensitivity measures as screening indicators of metabolic-associated fatty liver disease and liver fibrosis. Dig Dis Sci. (2024) 69:1430–43. doi: 10.1007/s10620-024-08309-9
14. Feng, Q, Bešević, J, Conroy, M, Omiyale, W, Woodward, M, Lacey, B, et al. Waist-to-height ratio and body fat percentage as risk factors for ischemic cardiovascular disease: a prospective cohort study from UK biobank. Am J Clin Nutr. (2024) 119:1386–96. doi: 10.1016/j.ajcnut.2024.03.018
15. Xuan, W, Liu, D, Zhong, J, Luo, H, and Zhang, X. Impacts of triglyceride glucose-waist to height ratio on diabetes incidence: a secondary analysis of a population-based longitudinal data. Front Endocrinol (Lausanne). (2022) 13:949831. doi: 10.3389/fendo.2022.949831
16. Zhou, J, Huang, H, Huang, H, Peng, J, Chen, W, Chen, F, et al. Association of triglyceride-glucose index and its combination with adiposity-related indices with the incidence of myocardial infarction: a cohort study from the UK biobank. Int J Obes. (2024). doi: 10.1038/s41366-024-01612-5
17. Feng, X, Zhu, J, Hua, Z, Yao, S, and Tong, H. Comparison of obesity indicators for predicting cardiovascular risk factors and multimorbidity among the Chinese population based on ROC analysis. Sci Rep. (2024) 14:20942. doi: 10.1038/s41598-024-71914-1
18. Li, S, An, L, Fu, Z, Zhang, W, and Liu, H. Association between triglyceride-glucose related indices and all-cause and cause-specific mortality in the general population: a cohort study. Cardiovasc Diabetol. (2024) 23:286. doi: 10.1186/s12933-024-02390-0
19. Peery, AF, Crockett, SD, Murphy, CC, Jensen, ET, Kim, HP, Egberg, MD, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2021. Gastroenterology. (2022) 162:621–44. doi: 10.1053/j.gastro.2021.10.017
20. Liu, X, Yan, G, Xu, B, and Sun, M. Association between monocyte-to-high-density lipoprotein-cholesterol ratio and gallstones in U.S. adults: findings from the National Health and nutrition examination survey 2017-2020. Lipids Health Dis. (2024) 23:173. doi: 10.1186/s12944-024-02166-1
21. Jackson, SS, Graubard, BI, Gabbi, C, and Koshiol, J. Association with menopausal hormone therapy and asymptomatic gallstones in US women in the third National Health and nutrition examination study. Sci Rep. (2024) 14:191. doi: 10.1038/s41598-023-50509-2
22. Yuk, JS, and Park, JY. Menopausal hormone therapy increases the risk of gallstones: health insurance database in South Korea (HISK)-based cohort study. PLoS One. (2023) 18:e0294356. doi: 10.1371/journal.pone.0294356
23. Politano, SA, Hamiduzzaman, N, and Alhaqqan, D. Diseases of the gallbladder and biliary tree. Prim Care. (2023) 50:377–90. doi: 10.1016/j.pop.2023.03.004
24. Su, PY, Hsu, YC, Cheng, YF, Kor, CT, and Su, WW. Strong association between metabolically-abnormal obesity and gallstone disease in adults under 50 years. BMC Gastroenterol. (2019) 19:117. doi: 10.1186/s12876-019-1032-y
25. Zhang, C, Dai, W, Yang, S, Wu, S, and Kong, J. Resistance to cholesterol gallstone disease: hepatic cholesterol metabolism. J Clin Endocrinol Metab. (2024) 109:912–23. doi: 10.1210/clinem/dgad528
26. Carey, MC, and Paigen, B. Epidemiology of the American Indians' burden and its likely genetic origins. Hepatology. (2002) 36:781–91. doi: 10.1053/jhep.2002.36545
27. Jiang, L, Du, J, Wang, J, and Ding, J. Sex-specific differences in the associations of metabolic syndrome or components with gallstone disease in Chinese euthyroid population. Sci Rep. (2023) 13:1081. doi: 10.1038/s41598-023-28088-z
28. Portincasa, P, Di Ciaula, A, Bonfrate, L, Stella, A, Garruti, G, and Lamont, JT. Metabolic dysfunction-associated gallstone disease: expecting more from critical care manifestations. Intern Emerg Med. (2023) 18:1897–918. doi: 10.1007/s11739-023-03355-z
29. Lim, J, Wirth, J, Wu, K, Giovannucci, E, Kraft, P, Turman, C, et al. Obesity, adiposity, and risk of symptomatic gallstone disease according to genetic susceptibility. Clin Gastroenterol Hepatol. (2022) 20:e1083–120. doi: 10.1016/j.cgh.2021.06.044
30. Bays, HE, Kirkpatrick, CF, Maki, KC, Toth, PP, Morgan, RT, Tondt, J, et al. Obesity, dyslipidemia, and cardiovascular disease: a joint expert review from the obesity medicine association and the National Lipid Association 2024. J Clin Lipidol. (2024) 18:e320–50. doi: 10.1016/j.jacl.2024.04.001
31. Portincasa, P, Bonfrate, L, Wang, DQ, Frühbeck, G, Garruti, G, and Di Ciaula, A. Novel insights into the pathogenic impact of diabetes on the gastrointestinal tract. Eur J Clin Investig. (2022) 52:e13846. doi: 10.1111/eci.13846
32. Mulita, F, Lampropoulos, C, Kehagias, D, Verras, GI, Tchabashvili, L, Kaplanis, C, et al. Long-term nutritional deficiencies following sleeve gastrectomy: a 6-year single-Centre retrospective study. Prz Menopauzalny. (2021) 20:170–6. doi: 10.5114/pm.2021.110954
33. Ratheesh, R, Ulrich, MT, Ghozy, S, Al-Jaboori, M, and Nayak, SS. The association between diabetes and gallstones: a nationwide population-based cohort study. Prz Gastroenterol. (2023) 18:292–9. doi: 10.5114/pg.2023.131395
34. Liao, KF, Chuang, HY, and Lai, SW. Metformin use correlates with reduced risk of gallstones in diabetic patients: a 12-year follow-up study. Front Pharmacol. (2017) 8:765. doi: 10.3389/fphar.2017.00765
35. Turecky, L, Kupcova, V, Durfinova, M, and Uhlikova, E. Serum butyrylcholinesterase activities in patients with non-alcoholic fatty liver disease. Comparison with liver proteosynthetic function and liver fibrosis. Bratisl Lek Listy. (2021) 122:689–94. doi: 10.4149/BLL_2021_110
36. Verras, GI, and Mulita, F. Butyrylcholinesterase levels correlate with surgical site infection risk and severity after colorectal surgery: a prospective single-center study. Front Surg. (2024) 11:1379410. doi: 10.3389/fsurg.2024.1379410
37. Han, XD, Li, YJ, Wang, P, Han, XL, Zhao, MQ, Wang, JF, et al. Insulin resistance-varying associations of adiposity indices with cerebral perfusion in older adults: a population-based study. J Nutr Health Aging. (2023) 27:219–27. doi: 10.1007/s12603-023-1894-2
38. Wu, T, Liao, Z, Wang, J, and Liu, M. The accumulative effect of multiple postnatal risk factors with the risk of being overweight/obese in late childhood. Nutrients. (2024) 16:1536. doi: 10.3390/nu16101536
39. Paajanen, TA, Oksala, NK, Kuukasjärvi, P, and Karhunen, PJ. Short stature is associated with coronary heart disease: a systematic review of the literature and a meta-analysis. Eur Heart J. (2010) 31:1802–9. doi: 10.1093/eurheartj/ehq155
Keywords: gallstone, triglyceride glucose-waist height ratio, cross-sectional study, abdominal obesity, metabolic syndrome, National Health and Nutrition Examination Survey
Citation: Wang J, Chen S, Chen X, Qin C, Hu J, Zeng X, Luo H, Yang P, Luo H, Yuan C, Shi R and Wang D (2024) Higher triglyceride glucose-waist height ratio index is associated with higher prevalence of gallstone: a population-based study. Front. Med. 11:1481620. doi: 10.3389/fmed.2024.1481620
Edited by:
Joel E. Lavine, Columbia University, United StatesReviewed by:
Francesk Mulita, General Hospital of Eastern Achaia-Unit of Aigio, GreeceTevfiktolga Sahin, İnönü University, Türkiye
Ivan Šoša, University of Rijeka, Croatia
Copyright © 2024 Wang, Chen, Chen, Qin, Hu, Zeng, Luo, Yang, Luo, Yuan, Shi and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Decai Wang, ZGVjYWl3YW5nXzIwMjBAMTYzLmNvbQ==; Ruizi Shi, c2hpcnVpemkxMjNAeWVhaC5uZXQ=
†These authors have contributed equally to this work and share first authorship
 Jianjun Wang
Jianjun Wang Sirui Chen1†
Sirui Chen1† Xintao Zeng
Xintao Zeng Ruizi Shi
Ruizi Shi Decai Wang
Decai Wang