- 1Department of Osteoporosis, The First People’s Hospital of Kunshan Affiliated with Jiangsu University, Kunshan, China
- 2Department of Graduate Office, The First People’s Hospital of Kunshan Affiliated with Jiangsu University, Kunshan, China
- 3Department of Anesthesiology, The First People’s Hospital of Kunshan Affiliated with Jiangsu University, Kunshan, China
Background: This study aims to explore the association between sleep duration and the prevalence of chronic musculoskeletal pain (CMP).
Methods: A cross-sectional study was conducted using data from the National Health and Nutrition Examination Survey (NHANES) 2009–2010, which involved multiple centers across the United States. The study included 3,904 adults selected based on age and complete data availability. Demographic variables such as gender, age, race, and socioeconomic status (represented by the poverty-to-income ratio) were considered.
Results: Of the participants, 1,595 reported less than 7 h of sleep, 2,046 reported 7–8 h, and 263 reported more than 9 h of sleep. Short sleep duration was associated with higher odds of CMP (OR, 1.611, 95% CI: 1.224–2.120, p = 0.005). Long sleep duration also showed a higher prevalence (OR, 1.751; 95% CI, 0.923 to 3.321; p = 0.059), although this result was not statistically significant. A U-shaped relationship emerged (Effective degree of freedom (EDF) = 3.32, p < 0.001), indicating that 7 h of sleep was associated with the lowest odds of CMP. In individuals with sleep durations less than 7 h, each hour increment correlated with 22.8% reduced odds of CMP (OR, 0.772; 95% CI, 0.717–0.833; p = 0.002). Beyond 7 h, each hour increment was associated with 38.9% increased odds of CMP (OR, 1.389; 95% CI, 1.103–1.749; p = 0.049).
Conclusion: The findings suggest that both insufficient and excessive sleep durations are linked to a higher prevalence of CMP, highlighting the importance of optimal sleep duration for musculoskeletal health.
1 Introduction
Chronic musculoskeletal pain (CMP) affects over 20–33% of the global population, according to the World Health Organization (WHO), resulting in significant health and socio-economic burdens (1). Sleep plays a crucial role in maintaining physical well-being, with an optimal duration generally ranging from 7 to 9 h (2–4). Poor sleep habits are linked to various adverse health effects, including cardiovascular events (2, 5, 6), diabetes mellitus (DM) (5), Alzheimer’s Disease (4, 7), and increased mortality risk (2).
In the United States, about one-third of the population frequently experiences sleep difficulties, a condition even more prevalent among those with concurrent pain symptoms (8, 9). Observational data indicate a link between sleep disturbances and the onset, progression, and persistence of pain symptoms (8, 10–12). Chronic pain and sleep disturbances often co-occur, highlighting a clinically important reciprocal relationship. Various epidemiological studies have indicated that poor sleep quality and not getting enough sleep are correlated with a higher prevalence of chronic pain (10, 13). Conversely, experiencing chronic pain can also disrupt an individual’s sleep patterns (14).
Understanding the potential mechanisms behind this reciprocal relationship is crucial for developing new treatments for chronic pain. However, the precise relationship between sleep duration and chronic pain remains unclear. Most studies suggest a link between short sleep duration and higher prevalence of chronic pain (14), but research demonstrating a connection between excessive sleep and chronic pain is limited. This study aims to conduct a large-scale population study to explore the relationship between sleep duration and chronic pain by analyzing retrospective data from the National Health and Nutrition Examination Survey (NHANES). Specifically, we assessed the link between self-reported sleep duration and the presence of chronic pain and investigate the potential effects of age, gender, Body Mass Index (BMI), race, poverty-to-income ratio (PIR), alcohol use, smoking, DM and sleep disorders. This research offers valuable insights into understanding the intricate relationship between sleep and chronic pain, with implications for chronic pain prevention and treatment.
2 Methods
2.1 Study population
The protocols for NHANES, a cross-sectional survey, were approved by the ethics review board of the National Center for Health Statistics, and all participants provided written informed consent. It was in accordance with the policy of the National Institutes of Health that analysis conducted using de-identified data not directly involving participants was not classified as a human subject study, thus not requiring institutional review board review.
NHANES participants undergo demographic assessments, laboratory examinations, and extensive interviews, covering measures of sex, age, race, PIR, clinical profiles, and personal behaviors. The sampling structure of NHANES allows it to effectively represent the non-institutionalized population of the United States after adjustments for sex, age, race, and ethnicity were made. We primarily focused on two indicators, including sleep behavior and musculoskeletal pain. Data from NHANES 2009–2010 which included an arthritis questionnaire were obtained. We excluded 48 participants who were pregnant. Participants missing information on sleep duration, pain, or other important covariates (e.g., smoking, alcohol consumption, diabetes, PIR) were excluded. Finally, data from 3,904 participants were included in the analysis (Supplementary Figure S1).
2.2 Measurement
2.2.1 Outcome ascertainment
CMP is defined as pain persisting or recurring for more than 3 months (15). To define this group, pain-related questions from the Arthritis Questionnaire Section (ARQ) provides interview data were utilized. Participants were classified into chronic musculoskeletal pain group if they reported experiencing “neck pain,” “upper back pain,” “mid back pain,” “low back pain,” “buttocks pain,” “hip pain,” or “rib cage pain” for at least three months.
2.2.2 Exposure measurement
Participants provided self-reported information regarding their typical sleep duration on weekdays or workdays. Between 2009 and 2010, NHANES participants were requested to disclose their usual sleep duration on weekdays or workdays with the question: “How much sleep do you usually get at night on weekdays or workdays?” The distribution of the number of participants in each sleep duration group is shown in Supplementary Figure S2. The sleep duration data was segmented into three categories: less than 7 h, 7 to 8 h, and 9 h or more.
2.2.3 Covariate assessment
Covariates were selected based on existing literature. Standardized questionnaires were utilized to gather data on various factors, including: age, sex, race, education level, PIR, BMI, waist circumference, smoking habits, alcohol consumption, hypertension, diabetes, sleep disorders.
Race was classified as Mexican American, other Hispanic, non-Hispanic white, non-Hispanic black, or other. Education level was categorized as below 12th grade, high school graduate or equivalent, and college or above.
Height, weight, and blood pressure were measured following established protocols. BMI was computed by dividing weight in kilograms by height in meters squared (kg/m^2).
Participants were grouped as never smokers, former smokers or current smokers based on their responses regarding smoking history and current behavior. Alcohol consumption status was determined by the question “Have you had at least 12 drinks of any type of alcoholic beverage in any one year?.” Hypertension was identified by self-reporting a history of high blood pressure diagnosis. Diabetes was established through self-reporting a diagnosis of diabetes or sugar diabetes, excluding gestational diabetes. Sleep Disorders was defined based on the questions “Have you ever told a doctor you had trouble sleeping?” and “Have you ever been told by a doctor you have a sleep disorder?.” These covariates were included to account for potential confounding variables in the relationship between sleep duration and pain.
2.3 Statistical analysis
This study utilized sample weights provided by NHANES for weighted analysis to accurately reflect the actual distribution of the U.S. population, ensuring a high level of accuracy and representativeness in assessing the connection between sleep duration and chronic pain. The data was presented as survey-weighted mean (95% confidence interval [CI]) for continuous variables and survey-weighted percentage (95% CI) for categorical variables. The population with missing data in the variables was excluded from the analysis. A comparison of baseline characteristics was conducted among participants with different sleep durations (< 7 h, 7 to 8 h, and ≥ 9 h) using one-way ANOVA for continuous variables and the chi-square test for categorical variables.
We selected these confounders on the basis of their association with the outcomes of interest or changes in effect estimates of more than 10% (16). After considering the clinical significance, we adjusted for the following covariates: age (years), sex, BMI, race, PIR, alcohol consumption, smoking habits, diabetes, and sleep disorders. Odds ratios (ORs) and 95% confidence intervals (CIs) were computed for prevalent CMP and low back pain (LBP) using weighted logistic regression model. To further assess the impact of excluding missing data, we conducted a sensitivity analysis. We compared the results from the complete dataset with those from the dataset after excluding individuals with missing data. To assess the nonlinear relationship between sleep duration and prevalent CMP and LBP, a generalized additive model (GAM) was employed. The effective degrees of freedom (EDF) provide insight into the curvature of the smoothing curve (17, 18). An EDF value of 1 indicates a linear relationship, while an EDF value greater than 1 suggests a more complex relationship between chronic pain and sleep duration. By analyzing the smoothing curve, a weighted two-piecewise linear regression model (19, 20) was developed to identify any potential threshold effect. The model adjusted for possible confounders, and the results were presented as odds ratios (ORs) with corresponding 95% CIs. Additionally, we conducted stratified analyses for gender, age, smoking, BMI, diabetes, and sleep disorders to validate the results.
All analyses were conducted using the R statistical software (The R Foundation1) and EmpowerStats software (X&Y solutions, Inc. Boston MA2) running on R software. A two-sided p-value < 0.05 was considered statistically significant.
3 Results
3.1 Baseline characteristics of the study participants
Among the 3,904 participants in the study, 1,956 were male and 1,948 were female. Sleep duration is categorized into three distinct groups: those sleeping less than 7 h, those sleeping 7 to 8 h, and those sleeping 9 h or more. Compared with the group that slept for 7 to 8 h, participants with shorter sleep durations were more often male, had a lower PIR, and exhibited higher BMI or waist circumferences. They were also more likely to smoke and to have comorbid hypertension, diabetes, and sleep disturbances. In contrast, participants with longer sleep durations, as opposed to the 7–8 h group, were predominantly female, had a lower PIR, and had lower BMI or waist circumferences. They were also smokers and frequently had diabetes, but less frequently had hypertension and reported alcohol use. A detailed summary of the baseline characteristics of the subjects is provided in Table 1.
3.2 Impact of short and long sleep durations on chronic musculoskeletal pain and low back pain
Table 2 depicts the prevalence of pain in different anatomical regions. Both longer sleep durations (≥9 h) and shorter sleep durations (<7 h), compared to the standard sleep duration (7–8 h), are linked to increased pain prevalence across all examined anatomical regions (neck, upper back, mid back, low back, buttocks or hips). Table 3 shows the results of weighted logistic regression model construction between sleep duration and CMP and LBP, detailing odds ratios (ORs) and 95% CIs for both conditions across short and long sleep durations versus the normal sleep duration. In the unadjusted model, short sleep duration was associated with 97% higher odds of CMP (OR = 1.970 [95% CI 1.648, 2.354], p < 0.001), and long sleep duration with 79% higher odds compared to normal sleep duration (OR = 1.797 [95% CI 1.201, 2.690], p = 0.013). For LBP, the ORs were 1.844 ([95% CI 1.527, 2.228], p < 0.001) for short sleepers and 1.911 ([95% CI 1.233, 2.963], p = 0.012) for long sleepers. After adjusting for confounders, the adjusted ORs for CMP were 1.611 (95% CI 1.224, 2.120, p = 0.005) for short and 1.751 (95% CI 0.923, 3.321, p = 0.059) for long sleep durations. For LBP, the adjusted ORs were 1.428 (95% CI 1.085, 1.879, p = 0.015) for short and 1.818 (95% CI 0.883, 3.741, p = 0.069) for long sleep durations. Results derived from the analysis of data with missing covariates corroborated the findings observed in the full dataset of the study (Supplementary Table S1).
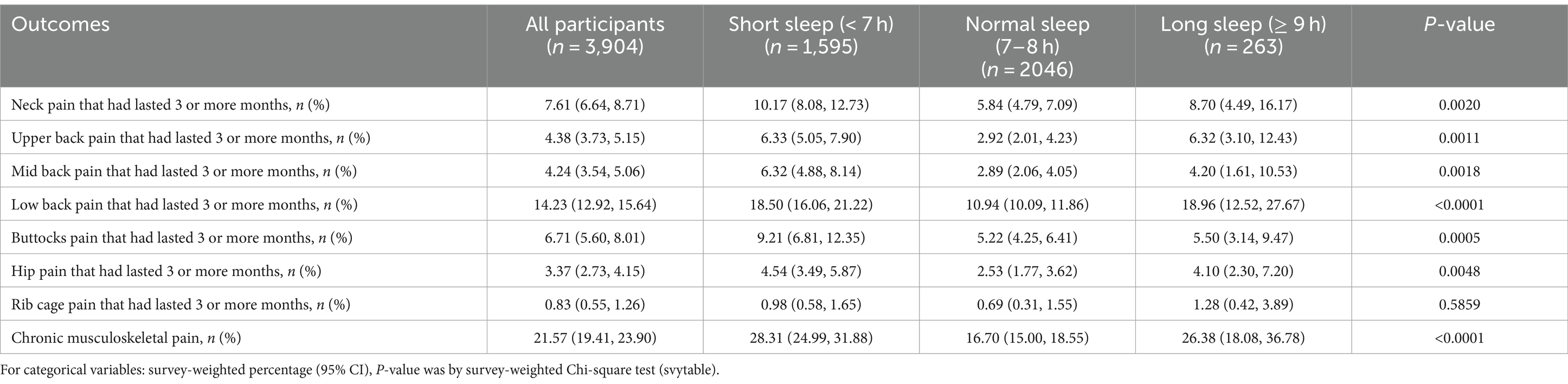
Table 2. Pain prevalence in different body regions among participants with different sleep durations.
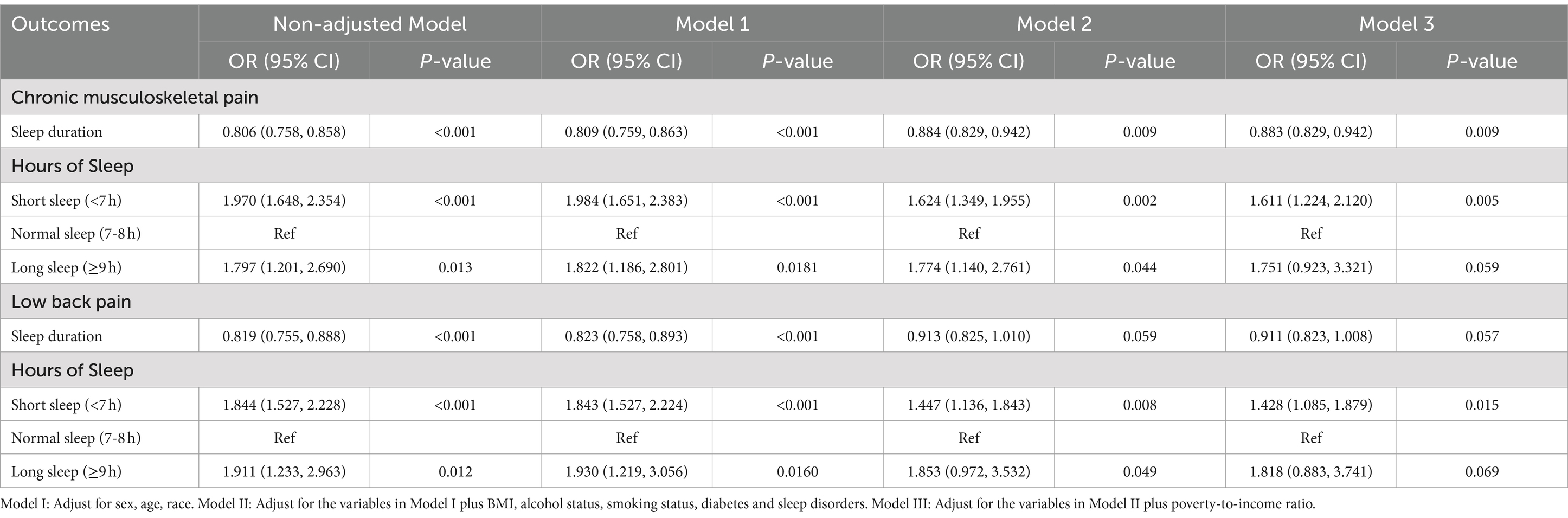
Table 3. Results of multiple logistic regressions on the association between sleep duration and pain outcomes.
3.3 Threshold effect analysis for sleep duration on chronic musculoskeletal pain and low back pain
Figure 1 illustrates a U-shaped relationship between CMP (EDF = 3.32, p < 0.001), LBP (EDF = 2.86, p < 0.001), and sleep duration post adjustment for confounders based on the generalized additive models. We further conducted a threshold effect analysis using the cut-off value of 7 h and found that the odds of CMP decreased with sleep duration until it reached a minimum at 7 h (OR = 0.772 [95% CI 0.717, 0.833], p = 0.002). Conversely, exceeding 7 h of sleep led to a significant increase in CMP odds (OR = 1.389 [95% CI 1.103, 1.749], p = 0.049) (Table 4). Similarly, the odds of LBP decreased with sleep duration until it reached a minimum at 7 h (OR = 0.776 [95% CI 0.663, 0.906], p = 0.006). However, surpassing 7 h of sleep resulted in an odds elevation (OR = 1.419 [95% CI 0.904, 2.228], p = 0.069).
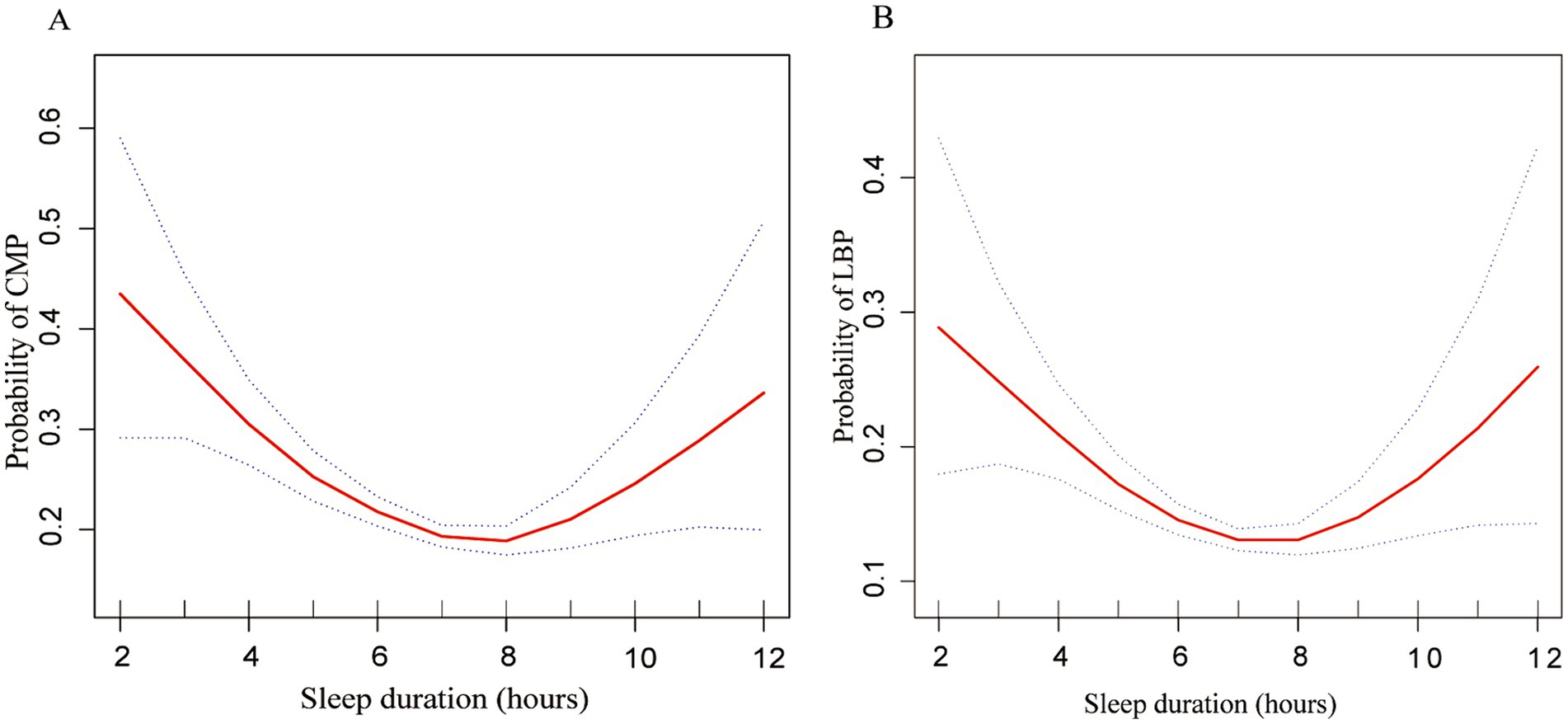
Figure 1. Multivariate-adjusted spline curves for relationship of sleep duration with chronic musculoskeletal pain and low back pain. (A) Probability of CMP and (B) probability of LBP. The solid line represents the estimated probability of CMP and LBP, and the dotted lines show point-wise 95% confidence intervals. These estimates are adjusted for sex, age, race, PIR, BMI, alcohol consumption, smoking habits, diabetes and sleep disorders. CMP, chronic musculoskeletal pain; LBP, low back pain; PIR, Poverty-to-Income Ratio.
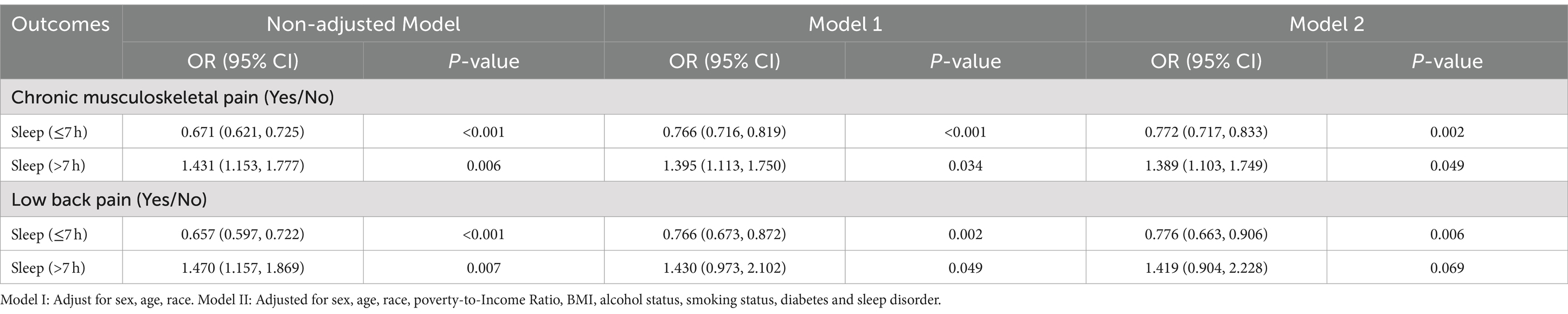
Table 4. Threshold effect analysis of sleep duration on chronic musculoskeletal pain and low back pain.
When stratified by gender, age, BMI, smoking, diabetes, and sleep disorders, a curvilinear relationship between sleep duration and musculoskeletal pain was still observed. Female, aged 40–65, smokers, obese individuals, those with diabetes, and individuals with sleep disorders have a high prevalence of CMP (Figure 2).
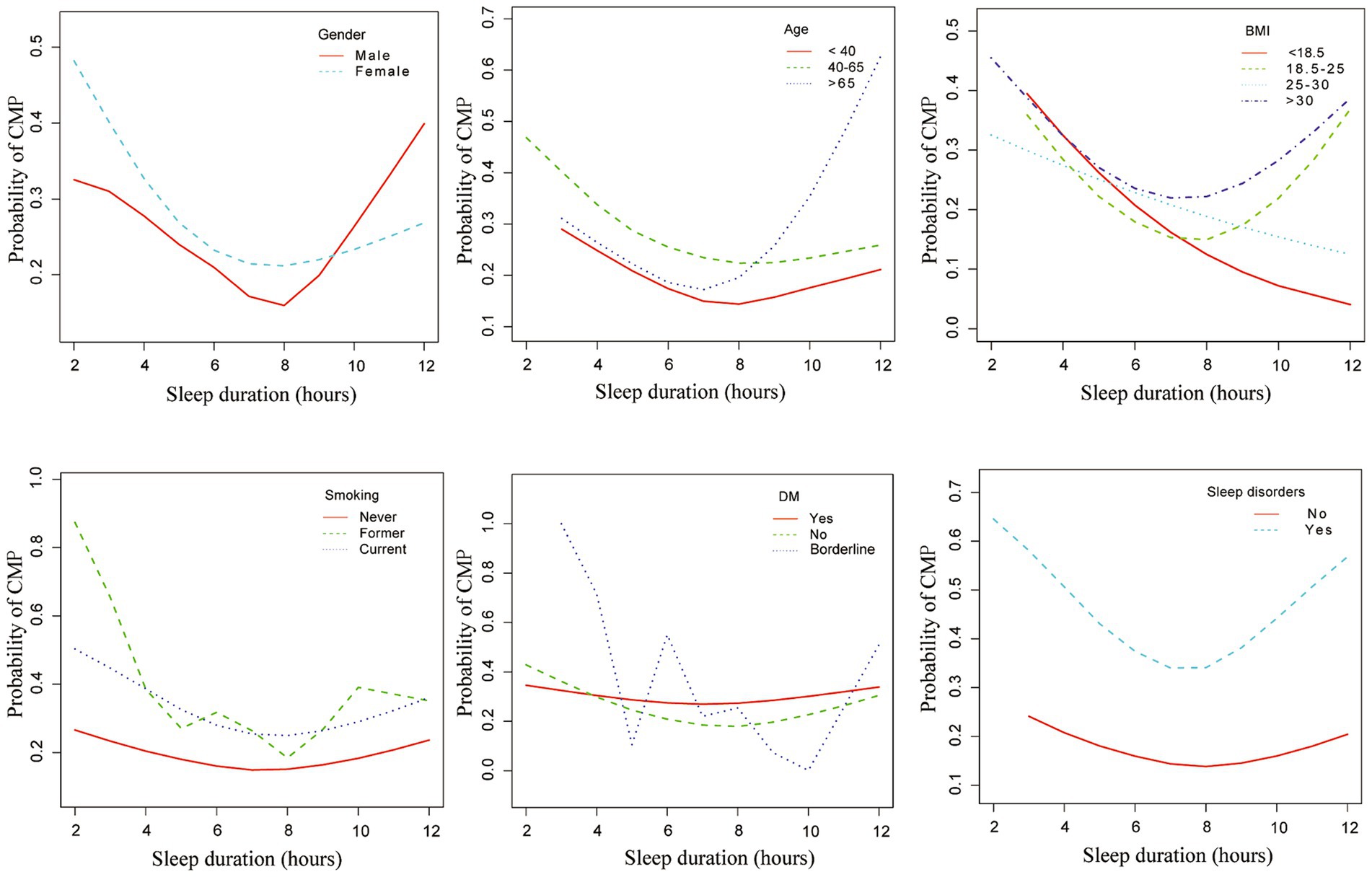
Figure 2. Associations between sleep duration and chronic musculoskeletal pain across different subgroups. This figure illustrates the associations between sleep duration and chronic musculoskeletal pain, stratified by gender, age, BMI, smoking status, diabetes, and sleep disorders. Adjusted for age (years), sex, race, PIR, BMI, alcohol consumption, smoking habits, diabetes and sleep disorders. CMP, chronic musculoskeletal pain; PIR, Poverty-to-Income Ratio.
4 Discussion
In this population-based cross-sectional investigation, we discovered an independent relationship between sleep duration and a higher prevalence of chronic musculoskeletal pain. Our analysis revealed a non-linear correlation between sleep duration and pain prevalence, with significant increases in pain odds observed at extreme sleep durations. The optimal sleep duration for mitigating pain odds was identified as 7 h, highlighting the importance of maintaining this balance to reduce the likelihood of chronic musculoskeletal pain.
Prior research has primarily focused on the adverse effects of insufficient sleep, with less attention given to the dangers linked with excessive sleep. Nevertheless, recent studies, including this one, have identified a U-shaped association between sleep duration and various health outcomes, including chest pain (21) and chronic musculoskeletal pain (22). This study’s results align with prior research, highlighting that both insufficient and excessive sleep are associated with higher odds of pain.
In our study, we observed a U-shaped relationship between sleep duration and the prevalence of CMP, with 7 h of sleep being associated with the lowest odds. This finding is consistent with other research demonstrating that deviations from the optimal sleep duration of 7 h are linked to various adverse health outcomes. For example, studies have shown that both insufficient and excessive sleep are associated with increased all-cause mortality (2) and accelerated phenotypic aging (23). These findings suggest that maintaining an optimal sleep duration is crucial not only for reducing the prevalence of chronic pain but also for overall health and longevity.
It is reported that the relationship between pain and sleep is complex and bidirectional: pain can disrupt sleep, while insufficient or interrupted sleep can lower pain thresholds and increase spontaneous pain levels (14). The underlying neurochemical mechanisms involved in this two-way relationship may encompass various components of the opioid, monoaminergic, orexinergic, immune, melatonin, and endocannabinoid systems. Additionally, the hypothalamic–pituitary–adrenal axis, adenosinergic signaling, and nitric oxide pathways are also thought to be implicated (14). Musculoskeletal pain is more prevalent and prone to chronicity compared to headaches or abdominal pain. Previous research has indicated that short-term sleep deprivation disrupts the internal redox balance of the body (24). Furthermore, sleep is thought to not only conserve energy but also facilitate the removal of toxic proteins and metabolic byproducts through different pathways (25). Sleep deprivation leads to oxidative stress in glial mitochondria and lipid droplet accumulation, necessitating overnight sleep for the induction of glial cell and neuronal mitochondrial autophagy. Sleep disruption has been associated with a reduction in N3 sleep while studies have indicated that partial night sleep deprivation can trigger inflammation in the body (26). The responsible mechanisms of the association among excessive sleep and musculoskeletal pain remain to be explored. But the present study demonstrates that individuals sleeping less than 7 h and 9 h or more have elevated levels of CRP and neutrophil count, indicating increased inflammation (Unpublished data). Previous research has demonstrated that CMP can lead to cognitive impairment and accelerated aging in the hippocampal region (27), supporting the rationale for investigating the association between sleep duration and CMP prevalence.
The study identified findings of borderline significance regarding the association between long sleep duration and musculoskeletal pain. Although the p-value of 0.059 is close to the conventional threshold for statistical significance, it suggests that further research is needed to substantiate this finding. Given the potential implications for public health and clinical practice, we recommend that future studies should consider larger sample sizes and longer follow-up periods.
Our research uncovered a more pronounced influence of sleep disturbances on pain perception in women compared to men. The underlying mechanisms behind how sleep affects pain perception, particularly in females, remain unclear. One hypothesis is that there may be biological distinctions in the way pain is regulated, driven by factors such as hormones and genetics, leading to divergent functional neurological changes in females compared to males when experiencing sleep issues (28).
The present study found a higher prevalence of musculoskeletal pain in populations with cigarette smoking. Smoking and pain have a complex relationship. Research indicates that smoking can worsen chronic pain conditions. Smokers with chronic pain tend to report higher pain intensity, functional impairment, and psychological symptoms compared to non-smokers (29). The prevalence of pain was also higher among former smokers compared to never smokers. While nicotine can initially have analgesic effects by stimulating the body’s natural pain relief system, chronic exposure to nicotine and tobacco smoke can lead to increased pain sensitivity and the need for more analgesic medication (30, 31). Smoking can impair the body’s ability to heal, decrease oxygen delivery, and lead to degenerative diseases that cause chronic pain (32). Additionally, smoking is associated with a higher odds of developing conditions like rheumatoid arthritis, which causes chronic joint pain and stiffness (32).
The present study found a higher prevalence of pain among adults aged 40–65 years compared to those under 40 years and over 65 years of age, excluding prolonged sleep durations. Several factors may contribute to the peak in pain prevalence within this middle-aged group: Many chronic diseases like osteoarthritis and degenerative spinal disease, which can lead to chronic pain, have a higher prevalence during the ages of 40–65 years (33). Adults in this age range often have high occupational stress and decreasing physical activity, predisposing them to musculoskeletal disorders (34). The perimenopausal period in women also falls within this age bracket, and hormone fluctuations may lower pain thresholds (35). Although older adults over 65 years have a high frequency of musculoskeletal conditions, pain thresholds gradually increase and pain perception declines in this elderly population (36).
5 Limitation
Our study boasts certain strengths, such as a relatively large sample size and adjustments for factors closely associated with musculoskeletal pain, such as smoking and alcohol consumption. However, our research also carries limitations. Firstly, the cross-sectional design of our study precludes causal inferences; thus, the directionality between sleep duration and pain requires further investigation through longitudinal or interventional research. Additionally, baseline self-reported sleep quality may be inaccurate, as individuals who self-report sleep disturbances are more likely to inaccurately estimate their sleep onset latency and total sleep time. In the future, both self-reported data and objective measurement methods such as actigraphs or sleep diaries, should be employed concurrently to enhance the accuracy of the data.
Additionally, our study solely focused on sleep duration, overlooking disruptions in sleep quality and patterns, such as fragmentation or shallow sleep, which are closely associated with heightened pain perception. Future research should incorporate monitoring and analyzing sleep patterns to comprehensively understand the relationship between sleep and pain. This study excluded participants with missing covariate data, which may result in a sample that does not fully represent the broader population. Consequently, despite our efforts to control for confounding factors in the analysis, selection bias may still influence the interpretation of our results. The study results are only applicable to the population aged 20–69 with available pain data, indicating that our findings are limited to this specific demographic in the United States.
6 Conclusion
To summarize, the duration of sleep was found to be linked to a higher occurrence of CMP in this study, which utilized data from the NHANES conducted between 2009 and 2010. It was observed that not only insufficient sleep, but also excess sleep, can contribute to a higher prevalence of experiencing pain.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the ethics review board of the National Center for Health Statistics. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because The protocols for the National Health and Nutrition Examination Survey (NHANES)were approved by the ethics review board of the National Center for Health Statistics, and all participants provided written informed consent.
Author contributions
CL: Methodology, Supervision, Writing – review & editing. HH: Data curation, Formal analysis, Writing – review & editing. QX: Conceptualization, Validation, Writing – original draft. LZ: Data curation, Supervision, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. LZ reports funding from the Project of Suzhou Science and Technology (No. SKY2022079), the Kunshan Science and Technology Development Fund (No. KS2311), and Medical Health Science and Technology Innovation Project of Kunshan First People’s Hospital (KSKFQYLWS2023022). CL reports funding from Suzhou Municipal Science and Technology Development Plan (Construction of Key Municipal Laboratories) (SZS2024018) and Suzhou City Major Disease Multicenter Clinical Research Project (DZXYJ202312).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1461785/full#supplementary-material
Footnotes
References
1. El-Tallawy, SN, Nalamasu, R, Salem, GI, LeQuang, JAK, Pergolizzi, JV, and Christo, PJ. Management of Musculoskeletal Pain: an update with emphasis on chronic musculoskeletal pain. Pain Ther. (2021) 10:181–209. doi: 10.1007/s40122-021-00235-2
2. Svensson, T, Saito, E, Svensson, AK, Melander, O, Orho-Melander, M, Mimura, M, et al. Association of Sleep Duration with all-and Major-Cause Mortality among Adults in Japan, China, Singapore, and Korea. JAMA Netw Open. (2021) 4:e2122837. doi: 10.1001/jamanetworkopen.2021.22837
3. Hirshkowitz, M, Whiton, K, Albert, SM, Alessi, C, Bruni, O, DonCarlos, L, et al. National Sleep Foundation's updated sleep duration recommendations: final report. Sleep Health. (2015) 1:233–43. doi: 10.1016/j.sleh.2015.10.004
4. Li, Y, Sahakian, BJ, Kang, J, Langley, C, Zhang, W, Xie, C, et al. The brain structure and genetic mechanisms underlying the nonlinear association between sleep duration, cognition and mental health. Nat Aging. (2022) 2:425–37. doi: 10.1038/s43587-022-00210-2
5. Cui, H, Xu, R, Wan, Y, Ling, Y, Jiang, Y, Wu, Y, et al. Relationship of sleep duration with incident cardiovascular outcomes: a prospective study of 33,883 adults in a general population. BMC Public Health. (2023) 23:124. doi: 10.1186/s12889-023-15042-x
6. Zhou, L, Yu, K, Yang, L, Wang, H, Xiao, Y, Qiu, G, et al. Sleep duration, midday napping, and sleep quality and incident stroke: the Dongfeng-Tongji cohort. Neurology. (2020) 94:e345–56. doi: 10.1212/WNL.0000000000008739
7. Lee, S . Naturally occurring consecutive sleep loss and day-to-day trajectories of affective and physical well-being. Ann Behav Med. (2022) 56:393–404. doi: 10.1093/abm/kaab055
8. Finan, PH, Goodin, BR, and Smith, MT. The association of sleep and pain: an update and a path forward. J Pain. (2013) 14:1539–52. doi: 10.1016/j.jpain.2013.08.007
9. Morin, CM, LeBlanc, M, Daley, M, Gregoire, JP, and Mérette, C. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. (2006) 7:123–30. doi: 10.1016/j.sleep.2005.08.008
10. Afolalu, EF, Ramlee, F, and Tang, NKY. Effects of sleep changes on pain-related health outcomes in the general population: a systematic review of longitudinal studies with exploratory meta-analysis. Sleep Med Rev. (2018) 39:82–97. doi: 10.1016/j.smrv.2017.08.001
11. Mundal, I, Gråwe, RW, Bjørngaard, JH, Linaker, OM, and Fors, EA. Psychosocial factors and risk of chronic widespread pain: an 11-year follow-up study—the HUNT study. Pain. (2014) 155:1555–61. doi: 10.1016/j.pain.2014.04.033
12. Varallo, G, Giusti, EM, Manna, C, Castelnuovo, G, Pizza, F, Franceschini, C, et al. Sleep disturbances and sleep disorders as risk factors for chronic postsurgical pain: a systematic review and meta-analysis. Sleep Med Rev. (2022) 63:101630. doi: 10.1016/j.smrv.2022.101630
13. Mills, SEE, Nicolson, KP, and Smith, BH. Chronic pain: a review of its epidemiology and associated factors in population-based studies. Br J Anaesth. (2019) 123:e273–83. doi: 10.1016/j.bja.2019.03.023
14. Haack, M, Simpson, N, Sethna, N, Kaur, S, and Mullington, J. Sleep deficiency and chronic pain: potential underlying mechanisms and clinical implications. Neuropsychopharmacology. (2020) 45:205–16. doi: 10.1038/s41386-019-0439-z
15. Raja, SN, Carr, DB, Cohen, M, Finnerup, NB, Flor, H, Gibson, S, et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. (2020) 161:1976–82. doi: 10.1097/j.pain.0000000000001939
16. Jaddoe, VW, de Jonge, LL, Hofman, A, Franco, OH, Steegers, EA, and Gaillard, R. First trimester fetal growth restriction and cardiovascular risk factors in school age children: population based cohort study. BMJ. (2014) 348:g14. doi: 10.1136/bmj.g14
17. Smiley, A, King, D, and Bidulescu, A. The association between sleep duration and metabolic syndrome: the NHANES 2013/2014. Nutrients. (2019) 11:2582. doi: 10.3390/nu11112582
18. Smiley, A, King, D, Harezlak, J, Dinh, P, and Bidulescu, A. The association between sleep duration and lipid profiles: the NHANES 2013-2014. J Diabetes Metab Disord. (2019) 18:315–22. doi: 10.1007/s40200-019-00415-0
19. Yu, X, Cao, L, and Yu, X. Elevated cord serum manganese level is associated with a neonatal high ponderal index. Environ Res. (2013) 121:79–83. doi: 10.1016/j.envres.2012.11.002
20. Yu, X, Chen, J, Li, Y, Liu, H, Hou, C, Zeng, Q, et al. Threshold effects of moderately excessive fluoride exposure on children's health: a potential association between dental fluorosis and loss of excellent intelligence. Environ Int. (2018) 118:116–24. doi: 10.1016/j.envint.2018.05.042
21. Chen, W, Wang, JP, Wang, ZM, Hu, PC, and Chen, Y. Association between sleep duration and chest pain in US adults: a cross-sectional study. Front Public Health. (2022) 10:952075. doi: 10.3389/fpubh.2022.952075
22. Bonvanie, IJ, Oldehinkel, AJ, Rosmalen, JGM, and Janssens, KAM. Sleep problems and pain: a longitudinal cohort study in emerging adults. Pain. (2016) 157:957–63. doi: 10.1097/j.pain.0000000000000466
23. You, Y, Chen, Y, Liu, R, Zhang, Y, Wang, M, Yang, Z, et al. Inverted U-shaped relationship between sleep duration and phenotypic age in US adults: a population-based study. Sci Rep. (2024) 14:6247. doi: 10.1038/s41598-024-56316-7
24. Trivedi, MS, Holger, D, Bui, AT, Craddock, TJA, and Tartar, JL. Short-term sleep deprivation leads to decreased systemic redox metabolites and altered epigenetic status. PLoS One. (2017) 12:e0181978. doi: 10.1371/journal.pone.0181978
25. Haynes, PR, Pyfrom, ES, Li, Y, Stein, C, Cuddapah, VA, Jacobs, JA, et al. A neuron-glia lipid metabolic cycle couples daily sleep to mitochondrial homeostasis. Nat Neurosci. (2024) 27:666–78. doi: 10.1038/s41593-023-01568-1
26. Irwin, MR, Olmstead, R, Bjurstrom, MF, Finan, PH, and Smith, MT. Sleep disruption and activation of cellular inflammation mediate heightened pain sensitivity: a randomized clinical trial. Pain. (2023) 164:1128–37. doi: 10.1097/j.pain.0000000000002811
27. Zhao, W, Zhao, L, Chang, X, Lu, X, and Tu, Y. Elevated dementia risk, cognitive decline, and hippocampal atrophy in multisite chronic pain. Proc Natl Acad Sci USA. (2023) 120:e2215192120. doi: 10.1073/pnas.2215192120
28. Mogil, JS . Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. (2012) 13:859–66. doi: 10.1038/nrn3360
29. Khan, JS, Hah, JM, and Mackey, SC. Effects of smoking on patients with chronic pain: a propensity-weighted analysis on the collaborative health outcomes information registry. Pain. (2019) 160:2374–9. doi: 10.1097/j.pain.0000000000001631
30. Ditre, JW, Brandon, TH, Zale, EL, and Meagher, MM. Pain, nicotine, and smoking: research findings and mechanistic considerations. Psychol Bull. (2011) 137:1065–93. doi: 10.1037/a0025544
31. Chen, D, Shen, L, Zhang, YZ, Kan, BF, Lou, QQ, Long, DD, et al. Chronic nicotine exposure elicits pain hypersensitivity through activation of dopaminergic projections to anterior cingulate cortex. Br J Anaesth. (2024) 132:735–45. doi: 10.1016/j.bja.2023.12.034
32. Hutchinson, D, Shepstone, L, Moots, R, Lear, JT, and Lynch, MP. Heavy cigarette smoking is strongly associated with rheumatoid arthritis (RA), particularly in patients without a family history of RA. Ann Rheum Dis. (2001) 60:223–7. doi: 10.1136/ard.60.3.223
33. Domenichiello, AF, and Ramsden, CE. The silent epidemic of chronic pain in older adults. Prog Neuro-Psychopharmacol Biol Psychiatry. (2019) 93:284–90. doi: 10.1016/j.pnpbp.2019.04.006
34. Stults-Kolehmainen, MA, and Sinha, R. The effects of stress on physical activity and exercise. Sports Med. (2014) 44:81–121. doi: 10.1007/s40279-013-0090-5
35. Ai, Z, Tang, C, Peng, P, Wen, X, and Tang, S. Prevalence and influencing factors of chronic pain in middle-aged and older adults in China: results of a nationally representative survey. Front Public Health. (2023) 11:1110216. doi: 10.3389/fpubh.2023.1110216
Keywords: sleep duration, chronic musculoskeletal pain, low back pain, NHANES, cross-sectional study
Citation: Li C, Huang H, Xia Q and Zhang L (2024) Association between sleep duration and chronic musculoskeletal pain in US adults: a cross-sectional study. Front. Med. 11:1461785. doi: 10.3389/fmed.2024.1461785
Edited by:
Frauke Musial, UiT The Arctic University of Norway, NorwayReviewed by:
Abbas Smiley, University of Rochester, United StatesLi-Hua Chen, Nantong University, China
Copyright © 2024 Li, Huang, Xia and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Zhang, emhhbmdsaTcxODJAbmptdS5lZHUuY24=
†These authors have contributed equally to this work
 Chong Li1†
Chong Li1† Li Zhang
Li Zhang