- 1Department of Clinical Pharmacy, Marmara University Faculty of Pharmacy, Istanbul, Türkiye
- 2Department of Clinical Pharmacy, Prof. Dr. Cemil Taşcıoğlu City Hospital, Istanbul, Türkiye
- 3Department of Pulmonary and Intensive Care Unit, Marmara University Faculty of Medicine, Istanbul, Türkiye
Background: Critically ill intensive care unit (ICU) patients often face life-threatening drug-related problems (DRPs) and malnutrition. Clinical pharmacists (CPs) play a crucial role in mitigating these issues and improving outcomes.
Aim: This study was designed to detect, prevent, reduce or resolve nutrition-related problems (NRPs) and DRPs in intensive care patients with renal dysfunction through clinical pharmacy services.
Method: This 9-month, prospective, non-randomized, controlled study was conducted in the ICU. During the intervention period (IP), CP recommendations addressing NRPs and DRPs were provided to the healthcare team. NRPs were evaluated using an expert-developed enteral nutrition consensus protocol, while DRPs were classified according to the Pharmaceutical Care Network Europe (PCNE) Classification for Drug-Related Problems Version 9.1.
Results: The study included 60 patients with a median age of 73 years (IQR: 60.5–80). A total of 504 DRPs (8.4 per patient) were identified across all patients. DRPs were decreased by 50% during the IP compared to the observation period (OP) (p < 0.001). The most common causes of DRPs were ‘too low a drug dose’ (22.2%), ‘drug–drug interactions’ (17%), and ‘too high a drug dose’ (16.4%). Of the recommendations made to the prescribing physician, 140 (97.9%) were accepted. In the IP, targeted calorie and protein supplementation was fully achieved in more patients (p < 0.05). The most common recommendations included ‘changes in the rate of nutrition’ (66.7%), ‘vitamin supplementation’ (16.7%), and ‘changes in enteral nutrition products’ (7.7%).
Conclusion: This study highlights the high incidence of DRPs and malnutrition risk in ICU patients with renal dysfunction, emphasizing the vital role of clinical pharmacists. Their collaboration with healthcare professionals significantly reduced both DRPs and NRPs.
1 Introduction
Critically ill patients requiring treatment in the intensive care unit (ICU) often suffer from potentially life-threatening drug-related problems (DRPs). The Pharmaceutical Care Network Europe (PCNE) defines a DRP as “an event or circumstance involving drug therapy that actually or potentially interferes with desired health outcomes” (https://www.pcne.org/working-groups/2/drug-related-problem-classification, Accessed September 10, 2022). Due to the complexity of critical patient care and intricate treatment protocols, the rate of DRPs is higher in the ICU compared to other medical services (1). Renal dysfunction is a risk factor that increases DRP rates in the ICU (2–4). The global incidence of acute kidney injury (AKI) is reported to be 22% (5), while it rises to 57% in the ICU (6). Patients with impaired renal function in the ICU have been shown to require more pharmaceutical interventions than those with normal renal function (7). Involvement of clinical pharmacists (CPs) as pharmacotherapy specialists in routine patient care, in collaboration with other healthcare professionals contributes to improved patient outcomes by reducing DRP rates (8).
In addition to DRPs, the nutritional needs of critically ill patients are complex and vary according to the stage of their illness (9, 10). Malnutrition can occur in critically ill patients at a rate ranging from 38 to 78% (9). It is associated with increased infectious complications, multi-organ dysfunction, prolonged hospitalization, and high mortality (11). Currently, many health centers manage nutritional therapy through multidisciplinary teams, with nutritional support pharmacists playing a vital role in maintaining and improving patients’ optimal nutritional status. The primary function of the pharmacist in the team is to provide nutritional therapy tailored to each patient’s needs (12). This study was designed to assess the effect of clinical pharmacy services in identification, prevention, and resolution of DRPs and nutrition-related problems (NRPs) in intensive care patients with renal dysfunctions.
2 Materials and methods
2.1 Ethical approval
This study received ethical approval from the Clinical Research Ethics Committee (approval no: 09.202211565, date: 02.09.2022). All procedures adhered to the ethical standards of the University of Siena and the principles of the 1964 Declaration of Helsinki and its subsequent amendments.
2.2 Setting and study design
The study was a prospective, observational study conducted in an 8-bed internal ICU of a university hospital between November 1, 2022, and July 21, 2023. The study consisted of two phases: a 4-month observation period (OP) and a 4-month intervention period (IP). There was a 1-month lag-period (LP) between the two phases to allow for the discharge of OP patients from the ICU.
2.3 Participants
For the sample size of the study, it was determined that at least 26 patients should be included in each group, based on a calculation using a standard deviation of 1, an alpha level of 0.05, and a power of 95%. This calculation was based on literature indicating that DRP rates could be reduced from 1.96 to 0.94 (approximately 50%) per patient following CP recommendations. A total of 60 patients were included, with at least 30 in each group, taking into account the 15% margin for potential dropout (13).
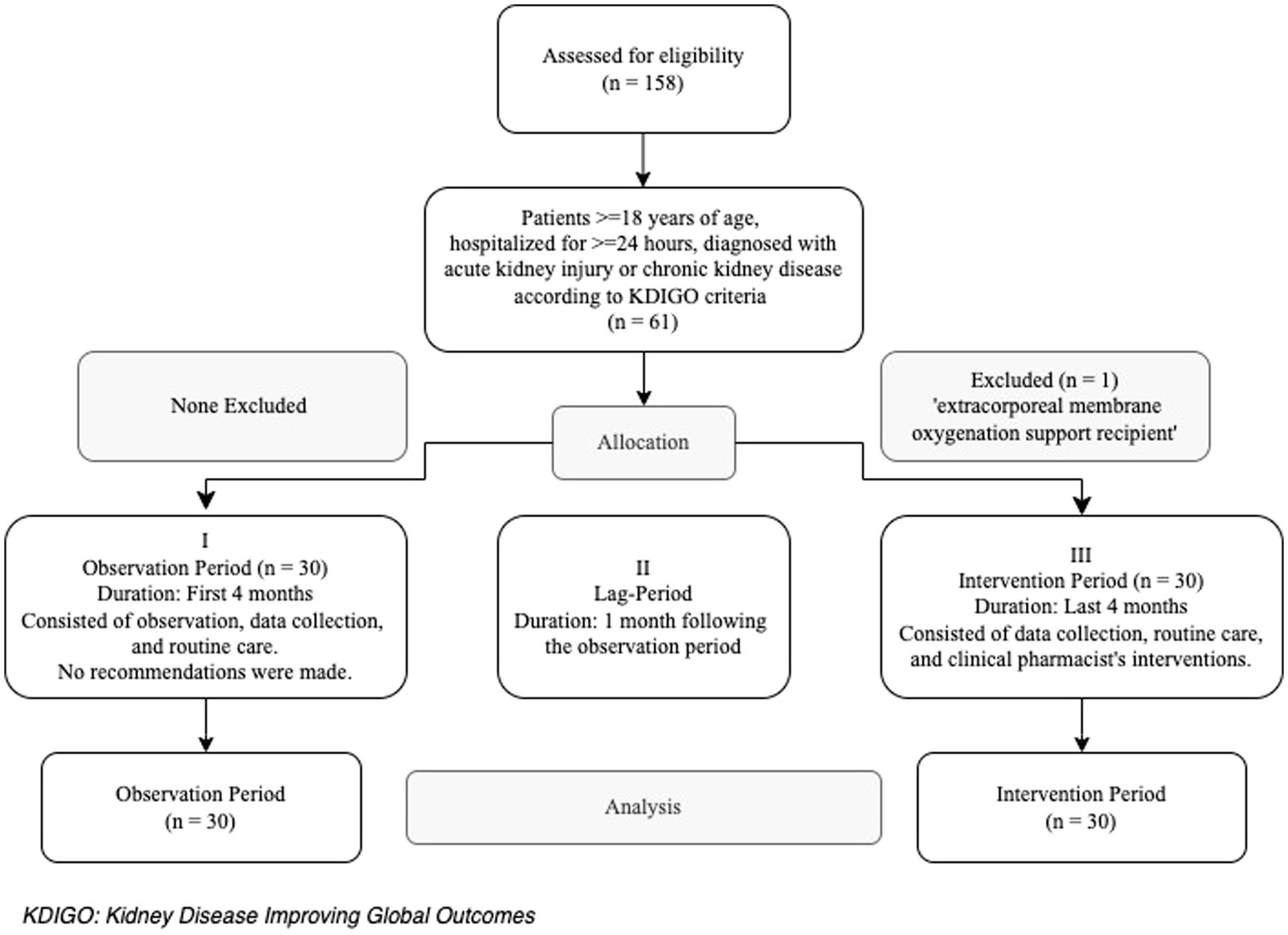
Inclusion criteria were patients aged ≥18 years, hospitalized for ≥24 h in the ICU and diagnosed with AKI or chronic kidney disease (CKD) according to Kidney Disease Improving Global Outcomes (KDIGO) criteria (14, 15). The exclusion criteria was the receipt of extracorporeal membrane oxygenation support (Figure 1). Patients and/or their surrogate family members were informed about the study and invited to participate. The first 30 patients from each group (OP and IP) who met the inclusion criteria and provided informed consent were included to the study. Both written and verbal consents were obtained from those who accepted to participate.
2.4 Study design
This prospective, observational study consisted of two phases: a 4-month observation period (OP) and a 4-month intervention period (IP), with a 1-month lag-period (LP) in between, to allow for the OP patients discharge from the ICU.
Throughout the study, the CP collaborated with the same attending physician, who is a professor of intensive care medicine. During both phases, the CP collected and recorded patients’ demographic and clinical data, including medical history, medications, laboratory values, microbiology culture results, nutritional status, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, Nutrition Risk in Critically ill (NUTRIC) score. On weekdays, the CP participated in routine patient rounds, regularly reviewing physician orders and patients to identify of any manifest and/or potential DRPs and NRPs. Patients were followed until discharge or death.
During the OP, patients received routine ICU care. The CP conducted medication and nutrition review to identify DRPs and NRPs, but did not implement any interventions. Routine ICU care included dietician consultations and general visits once weekly.
During the LP, the CP reviewed the most common DRPs and NRPs encountered during the OP and provided face-to-face training to the healthcare team, which consisted of the attending physician, ICU specialists, and resident physicians. This training covered the importance, prevention, and resolution of these problems. The ICU healthcare team was also informed about the enteral nutrition (EN) protocol that was developed by the expert team. These training sessions were repeated whenever new residents joined the team and as needed.
During the IP, besides the routine ICU care, the CP continued to provide medication and nutrition reviews for DRPs and NRPs and made recommendations to the attending physician, regarding the identified issues.
2.5 Main outcome measures
The primary outcomes of the study included the frequencies of DRPs, the number of DRPs prevented or resolved during the IP, and assessment of nutritional parameters (e.g., NUTRIC score, protein and calorie intake in the first 7 days and after the 7th day, time to reach target nutrition, complications, and vitamin supplements) under the protocol. Secondary outcomes included a description of medication groups involved in DRPs and identification of DRP risk factors.
2.6 Identification of DRPs
DRPs were classified using the Turkish version of the PCNE Classification for Drug-Related Problems V9.1 (https://www.pcne.org/working-groups/2/drug-related-problem-classification, Accessed September 10, 2022). UpToDate® and Micromedex® database were utilized to provide information on indications, contraindications, dosages (considering renal impairment, hepatic impairment, older adults and obesity), administration, adverse reactions, monitoring parameters, and pharmacology of the drugs. The Sanford Guide to Antimicrobial Therapy was also used for information on antimicrobial drugs.
Potential drug–drug interactions (pDDIs) were identified using the UpToDate® database. Major and contraindicated interactions were recorded as DRPs, with clinical significance were determined collaboratively by the attending physician and the CP. ‘Not clinically significant’ pDDIs were defined as at least one of the following: ‘interactions that could not be avoided in the ICU and/or the stated risk does not apply to the patient and/or no change in treatment or administration modality is required’. No recommendation was made regarding ‘not clinically significant’ pDDIs.
2.7 Identification of NRPs
In this study, only EN-related problems were assessed, as the patient population rarely received parenteral nutrition. The attending physician and the CP defined the NRPs as ‘errors or complications related to EN therapy, such as issues with timing, method, rate of administration, and choice of nutritional product, which could prevent the achievement of desired nutritional goals’.
The evaluation of NRPs in critically ill patients with renal dysfunction utilized an EN protocol (Supplementary Table S1) developed from the most current guidelines from the European Society for Clinical Nutrition and Metabolism (ESPEN) and the American Society for Parenteral and Enteral Nutrition (ASPEN) (16–19). This protocol was created through consensus among an expert team, consisting of two clinical pharmacy specialists, an intensive care medicine specialist, and an assistant clinical pharmacist.
Nutritional status was assessed using parameters such as the NUTRIC score, EN duration, achievement of calorie and protein goals in the first 7 days, and after the 7th day, as well as the time taken to reach these goals.
2.8 Clinical pharmacist interventions
Recommendations made by the CP to address the identified DRPs and NRPs included adding or stopping medications, changing to alternative treatments, changing routes of administration, dose adjustments, side effect management, therapeutic drug monitoring, or optimizing drug administration techniques. Recommendations were directed only to the prescribing physician.
A problem where the CP was consulted or intervened before a drug was prescribed, was recorded as a “prevented DRP”; a problem where the CP intervened before a prescribed drug was administered was recorded as a “potential DRP”; and a problem where the CP intervened after a drug was administered was recorded as a “manifest DRP”.
2.9 Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 29.0 (Armonk, New York: IBM Corp.). Continuous variables were expressed as median (interquartile range [IQR]), and nominal and ordinal variables were expressed as n (%). Normality of continuous variables was assessed using the Kolmogorov–Smirnov test. Differences between two groups for the non-normally distributed data were compared using the nonparametric Mann–Whitney U test. Chi-square tests were used to analyze the relationships between categorical data. The risk status of different clinical conditions for DRPs was determined by odds ratio (OR). All data are presented within 95% confidence intervals, and a p-value <0.05 was considered as statistically significant.
3 Results
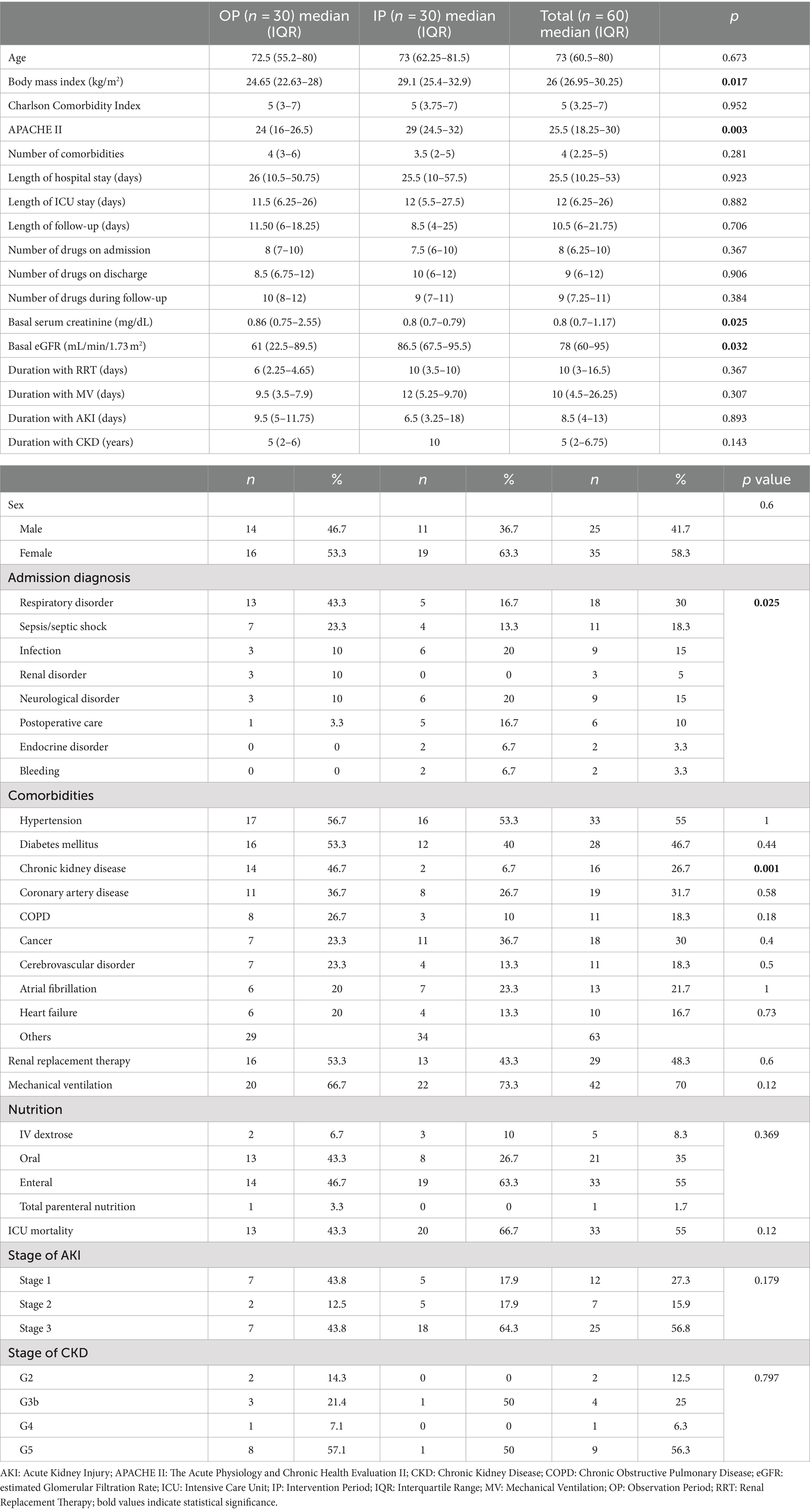
Out of the 158 patients hospitalized in the internal ICU during the OP and IP, 60 patients were included in the study (30 in the OP and 30 in the IP) (Figure 1). More than half of the patients were female, and the median (IQR) age was 73 (60.5–80) years. The most common comorbidities were hypertension (55%) and diabetes (46.7%), while the mortality rate was 55%. The prevalence of comorbidities other than CKD was similar in both groups (p > 0.05); however, the prevalence of CKD was higher in the OP patients compared to the IP patients (46.7% vs. 6.7%, respectively; p = 0.001). The APACHE II score was higher in the IP patients compared to the OP patients (24 vs. 29, p = 0.003). The most common reason for ICU admission was respiratory disorders in the IP (43.3%), while it was infection (20%) and neurologic disorders (20%) in the OP (p = 0.025). Of all patients, 27% had a diagnosis of CKD, 73% had developed AKI, and 55% received EN (Table 1).
A total of 504 DRPs were identified for all patients (8.4 DRPs per patient). The majority (98.3%) of patients had at least one DRP, with the number of DRPs per patient being 11.2 in the OP and 5.6 in the IP (p < 0.001). The most common causes of DRPs were “too low a drug dose” (C3.1; 22.2%), “an inappropriate combination of drugs with other drugs” (C1.3; 17%), and “too high a dose of a single active ingredient” (C3.2; 16.4%). In the IP, compared to the OP, the rates of DRP causes decreased significantly: by 67% for “no indication for the drug”, by 47% for “inappropriate combination of drugs with other drugs”, by 67% for “no or incomplete drug treatment despite existing indication”, by 45% for “low drug dose”, by 53% for “high drug dose”, by 67% for “too frequent dosing regimen”, by 100% for “not available prescribed drug”, and by 69% for “incorrect administration time” (coded as other reason) (p < 0.05, for all) (Table 2).
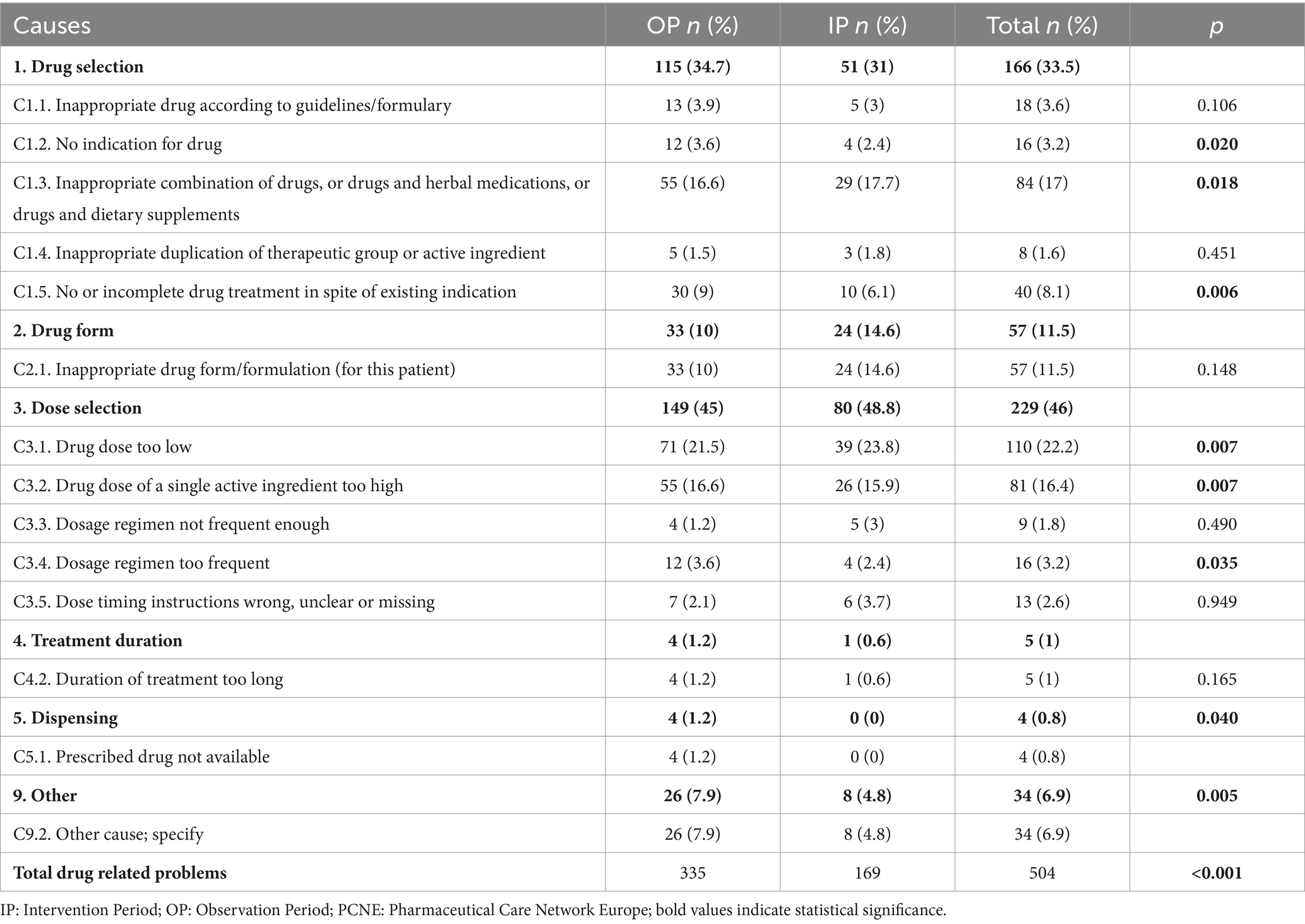
Table 2. Classification of identified drug-related problems according to the PCNE classification for drug-related problems version 9.1.
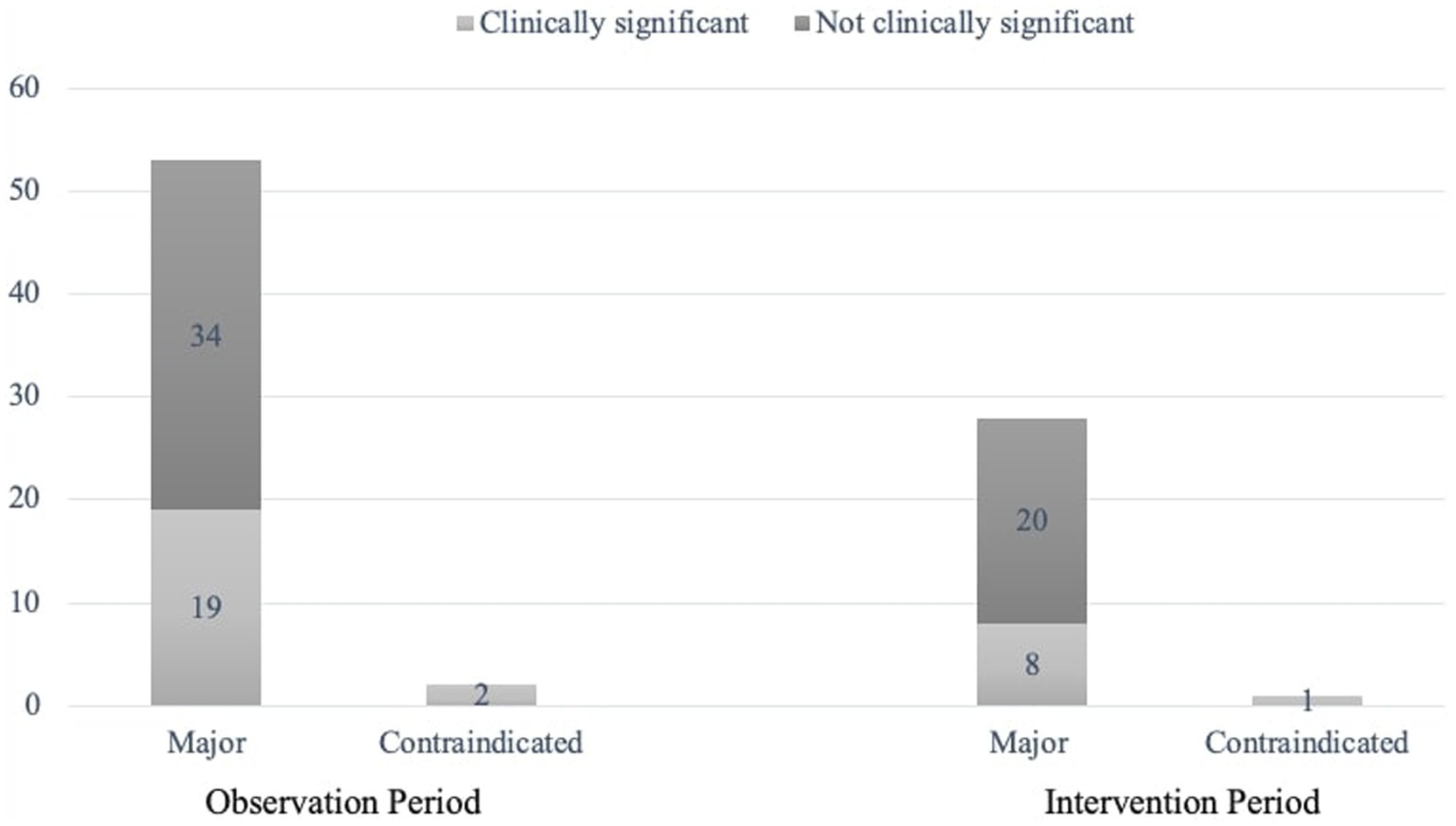
Intervention recommendations was proposed for the majority (84.6%, n = 143/169) of the DRPs identified in the IP. Most (88.5%, n = 23/26) of the DRPs for which intervention was not recommended, were related to “inappropriate combination of drugs with other drugs”. The frequency and clinical significance of pDDIs identified in both groups are shown in Figure 2. Of the recommendations made to the prescribing physician, 140 (97.9%) were accepted, and 87 DRPs were prevented and classified as “prevented DRPs.” In this study, 95.7% (134/140) of the accepted recommendations were fully implemented, two were partially implemented, and four were omitted.
When both periods were analyzed together, antimicrobial drugs (33.2%), nervous system drugs (21.4%), and digestive system and metabolism drugs (14.7%) were the most common groups associated with DRPs. The drugs most frequently involved in DRPs were enoxaparin (9.1%), meropenem (8.2%), vancomycin (7.7%), pantoprazole (6%), and piperacillin-tazobactam (5.2%). When pDDIs were analysed across both periods, the drugs most frequently involved in interactions were enoxaparin (14%), dexmedetomidine (12%), acetylsalicylic acid (7.6%), tramadol (6.4%), levetiracetam (5.7%), and valproic acid (5.7%).
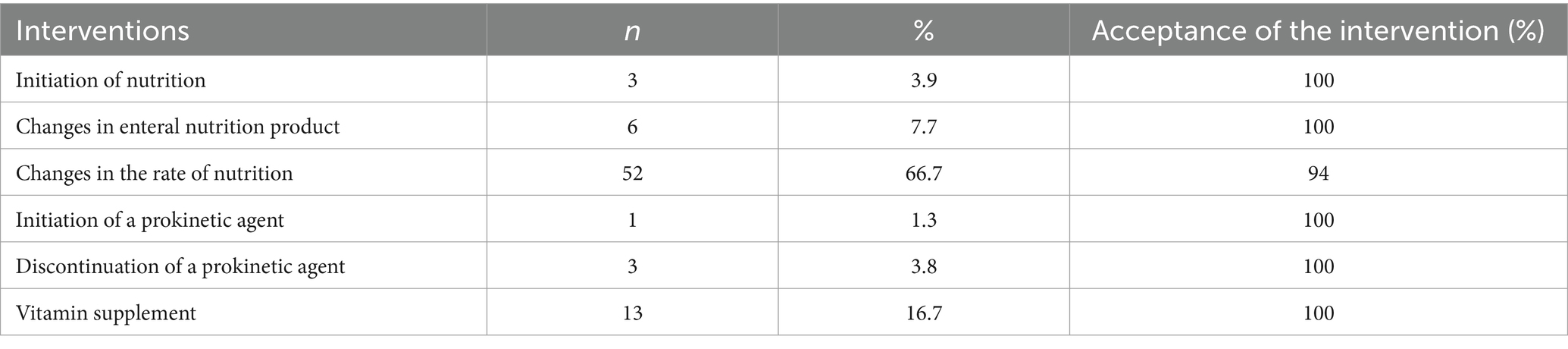
In the IP, targeted calorie and protein supplementation was fully achieved in more patients. Significant increases were observed in the percentages of patients reaching protein target in the first 7 days (p = 0.008), and calorie and protein targets after the 7th day (p = 0.007 and p < 0.001, respectively). Similarly, the amount of protein provided in the first 7 days (p = 0.034) and the amounts of calories and protein provided after the 7th day were significantly higher in IP compared to OP (p = 0.043 and p < 0.001, respectively). Serum vitamin levels (vitamin D, vitamin B12, folic acid) were measured in more patients in the IP (n = 13, 15, 16, respectively) compared to the OP (n = 1, 2, 2, respectively) and patients with low results were provided with the necessary supplements. Nutrition-related complications developed in 26.4% of the patients receiving EN, with the most common complications being diarrhea (12.1%) and vomiting (9.1%) (Table 3). A total of 78 recommendations were made to the ICU team to optimize clinical nutrition, and 96.2% of these recommendations were accepted. These recommendations included initiation of nutrition, changes in the rate of nutrition, addition or discontinuation of prokinetic agents, changes in EN products, and vitamin supplementation (Table 4).
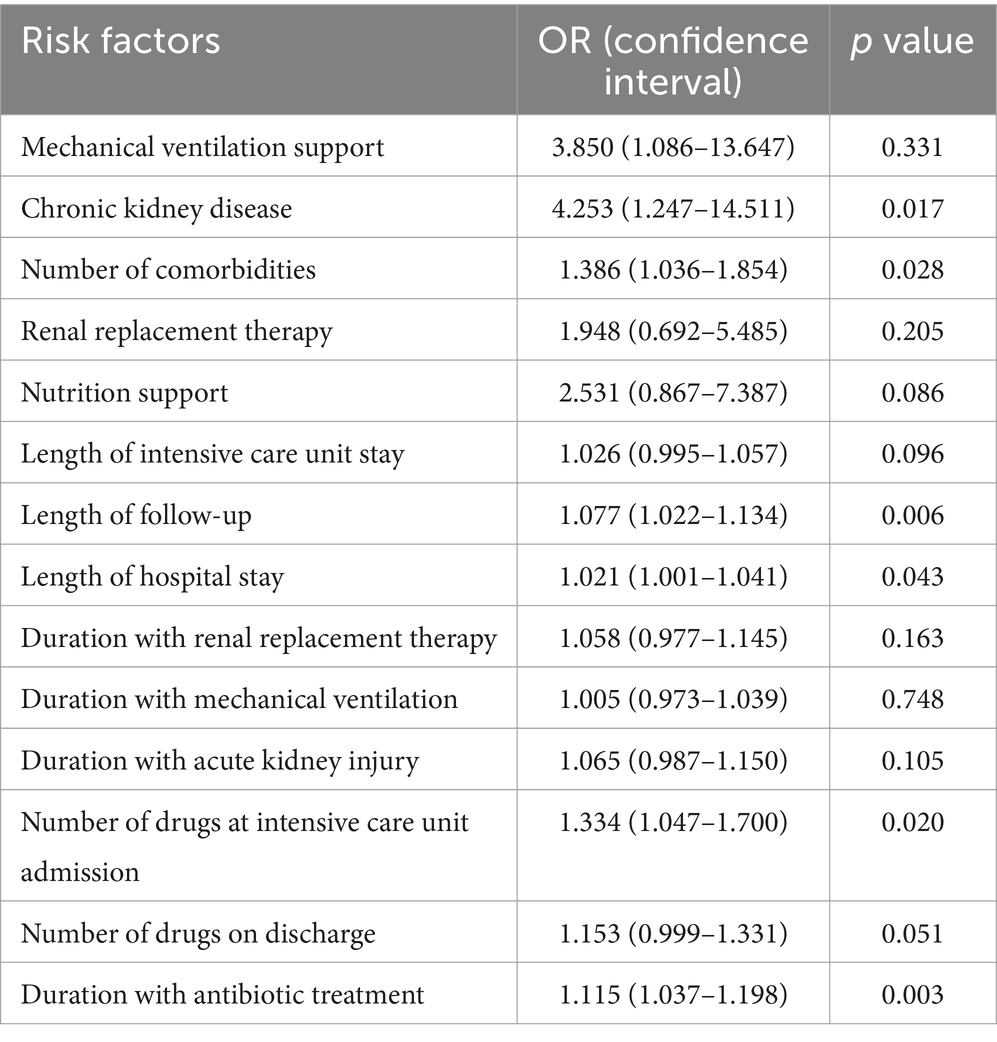
The risk factors that increase the number of DRPs for all periods were analyzed, and the risk ratios are presented in Table 5. DRP risk factors were identified as CKD, duration of antibiotic treatment, length of follow-up, number of comorbidities, length of hospital stay, and number of drugs at ICU admission (p < 0.05 for all), with CKD having the highest OR (CI) [4.253 (1.247–14.511)] among all.
4 Discussion
In our study, we evaluated the effect of clinical pharmacy services in identification, prevention, and resolution of DRPs and NRPs in intensive care patients with renal dysfunctions.
The reported DRP rate per patient in ICU studies ranges from 0.83 to 7.26 (3, 4, 20–23). The incidence of DRPs in patients with renal dysfunction is 1.63 to 9 times higher than in those with normal renal function (4, 7, 20, 23). Our study identified a higher number of DRPs than reported in the literature, reflecting our patient population’s renal dysfunction. We observed a significant decrease in the DRP rate per patient during the IP with CP involvement. While some controlled ICU studies noted a significant reduction in DRPs following CP intervention, others found no substantial differences between periods (4, 24–26). Variability in results may stem from factors such as differences in study settings, team composition changes, inadequate training, or prior experiences with CP.
In our study, dose selection emerged as the most frequent issue. This aligns with findings from Albayrak et al. and Chiang et al. where 30.5 and 30.9% of patients had renal dysfunction, respectively and dose selection was also a prevalent concern affecting 54.4 and 55.8% of patients, respectively (3, 7). Jiang et al. reported inappropriate drug frequency and dosing isuues in 37% of patients, with 74.7% of those with renal dysfunction or on renal replacement therapy (RRT) affected (20). In studies with lower renal dysfunction rates, drug selection was the predominant problem (22–60%) (4, 22, 23). These findings highlight the lack of awareness regarding necessary adjustments in drug dosing and frequency based on changing drug excretion rates, underscoring the importance of including CP in the multidisciplinary team to ensure proper dosing in patients with renal dysfunction.
We also evaluated the clinical significance of contraindicated and major interactions with input from both the CP and the ICU physician. Only 29 (34.5%) of the 84 pDDIs recorded necessitated changes in drug therapy. This emphasizes the need for evaluating drug interaction data on a patient-specific basis in collaboration with the CP. The total and clinically significant pDDI were higher in the IP (p = 0.024). This suggests that CP involvement in checking drug interactions prior to prescribing new medications effectively reduced the number of pDDIs. Aghili and Kasturirangan reported a similar rate of interactions requiring treatment adjustments at 31% (27). A systematic review indicated that 58% of ICU patients experience at least one pDDI, with not all interactions being clinically significant (28). Our study identified enoxaparin, dexmedetomidine, aspirin, tramadol, levetiracetam, and valproic acid as the most frequently involved drugs in interactions. In a systematic review and meta-analysis of 39 ICU studies, drugs most frequently involved in interactions were identified as aspirin, insulin, clopidogrel, furosemide, and omeprazole (28). Variability in reported drug interactions can be attributed to differences in population characteristics, treatment protocols, logistical issues, and the timing of studies.
Literature reports varying acceptance rates for CP recommendations, with figures ranging from 95 to 99.2%, similar to our findings, while others reported lower acceptance rates (67.3–93%) (3, 4, 7, 20–23, 26, 29, 30). High acceptance rates obsereved in this study may reflect the impact of a previous study evaluating clinical pharmacy services within the same ICU, and may be due to the fact that the recommendations were timely and tailored to patient needs.
Our findings, consistent with numerous studies, indicate that antimicrobial drugs frequently contribute to DRPs (22–81%) (7, 20, 22, 23, 30). Other drug categories commonly associated with DRPs included nervous system drugs (32.2%), gastrointestinal system drugs (18.7–27.4%), and antithrombotics (9.6–13.1%) (4,2 8). It is not surprising that the most commonly utilized drug groups in the ICU also represent a significant portion of DRPs (31, 32).
Many studies have explored factors contributing to DRPs and identified patient groups that should be prioritized by the CP. The risk factors identified in Table 5 align with those reported in other ICU studies (4, 21, 23, 25). Notably, CKD significantly increased the number of DRPs. However, the higher CKD prevalence in the OP patients compared to the IP patients (46.7% vs. 6.7%, respectively; p = 0.001) and the 50% drop in the frequency of DRPs in the IP compared to the OP may have influenced this outcome. Body mass index, which was significantly higher in the IP patients was not found to impact DRP presence (p = 0.426).
Evaluation of patients’ nutritional characteristics revealed high NUTRIC scores, indicating that many were at significant risk of malnutrition and are likely to benefit from aggressive nutritional therapy to improve mortality outcomes. Similar to the literature, the daily calorie and protein intake during the OP was much lower than targets. Contributing factors may include a greater focus on medical treatment, insufficient nutrition monitoring, and a lack of systemic management of nutritional support by the nutritional support team (33, 34). With highly accepted CP recommendations, more patients in IP transitioned to targeted nutrition, resulting in significant increases in the percentage achieving target calorie and protein support. It has been shown in the literature that establishing a nutritional support team, inclusive of pharmacists, or implementing a nutritional protocol leads to improved outcomes in reaching target calorie and protein values, and shortened timelines for nutrition initiation (35, 36). In our study center, the absence of protocol-based nutritional follow-up accentuates the necessity for CP involvement in a high-risk environment for malnutrition, highlighting its role in enhancing critical patient care.
4.1 Strengths and weaknesses
This study is significant as it focuses on patients with renal dysfunction, a population where DRPs are prevalent in ICUs in our country. It categorizes DRPs and NRPs while evaluating the effects of CP services. To our knowledge, no similar study exists in our country.
However, our study has limitations, including its conduct in a single center with a relatively small sample size and the lack of evaluation of parenteral nutrition, which was administered to very few patients.
4.2 Further research
Future randomized controlled trials will be conducted under CP leadership, and current consensus reports will be established across different disciplines for critically ill patient populations. This will further elucidate the importance of CP.
5 Conclusion
In summary, this study examines the impact of CP on evidence-based services for ICU patients with renal dysfunction. The healthcare team highly accepted and implemented recommendations for identifying, resolving, and preventing NRPs and DRPs. The involvement of CP in the ICU team, participating in rounds with other healthcare professionals, significantly reduces DRP frequency and enhances the achievement of nutritional goals.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Marmara Üniversitesi Tıp Fakültesi Klinik Araştırmalar Etik Kurulu. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
BÖ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft. YA: Conceptualization, Data curation, Methodology, Writing – original draft. SA: Methodology, Writing – review & editing. SK: Project administration, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1473719/full#supplementary-material
References
1. Cullen, DJ, Sweitzer, BJ, Bates, DW, Burdick, E, Edmondson, A, and Leape, LL. Preventable adverse drug events in hospitalized patients: a comparative study of intensive care and general care units. Crit Care Med. (1997) 25:1289–97. doi: 10.1097/00003246-199708000-00014
2. Abdelaziz, K, and Abdelrahim, ME. Identification and categorisation of drug-related problems on admission to an adult intensive care unit. Eur J Hosp Pharm. (2015) 22:138–41. doi: 10.1136/ejhpharm-2014-000566
3. Albayrak, A, Başgut, B, Bıkmaz, GA, and Karahalil, B. Clinical pharmacist assessment of drug-related problems among intensive care unit patients in a Turkish university hospital. BMC Health Serv Res. (2022) 22:79. doi: 10.1186/s12913-022-07494-5
4. Ayhan, YE, Karakurt, S, and Sancar, M. The effect of the clinical pharmacist in minimizing drug-related problems and related costs in the intensive care unit in Turkey: A non-randomized controlled study. J Clin Pharm Ther. (2022) 47:1867–74. doi: 10.1111/jcpt.13784
5. Susantitaphong, P, Cruz, DN, Cerda, J, Abulfaraj, M, Alqahtani, F, Koulouridis, I, et al. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. (2013) 8:1482–93. doi: 10.2215/CJN.00710113
6. Hoste, EA, Bagshaw, SM, Bellomo, R, Cely, CM, Colman, R, Cruz, DN, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. (2015) 41:1411–23. doi: 10.1007/s00134-015-3934-7
7. Chiang, LH, Huang, YL, and Tsai, TC. Clinical pharmacy interventions in intensive care unit patients. J Clin Pharm Ther. (2021) 46:128–33. doi: 10.1111/jcpt.13265
8. Stemer, G, and Lemmens-Gruber, R. Clinical pharmacy activities in chronic kidney disease and end-stage renal disease patients: a systematic literature review. BMC Nephrol. (2011) 12:1–12. doi: 10.1186/1471-2369-12-35
9. Lew, CCH, Yandell, R, Fraser, RJ, Chua, AP, Chong, MFF, and Miller, M. Association between malnutrition and clinical outcomes in the intensive care unit: a systematic review. J Parenter Enter Nutr. (2017) 41:744–58. doi: 10.1177/0148607115625638
10. White, JV, Guenter, P, Jensen, G, Malone, A, and Schofield, MAcademy of Nutrition and Dietetics Malnutrition Work Group, et al. Consensus statement of the academy of nutrition and dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. (2012) 112:730–8. doi: 10.1016/j.jand.2012.03.012
11. McClave, SA, Taylor, BE, Martindale, RG, Warren, MM, Johnson, DR, Braunschweig, C, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. (2016) 40:159–211. doi: 10.1177/0148607115621863
12. Tucker, A, Ybarra, J, Bingham, A, Blackmer, A, Curtis, C, Mattox, T, et al. American society for parenteral and enteral nutrition (a.s.p.e.N.) standards of practice for nutrition support pharmacists. Nutr Clin Pract. (2015) 30:139–46. doi: 10.1177/0884533614550318
13. Song, Y-K, Jeong, S, Han, N, Na, H, Jang, HY, Sohn, M, et al. Effectiveness of clinical pharmacist service on drug-related problems and patient outcomes for hospitalized patients with chronic kidney disease: A randomized controlled trial. J Clin Med. (2021) 10:1788. doi: 10.3390/jcm10081788
14. Andrassy, KM. Comments on 'KDIGO 2012 clinical practice guideline for the evaluation and Management of Chronic Kidney Disease'. Kidney Int. (2013) 84:622–3. doi: 10.1038/ki.2013.243
15. Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. (2012) 120:c179–84. doi: 10.1159/000339789
16. Compher, C, Bingham, AL, McCall, M, Patel, J, Rice, TW, Braunschweig, C, et al. Guidelines for the provision of nutrition support therapy in the adult critically ill patient: the American Society for Parenteral and Enteral Nutrition. JPEN J Parenter Enteral Nutr. (2022) 46:12–41. doi: 10.1002/jpen.2267
17. Fiaccadori, E, Sabatino, A, Barazzoni, R, Carrero, JJ, Cupisti, A, De Waele, E, et al. ESPEN guideline on clinical nutrition in hospitalized patients with acute or chronic kidney disease. Clin Nutr. (2021) 40:1644–68. doi: 10.1016/j.clnu.2021.01.028
18. Kreymann, KG, Berger, MM, Deutz, NE, Hiesmayr, M, Jolliet, P, Kazandjiev, G, et al. ESPEN guidelines on enteral nutrition: intensive care. Clin Nutr. (2006) 25:210–23. doi: 10.1016/j.clnu.2006.01.021
19. Singer, P, Blaser, AR, Berger, MM, Alhazzani, W, Calder, PC, Casaer, MP, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. (2019) 38:48–79. doi: 10.1016/j.clnu.2018.08.037
20. Jiang, SP, Chen, J, Zhang, XG, Lu, XY, and Zhao, QW. Implementation of pharmacists' interventions and assessment of medication errors in an intensive care unit of a Chinese tertiary hospital. Ther Clin Risk Manag. (2014) 10:861–6. doi: 10.2147/TCRM.S69585
21. Kim, JM, Park, SJ, Sohn, YM, Lee, YM, Yang, CS, Gwak, HS, et al. Development of clinical pharmacy services for intensive care units in Korea. Springerplus. (2014) 3:34. doi: 10.1186/2193-1801-3-34
22. Li, XX, Zheng, SQ, Gu, JH, Huang, T, Liu, F, Ge, QG, et al. Drug-related problems identified during pharmacy intervention and consultation: implementation of an intensive care unit pharmaceutical care model. Front Pharmacol. (2020) 11:571906. doi: 10.3389/fphar.2020.571906
23. Martins, RR, Silva, LT, and Lopes, FM. Impact of medication therapy management on pharmacotherapy safety in an intensive care unit. Int J Clin Pharm. (2019) 41:179–88. doi: 10.1007/s11096-018-0763-0
24. Abu-Oliem, A, Al-Sharayri, M, and Hakuz, N. A clinical trial to investigate the role of clinical pharmacist in resolving/preventing drug related problems in ICU patients who receive anti-infective therapy. Jordan. J Pharm Sci. (2013) 6:292–8. doi: 10.12816/0001507
25. Klopotowska, JE, Kuiper, R, Van Kan, HJ, De Pont, A-C, Dijkgraaf, MG, Lie-A-Huen, L, et al. On-ward participation of a hospital pharmacist in a Dutch intensive care unit reduces prescribing errors and related patient harm: an intervention study. Crit Care. (2010) 14:R174. doi: 10.1186/cc9278
26. Kucukarslan, SN, Corpus, K, Mehta, N, Mlynarek, M, Peters, M, Stagner, L, et al. Evaluation of a dedicated pharmacist staffing model in the medical intensive care unit. Hosp Pharm. (2013) 48:922–30. doi: 10.1310/hpj4811-922
27. Aghili, M, and Kasturirangan, MN. Management of Drug-Drug Interactions among critically ill patients with chronic kidney disease: impact of clinical Pharmacist's interventions. Indian J Crit Care Med. (2021) 25:1226–31. doi: 10.5005/jp-journals-10071-23919
28. Fitzmaurice, MG, Wong, A, Akerberg, H, Avramovska, S, Smithburger, PL, Buckley, MS, et al. Evaluation of potential drug-drug interactions in adults in the intensive care unit: A systematic review and Meta-analysis. Drug Saf. (2019) 42:1035–44. doi: 10.1007/s40264-019-00829-y
29. Bosma, BE, van den Bemt, P, Melief, P, van Bommel, J, Tan, SS, and Hunfeld, NGM. Pharmacist interventions during patient rounds in two intensive care units: clinical and financial impact. Neth J Med. (2018) 76:115–24.
30. Chapuis, C, Albaladejo, P, Billon, L, Catoire, C, Chanoine, S, Allenet, B, et al. Integrating a pharmacist into an anaesthesiology and critical care department: is this worthwhile? Int J Clin Pharm. (2019) 41:1491–8. doi: 10.1007/s11096-019-00909-0
31. Bonmarchand, G, Czernichow, P, Chrétien, P, Massari, P, Lecomte, F, Hantute, N, et al. Drugs used in a medical intensive care unit. Ann Fr Anesth Reanim. (1986) 5:497–501. doi: 10.1016/s0750-7658(86)80036-3
32. Mittal, N, Mittal, R, Singh, I, Shafiq, N, and Malhotra, S. Drug utilisation study in a tertiary care center: recommendations for improving hospital drug dispensing policies. Indian J Pharm Sci. (2014) 76:308–14.
33. Hwang, HS, Lee, SH, Lee, H, Kim, KS, Chung, SJ, and Lee, JG. Effects of nutrition consultation on nutritional status in critically ill surgical patients. J Korean Soc Parent Enteral Nutr. (2015) 7:28–34. doi: 10.15747/jcn.2015.7.1.28
34. Alberda, C, Gramlich, L, Jones, N, Jeejeebhoy, K, Day, AG, Dhaliwal, R, et al. The relationship between nutritional intake and clinical outcomes in critically ill patients: results of an international multicenter observational study. Intensive Care Med. (2009) 35:1728–37. doi: 10.1007/s00134-009-1567-4
35. Lee, H, Ryu, K, Sohn, Y, Kim, J, Suh, GY, and Kim, E. Impact on patient outcomes of pharmacist participation in multidisciplinary critical care teams: A systematic review and Meta-analysis. Crit Care Med. (2019) 47:1243–50. doi: 10.1097/ccm.0000000000003830
36. Mackenzie, SL, Zygun, DA, Whitmore, BL, Doig, CJ, and Hameed, SM. Implementation of a nutrition support protocol increases the proportion of mechanically ventilated patients reaching enteral nutrition targets in the adult intensive care unit. JPEN J Parenter Enteral Nutr. (2005) 29:74–80. doi: 10.1177/014860710502900274
Keywords: clinical pharmacist, intensive care unit, renal dysfunction, drug-related problem, nutrition
Citation: Özgan B, Ayhan YE, Apikoglu S and Karakurt S (2024) Clinical pharmacist interventions in nutrition-and drug-related problems in critically ill patients with renal dysfunction: a non-randomized controlled study. Front. Med. 11:1473719. doi: 10.3389/fmed.2024.1473719
Edited by:
Hao Li, Shanghai Jiao Tong University, ChinaReviewed by:
Zhiyan Zhan, Shanghai Children’s Medical Center, ChinaFarshid Sadeghipour, Lausanne University Hospital, Switzerland
Copyright © 2024 Özgan, Ayhan, Apikoglu and Karakurt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Betül Özgan, YmV0dWwub3pnYW5AbWFybWFyYS5lZHUudHI=
 Betül Özgan
Betül Özgan Yunus Emre Ayhan2
Yunus Emre Ayhan2