- 1Cystic Fibrosis Center of Verona, Azienda Ospedaliera Universitaria Integrata, Verona, Italy
- 2Pediatric Division, Department of Surgery, Dentistry, Pediatrics and Gynaecology, University of Verona, Verona, Italy
- 3Pediatric Division, University Hospital of Verona, Verona, Italy
- 4Institute of Translational Pharmacology (IFT), National Research Council (CNR), Palermo, Italy
Wheezing is a common condition in childhood, and its prevalence has increased in the last decade. Up to one-third of preschoolers develop recurrent wheezing, significantly impacting their quality of life and healthcare resources. Artificial Intelligence (AI) technologies have recently been applied in paediatric allergology and pulmonology, contributing to disease recognition, risk stratification, and decision support. Additionally, the COVID-19 pandemic has shaped healthcare systems, resulting in an increased workload and the necessity to reduce access to hospital facilities. In this view, AI and Machine Learning (ML) approaches can help address current issues in managing preschool wheezing, from its recognition with AI-augmented stethoscopes and monitoring with smartphone applications, aiming to improve parent-led/self-management and reducing economic and social costs. Moreover, in the last decade, ML algorithms have been applied in wheezing phenotyping, also contributing to identifying specific genes, and have been proven to even predict asthma in preschoolers. This minireview aims to update our knowledge on recent advancements of AI applications in childhood wheezing, summarizing and discussing the current evidence in recognition, diagnosis, phenotyping, and asthma prediction, with an overview of home monitoring and tele-management.
Introduction
Wheezing is a musical sound, high-pitched and continuous, emitted from the chest during exhalation and resulting from the narrowing of the intrathoracic airway and expiratory flow limitation (1). The prevalence of wheezing disorders in preschool children varies worldwide and appears to have increased during the last decade (2). It is estimated that about one in three children experiences wheezing during the first 3 years of life (3). Viral infections trigger most wheezing episodes, involving up to 30–50% of preschool children (4). Generally, such episodes are mild and transient. However, one-third of preschoolers develop recurrent wheezing, which is defined as four or more episodes in the previous year (5). Recurrent wheezing has a significant impact on quality of life as well as on healthcare resources (6). Indeed, the economic burden of wheezing for the European Union is estimated at EUR 5.2 billion (7).
Artificial intelligence (AI) and machine learning (ML) encompass approaches such as data mining methodologies, predictive analytics, and advanced statistics for pattern recognition and neurocomputing (8). The application of AI technologies in paediatric allergology and pulmonology has increased, contributing to disease detection, risk profiling, and decision support (9, 10). Additionally, the COVID-19 pandemic has shaped healthcare systems, resulting in an increased workload and the necessity to reduce access to hospital facilities. Indeed, several studies have investigated the applications of AI and ML during the COVID-19 pandemic (11). Overall, such approaches could help address current issues in managing preschool wheezing, including phenotyping and improving parent-led/self-management, while reducing economic and social costs.
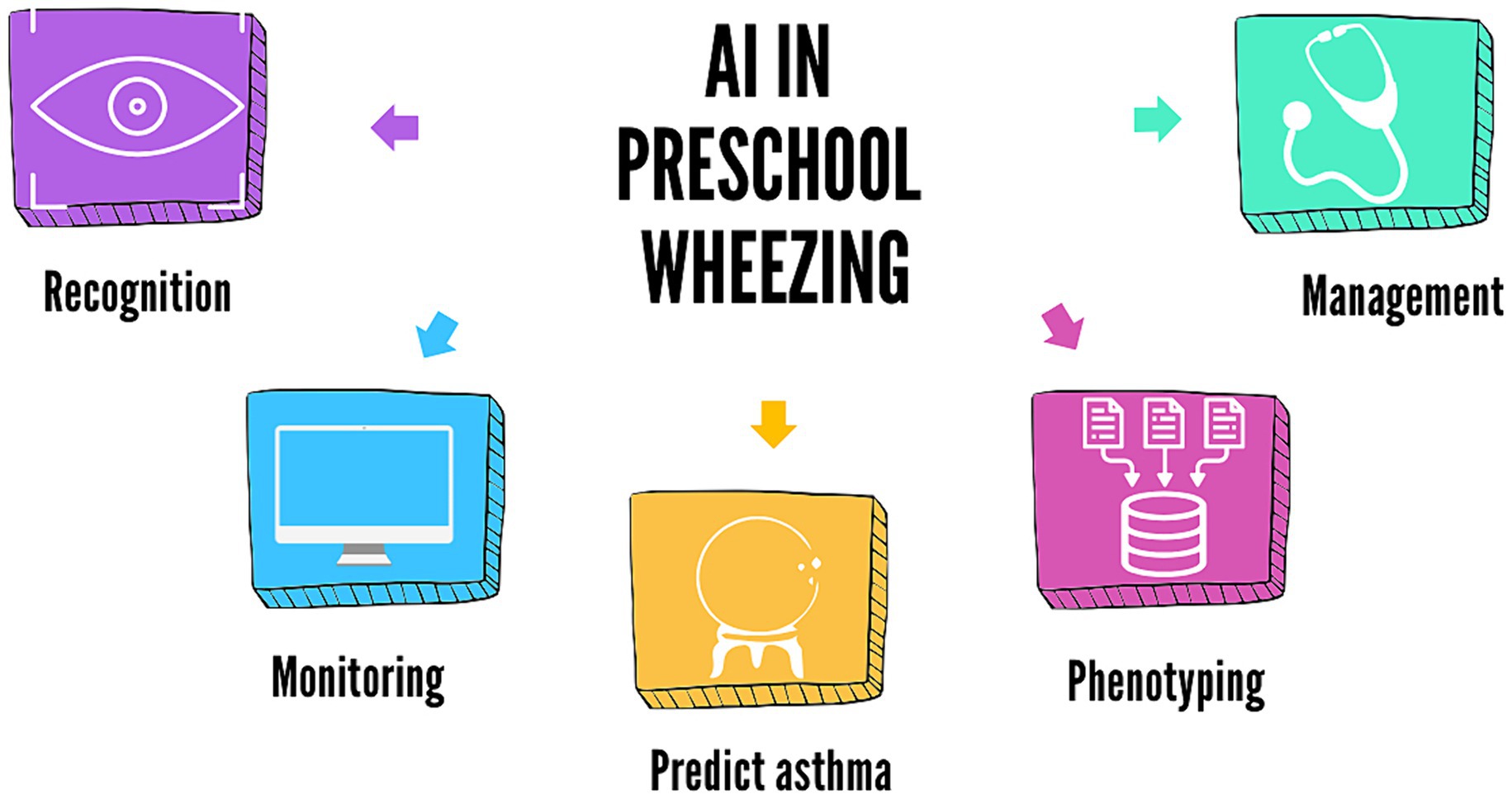
This minireview aims to summarize and discuss the current evidence on possible applications of AI in recognizing and monitoring wheezing in children and predicting future asthma development, progressing from deep phenotyping to patient-tailored management (Figure 1). We conducted a literature search in the PubMed database, selecting articles published over the last 10 years. We used medical subject headings (MeSH terms) and free-text terms related to wheezing, machine learning, artificial intelligence, and asthma and limited the search to clinical trials, randomized controlled trials, meta-analyses, and systematic reviews. Additionally, we manually consulted the reference lists of the retrieved articles. Manuscripts were selected by the authors (L.V. and S.M.), considering full manuscripts published in English in peer-reviewed journals.
Can artificial intelligence recognize and monitor wheezing in children?
Parents and doctors often use “wheezing” to describe various respiratory sounds, such as crackles (12). The cheapest and non-invasive method for assessing wheezing is auscultation using a phonendoscope, which is operator-dependent and does not allow recording. Therefore, there is an increasing demand for an automatic, more objective, shareable, and reproducible method to assist doctors in diagnosing and monitoring patients with respiratory diseases (13).
The electronic stethoscope is an innovative version of the classic model, offering the ability to record and store chest sounds, allowing remote access. Many devices enable sound amplification, which is helpful for teaching (13). However, the digital data collected are subject to human interpretation and inter-operator variability, challenges that can be addressed by AI and ML technologies. These technologies have demonstrated good accuracy in recognizing respiratory sounds, particularly wheezing in children (Supplementary Table S1).
AI-assisted home stethoscopes can provide reliable information on asthma exacerbations. A recent study evaluated StethoMe for the automatic detection of pathological lung sounds (wheezes, crackles, and rhonchi) at home in 90 patients (0–18 years), demonstrating its efficacy in identifying asthma exacerbation across all ages (14). StethAid® is a device with a decision support system based on deep learning, an artificial neural network technology, used in an emergency department to recognize wheezing (15). The device recorded lung sounds from patients aged 2–18 years experiencing asthma exacerbation. These recordings were converted into spectrograms, serving as input for two deep learning models: ResNet-18 and Harmonic Networks. Both models were trained and validated to identify wheezing sounds from clear breathing sounds with good sensitivity, specificity, and accuracy. Specifically, ResNet-18 achieved 77% sensitivity, 70.1% specificity and 73.9% accuracy, while Harmonic Networks achieved 83.7% sensitivity, 84.4% specificity, and 84% accuracy.
A recent study by Ajay Kevat et al. (16) demonstrated high accuracy in recognizing children’s lung sounds using AI-enhanced digital stethoscopes, although differences were noted between various devices. AI stethoscopes can store diaries of wheezing episodes, enabling remote monitoring (14). Limitations of these devices include high cost, complexity of use, incompatibility with software and/or operating systems, and technical constraints (e.g., limited data memory, duration of autonomy, and varying frequency characteristics) (15).
Another device for analyzing lung sounds is PulmoTrack® (17–19), which utilizes chest sensors with an external microphone to capture and cancel environmental noises. Its effectiveness was evaluated in a study (17) involving 120 infants during sleep, demonstrating that computerized wheezing detection is more objective, non-invasive, and standardized compared to medical auscultation. The device also proved beneficial in intensive care settings for managing patients with wheezing (20).
The HWZ-1000 T device (Omron Healthcare Corporation, Kyoto, Japan) was evaluated in a study (21) involving 374 children. Wheeze was detected by auscultation with a stethoscope and recorded using the wheeze recognition algorithm device (HWZ-1000T), based on the sound characteristics of wheezing. The device accurately identified wheezing (sensitivity 96.6%, specificity 98.5%, positive predictive value 98.3%, and negative predictive value 97.0%).
In the study by Dramburg et al. (22), 20 infants and preschool children (9–72 months) diagnosed with wheezing in the past year were recruited. All their families were requested to use the WheezeScan® digital wheeze detector (OMRON Healthcare Co., Ltd.) twice daily and simultaneously monitor the child’s respiratory symptoms through a smartphone clinical diary for 30 days. The results were displayed on an integrated screen that could be transmitted via Bluetooth to a PC or mobile device (e.g., smartphone or tablet). The study concluded that using the WheezeScan® Detector is straightforward and safe for children with wheezing. The support of a digital wheezing detector enhances parents’ self-efficacy in managing asthma and wheezing, boosting their confidence in handling their child’s wheezing at home. The WheezeScan® demonstrated good sensitivity (83.3%) and specificity (100%) in wheezing recognition, albeit with limited visits (22). In a more recent study (23), the analysis of WheezeScan® revealed no significant differences in wheeze control between study groups, with no impact on quality of life and minimal differences in parental efficacy in wheezing management.
Smartphone devices also play a role in integrating AI in Medical Practice. The ResAppDx® algorithm (24) analyses cough using a microphone integrated into the smartphone, alongside symptoms reported by the patient and/or parents. Automated cough analysis has demonstrated good diagnostic accuracy for common childhood respiratory diseases and it is non-invasive and feasible even in resource-limited environments (24). Another smartphone-based algorithm for detecting cough sounds was evaluated (25), comparing training data that included recordings of children coughing and ambient audio with everyday noises. The algorithm achieved an accuracy of 99.7% and a specificity of 99.96% when tested on the coughs of 21 children between 0 and 16 years hospitalized for lung diseases. This suggests that smartphone applications can be used for clinical follow-up and as a digital endpoint in clinical trials (25).
AI algorithms for wheezing recognition have some limitations, such as difficulty in correlating certain respiratory sounds with specific illnesses and considering that paediatric cough sounds vary with age due to respiratory and vocal system development. It should also be acknowledged that digital devices remain limited when compared to traditional lung auscultation for patients with severe airflow obstruction, who may have silent lungs without wheezing (19), and that the effectiveness of a digital device can be influenced by various factors such as age and cultural background. In conclusion, while many studies highlight the effectiveness and applicability of AI digital devices in detecting wheezing, others have yet to achieve similar results. Therefore, more studies will be necessary to assess their effectiveness in recognizing wheezing in children.
Can artificial intelligence predict asthma outcomes in children with wheezing?
Most children with asthma experience symptoms in early life, but these are typically transient, often disappearing by school age (6–13 years) (26). Therefore, it can be challenging to differentiate asthma from other wheezing disorders at this age (26). Over the last few decades, considerable effort has been dedicated to predicting asthma in children to identify earlier those at high risk and provide them with the best treatment option (26).
Previous studies based on population birth cohorts have identified distinct wheezing phenotypes (clusters) with associated early-life factors and outcomes, paving the path to predict wheezing trajectory and thereby develop targeted management (27). In this context, predictive models have been developed, considering various risk factors associated with asthma development, such as parental history of atopy and asthma, eczema and atopic dermatitis, allergic sensitization, and eosinophilia. However, they have rarely included environmental exposures and socioeconomic status (28). One of the most widely used models is the Asthma Predictive index (API) (29) and its modified version (mAPI) (30). Other models, such as the Isle of Wight score (31) and the Prevention and Incidence of Asthma and Mite Allergy (PIAMA) risk score, have also been developed (32); however, they have included children with recurrent chest infections, potentially misreporting episodes of wheezing (33).
Other predictive tools include the Leicester asthma prediction tool (34), the University of Connecticut (ucAPI) (35), and the Asthma Detection and Monitoring (ademAPI) (36), which also incorporate predictors such as 10 exhaled breath condensate biomarkers, 17 volatile organic compounds, and 31 genes. Although the ademAPI is the most comprehensive and sophisticated model, demonstrating reasonable specificity (88%) and sensitivity (90%), as well as the best positive and negative LRs (8.8 and 0.13, respectively), compared to other predictive models (33), its implementation in clinical practice remains challenging due to high cost (33). Even the original API, which includes only four items and a blood sample for eosinophil count, shows a good positive LR but a low negative LR, making it less effective in ruling out asthma (33). One of the more recent models is the CHILDhood Asthma Risk Tool (CHART) (37), which can identify children from 2 years of age at high risk of persistent wheezing and likely to develop asthma. Thanks to its simplicity, CHART could be used as a screening tool in primary care.
Overall, the models mentioned above indicate that the wheeze pattern alone cannot predict asthma progression. For this purpose, ML approaches have demonstrated better predictive performance and generalizability compared to regression-based models (27). In this context, artificial neural networks (ANNs) constitute a type of AI technique that learns the potential relationship between input–output mapping from a given dataset without prior knowledge or assumptions about the data distribution (38). This sets them apart from common statistical tests and makes them suitable for classification and prediction tasks.
One of the initial studies in this research field (26) employed Principal Component Analysis (PCA) for feature extraction, followed by the Least Square Support Vector Machine (LSSVM) classifier for pattern classification, resulting in a ML model with an accuracy of 95.54% in predicting asthma. More recently, a study (39) from the Isle of Wright birth cohort applied ML approaches to predict school-age asthma (at the age of 10 years) in early life (Childhood Asthma Prediction in Early life, CAPE model) and at preschool age (Childhood Asthma Prediction at Preschool age, CAPP model). Recursive Feature Elimination (RFE) with a random forest algorithm was used for feature selection. Seven ML classifiers were then implemented to identify the best classification algorithm: two Support Vector Machines (SVM), a decision tree, a random forest, Naive Bayes, Multilayer Perceptron, and K-Nearest Neighbours. Finally, the models were also validated in the Manchester Asthma and Allergy Study (MAAS) cohort. The SVM algorithms demonstrated the best performance for CAPE and CAPP, showing excellent sensitivity in predicting persistent wheezing. Interestingly, the study was implemented by incorporating genetic and epigenetic information (40), which marginally improved performance and indicated that genetic and epigenetic markers for the broader phenotype of “diagnosed with asthma” are unlikely to have clinical utility (41).
A limitation of using the scores mentioned above is the challenge of ruling out asthma rather than identifying it. However, in clinical practice, they can assist in identifying patients at high risk of developing asthma who are likely to respond to ICS, as shown in a latent class analysis (LCA) (42). This analysis showed that ICS treatment reduced exacerbations in children with persistent wheezing and conditions such as “sensitization with indoor pet exposure” and “multiple sensitization and eczema.”
Ultimately, the global diffusion of electronic health records (EHRs) created a need for automated chart review to diagnose asthma in children. Kaur et al. (43) developed a natural language processing (NLP) algorithm to identify children meeting API criteria. This NLP-API predicted asthma in preschoolers with a sensitivity of 86%, specificity of 98%, positive predictive value of 88%, and negative predictive value of 98%. Such an index has the potential to be utilized by healthcare systems to identify children meeting API criteria, even in early childhood (e.g., < 3 years old), thereby improving access to preventive and therapeutic interventions for asthma and monitoring their outcomes (9, 43).
Nonetheless, using AI algorithms and ML for predicting asthma outcomes in children may raise potential ethical concerns. Firstly, AI algorithms are trained on a large volume of personal data from EHRs, including clinical, imaging, and even genomic data, so it appears clear that ensuring privacy is critical, while overprotection of the data collection, usage, and sharing can slow down the innovation in AI training (44). To overcome this important limitation and preserve privacy, new techniques are emerging in AI such as the generation of synthetic data that mirrors the real-world dataset, but even this approach can not ensure full privacy, especially in small datasets, as patients from a specific region (45) or in a particular age range. Moreover, if AI algorithms are trained in a limited dataset, they can inadvertently present some gender, socioeconomic, and ethnic bias, that can exacerbate health inequalities in underrepresented social groups (44, 46), resulting in incorrect predictions, and leading to misdiagnosis when these biases are not corrected or prevented during the elaboration of the training dataset (44).
For these reasons, taking also in consideration that AI can actually make mistakes, AI can not be held morally accountable, having a role only as a decision support aid for clinicians (44). If used in clinical practice to provide therapeutic recommendations, to inform prognosis or risk of future events, informed consent should be provided to patients, explaining to them if AI has been used, clarifying which type of AI and how it was involved in the decision process, informing also about potential pitfalls (47).
Can artificial intelligence identify wheezing endotypes in preschool children?
Wheezing has been classified into different phenotypes since the first population-based cohort studies aimed to understand its heterogeneity (41).
The initial study was the Tucson Children’s Respiratory Study (5), which identified three patterns of preschool wheezing (early transient, late-onset, and persistent), each associated with different risk factors. Subsequent studies have further defined additional phenotypes and temporal patterns, such as the Avon Longitudinal Study of Parents and Children (ALSPAC) (4, 48), the Prevention and Incidence of Asthma and Mite Allergy (PIAMA) birth cohort (49), the Viva project (50).
This approach assumes that patterns of symptoms and/or biomarkers assessed in longitudinal or cross-sectional studies reflect the underlying mechanisms, leading to the identification of asthma endotypes, but this assumption is uncertain (51).
ML approaches such as LCA have also been used in preschool wheezing (4, 52–54) and childhood asthma (55, 56).
An interesting study (57) focused on the longitudinal trajectory of wheezing exacerbations using an ML approach (k-means clustering), which identified two types of trajectories from birth to adolescence. The k-means clustering revealed that a shorter duration of breastfeeding was one of the early risk factors for frequent exacerbations. Additionally, children with frequent exacerbations showed increased airway resistance and, at 8 years of age, a lower lung function with higher FeNO levels, with evolution to asthma at 16 years of age.
ML approaches have also contributed to identifying specific genes, as demonstrated by Lin et al. (58), who employed Weighted Gene Co-expression Network Analysis (WGCNA) to identify gene co-expression modules associated with pediatric asthma. They subsequently used ML algorithms (random forest, lasso regression algorithm, and support vector machine with recursive feature elimination) to classify asthma cases and controls based on the 11 identified genes that can potentially explain the pathophysiology of difficult asthma and serve as biomarkers for diagnosis and targets for future advanced treatments.
Notably, as Saglani et al. (51) highlighted, we should be cautious about assuming that clusters identified in these studies represent “true” wheezing endotypes. The limitations of these studies include the identification of different risk factors for the same disease (wheezing) using the same technique (LCA), differences in the characteristics of the wheezing trajectories, the temporal description of wheezing in these clusters that may not align with the temporal presentation of symptoms, and ultimately, the diverse pathological mechanisms that can lead to wheezing within the same cluster. For example, persistent wheezing can arise from recurrent airway infections due to impaired immunological responses or from allergen sensitization and exposure (51).
Considering these limitations, ML has identified more intermediate phenotypes with one certainty across all studies: all wheezing phenotypes, even the transient ones, lead to impaired lung function in early adulthood (41). Moreover, the results obtained so far need validation in further longitudinal studies involving larger populations of preschool children.
Conclusion
The applications of AI in preschool wheezing have encompassed various research topics, including phenotyping, delineating trajectories using data from EHR, predicting future asthma development and exacerbations, and identifying early risk factors and genetic markers. There are also several applications for clinical practice, such as wheezing recognition using AI-augmented stethoscopes or smartphones and telemonitoring (59).
Although AI could support clinicians in their daily practice, some questions must be addressed, especially when caring for children. Regulatory requirements are of foremost importance in protecting sensitive data and maintaining privacy. Additionally, AI approaches and their results must be rigorously validated before we adopt them in our routines.
In conclusion, AI could enhance the management of preschool wheezing, from recognition to identifying children potentially at high risk of exacerbation and asthma, thereby enabling an AI-tailored treatment. Furthermore, we should consider AI’s utility in case of future pandemics, particularly in telemonitoring and telemanagement. However, we must be mindful of its limitations and work to address them to ensure the safety of children’s data.
Author contributions
LV: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. SM: Methodology, Writing – original draft, Writing – review & editing. MP: Writing – original draft, Writing – review & editing. MZ: Writing – original draft, Writing – review & editing. LT: Writing – original draft, Writing – review & editing. GP: Writing – original draft, Writing – review & editing. GF: Conceptualization, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The authors declare that financial support for publication was received by University of Verona.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor AL declared past co-authorships with the authors MP, GP, and GF.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1460050/full#supplementary-material
References
1. Tenero, L, Tezza, G, Cattazzo, E, and Piacentini, G. Wheezing in preschool children. Early Hum Dev. (2013) 89:S13–7. doi: 10.1016/j.earlhumdev.2013.07.017
2. Bloom, CI, Franklin, C, Bush, A, Saglani, S, and Quint, JK. Burden of preschool wheeze and progression to asthma in the UK: population-based cohort 2007 to 2017. J Allergy Clin Immunol. (2021) 147:1949–58. doi: 10.1016/j.jaci.2020.12.643
3. Bisgaard, H, and Szefler, S. Prevalence of asthma-like symptoms in young children. Pediatr Pulmonol. (2007) 42:723–8. doi: 10.1002/ppul.20644
4. Henderson, J, Granell, R, Heron, J, Sherriff, A, Simpson, A, Woodcock, A, et al. Associations of wheezing phenotypes in the first 6 years of life with atopy, lung function and airway responsiveness in mid-childhood. Thorax. (2008) 63:974–80. doi: 10.1136/thx.2007.093187
5. Martinez, FD, Wright, AL, Taussig, LM, Holberg, CJ, Halonen, M, and Morgan, WJ. Asthma and wheezing in the first six years of life. N Engl J Med. (1995) 332:133–8. doi: 10.1056/NEJM199501193320301
6. Moorman, JE, Akinbami, LJ, Bailey, CM, Zahran, HS, King, ME, Johnson, CA, et al. National surveillance of asthma: United States, 2001-2010. Vital Health Stat. (2012) 3:1–58.
7. Van Den Akker-van Marle, ME, Bruil, J, and Detmar, SB. Evaluation of cost of disease: assessing the burden to society of asthma in children in the European Union. Allergy. (2005) 60:140–9. doi: 10.1111/j.1398-9995.2005.00692.x
8. Ferrante, G, Licari, A, Marseglia, GL, and La Grutta, S. Artificial intelligence as an emerging diagnostic approach in paediatric pulmonology. Respirology. (2020) 25:1029–30. doi: 10.1111/resp.13842
9. Ferrante, G, Licari, A, Fasola, S, Marseglia, GL, and La Grutta, S. Artificial intelligence in the diagnosis of pediatric allergic diseases. Pediatr Allergy Immunol. (2021) 32:405–13. doi: 10.1111/pai.13419
10. Cilluffo, G, Fasola, S, Ferrante, G, Licari, A, Marseglia, GR, Albarelli, A, et al. Machine learning: A modern approach to pediatric asthma. Pediatr Allergy Immunol. (2022) 33:34–7. doi: 10.1111/pai.13624
11. Zaeri, N. Artificial intelligence and machine learning responses to COVID-19 related inquiries. J Med Eng Technol. (2023) 47:301–20. doi: 10.1080/03091902.2024.2321846
12. Elphick, HE, Ritson, S, Rodgers, H, and Everard, ML. When a “wheeze” is not a wheeze: acoustic analysis of breath sounds in infants. Eur Respir J. (2000) 16:593–7. doi: 10.1034/j.1399-3003.2000.16d04.x
13. Shaharum, SM, Sundaraj, K, and Palaniappan, R. A survey on automated wheeze detection systems for asthmatic patients. Bosn J Basic Med Sci. (2012) 12:242–55. doi: 10.17305/bjbms.2012.2447
14. Emeryk, A, Derom, E, Janeczek, K, Kuźnar-Kamińska, B, Zelent, A, Łukaszyk, M, et al. Home monitoring of asthma exacerbations in children and adults with use of an AI-aided stethoscope. Ann Fam Med. (2023) 21:517–25. doi: 10.1370/afm.3039
15. Arjoune, Y, Nguyen, TN, Salvador, T, Telluri, A, Schroeder, JC, Geggel, RL, et al. StethAid: A digital auscultation platform for pediatrics. Sensors. (2023) 23:5750. doi: 10.3390/s23125750
16. Kevat, A, Kalirajah, A, and Roseby, R. Artificial intelligence accuracy in detecting pathological breath sounds in children using digital stethoscopes. Respir Res. (2020) 21:253. doi: 10.1186/s12931-020-01523-9
17. Puder, LC, Fischer, HS, Wilitzki, S, Usemann, J, Godfrey, S, and Schmalisch, G. Validation of computerized wheeze detection in young infants during the first months of life. BMC Pediatr. (2014) 14:257. doi: 10.1186/1471-2431-14-257
18. Puder, LC, Wilitzki, S, Bührer, C, Fischer, HS, and Schmalisch, G. Computerized wheeze detection in young infants: comparison of signals from tracheal and chest wall sensors. Physiol Meas. (2016) 37:2170–80. doi: 10.1088/0967-3334/37/12/2170
19. Bentur, L, Beck, R, Shinawi, M, Naveh, T, and Gavriely, N. Wheeze monitoring in children for assessment of nocturnal asthma and response to therapy. Eur Respir J. (2003) 21:621–6. doi: 10.1183/09031936.03.00036302
20. Prodhan, P, Dela Rosa, RS, Shubina, M, Haver, KE, Matthews, BD, Buck, S, et al. Wheeze detection in the pediatric intensive care unit: comparison among physician, nurses, respiratory therapists, and a computerized respiratory sound monitor. Respir Care. (2008) 53:1304–9.
21. Habukawa, C, Ohgami, N, Arai, T, Makata, H, Tomikawa, M, Fujino, T, et al. Wheeze recognition algorithm for remote medical care device in children: validation study. JMIR Pediatr Parent. (2021) 4:e28865. doi: 10.2196/28865
22. Dramburg, S, Dellbrügger, E, van Aalderen, W, and Matricardi, PM. The impact of a digital wheeze detector on parental disease management of pre-school children suffering from wheezing-a pilot study. Pilot Feasibility Stud. (2021) 7:185. doi: 10.1186/s40814-021-00917-w
23. Do, YH, van Aalderen, W, Dellbrügger, E, Grenzbach, C, Grigg, J, Grittner, U, et al. Clinical efficacy and satisfaction of a digital wheeze detector in a multicentre randomised controlled trial: the WheezeScan study. ERJ Open Res. (2024) 10:00518–2023. doi: 10.1183/23120541.00518-2023
24. Moschovis, PP, Sampayo, EM, Cook, A, Doros, G, Parry, BA, Lombay, J, et al. The diagnosis of respiratory disease in children using a phone-based cough and symptom analysis algorithm: the smartphone recordings of cough sounds 2 (SMARTCOUGH-C 2) trial design. Contemp Clin Trials. (2021) 101:106278. doi: 10.1016/j.cct.2021.106278
25. Kruizinga, MD, Zhuparris, A, Dessing, E, Krol, FJ, Sprij, AJ, Doll, R-J, et al. Development and technical validation of a smartphone-based pediatric cough detection algorithm. Pediatr Pulmonol. (2022) 57:761–7. doi: 10.1002/ppul.25801
26. Chatzimichail, E, Paraskakis, E, Sitzimi, M, and Rigas, A. An intelligent system approach for asthma prediction in symptomatic preschool children. Comput Math Methods Med. (2013) 2013:240182. doi: 10.1155/2013/240182
27. Salehian, S, Fleming, L, Saglani, S, and Custovic, A. Phenotype and endotype based treatment of preschool wheeze. Expert Rev Respir Med. (2023) 17:853–64. doi: 10.1080/17476348.2023.2271832
28. Smit, HA, Pinart, M, Antó, JM, Keil, T, Bousquet, J, Carlsen, KH, et al. Childhood asthma prediction models: a systematic review. Lancet Respir Med. (2015) 3:973–84. doi: 10.1016/S2213-2600(15)00428-2
29. Castro-Rodríguez, JA, Holberg, CJ, Wright, AL, and Martinez, FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. (2000) 162:1403–6. doi: 10.1164/ajrccm.162.4.9912111
30. Chang, TS, Lemanske, RF, Guilbert, TW, Gern, JE, Coen, MH, Evans, MD, et al. Evaluation of the modified asthma predictive index in high-risk preschool children. J Allergy Clin Immunol Pract. (2013) 1:152–6. doi: 10.1016/j.jaip.2012.10.008
31. Kurukulaaratchy, RJ, Matthews, S, Holgate, ST, and Arshad, SH. Predicting persistent disease among children who wheeze during early life. Eur Respir J. (2003) 22:767–71. doi: 10.1183/09031936.03.00005903
32. Caudri, D, Wijga, A, Schipper, CM, Hoekstra, M, Postma, DS, Koppelman, GH, et al. Predicting the long-term prognosis of children with symptoms suggestive of asthma at preschool age. J Allergy Clin Immunol. (2009) 124:903–910.e7. doi: 10.1016/j.jaci.2009.06.045
33. Castro-Rodriguez, JA, Cifuentes, L, and Martinez, FD. Predicting asthma using clinical indexes. Front Pediatr. (2019) 7:320. doi: 10.3389/fped.2019.00320
34. Pescatore, AM, Dogaru, CM, Duembgen, L, Silverman, M, Gaillard, EA, Spycher, BD, et al. A simple asthma prediction tool for preschool children with wheeze or cough. J Allergy Clin Immunol. (2014) 133:111–3. doi: 10.1016/j.jaci.2013.06.002
35. Amin, P, Levin, L, Epstein, T, Ryan, P, LeMasters, G, Khurana Hershey, G, et al. Optimum predictors of childhood asthma: persistent wheeze or the asthma predictive index? J Allergy Clin Immunol Pract. (2014) 2:709–15. doi: 10.1016/j.jaip.2014.08.009
36. Klaassen, EMM, Van De Kant, KDG, Jöbsis, Q, Van Schayck, OCP, Smolinska, A, Dallinga, JW, et al. Exhaled biomarkers and gene expression at preschool age improve asthma prediction at 6 years of age. Am J Respir Crit Care Med. (2015) 191:201–7. doi: 10.1164/rccm.201408-1537OC
37. Reyna, ME, Dai, R, Tran, MM, Breton, V, Medeleanu, M, Lou, WYW, et al. Development of a symptom-based tool for screening of children at high risk of preschool asthma. JAMA Netw Open. (2022) 5:e2234714. doi: 10.1001/jamanetworkopen.2022.34714
38. Chatzimichail, E, Paraskakis, E, and Rigas, A. Predicting asthma outcome using partial Least Square regression and artificial neural networks. Adv Artif Intellig. (2013) 2013:1–7. doi: 10.1155/2013/435321
39. Kothalawala, DM, Murray, CS, Simpson, A, Custovic, A, Tapper, WJ, Arshad, SH, et al. Development of childhood asthma prediction models using machine learning approaches. Clin Transl Allergy. (2021) 11:e12076. doi: 10.1002/clt2.12076
40. Kothalawala, DM, Kadalayil, L, Curtin, JA, Murray, CS, Simpson, A, Custovic, A, et al. Integration of genomic risk scores to improve the prediction of childhood asthma diagnosis. J Pers Med. (2022) 12:75. doi: 10.3390/jpm12010075
41. Custovic, D, Fontanella, S, and Custovic, A. Understanding progression from pre-school wheezing to school-age asthma: can modern data approaches help? Pediatr Allergy Immunol. (2023) 34:e14062. doi: 10.1111/pai.14062
42. Fitzpatrick, AM, Bacharier, LB, Guilbert, TW, Jackson, DJ, Szefler, SJ, Beigelman, A, et al. Phenotypes of recurrent wheezing in preschool children: identification by latent class analysis and utility in prediction of future exacerbation. J Allergy Clin Immunol Pract. (2019) 7:915–924.e7. doi: 10.1016/j.jaip.2018.09.016
43. Kaur, H, Sohn, S, Wi, C-I, Ryu, E, Park, MA, Bachman, K, et al. Automated chart review utilizing natural language processing algorithm for asthma predictive index. BMC Pulm Med. (2018) 18:34. doi: 10.1186/s12890-018-0593-9
44. Van Breugel, M, Fehrmann, RSN, Bügel, M, Rezwan, FI, Holloway, JW, Nawijn, MC, et al. Current state and prospects of artificial intelligence in allergy. Allergy. (2023) 78:2623–643. doi: 10.1111/all.15849
45. Wang, Z, Myles, P, and Tucker, A. Generating and evaluating cross‐sectional synthetic electronic healthcare data: preserving data utility and patient privacy. Comput Intell. (2021) 37:819–51. doi: 10.1111/coin.12427
46. Al Meslamani, AZ. How AI is advancing asthma management? Insights into economic and clinical aspects. J Med Eco. (2023) 26:1489–494. doi: 10.1080/13696998.2023.2277072
47. Molfino, NA, Turcatel, G, and Riskin, D. Machine learning approaches to predict asthma exacerbations: a narrative review. Adv Ther. (2024) 41:534–552. doi: 10.1007/s12325-023-02743-3
48. Sherriff, A, Peters, TJ, Henderson, J, and Strachan, DALSPAC Study Team. Avon longitudinal study of parents and children. Risk factor associations with wheezing patterns in children followed longitudinally from birth to 3(1/2) years. Int J Epidemiol. (2001) 30:1473–84. doi: 10.1093/ije/30.6.1473
49. Savenije, OE, Granell, R, Caudri, D, Koppelman, GH, Smit, HA, Wijga, A, et al. Comparison of childhood wheezing phenotypes in 2 birth cohorts: ALSPAC and PIAMA. J Allergy Clin Immunol. (2011) 127:1505–1512.e14. doi: 10.1016/j.jaci.2011.02.002
50. Sordillo, JE, Coull, BA, Rifas-Shiman, SL, Wu, AC, Lutz, SM, Hivert, M-F, et al. Characterization of longitudinal wheeze phenotypes from infancy to adolescence in project viva, a prebirth cohort study. J Allergy Clin Immunol. (2020) 145:716–719.e8. doi: 10.1016/j.jaci.2019.10.026
51. Saglani, S, and Custovic, A. Childhood asthma: advances using machine learning and mechanistic studies. Am J Respir Crit Care Med. (2019) 199:414–22. doi: 10.1164/rccm.201810-1956CI
52. Hose, AJ, Depner, M, Illi, S, Lau, S, Keil, T, Wahn, U, et al. Latent class analysis reveals clinically relevant atopy phenotypes in 2 birth cohorts. J Allergy Clin Immunol. (2017) 139:1935–1945.e12. doi: 10.1016/j.jaci.2016.08.046
53. Bacharier, LB, Beigelman, A, Calatroni, A, Jackson, DJ, Gergen, PJ, O’Connor, GT, et al. Longitudinal phenotypes of respiratory health in a high-risk urban birth cohort. Am J Respir Crit Care Med. (2019) 199:71–82. doi: 10.1164/rccm.201801-0190OC
54. Belgrave, DCM, Simpson, A, Semic-Jusufagic, A, Murray, CS, Buchan, I, Pickles, A, et al. Joint modeling of parentally reported and physician-confirmed wheeze identifies children with persistent troublesome wheezing. J Allergy Clin Immunol. (2013) 132:575–583.e12. doi: 10.1016/j.jaci.2013.05.041
55. Fitzpatrick, AM, Bacharier, LB, Jackson, DJ, Szefler, SJ, Beigelman, A, Cabana, M, et al. Heterogeneity of mild to moderate persistent asthma in children: confirmation by latent class analysis and association with 1-year outcomes. J Allergy Clin Immunol Pract. (2020) 8:2617–2627.e4. doi: 10.1016/j.jaip.2020.02.032
56. Su, M-W, Lin, W-C, Tsai, C-H, Chiang, B-L, Yang, Y-H, Lin, Y-T, et al. Childhood asthma clusters reveal neutrophil-predominant phenotype with distinct gene expression. Allergy. (2018) 73:2024–32. doi: 10.1111/all.13439
57. Deliu, M, Fontanella, S, Haider, S, Sperrin, M, Geifman, N, Murray, C, et al. Longitudinal trajectories of severe wheeze exacerbations from infancy to school age and their association with early-life risk factors and late asthma outcomes. Clin Exp Allergy. (2020) 50:315–24. doi: 10.1111/cea.13553
58. Lin, K, Wang, Y, Li, Y, and Wang, Y. Identification of biomarkers associated with pediatric asthma using machine learning algorithms: A review. Medicine. (2023) 102:e36070. doi: 10.1097/MD.0000000000036070
Keywords: wheezing, machine learning, artificial intelligence, asthma, digital health
Citation: Venditto L, Morano S, Piazza M, Zaffanello M, Tenero L, Piacentini G and Ferrante G (2024) Artificial intelligence and wheezing in children: where are we now? Front. Med. 11:1460050. doi: 10.3389/fmed.2024.1460050
Edited by:
Amelia Licari, University of Pavia, ItalyReviewed by:
Sara Manti, University of Messina, ItalyCopyright © 2024 Venditto, Morano, Piazza, Zaffanello, Tenero, Piacentini and Ferrante. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michele Piazza, bWljaGVsZS5waWF6emFAdW5pdnIuaXQ=
 Laura Venditto
Laura Venditto Sonia Morano2
Sonia Morano2 Michele Piazza
Michele Piazza Marco Zaffanello
Marco Zaffanello Laura Tenero
Laura Tenero Giorgio Piacentini
Giorgio Piacentini Giuliana Ferrante
Giuliana Ferrante