- 1Hepatobiliary Pancreatic Surgery, Banan Hospital Affiliated of Chongqing Medical University, Chongqing, China
- 2Xiangya School of Medicine, Central South University, Changsha, China
- 3College of Education, Wenzhou University, Wenzhou, China
Background: This study assesses the worldwide cardiovascular disease (CVD) burden attributed to air pollution, utilizing data from the Global Burden of Disease Study 2021.
Methods: We explored the impact of air pollution on CVDs globally, regionally, and nationally, while considering correlations with age, gender, and socio-demographic index (SDI). A decomposition analysis was conducted to discern the contributions of aging, population growth, and epidemiological shifts to the changes in disability-adjusted life years (DALYs) from 1990 to 2021. Additionally, an ARIMA model was used to forecast the future CVD burden through 2050.
Results: In 2021, air pollution was responsible for approximately 2.46 million deaths and 58.3 million disability-adjusted life years (DALYs) attributable to CVDs, with a discernible decrease over the period studied. The greatest impacts were observed in individuals aged 75–79 and over 80, particularly among males. The decomposition analysis indicated that shifts in epidemiology were the primary factors driving these changes. Future projections suggest potential increases in mortality and DALY rates in regions with low and high-middle SDI, alongside rising age-standardized death and mortality rates in high SDI areas.
Conclusion: These findings underscore the urgency of implementing targeted CVD prevention and air pollution control strategies to mitigate the impact on public health.
1 Introduction
Air pollution, a complex mixture of particulate matter and gases, is associated with approximately 8.8 million additional deaths annually. Nearly half of these deaths are linked to external environmental pollutants, while the remainder result from indoor pollution, substantially increasing the global health burden (1, 2). According to the World Health Organization (WHO) and the Global Burden of Disease Study, air pollution ranks as the fourth leading global cause of death and disease, just behind high blood pressure, tobacco use, and dietary risks (3). While the effects of air pollution on respiratory health are well-known, it is important to emphasize that cardiovascular diseases (CVDs) account for half of the deaths associated with air pollution (2). Additionally, solid evidence worldwide shows that approximately 20% of CVD deaths are related to exposure to air pollutants such as PM2.5, PM10, ozone (O3), and nitrogen dioxide (NO2) (4, 5). Beyond traditional risk factors, the impact of environmental factors like ambient air pollution is increasingly recognized as critical (6).
Extensive epidemiological studies demonstrate that air pollution aggravates cardiovascular risk factors including hyperlipidemia, hypertension, atherosclerotic changes, and diabetes, thus elevating the risk of cardiovascular conditions such as ischemic heart diseases, heart failure, and strokes (7–9). Several mechanisms, including oxidative stress induction, inflammation, disturbances in autonomic and neuroendocrine functions, increased vasoconstriction and coagulation, and particulate matter penetration into the bloodstream, are proposed to explain the connection between air pollution and CVDs (10, 11). Consequently, the link between air pollution and cardiovascular health has emerged as a critical issue in environmental and public health, necessitating comprehensive research to effectively identify and address this challenge.
In this research, we examined the impact of air pollution on the cardiovascular health burden, analyzing trends in mortality and DALYs across different age groups, genders, and socio-demographic indices from 1990 to 2021. We also projected future trends using the autoregressive integrated moving average (ARIMA) model, validated by multiple prior studies (12, 13). These results are intended to aid in decision-making regarding the prevention of cardiovascular diseases and the management of air pollution.
2 Method
2.1 Study data
Data for our study were obtained from the Global Burden of Disease Study (GBD) 2021, which is available at http://ghdx.healthdata.org/gbd-results-tool. The GBD 2021 provides a comprehensive assessment of 369 diseases and injuries, along with 87 risk factors across 204 countries spanning from 1990 to 2021 (2).
The specific methodologies employed to calculate the burden of cardiovascular diseases (CVDs) are elaborated in additional references [14, 15]. This summary describes the approach adopted in GBD 2021. Sources for CVD mortality data included vital registration systems, surveillance data, and verbal autopsies. This vital registration data was then refined to enhance accuracy, addressing data gaps and miscoding issues (16). These adjusted datasets were analyzed using the Cause of Death Ensemble model (CODEm), which generated estimates of CVD mortality segmented by location, year, age, and gender (15, 17). Furthermore, a comparative risk assessment was conducted to identify principal risk factors for CVDs. The population attributable fraction (PAF) was computed to quantify the contribution of air pollution to the CVD burden. Estimates for CVD mortality and disability-adjusted life years (DALYs) related to air pollution were derived by applying these specific PAFs to the mortality and DALY figures across different demographics (2).
DALYs serve as an inclusive metric of disease impact, combining years lost due to premature mortality (YLLs) and years lived with disability (YLDs). YLLs were calculated by multiplying the number of deaths from cardiovascular diseases in each age group by the remaining life expectancy for that age group. YLDs were determined by multiplying the prevalence of CVDs by the severity-adjusted disability weights (DWs) (16). The Socio-Demographic Index (SDI), a composite indicator ranging from 0 (worst) to 100 (best), was derived from three factors: the total fertility rate for individuals under 25 years old (TFU25), average educational attainment for those over 15 years old (EDU15+), and adjusted per capita income. Based on the SDI, the 204 countries and territories were classified into five categories: low SDI, low-middle SDI, middle SDI, high-middle SDI, and high SDI (16).
2.2 Risk factor estimate
In the Global Burden of Disease (GBD) study, the estimation of risk factors is guided by the comparative risk assessment (CRA) framework. This approach begins by determining the relative risk (RR) of specific health outcomes associated with exposure to risk factors, utilizing meta-regression and systematic reviews. Subsequent steps involve Bayesian statistical models, such as spatio-temporal Gaussian process regression (ST-GPR) and disease model meta-regression (DisMod-MR), to estimate the levels and distribution of exposure for each risk factor. Additionally, theoretical minimum risk exposure levels (TMREL) are established. These represent the exposure levels that would ideally minimize health risks. Based on these assessments, population attributable fractions (PAF) and summary exposure values (SEV) are calculated. These metrics indicate the potential changes in health outcomes that could result if exposures were reduced to TMREL. These calculations are essential for assessing the disease burden attributable to various risk factors.
2.3 Statistical analysis
Age-standardized rates (ASR) were utilized to normalize mortality and DALY rates across nations with varying age distributions and demographic profiles. A linear model was applied to the natural logarithm of these rates over time, formulated as y = α + βx + ϵy, where xxx represents the year, and y is the natural logarithm of the rate. The estimated annual percentage change (EAPC) was calculated as 100 × (eβ − 1)100, along with a 95% confidence interval (95% CI). An increase in ASR was identified when both the EAPC and the lower boundary of the 95% CI were positive. Conversely, a decrease was noted if the EAPC and the upper boundary of the 95% CI were negative. If neither condition was met, ASR was considered stable during the study period (18, 19).
The relationship between ASR and the socio-demographic index (SDI) was examined using a Gaussian process regression framework with Loess smoothing and assessed through Spearman rank order correlation tests (18, 20). A decomposition analysis quantified the impacts of aging populations, population growth, and epidemiological shifts on overall DALY changes from 1990 to 2021, with methodologies detailed in earlier publications (21).
Additionally, the ARIMA model was employed to evaluate the influence of air pollution on CVD trends and forecast global, regional, and national trends from 2020 to 2050. Known formally as the “integral moving average autoregressive model,” the ARIMA model integrates differential, integral, moving average, and autoregressive components. In the ARIMA model (p, d, q), “AR” signifies autoregressive, with ppp denoting the number of autoregressive terms; “MA” represents the moving average component, with qqq as the number of moving average terms; and ddd refers to the number of differencing steps to achieve stationarity (22). Model selection was optimized using the Akaike information criterion (AIC) and the Bayesian information criterion (BIC).
A 95% uncertainty interval (UI) was calculated for all measurements. Rates were expressed per 100,000 individuals. Data management, analysis, and visualization were performed using R software version 4.3.2.
3 Result
3.1 Spatiotemporal patterns of CVD attributable to air pollution
In 2021, CVD led to about 19.41 million deaths and 428.33 million disability-adjusted life years (DALYs). Among them, air pollution led to approximately 2.46 million deaths and 58.30 million DALYs due to cardiovascular diseases (CVDs), with an age-standardized mortality rate (ASMR) of 53.62 (95% UI, 42.70–64.57) and an age-standardized DALY rate (ASDR) of 1161.77 (95% UI, 939.61–1380.37) per 100,000 population. Over the last thirty years, the CVD burden from air pollution has shown a significant decline (Tables 1, 2; Supplementary Table S1).
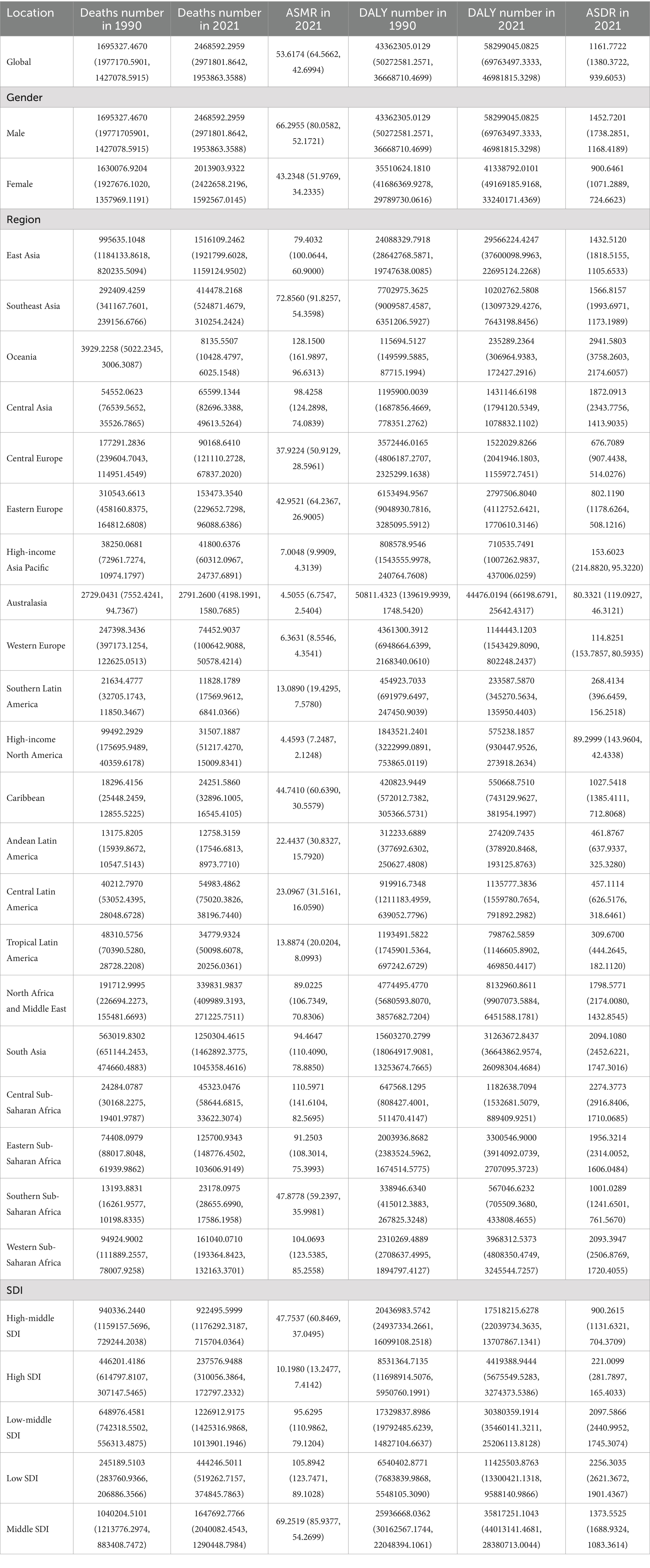
Table 1. Global and regional deaths and DALYs of CVDs attributable to air pollution in 1990 and 2021 in 27 global regions and different genders.
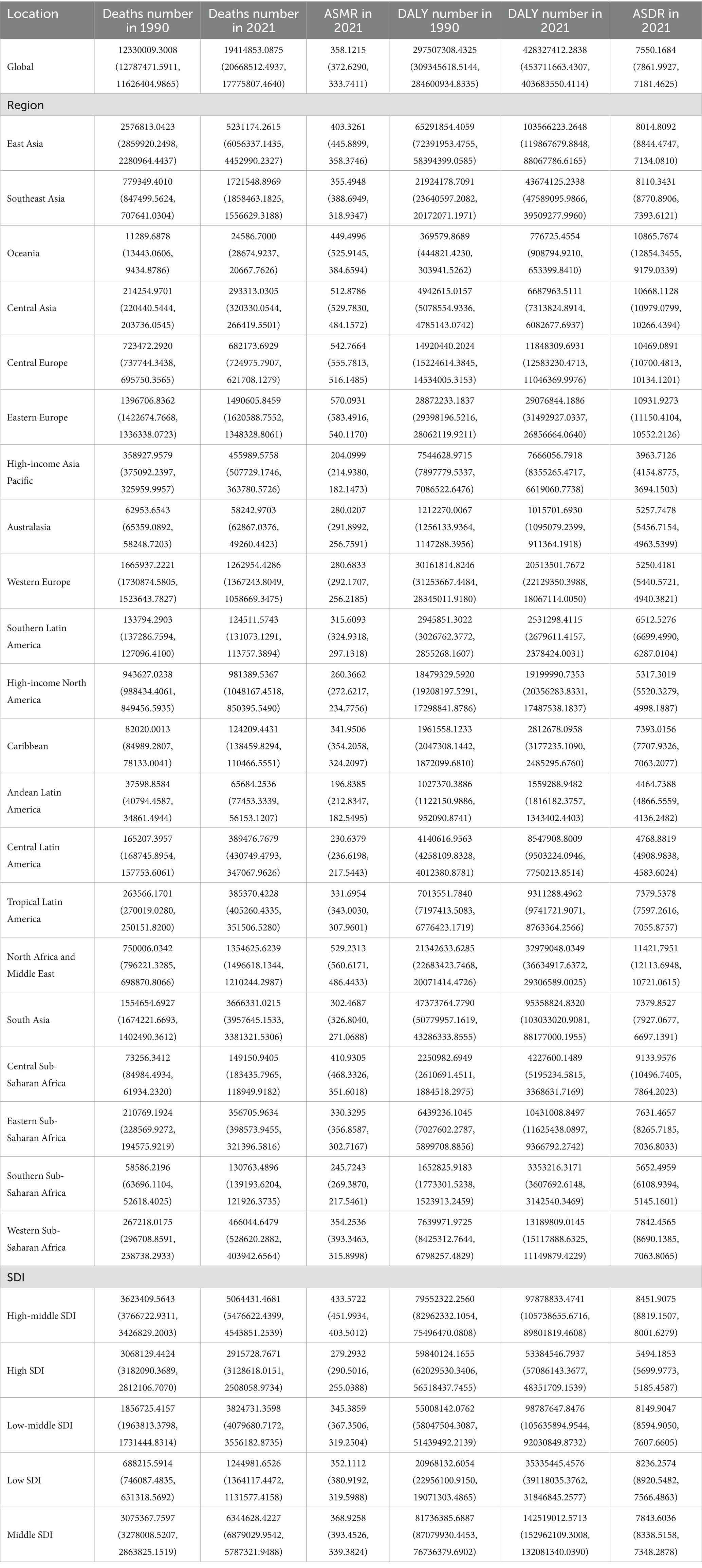
Table 2. Global and regional deaths and DALYs of CVDs in 1990 and 2021 in 27 global regions and different genders.
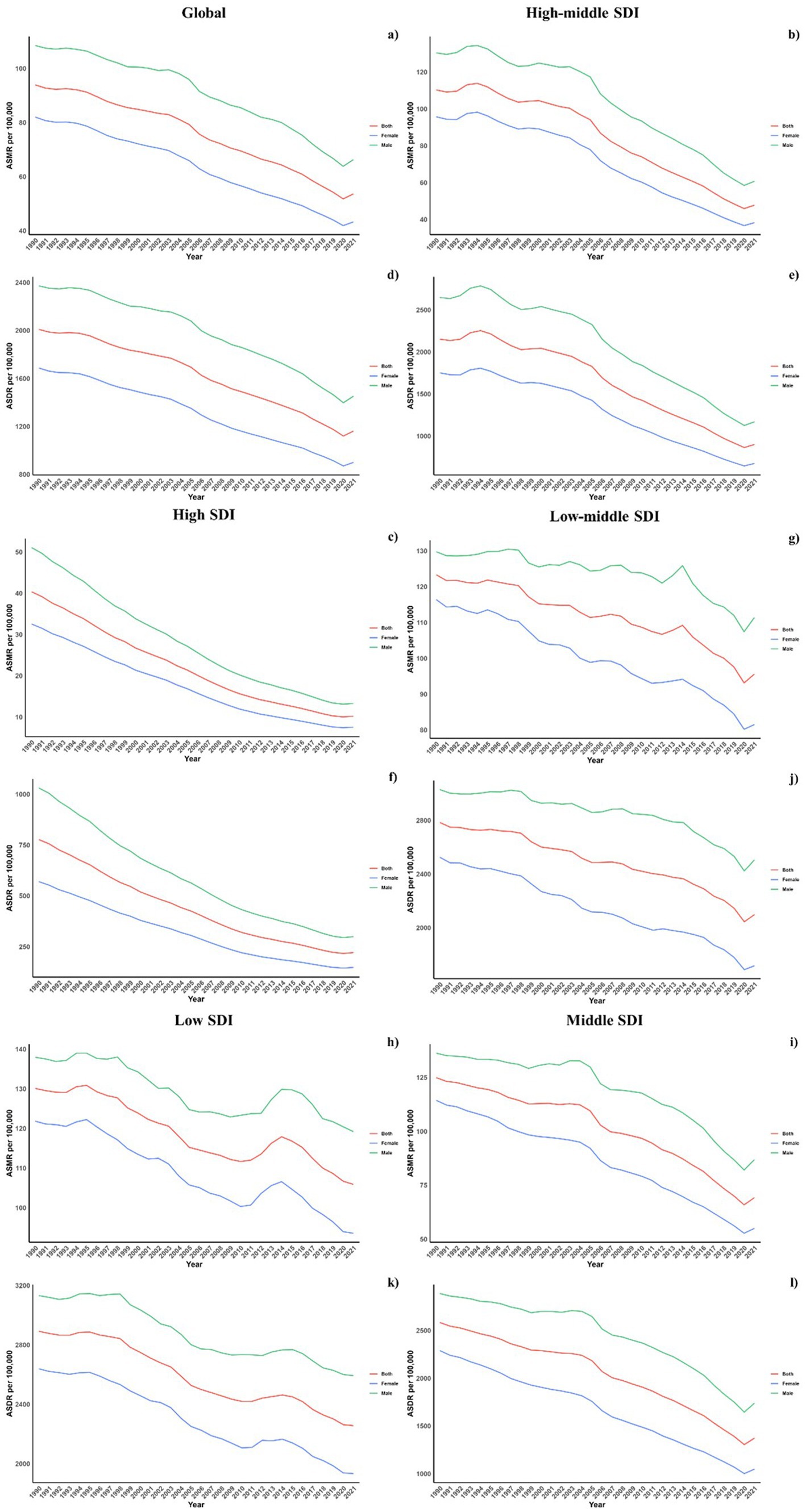
Regarding socio-demographic index (SDI) regions, higher SDI regions experienced a notably lower CVD burden due to air pollution. In contrast, the low, low-middle, and middle SDI regions observed substantial increases in CVD burdens linked to air pollution. All SDI regions recorded slight reductions in both ASMR and ASDR due to air pollution. This spatiotemporal pattern of CVD burden was aligned with this (Table 1; Supplementary Table S1; Table 2, and Figure 1).
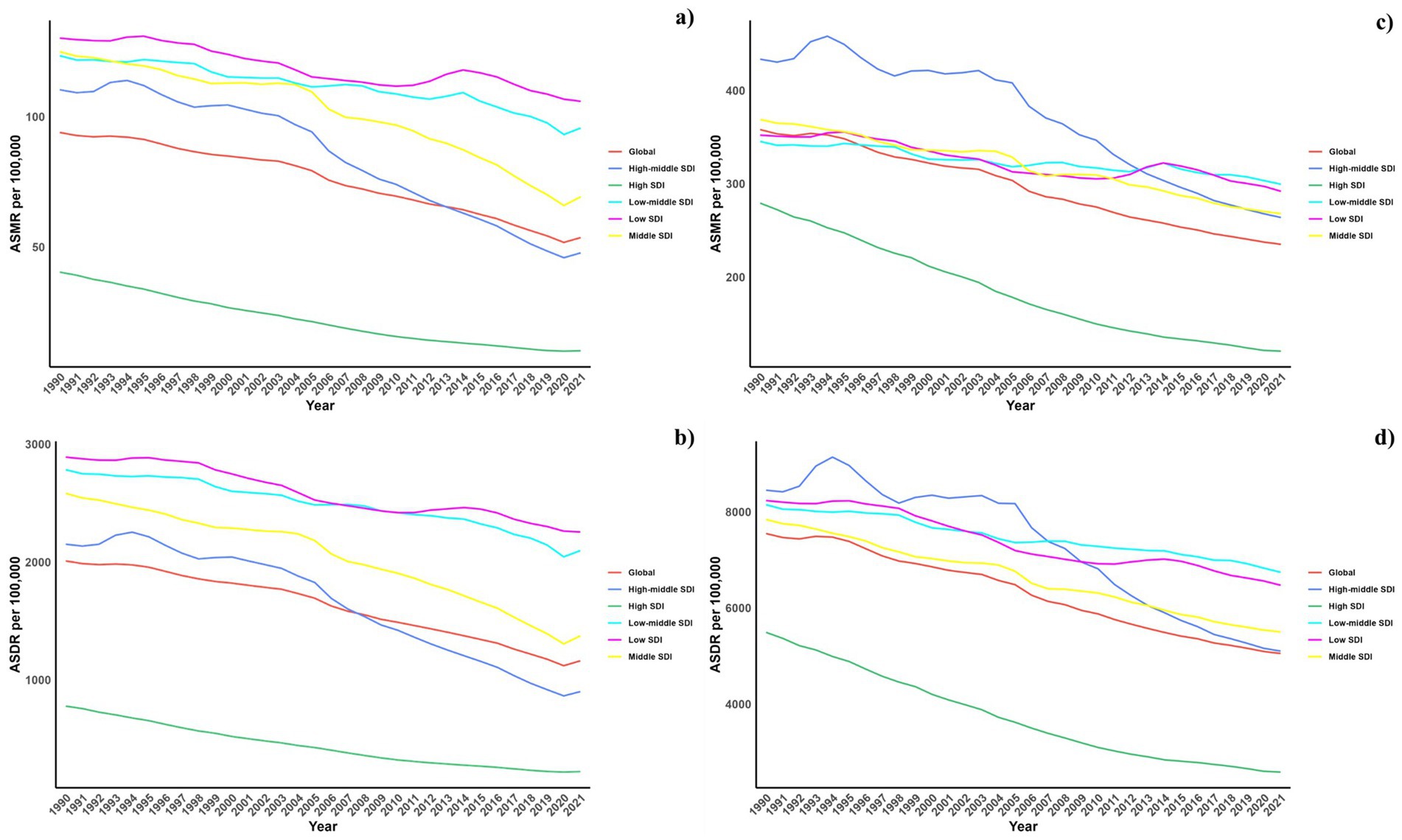
Figure 1. Temporal trends of ASMR and ASDR of CVDs attributable to air pollution (a,b) and total CVD burden (c,d) from 1990 to 2021 in different SDI regions.
Regionally, East Asia, Southeast Asia, and East Asia reported the highest CVD burdens from air pollution, marked by the greatest numbers of deaths and DALYs. Conversely, Oceania, Central Sub-Saharan Africa, and Western Sub-Saharan Africa recorded the highest rates of ASDR and ASMR. While increases in ASMR due to air pollution were noted across all regions, decreases were seen in the European and American regions, except in Central Latin America. Similarly, ASDR trends generally increased, except in high-income Asia Pacific and Australasia where decreases were observed (Tables 1, 2). This spatiotemporal pattern of CVD burden was aligned with this.
Nationally, in 2021, the ASDR of CVDs related to air pollution varied widely worldwide, with the highest rates noted in countries across North Africa, the Middle East, and Central Asia (Figure 2). From 1990 to 2019, most nations saw a decline in ASDR linked to air pollution, with the exception of 15 countries including Honduras [Estimate (95% UI): 0.1202 (0.3439, −0.1029)], Libya [Estimate (95% UI): 0.7887 (1.1774, 0.4014)], United Arab Emirates [Estimate (95% UI): −0.7593 (−0.2459, −1.2701)], Kenya [Estimate (95% UI): 0.4531 (0.6890, 0.2178)], Mozambique [Estimate (95% UI): 0.9241 (1.1264, 0.7222)], Lesotho [Estimate (95% UI): 2.1309 (2.6863, 1.5785)], Zimbabwe [Estimate (95% UI): 1.9544 (2.5467, 1.3656)], Burkina Faso [Estimate (95% UI): 0.0403 (0.1177, −0.0370)], Cameroon [Estimate (95% UI): 0.0070 (0.4280, −0.4121)], Chad [Estimate (95% UI): 0.1475 (0.3563, −0.0608)], Gambia [Estimate (95% UI): 0.1015 (0.2334, −0.0303)], Guinea [Estimate (95% UI): 0.3354 (0.4689, 0.2021)], Sierra Leone [Estimate (95% UI): 0.0179 (0.2127, −0.1765)], Northern Mariana Islands [Estimate (95% UI): 0.1044 (0.7569, −0.5440)], and Palau [Estimate (95% UI): 0.0144 (0.8653, −0.8294)]. The spatial and temporal trends of ASMR paralleled those of ASDR (Table 1; Supplementary Table S1, and Figures 2, 3). This spatiotemporal pattern of CVD burden was aligned with this.
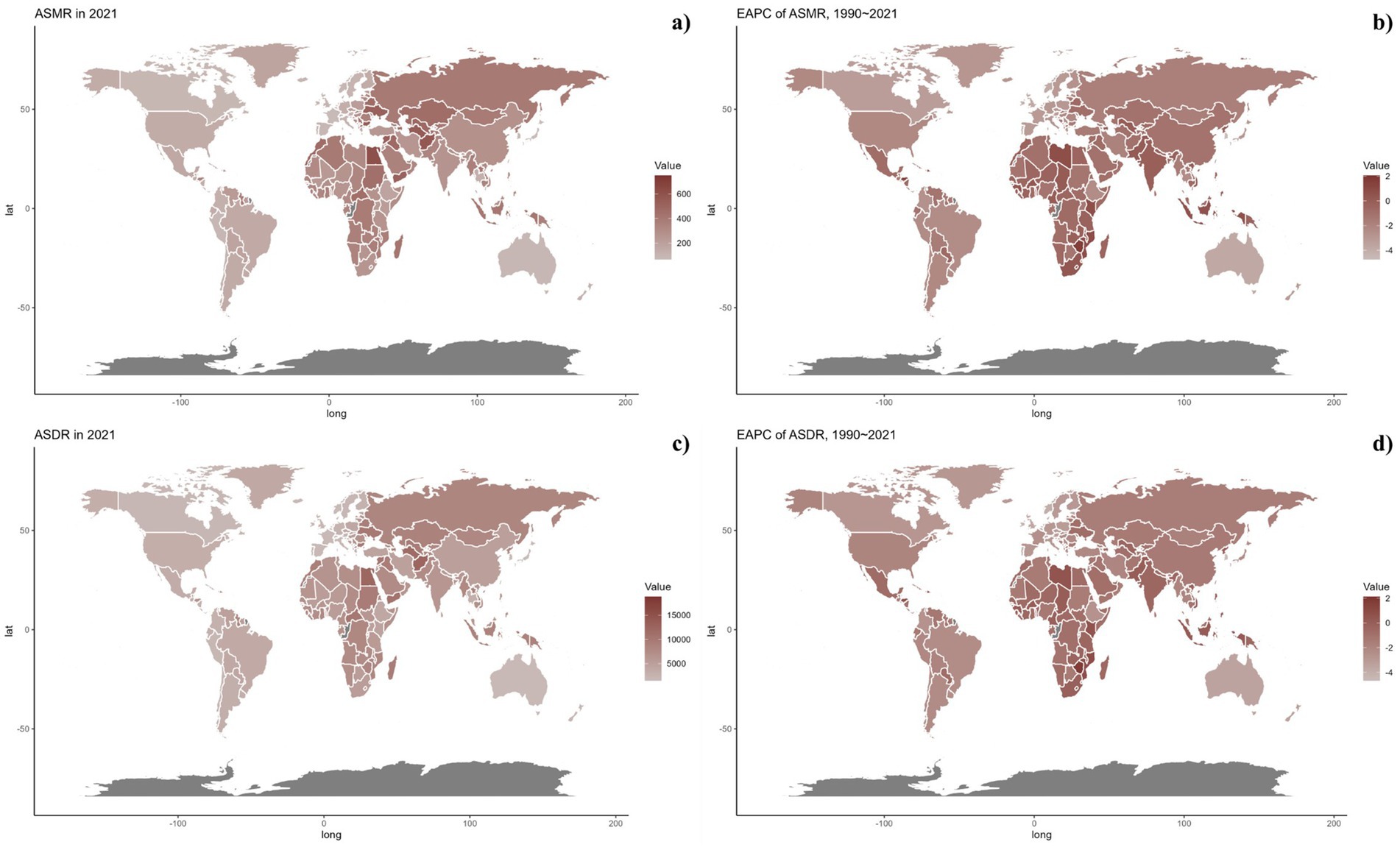
Figure 2. Global distribution of ASMR (a) and ASDR (c) of CVDs for both sexes in 2021 in 204 countries and territories. EAPC of ASMR (b) and ASDR (d) of CVDs from 1990 to 2021 in 204 countries and territories.
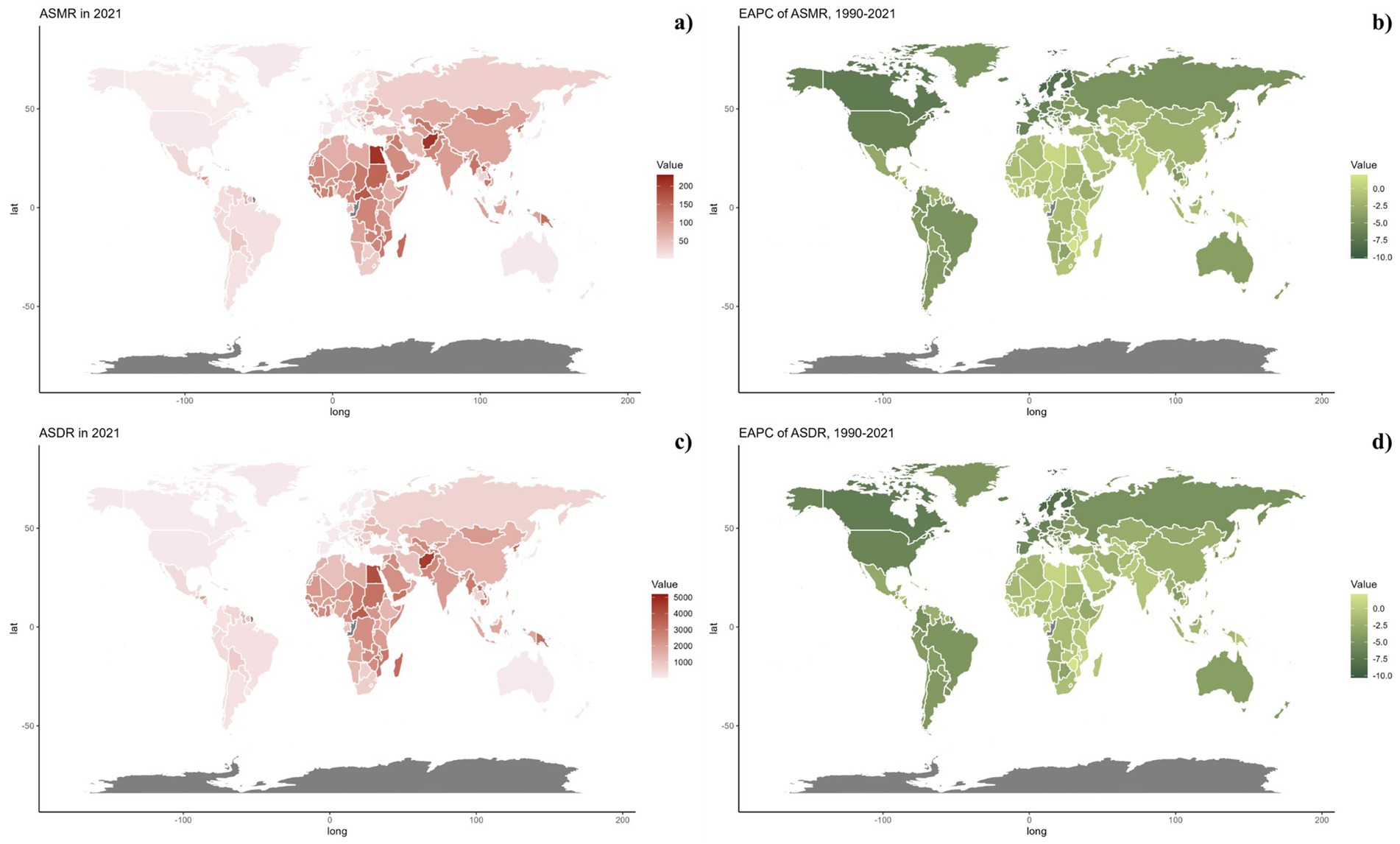
Figure 3. Global distribution of ASMR (a) and ASDR (c) of CVDs attributable to air pollution for both sexes in 2021 in 204 countries and territories. EAPC of ASMR (b) and ASDR (d) of CVDs attributable to air pollution from 1990 to 2021 in 204 countries and territories.
3.2 Age and sex patterns
Figure 4 displays the age-specific global mortality and DALY rates for CVDs in 2021, along with their changes from 1990 to 2021. These rates demonstrate a J-shaped distribution, showing a rise in mortality and DALY rates among individuals under 75, with a notable escalation in the 75–79 and 80+ age brackets. In all age categories, males consistently exhibited higher mortality rates due to air pollution compared to females. Likewise, the DALY rates linked to air pollution were greater for all age groups. Similarly, it can be also found that for the total CVD burden, males also suffered more (Figure 5).
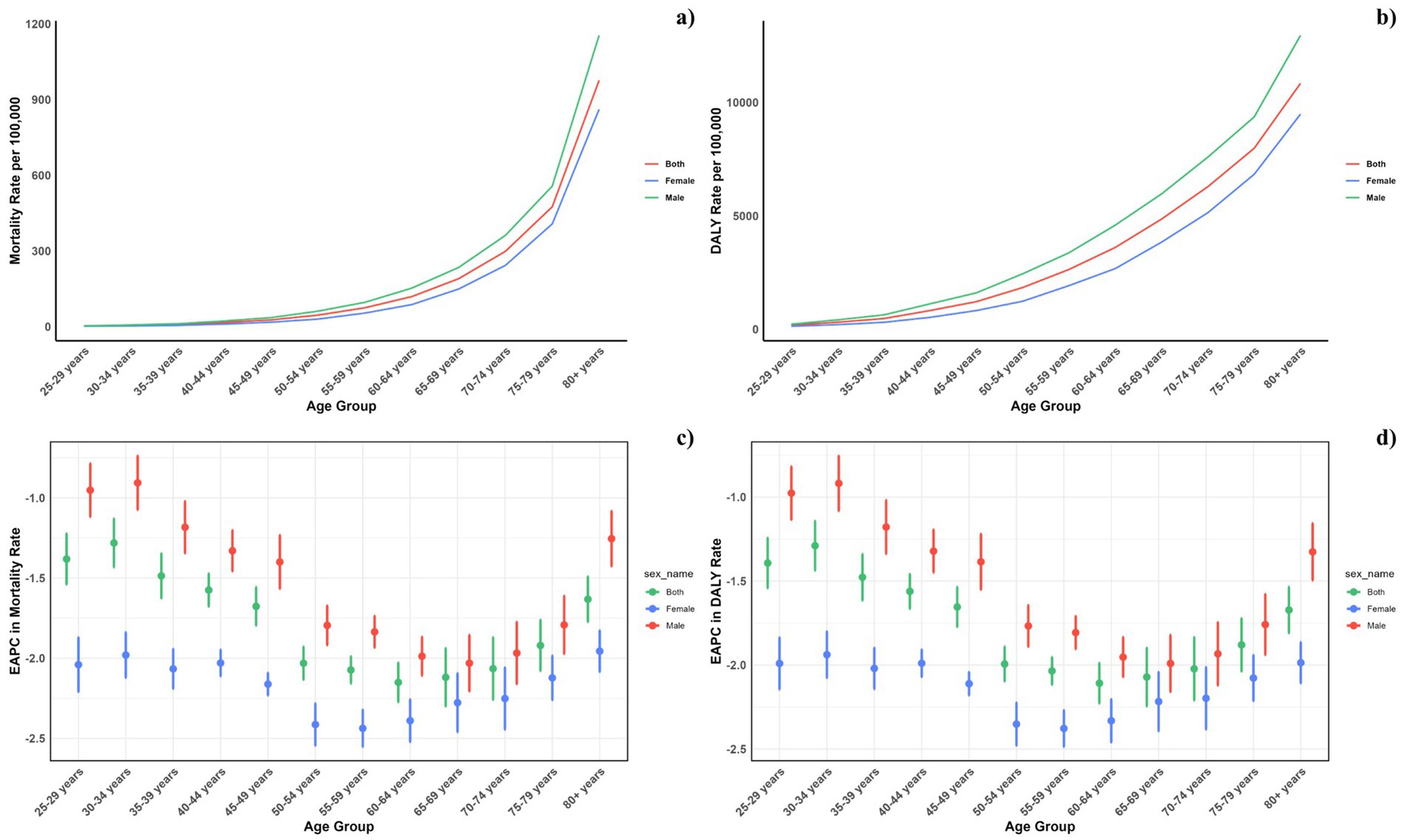
Figure 4. Age-specific rates of global deaths (a) and DALYs (b) of CVDs attributable to air pollution, by sex, in 2021 and the corresponding EAPC of global deaths (c) and DALYs (d) from 1990 to 2021.
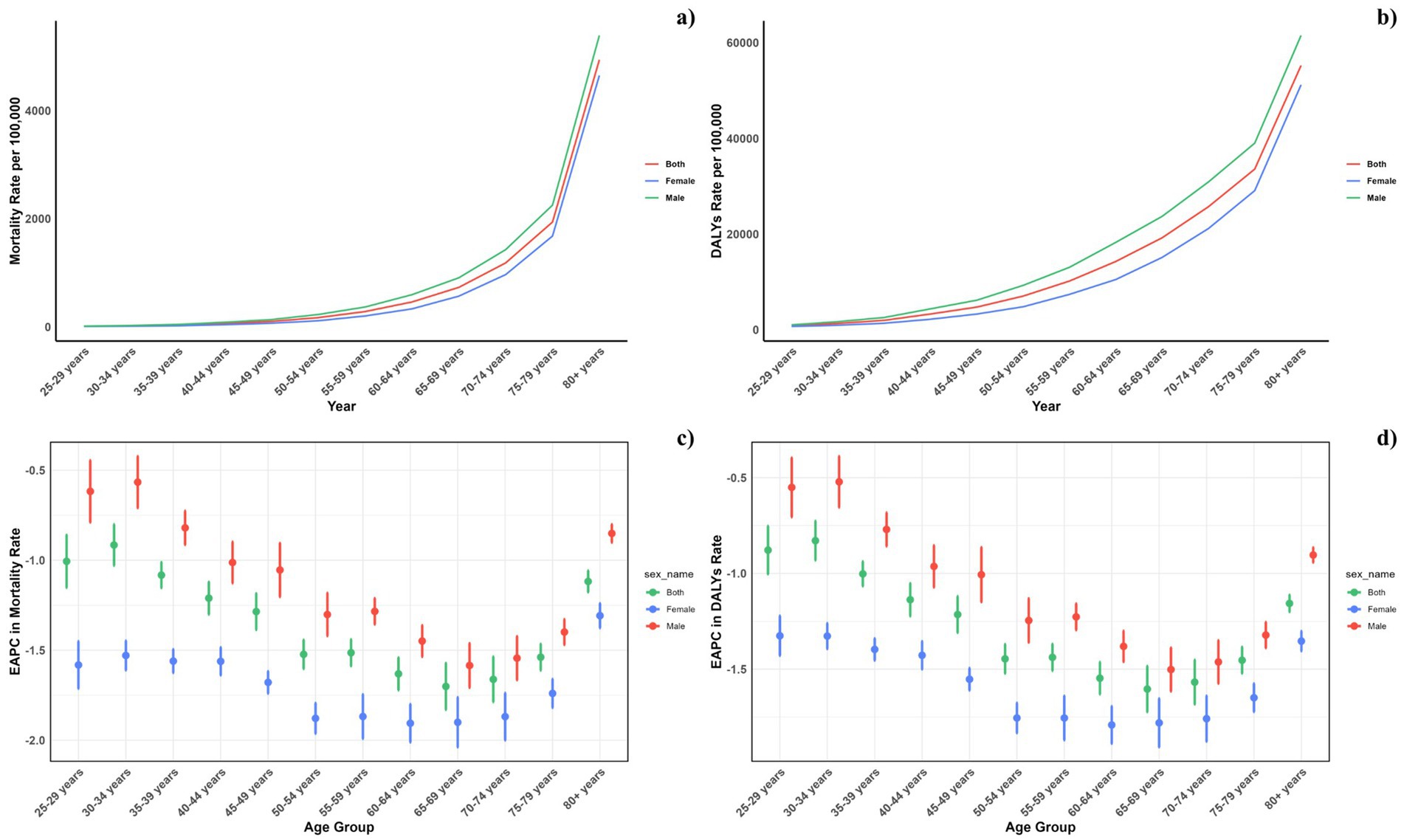
Figure 5. Age-specific rates of global deaths (a) and DALYs (b) of CVDs, by sex, in 2021 and the corresponding EAPC of global deaths (c) and DALYs (d) from 1990 to 2021.
Across all SDI regions, males reported higher mortality and DALY rates, with the gender disparity persisting uniformly across these regions (Figure 6).
3.3 Association with the socio-demographic index
Figure 7 illustrates the comparison between the observed and projected age-standardized DALY (ASDR) and mortality rates (ASMR) attributable to air pollution against socio-demographic index (SDI) values at both regional and national levels from 1990 to 2021. An inverse relationship was noted between ASDR and SDI, suggesting a decline in burden as SDI increased. Regions such as Eastern Europe, Oceania, Central Asia, Central Europe, North Africa, East Asia, the Middle East, and Southeast Asia displayed higher than expected ASDR during this period. Additionally, observed ASDR values exceeded projections in Central Latin America, Tropical Latin America, Southern Latin America, and the Caribbean. The pattern for observed versus expected ASMR based on SDI at regional levels mirrored the ASDR findings. Figure 7 also depict the observed versus expected ASDR and ASMR at the national level for 2019, showing a similar inverse correlation between these rates and SDI values, both regionally and nationally.
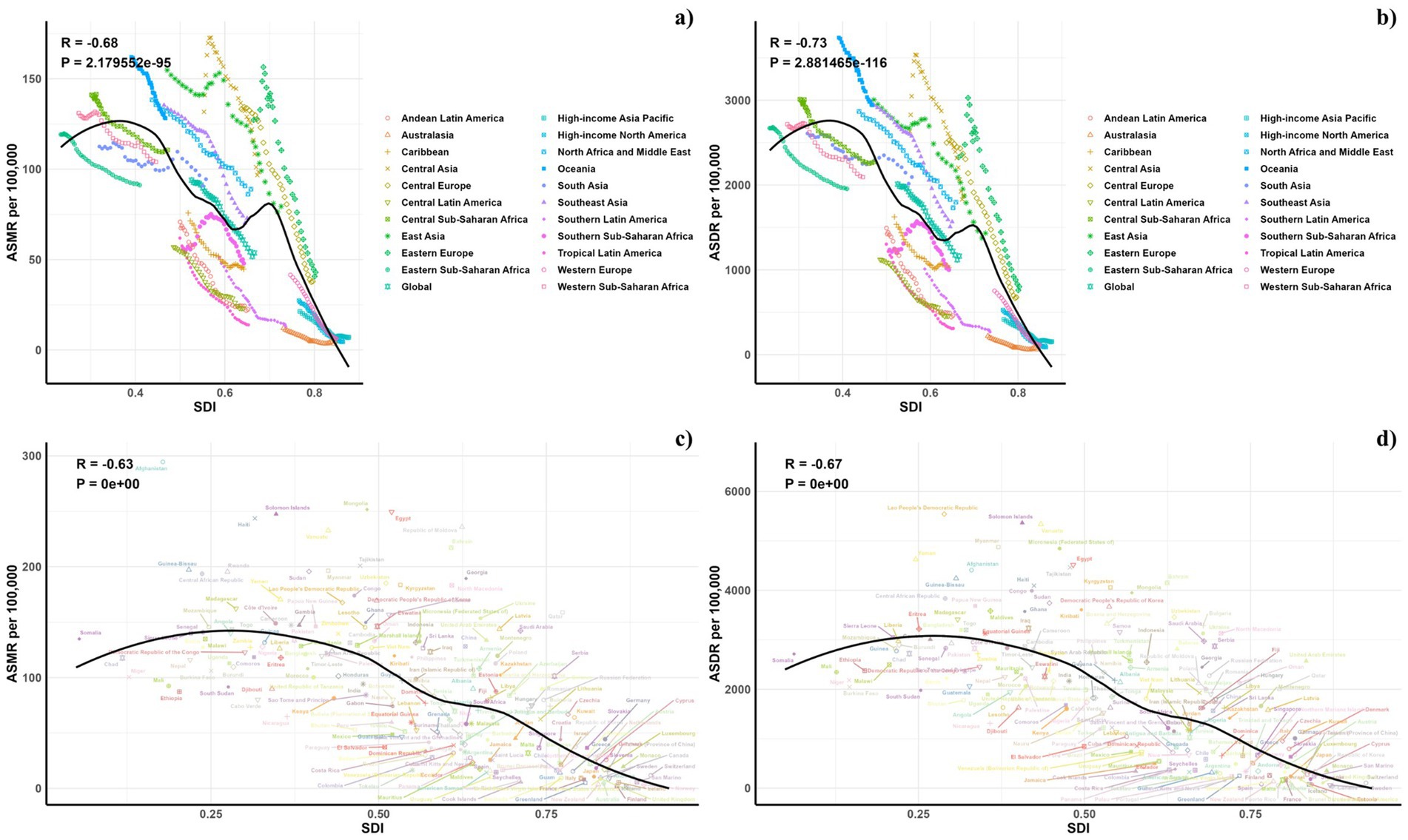
Figure 7. Correlations between ASMR (a,c) and ASDR (b,d) of CVDs attributable to air pollution and SDI at the regional level (a,b) and the national level (c,d).
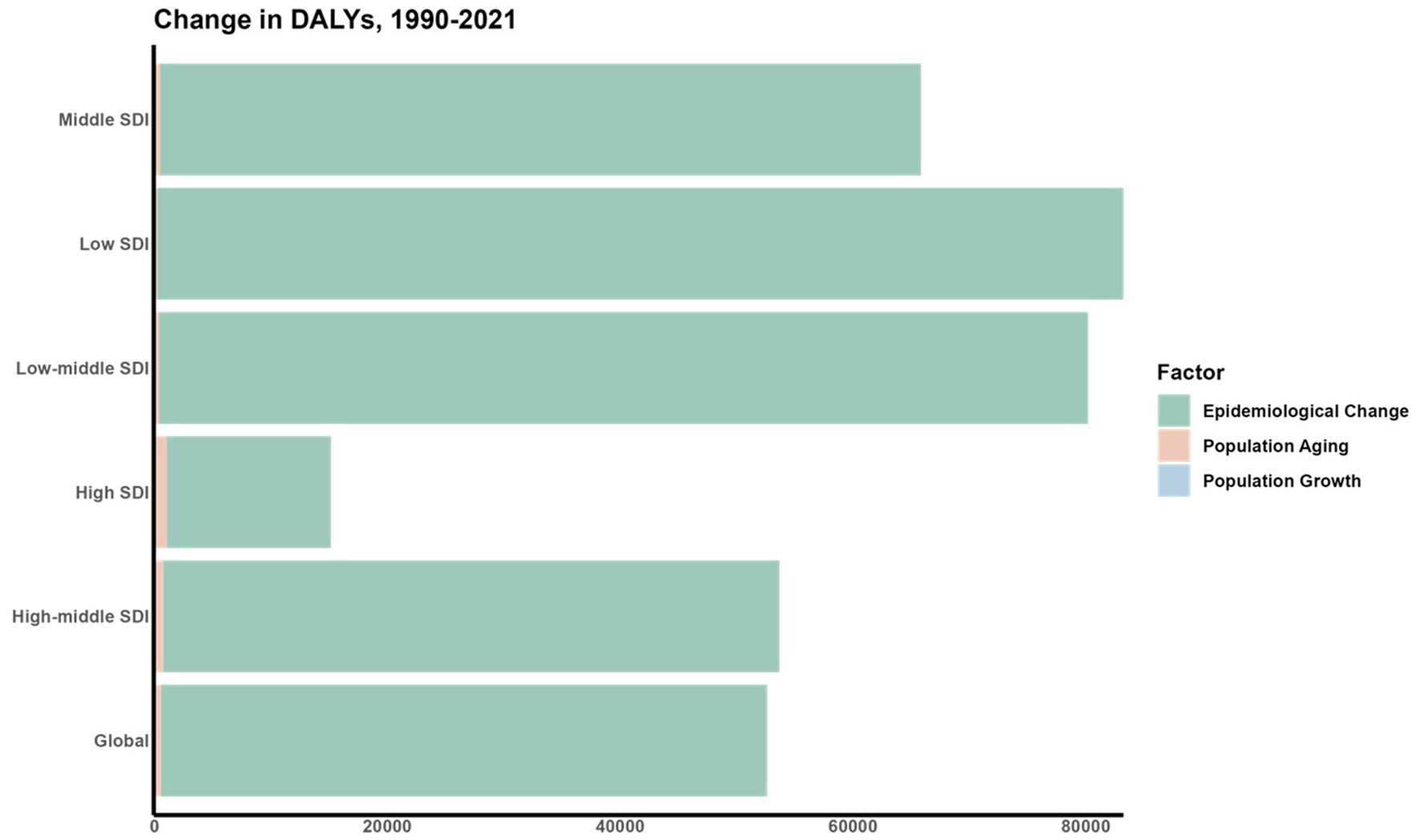
3.4 Decomposition analysis of the change in DALYs
A decomposition analysis was conducted to assess the impact of three key factors: aging populations, demographic growth, and shifts in epidemiology, on the variation in DALYs from 1990 to 2021 (Figure 8). In tracking the trends of ASDR, shifts in epidemiological factors were identified as major contributors to the fluctuations in DALYs for CVDs linked to air pollution across various SDI regions.
3.5 Forecasts for the mortality, DALYs rate, ASMR and ASDR of CVD attributable to air pollution in global (2022–2050)
Forecasts for mortality rates, DALY rates, ASMR, and ASDR of CVDs linked to air pollution are presented in Figures 9–11. At the regional level, increases are anticipated in both low and high-middle SDI regions for mortality and DALYs rate, whereas only the high SDI regions are expected to see rises in ASDR and ASMR. Nationally, the distribution is projected to remain consistent in 2030 and 2050. Nonetheless, the projected burden of CVDs due to air pollution is significantly greater in China compared to other countries for both 2030 and 2050.
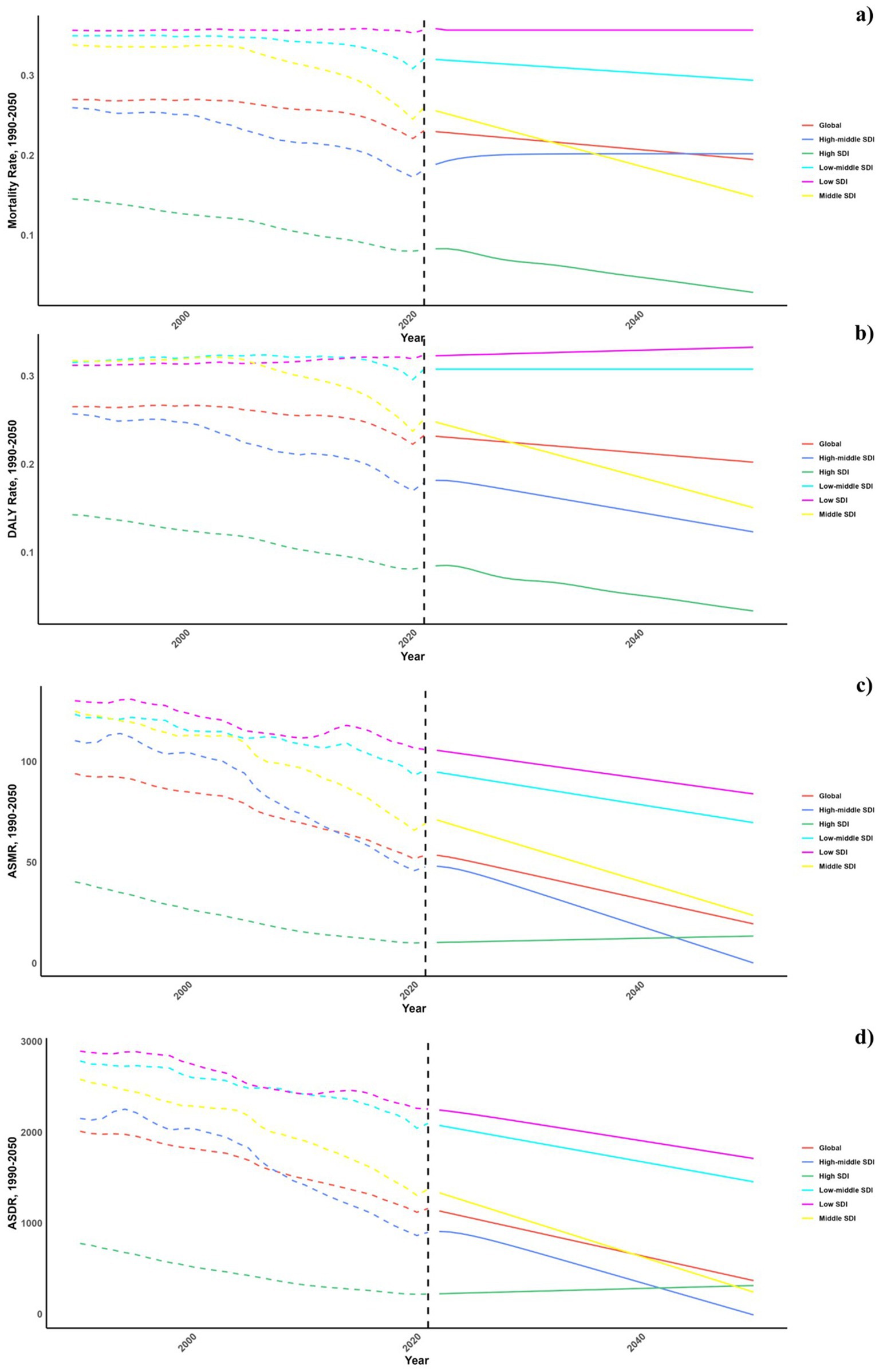
Figure 9. Estimated trends of mortality rate (a), DALYs rate (b), ASMR (c) and ASDR (d), 1990–2050 at the regional level.
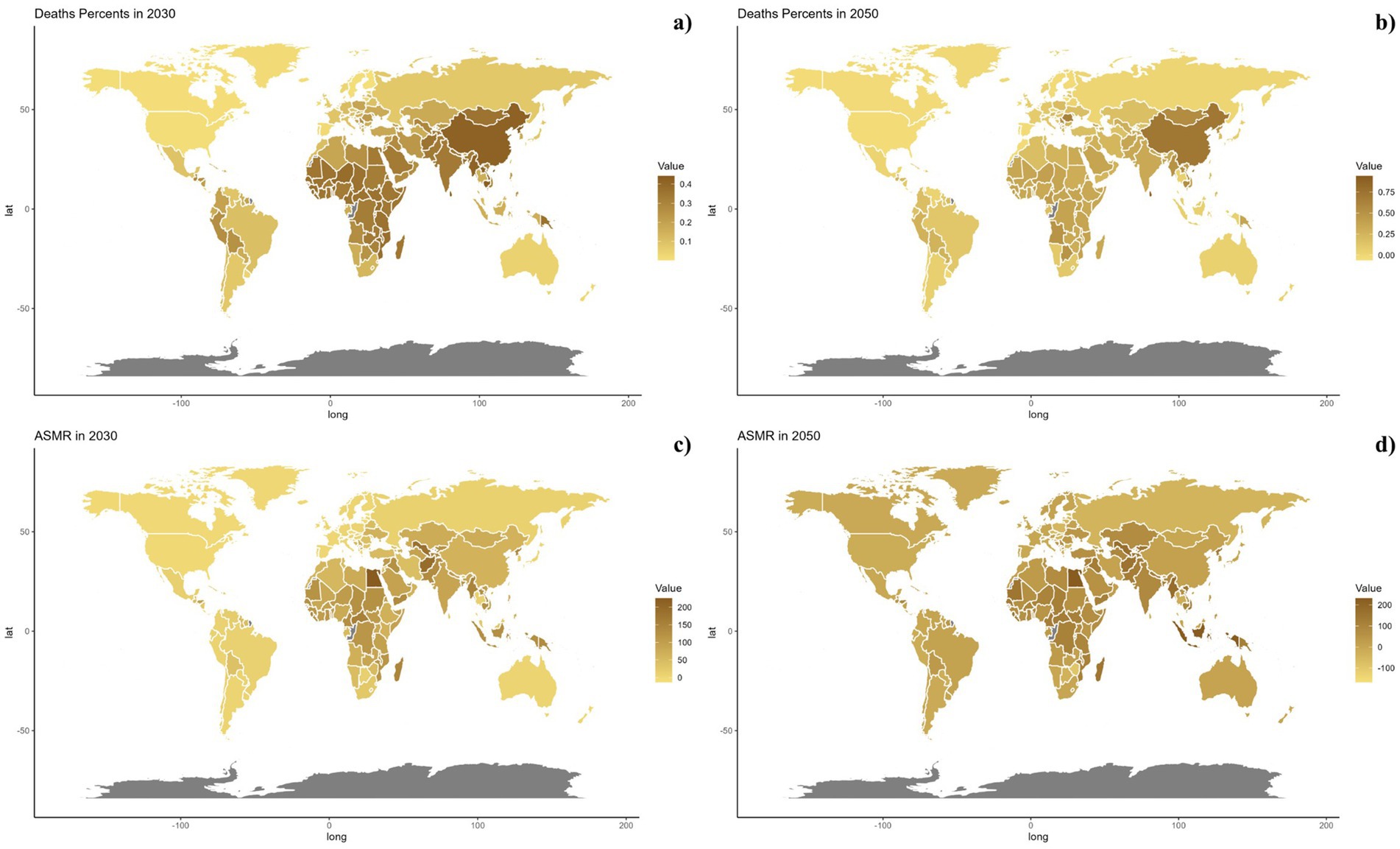
Figure 10. Estimated trends of mortality rate (a,b) and ASMR (c,d) in 2030 (a,c) and 2050 (b,d) at the national level.
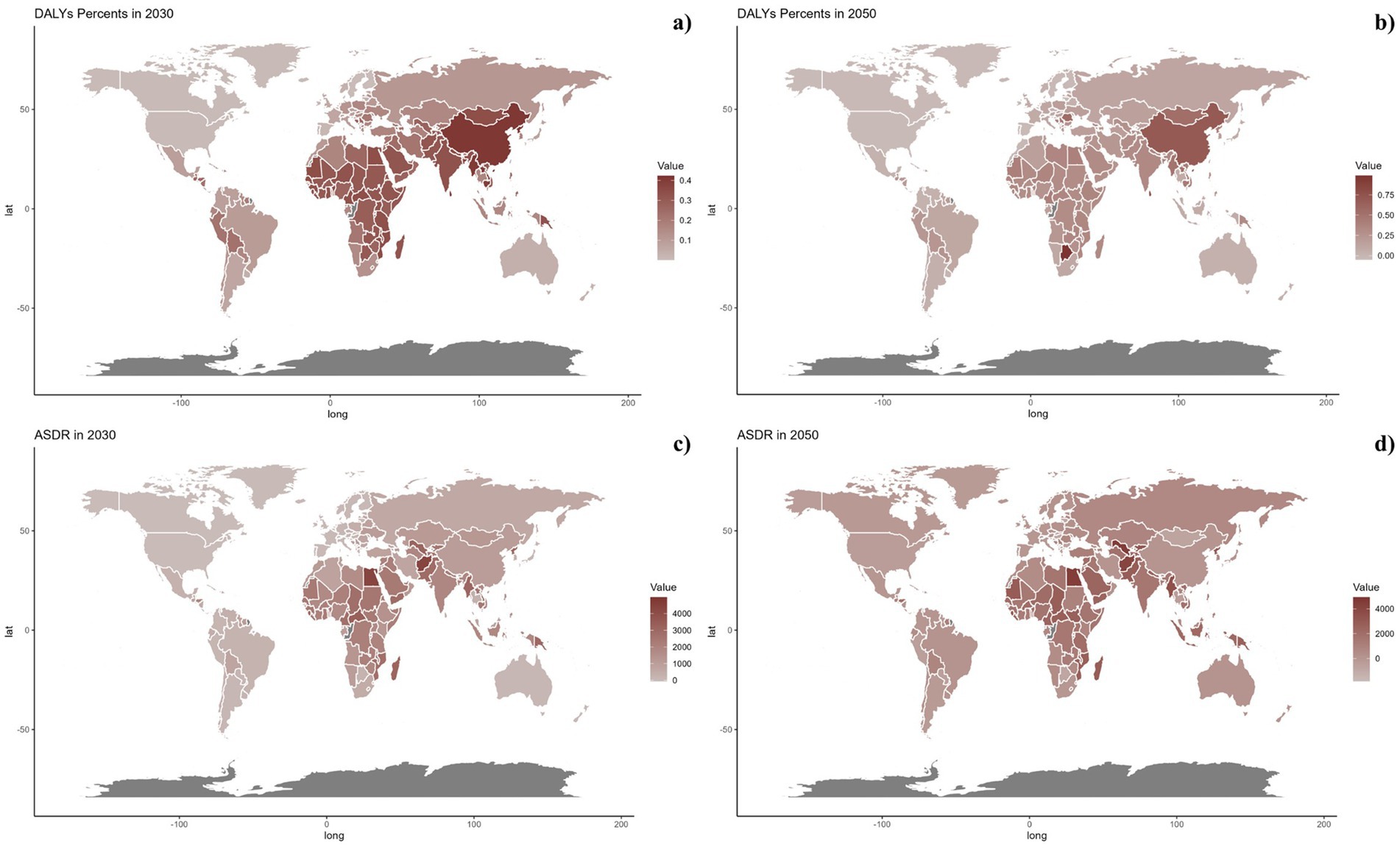
Figure 11. Estimated trends of DALYs rate (a,b) and ASDR (c,d) in 2030 (a,c) and 2050 (b,d) at the national level.
4 Discussion
This research evaluated the global burden of cardiovascular diseases (CVD) attributable to air pollution from 1990 to 2021, uncovering a significant decline over the period studied. The age groups most impacted were those below 75–79 and above 80, with males showing higher incidence rates. A decomposition analysis revealed that epidemiological shifts were the primary factors driving these changes. Predictions indicate increasing burdens in regions with low and high-middle socio-demographic index (SDI), along with rises in age-standardized death rate (ASDR) and age-standardized mortality rate (ASMR) in high SDI areas. To our knowledge, this is the first comprehensive assessment of the CVD impact from air pollution.
Key factors contributing to health-related adverse effects from air pollution include ozone and fine particulate matter. These pollutants affect bodily systems through mechanisms like oxidative stress, inflammation, and disturbances in endothelial and autonomic functions. The cardiovascular health impacts of air pollution can be traced from the initial physiological responses to pollutants, through their transmission, to their effects on target organs (23).
Primary reactions occurring in the lungs involve oxidative stress, local inflammation, and receptor activations initiated by pollutants such as PM2.5. These pollutants are ingested by alveolar macrophages through respiratory phagocytosis, leading to prolonged inflammatory responses in other tissues. The presence of pollutants activates inflammatory pathways involving NOD-like and Toll-like receptors, and the generation of reactive oxygen species (24–29).
Exposure to air pollution has been linked to systemic inflammation, primarily driven by the production of biological intermediates such as oxidized phospholipids and 7-ketocholesterol. These intermediates activate the TLR4, NOX2, and NCF1 pathways, leading to vascular and systemic inflammation (28). Furthermore, 7-ketocholesterol plays a role in promoting thrombosis, atherosclerosis, and endothelial dysfunction through CD36 pathways (30). Prolonged inhalation of PM2.5 escalates the production of reactive oxygen species (ROS) and inflammatory infiltration in the vasculature, potentially resulting in diastolic dysfunction, increased cardiac afterload, and compromised coronary reserve, which can culminate in cardiac complications such as left ventricular hypertrophy and fibrosis.
In vivo studies show that PM2.5 affects plasma tissue plasminogen activator levels, influencing thrombus formation. Variability in the composition and concentration of air pollution may explain discrepancies in research findings. PM2.5 also impacts the central nervous system, potentially causing autonomic dysfunction or adrenal activation through stimulation of the olfactory nerve (31–33). Exposure to ozone may similarly activate the adrenal axis (34), potentially leading to conditions like insulin resistance or hypertension. Moreover, while PM2.5 is known to induce thrombo-inflammatory responses and affect DNA methylation, its complete impact on genomic methylation and chromatin structure is yet to be fully elucidated (35, 36).
Chronic exposure to environmental pollutants can lead to lasting organ changes, thereby increasing vulnerability to cardiometabolic diseases such as diabetes, hypertension, and renal disease, while also accelerating atherosclerosis (37). Studies have linked even low-level, prolonged exposure to PM2.5 with a heightened risk of cardiovascular mortality, noting that increases in PM2.5 levels are associated with rising risks of cardiovascular death (38–41). In contrast, ozone exposure exhibits a weaker connection with CVD mortality (42). Additionally, prolonged exposure to PM2.5 correlates with greater carotid intima-media thickness, which suggests advancing atherosclerosis and elevated blood pressure, though these relationships remain subject to debate (23, 43–47). PM2.5 exposure also heightens the risk of severe cardiac events in individuals with pre-existing heart conditions and contributes to the development of diabetes and insulin resistance (29, 48–50).
Previous studies have highlighted significant variations in the CVD burden from air pollution across BRICS nations (51). In 2019, Brazil’s age-standardized rate of CVD attributable to air pollution was approximately five times lower than India’s. During the observation period, Brazil saw the most significant decrease in CVD burden from air pollution and household air pollution (HAP) from solid fuels. Brazilian authorities have implemented measures to reduce emissions from both stationary and mobile sources, leading to a noticeable decline in air pollutants from 1996 to 2009 (52, 53). Initiatives like the Family Health Program and integrated approaches to managing non-communicable diseases have also contributed to reducing the CVD burden (53, 54).
Russia has seen a notable reduction in the CVD burden, especially from ambient particulate matter pollution (51). Since 1990, the WHO European Centre for Environment and Health launched programs to improve air quality and control pollution, including monitoring its health effects in the European Region, which includes Russia (55). In 2005, WHO organized a workshop in Eastern Europe, the Caucasus, and Central Asia (EECCA) to develop strategies to mitigate air pollution’s health impacts in EECCA nations, including Russia (56).
Conversely, South Africa has experienced a rising trend in all-ages CVD fatalities and a slow decline in age-standardized CVD mortality linked to air pollution (51). A recent report highlighted that pollution continues to pose significant health and economic risks in low-and middle-income countries (LMICs), with ambient air pollution remaining the main cause of pollution-related illnesses and deaths in Africa. Consequently, the incidence of non-communicable disease (NCD) deaths related to air pollution is rising in many African nations (57–59).
To combat CVDs, controlling air pollution is critical. Governments should enforce air quality standards and establish mechanisms for monitoring, enforcing, and ensuring accountability in pollution management. Technological advances in industry and sustainable energy initiatives like solar power are essential. Researchers should focus on creating and evaluating methods to mitigate air pollution’s effects on cardiovascular health. Public education on pollution risks and personal health measures such as using air filters and consuming antioxidants are necessary. Future strategies might include integrating data from multiple environmental sources and fostering cross-disciplinary research to refine pollution risk assessments. Clinicians should guide those at risk to maintain a diet rich in antioxidants and fresh produce.
The COVID-19 pandemic’s lockdown measures significantly impacted air quality in various regions around the world. Research shows that due to reduced traffic flow and the suspension of certain industrial activities, many cities experienced a marked improvement in air pollution levels in a short period. For instance, in 2020, several major cities globally reported significant reductions in the concentrations of nitrogen oxides (NOx) and fine particulate matter (PM2.5). This improvement in air quality, particularly among the working-age population that is at high risk for cardiovascular diseases, may have helped reduce the incidence of cardiovascular events (60).
Further studies indicate a close association between improved air quality and the reduction in acute cardiovascular events. For example, during the pandemic, there was a decrease in emergency room visits and hospitalizations for various cardiovascular diseases, although this may also be influenced by changes in people’s healthcare-seeking behaviors during the pandemic. Additionally, the reduction in outdoor activities during the pandemic could have lessened the health risks associated with air pollution for individuals with respiratory and cardiovascular conditions (61).
However, the positive impacts brought about by the lockdown measures may be temporary. As economic activities gradually resume, air pollution levels could quickly return to pre-pandemic levels or even exceed them in some areas. Monitoring and researching air quality in the post-pandemic and post-pandemic periods will be crucial for understanding long-term trends and developing appropriate responses (62).
Our study faces a few significant limitations. First, there is a lack of primary data from underdeveloped regions, especially in Sub-Saharan Africa, where estimates were largely derived through mathematical models, resulting in broad uncertainty ranges. Second, air pollution consists of a complex blend of multiple components, each with distinct physicochemical characteristics and toxicological effects, which differ widely by geographic area and season.
5 Conclusion
This research conducted a detailed evaluation of the global CVD burden due to air pollution from 1990 to 2021, noting a substantial reduction in this burden over time. The most affected age groups were those under 75–79 and those over 80, with men experiencing a higher impact. The analysis indicated that changes in epidemiology were the primary drivers of these trends. Moreover, future projections suggest an increase in both ASDR and ASMR in low and high-middle SDI regions, with only high SDI regions expected to see rises.
This highlights a critical need for policymakers to develop and enhance targeted preventive strategies for specific demographic groups to mitigate the CVD burden linked to air pollution moving forward.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
QM: Formal analysis, Writing – original draft. XaZ: Data curation, Formal analysis, Investigation, Writing – original draft. XiZ: Visualization, Writing – original draft. YK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1472996/full#supplementary-material
References
1. Lelieveld, J, Pozzer, A, Pöschl, U, Fnais, M, Haines, A, and Münzel, T. Loss of life expectancy from air pollution compared to other risk factors: a worldwide perspective. Cardiovasc Res. (2020) 116:1910–7. doi: 10.1093/cvr/cvaa025
2. GBD 2019 Risk Factors Collaborators . Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396:1223–49. doi: 10.1016/S0140-6736(20)30752-2
3. Brauer, M, Casadei, B, Harrington, RA, Kovacs, R, and Sliwa, KWHF Air Pollution Expert Group. Taking a stand against air pollution-the impact on cardiovascular disease: a joint opinion from the World Heart Federation, American College of Cardiology, American Heart Association, and the European Society of Cardiology. Circulation. (2021) 143:e800–4. doi: 10.1161/CIRCULATIONAHA.120.052666
4. Macchi, C, Sirtori, CR, Corsini, A, Mannuccio Mannucci, P, and Ruscica, M. Pollution from fine particulate matter and atherosclerosis: a narrative review. Environ Int. (2023) 175:107923. doi: 10.1016/j.envint.2023.107923
5. Newby, DE, Mannucci, PM, Tell, GS, Baccarelli, AA, Brook, RD, Donaldson, K, et al. Expert position paper on air pollution and cardiovascular disease. Eur Heart J. (2015) 36:83–93b. doi: 10.1093/eurheartj/ehu458
6. Lan, Y, and Wu, S. Impacts of environmental insults on cardiovascular aging. Curr Environ Health Rep. (2022) 9:11–28. doi: 10.1007/s40572-022-00335-x
7. Rajagopalan, S, Al-Kindi, SG, and Brook, RD. Air pollution and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. (2018) 72:2054–70. doi: 10.1016/j.jacc.2018.07.099
8. Kong, Y, Zhu, X, Yang, Y, Xu, H, Ma, L, and Zuo, Y. Knowledge, attitudes, practice, and public health education demand regarding PARI prevention: a cross-sectional study among Chinese undergraduates. Front Public Health. (2024) 12:1387789. doi: 10.3389/fpubh.2024.1387789
9. Orellano, P, Reynoso, J, Quaranta, N, Bardach, A, and Ciapponi, A. Short-term exposure to particulate matter (PM10 and PM2.5), nitrogen dioxide (NO2), and ozone (O3) and all-cause and cause-specific mortality: systematic review and meta-analysis. Environ Int. (2020) 142:105876. doi: 10.1016/j.envint.2020.105876
10. Brook, RD, Rajagopalan, S, Pope, CA 3rd, Brook, JR, Bhatnagar, A, Diez-Roux, AV, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 21:2331–78. doi: 10.1161/CIR.0b013e3181dbece1
11. Kong, YZ, Xu, HT, Li, CY, Yang, Y, Zhu, XY, and Zuo, Y. Construction of PARI public health education programs for Chinese undergraduates: a Delphi study. Front Public Health. (2024) 12:1390011. doi: 10.3389/fpubh.2024.1390011
12. Wafa, HA, Marshall, I, Wolfe, CDA, Xie, W, Johnson, CO, Veltkamp, R, et al. Burden of intracerebral haemorrhage in Europe: forecasting incidence and mortality between 2019 and 2050. Lancet Reg Health Eur. (2024) 38:100842. doi: 10.1016/j.lanepe.2024.100842
13. Gong, Y, Jiang, Q, Zhai, M, Tang, T, and Liu, S. Thyroid cancer trends in China and its comparative analysis with G20 countries: projections for 2020–2040. J Glob Health. (2024) 14:04131. doi: 10.7189/jogh.14.04131
14. GBD 2019 LRI Collaborators . Age-sex differences in the global burden of lower respiratory infections and risk factors, 1990–2019: results from the Global Burden of Disease Study 2019. Lancet Infect Dis. (2022) 22:1626–47. doi: 10.1016/S1473-3099(22)00510-2
15. Safiri, S, Mahmoodpoor, A, Kolahi, AA, Nejadghaderi, SA, Sullman, MJM, Mansournia, MA, et al. Global burden of lower respiratory infections during the last three decades. Front Public Health. (2023) 10:1028525. doi: 10.3389/fpubh.2022.1028525
16. GBD 2019 Diseases and Injuries Collaborators . Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
17. Foreman, KJ, Lozano, R, Lopez, AD, and Murray, CJ. Modeling causes of death: an integrated approach using CODEm. Popul Health Metrics. (2012) 10:1. doi: 10.1186/1478-7954-10-1
18. Jin, X, Ren, J, Li, R, Gao, Y, Zhang, H, Li, J, et al. Global burden of upper respiratory infections in 204 countries and territories, from 1990 to 2019. EClinicalMedicine. (2021) 37:100986. doi: 10.1016/j.eclinm.2021.100986
19. Lu, H, Tan, Z, Liu, Z, Wang, L, Wang, Y, Suo, C, et al. Spatiotemporal trends in stroke burden and mortality attributable to household air pollution from solid fuels in 204 countries and territories from 1990 to 2019. Sci Total Environ. (2021) 775:145839. doi: 10.1016/j.scitotenv.2021.145839
20. Safiri, S, Kolahi, AA, Hoy, D, Buchbinder, R, Mansournia, MA, Bettampadi, D, et al. Global, regional, and national burden of neck pain in the general population, 1990–2017: systematic analysis of the Global Burden of Disease Study 2017. BMJ. (2020) 368:m791. doi: 10.1136/bmj.m791
21. Hu, J, Ke, R, Teixeira, W, Dong, Y, Ding, R, Yang, J, et al. Global, regional, and national burden of CKD due to glomerulonephritis from 1990 to 2019: a systematic analysis from the Global Burden of Disease Study 2019. Clin J Am Soc Nephrol. (2023) 18:60–71. doi: 10.2215/CJN.0000000000000017
22. Zhu, B, Wang, Y, Zhou, W, Jin, S, Shen, Z, Zhang, H, et al. Trend dynamics of gout prevalence among the Chinese population, 1990–2019: a joinpoint and age-period-cohort analysis. Front Public Health. (2022) 10:1008598. doi: 10.3389/fpubh.2022.1008598
23. Al-Kindi, SG, Brook, RD, Biswal, S, and Rajagopalan, S. Environmental determinants of cardiovascular disease: lessons learned from air pollution. Nat Rev Cardiol. (2020) 17:656–72. doi: 10.1038/s41569-020-0371-2
24. Shoenfelt, J, Mitkus, RJ, Zeisler, R, Spatz, RO, Powell, J, Fenton, MJ, et al. Involvement of TLR2 and TLR4 in inflammatory immune responses induced by fine and coarse ambient air particulate matter. J Leukoc Biol. (2009) 86:303–12. doi: 10.1189/jlb.1008587
25. Becker, S, Fenton, MJ, and Soukup, JM. Involvement of microbial components and Toll-like receptors 2 and 4 in cytokine responses to air pollution particles. Am J Respir Cell Mol Biol. (2002) 27:611–8. doi: 10.1165/rcmb.4868
26. Cheng, Z, Kong, Y, Yang, W, Xu, H, Tang, D, and Zuo, Y. Association between serum copper and blood glucose: a mediation analysis of inflammation indicators in the NHANES (2011–2016). Front Public Health. (2024) 12:1401347. doi: 10.3389/fpubh.2024.1401347
27. Xu, X, Yavar, Z, Verdin, M, Ying, Z, Mihai, G, Kampfrath, T, et al. Effect of early particulate air pollution exposure on obesity in mice: role of p47phox. Arterioscler Thromb Vasc Biol. (2010) 30:2518–27. doi: 10.1161/ATVBAHA.110.215350
28. Kampfrath, T, Maiseyeu, A, Ying, Z, Shah, Z, Deiuliis, JA, Xu, X, et al. Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circ Res. (2011) 108:716–26. doi: 10.1161/CIRCRESAHA.110.237560
29. Münzel, T, Sørensen, M, Gori, T, Schmidt, FP, Rao, X, Brook, FR, et al. Environmental stressors and cardio-metabolic disease: part II-mechanistic insights. Eur Heart J. (2017) 38:557–64. doi: 10.1093/eurheartj/ehw294
30. Rao, X, Zhong, J, Maiseyeu, A, Gopalakrishnan, B, Villamena, FA, Chen, LC, et al. CD36-dependent 7-ketocholesterol accumulation in macrophages mediates progression of atherosclerosis in response to chronic air pollution exposure. Circ Res. (2014) 115:770–80. doi: 10.1161/CIRCRESAHA.115.304666
31. Münzel, T, Sørensen, M, Hahad, O, Nieuwenhuijsen, M, and Daiber, A. The contribution of the exposome to the burden of cardiovascular disease. Nat Rev Cardiol. (2023) 20:651–69. doi: 10.1038/s41569-023-00873-3
32. Zhang, D, Ma, Q, Wang, Z, Zhang, M, Guo, K, Wang, F, et al. β2-adrenoceptor blockage induces G1/S phase arrest and apoptosis in pancreatic cancer cells via Ras/Akt/NFκB pathway. Mol Cancer. (2011) 10:146. doi: 10.1186/1476-4598-10-146
33. Stafoggia, M, Oftedal, B, Chen, J, Rodopoulou, S, Renzi, M, Atkinson, RW, et al. Long-term exposure to low ambient air pollution concentrations and mortality among 28 million people: results from seven large European cohorts within the ELAPSE project. Lancet Planet Health. (2022) 6:e9–e18. doi: 10.1016/S2542-5196(21)00277-1
34. Jiang, Y, Huang, J, Li, G, Wang, W, Wang, K, Wang, J, et al. Ozone pollution and hospital admissions for cardiovascular events. Eur Heart J. (2023) 44:1622–32. doi: 10.1093/eurheartj/ehad091
35. Bind, MA, Lepeule, J, Zanobetti, A, Gasparrini, A, Baccarelli, A, Coull, BA, et al. Air pollution and gene-specific methylation in the normative aging study: association, effect modification, and mediation analysis. Epigenetics. (2014) 9:448–58. doi: 10.4161/epi.27584
36. Wang, T, Pehrsson, EC, Purushotham, D, Li, D, Zhuo, X, Zhang, B, et al. The NIEHS TaRGET II Consortium and environmental epigenomics. Nat Biotechnol. (2018) 36:225–7. doi: 10.1038/nbt.4099
37. Dwivedi, AK, Vishwakarma, D, Dubey, P, and Reddy, SY. Air pollution and the heart: updated evidence from meta-analysis studies. Curr Cardiol Rep. (2022) 24:1811–35. doi: 10.1007/s11886-022-01819-w
38. Lu, F, Xu, D, Cheng, Y, Dong, S, Guo, C, Jiang, X, et al. Systematic review and meta-analysis of the adverse health effects of ambient PM2.5 and PM10 pollution in the Chinese population. Environ Res. (2015) 136:196–204. doi: 10.1016/j.envres.2014.06.029
39. Krewski, D, Jerrett, M, Burnett, RT, Ma, R, Hughes, E, Shi, Y, et al. Extended follow-up and spatial analysis of the American Cancer Society study linking particulate air pollution and mortality. Res Rep Health Eff Inst. (2009) 140:5–114.
40. Crouse, DL, Peters, PA, van Donkelaar, A, Goldberg, MS, Villeneuve, PJ, Brion, O, et al. Risk of nonaccidental and cardiovascular mortality in relation to long-term exposure to low concentrations of fine particulate matter: a Canadian national-level cohort study. Environ Health Perspect. (2012) 120:708–14. doi: 10.1289/ehp.1104049
41. Burnett, R, Chen, H, Szyszkowicz, M, Fann, N, Hubbell, B, Pope, CA 3rd, et al. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc Natl Acad Sci USA. (2018) 115:9592–7. doi: 10.1073/pnas.1803222115
42. Lim, CC, Hayes, RB, Ahn, J, Shao, Y, Silverman, DT, Jones, RR, et al. Long-term exposure to ozone and cause-specific mortality risk in the United States. Am J Respir Crit Care Med. (2019) 200:1022–31. doi: 10.1164/rccm.201806-1161OC
43. Provost, EB, Madhloum, N, Int Panis, L, De Boever, P, and Nawrot, TS. Carotid intima-media thickness, a marker of subclinical atherosclerosis, and particulate air pollution exposure: the meta-analytical evidence. PLoS One. (2015) 10:e0127014. doi: 10.1371/journal.pone.0127014
44. Kaufman, JD, Adar, SD, Barr, RG, Budoff, M, Burke, GL, Curl, CL, et al. Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the multi-ethnic study of atherosclerosis and air pollution): a longitudinal cohort study. Lancet. 388:696–704. doi: 10.1016/S0140-6736(16)00378-0
45. Cai, Y, Zhang, B, Ke, W, Feng, B, Lin, H, Xiao, J, et al. Associations of short-term and long-term exposure to ambient air pollutants with hypertension: a systematic review and meta-analysis. Hypertension. (2016) 68:62–70. doi: 10.1161/HYPERTENSIONAHA.116.07218
46. Yang, BY, Qian, Z, Howard, SW, Vaughn, MG, Fan, SJ, Liu, KK, et al. Global association between ambient air pollution and blood pressure: a systematic review and meta-analysis. Environ Pollut. (2018) 235:576–88. doi: 10.1016/j.envpol.2018.01.001
47. Pedersen, M, Stayner, L, Slama, R, Sørensen, M, Figueras, F, Nieuwenhuijsen, MJ, et al. Ambient air pollution and pregnancy-induced hypertensive disorders: a systematic review and meta-analysis. Hypertension. (2014) 64:494–500. doi: 10.1161/HYPERTENSIONAHA.114.03545
48. Huang, M, Chen, J, Yang, Y, Yuan, H, Huang, Z, and Lu, Y. Effects of ambient air pollution on blood pressure among children and adolescents: a systematic review and meta-analysis. J Am Heart Assoc. (2021) 10:e017734. doi: 10.1161/JAHA.120.017734
49. Folino, F, Buja, G, Zanotto, G, Marras, E, Allocca, G, Vaccari, D, et al. Association between air pollution and ventricular arrhythmias in high-risk patients (ARIA study): a multicentre longitudinal study. Lancet Planet Health. (2017) 1:e58–64. doi: 10.1016/S2542-5196(17)30020-7
50. Münzel, T, Sørensen, M, Gori, T, Schmidt, FP, Rao, X, Brook, J, et al. Environmental stressors and cardio-metabolic disease: part I-epidemiologic evidence supporting a role for noise and air pollution and effects of mitigation strategies. Eur Heart J. (2017) 38:550–6. doi: 10.1093/eurheartj/ehw269
51. Nawsherwan, SAK, Mubarik, S, Le, Z, Akbar, F, and Wang, Y. Epidemiological trends and age-period-cohort effects on cardiovascular diseases burden attributable to ambient air pollution across BRICS. Sci Rep. (2024) 14:11464. doi: 10.1038/s41598-024-62295-6
52. Andrade, MF, Kumar, P, de Freitas, ED, Ynoue, RY, Martins, J, Martins, LD, et al. Air quality in the megacity of São Paulo: evolution over the last 30 years and future perspectives. Atmos Environ. (2017) 159:66–82. doi: 10.1016/j.atmosenv.2017.03.051
53. Carvalho, VSB, Freitas, ED, Martins, LD, Martins, JA, Mazzoli, CR, and de Fátima Andrade, M. Air quality status and trends over the metropolitan area of São Paulo, Brazil as a result of emission control policies. Environ Sci. (2015) 47:68–79. doi: 10.1016/j.envsci.2014.11.001
54. Schmidt, M, Duncan, BB, Azevedo e Silva, G, Menezes, AM, Monteiro, CA, Barreto, SM, et al. Chronic non-communicable diseases in Brazil: burden and current challenges. Lancet. (2011) 377:1949–61. doi: 10.1016/S0140-6736(11)60135-9
55. Mücke, HG . Air quality management in the WHO European region—results of a quality assurance and control programme on air quality monitoring (1994–2004). Environ Int. (2008) 34:648–53. doi: 10.1016/j.envint.2007.12.008
56. World Health Organization. Regional Office for Europe . Health basis for air quality management in Eastern Europe, Caucasus and Central Asia: report from a WHO consultative meeting. Moscow: Russian Federation (2005).
57. Fuller, R, Landrigan, PJ, Balakrishnan, K, Bathan, G, Bose-O'Reilly, S, Brauer, M, et al. Pollution and health: a progress update. Lancet Planet Health. (2022) 6:e535–47. doi: 10.1016/S2542-5196(22)00090-0
58. Katoto, PDMC, Byamungu, L, Brand, AS, Mokaya, J, Strijdom, H, Goswami, N, et al. Ambient air pollution and health in Sub-Saharan Africa: current evidence, perspectives and a call to action. Environ Res. (2019) 173:174–88. doi: 10.1016/j.envres.2019.03.029
59. Fisher, S, Bellinger, DC, Cropper, ML, Kumar, P, Binagwaho, A, Koudenoukpo, JB, et al. Air pollution and development in Africa: impacts on health, the economy, and human capital. Lancet Planet. Health. (2021) 5:e681–8. doi: 10.1016/S2542-5196(21)00201-1
60. Liu, C, Huang, Z, Huang, J, Liang, C, Ding, L, Lian, X, et al. Comparison of PM2.5 and CO2 concentrations in large cities of China during the COVID-19 lockdown. Adv Atmos Sci. (2022) 39:861–75. doi: 10.1007/s00376-021-1281-x
61. Dutheil, F, Baker, JS, and Navel, V. COVID-19 as a factor influencing air pollution? Environ Pollut. (2020) 263:114466. doi: 10.1016/j.envpol.2020.114466
Keywords: cardiovascular diseases, air pollution, mortality forecasting, epidemiology, disease burden
Citation: Mao Q, Zhu X, Zhang X and Kong Y (2024) Effect of air pollution on the global burden of cardiovascular diseases and forecasting future trends of the related metrics: a systematic analysis from the Global Burden of Disease Study 2021. Front. Med. 11:1472996. doi: 10.3389/fmed.2024.1472996
Edited by:
Yashendra Sethi, PearResearch, IndiaReviewed by:
HuangHsi Chen, Chung Shan Medical University Hospital, TaiwanYiwei Liu, Shanghai Children’s Medical Center, China
Rana Bayakly, Georgia Department of Public Health, United States
Copyright © 2024 Mao, Zhu, Zhang and Kong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuzhe Kong, Y3N1eXV6aGVrb25nQGZveG1haWwuY29t
 Qingsong Mao
Qingsong Mao Xiaoyi Zhu2
Xiaoyi Zhu2 Yuzhe Kong
Yuzhe Kong