
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 01 November 2024
Sec. Geriatric Medicine
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1453502
This article is part of the Research Topic Endocrinology, Lipids, and Disease: Unraveling the Links View all 13 articles
Background: Observational studies have demonstrated a correlation between thyroid dysfunction and osteoporosis (OP); however, the underlying causality has yet to be fully elucidated.
Methods: The necessary dataset was sourced from public databases. Initially, instrumental variables (IVs) were selected based on three primary hypotheses. Subsequently, Cochran’s Q test was employed to exclude IVs exhibiting heterogeneity. The MR-PRESSO test and the leave-one-out sensitivity test were further applied to detect potential pleiotropy. Inverse variance was utilized for the analysis. This study primarily utilized the inverse variance weighted (IVW) model for Mendelian analysis. Since Type 1 diabetes mellitus can also contribute to the development of osteoporosis, this study additionally employed multivariate Mendelian analysis. Furthermore, 249 circulating metabolites were selected for mediation analysis in the Mendelian randomization framework.
Results: In this study, the two-sample Mendelian randomization (MR) analysis primarily employed the random-effects IVW model and demonstrated a causal relationship between hypothyroidism (OR = 1.092, 95% CI: 1.049–1.137, p < 0.001) and hyperthyroidism (OR = 1.080, 95% CI: 1.026–1.137, p = 0.003) with the risk of OP. No causal relationships were identified between FT3, FT4, TSH, and the risk of OP (p > 0.05). The results of the multivariate Mendelian randomization (MVMR) analysis indicated that hyperthyroidism was no longer a risk factor for OP (OR = 0.984, 95% CI: 0.918–1.055, p = 0.657), whereas hypothyroidism persisted as a risk factor (OR = 1.082, 95% CI: 1.021–1.147, p = 0.008). The mediated Mendelian randomization analysis revealed that hypothyroidism may exert an indirect effect on OP via triglycerides in large VLDL, mediating approximately 2.47% of the effect.
Conclusion: This study identifies a potential link between hypothyroidism and OP, possibly mediated indirectly via triglyceride levels in large VLDL. Further investigations are required to elucidate the direct or indirect causal mechanisms underlying this association.
American scholars project that by 2025, more than 3 million fractures will occur among osteoporosis patients in the United States, with associated medical costs expected to reach $25.3 billion (1). Osteoporosis (OP), a hallmark of aging, is a systemic metabolic bone disease characterized by decreased bone mineral density and increased fracture susceptibility, and, from a cytological perspective, by an imbalance in the functions of osteoblasts, osteoclasts, and adipocytes (2, 3). Multiple factors contribute to the development of OP, including insufficient calcium intake, smoking, alcohol consumption, long-term use of certain medications, and the presence of specific diseases (4).
Hyperthyroidism and hypothyroidism are the most prevalent thyroid dysfunctions, each influencing various physiological functions. In the United States, hypothyroidism affects approximately 5% of the population, while hyperthyroidism affects nearly 1% (5). Numerous observational studies have demonstrated an association between thyroid dysfunction and OP (6); however, the causal relationships between hyperthyroidism, hypothyroidism, TSH, FT4, FT3, and OP remain unclear.
To elucidate the causal relationship, Mendelian randomization (MR) analysis was conducted in this study. MR analysis is a widely used method that evaluates potential causal relationships between exposure factors and outcomes using instrumental variables (IVs) and is less susceptible to confounders, thereby producing more reliable results (7). Therefore, this study employed MR analysis to investigate the potential causal relationship between thyroid dysfunction, thyroid function, and the risk of OP.
Patients with type 1 diabetes have a higher likelihood of developing thyroid dysfunction, and type 1 diabetes is also a known risk factor for OP. Therefore, this study incorporated type 1 diabetes into the multivariate Mendelian randomization (MVMR) analysis (8). This was done to explore the effect of adjusting for type 1 diabetes on OP, and subsequently to assess whether a causal relationship exists between thyroid dysfunction and OP.
Metabolomics is an emerging field of research that offers novel insights into how metabolites influence various physiological systems, particularly the endocrine system (9). The metabolome consists of metabolites and their associated metabolic pathways (10). A study analyzed plasma metabolites from 41 individuals with subclinical hypothyroidism, 45 hypothyroid patients, and 40 euthyroid individuals using metabolomics and machine learning algorithms. The findings revealed that the metabolic patterns of the hypothyroid and subclinical hypothyroid groups differed significantly from those of the euthyroid group. Specifically, primary bile acid biosynthesis, lysine degradation, tryptophan metabolism, steroid hormone biosynthesis, and purine metabolism were significantly impacted (11). However, few studies have focused on circulating metabolites in plasma.
Several observational studies have demonstrated an association between hypothyroidism and dyslipidemia, as well as a link between dyslipidemia and OP (12, 13). However, it remains unclear whether hypothyroidism influences the development of OP through circulating metabolites. Therefore, this study will explore this question using mediated Mendelian randomization analysis.
Our data were sourced from the widely recognized IEU OpenGWAS database, the ThyroidOmics Consortium, and the FinnGen database (Table 1). As these databases were externally developed, ethical approval was not required. In this study, hyperthyroidism, hypothyroidism, TSH, FT4, and FT3 were used as exposure variables, with OP as the outcome variable. An MR analysis was conducted to investigate potential causal relationships between these factors (Figure 1).
In MR analysis, instrumental variables are genetic variants, often single nucleotide polymorphisms (SNPs), used to study the impact of genetic variation (14). The screening criteria were as follows: (1) instrumental variables must be strongly correlated with exposure variables. We set a significance threshold of p < 5 × 10^-8 to identify SNPs with strong associations. (2) Instrumental variables must be independent, meaning they are not correlated with confounders (15). Linkage disequilibrium among SNPs was addressed by setting kb = 10,000 and r2 ≤ 0.001 (16). (3) Instrumental variables should affect the outcome exclusively through the exposure variables (17). The F-statistic assesses the strength of the correlation between instrumental variables and exposure factors. Thus, we calculated the F-statistic for each exposure-related SNP (F = β2/SE2) (18), where β represents the effect estimate of the exposure factor and SE is the standard error. We selected IVs with F > 10 to reduce bias from weak instruments (19). Subsequently, we identified SNPs where the exposure and outcome overlapped to remove palindromic SNPs and misaligned alleles.
To determine whether a causal relationship exists between genetic exposure and outcome, we employed several commonly used MR methods, including inverse-variance weighted (IVW), MR-Egger regression, weighted median (WM), weighted mode techniques, and MR-PRESSO. IVW is widely regarded as the primary statistical method in MR, and thus, this study adopts IVW as the main analysis approach. The fixed-effects IVW model operates under the assumption that all instrumental variables (IVs) are valid, whereas the random-effects IVW model assumes that not all IVs are valid (20). Therefore, we initially conducted a heterogeneity test using R. If heterogeneity was detected (i.e., p < 0.05), the random-effects model was applied (21). Subsequently, a pleiotropy test was conducted, and if p > 0.05, it indicated that the likelihood of pleiotropy was minimal or nonexistent and could be disregarded (22). Additionally, the intercept of the MR-Egger regression was not statistically significant, and the funnel plot was symmetric around zero, further confirming the absence of pleiotropy. A Cochran Q test was also conducted to assess SNP heterogeneity, which was statistically significant at p < 0.05 (23).In this study, we used the “TwoSampleMR,” “MendelianRandomization,” and “MR-PRESSO” packages in R (version 4.4.0) for MR analysis (24).
Data on 249 circulating metabolites, collected and provided by Nightingale Health, were obtained from the IEU Open-GWAS Project public database (25). Circulating metabolites were used as mediators to decompose the direct and indirect effects of hypothyroidism on OP through a two-step Mendelian randomization (TSMR) approach. The total effect of hypothyroidism on OP was denoted as TE, the effect of hypothyroidism on circulating metabolites as β1, and the effect of circulating metabolites on OP as β2. The indirect effect (IE), representing the causal pathway through which hypothyroidism influences the occurrence of OP via the mediator, was estimated using the product of coefficients method (β1 × β2). Accordingly, the proportion of the total effect mediated by the circulating metabolites was calculated as “indirect effect/total effect (IE/TE),” with the direct effect (DE) determined as TE - IE (26). Confidence intervals (CIs) for the mediated proportions were calculated using the delta method (27).
In this study, based on the selection criteria for instrumental variables, SNPs without a palindromic structure, no linkage disequilibrium, and an F-statistic >10 were selected. Specifically, 104, 11, 6, 60, and 161 SNPs were selected as valid instrumental variables (IVs) for hypothyroidism, hyperthyroidism, FT3, FT4, and TSH, respectively.
To investigate the effects of thyroid disease and thyroid function on OP, a TSMR analysis was conducted. Specifically, we examined the causal relationships between hypothyroidism, hyperthyroidism, FT3, FT4, TSH, and the risk of OP.
The results indicated a causal relationship between hypothyroidism and an increased risk of OP (IVW: OR = 1.092, 95% CI: 1.049–1.137, p < 0.001). A causal relationship was also found between hyperthyroidism and OP risk (IVW: OR = 1.080, 95% CI: 1.026–1.137, p = 0.003). However, no causal relationships were observed between FT3, FT4, TSH, and OP risk (p > 0.05) (Figure 2). The intercept of the MR-Egger regression was not statistically significant, and the symmetry of the funnel plot around zero suggested that the selected instrumental variables were minimally, affected by horizontal pleiotropy (Figure 3). A multiplicity test conducted using R software showed that all p-values were greater than 0.05, indicating negligible or no multiplicity effects among the instrumental variables. Leave-one-out sensitivity analysis revealed that all SNPs in the hyperthyroidism and hypothyroidism groups were on one side of the distribution. The p-values from the heterogeneity test were greater than 0.05, indicating no biased SNPs (Figure 4).
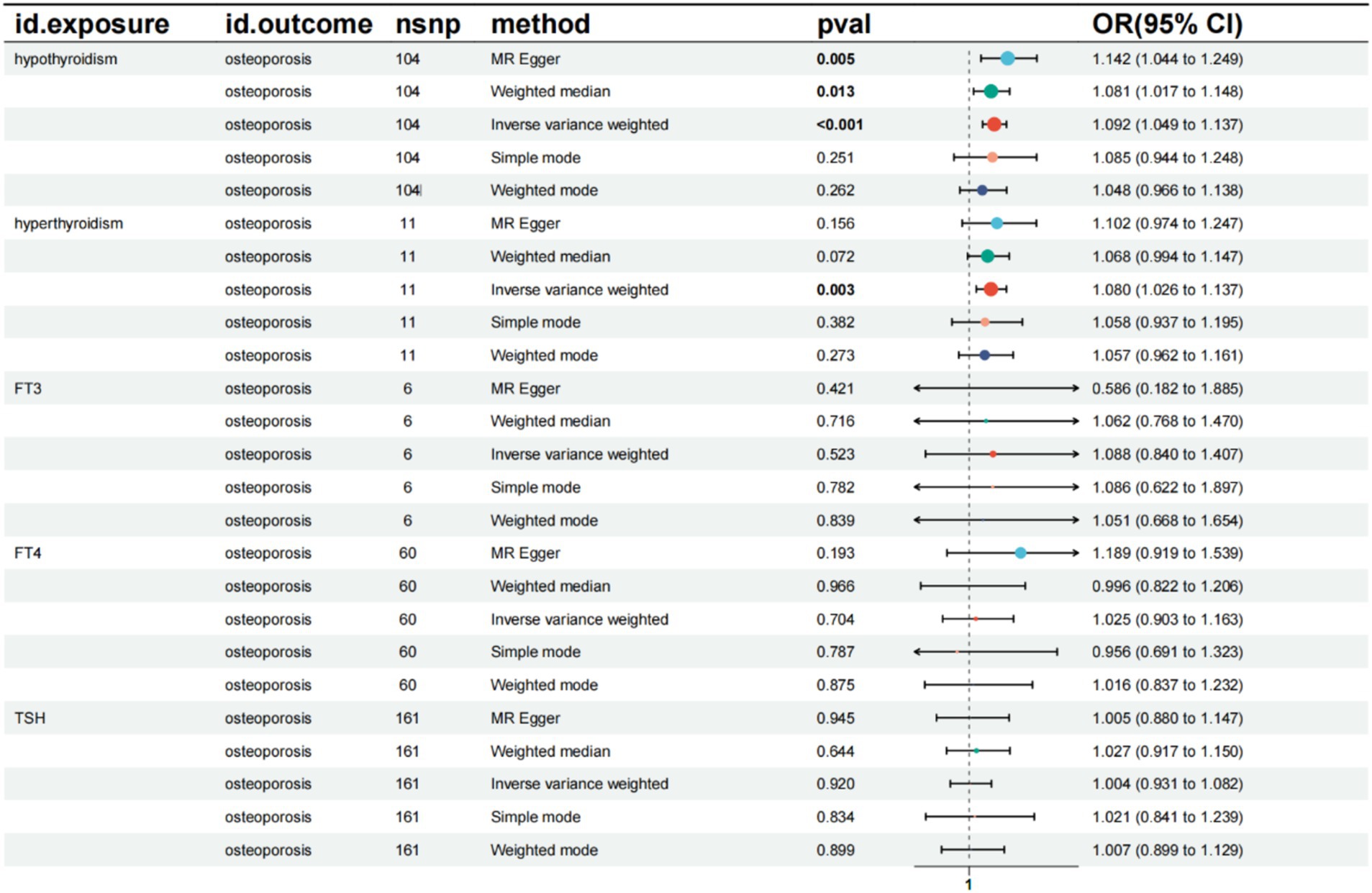
Figure 2. Summary of the five Mendelian randomization analysis methods. CI, confidence interval; OR, odds ratio; TSH, Thyroid stimulating hormone.
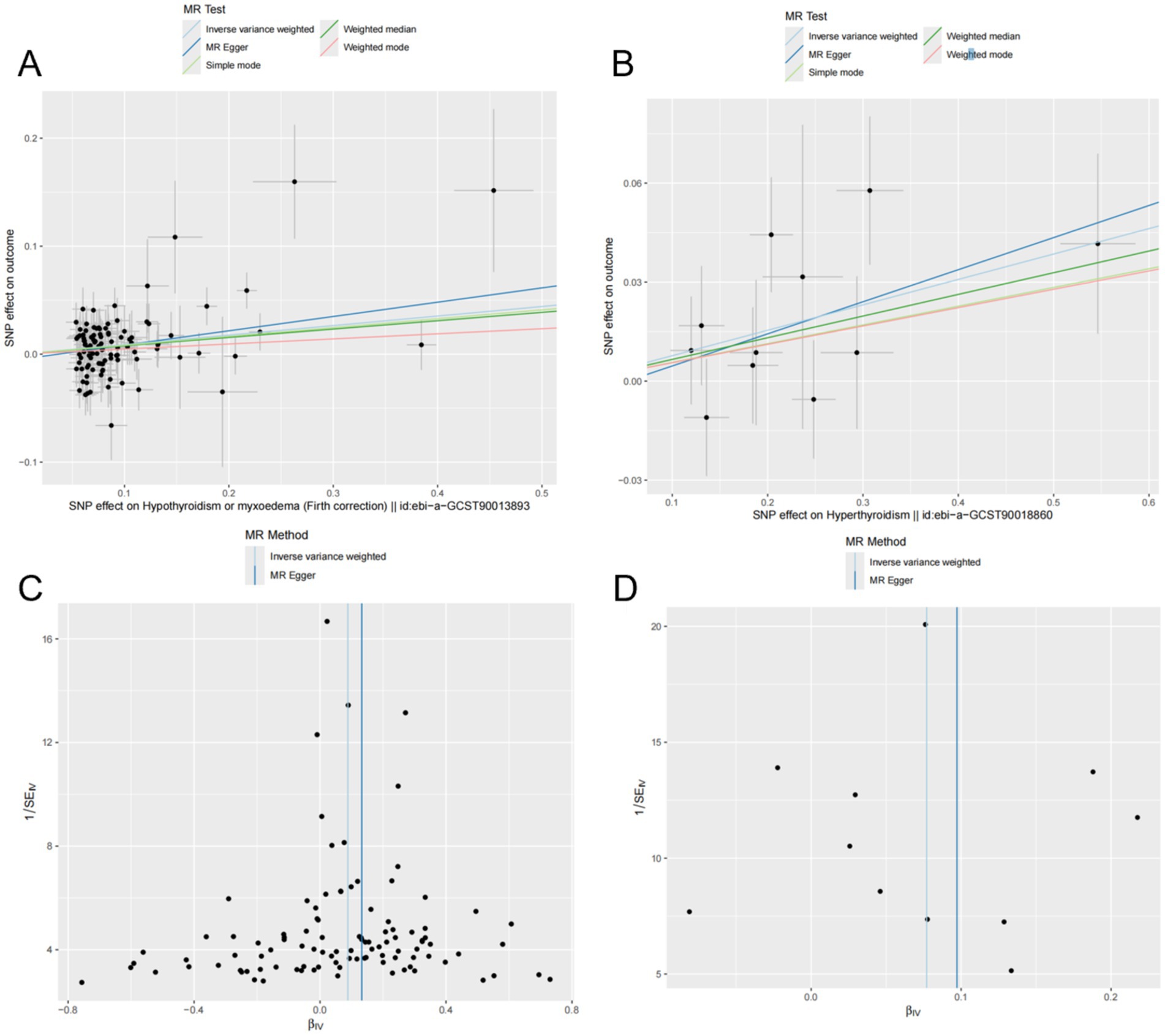
Figure 3. Mendelian randomization (MR) analyses of hyperthyroidism, hypothyroidism, and OP were conducted using multiple models. (A,B) Scatter plots depict the potential causal relationships between hypothyroidism (A), hyperthyroidism (B), and OP, where the slopes represent the estimated magnitude of causality. (C,D) Funnel plots present heterogeneity tests, highlighting causal effect estimates of hypothyroidism (C) and hyperthyroidism (D) on OP. The lines represent causal effect estimates derived from the inverse variance weighting and MR-Egger models.
Diabetes mellitus is a known risk factor for OP and fractures, with patients with type 1 diabetes mellitus having an elevated risk of developing OP (28). Therefore, type 1 diabetes was included in this study for MVMR analysis. The results indicated that hyperthyroidism was no longer a risk factor for OP (OR = 0.984, 95% CI: 0.918–1.055, p = 0.657). However, hypothyroidism remained a risk factor for OP (OR = 1.082, 95% CI: 1.021–1.147, p = 0.008), and type 1 diabetes also emerged as a risk factor for OP (OR = 1.049, 95% CI: 1.029–1.069, p < 0.001) (Figure 5).
We investigated the effect of hypothyroidism on circulating metabolites in 249 individuals and identified a significant correlation between hypothyroidism and 59 circulating metabolites after applying FDR correction (p < 0.05) (29). Hypothyroidism was found to be a risk factor for citrate (OR = 1.021, 95% CI: 1.001–1.032, p = 0.005) and creatinine (OR = 1.018, 95% CI: 1.006–1.030, p = 0.017). It was also inversely associated with ApoA1 (OR = 0.980, 95% CI: 0.966–0.995, p = 0.038), cholines (OR = 0.976, 95% CI: 0.962–0.990, p = 0.011), the concentration of large high-density lipoprotein particles (HDL) (OR = 0.975, 95% CI: 0.960–0.990, p = 0.013), large cholesteryl esters in LDL (OR = 0.981, 95% CI: 0.965–0.996, p = 0.047), cholesteryl esters in large very-low-density lipoproteins (VLDL) (OR = 0.983, 95% CI: 0.970–0.997, p = 0.047), triglycerides in large VLDL (OR = 0.982, 95% CI: 0.969–0.996, p = 0.045), linoleic acid (LA) (OR = 0.970, 95% CI: 0.951–0.990, p = 0.018), low-density lipoprotein cholesterol (LDL) (OR = 0.981, 95% CI: 0.966–0.994, p = 0.032), free cholesterol in medium VLDL (OR = 0.981, 95% CI: 0.968–0.996, p = 0.039), concentration of medium VLDL particles (OR = 0.980, 95% CI: 0.968–0.993, p = 0.020), and phospholipids in small HDL (OR = 0.971, 95% CI: 0.960–0.985, p = 0.002), along with other protective factors. None of the selected SNPs exhibited significant horizontal pleiotropy (p > 0.05) or heterogeneity (p < 0.05). A Mendelian randomization analysis was then conducted with these 59 circulating metabolites as exposure variables and OP as the outcome variable. A causal effect was found only for triglycerides in large VLDL (OR = 0.885, 95% CI: 0.787–0.995, p = 0.042). Subsequently, a mediated Mendelian randomization analysis was performed, revealing that hypothyroidism exerted an indirect effect on OP through triglycerides in large VLDL, mediating 2.47% of the total effect (Figure 6).
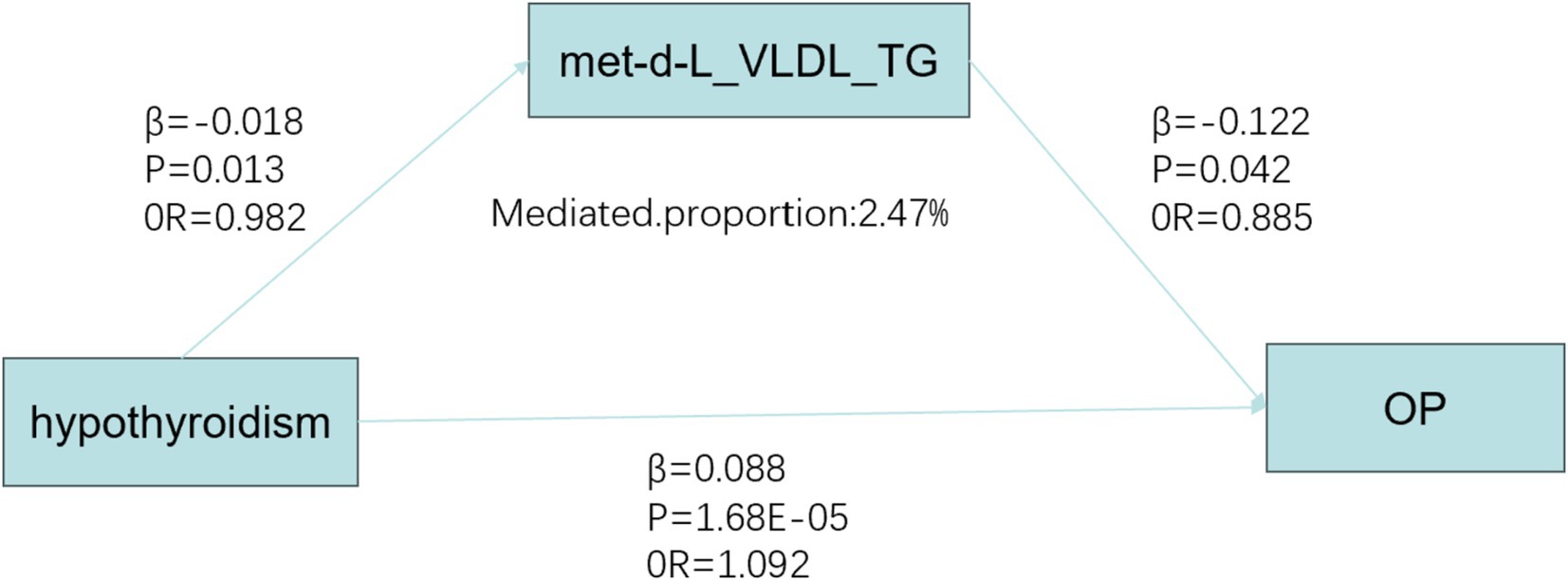
Figure 6. Mediated Mendelian analysis. OP, osteoporosis; OR, odds ratio; met-d-L_VLDL_TG, Triglycerides in large very low-density lipoprotein.
In this study, we employed univariate and MVMR analyses, along with mediation analyses, to evaluate the relationships between hypothyroidism, circulating metabolites, and OP. Our findings demonstrated that hypothyroidism and hyperthyroidism acted as risk factors for OP in two-sample MR analyses. However, after adjusting for confounders, including diabetes mellitus, in MVMR models, hyperthyroidism was no longer a significant risk factor. Furthermore, hypothyroidism may elevate the risk of OP by altering blood metabolite levels, with triglycerides in large VLDL potentially playing a mediating role, contributing to approximately 2.7% of the overall association between hypothyroidism and OP risk.
Thyroid hormone promotes the growth of long bones, stimulates the growth and differentiation of osteoblasts, and increases osteoclast activity (30). Yang et al. investigated the relationship between thyroid autoimmune diseases and OP within the normal range of TSH. Their results indicated that thyroid peroxidase antibodies (TPO) levels were significantly higher in the serum of patients with OP compared to those without the condition (p < 0.05) (31). TPO is a key indicator for diagnosing thyroid autoimmune disorders, which are most commonly associated with hyperthyroidism and hypothyroidism. These findings suggest a relationship between hyperthyroidism, hypothyroidism, and OP, which is consistent with the genetic conclusions drawn in this study. We propose that hypothyroidism is a risk factor for OP, a viewpoint shared by other scholars (32). Some studies have found that higher serum concentrations of TSH are associated with increased bone density and a lower likelihood of developing OP. The proposed mechanism is that TSH promotes osteoblast differentiation and proliferation. Additionally, a correlation has been identified between elevated FT3 and FT4 levels and an increased risk of fractures and reduced bone density (33). Studies have also shown that in older adults with normal TSH levels, a positive correlation between TSH concentration and OP still exists (34). In this study, from a genetic perspective, we did not find a definitive causal relationship between TSH and OP. This may be due to population selection bias in the data used, indicating the need for further research. Some scholars analyzed 3,338 men without thyroid or bone diseases and found no association between TSH, FT4, and either fracture or bone mineral density, which is consistent with our findings (35). In this study, we also included type 1 diabetes mellitus in the multivariate Mendelian analysis and found that both hypothyroidism and type 1 diabetes mellitus were risk factors for OP, though the underlying mechanism remains unclear. A possible explanation is a synergistic effect between hypothyroidism and type 1 diabetes.
Mediated MR approaches enable the investigation of potential causal pathways linking genetic risk factors to disease outcomes via specific biomarkers or metabolic pathways. In this study, we employed the genetic instrumental variable for hypothyroidism to evaluate its indirect influence on OP risk, mediated through circulating metabolites. We found that hypothyroidism may have an indirect effect on OP via triglycerides in large VLDL, though the underlying mechanism remains unknown. In a study involving patients in the early post-thyroidectomy phase, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), and the TC/HDL-C ratio were elevated in the thyroid hormone-naïve group compared to the control group (36). Cai et al. (37) analyzed 27 pregnant women with hypothyroidism and 28 healthy pregnant women, and found that plasma lipid levels in the hypothyroid group were significantly higher than in the healthy group. Zhang et al. (38) conducted a TSMR analysis and identified a causal relationship between dyslipidemia and OP, where VLDL was found to be a protective factor for OP (OR = 0.948), consistent with our findings. They further suggested that this result might be related to abnormal methylation gene modifications. In a study investigating the relationship between triglyceride-glucose (TyG) index and bone mass, findings indicated a significant association between elevated TyG and an increased risk of OP (39). Elevated triglycerides (TG) in VLDL may disrupt the balance between bone formation and resorption, but further research is required to elucidate the precise role of these metabolites in bone health.
Our study has several strengths, including multiple data sources, a large sample size, and the use of heterogeneity, multiplicity, sensitivity analyses, and FDR correction to ensure the robustness of the findings. Notably, this study is the first to use circulating metabolites as mediators to investigate the indirect effect of hypothyroidism on OP, the first to explore the causal effect of FT3 on OP, and the first to examine the impact of MVMR analyses on type 1 diabetes mellitus and thyroid disease in relation to the causal effect on OP.
Limitations of this study include: first, the study population was European, which may not be representative of the global population. Second, the only intervening variable analyzed was type 1 diabetes mellitus, while many other factors contribute to the development of OP, suggesting that future studies should incorporate additional variables for multivariate analysis. Third, due to the lack of distinction between OP subtypes in publicly available GWAS data on functional outcomes in hypothyroidism, it is currently not feasible to evaluate functional outcomes across specific OP subtypes. Fourthly, we only used summary data and did not assess and analyze other factors such as different age and gender. Moreover, full-scale clinical trials are necessary to validate these clinical conclusions. Therefore, a more comprehensive GWAS database and further trials are needed to clarify the mechanisms by which thyroid disease influences OP through lipid metabolism.
In this study, we evaluated the causal relationships between hypothyroidism, hyperthyroidism, FT3, FT4, TSH, and OP using TSMR analysis. Our findings suggest that hypothyroidism is a risk factor for OP, while a causal relationship between FT3, FT4, TSH, and OP has not yet been supported, and whether hyperthyroidism is a risk factor for OP requires further investigation. We also conducted a mediation analysis and found that hypothyroidism may have an indirect effect on OP through triglycerides in large VLDL, with a mediation proportion of 2.47%. This study highlights the importance of reminding patients with thyroid disease, especially those with concomitant dyslipidemia, and clinicians that lipid control should be emphasized in the treatment of thyroid disorders. Additionally, proactive measures should be taken to prevent the development and progression of osteoporosis.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number (s) can be found in the article/Supplementary material.
The study did not require ethical approval according to local legislation and institutional requirements, and no written informed consent was obtained from participants or their legal guardians/next of kin in accordance with these guidelines.
PL: Validation, Investigation, Formal analysis, Conceptualization, Writing – original draft, Methodology, Writing – review & editing, Data curation. YQ: Software, Resources, Methodology, Investigation, Formal analysis, Conceptualization, Writing – original draft, Writing – review & editing, Data curation. MeZ: Writing – original draft, Visualization, Validation, Supervision, Conceptualization, Writing – review & editing, Data curation. YZ: Writing – original draft, Visualization, Validation, Supervision, Methodology, Writing – review & editing, Data curation. NY: Writing – original draft, Visualization, Validation, Supervision, Formal analysis, Data curation, Conceptualization, Writing – review & editing. MiZ: Methodology, Conceptualization, Writing – review & editing. WW: Visualization, Validation, Supervision, Project administration, Data curation, Writing – review & editing, Writing – original draft. ML: Visualization, Validation, Investigation, Formal analysis, Conceptualization, Writing – review & editing, Supervision, Project administration, Writing – original draft, Methodology, Data curation.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Appreciation is also due to the MRC IEU Open GWAS project and the FinnGen database for their valuable contributions in making data accessible to the public.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1453502/full#supplementary-material
1. Burge, R, Dawson-Hughes, B, Solomon, DH, Wong, JB, King, A, and Tosteson, A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. (2007) 22:465–75. doi: 10.1359/jbmr.061113
2. Letarouilly, JG, Broux, O, and Clabaut, A. New insights into the epigenetics of osteoporosis. Genomics. (2019) 111:793–8. doi: 10.1016/j.ygeno.2018.05.001
3. Aibar-Almazán, A, Voltes-Martínez, A, Castellote-Caballero, Y, Afanador-Restrepo, DF, Carcelén-Fraile, MC, and López-Ruiz, E. Current status of the diagnosis and Management of Osteoporosis. Int J Mol Sci. (2022) 23:9465. doi: 10.3390/ijms23169465
4. Leboff, MS, Greenspan, SL, Insogna, KL, Lewiecki, EM, Saag, KG, Singer, AJ, et al. The clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. (2022) 33:2049–102. doi: 10.1007/s00198-021-05900-y
5. Vanderpump, MP. The epidemiology of thyroid disease. Br Med Bull. (2011) 99:39–51. doi: 10.1093/bmb/ldr030
6. Lee, K, Lim, S, Park, H, Woo, HY, Chang, Y, Sung, E, et al. Subclinical thyroid dysfunction, bone mineral density, and osteoporosis in a middle-aged Korean population. Osteoporos Int. (2020) 31:547–55. doi: 10.1007/s00198-019-05205-1
7. Gao, N, Kong, M, Li, X, Wei, D, Zhu, X, Hong, Z, et al. Systemic lupus erythematosus and cardiovascular disease: a Mendelian randomization study. Front Immunol. (2022) 13:908831. doi: 10.3389/fimmu.2022.908831
8. Nederstigt, C, Corssmit, EP, De Koning, EJ, and Dekkers, OM. Incidence and prevalence of thyroid dysfunction in type 1 diabetes. J Diabetes Complicat. (2016) 30:420–5. doi: 10.1016/j.jdiacomp.2015.12.027
9. Rinschen, MM, Ivanisevic, J, Giera, M, and Siuzdak, G. Identification of bioactive metabolites using activity metabolomics. Nat Rev Mol Cell Biol. (2019) 20:353–67. doi: 10.1038/s41580-019-0108-4
10. Wishart, DS. Metabolomics for investigating physiological and pathophysiological processes. Physiol Rev. (2019) 99:1819–75. doi: 10.1152/physrev.00035.2018
11. Shao, F, Li, R, Guo, Q, Qin, R, Su, W, Yin, H, et al. Plasma metabolomics reveals systemic metabolic alterations of subclinical and clinical hypothyroidism. J Clin Endocrinol Metab. (2022) 108:13–25. doi: 10.1210/clinem/dgac555
12. Wei, X, Zhang, Y, Sun, C, Qi, B, Huang, X, Chen, M, et al. Elucidating the relationship between dyslipidemia and osteoporosis: a multicenter, prospective cohort study protocol. Front Cardiovasc Med. (2022) 9:901786. doi: 10.3389/fcvm.2022.901786
13. Han, Y, Wang, C, Zhang, L, Zhu, J, Zhu, M, Li, Y, et al. Menopausal impact on the association between thyroid dysfunction and lipid profiles: a cross-sectional study. Front Endocrinol. (2022) 13:853889. doi: 10.3389/fendo.2022.853889
14. Larsson, SC, Butterworth, AS, Burgess, S, Zhu, J, Zhu, M, and Li, Y. Mendelian randomization for cardiovascular diseases: principles and applications. Eur Heart J. (2023) 44:4913–24. doi: 10.1093/eurheartj/ehad736
15. Burgess, S, and Labrecque, JA. Mendelian randomization with a binary exposure variable: interpretation and presentation of causal estimates. Eur J Epidemiol. (2018) 33:947–52. doi: 10.1007/s10654-018-0424-6
16. Myers, TA, Chanock, SJ, and Machiela, MJ. LdlinkR: an R package for rapidly calculating linkage disequilibrium statistics in diverse populations. Front Genet. (2020) 11:157. doi: 10.3389/fgene.2020.00157
17. Van Kippersluis, H, and Rietveld, CA. Pleiotropy-robust Mendelian randomization. Int J Epidemiol. (2018) 47:1279–88. doi: 10.1093/ije/dyx0002
18. Li, H, Pan, X, Zhang, S, Shen, X, Li, W, Shang, W, et al. Association of autoimmune diseases with the occurrence and 28-day mortality of sepsis: an observational and Mendelian randomization study. Crit Care. (2023) 27:476. doi: 10.1186/s13054-023-04763-5
19. Fang, P, Liu, X, Qiu, Y, Wang, Y, Wang, D, Zhao, J, et al. Exploring causal correlations between inflammatory cytokines and ankylosing spondylitis: a bidirectional mendelian-randomization study. Front Immunol. (2023) 14:1285106. doi: 10.3389/fimmu.2023.1285106
20. Lin, Z, Deng, Y, and Pan, W. Combining the strengths of inverse-variance weighting and egger regression in Mendelian randomization using a mixture of regressions model. PLoS Genet. (2021) 17:e1009922. doi: 10.1371/journal.pgen.1009922
21. Zhou, J, Ye, Z, Wei, P, Yi, F, Ouyang, M, Xiong, S, et al. Effect of basal metabolic rate on osteoporosis: a Mendelian randomization study. Front Public Health. (2023) 11:1096519. doi: 10.3389/fpubh.2023.1096519
22. Ding, M, Zhang, Z, Chen, Z, Song, J, Wang, B, and Jin, F. Association between periodontitis and breast cancer: two-sample Mendelian randomization study. Clin Oral Investig. (2023) 27:2843–9. doi: 10.1007/s00784-023-04874-x
23. Nagayoshi, M, Hishida, A, Shimizu, T, Kato, Y, Kubo, Y, Okada, R, et al. Bmi and Cardiometabolic traits in Japanese: a Mendelian randomization study. J Epidemiol. (2024) 34:51–62. doi: 10.2188/jea.JE20220154
24. Hemani, G, Zheng, J, Elsworth, B, Wade, KH, Haberland, V, Baird, D, et al. The Mr-Base platform supports systematic causal inference across the human phenome. elife. (2018) 7:e34408. doi: 10.7554/eLife.34408
25. Ritchie, SC, Surendran, P, Karthikeyan, S, Lambert, SA, Bolton, T, Pennells, L, et al. Quality control and removal of technical variation of Nmr metabolic biomarker data in ~120,000 Uk biobank participants. Sci Data. (2023) 10:64. doi: 10.1038/s41597-023-01949-y
26. Carter, AR, Sanderson, E, Hammerton, G, Richmond, RC, Davey Smith, G, Heron, J, et al. Mendelian randomisation for mediation analysis: current methods and challenges for implementation. Eur J Epidemiol. (2021) 36:465–78. doi: 10.1007/s10654-021-00757-1
27. Mackinnon, DP, Lockwood, CM, Hoffman, JM, West, SG, and Sheets, V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. (2002) 7:83–104. doi: 10.1037/1082-989X.7.1.83
28. Vilaca, T, and Eastell, R. Antiresorptive versus anabolic therapy in managing osteoporosis in people with type 1 and type 2 diabetes. Jbmr Plus. (2023) 7:e10838. doi: 10.1002/jbm4.10838
29. Deng, K, Shuai, M, Zhang, Z, Jiang, Z, Fu, Y, Shen, L, et al. Temporal relationship among adiposity, gut microbiota, and insulin resistance in a longitudinal human cohort. BMC Med. (2022) 20:171. doi: 10.1186/s12916-022-02376-3
30. Kenkre, JS, and Bassett, J. The bone remodelling cycle. Ann Clin Biochem. (2018) 55:308–27. doi: 10.1177/0004563218759371
31. Yang, L, Wang, H, Guo, J, Zheng, G, Wei, D, and Zhang, T. Low Normal Tsh levels and thyroid autoimmunity are associated with an increased risk of osteoporosis in Euthyroid postmenopausal women. Endocr Metab Immune Disord Drug Targets. (2021) 21:859–65. doi: 10.2174/1871530320666200810144506
32. González-Rodríguez, LA, Felici-Giovanini, ME, and Haddock, L. Thyroid dysfunction in an adult female population: a population-based study of Latin American vertebral osteoporosis study (Lavos) - Puerto Rico site. P R Health Sci J. (2013) 32:57–62.
33. Deng, T, Zhang, W, Zhang, Y, Zhang, M, Huan, Z, Yu, C, et al. Thyroid-stimulating hormone decreases the risk of osteoporosis by regulating osteoblast proliferation and differentiation. BMC Endocr Disord. (2021) 21:49. doi: 10.1186/s12902-021-00715-8
34. Leader, A, Ayzenfeld, RH, Lishner, M, Cohen, E, Segev, D, and Hermoni, D. Thyrotropin levels within the lower normal range are associated with an increased risk of hip fractures in euthyroid women, but not men, over the age of 65 years. J Clin Endocrinol Metab. (2014) 99:2665–73. doi: 10.1210/jc.2013-2474
35. Siru, R, Alfonso, H, Chubb, SAP, Golledge, J, Flicker, L, and Yeap, BB. Subclinical thyroid dysfunction and circulating thyroid hormones are not associated with bone turnover markers or incident hip fracture in older men. Clin Endocrinol. (2018) 89:93–9. doi: 10.1111/cen.13615
36. Al-Janabi, G, Hassan, HN, and Al-Fahham, A. Biochemical changes in patients during hypothyroid phase after thyroidectomy. J Med Life. (2022) 15:104–8. doi: 10.25122/jml-2021-0297
37. Cai, Y, Xu, Y, Ban, Y, Li, J, Sun, Z, Zhang, M, et al. Plasma lipid profile and intestinal microflora in pregnancy women with hypothyroidism and their correlation with pregnancy outcomes [J]. Front Endocrinol. (2021) 12:792536. doi: 10.3389/fendo.2021.792536
38. Zhang, Z, Duan, Y, and Huo, J. Lipid metabolism, methylation aberrant, and osteoporosis: a multi-omics study based on Mendelian randomization. Calcif Tissue Int. (2024) 114:147–56. doi: 10.1007/s00223-023-01160-6
Keywords: circulating metabolites, Mendelian randomization, mediation analysis thyroid dysfunction, osteoporosis, thyroid-related hormones
Citation: Leng P, Qiu Y, Zhou M, Zhu Y, Yin N, Zhou M, Wu W and Liu M (2024) Hypothyroidism correlates with osteoporosis: potential involvement of lipid mediators. Front. Med. 11:1453502. doi: 10.3389/fmed.2024.1453502
Received: 23 June 2024; Accepted: 15 October 2024;
Published: 01 November 2024.
Edited by:
Prem Prakash Kushwaha, Case Western Reserve University, United StatesCopyright © 2024 Leng, Qiu, Zhou, Zhu, Yin, Zhou, Wu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Liu, cmFob3Nqb2ludEB3bXUuZWR1LmNvbQ==; Weili Wu, d2VpbGk4OTZAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.