- 1Department of Obstetrics and Gynecology, Kawasaki Medical School, Okayama, Japan
- 2Fukushima Medical Center for Children and Women, Fukushima Medical University, Fukushima, Japan
- 3Department of Obstetrics and Gynecology, Koujin Hospital, Kagawa, Japan
- 4Department of Obstetrics and Gynecology, Koike Hospital, Hiroshima, Japan
Background: Robotic simple hysterectomy (RSH) is the most common robotic gynecologic surgery in the United States. Uterine manipulators are commonly used to handle the uterus during laparoscopic surgery, but few studies have examined their necessity in RSH. This study retrospectively compares RSH cases with and without the use of manipulators, and identifies predictors for their intraoperative use.
Materials and methods: This retrospective cohort study included patients undergoing RSH for benign pathologies at Kawasaki Medical School from October 2020 to December 2022. Patients with malignancies were excluded. The robotic surgeries were performed by three skilled surgeons using the four-arm da Vinci Xi surgical system. Data on perioperative and operative parameters were collected, including age, body mass index (BMI), history of abdominal surgery, disease type, presence of ovarian cysts, and operative time. Statistical analyses were performed using EZR software, with multivariate logistic regression to identify predictive factors for uterine manipulator use.
Results: The study included 113 patients who underwent RSH without a uterine manipulator and 58 with one. Patients without a manipulator were older, while those with a manipulator had higher BMIs and a higher prevalence of ovarian chocolate cysts and Douglas obliteration. Operating time was shorter without a manipulator. Independent predictors for manipulator use were higher BMI, presence of ovarian endometrioid cysts, and Douglas obliteration.
Conclusion: RSH without a uterine manipulator is feasible and can reduce the need for surgical assistants. Predictors for manipulator use include higher BMI, ovarian cysts, and Douglas obliteration. The use of a fourth robotic arm can enhance surgical independence and resource efficiency. Further research is needed to assess the long-term cost-effectiveness and outcomes of this approach.
1 Introduction
Hysterectomy is the most frequent surgical procedure performed on women with uterine benign diseases accounting for approximately 90% of hysterectomies (1). Before surgical robots, laparoscopy was the only minimally invasive option, limited by its steep learning curve and need for advanced training. Since the United States Food and Drug Administration approved the da Vinci robot (Intuitive Surgical) in 2005, advancements in robotic technology have greatly increased its use in gynecologic surgeries. At present, robotic simple hysterectomy (RSH) is now the most common robotic gynecologic surgeries in the United States (2, 3).
The greatest advantage of robotic surgery compared with laparotomy or laparoscopy is the saving in human resources. The equipment for RSH is slightly more expensive per procedure than for total laparoscopic hysterectomy (TLH), but performing 45 or more RSH procedures becomes more cost-effective than TLH (4, 5). The use of uterine manipulators is well established and it is clear that uterine manipulators offer the easiest way to handle the uterus during surgery (6). TLH without a uterine manipulator has been reported to reduce operative time and the need for a pelvic assistant (7). However, few studies have examined whether manipulators are necessary for RSH. In this study, we aimed to retrospectively compare cases of RSH with and without the manipulator and identify predictors for the intraoperative use of manipulators.
2 Materials and methods
2.1 Study design and data collection
This study was reviewed and approved by the Human Research Ethics Committee of Kawasaki Medical School (trial registration no.: 5043-03). After institutional review board approval, this study was designed as a retrospective cohort study. Patients who underwent RSH at the Women’s Medical Center, Kawasaki Medical School from October 2020 and December 2022 were included. Inclusion criteria encompassed RSH for benign pathologies, including fibroids, adenomyosis, cervical diseases (such as high-grade cervical intraepithelial neoplasia), and endometrial diseases (such as endometrial hyperplasia without atypia). All patients provided consent before the procedure. Patients with indications of malignancy were excluded from this study.
The robotic surgeries were performed by three skilled surgeons (surgeons A, B, and C). Surgeon A was awarded a class B International license by The Japanese Society for Robotic Surgery and is a proctor in The Japan Society for Endoscopic Surgery and Intuitive Surgical; Surgeon B holds a class B domestic license from The Japanese Society for Robotic Surgery and is a proctor in Intuitive Surgical; and Surgeon C is a proctor in Intuitive Surgical. Each surgery was assisted by a gynecological resident who had at least 1 year of experience in robotic surgeries, having participated in more than 30 cases.
We collected the data regarding perioperative parameters: age, body mass index (BMI), history of abdominal surgery, types of disease, presence of ovarian chocolate cysts, presence of pouch of Douglas obliteration, and operative parameters as follows: estimated blood loss, operating time defined as the time from skin incision to skin closure for RSH, concomitant procedures, and uterine weight excised.
2.2 Surgical procedures
Under general anesthesia, the patient was placed in a lithotomy position with Trendelenburg tilt. All robotic procedures utilized the four-arm da Vinci Xi surgical system (Intuitive Surgical Inc., Sunnyvale, CA, USA). Initially, an 8-mm endoscope port trocar was inserted 3 cm above the umbilicus using the direct closed method to establish pneumoperitoneum. Three 8-mm robotic ports were then inserted under direct vision on the same horizontal line, spaced 8 cm apart at the level of the endoscope port. The second arm managed the endoscope, while the first arm operated fenestrated bipolar forceps and the third arm managed Maryland bipolar forceps using the double bipolar technique (8). The fourth arm utilized Cadiere forceps to manipulate the uterus, eliminating the need for a uterine manipulator. Suturing was performed using the Suture Cut needle driver on the Maryland bipolar forceps on the third arm, which enabled both suturing and cutting of threads. For suction and intra-abdominal needle transport, the Probe Plus II (Ethicon, Tokyo, Japan) was introduced through the third arm. However, when it was necessary to pull the uterus or bowel toward the head to ensure a clear surgical field, a uterine manipulator was used.
The hysterectomy procedure followed our standard operating procedure for conventional RSH (9). Briefly, the round ligament was transected initially, followed by dissection of the broad ligament anteriorly and posteriorly using the double bipolar method with the Maryland bipolar and fenestrated bipolar forceps. Dissection of the bladder from the proximal vagina was followed by incision and expansion of the peritoneum of vesico-uterine pouch to identify and ligate the ureter and uterine artery, including the ureter-uterine artery crossover point, with 2-0 Vicryl (Ethicon, Tokyo, Japan) sutures. The uterine artery and ascending branches of the uterine vessels were ligated at two points with C 2-0 Vicryl (Ethicon, Tokyo, Japan) and coagulated using the fenestrated bipolar forceps before transecting with the Maryland bipolar forceps. The cardinal ligaments were transected, and colpotomy was performed with the Maryland bipolar forceps, followed by vaginal extraction of the uterus. A large uterus was divided into removable segments and extracted vaginally. Closure of the vaginal cuff was achieved using interrupted 0-Vicryl (Ethicon, Tokyo, Japan) sutures. A video clip summarizing the RSH technique is available (Supplementary Video 1).
2.3 Statistical analysis
Statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) (10). Data was represented as median and interquartile ranges (IQR) for non-parametric variables, and categorical variables are described as frequency and percentage and compared between the groups using Mann–Whitney’s U test (for numeric non-parametric variables) or Fisher’s exact test (for categorical variables). Multiple logistic regression analyses were performed to investigate potential influencing factors to evaluate RSH with a uterine manipulator, employing a forward stepwise methodology to identify independent predictive factors. Specifically, odds ratios (ORs) and 95% confidence intervals (CIs) were calculated in the multivariate analyses, controlling for potential confounders such as age, BMI, history of abdominal surgery, types of diseases, location of fibroids, console surgeons, presence of ovarian endometrioid cysts, presence of pouch of Douglas obliteration, and extracted uterine weight. These factors relate to securing the operative field before or at the start of surgery. Factors such as operative time and operative blood loss, which occur after surgery begins, were excluded as adjustment factors because they were not related to whether manipulators were used. However, for console surgeons, there was a significant difference in the number of RSH surgeries among surgeons. Therefore, console surgeon was included as an adjustment factor. Statistical significance was set at P < 0.05.
3 Results
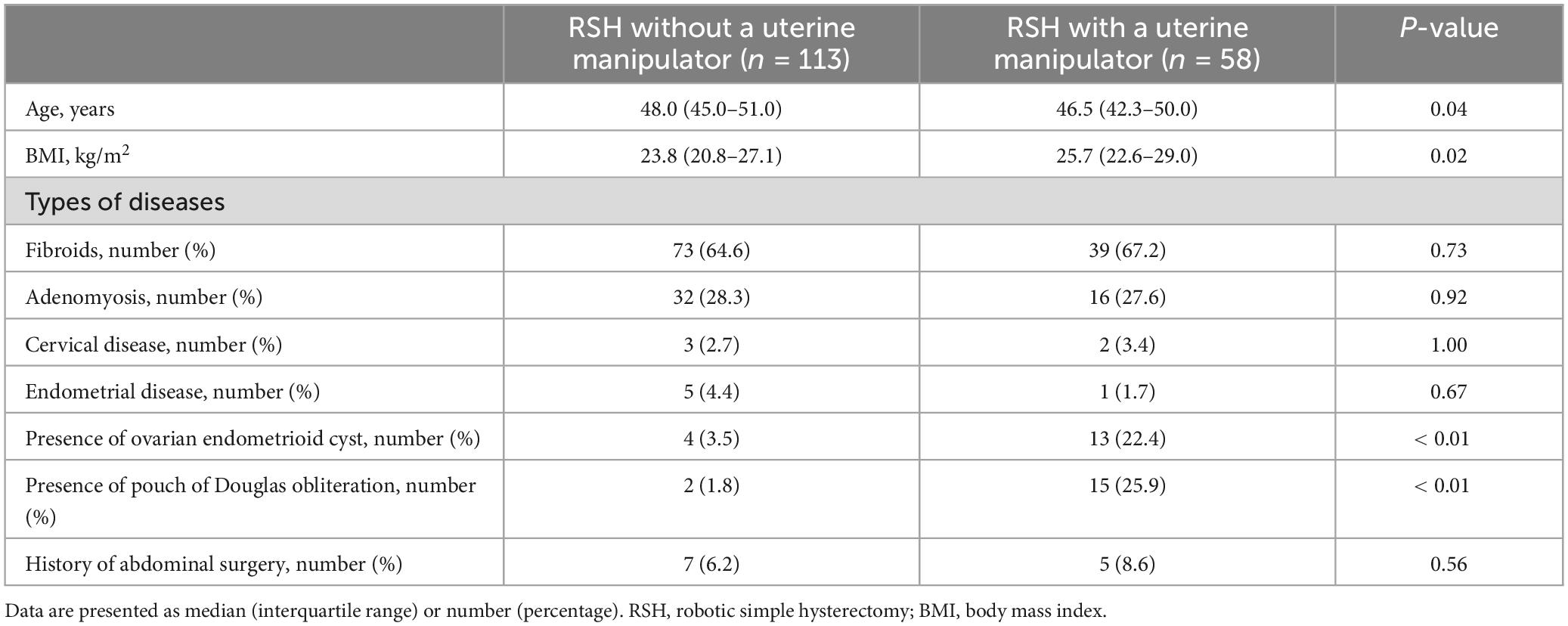
The characteristics of patients who underwent RSH without a manipulator (113 patients) and RSH with a manipulator (58 patients) are summarized in Table 1. Patients in RSH without a manipulator were significantly older than those in RSH with a manipulator (median 48.0, IQR 45.0–51.0 versus median 46.5, IQR 42.3–50.0, P = 0.04), and BMI was significantly higher in RSH with a manipulator than in RSH without a manipulator (median 25.7, IQR 22.6–29.0 versus median 23.8, IQR 20.8–27.1, P = 0.02). There were no statistically significant differences in the types of benign disease excluding the presence of cervical or broad ligament fibroid between the groups. The percentage of cervical or broad ligament fibroids was significantly higher in the RSH with a manipulator group than that in RSH without a manipulator group (13.8 versus 4.4%, P = 0.03). There were no statistically significant differences in the history of abdominal surgery between the groups. The percentage of presence of ovarian chocolate cysts was significantly higher in the RSH with a manipulator group than in the RSH with a uterine manipulator group (22.4 versus 3.5%, P < 0.01). The percentage of pouch of Douglas obliteration was significantly higher in the RSH with a manipulator group than in the RSH with a uterine manipulator group (25.9 versus 1.8%, P < 0.01).
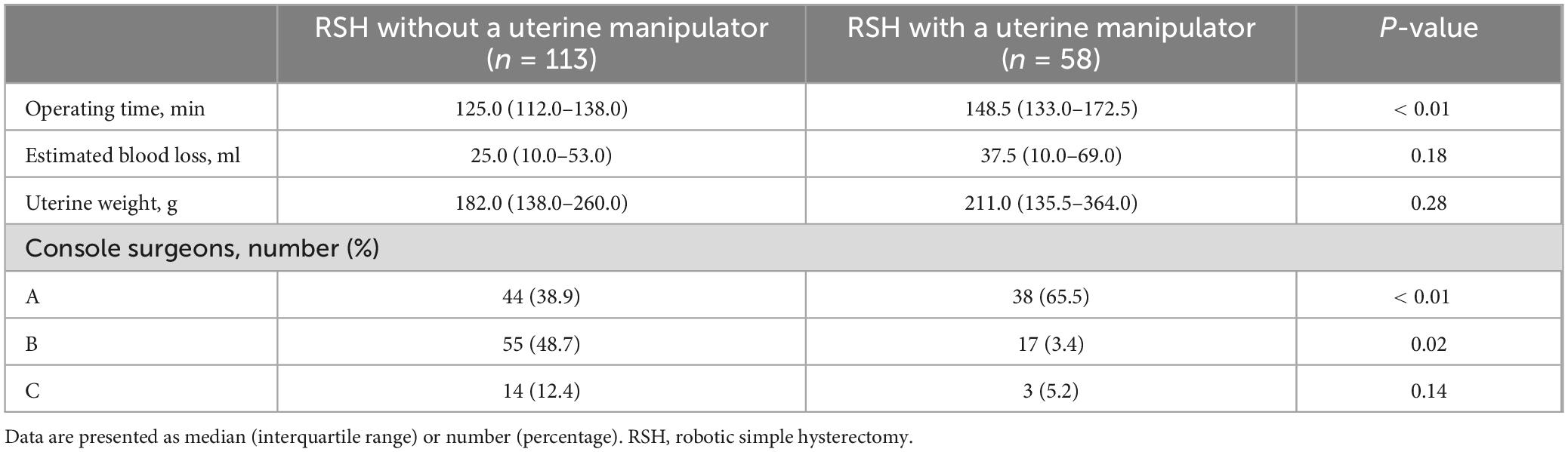
The surgical outcomes of RSH without a uterine manipulator and RSH with a uterine manipulator are summarized in Table 2. Operating time was significantly shorter in RSH without a uterine manipulator than in RSH with a uterine manipulator (125.0, IQR 112.0–138.0 versus 148.5, IQR 133.0–172.5). Both estimated blood loss and uterine weight were not significantly different between the groups. For console Surgeon A, the percentage of RSH with a uterine manipulator was significantly higher than that of RSH without a uterine manipulator (65.6 versus 38.9%, P < 0.01). For console Surgeon B, the percentage of RSH with a uterine manipulator was significantly lower than that of RSH without a uterine manipulator (3.4 versus 48.7%, P = 0.02). For console Surgeon C, there was no significant difference in the percentage of RSH with and without a uterine manipulator.
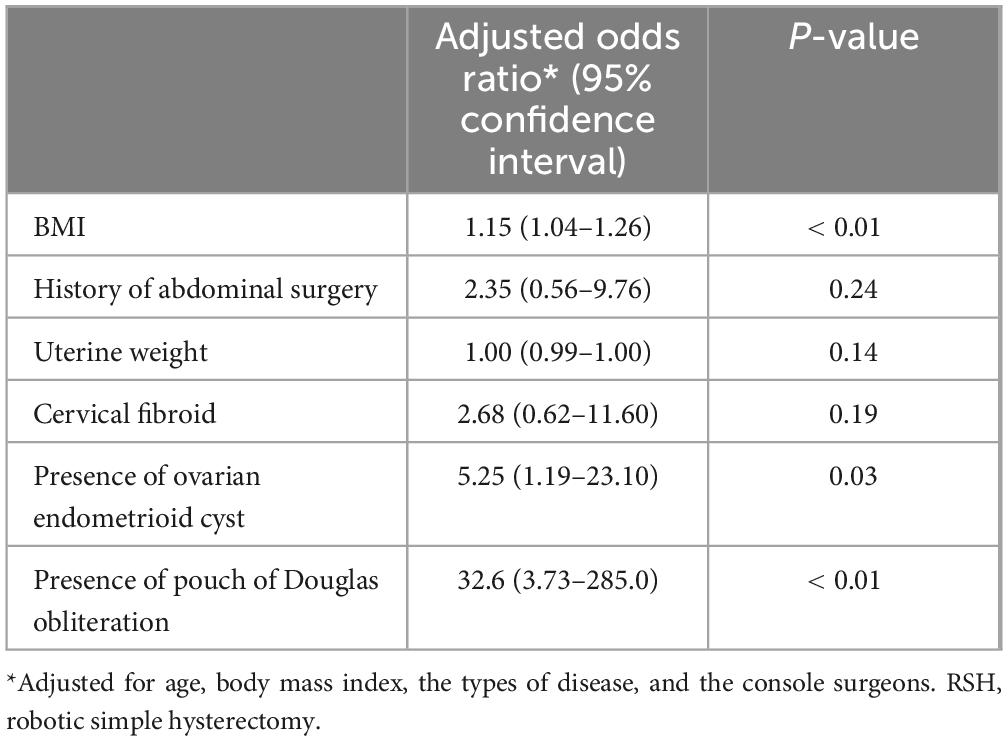
Predictive factors for uterine manipulator use during RSH are summarized in Table 3. Multivariate logistic analysis showed that BMI (adjusted OR: 1.15, 95% CI 1.04–1.26, P < 0.01), the presence of ovarian endometrioid cysts (OR: 5.25, 95% CI 1.19–23.10, P = 0.03), and the presence of pouch of Douglas obliteration (OR: 32.6, 95% CI 3.73–285.00, P < 0.041) were independent predictive factors for uterine manipulator use during RSH. Adjusting explanatory variables, history of abdominal surgery, uterine weight, and cervical or broad ligament fibroids were not significant predictors for uterine manipulator use during RSH.
4 Discussion
In this study, we showed for the first time that independent predictors of uterine manipulator use during RSH were BMI, presence of endometrial cysts in the ovary, and Douglas obliteration.
The use of manipulators for RSH is the technique derived from imitation methods of the TLH. In TLH procedures, several studies have highlighted the importance of uterine manipulators in reducing complications during hysterectomy (6, 11). In fact, a uterine manipulator is routinely used as the gold standard in TLH to allow better exposure of the anatomical spaces, consequently lower the overall complication rate, and to prevent bowel and ureteral injuries (12, 13). On the other hand, recently, the use of uterine manipulators has been increasingly discouraged in laparoscopic and robotic surgery. The use of the uterine manipulator in TLH might be avoided in malignant tumor because it is suggested to increase concerns regarding the dissemination of malignant cells to the vaginal cuff and the peritoneal cavity through the fallopian tubes when the uterine manipulator is used for endometrial carcinoma (14). Therefore, there have been many reports of TLH without manipulators in recent years (15), although TLH without the use of a manipulator is a more demanding surgical procedure (16–19). This is because the learning curve for performing TLH without a uterine manipulator is likely to be longer (20, 21). Because robotic surgery has advantages such as a short learning curve, even in RSH, uterine manipulators tend not to be used for endometrial cancer (22).
Recent studies on uterine manipulators in gynecological surgery provide mixed insights. Ongoing research may provide clearer guidelines for their optimal use in various gynecological surgeries. To date, there is no conclusion to the debate on whether a uterine manipulator should or should not be used for TLH, although recent studies on uterine manipulators in gynecological surgery provide mixed insights. In particular, some reports of surgical approaches for a TLH without a uterine manipulator (23) raised concerns over the use of a uterine manipulator for uterine cancers during TLH (24, 25). The meta-analysis by Scutiero et al. (26) suggests that while manipulators don’t significantly impact overall or disease-free survival in endometrial cancer cases, they may increase the risk of positive peritoneal cytology. The prospective randomized trial found that the use of a uterine manipulator during minimally invasive staging for early-stage endometrial cancer does not significantly impact lymph vascular space invasion or patient outcomes (27). Therefore, uterine manipulators are expected to be in increasing demand. On the other hand, Cianci et al.’s (28, 29) reviews highlight the importance of tailoring surgical approaches to individual cases, especially for fibroid treatment and large uterine. Their study on laparoscopic hysterectomy conversions indicates that uterine size significantly affects procedural success, with manipulators potentially less effective for very large uterine (28, 29). Overall, while uterine manipulators offer benefits in visualization and ease of minimally invasive procedures, their use should be carefully considered based on factors like uterine size, cancer risk, and patient characteristics. Hence, it is the new era to adapt to hysterectomy even if a manipulator was not available. Zygouris et al. (15) concluded the feasible and safe technique using grasping forceps for uterine manipulation instead of a uterine manipulator from a large clinical study, if performed by well-trained, experienced laparoscopic surgeons. Moreover, Abdel Khalek et al. (11) advise that surgeons performing TLH should decide on a case-by-case basis whether a uterine manipulator is necessary and, if so, which type best suits the procedure.
The study by Gallotta et al. (30) demonstrates that robotic surgery (RS) is a feasible and safe option for elderly (65–74 years) and very elderly (≥ 75 years) women undergoing gynecologic oncologic procedures. While their study doesn’t specifically address uterine manipulators, the precision of robotic systems may reduce the need for extensive manipulation, potentially benefiting older patients with more fragile tissues (30). Although there were no elderly patients in this study, it may provide the basis for further development into robotic sacrocolpopexy does not use manipulators if this study has advanced and establish as a manipulator-free RSH.
Robotic surgery is considered as advantageous for reducing human resources, but an assistant is still needed. When a uterine manipulator is used, at least two assistants are required. Additionally, the assistant must work in a confined space with the patient’s legs spread, causing significant stress during surgery. Determining whether a uterine manipulator can be used before or immediately after surgery helps reduce the number of assistants needed for the procedure. Perrone et al. (31) described that RSH with the assistance of the fourth robotic arm instead of a uterine manipulator may reduce the need for uterine manipulation because the operating time was not different from TLH without a uterine manipulator done by the experts. Our technique of RSH without a uterine manipulator is consistent with the very same concept. Recently, Barger et al. (32) described that adding a fourth robotic arm to the standard three port setup can markedly improve robotic hysterectomy without a uterine manipulator. In this study, RSH utilizing four arms also allowed us to complete the hysterectomy without a uterine manipulator; however, even with four arms, a manipulator was necessary in cases with increased BMI, the presence of ovarian endometrioid cysts, and the presence of pouch of Douglas obliteration. In those cases, the console surgeon’s independence may be somewhat diminished, as an additional surgical assistant is required. On the other hand, reducing human resources for surgery would deprive fellows or residents of the opportunity to enter robotic surgery. In fact, Hall et al. (33) reported that few fellows were deemed competent enough to independently operate the robot with only 15% being able to perform an entire hysterectomy. Therefore, recent curriculum for OB/Gyn residents and fellows reduces barriers by providing protected time away from clinical duties to provide a reproducible platform for the early acquisition of advanced robotic skills outside of the operating room to standardize mastery-based training for the next generation of robotic surgeons (34).
The difficulty of performing a hysterectomy increases when fibroids are located in the cervix or the broad ligament. In laparoscopic surgery, where manual traction is not possible, the location of the fibroids particularly affects the surgical difficulty. Our study found no association between the use of uterine manipulators and cervical or broad ligament fibroids when adjusted for explanatory variables. Of the 13 cases with cervical or broad ligament fibroids, eight were performed by Surgeon A, five by Surgeon B, and none by Surgeon C (data not shown). These results may be influenced by differences in surgeon experience and should be interpreted with caution.
Although this is a robot-specific study, it may be necessary to consider whether robotic surgery is more significant than laparoscopic surgery. In a previous review, RSH had a longer operating time than TLH (35). However, in the recent RCT-only meta-analysis, there was no difference in operating time between RSH and TLH, and those are now rated as equivalent operative techniques (36). Both the decade of surgeons’ experience and robotic development realized the democratization and widespread of robotic surgery and might reduce the operating time. However, there is no doubt that robotic surgery will surpass laparoscopic surgery in the future. Although the current challenge lies in the training of surgeons and the development of the operating room of the future, In the era of digital surgery, robotic platforms serve as computer interfaces capable of integrating various real-time data analysis modalities, and the next decade enables advanced systems to provide augmented surgical vision through augmented reality, improved surgical decisions using artificial intelligence, and enhanced surgical maneuvers through the advancement of robotic instruments (37). In addition, recent meta-analysis demonstrates that 3D vision systems offer significant advantages over 2D systems in laparoscopic surgery, particularly in terms of improved depth perception, precision, and task completion time (38). All robotic systems always include 3D vision systems, and could be especially beneficial for the training of surgeons to enhance spatial awareness and precision afforded by 3D vision. Pavone et al. (39) have systematically studied the advantages and advancements of robotic platforms in gynecological surgery, and the potential benefits of robotic approaches are sufficient strength for endometriosis surgery (40) and superior to laparoscopic approaches for severe cases such as deep endometriosis through a meta-analysis (41). Furthermore, those foundations are more established when they combine structured training in skill development, novel techniques such as ultrasound-guided robotic surgical procedures, and the integration of imaging technologies (42, 43). Therefore, the potential benefits in the next decade would also depend on the widespread adoption of robotic surgery systems in gynecological surgery settings emphasizing technological advancements, comparative effectiveness, training methodologies, and the integration of imaging techniques.
Our study had several limitations. First, the data may have incomplete information that was not fulfilled in the patient record because of the retrospective nature of the study, which limits the generalizability of our findings. In this retrospective study, there was the possible allocation biases arising from the retrospective comparison between RSH without a uterine manipulator and RSH with a uterine manipulator because of the non-randomized nature of the study design. Therefore, we further need to analyze with a propensity-matched analysis to decrease biases arising from different covariates if both cases are increased, or further prospective trials are needed to confirm our results. Second, three surgeons from our robotic surgical team performed the surgeries. Although most surgeons in the team were trained at the same institution, biases resulting from individual surgeon differences cannot be excluded. Moreover, the most proficient surgeon (Surgeon A) required a uterine manipulator for RSH, which may have resulted in an unbalanced distribution of surgical difficulty and may have impacted the statistical analysis. Third, In the current landscape, other robotic systems have been developed, and the Hugo™ RAS (Medtronic, Minneapolis, MN, USA) or hinotori™ system (Medicaroid Corporation, Kobe, Japan) for hysterectomy has demonstrated effectiveness (44–46). It has raised the possibility of different results to operate with other robotic systems, although Matsuura et al. (47) described that surgeons who are already proficient in performing robotic surgery with da Vinci X can safely perform surgeries with the new models when three robotic systems were compared. The study’s main strength is its description of a four-arm approach to hysterectomy using the da Vinci Xi without a uterine manipulator, which enabled minimal dependence on an assistant. Although previous reports have shown the superiority of a four-arm hysterectomy approach for malignant gynecological diseases (48), this is the first study to demonstrate the superiority of this hysterectomy approach for benign gynecological diseases. Furthermore, this report seems worthwhile because pelvic occupying diseases, such as enlarged uterine fibroids, are more difficult to treat robotically than with radical total hysterectomy.
In conclusion, the routine use of a fourth robotic arm during RSH provides the operating surgeon with greater independence during critical phases of the procedure without the requirement of a uterine manipulator and assistant. This advantage translates into non-dependence on an assistant and the conservation of human resources as well as medical resources such as a uterine manipulator. Although the initial investment in robotic surgical systems is high, we need further longitudinal research on whether shorter hospital stays, reduced postoperative complications, and quicker recovery times, can significantly lower overall healthcare costs.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: Raw data were generated at Kawasaki Medical School. Derived data supporting the findings of this study are available from the corresponding author YO on request. Requests to access these datasets should be directed to eW9zaGltb25AbWVkLmthd2FzYWtpLW0uYWMuanA=.
Ethics statement
The studies involving humans were reviewed and approved by the Human Research Ethics Committee of Kawasaki Medical School (trial registration no.: 5086-01). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
SK: Data curation, Methodology, Writing – original draft, Writing – review & editing. KO: Writing – original draft, Writing – review & editing. YO: Conceptualization, Supervision, Writing – review & editing. TT: Writing – original draft, Writing – review & editing. HF: Data curation, Methodology, Writing – review & editing. KT: Data curation, Methodology, Writing – review & editing. HO: Data curation, Methodology, Writing – review & editing. YM: Data curation, Methodology, Writing – review & editing. WS: Data curation, Methodology, Writing – review & editing. MSu: Formal analysis, Writing – review & editing. TM: Supervision, Writing – review & editing. EK: Supervision, Writing – review & editing. MSh: Supervision, Writing – review & editing. KS: Supervision, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank all the staff in the operating room at Kawasaki Medical School and all the nurses who cared for our postoperative patients. We are also very grateful to M. S. Yoshimi Harada, secretary of the Department of Obstetrics and Gynecology at Kawasaki Medical School.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1462632/full#supplementary-material
References
1. Garry R. Health economics of hysterectomy. Best Pract Res Clin Obstet Gynaecol. (2005) 19:451–65.
2. Cohen S, Ajao M, Clark N, Vitonis A, Einarsson J. Outpatient hysterectomy volume in the United States. Obstet Gynecol. (2017) 130:130–7.
3. Gitas G, Alkatout I, Mettler L, Abdusattarova K, Ertan A, Rody A, et al. Incidence of unexpected uterine malignancies after electromechanical power morcellation: A retrospective multicenter analysis in Germany. Arch Gynecol Obstet. (2020) 302:447–53. doi: 10.1007/s00404-020-05620-4
4. Kaaki B, Lewis E, Takallapally S, Cleveland B. Direct cost of hysterectomy: Comparison of robotic versus other routes. J Robot Surg. (2020) 14:305–10.
5. Ghomi A, Nolan W, Sanderson D, Sanderson R, Schwander B, Feldstein J. Robotic hysterectomy compared with laparoscopic hysterectomy: Is it still more costly to perform? J Robot Surg. (2022) 16:537–41. doi: 10.1007/s11701-021-01273-w
6. van den Haak L, Alleblas C, Nieboer T, Rhemrev J, Jansen F. Efficacy and safety of uterine manipulators in laparoscopic surgery: A review. Arch Gynecol Obstet. (2015) 292:1003–11. doi: 10.1007/s00404-015-3727-9
7. Gendia A, Donlon N, Kamran WM. A novel approach to minimally invasive hysterectomy without the use of a uterine manipulator: Kamran’s TLH technique. Gynecol Surg. (2020) 17:14. doi: 10.1186/s10397-020-01078-z
8. Katsuno H, Hanai T, Endo T, Morise Z, Uyama I. The double bipolar method for robotic total mesorectal excision in patients with rectal cancer. Surg Today. (2022) 52:978–85. doi: 10.1007/s00595-021-02418-y
9. Ota Y, Ota K, Takahashi T, Suzuki S, Sano R, Shiota M. Robotic-assisted total hysterectomy with low pneumoperitoneal pressure (6 mmHg) and use of surgical plume evacuator system to minimize potential airborne particles according to the joint statement on minimally invasive gynecologic surgery during the COVID-19 pandemic: A case report from Japan. Gynecol Minim Invasive Ther. (2022) 11:127–30. doi: 10.4103/gmit.Gmit_131_20
10. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’for medical statistics. Bone Marrow Transplant. (2013) 48:452–8.
11. Abdel Khalek Y, Bitar R, Christoforou C, Garzon S, Tropea A, Biondi A, et al. Uterine manipulator in total laparoscopic hysterectomy: Safety and usefulness. Updates Surg. (2020) 72:1247–54. doi: 10.1007/s13304-019-00681-w
12. Aarts J, Nieboer T, Johnson N, Tavender E, Garry R, Mol B, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. (2015) 2015:CD003677.
13. Elkington N, Chou DA. review of total laparoscopic hysterectomy: Role, techniques and complications. Curr Opin Obstet Gynecol. (2006) 18:380–4.
14. Meng Y, Liu Y, Lin S, Cao C, Wu P, Gao P, et al. The effects of uterine manipulators in minimally invasive hysterectomy for endometrial cancer: A systematic review and meta-analysis. EurJ Surg Oncol. (2020) 46:1225–32. doi: 10.1016/j.ejso.2020.03.213
15. Zygouris D, Chalvatzas N, Gkoutzioulis A, Anastasiou G, Kavallaris A. Total laparoscopic hysterectomy without uterine manipulator. A retrospective study of 1023 cases. Eur J Obstet Gynecol Reprod Biol. (2020) 253:254–8. doi: 10.1016/j.ejogrb.2020.08.035
16. Donnez O, Donnez JA. series of 400 laparoscopic hysterectomies for benign disease: A single centre, single surgeon prospective study of complications confirming previous retrospective study. BJOG. (2010) 117:752–5.
17. Mueller A, Oppelt P, Ackermann S, Binder H, Beckmann M. The Hohl instrument for optimizing total laparoscopic hysterectomy procedures. J Minim Invasive Gynecol. (2005) 12:432–5.
18. Schollmeyer T, Elessawy M, Chastamouratidhs B, Alkatout I, Meinhold-Heerlein I, Mettler L, et al. Hysterectomy trends over a 9-year period in an endoscopic teaching center. Int J Gynecol Obstet. (2014) 126:45–9. doi: 10.1016/j.ijgo.2013.12.017
19. Terzi H, Biler A, Demirtas O, Guler O, Peker N, Kale A. Total laparoscopic hysterectomy: Analysis of the surgical learning curve in benign conditions. Int J Surg. (2016) 35:51–7.
20. Mavrova R, Radosa J, Wagenpfeil G, Hamza A, Solomayer E, Juhasz-Böss I. Learning curves for laparoscopic hysterectomy after implementation of minimally invasive surgery. Int J Gynecol Obstet. (2016) 134:225–30.
21. Twijnstra A, Blikkendaal M, Kolkman W, Smeets M, Rhemrev J, Jansen F. Implementation of laparoscopic hysterectomy: Maintenance of skills after a mentorship program. Gynecol Obstet Invest. (2010) 70:173–8. doi: 10.1159/000316266
22. Ito H, Moritake T, Isaka K. Does the use of a uterine manipulator in robotic surgery for early-stage endometrial cancer affect oncological outcomes? Int J Med Robot Comput Assist Surg. (2022) 18:e2443. doi: 10.1002/rcs.2443
23. Kavallaris A, Chalvatzas N, Kelling K, Bohlmann M, Diedrich K, Hornemann A. Total laparoscopic hysterectomy without uterine manipulator: Description of a new technique and its outcome. Arch Gynecol Obstet. (2011) 283:1053–7. doi: 10.1007/s00404-010-1494-1
24. Köhler C, Hertel H, Herrmann J, Marnitz S, Mallmann P, Favero G, et al. Laparoscopic radical hysterectomy with transvaginal closure of vaginal cuff–a multicenter analysis. Int J Gynecol Cancer. (2019) 29:845–50. doi: 10.1136/ijgc-2019-000388
25. Köhler C, Schneider A, Marnitz S, Plaikner A. The basic principles of oncologic surgery during minimally invasive radical hysterectomy. J Gynecol Oncol. (2020) 31:e33. doi: 10.3802/jgo.2020.31.e33
26. Scutiero G, Vizzielli G, Taliento C, Bernardi G, Martinello R, Cianci S, et al. Influence of uterine manipulator on oncological outcome in minimally invasive surgery of endometrial cancer: A systematic review and meta-analysis. Eur J Surg Oncol. (2022) 48:2112–8. doi: 10.1016/j.ejso.2022.05.034
27. Gueli Alletti S, Perrone E, Fedele C, Cianci S, Pasciuto T, Chiantera V, et al. A multicentric randomized trial to evaluate the ROle of Uterine MANipulator on laparoscopic/robotic hysterectomy for the treatment of early-stage endometrial cancer: The ROMANHY trial. Front Oncol. (2021) 11:720894. doi: 10.3389/fonc.2021.720894
28. Cianci S, Gulino F, Palmara V, La Verde M, Ronsini C, Romeo P, et al. Exploring surgical strategies for uterine fibroid treatment: A comprehensive review of literature on open and minimally invasive approaches. Medicina (Kaunas). (2023) 60:64. doi: 10.3390/medicina60010064
29. Cianci S, Gueli Alletti S, Rumolo V, Rosati A, Rossitto C, Cosentino F, et al. Total laparoscopic hysterectomy for enlarged uteri: Factors associated with the rate of conversion to open surgery. J Obstet Gynaecol. (2019) 39:805–10. doi: 10.1080/01443615.2019.1575342
30. Gallotta V, Conte C, D’Indinosante M, Federico A, Biscione A, Vizzielli G, et al. Robotic surgery in elderly and very elderly gynecologic cancer patients. J Minim Invasive Gynecol. (2018) 25:872–7. doi: 10.1016/j.jmig.2018.01.007
31. Perrone E, Capasso I, Pasciuto T, Gioè A, Alletti S, Restaino S, et al. Laparoscopic vs. robotic-assisted laparoscopy in endometrial cancer staging: Large retrospective single-institution study. J Gynecol Oncol. (2021) 32:e45. doi: 10.3802/jgo.2021.32.e45
32. Barger A, Haworth L, Bennett M, Hudgens J, Woo J. The 4th arm solution: An easy answer to the robotic hysterectomy without a uterine manipulator. Am J Obstet Gynecol. (2024) 230:S1296.
33. Hall E, Bregar A, Robison K, Ruhotina M, Raker C, Wohlrab K. Ready for the robot? A cross-sectional survey of OB/GYN fellowship directors’ experience and expectations of their incoming fellow’s robotic surgical skills. J Robot Surg. (2021) 15:723–9. doi: 10.1007/s11701-020-01160-w
34. Ramirez Barriga M, Rojas A, Roggin K, Talamonti M, Hogg M. Development of a two-week dedicated robotic surgery curriculum for general surgery residents. J Surg Educ. (2022) 79:861–6. doi: 10.1016/j.jsurg.2022.02.015
35. Weinberg L, Rao S, Escobar P. Robotic surgery in gynecology: An updated systematic review. Obstet Gynecol Int. (2011) 2011:852061. doi: 10.1155/2011/852061
36. Lenfant L, Canlorbe G, Belghiti J, Kreaden U, Hebert A, Nikpayam M, et al. Robotic-assisted benign hysterectomy compared with laparoscopic, vaginal, and open surgery: A systematic review and meta-analysis. J Robot Surg. (2023) 17:2647–62. doi: 10.1007/s11701-023-01724-6
37. Lecointre L, Verde J, Goffin L, Venkatasamy A, Seeliger B, Lodi M, et al. Robotically assisted augmented reality system for identification of targeted lymph nodes in laparoscopic gynecological surgery: A first step toward the identification of sentinel node: Augmented reality in gynecological surgery. Surg Endosc. (2022) 36:9224–33. doi: 10.1007/s00464-022-09409-1
38. Restaino S, Scutiero G, Taliento C, Poli A, Bernardi G, Arcieri M, et al. Three-dimensional vision versus two-dimensional vision on laparoscopic performance of trainee surgeons: A systematic review and meta-analysis. Updates Surg. (2023) 75:455–70. doi: 10.1007/s13304-023-01465-z
39. Pavone M, Baroni A, Taliento C, Goglia M, Lecointre L, Rosati A, et al. Robotic platforms in gynaecological surgery: Past, present, and future. Facts Views Vis Obgyn. (2024) 16:163–72. doi: 10.52054/fvvo.16.2.024
40. Pavone M, Seeliger B, Alesi M, Goglia M, Marescaux J, Scambia G, et al. Initial experience of robotically assisted endometriosis surgery with a novel robotic system: First case series in a tertiary care center. Updates Surg. (2024) 76:271–7. doi: 10.1007/s13304-023-01724-z
41. Pavone M, Baroni A, Campolo F, Goglia M, Raimondo D, Carcagnì A, et al. Robotic assisted versus laparoscopic surgery for deep endometriosis: A meta-analysis of current evidence. J Robot Surg. (2024) 18:212. doi: 10.1007/s11701-024-01954-2
42. Seeliger B, Pavone M, Schröder W, Krüger C, Bruns C, Scambia G, et al. Skill progress during a dedicated societal robotic surgery training curriculum including several robotic surgery platforms. Surg Endosc. (2024) 2:1–8. doi: 10.1007/s00464-024-11128-8
43. Pavone M, Seeliger B, Teodorico E, Goglia M, Taliento C, Bizzarri N, et al. Ultrasound-guided robotic surgical procedures: A systematic review. Surg Endosc. (2024) 38:2359–70. doi: 10.1007/s00464-024-10772-4
44. Monterossi G, Pedone Anchora L, Gueli Alletti S, Fagotti A, Fanfani F, Scambia G. The first European gynaecological procedure with the new surgical robot Hugo™ RAS. A total hysterectomy and salpingo-oophorectomy in a woman affected by BRCA-1 mutation. Facts Views Vis Obgyn. (2022) 14:91–4. doi: 10.52054/fvvo.14.1.014
45. Togami S, Higashi T, Tokudome A, Fukuda M, Mizuno M, Yanazume S, et al. The first report of surgery for gynecological diseases using the hinotori™ surgical robot system. Japan J Clin Oncol. (2023) 53:1034–7. doi: 10.1093/jjco/hyad105
46. Pavone M, Alesi M, Scambia G, Ianieri M. Robot-assisted radical hysterectomy and bilateral salpingectomy with bilateral postero-lateral parametrectomy and cecum resection for deep endometriosis with the new Hugo™ RAS system. (2020). Available online at: https://websurg.com/en/doi/vd01en7241/
47. Matsuura M, Nagao S, Kurokawa S, Tamate M, Akimoto T, Saito T. Early outcomes of three new robotic surgical systems in patients undergoing hysterectomy. Updates Surg. (2024): doi: 10.1007/s13304-024-01891-7 [Epub ahead of print].
Keywords: robotic simple hysterectomy, robot-assisted simple hysterectomy, uterine manipulator, da Vinci Xi surgical system, pouch of Douglas obliteration, operative assistant
Citation: Kawamura S, Ota K, Ota Y, Takahashi T, Fujiwara H, Tasaka K, Okamoto H, Morimoto Y, Saito W, Sugihara M, Matsuyama T, Koike E, Shiota M and Shimoya K (2024) Identifying key predictors for uterine manipulator use in robotic simple hysterectomy: a retrospective cohort analysis. Front. Med. 11:1462632. doi: 10.3389/fmed.2024.1462632
Received: 10 July 2024; Accepted: 26 August 2024;
Published: 11 September 2024.
Edited by:
Rafał Watrowski, Helios Hospital Müllheim, GermanyReviewed by:
Giuseppe Vizzielli, University of Udine, ItalyMatteo Pavone, Agostino Gemelli University Polyclinic (IRCCS), Italy
Copyright © 2024 Kawamura, Ota, Ota, Takahashi, Fujiwara, Tasaka, Okamoto, Morimoto, Saito, Sugihara, Matsuyama, Koike, Shiota and Shimoya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yoshiaki Ota, eW9zaGltb25AbWVkLmthd2FzYWtpLW0uYWMuanA=
†These authors share first authorship
‡ORCID: Kuniaki Ota, orcid.org/0000-0003-3984-975X; Yoshiaki Ota, orcid.org/0000-0003-4443-8365; Toshifumi Takahashi, orcid.org/0000-0003-0955-4248; Koichiro Shimoya, orcid.org/0000-0002-1623-2020
 Shogo Kawamura1†
Shogo Kawamura1† Kuniaki Ota
Kuniaki Ota