- 1Faculty of Medicine, Bogomolets National Medical University (NMU), Kyiv, Ukraine
- 2Faculty of Medicine, Islamic Azad University, Tehran Medical Sciences Branch, Tehran, Iran
- 3Faculty of Medicine, Tehran University of Medical Science (TUMS), Tehran, Iran
- 4Faculty of Medicine, Hamadan University of Medical Science (UMSHA), Hamadan, Iran
- 5Department of Biological Sciences in Sport, Faculty of Sport Sciences and Health, Shahid Beheshti University, Tehran, Iran
Background: Chronic kidney disease (CKD) affects 10% of the global population and leads to end-stage renal disease (ESRD). Hemodialysis is a common treatment for ESRD, but patients often have low carnitine levels, leading to dyslipidemia, a risk factor for cardiovascular disease and the leading cause of mortality. This study aimed to assess the effects of L-carnitine on lipid profiles in adult hemodialysis patients.
Methods: A comprehensive search was conducted across the online databases from inception to June 2024 to identify randomized clinical trials (RCTs) evaluating the effects of L-carnitine on lipid profiles in hemodialysis patients. Data extraction and quality assessment were performed, focusing on primary outcomes, including changes in triglycerides (TG), total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and very low-density lipoprotein (VLDL), and secondary outcomes including blood pressure (BP) and body mass index (BMI).
Results: A total of 28 RCTs were eligible for the current systematic review, including 1,340 hemodialysis patients (671 intervention, 669 control). There were no significant differences in the mean change of TG (SMD: −0.006; 95% CI, −0.272 to 0.259; P = 0.95), TC (SMD: −0.086; 95% CI, −0.253 to −0.079; P = 0.29), HDL (SMD: 0.060; 95% CI, −0.057 to 0.177; P = 0.29), LDL (SMD: −0.075; 95% CI, −0.274 to 0.123; P = 0.43), VLDL (SMD: −0.064; 95% CI, −0.272 to 0.142; P = 0.51), BMI (SMD: −0.025; 95% CI, −0.139 to 0.088; P = 0.56), systolic BP (SMD: 0.055; 95% CI, −0.110 to 0.220; P = 0.43), and diastolic BP (SMD: −0.028; 95% CI, 0.156 to 0.099; P = 0.56). The same insignificant findings were observed after conducting a subgroup analysis based on the route of administration (intravenous vs. Oral).
Conclusion: L-carnitine supplementation does not significantly change and improve the serum lipid profile, including TG, TC, HDL, LDL, and VLDL levels. Additionally, it has no notable effects on BMI, systolic, or diastolic BP.
1 Introduction
Chronic kidney disease (CKD) is a progressive loss of kidney function and a permanent clinical syndrome that is known by the kidney’s failure to filter waste products and remove excessive fluid from the body (1). CKD is a prevalent condition that affects about 10% of the global population (2). CKD can progress to end-stage renal disease (ESRD), which is a considerable cause of reduced quality of life and premature mortality (3, 4). Although the overall mortality rate among the ESRD population has been improving over time, the mortality rate remains relatively high (up to 30%) within the initial year after transitioning from CKD to ESRD (5, 6). Hemodialysis (HD) is the most prevalent type of kidney replacement therapy globally and is known as a standard therapeutic option in patients with ESRD (7). Patients undergoing hemodialysis often have dyslipidemia, a known risk factor for cardiovascular disease (CVD) and the primary cause of death among HD patients (8, 9).
L-carnitine (LC) is a naturally occurring compound that plays a role in the metabolism of fatty acids. LC transports long-chain fatty acids into the mitochondrial matrix for energy conversion through β-oxidation, enabling cells to break down fat for stored energy (10). It also depletes acyl groups from mitochondria in tissues and improves adipokine concentration, potentially improving lipid profile and preventing related diseases (11–13). Patients undergoing dialysis frequently have deficiencies in carnitine. Serum-free carnitine levels in hemodialysis patients (HD) are much lower than in the general population (14). Carnitine deficiency contributes to the development of various pathological conditions, such as cardiac dysfunction, muscle weakness, and erythropoietin-resistant anemia in patients undergoing hemodialysis (15).
Numerous randomized clinical trials (RCTs) have been conducted to evaluate the efficacy of LC on lipid profile in patients undergoing HD; however, the contradictions between the reports are evident. Among them, some studies concluded that LC has a promising effect on lipid profile (16), whereas, others did not find acceptable evidence for the relationship between LC and the improvement of dyslipidemia in HD patients (17).
Research indicates that hemodialysis patients often suffer from carnitine deficiency (18–20). However, while several studies have explored the effects of LC in healthy populations, there is a lack of evidence regarding its impact on hemodialysis patients specifically. Given these discrepancies in the literature, this study aims to evaluate the effects of LC supplementation on the lipid profiles of hemodialysis patients by analyzing data from randomized controlled trials (RCTs). This review seeks to determine whether LC can be recommended as a therapeutic approach for managing dyslipidemia and improving cardiovascular function and overall health in this population.
2 Methods
This investigation was conducted according to the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (21).
The protocol for this systematic review has been registered with PROSPERO (the International Prospective Register of Systematic Reviews) under the registration number CRD42024555147.
2.1 Search strategy
A systematic search was conducted across PubMed, Web of Science, Scopus, and Embase databases, covering all available records from their inception to May 2024. The aim was to identify RCTs evaluating the effects of LC supplementation on the lipid profile of patients undergoing hemodialysis. The following MeSH and related search terms were used: (“L-Carnitine” OR Carnitine OR Levocarnitine OR Bicarnesine OR “Vitamin BT” OR “Acetate Free Biofiltration” AND “lipid profile” OR “blood lipid” OR “plasma lipid” OR “blood fat” OR Lipemia OR “Lipidemia OR Hyperlipemia OR Hyperlipidemia OR Hypolipemia OR Hypolipidemia OR Cholesterol OR Triglyceride OR Triacylglycerol OR lipoprotein OR lipoproteinemia OR HDL OR “high-density lipoprotein” OR “LDL” OR “low-density lipoprotein”) AND (Dialysis OR Hemodialysis OR Hemodialysis OR hemodiafiltration OR hemofiltration OR “renal replacement therapy” OR “kidney failure” OR “renal failure” OR “dialysis solutions” OR “chronic kidney disease” OR “CKD” OR “chronic renal disease” OR “CRD” OR “end-stage renal disease” OR “ESRD” OR “end-stage kidney disease” OR “ESKD”). In addition to electronic database searches, manual searches were performed using Google Scholar, and the reference lists of all relevant primary and secondary sources were screened to identify additional eligible studies and gray literature.
Two independent reviewers (S.R. and N.SH.) screened and assessed all retrieved studies for eligibility based on predefined inclusion and exclusion criteria. Any disagreements were resolved through consultation with a third reviewer (M.K.), who provided a final assessment to ensure the accuracy and completeness of the selected studies.
2.2 Inclusion and exclusion criteria
Two reviewers (M.K. & S.R.) assessed the retrieved articles separately to determine their eligibility. The framework for eligibility criteria in this systematic review study was formulated according to the Population, Intervention, Comparison, and Outcomes (PICO) criteria (22) (Table 1). Inclusion criteria for this systematic review and meta-analysis were as follows: (1) randomized controlled trials (RCTs) that investigated the effects of LC supplementation on lipid profiles in adult patients undergoing hemodialysis; (2) studies that reported specific outcomes related to lipid profiles, including total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and very low-density lipoprotein (VLDL); (3) studies published in peer-reviewed journals; and (4) studies published in English from inception to June 2024.
Exclusion criteria included: (1) studies involving populations other than adult patients undergoing hemodialysis; (2) non-randomized trials, observational studies, case reports, or reviews; (3) studies that did not include a control group; (4) trials that did not report relevant lipid profile outcomes; and (5) studies that assessed LC in combination with other interventions without a clear distinction of effects. These criteria ensured a focused analysis of the available evidence regarding the efficacy of LC supplementation on lipid profiles in the specified population.
2.3 Data extraction
Data extraction was performed independently by two reviewers using a standardized form to ensure consistency and accuracy. Key information extracted from each included study encompassed study characteristics (first author, year of publication, study design, sample size, and participant demographics), intervention details (dosage, route of administration, treatment duration, and control conditions), and primary outcomes related to lipid profiles, specifically TC, TG, HDL, LDL, and VLDL. Secondary outcomes, such as body mass index (BMI) and blood pressure (BP), were also documented where available. Data on study quality indicators, including risk of bias (e.g., random sequence generation, allocation concealment, blinding), were also collected to facilitate further analysis. Discrepancies between reviewers (S.P. & N.SH.) were resolved through discussion, with a third reviewer (M.K.) consulted if necessary, and the extracted data were compiled for analysis to evaluate the effects of LC supplementation on the lipid profiles of hemodialysis patients.
2.4 Quality assessment
The quality of RCTs was assessed for bias using the Cochrane risk of bias (RoB-1). Each study was rated as having low, some concerns, or high risk in various domains, including random sequence generation, allocation concealment, selective reporting, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and other biases (23, 24). Any disagreements were resolved through consensus.
2.5 Statistical analysis
The mean change and SD between baseline and last follow-up for TG, TC, HDL, LDL, VLDL, BMI, systolic BP, and diastolic BP in the intervention and control arms were extracted. The standardized mean difference (SMD) and 95% confidence interval (CI) used to compare the effect size (25). Some studies reported median and interquartile range (IQR) or median and range of variables. Luo et al.’s (26) and Wan et al.’s (27) methods were utilized to convert that report into mean and SD. Once the SD of the mean change was not reported directly, the following formula was used: SD change = square root [(SD baseline2 + SD final2) – (2 × 0.5 × SD baseline × SD final)] (28). Our meta-analyses employed a random-effects model using restricted maximum likelihood estimation. The between-study heterogeneity was assessed using Cochrane’s Q statistic and Hedges’ g I2 estimation (29). In the current analysis, we classified I2 values less than 25% as low heterogeneity, values between 25 and 50% as moderate heterogeneity, and values exceeding 50% as high heterogeneity. We also performed subgroup analysis based on the route of administration (RoA; IV vs Oral). Visual and statistical assessments using funnel plots and Begg’s and Egger’s tests were performed regarding the risk of publication bias (30, 31). Meta-regression analysis was conducted for variables reported in more than ten articles, including the year of publication, dosage of L-carnitine, and duration of treatment. All analyses were performed using R Statistical Software [v4.1.2; R Core Team (32)].
3 Results
3.1 Study selection
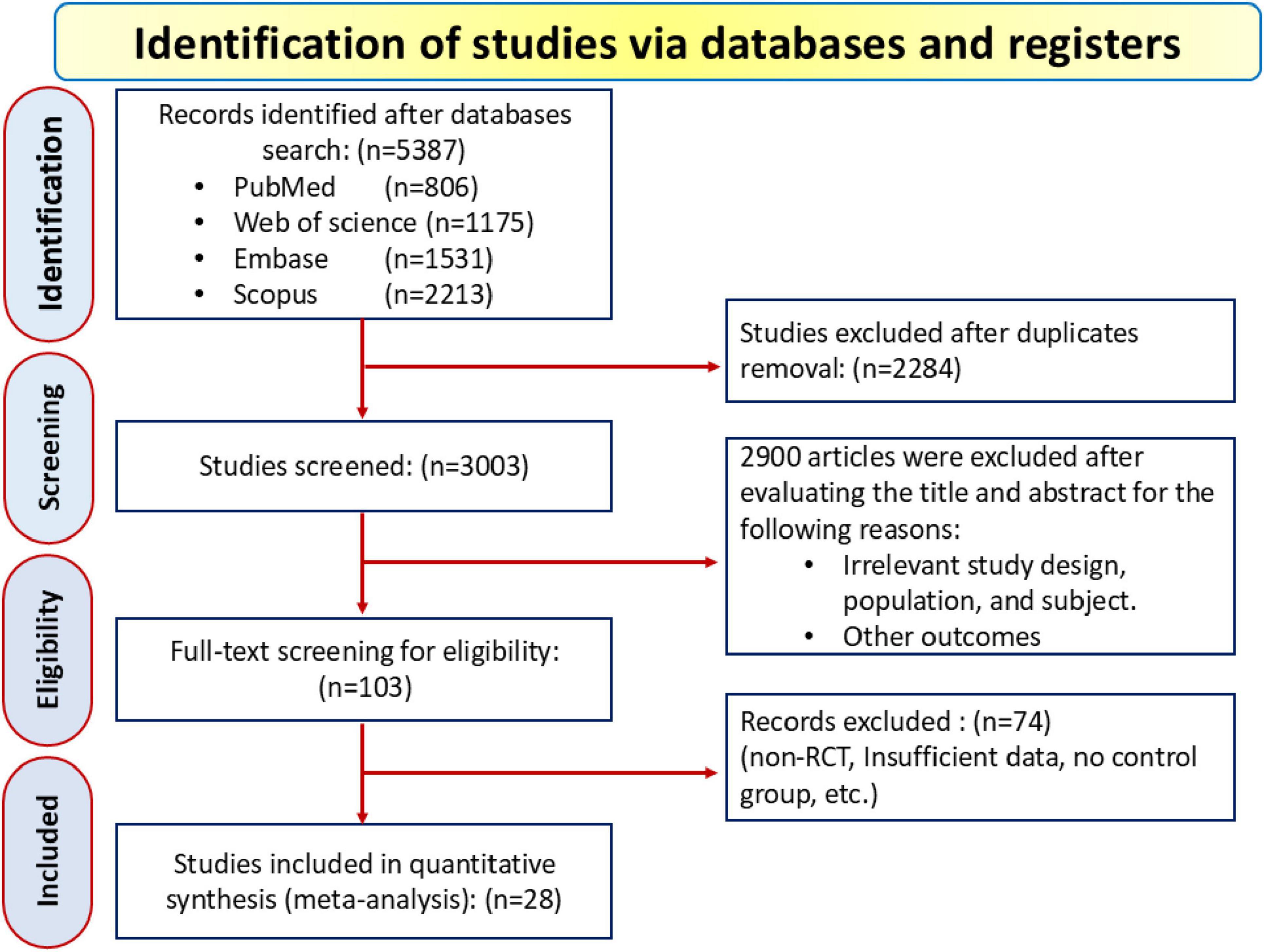
A total of 5,387 records were initially identified—806 from PubMed, 1,531 from Embase, 1,175 from Web of Science, and 2,213 from Scopus—and after removing 2,284 duplicates, 3,003 studies were screened based on title and abstract. During this process, 2,900 records were excluded for reasons including irrelevant study designs (e.g., observational studies, case reports), non-hemodialysis patients, lack of focus on LC supplementation, or absence of relevant lipid profile outcomes. This left 103 eligible articles for full-text review, with 28 RCT ultimately included in the final analysis, as depicted in the PRISMA flow diagram in Figure 1.
3.2 Study characteristics
Table 2 details the included studies. All the RCT included in the study were published between 1980 and 2024. A total of 1,340 patients under hemodialysis treatment were studied, including 671 cases in the intervention arm and 669 subjects in the control arm. The most common etiology of hemodialysis was ESRD (17 studies), followed by CRF (8 studies) and Uremic conditions (1 study). The etiology was not reported in 2 articles. The duration of the included articles ranged from 5 to 48 weeks, and the sample size ranged from 10 to 148 cases.
3.3 Risk of bias in studies
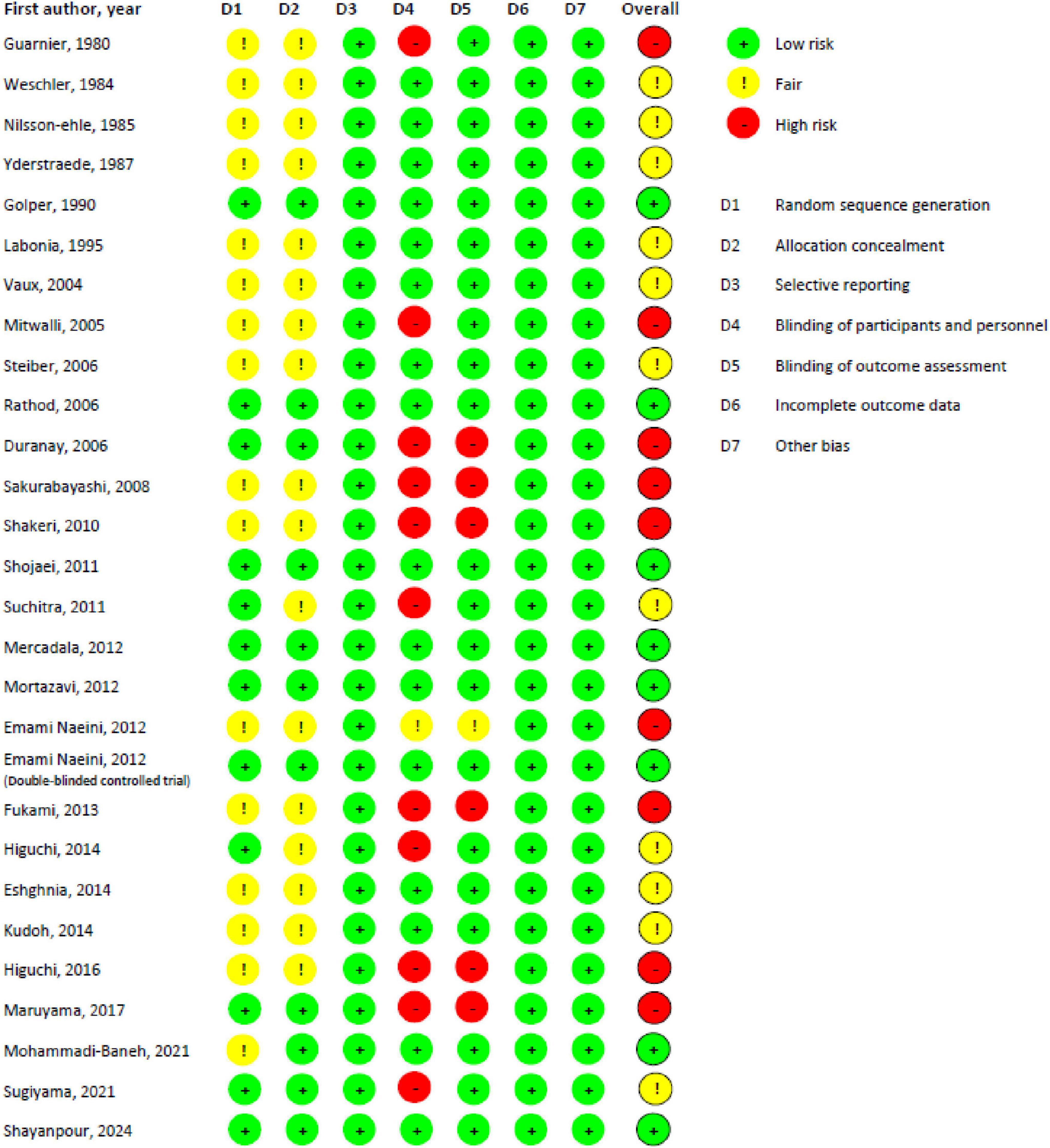
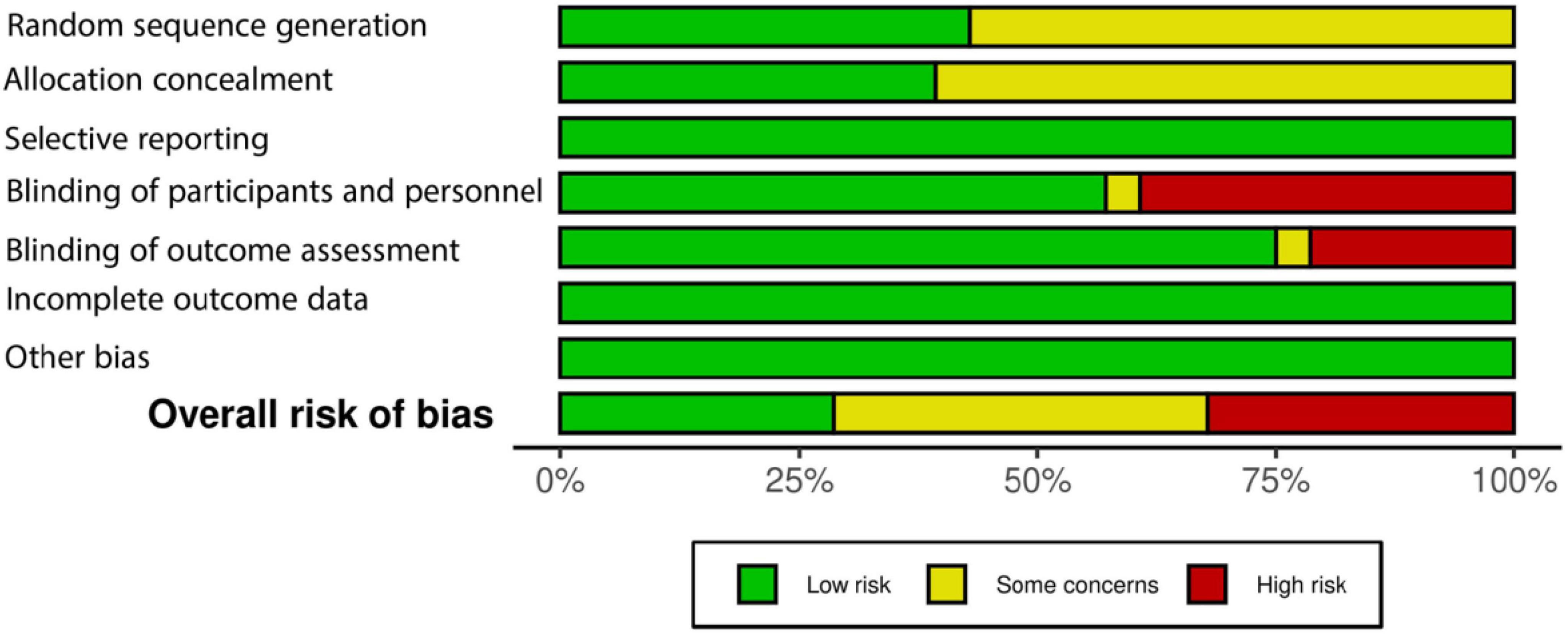
Figures 2, 3 present the risk of bias assessment results. Eleven studies contained some concerns about the risk of bias. Nine articles were classified as high risk, and eight studies as low risk of biased papers.
3.4 Effect of L-carnitine on triglycerides (TG)
Twenty-four articles were included that compared the efficacy of LC on TG levels versus placebo. Meta-analysis of these studies demonstrated (Figure 4) that there was no significant difference in the mean changes (follow-up from baseline) between groups (SMD, −0.006; 95% CI, −0.272 to 0.259; P = 0.95), and this analysis had a high heterogeneity (I2 = 73.5%). Additionally, there was no significant (P = 0.26) between-group difference in subgroup analysis based on the RoA (IV vs. oral). Furthermore, none of these two subgroups showed significant differences (Table 3) in the mean changes (IV: SMD, 0.12; 95% CI, −0.28 to 0.52; Oral: SMD, −0.01; 95% CI, −0.27 to 0.26), while heterogeneity remained high for both subgroups (75% and 65%, respectively). Further subgroup analyses based on the dosage and duration of treatment also demonstrated no significant SMD in any subgroups (Supplementary Figure 1).
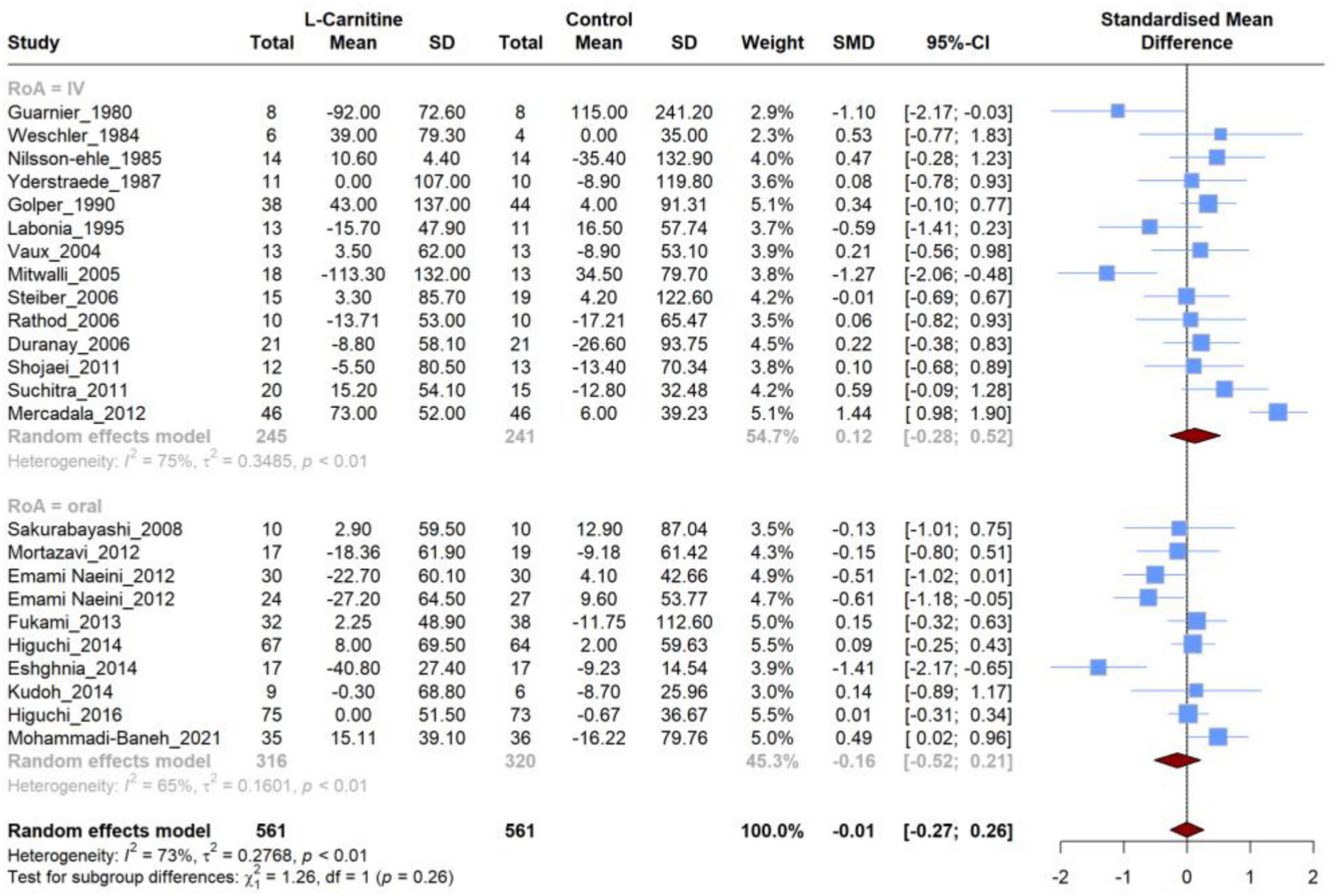
Figure 4. Forest plot for meta-analysis of triglycerides (TG) mean change in L-carnitine groups versus control groups.
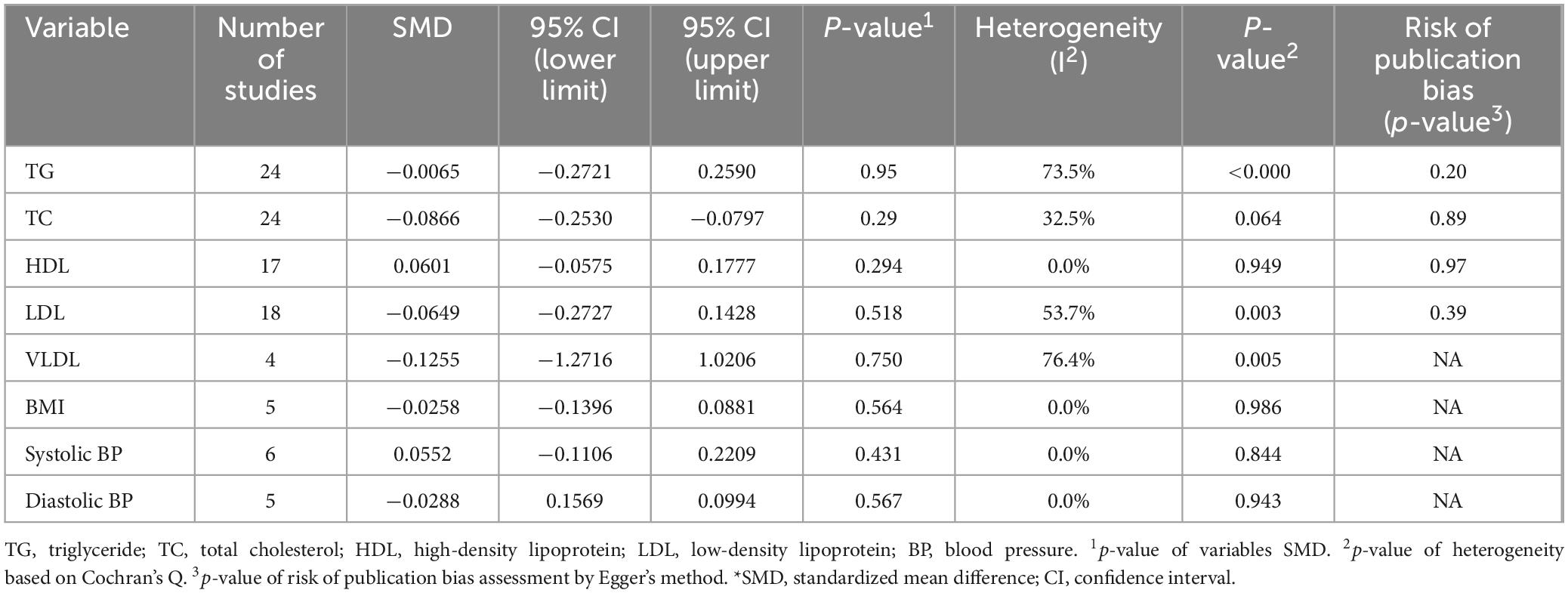
Table 3. Summary effects of L-carnitine and subgroup analysis on the route of L-carnitine supplementation on outcomes of interest among hemodialysis patients.
A meta-regression analysis was performed using several continuous variables, such as publication year, dosage, and treatment duration. Table 4 demonstrates the detailed result of this analysis. None of the variables were significantly associated with pooled effect size.
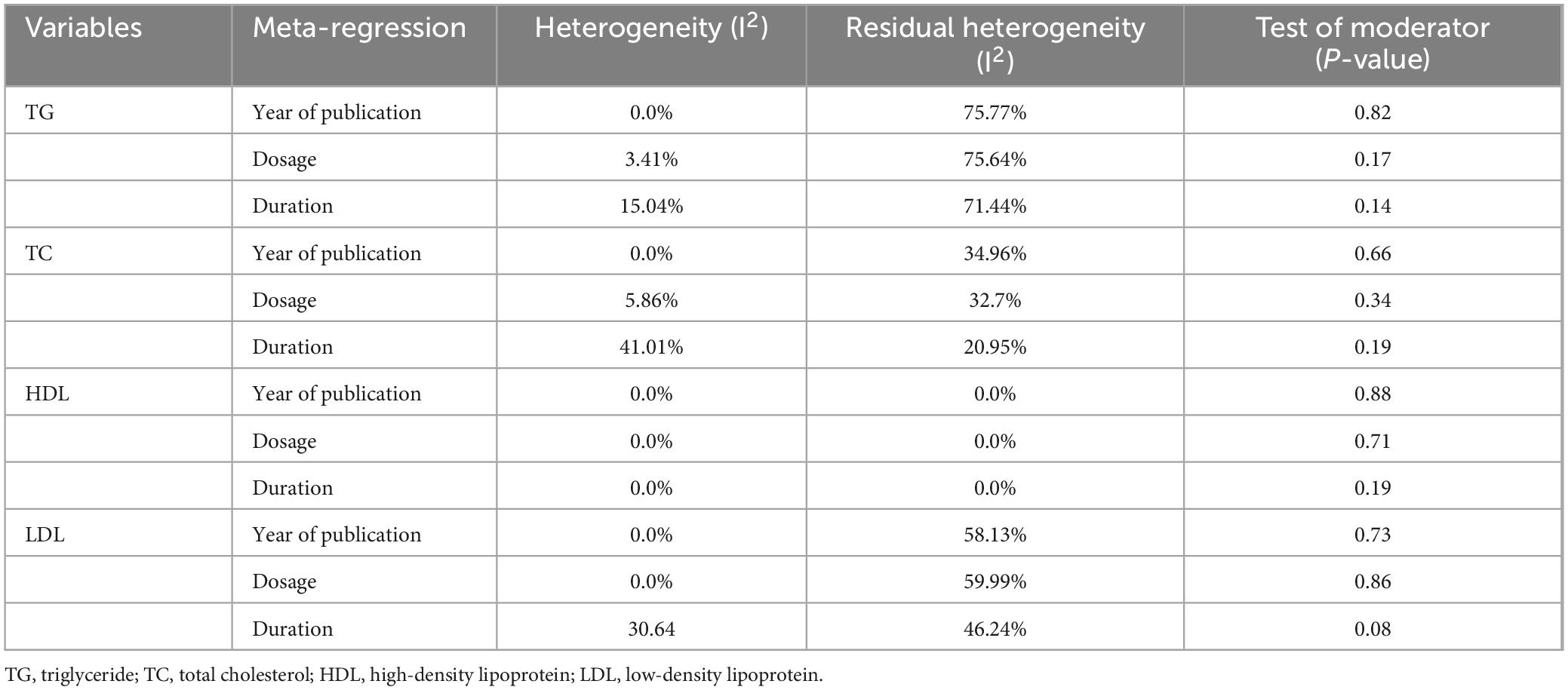
Table 4. Findings of meta-regression analysis of TG, TC, HDL, and LDL with year of publication, dosage of L-carnitine, and duration of treatment.
3.5 Effect of L-carnitine on total cholesterol (TC)
A total of 24 RCT were incorporated in the meta-analysis, all of which compared the effect of LC on TC levels to placebo. The meta-analysis of these studies resulted in no significant (SMD, −0.086; 95% CI, −0.253 to −0.079; P = 0.29) difference in the mean changes between groups (Figure 5). In addition, the heterogeneity of studies was moderate (I2 = 32.5%).
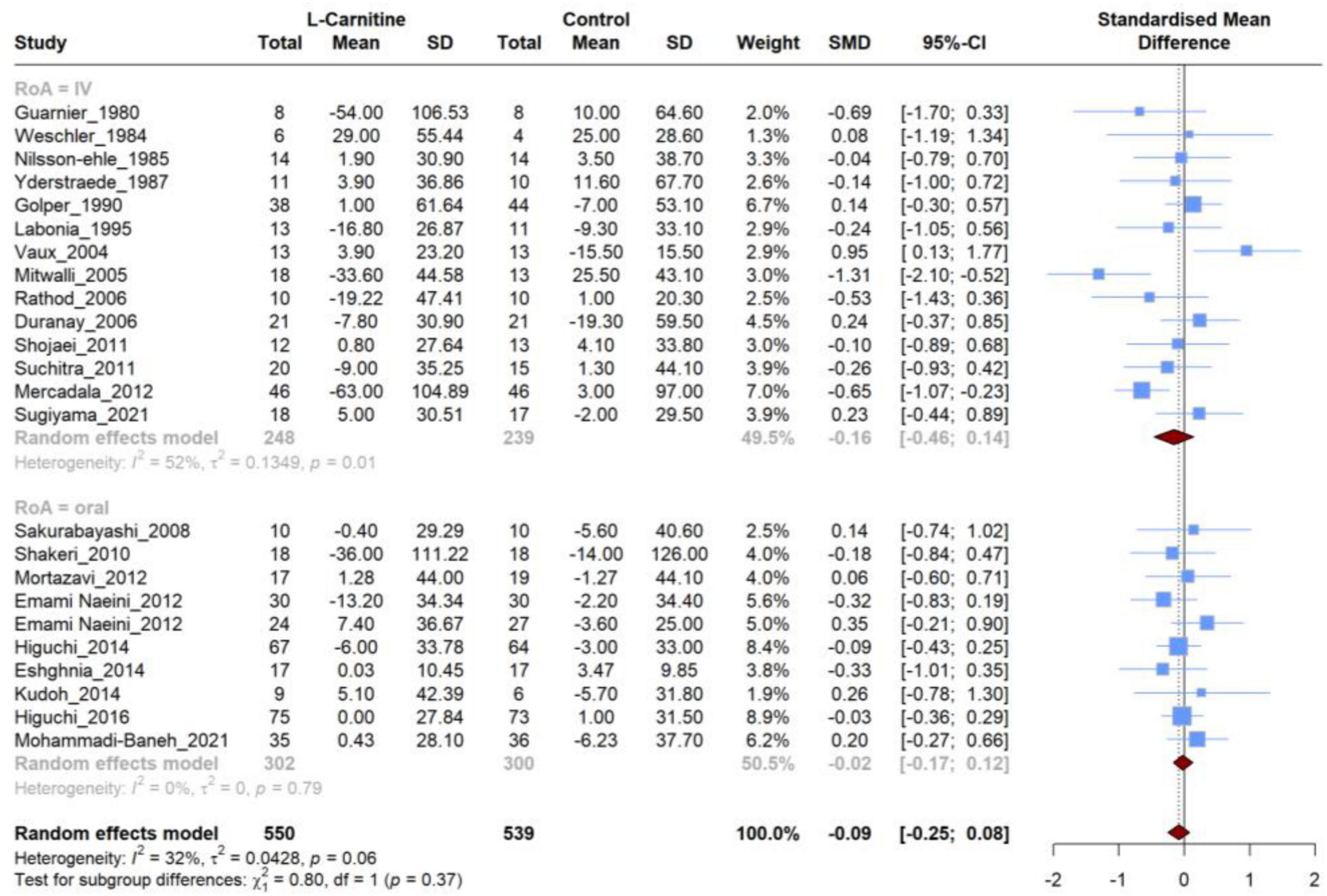
Figure 5. Forest plot for meta-analysis of total cholesterol (TC) mean change in L-carnitine groups versus control groups.
The subgroup analysis based on the RoA did not reveal significant differences between the groups (P = 0.37). Furthermore, neither of these two subgroups exhibited significant differences regarding the mean changes (IV: SMD, −0.16; 95% CI, −0.46 to 0.14; Oral: SMD, −0.02; 95% CI, −0.17 to 0.12). However, heterogeneity was high for the IV subgroup (I2 = 52%), and the oral subgroup contained homogeneous studies (I2 = 0%) (Table 3). Similar to the RoA, additional investigation based on subgroup analysis of dosage and duration of treatment revealed no significant SMD in subgroups (Supplementary Figure 2).
Results from meta-regression analysis showed that the duration of treatment accounted for 41.01% of heterogeneity among studies. In contrast, the moderator test for the duration was non-significant (P = 0.19). These findings showed that although the duration of treatment could explain some of the heterogeneity, it did not help predict the SMD between arms of studies. Furthermore, the year of publication and dosage were found not to be a source of heterogeneity (Table 4).
3.6 Effect of L-carnitine on HDL, LDL, VLDL
A meta-analysis of studies comparing LC and placebo effectiveness in modifying HDL, LDL, and VLDL levels was conducted separately, and it comprised 17,18 and 4 studies, respectively. Similar to the previous lipid profile variables, no significant difference was observed in the mean changes between LC and placebo for HDL levels (SMD, 0.060; 95% CI, −0.057 to 0.177; P = 0.29), LDL levels (SMD, −0.064; 95% CI, −0.272 to 0.142; P = 0.51), and VLDL (SMD, −0.125; 95% CI, −1.271 to 1.020; P = 0.75) (Figure 6). Regarding the HDL levels, there was no between-study heterogeneity (I2 = 0.0%). However, analysis of LDL and VLDL levels revealed high heterogeneity (I2 = 53.7% and 76.4%, respectively).
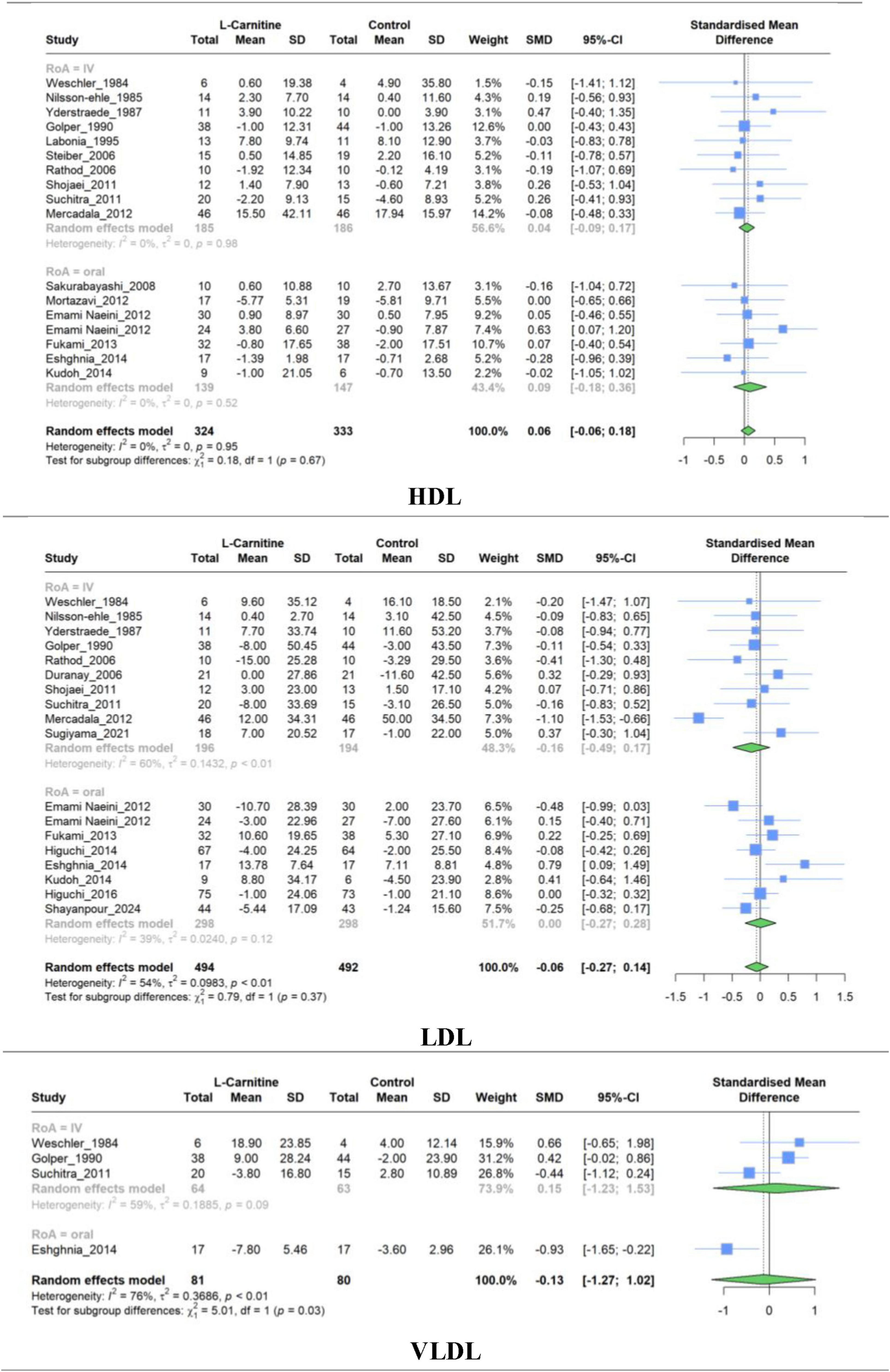
Figure 6. Forest plot for meta-analysis of HDL, LDL, and VLDV mean change in L-carnitine groups versus control groups.
Similar to the overall pooled effect for HDL studies, the subgroup synthesized results based on the RoA were non-significant (IV: SMD, 0.03; 95% CI, −0.09 to 0.15; Oral: SMD, 0.08; 95% CI, −0.18 to 0.36). Furthermore, no significant (P = 0.67) between-group differences were observed (Table 3).
Subgroup meta-analysis evaluating the efficacy of LC on LDL resulted in non-significant pooled effects of 10 studies with IV administration (SMD, −0.16; 95% CI, −0.48 to 0.16) and eight studies with oral administration (SMD, 0.00; 95% CI, −0.27 to 0.28). Supplementary Figures 3–5 represent subgroup analyses of dosage and treatment duration for HDL, LDL, and VLDL, respectively. The findings of subgroup analyses were similar to the overall results for each variable and revealed no significant SMD.
Meta-regression with the same variables (year of publication, dosage, and duration) was performed for HDL and LDL analysis. The results of the HDL analysis were non-significant, and the pooled estimate was not associated significantly with other variables. On the other hand, the variance between studies was partially attributed to the treatment duration, accounting for 30.64% of the heterogeneity. Nevertheless, the duration could not be a significant moderator of SMD between groups (P = 0.08). The outcomes of other variables’ meta-regression were non-significant (Table 4).
3.7 Effect of L-carnitine on BMI and BP
Meta-analysis of studies evaluating the effect of LC on BMI (5 RCT), systolic BP (6 RCT), and diastolic BP (5 RCT) was performed separately. The combined results show that there was no significant difference in the mean change between intervention and control groups for BMI (SMD, −0.025; 95% CI, −0.139 to 0.088; P = 0.56), systolic BP (SMD, 0.055; 95% CI, −0.110 to 0.220; P = 0.43), and diastolic BP (SMD, −0.028; 95% CI, 0.156 to 0.099; P = 0.56). The studies exhibited very low heterogeneity in all groups (I2 = 0.0%) (Figure 7). The results of subgroup analysis based on the RoA, treatment duration, and dosage were non-significant (Supplementary Figures 6–8).
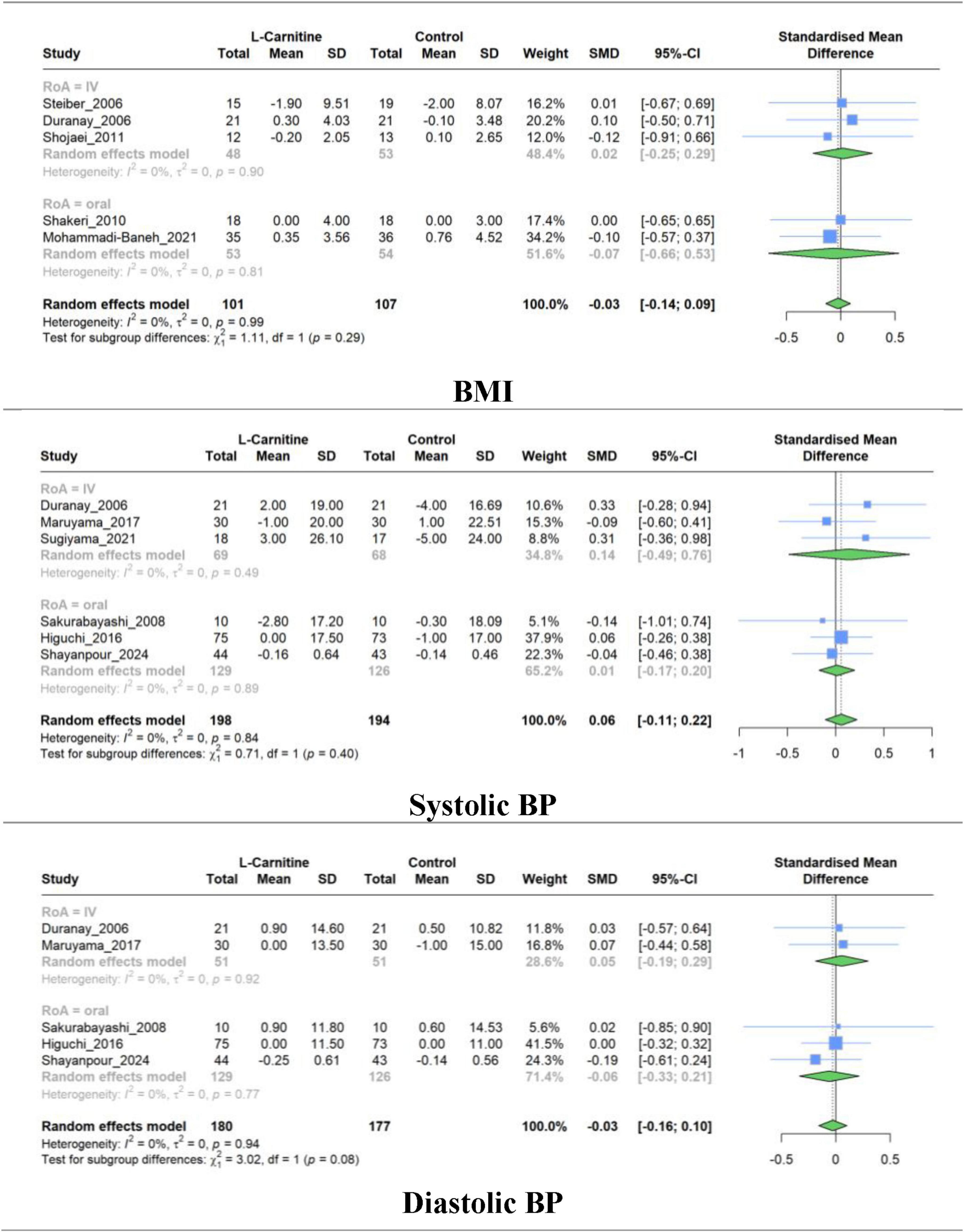
Figure 7. Forest plot for meta-analysis of BMI, systolic BP, and diastolic BP mean change in L-carnitine groups versus control groups.
3.8 Sensitivity analysis
Sensitivity analysis based on the leave-one-out method for TG, TC, and HDL studies showed that excluding articles did not significantly change heterogeneity or pooled results. However, the leave-one-out method’s finding in LDL revealed that the high between-study heterogeneity in the LDL group could be reduced significantly by removing Mercadal et al. (33) study. In contrast, removing any studies did not significantly change the LDL group pooled estimate (Figure 8).
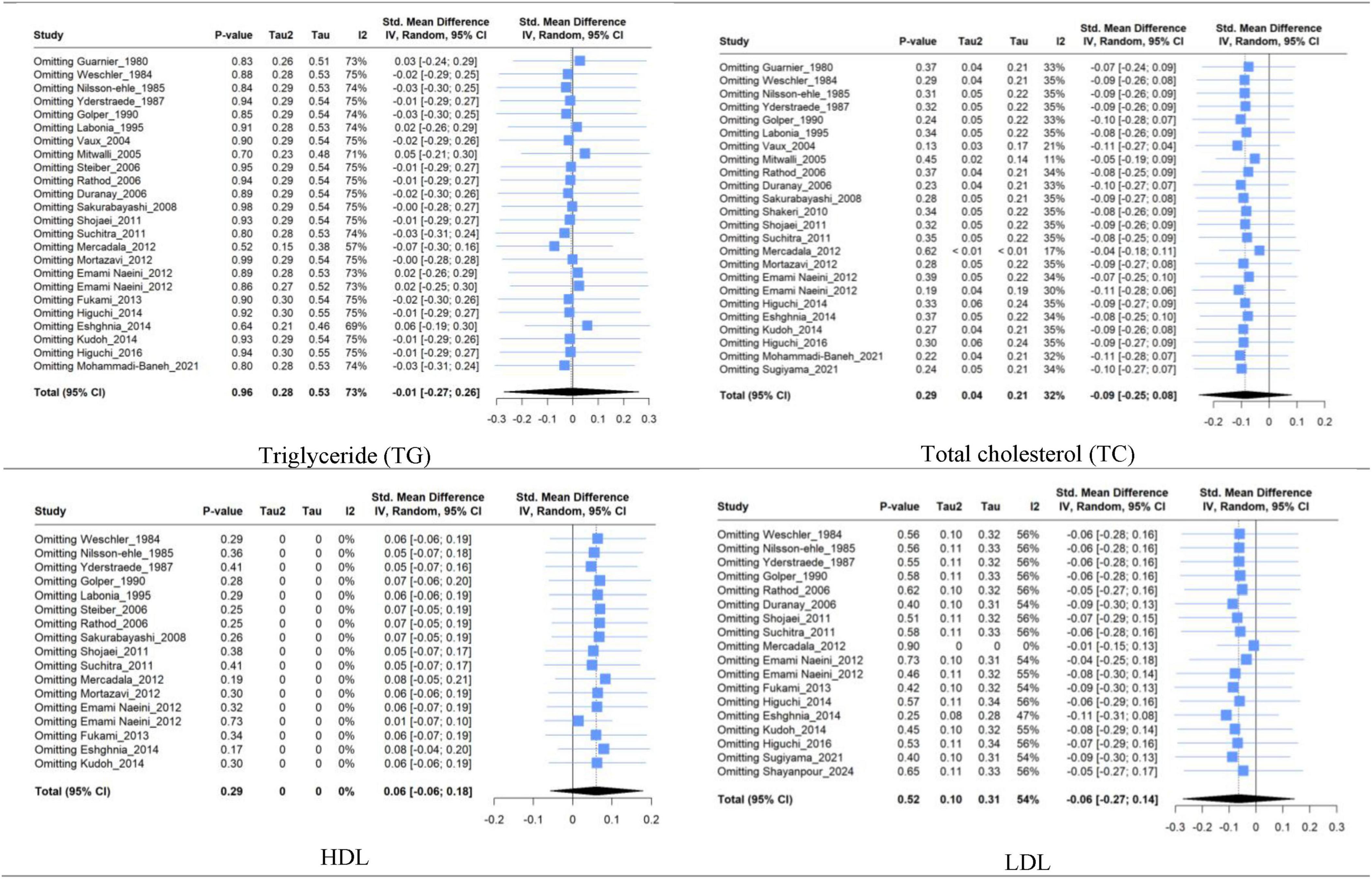
Figure 8. Forest plot for sensitivity analysis based on leave-one-out method of triglyceride (TG), total cholesterol (TC), high-density lipoprotein (HDL), and low-density lipoprotein (LDL) between mean change in L-carnitine groups versus control groups.
The Supplementary materials include a Sensitivity analysis based on the leave-one-out method for systolic BP, diastolic BP, and BMI (Supplementary Figure 9).
3.9 Assessment of publication bias
Regarding the risk of publication bias, the funnel plot’s visual assessment did not show asymmetry or risk of bias for any of the analyses (Figure 9 and Supplementary Figures 10–13). Moreover, for a meta-analysis with more than ten included studies, statistical tests (Begg’s and Egger’s methods) for assessing the risk of publication bias were used and confirmed no source of publication bias (Table 3).
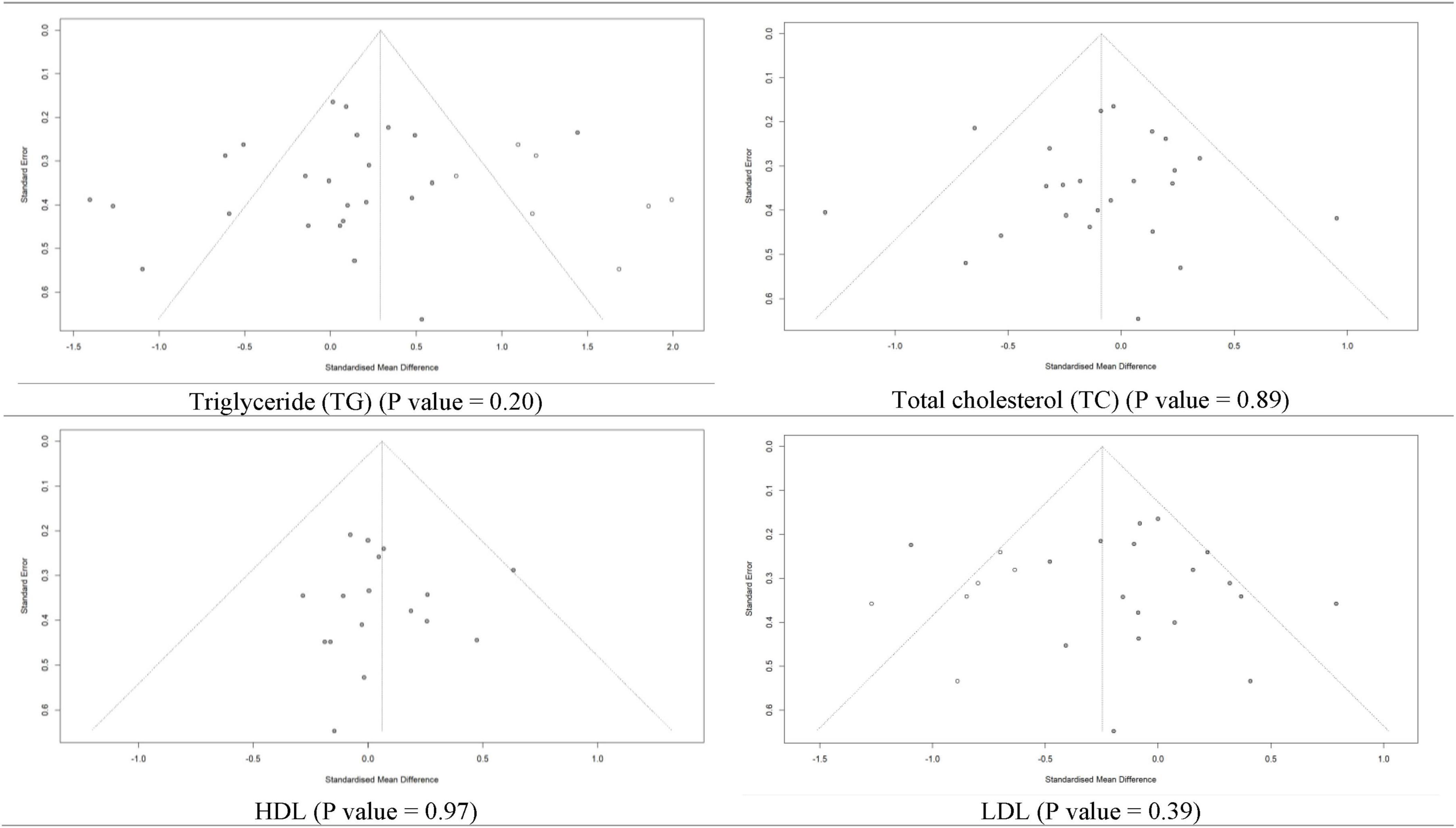
Figure 9. Funnel plot for risk of publication bias assessment based on trim and fill method of SMD of triglyceride, total cholesterol, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) between L-carnitine groups versus control groups with P-value of Egger’s test.
4 Discussion
In this meta-analysis, we showed that LC supplementation did not significantly change serum lipid levels, including TG, TC, HDL, LDL, or VLDL. While previous research has suggested that hemodialysis patients often have carnitine deficiency (14, 18–20), which may contribute to metabolic disturbances such as dyslipidemia, our findings indicate that LC supplementation alone is not effective in improving lipid metabolism or managing dyslipidemia in this specific population. These results highlight the need for further investigation into alternative therapeutic approaches for reducing cardiovascular risk in hemodialysis patients.
Carnitine has a crucial role in lipid metabolism, considering its involvement in the beta-oxidation of fatty acids and reducing the conversion of free fatty acids (FFA) to TG (34). Carnitine deficiency in hemodialysis individuals is linked to several factors, such as inadequate carnitine intake, reduced biosynthesis, and removal during hemodialysis (15, 35). Abnormal carnitine metabolism in these patients is correlated with various clinical conditions, particularly impaired cardiac function (36, 37). Cardiovascular diseases are a leading cause of death in chronic renal failure individuals on maintenance hemodialysis (38), which can be related to impairments in cardiac metabolism along with higher inflammation and oxidative stress status. This can lead to myocyte necrosis resulting from altered lipid metabolism and LC deficiency (39, 40). In this regard, numerous RCTs have explored the impact of LC supplementation on lipid profiles across various diseases, particularly in hemodialysis patients. However, the findings have been inconsistent with partly modest sample sizes (16).
Although previous studies have demonstrated that LC supplementation generally improves lipid profiles (41–43), but the effects of LC in Specific populations are controversial. The conflicting outcomes from various studies underscore the complexity of LC’s effects in different patient populations. This inconsistency may stem from variations in study design, dosage, and patient characteristics. For example, a similar study conducted by Huang et al. (16) analyzing twelve studies with a total of 391 hemodialysis patients which concluded that LC significantly lowers LDL but does not affect TC, HDL, or TG. In another study on patients with liver disease, Abbasnezhad et al. (34) Showed that LC reduces TC and TG but has no significant effect on HDL and LDL. Asbaghi et al. (44) and Vidal-Casariego et al. (45) showed that in type 2 diabetes patients, LC improves TC and LDL-C levels but has no significant effect on HDL-C and TG. Yang et al. (17) in a meta-analysis, reported that LC therapy did not improve oxidized LDL (SMD: 0.04, P = 0.87) or TC (-0.24, P = 0.33). Also, in a meta-analysis by Huang et al. (16), LC supplementation did not significantly reduce TC, HDL, VLDL, or serum TG. However, it significantly decreased LDL in hemodialysis patients (SMD: −0.29, P = 0.01). In another systematic review (46) of patients with CKD, LC was found to increase levels of TC and LDL significantly.
In our meta-analysis, subgroup analysis based on the RoA, dosage, and duration of LC supplementation revealed no significant effects on TC, TG, HDL, or VLDL levels in hemodialysis patients. However, a notable reduction in LDL was observed in patients receiving intravenous LC. Regarding the dosage of LC, Musazadeh et al. (41) and Askarpour et al. (42), which reported that higher LC doses, particularly above 2 g/day, improved lipid profiles by reducing TC, LDL, and TG while increasing HDL. The discrepancies suggest that factors such as dosage and RoA might play a critical role in LC’s lipid-modifying effects, warranting further investigation to better understand its impact in different patient populations and treatment regimens.
Our study found that LC supplementation had no significant effect on BMI in hemodialysis patients, and subgroup analysis based on the RoA also showed no notable impact. This result aligns with findings from several other studies across different patient populations and conditions. For instance, Abolfathi et al. (47) reported no significant changes in BMI or body weight in patients with non-alcoholic fatty liver disease (NAFLD) following LC supplementation, while Del Vecchio et al. (48) similarly found no effect of LC on body mass reduction. However, contrasting outcomes were observed in studies by Pooyandjoo et al. (49) and Talenezhad et al. (50), where LC supplementation led to significant BMI reductions, particularly in overweight or obese individuals. These mixed results suggest that the effect of LC on BMI may vary based on factors such as patient population, baseline body weight, and underlying health conditions, highlighting the need for further research to clarify LC’s role in weight management.
The effect of LC on BP has shown mixed results in various studies. In our study, LC supplementation had no significant impact on either systolic or diastolic BP in hemodialysis patients, and subgroup analysis based on the RoA also yielded insignificant results. However, a meta-analysis by Askarpour et al. (51) reported that LC supplementation significantly reduced diastolic BP in overweight and obese participants (-1.232 mmHg, P = 0.023) and in those receiving doses less than 2 g per day (-1.639 mmHg, P = 0.022). In contrast, Choi et al. (52) found a significant reduction in systolic BP with LC supplementation, indicating potential variability between studies. Additionally, Dong et al. (53) observed that oral LC significantly lowers both systolic and diastolic BP, suggesting a broader range of cardiovascular effects for LC. These inconsistencies point to the need for further research to better understand how factors like patient population, dosage, and underlying health conditions may influence LC’s impact on BP.
This study has several strengths and limitations. A key limitation is the moderate to high heterogeneity across the included studies, which indicates variability in study designs, patient populations, and intervention protocols. Such variability can impact the reliability of the pooled results. Additionally, discrepancies in findings from other LC supplementation meta-analyses may be attributed to differences in population characteristics, types of interventions, sample sizes, study quality, and measurement methods, complicating the assessment of LC’s effects across different patient groups. On the other hand, the study’s strengths include its comprehensive analysis of LC supplementation’s effects on lipid profiles, BMI, and blood pressure in hemodialysis patients, offering a detailed examination of this specific population. The low to moderate heterogeneity in most outcomes enhances the reliability of the findings, and subgroup analyses based on dosage, duration, and route of administration provide further insights. By incorporating more variables and recent studies, this meta-analysis delivers a broader understanding compared to previous reviews.
5 Conclusion
In conclusion, LC supplementation does not significantly improve the serum lipid profile, including TG, TC, HDL, LDL, and VLDL, in hemodialysis patients. It also has no notable impact on BMI, systolic, or diastolic blood pressure in this population. Despite LC’s known role in lipid metabolism, the variability in findings across studies suggests that its effects may differ depending on patient populations, study design, dosage, and other factors. To reduce variation and get a clearer picture of the effects of LC supplementation, especially on managing cardiovascular risk, future research should focus on larger, well-designed RCTs with more specific patient groups.
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
MK: Writing – review and editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. SP: Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. SMP: Writing – review and editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. PP: Writing – review and editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. NS: Writing – original draft, Visualization, Supervision, Data curation. SA: Writing – review and editing, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1454921/full#supplementary-material
Abbreviations
CKD, chronic kidney disease; LC, L-carnitine; ESRD, end-stage renal disease; RCT, randomized clinical trials; TG, triglycerides; TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDV, very low-density lipoprotein; BP, blood pressure; RoA, route of administration.
References
1. Gaitonde DY, Cook DL, Rivera IM. Chronic kidney disease: Detection and evaluation. Am Fam Phys. (2017) 96:776–83.
2. Stevens PE, Ahmed SB, Carrero JJ, Foster B, Francis A, Hall RK, et al. KDIGO 2024 Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. (2024) 105:S117–314.
3. Jin D-C, Yun SR, Lee SW, Han SW, Kim W, Park J, et al. Lessons from 30 years’ data of Korean end-stage renal disease registry, 1985–2015. Kidney Res Clin Pract. (2015) 34:132–9. doi: 10.1016/j.krcp.2015.08.004
4. Hashmi MF, Benjamin O, Lappin SL. End-Stage Renal Disease. Treasure Island, FL: StatPearls Publishing (2018).
5. Sharief S, Hsu C-Y. The transition from the pre-ESRD to ESRD phase of CKD: Much remains to be learned. Am J Kidney Dis. (2017) 69:8–10. doi: 10.1053/j.ajkd.2016.10.001
6. Himmelfarb J, Vanholder R, Mehrotra R, Tonelli M. The current and future landscape of dialysis. Nat Rev Nephrol. (2020) 16:573–85.
7. Bello AK, Okpechi IG, Osman MA, Cho Y, Htay H, Jha V, et al. Epidemiology of haemodialysis outcomes. Nat Rev Nephrol. (2022) 18:378–95.
8. Hasan YK, Alsultan M, Anan MT, Hassn Q, Basha K. The prevalence of dyslipidemia in patients on hemodialysis: A cross-sectional study from Syria. Ann Med Surg. (2023) 85:3838–44. doi: 10.1097/MS9.0000000000000931
9. Ahmed HM, Miller M, Nasir K, McEvoy JW, Herrington D, Blumenthal RS, et al. Primary low level of high-density lipoprotein cholesterol and risks of coronary heart disease, cardiovascular disease, and death: Results from the multi-ethnic study of atherosclerosis. Am J Epidemiol. (2016) 183:875–83.
10. Pekala J, Patkowska-Sokoła B, Bodkowski R, Jamroz D, Nowakowski P, Lochyński S, et al. L-carnitine–metabolic functions and meaning in humans life. Curr Drug Metab. (2011) 12:667–78. doi: 10.2174/138920011796504536
11. Wang Z-Y, Liu Y-Y, Liu G-H, Lu H-B, Mao C-Y. L-Carnitine and heart disease. Life Sci. (2018) 194:88–97.
12. Serban M-C, Sahebkar A, Mikhailidis DP, Toth PP, Jones SR, Muntner P, et al. Impact of L-carnitine on plasma lipoprotein (a) concentrations: A systematic review and meta-analysis of randomized controlled trials. Sci Rep. (2016) 6:19188. doi: 10.1038/srep19188
13. DiNicolantonio JJ, Lavie CJ, Fares H, Menezes AR, O’Keefe JH editors. L-carnitine in the secondary prevention of cardiovascular disease: Systematic review and meta-analysis. Mayo Clin Proc. (2013) 8:544–51.
14. Shimizu S, Takashima H, Tei R, Furukawa T, Okamura M, Kitai M, et al. Prevalence of carnitine deficiency and decreased carnitine levels in patients on peritoneal dialysis. Nutrients. (2019) 11:2645. doi: 10.3390/nu11112645
15. Kaneko S, Hirai K, Morino J, Minato S, Yanai K, Mutsuyoshi Y, et al. Association between carnitine deficiency and the erythropoietin resistance index in patients undergoing peritoneal dialysis: A cross-sectional observational study. Ren Fail. (2020) 42:146–53. doi: 10.1080/0886022X.2020.1719847
16. Huang H, Song L, Zhang H, Zhang H, Zhang J, Zhao W. Influence of L-carnitine supplementation on serum lipid profile in hemodialysis patients: A systematic review and meta-analysis. Kidney Blood Pressure Res. (2013) 38:31–41. doi: 10.1159/000355751
17. Yang S-K, Xiao L, Song P-A, Xu X, Liu F-Y, Sun L. Effect of L-carnitine therapy on patients in maintenance hemodialysis: A systematic review and meta-analysis. J Nephrol. (2014) 27:317–29. doi: 10.1007/s40620-013-0002-7
18. Golper TA, Ahmad S editors. L-carnitine administration to hemodialysis patients: Has its time come? Semin Dial. (1992) 5:94–8.
19. Bertoli M, Battistella PA, Vergani L, Naso A, Gasparotto ML, Romagnoli GF, et al. Carnitine deficiency induced during hemodialysis and hyperlipidemia: Effect of replacement therapy. Am J Clin Nutr. (1981) 34:1496–500. doi: 10.1093/ajcn/34.8.1496
20. Nishioka N, Luo Y, Taniguchi T, Ohnishi T, Kimachi M, Ng RC, et al. Carnitine supplements for people with chronic kidney disease requiring dialysis. Cochr Datab Syst Rev. (2022) 12:Cd013601.
21. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J Clin Epidemiol. (2021) 134:103–12.
22. Amir-Behghadami M, Janati A. Population, Intervention, Comparison, Outcomes and Study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg Med J. (2020) 37:387.
23. Hróbjartsson A, Boutron I, Turner L, Altman DG, Moher D. Assessing risk of bias in randomised clinical trials included in Cochrane Reviews: The why is easy, the how is a challenge. Cochr Datab Syst Rev. (2013) 2013:Ed000058. doi: 10.1002/14651858.ED000058
24. Wang D, Deng L, Zhang R, Zhou Y, Zeng J, Jiang H. Efficacy of intraosseous access for trauma resuscitation: A systematic review and meta-analysis. World J Emerg Surg. (2023) 18:17.
25. Takeshima N, Sozu T, Tajika A, Ogawa Y, Hayasaka Y, Furukawa TA. Which is more generalizable, powerful and interpretable in meta-analyses, mean difference or standardized mean difference? BMC Med Res Methodol. (2014) 14:30. doi: 10.1186/1471-2288-14-30
26. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. (2018) 27:1785–805.
27. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
28. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. Hoboken, NJ: Wiley (2011).
29. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60.
30. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101.
31. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34.
32. Lüdecke D, Ben-Shachar MS, Patil I, Waggoner P, Makowski D. Performance: an R package for assessment, comparison and testing of statistical models. J Open Source Softw. (2021) 6.
33. Mercadal L, Coudert M, Vassault A, Pieroni L, Debure A, Ouziala M, et al. L-carnitine treatment in incident hemodialysis patients: The multicenter, randomized, double-blinded, placebo-controlled CARNIDIAL trial. Clin J Am Soc Nephrol. (2012) 7:1836–42. doi: 10.2215/CJN.12431211
34. Abbasnezhad A, Hasanavand A, Falahi E, Kashkooli S, Asbaghi O, Choghakhori R. Effect of L-Carnitine supplementation on lipid profiles of patients with liver disease: A systematic review and meta-analysis. Prev Nutr Food Sci. (2020) 25:124–32. doi: 10.3746/pnf.2020.25.2.124
35. Kuwasawa-Iwasaki M, Io H, Muto M, Ichikawa S, Wakabayashi K, Kanda R, et al. Effects of L-Carnitine supplementation in patients receiving hemodialysis or peritoneal dialysis. Nutrients. (2020) 12:3371.
37. Ulinski T, Cirulli M, Virmani MA. The role of L-carnitine in kidney disease and related metabolic dysfunctions. Kidney Dial. (2023) 3:178–91.
38. Fan X, Li J, Bi ZY, Liang W, Wang FJ. Cause of death and influencing factors of chronic renal failure on maintenance hemodialysis. Pak J Med Sci. (2023) 39:1378–82.
39. Abdul-Hassan Mahdi S, Abdul-Aziz AA. The effect of L-Carnitine on cardiac output in patient with chronic kidney disease on regular hemodialysis. Indian J Forensic Med Toxicol. (2021) 15:1233–9.
40. Alhasaniah AH. L-carnitine: Nutrition, pathology, and health benefits. Saudi J Biol Sci. (2023) 30:103555.
41. Musazadeh V, Alinejad H, Esfahani NK, Kavyani Z, Keramati M, Roshanravan N, et al. The effect of L-carnitine supplementation on lipid profile in adults: An umbrella meta-analysis on interventional meta-analyses. Front Nutr. (2023) 10:1214734. doi: 10.3389/fnut.2023.1214734
42. Askarpour M, Hadi A, Symonds ME, Miraghajani M, Omid S, Sheikhi A, et al. Efficacy of L-carnitine supplementation for management of blood lipids: A systematic review and dose-response meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. (2019) 29:1151–67. doi: 10.1016/j.numecd.2019.07.012
43. Li Y, Xie Y, Qiu C, Yu B, Yang F, Cheng Y, et al. Effects of L-carnitine supplementation on glucolipid metabolism: A systematic review and meta-analysis. Food Funct. (2023) 14:2502–17. doi: 10.1039/d2fo02930h
44. Asbaghi O, Kashkooli S, Amini MR, Shahinfar H, Djafarian K, Clark CCT, et al. The effects of L-carnitine supplementation on lipid concentrations inpatients with type 2 diabetes: A systematic review and meta-analysis of randomized clinical trials. J Cardiovasc Thorac Res. (2020) 12:246–55. doi: 10.34172/jcvtr.2020.43
45. Vidal-Casariego A, Burgos-Peláez R, Martínez-Faedo C, Calvo-Gracia F, Valero-Zanuy M, Luengo-Pérez LM, et al. Metabolic effects of L-carnitine on type 2 diabetes mellitus: Systematic review and meta-analysis. Exp Clin Endocrinol Diabetes. (2013) 121:234–8.
46. Gholipur-Shahraki T, Feizi A, Mortazavi M, Badri S. Effects of carnitine on nutritional parameters in patients with chronic kidney disease: An updated systematic review and meta-analysis. J Res Pharm Pract. (2018) 7:57–68.
47. Abolfathi M, Mohd-Yusof BN, Hanipah ZN, Mohd Redzwan S, Yusof LM, Khosroshahi MZ. The effects of carnitine supplementation on clinical characteristics of patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. (2020) 48:102273. doi: 10.1016/j.ctim.2019.102273
48. Del Vecchio FB, Coswig VS, Galliano LM. Comment on ‘The effect of (l-)carnitine on weight loss in adults: A systematic review and meta-analysis of randomized controlled trials’. Obes Rev. (2017) 18:277–8. doi: 10.1111/obr.12488
49. Pooyandjoo M, Nouhi M, Shab-Bidar S, Djafarian K, Olyaeemanesh A. The effect of (L-)carnitine on weight loss in adults: A systematic review and meta-analysis of randomized controlled trials. Obes Rev. (2016) 17:970–6.
50. Talenezhad N, Mohammadi M, Ramezani-Jolfaie N, Mozaffari-Khosravi H, Salehi-Abargouei A. Effects of l-carnitine supplementation on weight loss and body composition: a systematic review and meta-analysis of 37 randomized controlled clinical trials with dose-response analysis. Clin Nutr ESPEN. (2020) 37:9–23. doi: 10.1016/j.clnesp.2020.03.008
51. Askarpour M, Hadi A, Dehghani Kari Bozorg A, Sadeghi O, Sheikhi A, Kazemi M, et al. Effects of l-carnitine supplementation on blood pressure: A systematic review and meta-analysis of randomized controlled trials. J Hum Hypertens. (2019) 33:725–34. doi: 10.1038/s41371-019-0248-1
52. Choi M, Park S, Lee M. L-Carnitine’s effect on the biomarkers of metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. Nutrients. (2020) 12:2795. doi: 10.3390/nu12092795
53. Dong JY, Qin LQ, Zhang Z, Zhao Y, Wang J, Arigoni F, et al. Effect of oral L-arginine supplementation on blood pressure: A meta-analysis of randomized, double-blind, placebo-controlled trials. Am Heart J. (2011) 162:959–65. doi: 10.1016/j.ahj.2011.09.012
54. Guarnieri G, Ranieri F, Toigo G, Vasile A, Ciman M, Rizzoli V, et al. Lipid-lowering effect of carnitine in chronically uremic patients treated with maintenance hemodialysis. Am J Clin Nutr. (1980) 33:1489–92. doi: 10.1093/ajcn/33.7.1489
55. Weschler A, Aviram M, Levin M, Better OS, Brook G. High dose of L-carnitine increases platelet aggregation and plasma triglyceride levels in uremic patients on hemodialysis. Nephron. (1984) 38:120–4. doi: 10.1159/000183292
56. Nilsson-Ehle P, Cederblad G, Fagher B, Monti M, Thysell H. Plasma lipoproteins, liver function and glucose metabolism in haemodialysis patients: Lack of effect of L-carnitine supplementation. Scand J Clin Lab Investig. (1985) 45:179–84. doi: 10.3109/00365518509160992
57. Yderstræde KB, Pedersen FB, Dragsholt C, Trostmann A, Laier E, Larsen H. The effect of L-carnitine on lipid metabolism in patients on chronic haemodialysis. Nephrol Dial Transpl. (1987) 1:238–41.
58. Golper TA, Wolfson M, Ahmad S, Hirschberg R, Kurtin P, Katz LA, et al. Multicenter trial of L-carnitine in maintenance hemodialysis patients. I. Carnitine concentrations and lipid effects. Kidney Int. (1990) 38:904–11. doi: 10.1038/ki.1990.289
59. Labonia WD. L-carnitine effects on anemia in hemodialyzed patients treated with erythropoietin. Am J Kidney Dis. (1995) 26:757–64.
60. Vaux E, Taylor D, Altmann P, Rajagopalan B, Graham K, Cooper R, et al. Effects of carnitine supplementation on muscle metabolism by the use of magnetic resonance spectroscopy and near-infrared spectroscopy in end-stage renal disease. Nephron Clin Pract. (2004) 97:c41–8. doi: 10.1159/000078399
61. Mitwalli AH, Al-Wakeel JS, Alam A, Tarif N, Abu-Aisha H, Rashed M, et al. L-carnitine supplementation in hemodialysis patients. Saudi J Kidney Dis Transpl. (2005) 16:17–22.
62. Steiber AL, Davis AT, Spry L, Strong J, Buss ML, Ratkiewicz MM, et al. Carnitine treatment improved quality-of-life measure in a sample of Midwestern hemodialysis patients. J Parenter Enteral Nutr. (2006) 30:10–5. doi: 10.1177/014860710603000110
63. Rathod R, Baig M, Khandelwal P, Kulkarni S, Gade P, Siddiqui S. Results of a single blind, randomized, placebo-controlled clinical trial to study the effect of intravenous L-carnitine supplementation on health-related quality of life in Indian patients on. Indian J Med Sci. (2006) 60:143–53.
64. Duranay M, Akay H, Yilmaz FM, Şeneş M, Tekeli N, Yücel D. Effects of L-carnitine infusions on inflammatory and nutritional markers in haemodialysis patients. Nephrol Dial Transpl. (2006) 21:3211–4. doi: 10.1093/ndt/gfl356
65. Sakurabayashi T, Miyazaki S, Yuasa Y, Sakai S, Suzuki M, Takahashi S, et al. L-carnitine supplementation decreases the left ventricular mass in patients undergoing hemodialysis. Circ J. (2008) 72:926–31.
66. Shakeri A, Tabibi H, Hedayati M. Effects of l-carnitine supplement on serum inflammatory cytokines, C-reactive protein, lipoprotein (a), and oxidative stress in hemodialysis patients with Lp (a) hyperlipoproteinemia. Hemodial Int. (2010) 14:498–504.
67. Shojaei M, Djalali M, Khatami M, Siassi F, Eshraghian M. Effects of carnitine and coenzyme Q10 on lipid profile and serum levels of lipoprotein (a) in maintenance hemodialysis patients on statin therapy. Iran J Kidney Dis. (2011) 5:114–8.
68. Suchitra M, Ashalatha V, Sailaja E, Rao AM, Reddy VS, Bitla AR, et al. The effect of L-carnitine supplementation on lipid parameters, inflammatory and nutritional markers in maintenance hemodialysis patients. Saudi J Kidney Dis Transpl. (2011) 22:1155–9.
69. Mortazavi M, Seirafian S, Eshaghian A, Ghassami M, Taheri S, Atapour A, et al. Associations of oral L-carnitine with hemoglobin, lipid profile, and albumin in hemodialysis patients. J Res Med Sci. (2012) 17(1 SPL. 1):S33–7.
70. Naini AE, Sadeghi M, Mortazavi M, Moghadasi M, Harandi AA. Oral carnitine supplementation for dyslipidemia in chronic hemodialysis patients. Saudi J Kidney Dis Transpl. (2012) 23:484–8.
71. Emami Naini A, Moradi M, Mortazavi M, Amini Harandi A, Hadizadeh M, Shirani F, et al. Effects of oral L-carnitine supplementation on lipid profile, anemia, and quality of life in chronic renal disease patients under hemodialysis: A randomized, double-blinded, placebo-controlled trial. J Nutr Metab. (2012) 2012:510483. doi: 10.1155/2012/510483
72. Fukami K, Yamagishi S-I, Sakai K, Kaida Y, Adachi T, Ando R, et al. Potential inhibitory effects of L-carnitine supplementation on tissue advanced glycation end products in patients with hemodialysis. Rejuvenation Res. (2013) 16:460–6. doi: 10.1089/rej.2013.1459
73. Higuchi T, Abe M, Yamazaki T, Mizuno M, Okawa E, Ando H, et al. Effects of levocarnitine on brachial-ankle pulse wave velocity in hemodialysis patients: A randomized controlled trial. Nutrients. (2014) 6:5992–6004. doi: 10.3390/nu6125992
74. Eshghinia S, Marjani A, Kor BEZ. Effects of oral L-carnitine supplementation on lipid profiles and anemia in patients under hemodialysis in gonbadkavoos, Iran. Annu Res Rev Biol. (2013) 4:1092–8.
75. Kudoh Y, Aoyama S, Torii T, Chen Q, Nagahara D, Sakata H, et al. The effects of oral L-carnitine supplementation on physical capacity and lipid metabolism in chronic hemodialysis patients. Nephron Extra. (2014) 4:33–41. doi: 10.1159/000360086
76. Higuchi T, Abe M, Yamazaki T, Okawa E, Ando H, Hotta S, et al. Levocarnitine improves cardiac function in hemodialysis patients with left ventricular hypertrophy: A randomized controlled trial. Am J Kidney Dis. (2016) 67:260–70. doi: 10.1053/j.ajkd.2015.09.010
77. Maruyama T, Higuchi T, Yamazaki T, Okawa E, Ando H, Oikawa O, et al. Levocarnitine injections decrease the need for erythropoiesis-stimulating agents in hemodialysis patients with renal anemia. Cardiorenal Med. (2017) 7:188–97. doi: 10.1159/000462983
78. Mohammadi-Baneh A, Rahimi E, Moradi G, Mafakheri Z, Khorshidi A. The effects of L-carnitine supplement on anemia in hemodialysis patients: A randomized clinical trial study. Chronic Dis J. (2021) 9:125–31.
79. Sugiyama M, Hazama T, Nakano K, Urae K, Moriyama T, Ariyoshi T, et al. Effects of reducing L-carnitine supplementation on carnitine kinetics and cardiac function in hemodialysis patients: A multicenter, single-blind, placebo-controlled, randomized clinical trial. Nutrients. (2021) 13:1900. doi: 10.3390/nu13061900
Keywords: L-carnitine, carnitine, serum lipid, hemodialysis, chronic kidney disease, nephropathy, meta-analysis
Citation: Karimi M, Pirzad S, Pourfaraji SMA, Parhizkar Roudsari P, Shirsalimi N and Ahmadizad S (2024) Effects of L-carnitine supplementation on lipid profile in adult patients under hemodialysis: a systematic review and meta-analysis of RCTs. Front. Med. 11:1454921. doi: 10.3389/fmed.2024.1454921
Received: 26 June 2024; Accepted: 20 November 2024;
Published: 02 December 2024.
Edited by:
Amani Kallel, Ministry of Public Health, TunisiaReviewed by:
Rizaldy Taslim Pinzon, Duta Wacana Christian University, IndonesiaThiago Santos Rosa, Catholic University of Brasilia (UCB), Brazil
Copyright © 2024 Karimi, Pirzad, Pourfaraji, Parhizkar Roudsari, Shirsalimi and Ahmadizad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mehdi Karimi, a2FyaW1pOTAxMEBnbWFpbC5jb20=; Sajad Ahmadizad, U19haG1hZGl6YWRAc2J1LmFjLmly
†ORCID: Mehdi Karimi, orcid.org/0009-0006-4388-0214; Samira Pirzad, orcid.org/0009-0003-9220-3891; Seyed Morteza Ali Pourfaraji, orcid.org/0009-0003-7712-9427; Peyvand Parhizkar Roudsari, orcid.org/0000-0003-3521-0959; Niyousha Shirsalimi, orcid.org/0000-0002-3337-942X; Sajad Ahmadizad, orcid.org/0000-0001-5859-3556
 Mehdi Karimi
Mehdi Karimi Samira Pirzad
Samira Pirzad Seyed Morteza Ali Pourfaraji
Seyed Morteza Ali Pourfaraji Peyvand Parhizkar Roudsari
Peyvand Parhizkar Roudsari Niyousha Shirsalimi4†
Niyousha Shirsalimi4† Sajad Ahmadizad
Sajad Ahmadizad