- 1Isra University Karachi-Campus, Karachi, Pakistan
- 2Jinnah Post Graduate Medical Centre, Karachi, Pakistan
- 3Nishtar Medical College, Multan, Pakistan
- 4Sri Aurobindo University, Indore, India
- 5Shaheed Mohtarma Benazir Bhutto Medical University, Larkana, Pakistan
- 6Bahria University Medical and Dental College, Karachi, Pakistan
- 7Dow University of Health Sciences, Karachi, Pakistan
Background: Labor induction is a common obstetric intervention, increasingly performed worldwide, often using prostaglandins like misoprostol and dinoprostone.
Objective: This study aims to compare the effectiveness and safety of intravaginal misoprostol versus dinoprostone for inducing labor, examining their impact on various maternal and neonatal outcomes.
Methods: A systematic review and meta-analysis were conducted using four databases—PubMed, Google Scholar, EBSCO, and the Cochrane Library—from January 2000 to April 2023. We included randomized controlled trials (RCTs) involving singleton pregnancies at term (37–42 weeks) with unfavorable cervices, where intravaginal misoprostol was compared to dinoprostone. Key outcomes evaluated for effectiveness included vaginal delivery within 24 h, overall vaginal delivery rate, and need for oxytocin augmentation. Safety outcomes assessed were tachysystole, uterine hyperstimulation, abnormal cardiotocography, NICU admissions, cesarean delivery, and APGAR scores. Risk ratios (RRs) and 95% confidence intervals (CIs) were calculated using a random-effects model in Review Manager (RevMan) version 5.4.1.
Results: Eight RCTs with a total of 1,801 participants (937 in the misoprostol group and 864 in the dinoprostone group) met the inclusion criteria. Misoprostol required a significantly less oxytocin augmentation than dinoprostone [RR = 0.83; 95% CI (0.71, 0.97), p = 0.02]. Other outcomes, including rates of cesarean delivery, uterine tachysystole, hyperstimulation, and NICU admissions, showed no significant differences between the two groups, indicating comparable safety and efficacy profiles.
Conclusion: This meta-analysis demonstrates that intravaginal misoprostol is an effective and safe alternative to dinoprostone for labor induction at term. Misoprostol achieved comparable efficacy and safety outcomes while requiring less oxytocin augmentation, supporting its potential as a practical induction agent in clinical settings.
Introduction
The delivery of a fetus can be induced by initiating intrauterine contractions using pharmacological or mechanical methods (1). Approximately 20% of all births are now intentionally induced through induction of labor (IOL), an increasingly common obstetric practice in modern obstetrics (2, 3), aimed at enhancing maternal and neonatal outcomes, especially when spontaneous labor may present risks. Common indications for labor induction include prolonged pregnancy (post-term), maternal conditions (e.g., hypertension, diabetes), and concerns about fetal well-being (e.g., intrauterine growth restriction) (4). Risks of stillbirth or neonatal death increase as gestation continues beyond term (around 40 weeks’ gestation), making timely induction a preventive measure (5). Evidence suggests that elective induction at 41 weeks—or potentially earlier under specific conditions—may lower the risks associated with cesarean delivery and complications like meconium-stained amniotic fluid (6). Labor induction success is often defined as achieving vaginal delivery within 24–48 h (7).
In recent years, the use of labor induction (IOL) has significantly increased, growing from 9.0% of all births in 1989 to 23% in 2012 (8). A 2012 study analyzing data from numerous hospitals across the United States discovered that over two-fifths (42.9%) of nulliparous women and slightly more than a third (31.8%) of multiparous women underwent labor induction (9). Pharmacological therapies, such as oxytocin and prostaglandins, are administered orally, vaginally, or intravenously to mature the cervix for labor induction. While oxytocin is effective for labor augmentation in women with favorable cervices, a ripening agent may be used when induction of labor is performed on women with unfavorable cervices (10–12). Other treatments designed to aid the induction process in cases of unfavorable cervix, such as membrane rupture, have been associated with reduced efficiency and higher failure rates (13).
Dinoprostone, a prostaglandin E2 analog, has traditionally been used to induce labor using either an intracervical gel or a vaginal insert (14). However, its use in resource-constrained settings is hindered by challenges such as cost and the requirement for cold storage (15). Misoprostol, a prostaglandin E1 analog originally used in the 1980s to manage and cure peptic ulcer disease (16), has been extensively studied in randomized clinical trials for its efficacy in gynecologic and obstetric procedures. It is utilized for inducing uterine contractions and cervical ripening to facilitate labor induction (17–19). Unlike dinoprostone, misoprostol is significantly more affordable, easier to administer, does not require cold storage, and is readily available even in resource-constrained countries, giving it a distinct advantage over dinoprostone (20).
Many clinical trials have investigated the effectiveness and safety of intravaginal misoprostol versus dinoprostone (17–19, 21–25), finding that misoprostol is more effective in minimizing the requirement for oxytocin augmentation in labor induction (26, 27). The meta-analysis conducted by Wang et al. (19) found comparable outcomes between the misoprostol and dinoprostone groups, showing no significant differences. However, it should be noted that their study included Saxena et al. (28) and Chitrakar et al. (29), who administered dinoprostone intracervically instead of vaginally, which goes against the specified inclusion criteria.
Considering these concerns, we conducted an updated meta-analysis to explore whether there are significant differences in various outcomes between the misoprostol and dinoprostone groups, contrasting with the nonsignificant findings reported by Wang et al. (19).
Methods
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines (30).
Literature search
A thorough search of PUBMED, Google Scholar, Ebsco, Cochrane Library, and CNKI was conducted from January 2000 to April 2023. The following combination of Medical Subject Heading (MeSH) terms and keywords were used in the database searches: “Misoprostol,” “Dinoprostone,” “Labor Induction,” “Intravaginally,” and “Term.” A detailed search strategy is presented in the Supplementary Table S1. Two independent reviewers thoroughly reviewed the titles, abstracts, full texts, and bibliographies of all identified studies separately to identify potentially relevant research. The assessment included a detailed examination of references in the relevant literature to identify appropriate studies, with no restrictions based on geographical location, ethnicity, or publication language. In cases of discrepancy, a third author was consulted to reach a consensus. Additionally, gray literature sources were searched to identify potential publications relevant to this study. A detailed search strategy is presented in the Supplementary Table S1.
Data extraction
Initially, two reviewers independently examined the titles and abstracts of publications that met the inclusion criteria, followed by a comprehensive review of the full texts. Subsequently, they extracted data from the eligible studies and documented it in an information extraction table. Two researchers independently collected the following information from each study included in the analysis: (a) the name and year of the study, (b) study design, (c) study location, (d) the number of patients in each group (misoprostol vs. dinoprostone), (e) general characteristics of the patients (age, gestational weeks, dosage, and mean birth weight), and (f) all outcomes of interest. Any discrepancies in data extraction were resolved through discussion or by consulting a third reviewer.
Inclusion and exclusion criteria
This study included only RCTs and adhered to strict eligibility criteria for research inclusion, with no restrictions on intervention dosage. The specific parameters are detailed below:
PICO
P: Population
Singleton pregnant women with live intrauterine gestations, unfavorable cervices, and a gestational period of 37 to 42 weeks.
I: Intervention
Intravaginal misoprostol.
C: Comparison
Intravaginal dinoprostone.
O: Outcome
Cesarean section rate, vaginal delivery rate, vaginal delivery within 24 h, incidences of uterine tachysystole (defined as at least six contractions in a 10-min period sustained over two consecutive 10-min intervals), hyperstimulation (defined as fetal heart rate abnormality associated with tachysystole), necessity for oxytocin augmentation, NICU admissions, abnormal cardiotocography readings, and APGAR scores below 7 at 5 min.
Studies were excluded for various reasons, including unsuitable design (such as non-randomization), lack of relevant data, involvement of animal models, or if they were case reports, editorials, reviews, conference abstracts, or duplicate publications.
Statistical analysis
Statistical analysis was conducted using Review Manager (RevMan) version 5.4.1, following The Cochrane Collaboration’s (2020) guidelines. For pooling categorical outcomes, risk ratios (RRs) and their corresponding 95% confidence intervals (CIs) were calculated using a random-effects meta-analysis approach. A random-effects meta-analysis was also performed for continuous outcomes to determine mean differences (MDs) and their 95% confidence intervals (CIs). Sensitivity analysis was conducted to address outcomes with severe heterogeneity. Funnel plots were not generated due to the presence of fewer than 10 studies. Higgins’ I2 statistics were used to quantify heterogeneity: I2 values of 25–50% indicated mild heterogeneity, 50–75% indicated moderate heterogeneity, and values greater than 75% indicated severe heterogeneity (31). To identify and address sources of heterogeneity, sensitivity analyses were planned to use the leave-one-out method. A p-value of 0.05 or less was considered statistically significant.
Risk of bias assessment
The risk of bias within individual studies was evaluated using the Cochrane Risk of Bias Tool, which examines potential sources of bias across multiple domains, including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other potential sources of bias. The risk of bias in each category was systematically classified as low, high, or unclear (32).
Results
Study selection, baseline, and characteristics overview
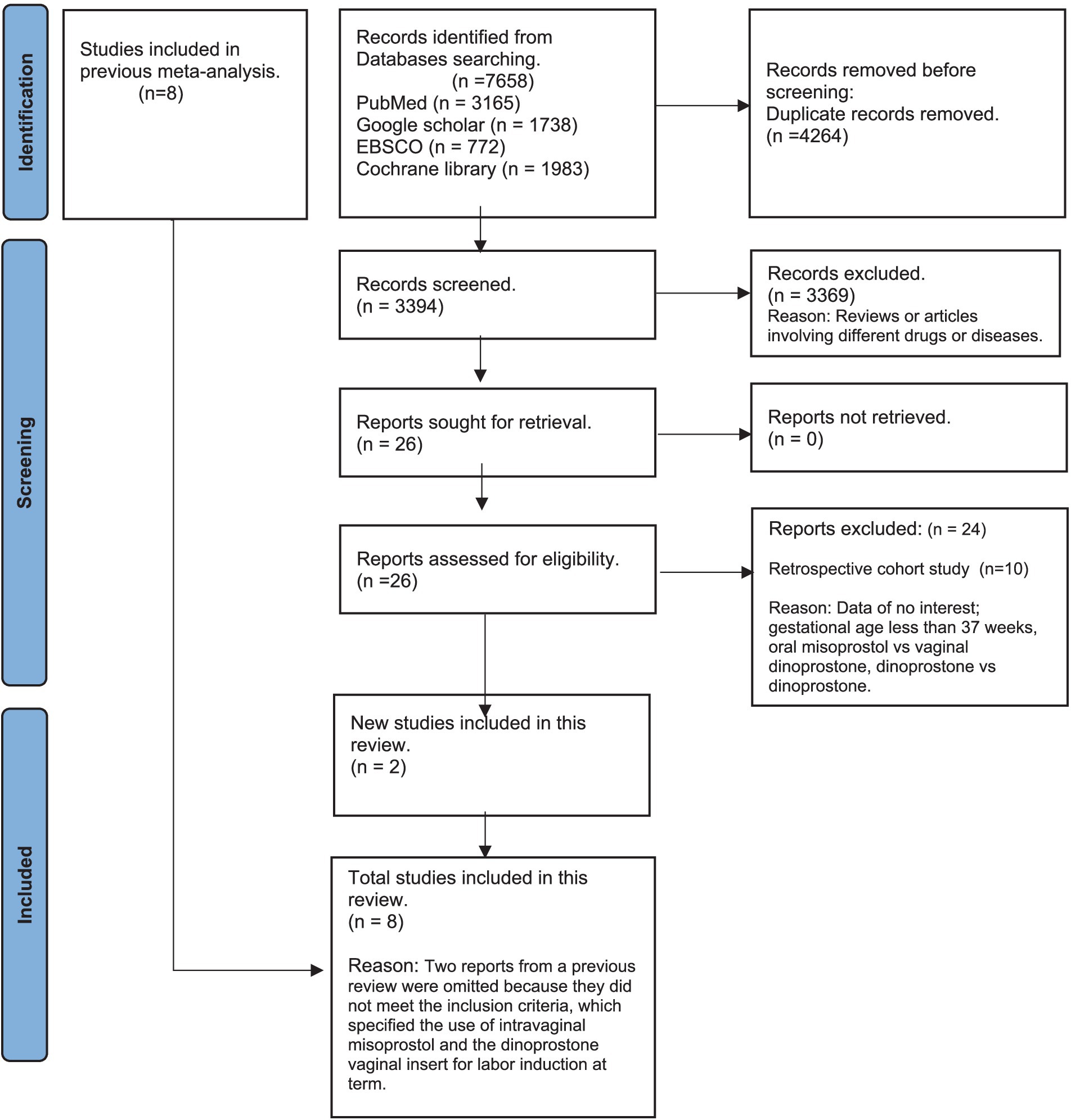
From an initial 7,658 search results across PubMed, Google Scholar, Cochrane Library, and EBSCO, we removed 4,264 duplicates, leaving 3,394 studies for screening. After excluding irrelevant studies, 26 full-text articles were reviewed. A full-text review of 26 studies followed, leading to the exclusion of 10 cohort studies (25, 33–41) and 14 studies due to non-relevant data, gestational age under 37 weeks, or inappropriate comparisons (21, 22, 42–53). Ultimately, eight RCTs were included in the analysis—six from Wang et al.’s meta-analysis (19) and two newly identified RCTs meeting our criteria (Figure 1).
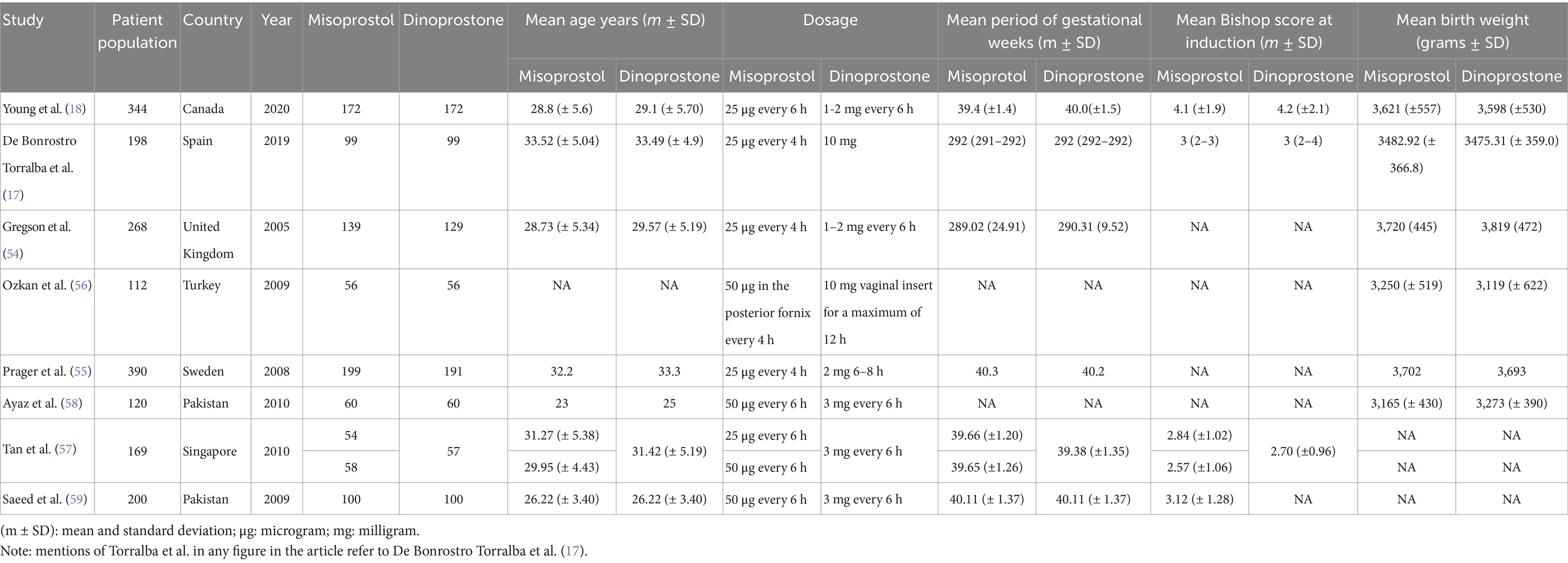
Table 1 details the baseline and study characteristics of the included trials. This analysis encompasses eight RCTs with a total of 1,801 participants. Of these, 937 received misoprostol, while 864 were in the dinoprostone group. The dosage and administration regimens for both drugs varied among the trials. In three studies (17, 54, 55), the misoprostol group received 25 micrograms (μg) every 4 h for a total of six doses. One trial administered up to five doses of 50 μg every 4 h (56), two trials gave up to two doses of 25 μg every 6 h (18, 57), two trials administered up to three doses of 50 μg every 6 h (58, 59), and another trial administered up to two doses of 50 μg every 6 h (57).
For the dinoprostone groups, two trials administered 1–2 milligrams (mg) every 6 h for 24 h (18, 54), one trial administered 2 mg for a maximum of four doses every 6 h (55), two trials administered a 10 mg vaginal insert for up to 12 h (17, 56), one trial administered 3 mg into the posterior vaginal fornix for up to two doses every 6 h (57), and two trials administered 3 mg into the posterior vaginal fornix for up to three doses every 6 h (58, 59).
Quality assessment
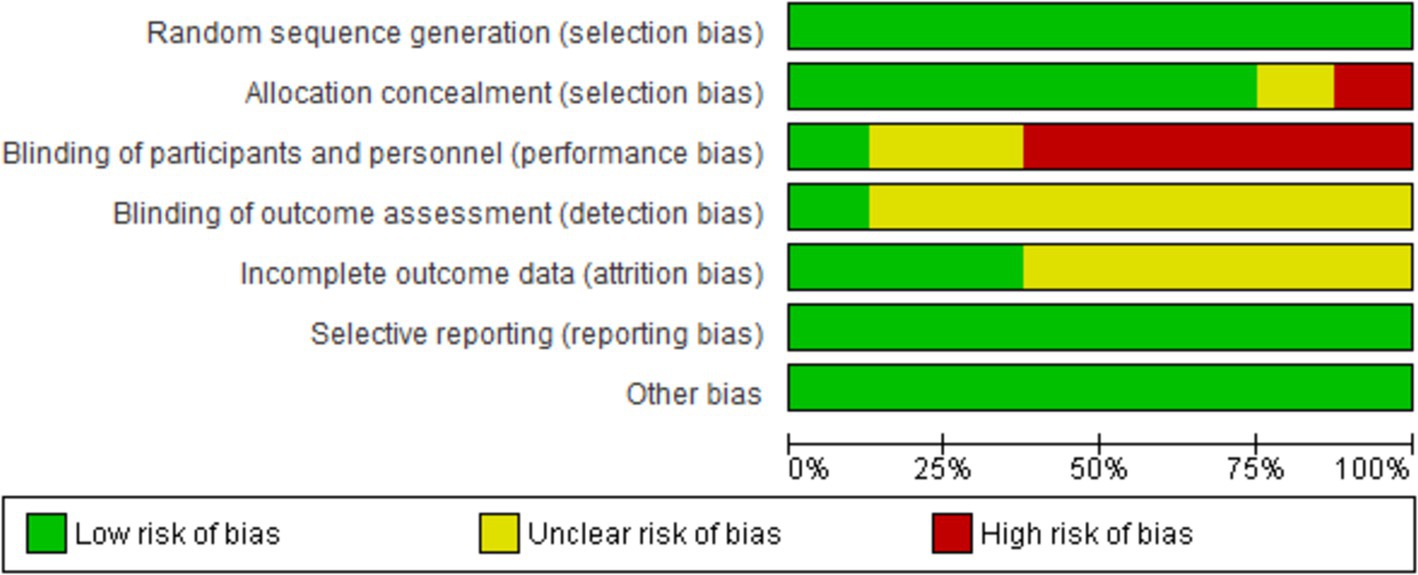
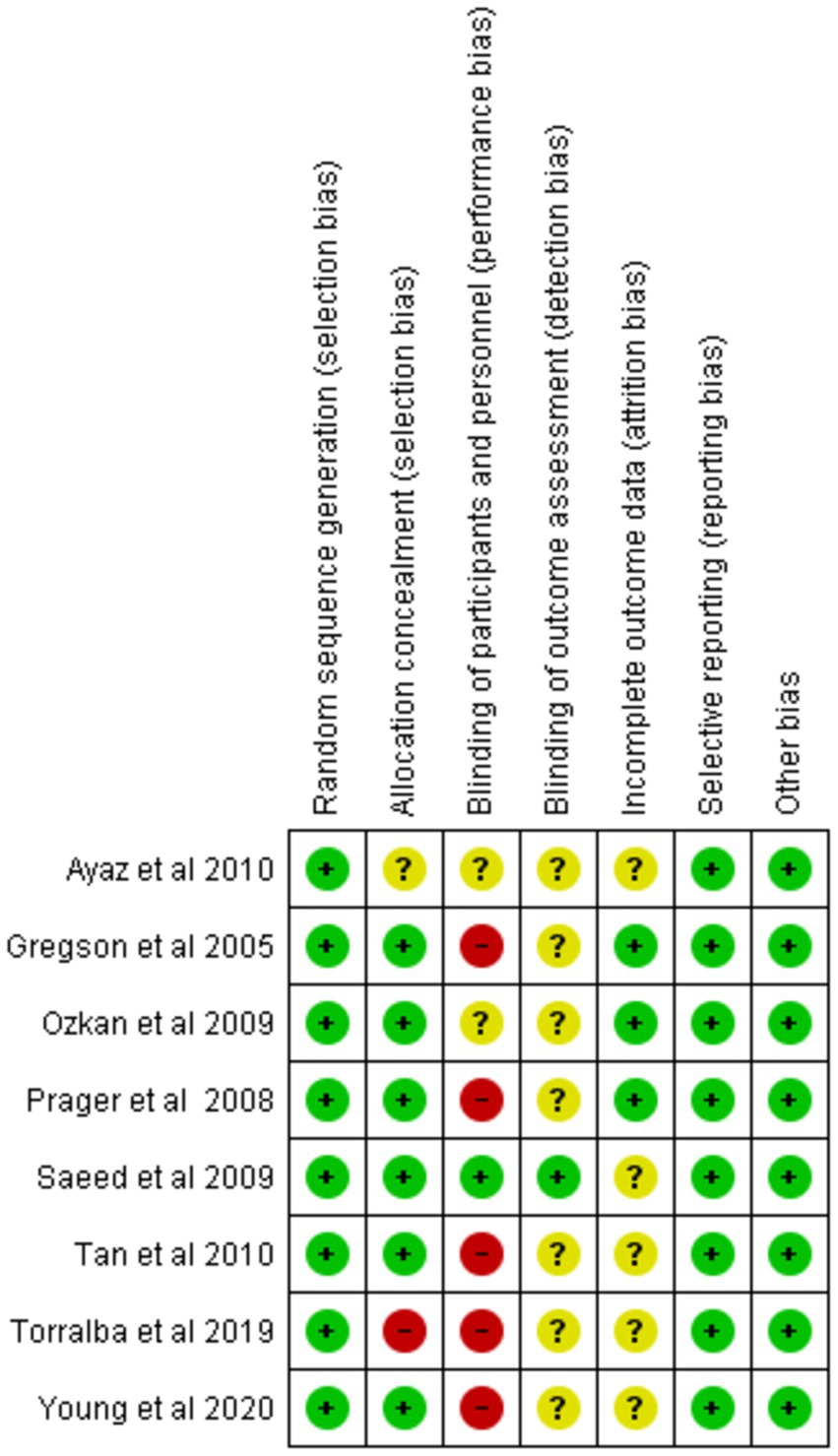
We evaluated the validity of the eight RCTs using the Cochrane Risk of Bias Tool. Overall, these studies were determined to be of excellent quality and exhibited a low risk of bias across all seven assessment categories, thereby enhancing the credibility of our findings. A comprehensive assessment is illustrated in Figures 2, 3.
Maternal outcomes
Vaginal delivery within 24 h
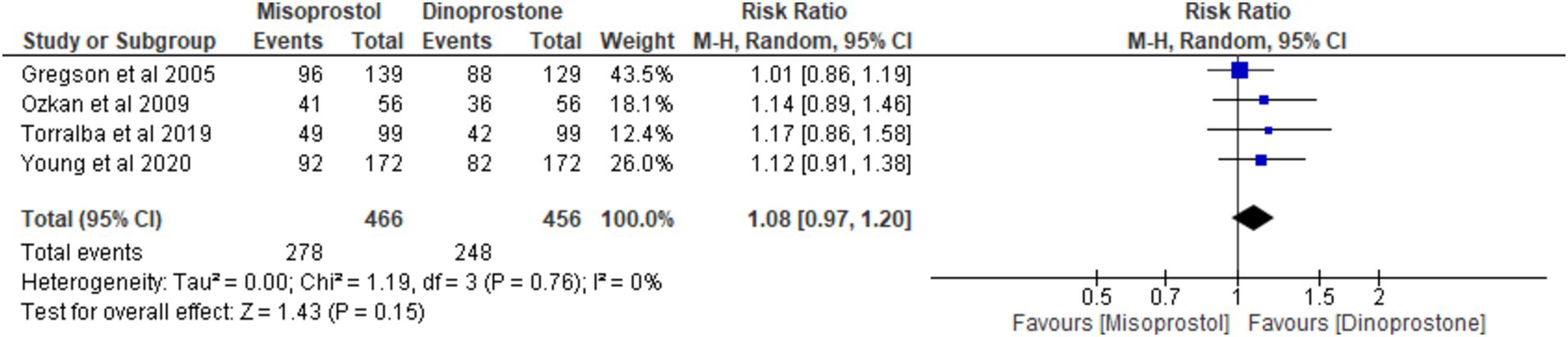
Four studies (17, 18, 54, 56) involving 922 patients reported on vaginal delivery within 24 h. Using a random-effects model to pool the results, no significant difference was found between misoprostol and dinoprostone in achieving vaginal delivery within 24 h [RR = 1.08; 95% CI (0.97, 1.20) p = 0.15]. Additionally, no statistically significant heterogeneity was observed among the studies (p = 0.76, I2 = 0%; Figure 4).
Cesarean delivery
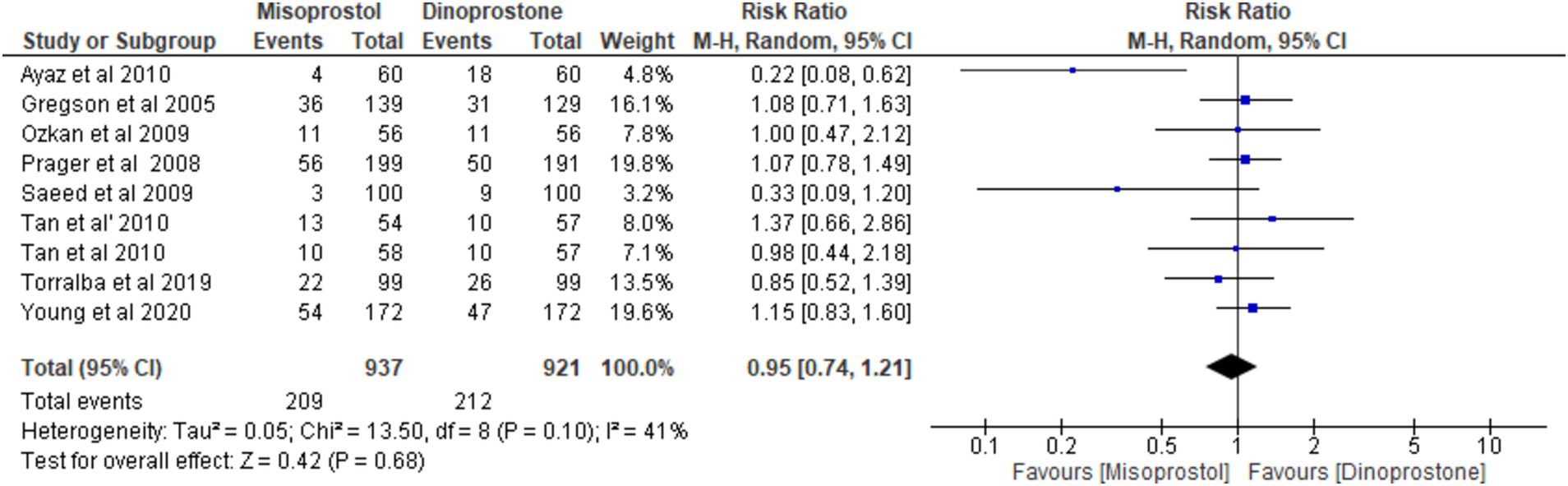
Eight studies (17, 18, 54–59), encompassing 1,858 patients, reported on cesarean delivery. Using a random-effects model to pool the combined effects, the results indicated no significant difference between the misoprostol and dinoprostone groups [RR = 0.95; 95% CI (0.74, 1.21) p = 0.68]. Additionally, there was no significant heterogeneity among the studies (p = 0.10, I2 = 41%; Figure 5).
Oxytocin augmentation
Five studies (17, 18, 54, 56, 59) involving 1,088 patients reported on oxytocin augmentation. Using a random-effects model to pool the combined effect, the results showed that the misoprostol group required significantly less oxytocin compared to the dinoprostone group [RR = 0.83; 95% CI (0.71, 0.97) p = 0.02]. Additionally, there was no significant heterogeneity observed among the studies (p = 0.26, I2 = 24%; Figure 6).
Uterine tachysystole
Five studies (18, 54, 56–58) involving 1,070 patients reported on the incidence of tachysystole. Using a random-effects model to pool the combined effect, the results showed that misoprostol was not significantly associated with a higher incidence of tachysystole compared to dinoprostone [RR = 1.27; 95% CI (0.76, 2.13) p = 0.36]. Additionally, no significant heterogeneity was observed among the studies (p = 0.13, I2 = 42%; Figure 7).
Vaginal delivery
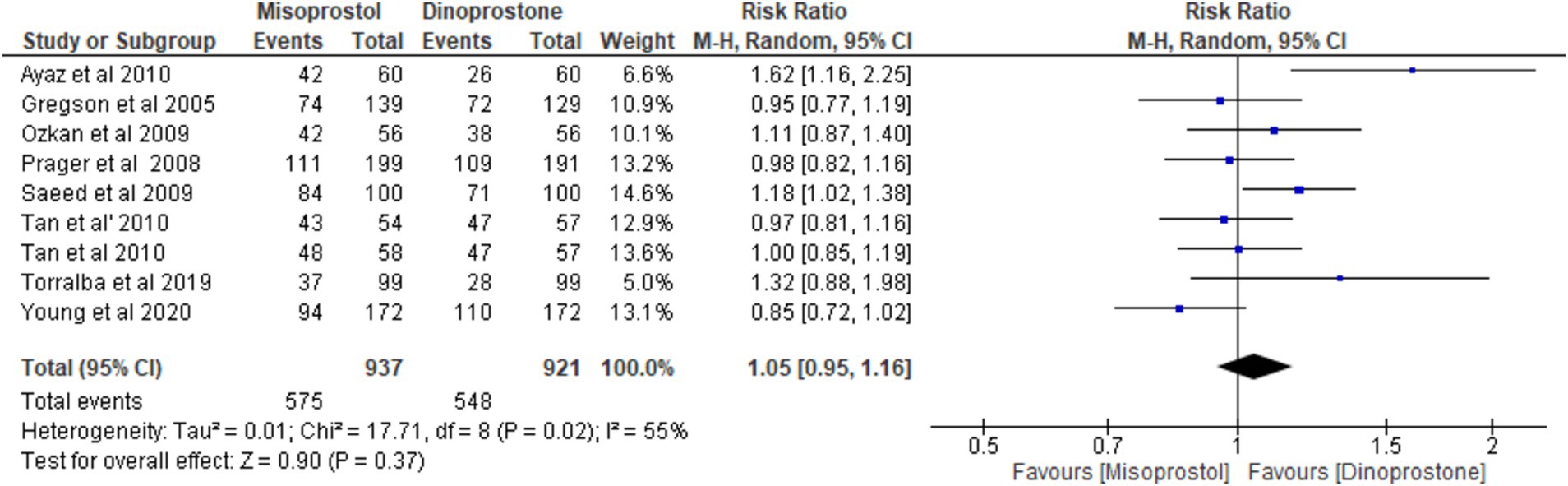
Eight studies (17, 18, 54–59) involving 1,858 patients reported on vaginal delivery outcomes. Using a random-effects model to pool the combined effect, the results indicated no significant difference in vaginal delivery rates between misoprostol and dinoprostone [RR = 1.05; 95% CI (0.95, 1.16) p = 0.37]. Moderate heterogeneity was observed among the studies (p = 0.02, I2 = 55%; Figure 8).
Instrumental delivery
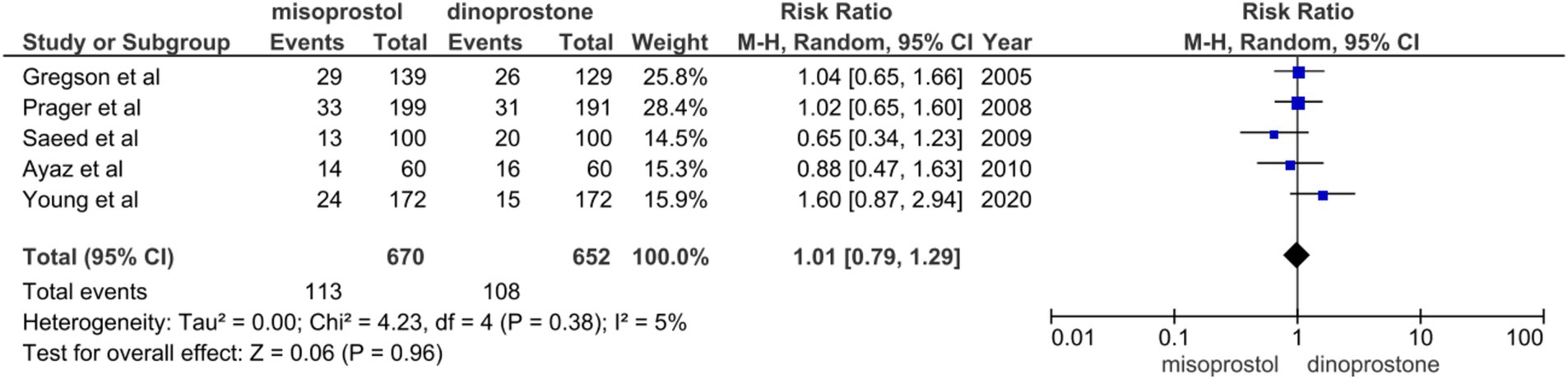
Five studies (14, 48, 49, 52, 53) involving 1,322 patients reported on instrumental delivery. A random-effects model was used to pool the combined effect, revealing no significant difference between the misoprostol and dinoprostone groups [RR = 1.01; 95% CI (0.79–1.29) p = 0.96]. Furthermore, no notable heterogeneity was detected among these studies (I2 = 5%; Figure 9).
Obstetrics outcomes
NICU admission
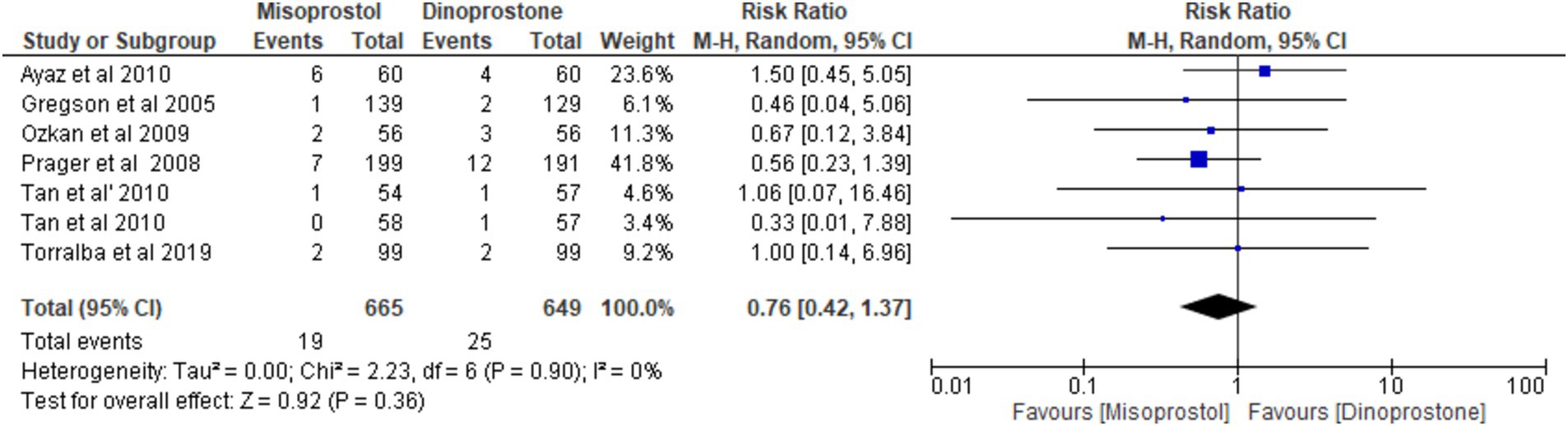
Six studies (17, 54–58), encompassing a total of 1,314 patients, reported on the incidence of NICU admissions. Employing a random-effects model to synthesize the data, the pooled results indicated no statistically significant difference between the two cohorts [RR = 0.76; 95% CI (0.42, 1.37) p = 0.36] (Figure 10). Furthermore, no notable heterogeneity was detected among these studies (p = 0.90, I2 = 0%; Figure 11).
APGAR score < 8 at 5 min
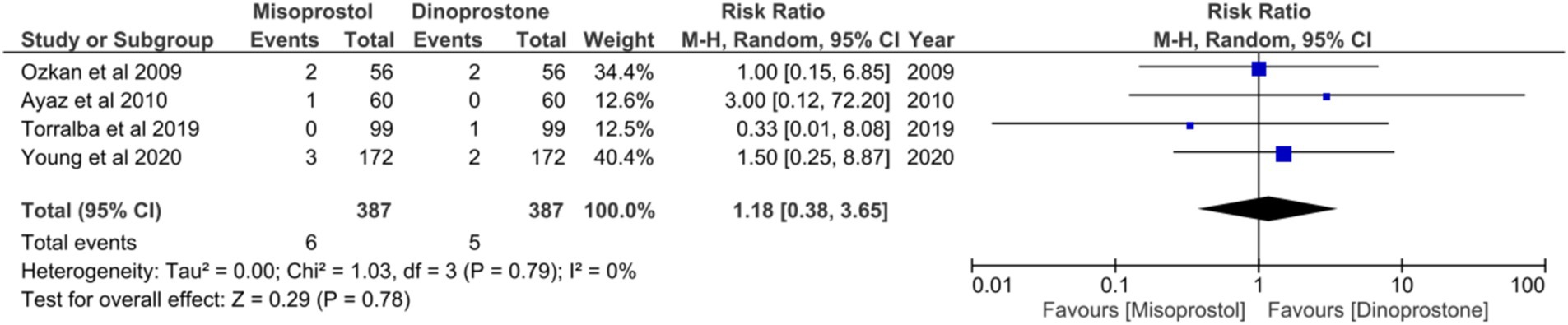
Four studies (17, 18, 54, 58), comprising a total of 774 patients, reported on APGAR scores below 8 in 5 min. No statistically significant heterogeneity was observed among these studies (p = 0.79, I2 = 0%). A random-effects model was employed to aggregate the combined effect, demonstrating no significant difference in the incidence of APGAR scores below 8 at 5 min between the two groups [RR = 1.18; 95% CI (0.38, 3.65) p = 0.78] (Figure 10).
Abnormal cardiotocograph
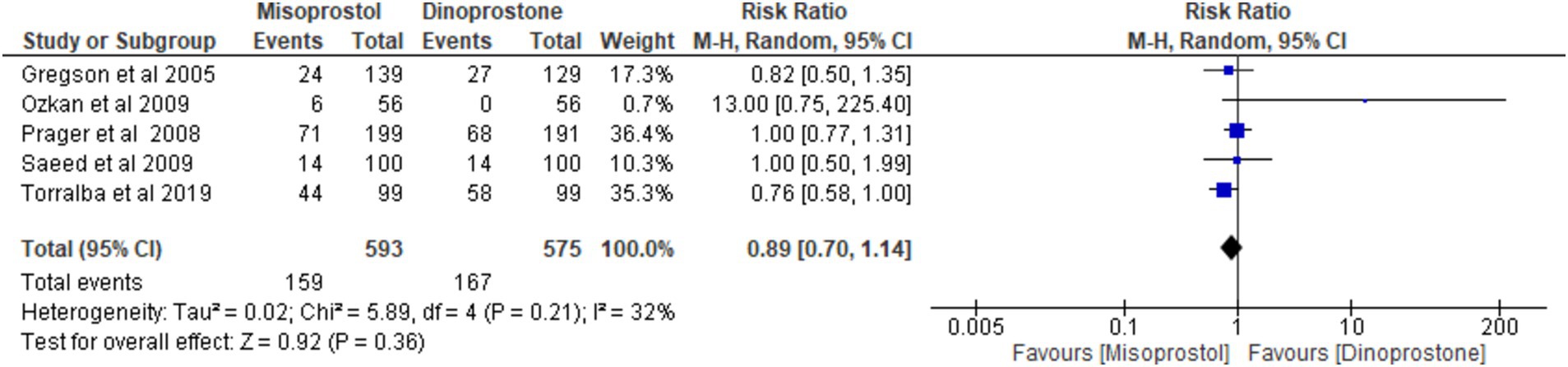
Five studies (17, 54–56, 59), encompassing 1,168 patients, reported on the incidence of abnormal cardiotocograph results. Using a random-effects model to pool the combined results, the analysis revealed no significant difference between misoprostol and dinoprostone [RR = 0.89; 95% CI (0.70), 1.14; p = 0.36]. No statistically significant heterogeneity was observed among the studies (p = 0.21, I2 = 32%; Figure 12).
Hyperstimulation
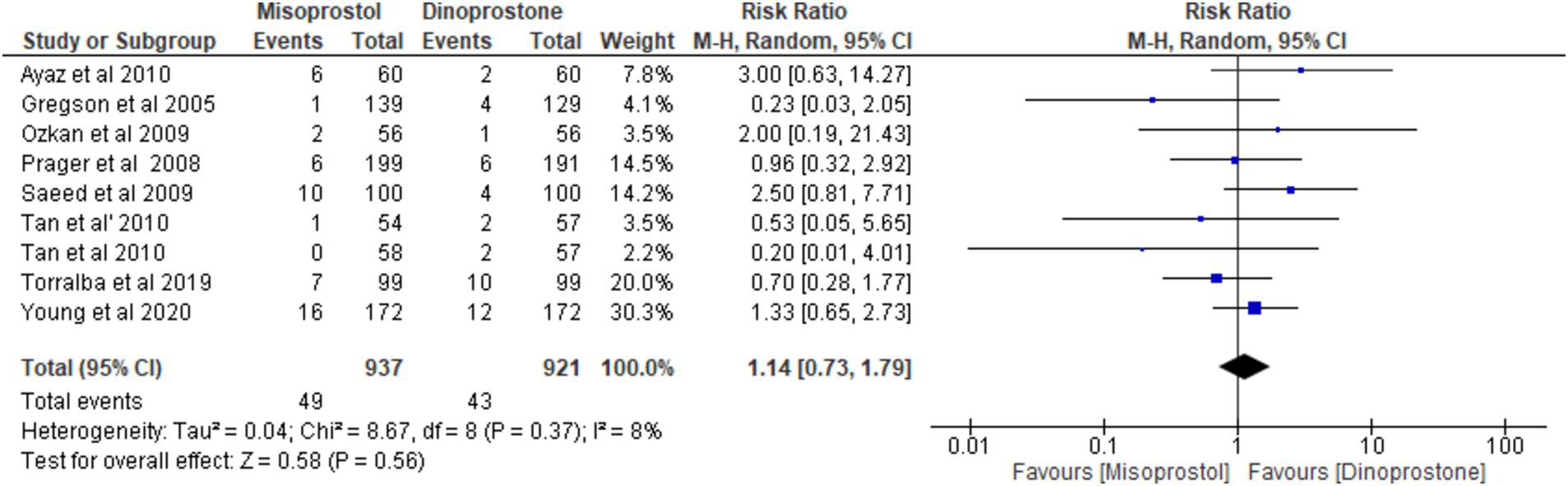
Eight studies (17, 18, 54–59), involving 1858 patients, reported the incidence of uterine hyperstimulation. A random-effects model was used to pool the combined effect, which indicated no significant difference between misoprostol and dinoprostone [RR = 1.14; 95% CI (0.73, 1.79) p = 0.56]. No significant heterogeneity was observed among the studies (p = 0.37, I2 = 8%).
Leave-one-out analysis
No outcomes exhibited significant heterogeneity except for vaginal delivery. The leave-one-out sensitivity analysis revealed that the rate of vaginal delivery was influenced by a single study, namely Ayaz et al. (58). Excluding this study led to a notable reduction in I2 values (p = 0.16; I2 = 34%) and altered the overall effect [RR = 1.01, 95% CI (0.93, 1.10), p = 0.74] (Supplementary Figure S1).
Discussion
This meta-analysis of eight RCTs comparing intravaginal misoprostol and dinoprostone for labor induction in women with unfavorable cervices at term found no significant differences between the two groups in key maternal and neonatal outcomes, such as vaginal delivery within 24 h, cesarean delivery, and overall vaginal delivery rates. While oxytocin augmentation was needed less frequently in the misoprostol group, other outcomes—including the incidence of uterine tachysystole, hyperstimulation, NICU admissions, low APGAR scores, and abnormal cardiotocograph readings—showed no notable differences between groups (Figure 13).
Wang et al.’s (19) study showed non- significant result in terms of oxytocin augmentation for dinoprostone group with a p value of (p = 0.11), while our meta-analysis clearly showed the significance need of oxytocin augmentation in dinoprostone group with a p value of (0.02). This could be attributed to the inclusion of two studies in the meta-analysis by Wang et al. (19) not aligning with inclusion criteria. This finding is supported by a study conducted by Meyer et al. (27) which states that misoprostol decreased the dose of oxytocin. Another meta-analysis by Liu et al. (60) comparing intravaginal misoprostol to intracervical dinoprostone concluded that the misoprostol group required less oxytocin augmentation than the dinoprostone group.
According to our findings, there were no appreciable changes in the two groups’ rate of Cesarean sections, which is consistent with meta-analysis by Wang et al. (19) and study by Wing et al. (61) that have reported inconsistent results regarding the impact of misoprostol on Cesarean section rates. Similarly, a study by Moodley et al. (62) also supports our findings by suggesting that neither intervention affects the rate of C-sections. Regarding vaginal delivery within 24 h, our results showed no significant difference, which is consistent with an observational study conducted by Moodley et al. (62). This lack of difference may be attributed to both interventions being equally efficient in promoting vaginal delivery. In addition to this, our study found out that there was no significant difference in instrumental delivery between the two groups. A recent comparative study by Sire et al. (63) aligns with the results of our analysis. However, a study conducted by Akhtar et al. (64) at a hospital in Pakistan shows that there is difference between two groups and use of dinoprostone shows greater incidence of instrumental delivery which could possibly be due to small sample size of the study.
Furthermore, this meta-analysis did not find a significant difference in hyperstimulation between the misoprostol and dinoprostone groups. This aligns with a randomized controlled trial conducted by Madaan et al. (65) which also found no significant difference between the two groups. In terms of neonatal outcomes, our study did not find any significant differences in NICU admissions, abnormal cardiotocographs, or APGAR scores below 7. These findings align with a previous meta-analysis conducted by Wang et al. (19). Another randomized controlled trial by Wing et al. (53) comparing dinoprostone vaginal insert with vaginal misoprostol insert also found no association between neonatal outcomes in treatment groups.
When compared to women who received dinoprostone treatment, women treated with misoprostol had a significantly lower rate of oxytocin augmentation (17, 18, 41, 54–59). This shows that misoprostol might be more efficient at accelerating the course of labor, hence minimizing the requirement for additional interventions. On the other hand, although not statistically significant, the occurrence of tachysystole was higher in women administered misoprostol (18, 54, 56–58). This may suggest that misoprostol may raise the incidence of tachysystole and could perhaps suggest that lower doses must be administered which calls for additional research. Furthermore, a review by Boulvain et al. (66), comparing misoprostol to other controls, also supports this association, suggesting that misoprostol is linked to uterine tachysystole. Additionally, Farah et al. (67) found that a higher dose of 50 μg misoprostol showed a greater incidence of uterine tachysystole. This could be explained by the slow decline in plasma concentration of misoprostol after reaching maximum levels, resulting in abnormal uterine contractions (68). The American College of Obstetricians and Gynecologists recommends a lower dose of 25 μg misoprostol due to these potential uterine contractile abnormalities (69–71).
Our study had several limitations. Firstly, our meta-analysis included only eight studies with a limited sample size. Despite an extensive search strategy, few studies met the inclusion criteria for the meta-analysis. Additionally, this meta-analysis considered only publications in English, which could introduce bias and exclude pertinent studies published in other languages. Secondly, the dosages of misoprostol and dinoprostone varied across the studies, potentially affecting the interpretation of the results. Finally, the meta-analysis focused solely on the short-term effects of labor induction. Long-term outcomes, such as neonatal morbidity and maternal complications beyond the first few weeks postpartum, were not assessed.
These limitations should be considered when interpreting the findings of this meta-analysis and applying them to clinical practice. Future trials with larger sample sizes, standardized dosing protocols, and comprehensive outcome reporting are necessary to gain a clearer understanding of the efficacy and safety of misoprostol compared to dinoprostone for labor induction at term.
Conclusion
In summary, our findings suggest that misoprostol and dinoprostone are comparably effective and safe for labor induction and misoprostol requires less oxytocin augmentation. The majority of analyzed outcomes exhibited low heterogeneity, indicating overall consistency among the included studies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
NL: Conceptualization, Methodology, Supervision, Writing – original draft. MH: Conceptualization, Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing. TA: Conceptualization, Data curation, Formal analysis, Writing – original draft. SKh: Data curation, Formal analysis, Methodology, Writing – original draft. AK: Data curation, Formal analysis, Methodology, Writing – original draft. MJ: Data curation, Software, Writing – original draft. MaK: Data curation, Formal analysis, Writing – original draft. AQ: Data curation, Project administration, Writing – original draft. SKu: Data curation, Writing – original draft. MuK: Data curation, Writing – original draft. FD: Data curation, Writing – original draft. KR: Data curation, Writing – original draft. AA: Data curation, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1459793/full#supplementary-material
References
1. ACOG Practice Bulletin No. 107: induction of labor. Obstet Gynecol. (2009) 114:386–97. doi: 10.1097/AOG.0b013e3181b48ef5
2. Martin, JA, Hamilton, BE, Ventura, SJ, Osterman, MJ, Kirmeyer, S, Mathews, TJ, et al. Births: final data for 2009. Natl Vital Stat Rep. 60:1–70.
3. Alfirevic, Z, Keeney, E, Dowswell, T, Welton, NJ, Dias, S, Jones, LV, et al. Labour induction with prostaglandins: a systematic review and network meta-analysis. BMJ. (2015) 350:350. doi: 10.1136/bmj.h217
4. Papalia, N, D’Souza, RD, and Hobson, SR. Optimal timing of labour induction in contemporary clinical practice. Best Pract Res Clin Obstet Gynaecol. (2022) 79:18–26. doi: 10.1016/j.bpobgyn.2021.12.002
5. Middleton, P, Shepherd, E, Morris, J, Crowther, CA, and Gomersall, JC. Induction of labour at or beyond 37 weeks’ gestation. Cochrane Database Syst Rev. (2020) 2020:945. doi: 10.1002/14651858.CD004945.pub5
6. Caughey, AB, Sundaram, V, Kaimal, AJ, Cheng, YW, Gienger, A, Little, SE, et al. Maternal and neonatal outcomes of elective induction of labor. Evid Rep Technol Assess (Full Rep). (2009) 176:1–257.
7. Stupar, ŽT, Novakov Mikić, A, Bogavac, M, Milatović, S, and Sekulić, S. Prediction of labor induction outcome using different clinical parameters. Srp Arh Celok Lek. (2013) 141:770–4. doi: 10.2298/SARH1312770T
8. Martin, JA, Hamilton, BE, Osterman, MJ, Curtin, SC, and Matthews, TJ. Births: final data for 2012. Natl Vital Stat Rep. (2013) 62:1–68.
9. Laughon, SK, Zhang, J, Grewal, J, Sundaram, R, Beaver, J, and Reddy, UM. Induction of labor in a contemporary obstetric cohort. Am J Obstet Gynecol. (2012) 206:486.e1–9. doi: 10.1016/j.ajog.2012.03.014
10. Stubbs, TM. Oxytocin for labor induction. Clin Obstet Gynecol. (2000) 43:489–94. doi: 10.1097/00003081-200009000-00009
11. Robinson, D, Campbell, K, Hobson, SR, MacDonald, WK, Sawchuck, D, and Wagner, B. 432c: induction of labour. J Obstet Gynaecol Can. (2023) 45:70–77.e3. doi: 10.1016/j.jogc.2022.11.009
12. Hofmeyr, GJ. Induction of labour with an unfavourable cervix. Best Pract Res Clin Obstet Gynaecol. (2003) 17:777–94. doi: 10.1016/S1521-6934(03)00037-3
13. Crane, JMG. Factors predicting labor induction success: a critical analysis. Clin Obstet Gynecol. (2006) 49:573–84. doi: 10.1097/00003081-200609000-00017
14. Hawkins, JS, and Wing, DA. Current pharmacotherapy options for labor induction. Expert Opin Pharmacother. (2012) 13:2005–14. doi: 10.1517/14656566.2012.722622
15. Church, S, Van Meter, A, and Whitfield, R. Dinoprostone compared with misoprostol for cervical ripening for induction of labor at term. J Midwifery Womens Health. (2009) 54:405–11. doi: 10.1016/j.jmwh.2009.03.006
16. Watkinson, G, and Akbar, F. Misoprostol in peptic ulcer disease. Prostaglandins. (1987) 33:78–92. doi: 10.1016/0090-6980(87)90051-7
17. De Bonrostro Torralba, C, Tejero Cabrejas, EL, Envid Lázaro, BM, Franco Royo, MJ, Roca Arquillué, M, and Campillos Maza, JM. Low-dose vaginal misoprostol vs vaginal dinoprostone insert for induction of labor beyond 41st week: A randomized trial. Acta Obstet Gynecol Scand. (2019) 98:913–9. doi: 10.1111/aogs.13556
18. Young, DC, Delaney, T, Anthony Armson, B, and Fanning, C. Oral misoprostol, low dose vaginal misoprostol, and vaginal dinoprostone for labor induction: randomized controlled trial. PLoS One. (2020) 15:245. doi: 10.1371/journal.pone.0227245
19. Wang, L, Zheng, J, Wang, W, Fu, J, and Hou, L. Efficacy and safety of misoprostol compared with the dinoprostone for labor induction at term: a meta-analysis. J Matern Fetal Neonatal Med. (2016) 29:1297–307. doi: 10.3109/14767058.2015.1046828
20. Shannon, CS, and Winikoff, B. Misoprostol: an emerging technology for women’s health—report of a seminar. Reprod Health (2004).
21. Gaudineau, A, Senat, MV, Ehlinger, V, Gallini, A, Morin, M, Olivier, P, et al. Induction of labor at term with vaginal misoprostol or a prostaglandin E2 pessary: a noninferiority randomized controlled trial. Am J Obstet Gynecol. (2021) 225:542.e1–8. doi: 10.1016/j.ajog.2021.04.226
22. Kawakita, T, Grantz, KL, Landy, HJ, Huang, CC, and Kominiarek, MA. Induction of labor in women with oligohydramnios: misoprostol compared with prostaglandin E2. Am J Perinatol. (2017) 34:204–10.
23. Chyu, JK, and Strassner, HT. Prostaglandin E2 for cervical ripening: a randomized comparison of Cervidil versus Prepidil. Am J Obstet Gynecol. (1997) 177:606–11. doi: 10.1016/S0002-9378(97)70153-4
24. Harms, K. Intravaginal misoprostol versus cervidil for cervical ripening in term pregnancies. Obstet Gynecol. (2001) 97:36S. doi: 10.1097/00006250-200104001-00086
25. Draycott, T, Van Der Nelson, H, Montouchet, C, Ruff, L, and Andersson, F. Reduction in resource use with the misoprostol vaginal insert vs the dinoprostone vaginal insert for labour induction: a model-based analysis from a United Kingdom healthcare perspective. BMC Health Serv Res. (2016) 16:1278. doi: 10.1186/s12913-016-1278-9
26. Hofmeyr, GJ, Gülmezoglu, AM, and Pileggi, C. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database Syst Rev. (2010) 2010:CD000941. doi: 10.1002/14651858.CD000941.pub2
27. Meyer, M, Pflum, J, and Howard, D. Outpatient misoprostol compared with dinoprostone gel for preinduction cervical ripening: a randomized controlled trial. Obstet Gynecol. (2005) 105:466–72. doi: 10.1097/01.AOG.0000152341.31873.d9
28. Saxena, P, Puri, M, Bajaj, M, Mishra, A, and Trivedi, SS. A randomized clinical trial to compare the efficacy of different doses of intravaginal misoprostol with intracervical dinoprostone for cervical ripening and labor induction. Eur Rev Med Pharmacol Sci. 15:759–63.
29. Chitrakar, NS. Comparison of Misoprostol versus Dinoprostone for pre-induction cervical ripening at-term. J Nepal Health Res Counc. (2012) 10:10–5.
30. Liberati, A, Altman, DG, Tetzlaff, J, Mulrow, C, Gøtzsche, PC, Ioannidis, JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
31. Higgins, JPT, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
32. Sterne, JAC, Savović, J, Page, MJ, Elbers, RG, Blencowe, NS, Boutron, I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019):l4898. doi: 10.1136/bmj.l4898
33. Hostinska, E, Lubusky, M, and Pilka, R. Prospective comparison of cervical ripening with double balloon cook catheter, misoprostol or dinoprostone in term singleton pregnancies. Ginekol Pol. (2022) 94:221–8. doi: 10.5603/GP.a2022.0023
34. Aghideh, FK, Mullin, PM, Ingles, S, Ouzounian, JG, Opper, N, Wilson, ML, et al. A comparison of obstetrical outcomes with labor induction agents used at term. J Matern Fetal Neonatal Med. (2014) 27:592–6. doi: 10.3109/14767058.2013.831066
35. Duro-Gómez, J, Garrido-Oyarzún, MF, Rodríguez-Marín, AB, de la Torre González, AJ, Arjona-Berral, JE, and Castelo-Branco, C. Efficacy and safety of misoprostol, dinoprostone and Cook’s balloon for labour induction in women with foetal growth restriction at term. Arch Gynecol Obstet. (2017) 296:777–81. doi: 10.1007/s00404-017-4492-8
36. Tsikouras, P, Koukouli, Z, Manav, B, Soilemetzidis, M, Liberis, A, Csorba, R, et al. Induction of labor in post-term nulliparous and parous women - potential advantages of misoprostol over Dinoprostone. Geburtshilfe Frauenheilkd. (2016) 76:785–92. doi: 10.1055/s-0042-105287
37. Górnisiewicz, T, Jaworowski, A, Zembala-Szczerba, M, Babczyk, D, and Huras, H. Analysis of intravaginal misoprostol 0.2 mg versus intracervical dinoprostone 0.5 mg doses for labor induction at term pregnancies. Ginekol Pol. (2017) 88:320–4. doi: 10.5603/GP.a2017.0060
38. Gornisiewicz, T, Huras, H, Kusmierska-Urban, K, and Galas, A. Pregnancy-related comorbidities and labor induction - the effectiveness and safety of dinoprostone compared to misoprostol. Ginekol Pol. (2021) 92:647–58. doi: 10.5603/GP.a2021.0092
39. Ting, NS, Ding, DC, and Wei, YC. Comparison of the Dinoprostone vaginal insert and Dinoprostone tablet for the induction of labor in Primipara: A retrospective cohort study. J Clin Med. (2022) 11:519. doi: 10.3390/jcm11123519
40. Suidan, RS, Rondon, KC, Apuzzio, JJ, and Williams, SF. Labor outcomes of obese patients undergoing induction of labor with misoprostol compared to dinoprostone. Am J Perinatol. (2015) 30:187–92. doi: 10.1055/s-0034-1381721
41. Benalcazar-Parra, C, Ye-Lin, Y, Garcia-Casado, J, Monfort-Orti, R, Alberola-Rubio, J, Perales, A, et al. Electrohysterographic characterization of the uterine myoelectrical response to labor induction drugs. Med Eng Phys. (2018) 56:27–35. doi: 10.1016/j.medengphy.2018.04.002
42. Osoti, A, Kibii, DK, Tong, TMK, and Maranga, I. Effect of extra-amniotic Foley’s catheter and vaginal misoprostol versus vaginal misoprostol alone on cervical ripening and induction of labor in Kenya, a randomized controlled trial. BMC Pregnancy Childbirth. (2018) 18:1793. doi: 10.1186/s12884-018-1793-2
43. Soilemetzidis, M, Pinidis, P, Tsagias, N, Ammari, A, Liberis, A, and Liberis, V. The effectiveness of misoprostol or dinoprostone in neonatal outcome after labour induction in post-term nulliparas. Clin Exp Obstet Gynecol. (2015) 42:649–52.
44. Reinhard, J, Rosler, R, Yuan, J, Schiermeier, S, Herrmann, E, Eichbaum, MH, et al. Prostaglandin E2 labour induction with intravaginal (Minprostin) versus intracervical (Prepidil) administration at term: randomized study of maternal and neonatal outcome and patient’s perception using the osgood semantic differential scales. Biomed Res Int. (2014) 2014:1–6. doi: 10.1155/2014/682919
45. Mounie, M, Costa, N, Gaudineau, A, Molinier, L, Vayssière, C, and Derumeaux, H. Cost-effectiveness analysis of vaginal misoprostol versus dinoprostone pessary: A non-inferiority large randomized controlled trial in France. Int J Gynaecol Obstet. (2022) 158:390–7. doi: 10.1002/ijgo.13999
46. Wang, X, Yang, A, Ma, Q, Li, X, Qin, L, and He, T. Comparative study of titrated oral misoprostol solution and vaginal dinoprostone for labor induction at term pregnancy. Arch Gynecol Obstet. (2016) 294:495–503. doi: 10.1007/s00404-015-4000-y
47. Lapuente-Ocamica, O, Ugarte, L, Lopez-Picado, A, Sanchez-Refoyo, F, Lasa, IL, Echevarria, O, et al. Efficacy and safety of administering oral misoprostol by titration compared to vaginal misoprostol and dinoprostone for cervical ripening and induction of labour: study protocol for a randomised clinical trial. BMC Pregnancy Childbirth. (2019) 19:2132. doi: 10.1186/s12884-018-2132-3
48. Rugarn, O, Tipping, D, Powers, B, and Wing, DA. Induction of labour with retrievable prostaglandin vaginal inserts: outcomes following retrieval due to an intrapartum adverse event. BJOG. (2017) 124:796–803. doi: 10.1111/1471-0528.14147
49. D’Souza, R, Doyle, O, Miller, H, Pillai, N, Angehrn, Z, Li, P, et al. Prediction of successful labor induction in persons with a low bishop score using machine learning: secondary analysis of two randomized controlled trials. Birth. (2023) 50:234–43. doi: 10.1111/birt.12691
50. Hostinská, E, Šinská, A, Ľubušký, M, and Pilka, R. Comparison of dinoprostone, misoprostol and amniotomy in labor induction. Ceska Gynekol. (2021) 86:368–73. doi: 10.48095/cccg2021368
51. Inal, HA, Ozturk Inal, ZH, Tonguc, E, and Var, T. Comparison of vaginal misoprostol and dinoprostone for cervical ripening before diagnostic hysteroscopy in nulliparous women. Fertil Steril. (2015) 103:1326–31. doi: 10.1016/j.fertnstert.2015.01.037
52. Mendez-Figueroa, H, Bicocca, MJ, Gupta, M, Wagner, SM, and Chauhan, SP. Labor induction with prostaglandin E1 versus E2: a comparison of outcomes. J Perinatol. (2021) 41:726–35. doi: 10.1038/s41372-020-00888-5
53. Wing, DA, Brown, R, Plante, LA, Miller, H, Rugarn, O, and Powers, BL. Misoprostol vaginal insert and time to vaginal delivery: a randomized controlled trial. Obstet Gynecol. (2013) 122:201–9. doi: 10.1097/AOG.0b013e31829a2dd6
54. Gregson, S, Waterstone, M, Norman, I, and Murrells, T. A randomised controlled trial comparing low dose vaginal misoprostol and dinoprostone vaginal gel for inducing labour at term. BJOG. (2005) 112:438–44. doi: 10.1111/j.1471-0528.2004.00496.x
55. Prager, M, Eneroth-Grimfors, E, Edlund, M, and Marions, L. A randomised controlled trial of intravaginal dinoprostone, intravaginal misoprostol and transcervical balloon catheter for labour induction. BJOG. (2008) 115:1443–50. doi: 10.1111/j.1471-0528.2008.01843.x
56. Özkan, S, ÇalIşkan, E, Doğer, E, Yücesoy, I, Özeren, S, and Vural, B. Comparative efficacy and safety of vaginal misoprostol versus dinoprostone vaginal insert in labor induction at term: a randomized trial. Arch Gynecol Obstet. (2009) 280:19–24. doi: 10.1007/s00404-008-0843-9
57. Tan, TC, Yan, SY, Chua, TM, Biswas, A, and Chong, YS. A randomised controlled trial of low-dose misoprostol and dinoprostone vaginal pessaries for cervical priming. BJOG. (2010) 117:1270–7. doi: 10.1111/j.1471-0528.2010.02602.x
58. Ayaz, A, Shaukat, S, Farooq, MU, Mehmood, K, Ahmad, I, and Bahoo, MLA. Induction of labor: a comparative study of intravaginal misoprostol and dinoprostone. Taiwan J Obstet Gynecol. (2010) 49:151–5. doi: 10.1016/S1028-4559(10)60032-0
59. Saeed, GA, Fakhar, S, Nisar, N, and Alam, AY. Misoprostol for term labor induction: a randomized controlled trial. Taiwan J Obstet Gynecol. (2011) 50:15–9. doi: 10.1016/j.tjog.2009.08.001
60. Liu, A, Lv, J, Hu, Y, Lang, J, Ma, L, and Chen, W. Efficacy and safety of intravaginal misoprostol versus intracervical dinoprostone for labor induction at term: a systematic review and meta-analysis. J Obstet Gynaecol Res. (2014) 40:897–906. doi: 10.1111/jog.12333
61. Wing, DA, and Lyons Gaffaney, CA. Vaginal misoprostol administration for cervical ripening and labor induction. Clin Obstet Gynecol. (2006) 49:627–41. doi: 10.1097/00003081-200609000-00021
62. Moodley, J, Venkatachalam, S, and Songca, P. Misoprostol for cervical ripening at and near term--a comparative study. S Afr Med J. (2003) 93:371–4.
63. Sire, F, Ponthier, L, Eyraud, JL, Catalan, C, Aubard, Y, and Coste, MP. Comparative study of dinoprostone and misoprostol for induction of labor in patients with premature rupture of membranes after 35 weeks. Sci Rep. (2022) 12:948. doi: 10.1038/s41598-022-18948-5
64. Akhtar, A, Talib, W, Shami, N, and Anwar, S. Induction of labour – a comparison between misoprostol and dinoprostone. Pak J Med Health Sci. (2011) 5:617–9.
65. Madaan, M, Agrawal, S, Puri, M, Nigam, A, Kaur, H, and Trivedi, SS. Is low dose vaginal misoprostol better than dinoprostone gel for induction of labor: a randomized controlled trial. J Clin Diagn Res. (2014) 8:OC31–4. doi: 10.7860/JCDR/2014/8101.4906
66. Boulvain, M, Kelly, AJ, Lohse, C, Stan, CM, and Irion, O. Mechanical methods for induction of labour. Cochrane Database Syst Rev. (2001):4. doi: 10.1002/14651858.CD001233
67. Farah, LA, Sanchez-Ramos, L, Rosa, C, Del Valle, GO, Gaudier, FL, Delke, I, et al. Randomized trial of two doses of the prostaglandin E1 analog misoprostol for labor induction. Am J Obstet Gynecol. (1997) 177:364–71. doi: 10.1016/S0002-9378(97)70199-6
68. Zieman, M, Fong, SK, Benowitz, NL, Banskter, D, and Darney, PD. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. (1997) 90:88–92. doi: 10.1016/S0029-7844(97)00111-7
69. ACOG Committee Opinion. American College of Obstetrician and Gynecologist. ACOG. Committee Opinion. Number 283, May 2003. New U.S. Food and Drug Administration labeling on Cytotec (misoprostol) use and pregnancy. Obstet Gynecol. (2003) 101:1049–50. doi: 10.1016/s0029-7844(03)00396-x
70. Hafeezullah, N, AlHilali, S, Alghulaydhawi, F, Edward, DP, Ahmad, S, and Malik, R. A preliminary comparison of the Aravind aurolab drainage implant with the Baerveldt glaucoma implant: A matched case-control study. Eur J Ophthalmol. (2021) 31:445–52. doi: 10.1177/1120672120912383
Keywords: misoprostol, dinoprostone, intravaginally, labor induction, term
Citation: Lakho N, Hyder M, Ashraf T, Khan S, Kumar A, Jabbar M, Kumari M, Qammar A, Kumar S, Kumari M, Deepak F, Raj K and Ali A (2024) Efficacy and safety of misoprostol compared with dinoprostone for labor induction at term: an updated systematic review and meta-analysis of randomized controlled trials. Front. Med. 11:1459793. doi: 10.3389/fmed.2024.1459793
Edited by:
Mattia Dominoni, San Matteo Hospital Foundation (IRCCS), ItalyReviewed by:
Shuhua Liu, Anhui Maternal and Child Health Hospital, ChinaKwabena Amo-Antwi, Kwame Nkrumah University of Science and Technology, Ghana
Copyright © 2024 Lakho, Hyder, Ashraf, Khan, Kumar, Jabbar, Kumari, Qammar, Kumar, Kumari, Deepak, Raj and Ali. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Azzam Ali, YXp6YW1hdGljODEwQGdtYWlsLmNvbQ==
 Nusrat Lakho1
Nusrat Lakho1 Taimoor Ashraf
Taimoor Ashraf Ajay Kumar
Ajay Kumar Madhurta Kumari
Madhurta Kumari Asfia Qammar
Asfia Qammar Sateesh Kumar
Sateesh Kumar Muskan Kumari
Muskan Kumari Fnu Deepak
Fnu Deepak Kapil Raj
Kapil Raj Azzam Ali
Azzam Ali