- 1Department of Critical Care Medicine, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, Guangdong, China
- 2Medical Intensive Care Unit, Sichuan Provincial Maternity and Child Health Care Hospital, Chengdu, Sichuan, China
- 3The Affiliated Women's and Children's Hospital of Chengdu Medical College, Chengdu, Sichuan, China
- 4Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China
Introduction: Delirium in patients with sepsis can be life-threatening. This study aims to investigate the impact of the use of beta-blockers on the occurrence of delirium in patients with sepsis in the ICU by utilizing a comprehensive dataset.
Methods: This is a cross-sectional study conducted using the data obtained from a single ICU in the USA. Patients diagnosed with sepsis and receiving beta-blockers were compared with those not receiving beta-blockers. Propensity score matching (PSM) and multiple regression analysis were employed to adjust for potential confounders.
Results: Among the 19,660 patients hospitalized for sepsis, the beta-blocker and non-user groups comprised 13,119 (66.73%) and 6,541 (33.27%) patients, respectively. Multivariable logistic regression models revealed a significant reduction of 60% in 7-day delirium for beta-blocker users (OR = 0.40, 95% CI: 0.37–0.43, p < 0.001), for 30-day delirium (OR = 0.32, 95% CI: 0.29–0.35, p < 0.001), and for 90-day delirium (OR = 0.33, 95% CI: 0.30–0.35, p < 0.001). The PSM results further strengthen the validity of these findings. An analysis of safety issues demonstrated that beta-blockers may have an impact on the risk of acute kidney injury. However, following PSM, the results are not considered robust. Furthermore, there was no discernible change in the odds of renal replacement therapy and the length of ICU stays.
Discussion: Our findings suggest a potential protective effect of beta-blockers against delirium in patients with sepsis. Nevertheless, the observational design limits causal inference, necessitating future randomized controlled trials to validate these findings.
1 Introduction
Delirium represents a prevalent form of organ dysfunction in critically ill adults and may substantially increase both morbidity and mortality rates. Some recent studies have indicated that more than half of all patients admitted to modern intensive care units (ICUs) will experience delirium at some point during their hospital stay (1–4). This is a critical issue owing to the independent association of delirium with an increase in mortality risk (5–7). Furthermore, the duration of delirium has been identified as the primary risk factor for subsequent cognitive impairment in individuals recovering from critical illness, often presenting as ICU-acquired delirium—a condition associated with substantial debilitation (3, 8–10). The onset of delirium is associated with prolonged hospitalizations and increased health care costs. Despite extensive research, however, no pharmacological agent has yet been developed that can show some efficacy in either treating or preventing delirium. Notably, current guidelines of the Society of Critical Care Medicine (SCCM) recommend against the routine use of dexmedetomidine, statins, or ketamine for preventing delirium in critically ill adults. These recommendations highlight the ongoing challenge of effectively managing delirium within ICU settings, necessitating further investigation into alternative therapeutic approaches (11).
Although the mechanism underlying delirium remains elusive, it has been suggested that delirium likely encompasses multiple pathways that are disrupted during critical illness, resulting in the impairment of normal cognitive function (12, 13). While various factors contributing to the pathophysiology of delirium have been recognized, numerous other aspects remain unidentified (14). Sepsis, arising from a dysregulated immune response after infection, is a significant public health concern (15). Various studies have suggested sepsis as one of the most imperative and robust risk factors for delirium (14, 16). Given the close association of both sepsis and delirium with increased morbidity and mortality rates (17–19), it becomes critical to prioritize the prevention and management of delirium in patients with sepsis.
Beta-blockers have been shown to be safe and effective in reducing 28-day mortality and controlling the ventricular rate in patients with sepsis post-fluid resuscitation, without any major adverse effects on tissue perfusion (20). Numerous systematic reviews have reported on beta-blocker therapy during sepsis (21–23). As beta-blockers play a pivotal role in sepsis management, their potential protective effects in patients with sepsis experiencing delirium should be investigated. Therefore, we herein conduct a retrospective study utilizing the Medical Information Mart for Intensive Care (MIMIC-IV) dataset for the 2008–2019 period. This investigation explores the association between the administration of beta-blockers and the onset of delirium in patients with sepsis.
2 Methods
We included patients with sepsis, both with and without exposure to beta-blockers, from the MIMIC-IV (version 2.2) database, a longitudinal single-center database covering the period spanning from 2008 to 2019 (24). Yi Yu, one of the authors, obtained permission to access the database (certificate ID: 6477678). The manuscript was prepared in accordance with the Guidelines for Strengthening the Reporting of Observational Studies in Epidemiology (25).
2.1 Study population and data extraction
Patients aged ≥18 years with a diagnosis of sepsis were included in the study. For patients with multiple ICU admissions, only the initial admission was considered. Patients with ICU stays shorter than 24 h or lacking essential information were excluded. Who received beta-blocker after the onset of delirium were also excluded. Delirium diagnosis was based on the Confusion Assessment Method for the ICU (CAM-ICU) criteria (26). Data on patient demographics, vital signs, laboratory results, comorbidities, clinical severity scores, and other admission details were collected.
2.2 Beta-blocker usage
Beta-blocker usage, defined as the use of beta-blockers at any time point, was determined by the presence of beta-blockers in the “Prescriptions” section of the MIMIC-IV database. This section mentioned beta-blockers such as acebutolol, atenolol, bisoprolol, esmolol, metoprolol, nadolol, nebivolol, propranolol, and timolol. The term “Pre-ICU” denotes the administration of beta-blockers exclusively prior to ICU admission, while “Post-ICU” signifies the use of beta-blockers exclusively following ICU admission. “Post + Pre ICU” refers to the utilization of beta-blockers both prior to and following admission to the ICU. “No use” indicates the absence of beta-blockers utilization.
2.3 Covariates
The risk factors for delirium among the patients with sepsis were documented (27, 28). The covariates analyzed included age, sex, body mass index (BMI), respiratory rate, white blood cell (WBC) count, hemoglobin level, platelet count, and glucose level (based on the first result upon admission to the ICU, or the average of multiple measurements over 24 h). The study collected data on health indicators, such as the SOFA score, and comorbid conditions, including cardiovascular diseases, kidney diseases, liver diseases, malignancy, neurological diseases, and chronic pulmonary diseases. Demographic information regarding race and marital status was also extracted. Multicollinearity was assessed by calculating the variance inflation factor (VIF) among variables involved. Multicollinearity was considered present if the VIF was greater than 2.
2.4 Outcome
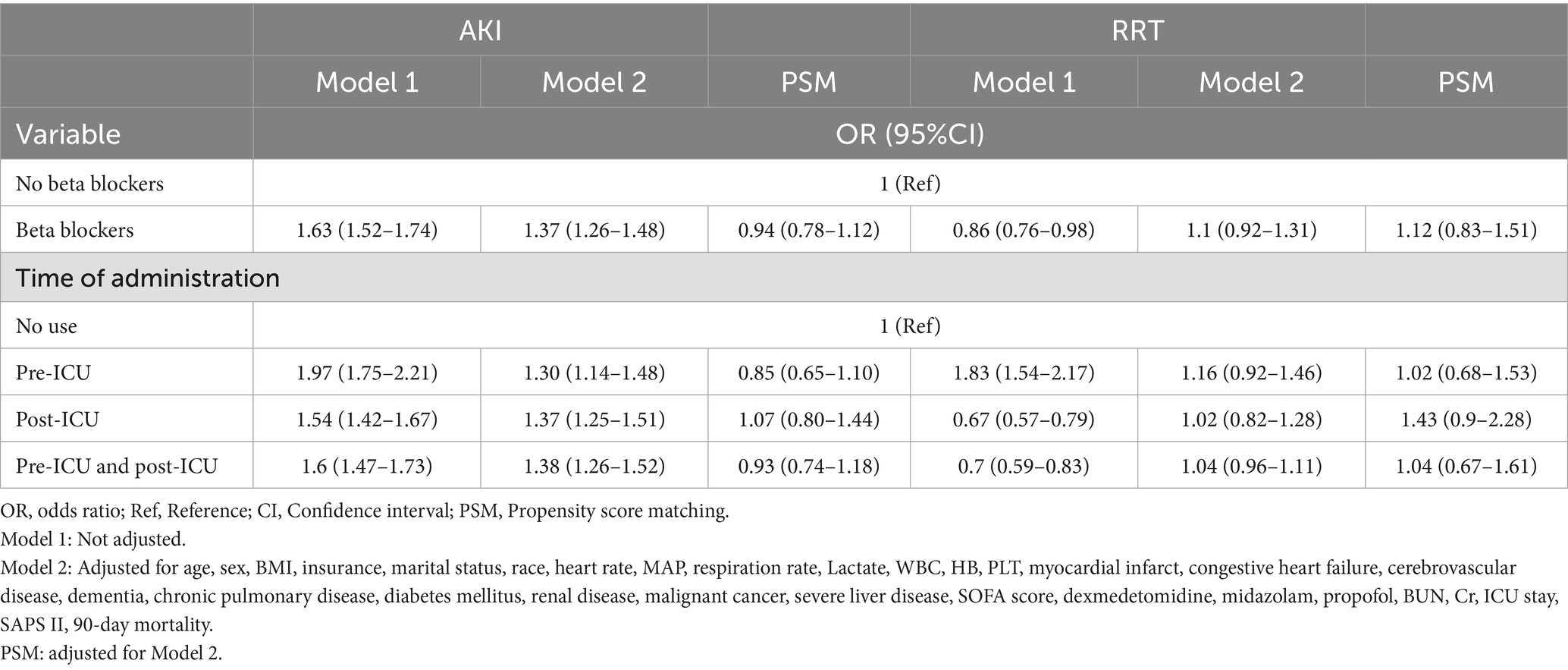
The primary outcome was the incidence of delirium on 7, 30, and 90 days. The secondary outcomes included the length of the ICU stay, acute kidney injury (AKI), and the requirement for renal replacement therapy (RRT).
2.5 Statistical analysis
Baseline characteristics of patients across different groups were analyzed. Categorical data were presented as frequencies (percentages), while continuous variables were expressed as mean ± standard deviation or median (interquartile range) as appropriate. Statistical analyses involved the analysis of variance or Wilcoxon rank-sum tests to evaluate differences in continuous variables between groups. Chi-squared or Fisher’s exact tests were utilized for comparing categorical variables among the study cohort. Furthermore, consistent formatting and citation styles were adhered to throughout the study.
Missing data, constituting approximately 5% of vital signs and laboratory parameters, were imputed using the median. Given the low rates of missing data (0.5–8%) for height and weight, no imputation method was applied. A multivariate logistic regression analysis was conducted to evaluate the specific association between the usage of beta-blockers and delirium. The adjusted logistic model incorporated various covariates across six models. Additional analyses, adjusting for pertinent covariates, encompassed subgroup and interaction analyses. Propensity score matching (PSM) was implemented via a 1:1 nearest neighbor matching algorithm with a caliper width of 0.1 to enhance methodological rigor. PSM was used to reduce possible confounding factors that could affect the results and infer the true effect of the beta-blocker usage. Furthermore, the covariates mentioned above were selected to generate the propensity score. For deriving mortality odds ratios (OR), a multivariate logistic regression model with a robust variance estimator was employed. Linear regression analyses were conducted to investigate the relationship between beta-blocker usage and the length of ICU stay, while logistic regression analyses were conducted to assess associations between AKI and the necessity for RRT.
All statistical analyses were performed using STATA software (version 17.0), R packages (The R Foundation)1, and Free Statistics Software version 1.8 (29). Multiple imputations were conducted to handle missing values in logistic regression and model development. Statistical significance was set at p < 0.05. Two-tailed tests were employed consistently throughout the study.
3 Results
3.1 Participants
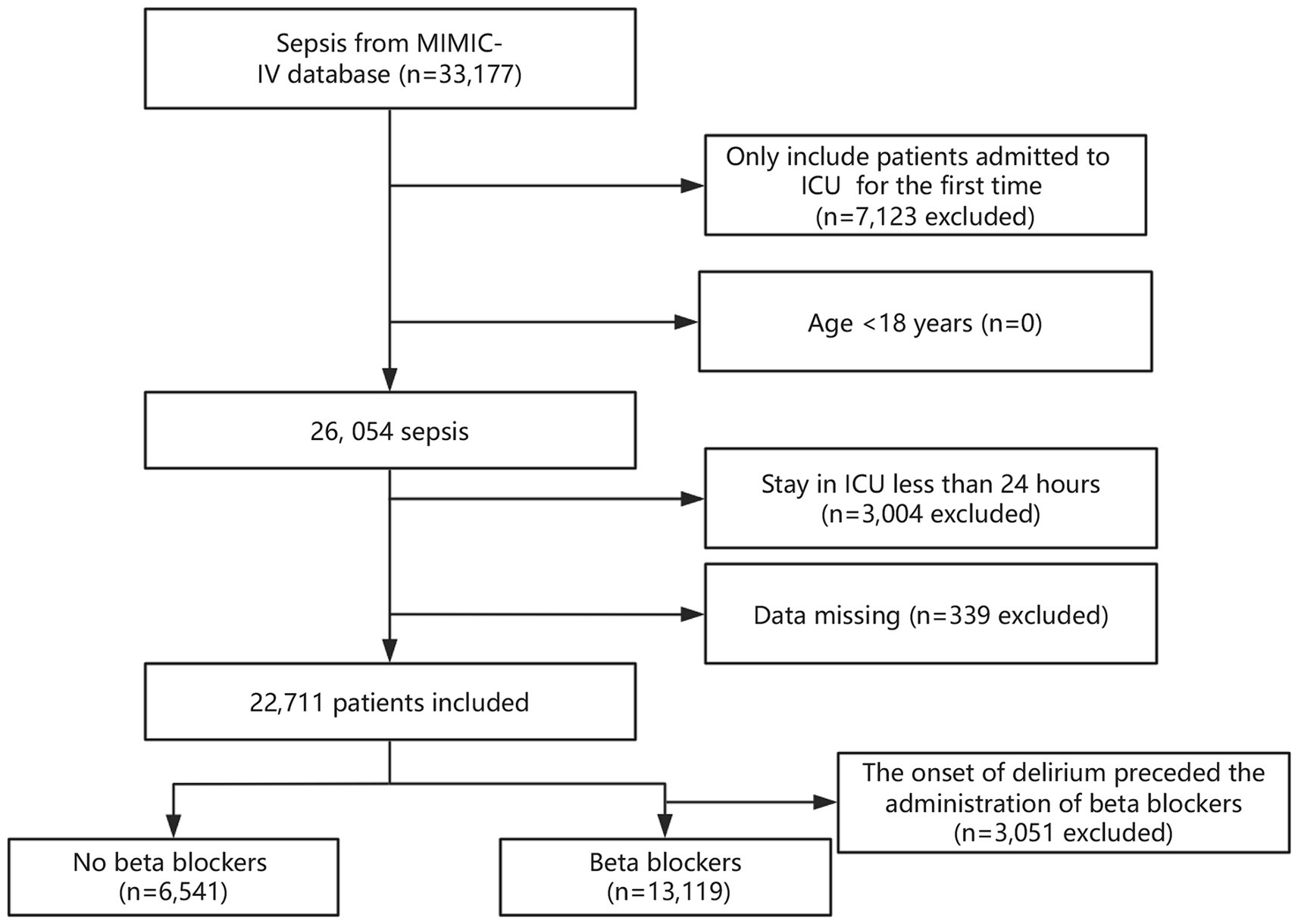
A total of 33,177 patients met the criteria for sepsis. After excluding duplicate ICU admissions, individuals under 18 years of age, and those with an ICU stay of <24 h, the final study consisted of 19,660 patients. Figure 1 depicts the details of participant selection.
3.2 Baseline characteristics
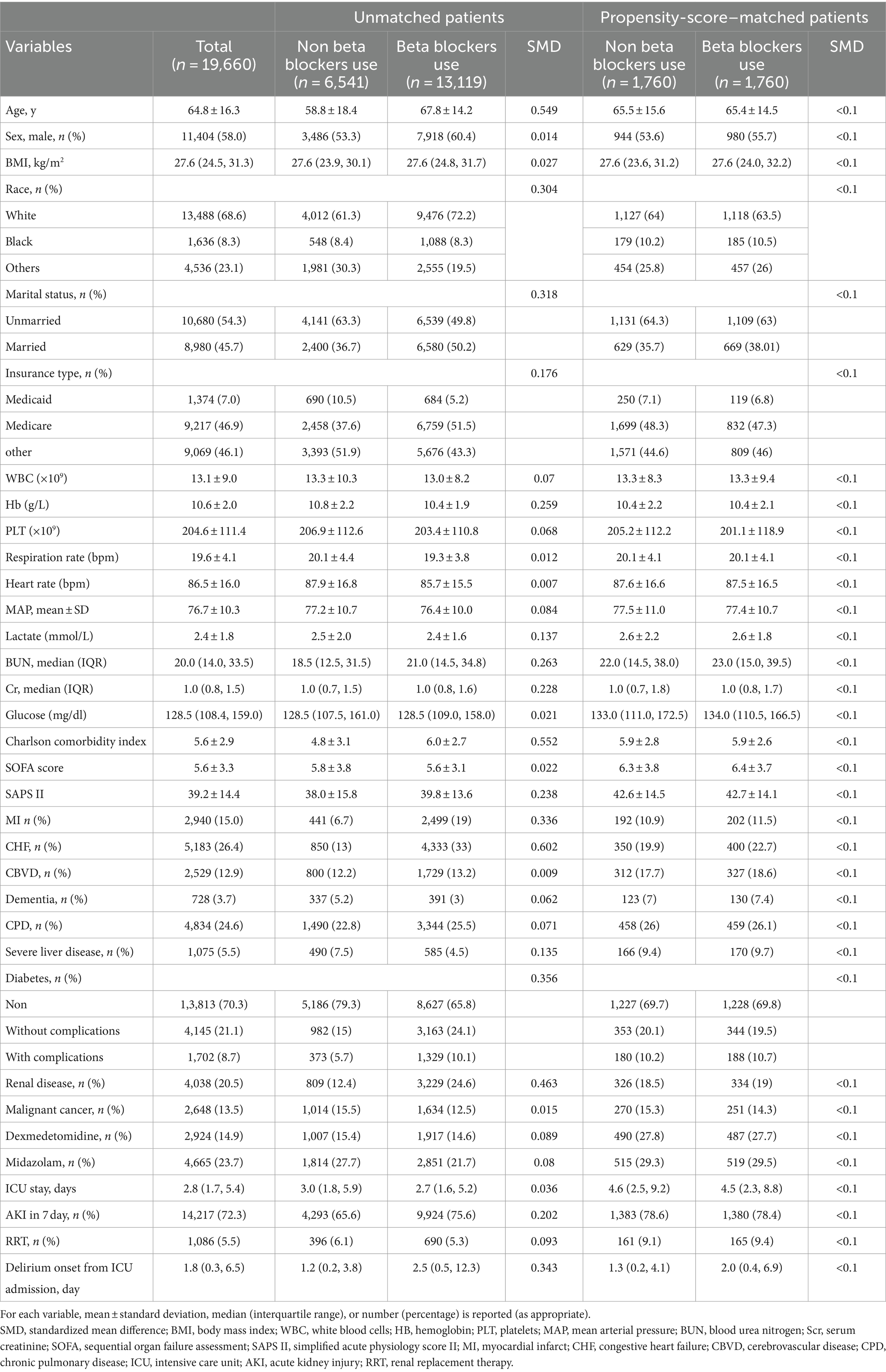
The study included 19,660 patients, 58% of whom were men, with a mean age of 64.8 ± 16.3 years. Table 1 details the baseline characteristics of the patient included. Between-group comparisons revealed notable differences: the non-beta blocker group tended to be younger, had a higher proportion of women, a lower Charlson Comorbidity Index, and higher heart rates. The pre-ICU beta-blocker was found to be less effective than the non-beta-blocker alternative. This discrepancy may be attributed to the abrupt cessation of beta blocker administration (30, 31).
3.3 Relationship between beta-blocker usage and delirium
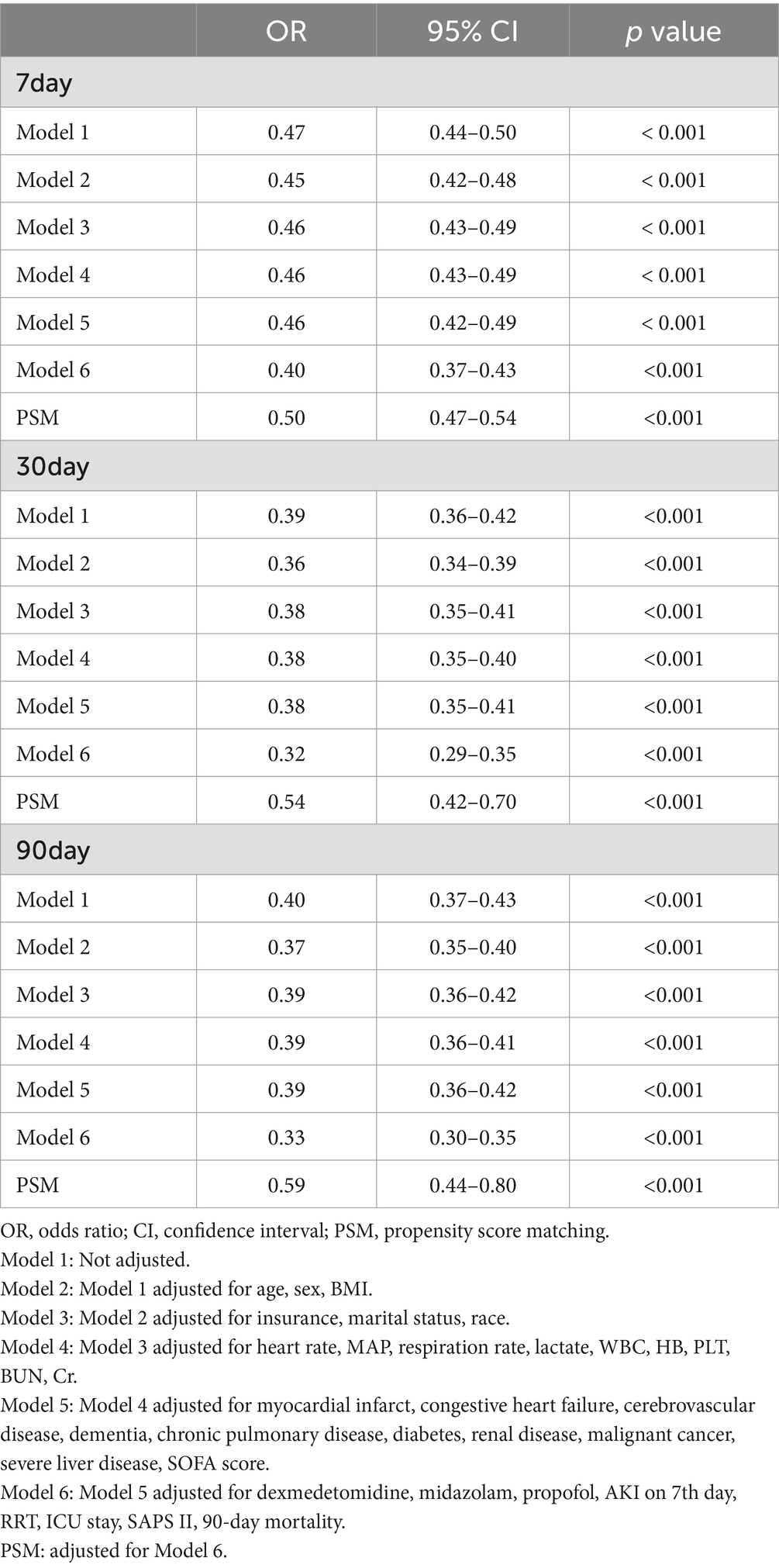
In the univariate analysis, the beta-blocker usage was significantly associated with lower delirium rates compared to those observed with non-beta blocker usage, with ORs of 0.47 (95% CI, 0.44–0.50, p < 0.001) for 7-day delirium, 0.39 (95% CI, 0.36–0.42, p < 0.001) for 30-day delirium, and 0.40 (95% CI, 0.37–0.43, p < 0.001) for 90-day delirium (Table 2). Subsequently, in the extended multivariate logistic regression analysis (Table 2), the beta-blocker usage consistently demonstrated significant ORs across all models (ranging from 0.32 to 0.47, p < 0.001 for all). Following adjustment for all covariates listed in Table 2, beta-blocker users showed a 60% reduction in the risk of 7-day delirium (OR = 0.40, 95% CI: 0.37–0.43, p < 0.001, model 6). Similarly, a 68% lower risk was observed for 30-day delirium (OR = 0.32, 95% CI: 0.29–0.35, p < 0.001, model 6) and a 67% lower risk was observed for 90-day delirium rates among beta-blocker users (OR = 0.33, 95% CI: 0.30–0.35, p < 0.001, model 6). These results highlight the robustness of the analytical models employed.
3.4 Relationship between beta-blocker usage and other outcomes
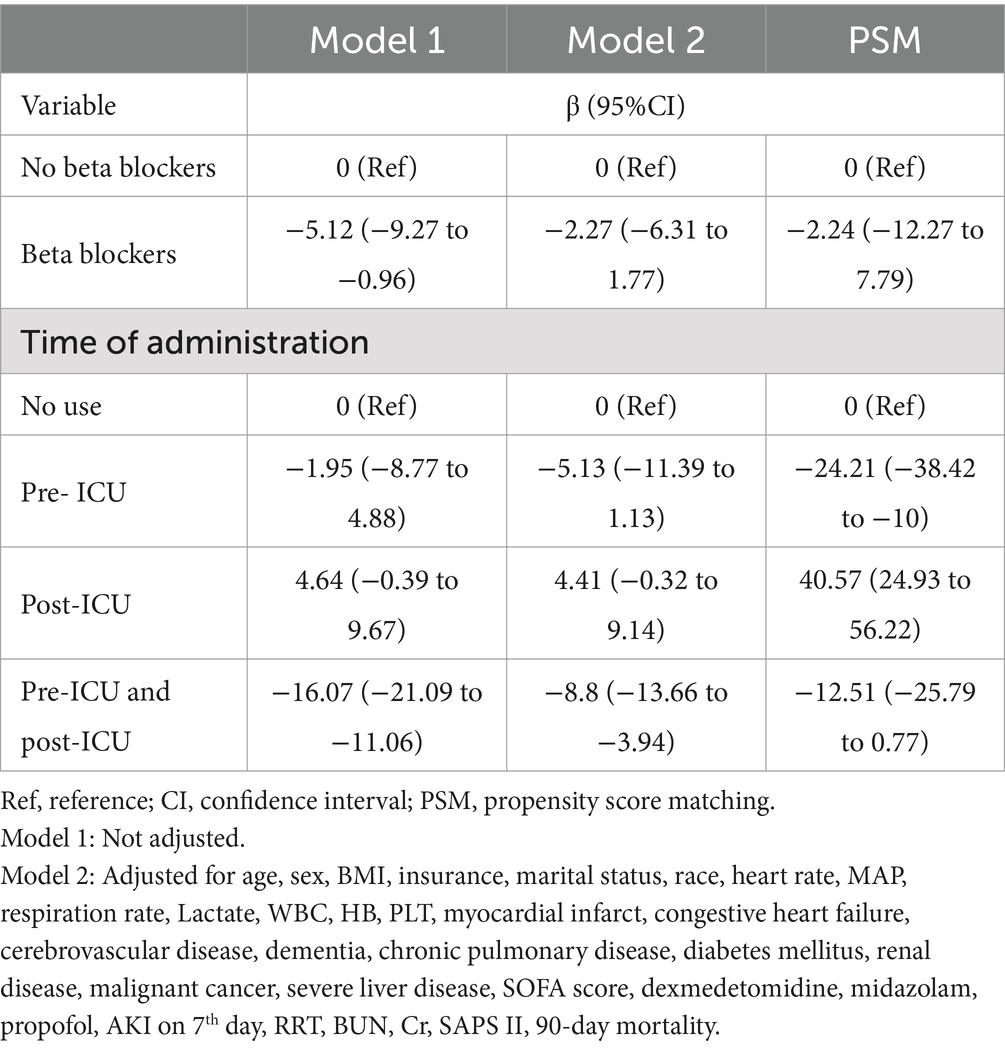
After controlling for all covariates from Tables 3, 4, the beta-blocker usage was not significantly related to the length of ICU stay (β = −2.27, 95% CI: −6.31 to 1.77). Our findings did not suggest that prolonged ICU stays can be attributed to beta-blockers (β = −2.24, 95% CI: −12.27 to 7.79) following PSM. Stratification of beta-blocker administration based on timing indicated that its initiation post-ICU admission was associated with a prolonged ICU duration, whereas pre-ICU initiation was linked to a reduction in ICU stays (Table 3).
Beta-blocker usage was associated with a 37% increase in the risk of AKI on the 7th day (OR = 1.37, 95% CI: 1.26–1.48). However, our findings did not suggest that beta-blocker usage elevates the risk of AKI (OR = 0.94, 95% CI: 0.78–1.12) after PSM. Additionally, beta-blocker usage did not correlate with an increased likelihood of requiring RRT (OR = 1.1, 95% CI: 0.92–1.31). Stratifying beta-blocker administration by timing indicated that beta-blocker usage was not associated with an increased likelihood of requiring RRT. The implementation of PSM reinforced the reliability of these findings (Table 4).
3.5 Subgroup and sensitivity analyses
Subgroup analysis confirmed the robust and consistent nature of our findings. Specifically, beta-blocker usage showed a more pronounced protective effect in individuals younger than 65 years, men, BMI ≥ 25 kg/m2 and SAPS II < 40. However, no other significant interactions were observed in subgroup analyses for 7-day delirium (P for interaction >0.05) (Figure 2). A similar trend was obtained with 30-day and 90-day delirium outcomes (Supplementary Figures S1, S2).
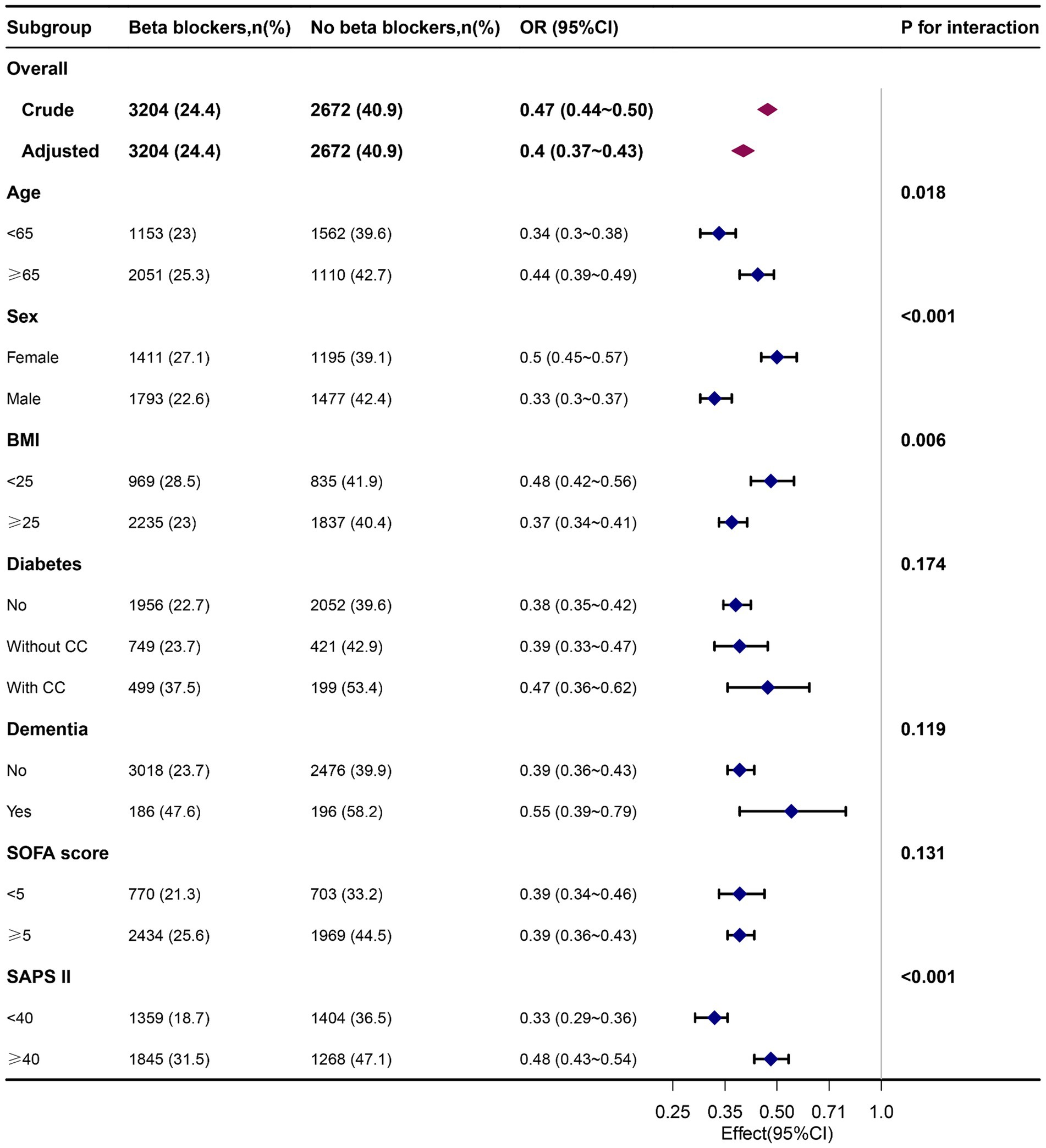
Figure 2. Associations of delirium in patients who received beta-blockers with those who did not receive them on the 7th day, by baseline characteristics. Each stratification was adjusted for all factors, excluding the stratified factor itself. OR, odd ratio; BMI, body mass index; CC, complication; SOFA, Sequential organ failure assessment.
Following PSM, the study consisted of 1,760 well-matched pairs, with no significant differences in key indicators between the two matched groups. The robustness of our findings is affirmed across logistic regression models. Specifically, among the users of beta-blockers, those initiating treatment post-ICU exhibited a lower OR (0.28, 95% CI, 0.25–0.31), whereas pre-ICU initiators showed a higher OR (1.1, 95% CI, 0.99–1.22). Patients using beta-blockers both pre- and post-ICU demonstrated a lower OR (0.55, 95% CI, 0.50–0.60), with all trends statistically significant (p < 0.001) for 7-day delirium. Similar patterns were observed for 30-day and 90-day delirium outcomes (Supplementary Table S1). A subgroup analysis of specific beta-blocker types revealed that metoprolol and atenolol consistently reduced delirium at 7, 30, and 90 days, findings that were robust across PSM analyses. However, other categories of beta-blockers did not consistently demonstrate significant effects across different time points and models (Supplementary Table S2). There are no significant differences in mortality between the groups after PSM (Supplementary Table S3).
4 Discussion
4.1 Main findings
Our study is the most comprehensive investigation to date on the impact of beta-blockers on delirium in patients with sepsis. We found that patients with sepsis who were administered beta-blockers had a lower adjusted prevalence of delirium at 7, 30, and 90 days compared to those who did not receive beta-blockers. Importantly, these results remained consistent even after adjusting for potential confounders using PSM. We did not observe any increase in the risk of AKI or need for RRT associated with beta-blocker usage, nor did we find a significant association with the length of the ICU stay. Our findings suggest a potential protective effect of beta-blockers against delirium in patients with sepsis.
4.2 Effects of beta-blocker usage on delirium in patients with sepsis
It has been suggested that beta-blockers mitigate the sepsis-induced hypermetabolic state, cardiac dysfunction, and coagulopathy (32). Our findings indicate that patients with sepsis administered beta-blockers had a decreased adjusted incidence of delirium at 7, 30, and 90 days compared to those who did not receive beta-blockers.
These findings are in contrast to those reported in previous studies. A retrospective cohort study of 490 patients, all aged ≥70 years, admitted for hip fracture revealed no link between beta-blocker administration and the onset of postoperative delirium (33). Similarly, a study of 2,648 patients who received beta-blockers within 24 h before cardiac surgery, controlled for potential confounders, did not identify an independent association between beta-blocker usage and postoperative delirium (34). In contrast, another study reported that the preoperative administration of beta-blockers increased the odds of postoperative delirium by 2.06 times among patients who underwent vascular surgery (35). Our study findings diverge from those of previous research, potentially owing to variations in the study population. Specifically, our study targeted patients with sepsis, in whom the effects of beta-blockers might differ from those in other patient cohorts. We theorize that beta-blockers may act by attenuating the inflammatory cascade response mediated by catecholamine surge and enhancing cytokine release (36–38). Previous studies have demonstrated that the administration of beta-blockers reduces the release of proinflammatory factors, such as interleukin-6 and tumor necrosis factor-α (39, 40). However, the previous studies did not address the temporal dimension of delirium or employ logistic regression analyses, unlike our study, which integrated both methodologies, thereby offering more holistic insights related to delirium. In addition, our study further categorized the administration of beta-blockers based on timing and classification.
Pre-ICU admission beta-blocker usage was associated with an increased risk of delirium, whereas post-ICU and combined pre- and post-ICU usage were linked to a decreased risk of delirium. These findings are consistent with the 2014 ACC/AHA Guidelines on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery, which advocate for the continuation of beta-blocker therapy in patients under regular preoperative treatment and its initiation in intermediate-to-high-risk patients (41).
4.3 Effects of beta-blocker usage on other outcomes for patients with sepsis
The use of beta-blockers significantly increased the risk of AKI by the 7th day (OR = 1.37, 95% CI: 1.26–1.48). However, no association was found with an increased use of RRT (OR = 1.1, 95% CI: 0.92–1.31). Importantly, our findings did not indicate that beta-blocker administration results in heightened AKI rates, increased RRT usage, or prolonged ICU stays following PSM.
Notably, the cardio-inhibitory effects of beta-blockers may compromise the cardiac compensatory reserve, which is essential for renal perfusion (42), thereby potentially precipitating renal failure. A study involving 2,972 adult recipients of living donor liver transplantation (LDLT) from January 2012 to July 2022 concluded, following PSM analyses, that there was no significant difference in the incidence of AKI between groups. Preoperative beta-blocker use did not correlate with AKI following LDLT (43). Although the impact of beta-blockers on AKI has been studied across diverse patient cohorts (44–46), the research remains limited on their influence on AKI incidence in patients with sepsis. In septic shock, esmolol-induced beta-blockade significantly enhances the pressure dependency of renal blood flow compared to renal perfusion pressure, potentially impairing renal autoregulation. Discontinuation of esmolol suggests the potential for the reversibility of this effect (47). Therefore, additional investigation is warranted to ascertain the potential contribution of beta-blockers to AKI and the need for RRT in patients with sepsis.
4.4 Strengths of our study
Our study has several major strengths. Firstly, we utilized a comprehensive and publicly accessible database, ensuring the reliability and comprehensiveness of our data. Secondly, to our knowledge, no prior research has specifically investigated the impact of beta-blocker usage on delirium risk in patients with sepsis. Our findings provide compelling and definitive evidence that beta-blocker administration significantly reduces the occurrence of delirium in this population. Thirdly, we employed multiple regression analyses and PSM to establish the robustness and credibility of our study outcomes. This rigorous analytical approach enhances the internal validity and credibility of our findings. Lastly, given the widespread use of beta-blockers for cardiovascular conditions, our results have implications and applicability beyond the septic patient population specifically.
4.5 Limitations of our study
This study represents the most comprehensive investigation to date on the use of beta-blockers in ICU patients with sepsis. However, it is subject to several limitations. A major constraint is the notable variability in beta-blocker dosages and treatment durations, potentially introducing heterogeneity into the reported data. Secondly, caution is advised in generalizing the implications of our study, as it was conducted using data from a single ICU facility in the USA. Thirdly, certain factors that could contribute to sepsis-related delirium were not accounted for in the available studies, such as the adequacy of antibiotic therapy, volume resuscitation, history of alcohol consumption, and phosphorus levels, making further analysis on these aspects unfeasible. Fourthly, due to the observational nature of this study, it did not employ the optimal methodology for assessing drug effects. As this study employed a cross-sectional design, the temporal interval between the administration of beta-blockers and the onset of delirium may have varied. Consequently, the statistical analyses may have been susceptible to the bias inherent in observational studies. Future randomized controlled trials should employ a more suitable approach. Despite these limitations, the large sample size and the use of PSM partially mitigate these constraints.
5 Conclusion
This analysis clearly demonstrates that administering beta-blockers decreases the occurrence of delirium in patients with sepsis. However, as this evidence mainly derives from non-randomized studies, there may be limitations in interpreting and applying these findings more broadly. Therefore, further research is necessary to thoroughly understand this relationship.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: all data in the article can be obtained from MlMIC-IV database (https://mimic.physionet.org/).
Ethics statement
The studies involving humans were approved by the studies involving human participants were reviewed and approved by the Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HO: Conceptualization, Data curation, Investigation, Software, Writing – original draft. XW: Formal analysis, Methodology, Writing – original draft. DD: Project administration, Validation, Writing – original draft. QW: Resources, Writing – review & editing. YY: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We express our sincere gratitude to the Free Statistics team for providing technical support and the provision of essential tools for our data analysis and visualization. Additionally, Yi Yu would like to extend a heartfelt thanks to all members of the Clinical Scientists team for their unwavering spiritual support and encouragement.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1458417/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | Associations of delirium in patients who received beta-blockers with those who did not receive them on the 30th day, by baseline characteristics. Each stratification was adjusted for all factors excluding the stratified factor itself.
SUPPLEMENTARY FIGURE S2 | Associations of delirium in patients who received beta-blockers with those who did not receive them on the 90th day by baseline characteristics. Each stratification was adjusted for all factors excluding the stratified factor itself.
Footnotes
References
1. Dubois, MJ, Bergeron, N, Dumont, M, Dial, S, and Skrobik, Y. Delirium in an intensive care unit: a study of risk factors. Intensive Care Med. (2001) 27:1297–304. doi: 10.1007/s001340101017
2. Ely, EW, Inouye, SK, Bernard, GR, Gordon, S, Francis, J, May, L, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (cam-Icu). JAMA. (2001) 286:2703–10. doi: 10.1001/jama.286.21.2703
3. Pandharipande, PP, Girard, TD, Jackson, JC, Morandi, A, Thompson, JL, Pun, BT, et al. Long-term cognitive impairment after critical illness. N Engl J Med. (2013) 369:1306–16. doi: 10.1056/NEJMoa1301372
4. Pisani, MA, Murphy, TE, Van Ness, PH, Araujo, KL, and Inouye, SK. Characteristics associated with delirium in older patients in a medical intensive care unit. Arch Intern Med. (2007) 167:1629–34. doi: 10.1001/archinte.167.15.1629
5. Pisani, MA, Kong, SY, Kasl, SV, Murphy, TE, Araujo, KL, and Van Ness, PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. (2009) 180:1092–7. doi: 10.1164/rccm.200904-0537OC
6. Lin, SM, Liu, CY, Wang, CH, Lin, HC, Huang, CD, Huang, PY, et al. The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med. (2004) 32:2254–9. doi: 10.1097/01.ccm.0000145587.16421.bb
7. Ely, EW, Shintani, A, Truman, B, Speroff, T, Gordon, SM, Harrell, FE Jr, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. (2004) 291:1753–62. doi: 10.1001/jama.291.14.1753
8. Sakusic, A, O'Horo, JC, Dziadzko, M, Volha, D, Ali, R, Singh, TD, et al. Potentially modifiable risk factors for long-term cognitive impairment after critical illness: a systematic review. Mayo Clin Proc. (2018) 93:68–82. doi: 10.1016/j.mayocp.2017.11.005
9. Wolters, AE, van Dijk, D, Pasma, W, Cremer, OL, Looije, MF, de Lange, DW, et al. Long-term outcome of delirium during intensive care unit stay in survivors of critical illness: a prospective cohort study. Crit Care. (2014) 18:R125. doi: 10.1186/cc13929
10. Girard, TD, Jackson, JC, Pandharipande, PP, Pun, BT, Thompson, JL, Shintani, AK, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. (2010) 38:1513–20. doi: 10.1097/CCM.0b013e3181e47be1
11. Devlin, JW, Skrobik, Y, Gelinas, C, Needham, DM, Slooter, AJC, Pandharipande, PP, et al. Clinical practice guidelines for the prevention and Management of Pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the Icu. Crit Care Med. (2018) 46:e825–73. doi: 10.1097/CCM.0000000000003299
12. Inouye, SK, Westendorp, RG, and Saczynski, JS. Delirium in elderly people. Lancet. (2014) 383:911–22. doi: 10.1016/S0140-6736(13)60688-1
13. van Gool, WA, van de Beek, D, and Eikelenboom, P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet. (2010) 375:773–5. doi: 10.1016/S0140-6736(09)61158-2
14. Atterton, B, Paulino, MC, Povoa, P, and Martin-Loeches, I. Sepsis associated delirium. Medicina (Kaunas). (2020) 56:5. doi: 10.3390/medicina56050240
15. Angus, DC, and van der Poll, T. Severe Sepsis and septic shock. N Engl J Med. (2013) 369:840–51. doi: 10.1056/NEJMra1208623
16. Sonneville, R, de Montmollin, E, Poujade, J, Garrouste-Orgeas, M, Souweine, B, Darmon, M, et al. Potentially modifiable factors contributing to Sepsis-associated encephalopathy. Intensive Care Med. (2017) 43:1075–84. doi: 10.1007/s00134-017-4807-z
17. Singer, M, Deutschman, CS, Seymour, CW, Shankar-Hari, M, Annane, D, Bauer, M, et al. The third international consensus definitions for Sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
18. Salluh, JI, Wang, H, Schneider, EB, Nagaraja, N, Yenokyan, G, Damluji, A, et al. Outcome of delirium in critically ill patients: systematic review and Meta-analysis. BMJ. (2015) 350:h2538. doi: 10.1136/bmj.h2538
19. Maclullich, AM, Anand, A, Davis, DH, Jackson, T, Barugh, AJ, Hall, RJ, et al. New horizons in the pathogenesis, assessment and Management of Delirium. Age Ageing. (2013) 42:667–74. doi: 10.1093/ageing/aft148
20. Li, J, Sun, W, Guo, Y, Ren, Y, Li, Y, and Yang, Z. Prognosis of Beta-adrenergic blockade therapy on septic shock and Sepsis: a systematic review and Meta-analysis of randomized controlled studies. Cytokine. (2020) 126:154916. doi: 10.1016/j.cyto.2019.154916
21. van Loon, LM, van der Hoeven, JG, and Lemson, J. Hemodynamic response to Beta-blockers in severe Sepsis and septic shock: a review of current literature. J Crit Care. (2019) 50:138–43. doi: 10.1016/j.jcrc.2018.12.003
22. Chacko, CJ, and Gopal, S. Systematic review of use of Beta-blockers in Sepsis. J Anaesthesiol Clin Pharmacol. (2015) 31:460–5. doi: 10.4103/0970-9185.169063
23. Sanfilippo, F, Santonocito, C, Morelli, A, and Foex, P. Beta-blocker use in severe Sepsis and septic shock: a systematic review. Curr Med Res Opin. (2015) 31:1817–25. doi: 10.1185/03007995.2015.1062357
24. Giesa, N, Heeren, P, Klopfenstein, S, Flint, A, Agha-Mir-Salim, L, Poncette, A, et al. Mimic-iv as a clinical data Schema. Stud Health Technol Inform. (2022) 294:559–60. doi: 10.3233/SHTI220522
25. von Elm, E, Altman, DG, Egger, M, Pocock, SJ, Gotzsche, PC, Vandenbroucke, JP, et al. The strengthening the reporting of observational studies in epidemiology (strobe) statement: guidelines for reporting observational studies. Lancet. (2007) 370:1453–7. doi: 10.1016/S0140-6736(07)61602-X
26. Gusmao-Flores, D, Salluh, JI, Chalhub, RA, and Quarantini, LC. The confusion assessment method for the intensive care unit (cam-Icu) and intensive care delirium screening checklist (Icdsc) for the diagnosis of delirium: a systematic review and Meta-analysis of clinical studies. Crit Care. (2012) 16:R115. doi: 10.1186/cc11407
27. Hernandez-Quiles, R, Merino-Lucas, E, Boix, V, Fernandez-Gil, A, Rodriguez-Diaz, JC, Gimeno, A, et al. Bacteraemia and quick Sepsis related organ failure assessment (Qsofa) are independent risk factors for long-term mortality in very elderly patients with suspected infection: retrospective cohort study. BMC Infect Dis. (2022) 22:248. doi: 10.1186/s12879-022-07242-4
28. Shankar-Hari, M, Harrison, DA, Ferrando-Vivas, P, Rubenfeld, GD, and Rowan, K. Risk factors at index hospitalization associated with longer-term mortality in adult Sepsis survivors. JAMA Netw Open. (2019) 2:e194900. doi: 10.1001/jamanetworkopen.2019.4900
29. Yu, Y, Yang, D, Wang, Q, and Li, J. Association between pre-Icu aspirin administration and Ards mortality in the mimic-iv database: a cohort study. Pulm Pharmacol Ther. (2024) 85:102288. doi: 10.1016/j.pupt.2024.102288
30. Morimoto, S, Shimizu, K, Yamada, K, Hiramitsu, S, and Hishida, H. Can Beta-blocker therapy be withdrawn from patients with dilated cardiomyopathy? Am Heart J. (1999) 138:456–9. doi: 10.1016/s0002-8703(99)70147-x
31. Prichard, BN, Tomlinson, B, Walden, RJ, and Bhattacharjee, P. The Beta-adrenergic blockade withdrawal phenomenon. J Cardiovasc Pharmacol. (1983) 5:S56–62. doi: 10.1097/00005344-198300051-00009
32. Suzuki, T, Suzuki, Y, Okuda, J, Kurazumi, T, Suhara, T, Ueda, T, et al. Sepsis-induced cardiac dysfunction and Beta-adrenergic blockade therapy for Sepsis. J Intensive Care. (2017) 5:22. doi: 10.1186/s40560-017-0215-2
33. Genet, B, Lamy, T, Cohen-Bittan, J, Glasman, P, Verny, M, Riou, B, et al. Lack of association between perioperative medication and postoperative delirium in hip fracture patients in an Orthogeriatric care pathway. J Am Med Dir Assoc. (2022) 23:623–630.e2. doi: 10.1016/j.jamda.2021.09.022
34. O'Neal, JB, Billings, F IV, Liu, X, Shotwell, M, Liang, Y, Shah, A, et al. Effect of preoperative Beta-blocker use on outcomes following cardiac surgery. Am J Cardiol. (2017) 120:1293–7. doi: 10.1016/j.amjcard.2017.07.012
35. Katznelson, R, Djaiani, G, Mitsakakis, N, Lindsay, TF, Tait, G, Friedman, Z, et al. Delirium following vascular surgery: increased incidence with preoperative Beta-blocker administration. Can J Anaesth. (2009) 56:793–801. doi: 10.1007/s12630-009-9148-0
36. Contenti, J, Occelli, C, Corraze, H, Lemoel, F, and Levraut, J. Long-term Beta-blocker therapy decreases blood lactate concentration in severely septic patients. Crit Care Med. (2015) 43:2616–22. doi: 10.1097/CCM.0000000000001308
37. Ince, C. To Beta block or not to Beta block; that is the question. Crit Care. (2015) 19:339. doi: 10.1186/s13054-015-1059-6
38. de Montmollin, E, Aboab, J, Mansart, A, and Annane, D. Bench-to-bedside review: Beta-adrenergic modulation in Sepsis. Crit Care. (2009) 13:230. doi: 10.1186/cc8026
39. Rough, J, Engdahl, R, Opperman, K, Yerrum, S, Monroy, MA, and Daly, JM. Beta2 Adrenoreceptor blockade attenuates the Hyperinflammatory response induced by traumatic injury. Surgery. (2009) 145:235–42. doi: 10.1016/j.surg.2008.09.013
40. Friese, RS, Barber, R, McBride, D, Bender, J, and Gentilello, LM. Could Beta blockade improve outcome after injury by modulating inflammatory profiles? J Trauma. (2008) 64:1061–8. doi: 10.1097/TA.0b013e3181684cf0
41. Fleisher, LA, Fleischmann, KE, Auerbach, AD, Barnason, SA, Beckman, JA, Bozkurt, B, et al. 2014 Acc/Aha guideline on perioperative cardiovascular evaluation and Management of Patients Undergoing Noncardiac Surgery: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. (2014) 64:e77–e137. doi: 10.1016/j.jacc.2014.07.944
42. Krag, A, Wiest, R, Albillos, A, and Gluud, LL. The window hypothesis: Haemodynamic and non-Haemodynamic effects of Beta-blockers improve survival of patients with cirrhosis during a window in the disease. Gut. (2012) 61:967–9. doi: 10.1136/gutjnl-2011-301348
43. Jeong, HW, Kim, JH, Han, SB, Kwon, HM, Jun, IG, Song, JG, et al. Impact of preoperative nonselective Beta-blocker use on acute kidney injury after living donor liver transplantation: propensity score analysis. Ann Hepatol. (2024) 29:101474. doi: 10.1016/j.aohep.2024.101474
44. Scheiner, B, Parada-Rodriguez, D, Bucsics, T, Schwabl, P, Mandorfer, M, Pfisterer, N, et al. Non-selective Beta-blocker treatment does not impact on kidney function in cirrhotic patients with varices. Scand J Gastroenterol. (2017) 52:1008–15. doi: 10.1080/00365521.2017.1329456
45. Kim, SG, Larson, JJ, Lee, JS, Therneau, TM, and Kim, WR. Beneficial and harmful effects of nonselective Beta blockade on acute kidney injury in liver transplant candidates. Liver Transpl. (2017) 23:733–40. doi: 10.1002/lt.24744
46. Serste, T, Njimi, H, Degre, D, Deltenre, P, Schreiber, J, Lepida, A, et al. The use of Beta-blockers is associated with the occurrence of acute kidney injury in severe alcoholic hepatitis. Liver Int. (2015) 35:1974–82. doi: 10.1111/liv.12786
Keywords: beta-blockers, delirium, sepsis, acute kidney injury, ICU stay, MIMIC-IV
Citation: Ouyang H, Wang X, Deng D, Wang Q and Yu Y (2024) Impact of beta-blocker usage on delirium in patients with sepsis in ICU: a cross-sectional study. Front. Med. 11:1458417. doi: 10.3389/fmed.2024.1458417
Edited by:
Hiroyoshi Takeuchi, Keio University, JapanReviewed by:
Junji Shiotsuka, Toronto General Hospital, CanadaNozomi Takahashi, University of British Columbia, Canada
Copyright © 2024 Ouyang, Wang, Deng, Wang and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Yu, eXV5aTI3QGd6dWNtLmVkdS5jbg==; Qianqian Wang, d2FuZ3FxNTNAbWFpbC5zeXN1LmVkdS5jbg==
†These authors have contributed equally to this work
 Honglian Ouyang1†
Honglian Ouyang1† Yi Yu
Yi Yu