- 1Department of Anesthesiology, First Hospital of Jilin University, Changchun, China
- 2Department of Hand and Foot Surgery, Orthopedics Center, First Hospital of Jilin University, Changchun, China
- 3Department of Urology Surgery, First Hospital of Jilin University, Changchun, China
Purpose: To evaluate the diagnostic performance of PSMA PET/CT, including [68Ga]Ga-PSMA-11 and [18F]DCFPyL, in comparison with the [99mTc]Tc-MDP bone scan (BS) in identifying bone metastases among prostate cancer patients.
Methods: A search was performed in the PubMed and Embase databases to locate pertinent publications from inception to February 12, 2024. The studies included were those that examined the diagnostic effectiveness of PSMA PET/CT (covering [68Ga]Ga-PSMA-11 and [18F]DCFPyL) compared to [99mTc]Tc-MDP BS in identifying bone metastases among prostate cancer patients. The quality of the selected studies was evaluated using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) checklist.
Results: The meta-analysis included nine articles involving 702 patients. The sensitivity of PSMA PET/CT was higher compared to [99mTc]Tc-MDP BS (0.98 vs. 0.85, P < 0.01), while the specificity of PSMA PET/CT was also higher than [99mTc]Tc-MDP BS (0.97 vs. 0.70,P < 0.01). In subgroup analysis, the sensitivity of [68Ga]Ga-PSMA-11 PET/CT was higher compared to [99mTc]Tc-MDP BS (0.98 vs. 0.86), while the specificity of [68Ga]Ga-PSMA-11 PET/CT was also higher than [99mTc]Tc-MDP BS (0.98 vs. 0.65).
Conclusion: Our meta-analysis demonstrates that PSMA PET/CT exhibits superior sensitivity and specificity in comparison with [99mTc]Tc-MDP BS for identifying bone metastases in prostate cancer patients. Further research with head-to-head design is necessary to validate these results and evaluate the clinical effectiveness of these imaging methods.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier PROSPERO CRD42024545112.
Introduction
Prostate cancer (PCa) is a prevalent malignancy among men, with bone metastases being a common and serious complication in its advanced stages (1, 2). While lymph nodes are the most common site for PCa metastasis, bone is the second most common site, affecting around 70% of patients with advanced disease (3). These metastases often lead to significant morbidity, including pain, fractures, and decreased quality of life, underscoring the critical need for early and accurate diagnosis (4, 5). Early detection of bone metastases is crucial for optimizing treatment plans and enhancing patient outcomes (6).
Traditional methods for diagnosing bone metastases in PCa include computed tomography (CT), magnetic resonance imaging (MRI), and biopsy (7). CT and MRI provide detailed anatomical information but may lack the sensitivity to detect early or small metastatic lesions (8, 9). Although biopsies are considered definitive, they are invasive and not always practical for assessing multiple sites (10). Consequently, these conventional tools have limitations in sensitivity, specificity, and overall diagnostic accuracy, prompting the need for more advanced imaging techniques (11).
Recent advancements have introduced PSMA PET/CT, which employs radiotracers such as [68Ga]Ga-PSMA-11 and [18F]DCFPyL, as a cutting-edge method for detecting PCa bone metastases. In contrast, the [99mTc]Tc-MDP BS remains a traditional and widely used technique in the diagnostic evaluation of these metastases (12). PSMA PET/CT is designed to target the PSMA protein, which is overexpressed in PCa cells, thereby providing a more targeted imaging approach (13). The radiotracers [68Ga]Ga-PSMA-11 and [18F]DCFPyL were approved by the FDA in December 2020 and May 2021, enhancing the specificity and effectiveness of PCa imaging (14). In contrast, [99mTc]Tc-MDP BS, a longstanding method for detecting bone metastases, function by highlighting areas of increased bone turnover (15). Despite their widespread use, there is ongoing debate regarding the diagnostic superiority of PSMA PET/CT compared to [99mTc]Tc-MDP BS, with conflicting evidence on their relative sensitivities and specificities (16).
This meta-analysis aims to conduct a head-to-head comparative evaluation of PSMA PET/CT (including [68Ga]Ga-PSMA-11 and [18F]DCFPyL) and [99mTc]Tc-MDP BS in diagnosing bone metastases about PCa.
Materials and methods
The meta-analysis adhered to the guidelines set by the Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy (PRISMA-DTA) (17). The protocol has been registered with PROSPERO under the registration number CRD42024545112. Additionally, we have provided the PRISMA checklist as Supplementary Table 1, which is now referenced in the manuscript.
Search strategy
A thorough search was performed in the PubMed and Embase databases to locate available publications from 2006 to February 12, 2024. The search used the keywords: (“Prostatic Neoplasms” OR “Prostatic Cancers” OR “Prostatic Cancer” OR “Prostate Cancers” OR “Prostate Cancer” OR “Prostatic Neoplasm” OR “Prostate Neoplasm” OR “Prostate Neoplasms” OR “Prostate tumor” OR “prostatic tumor”) AND (“Positron Emission Tomography Computed Tomography” OR “positron emission tomography/computed tomography” OR “PET/CT”) AND (“Bone scan” OR “Bone scintigraphy”) AND (“Bone metastasis” OR “Bone metastases”). No language or other filters were applied during the search. Supplementary Table 2 provides more details. Additionally, the reference lists of the included studies were manually reviewed to uncover further relevant studies. The search and study selection process was conducted independently by two reviewers. One reviewer identified seven of the nine included studies, while the other identified all nine, resulting in an overlap rate of approximately 78%. Discrepancies were resolved through discussion or by consulting a third reviewer to ensure accurate aggregation and inclusion of studies.
Inclusion and exclusion criteria
The inclusion criteria for our study were defined as follows: (1) Population (P): Individuals diagnosed with PCa; (2) Intervention (I): Utilization of PSMA PET/CT ([68Ga]Ga-PSMA-11 and [18F]DCFPyL) for the assessment of bone metastasis; (3) Comparator (C): Employment of [99mTc]Tc-MDP BS for the evaluation of bone metastasis; (4) Outcomes (O): The primary outcomes were sensitivity and specificity in patient-based analysis; (5) Study design (S): Both retrospective and prospective studies were included in the analysis.
Duplicated studies, case reports, abstracts, letters, reviews, meta-analyses, clearly irrelevant titles and abstracts, no head-to-head comparison and data not available were excluded.
Quality assessment
The quality of the included articles was assessed by two researchers independently utilizing the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool (18). This tool covers four domains: (1) patient selection, (2) index test, (3) reference standard, and (4) flow and timing. The risk of bias for each domain was classified as “high risk”, “low risk”, or “unclear risk”. For risk of bias, patient selection was rated as low risk if consecutive patients were included, high risk if there were inappropriate exclusions, and unclear if not specified. The index test was rated as low risk if the cut-off value was pre-specified, high risk if determined post hoc, and unclear if not reported. The reference standard was rated as low risk if diagnosed by two or more physicians or if pathology plus imaging was used, high risk if only one physician, and unclear if not reported. Flow and timing was rated as low risk if the time interval was less than three months, high risk if more than three months, and unclear if not described. For applicability concerns, patient selection was rated as high risk if the study population differed from our meta-analysis criteria, low risk if consistent, and unclear if not specified, while the index test and reference standard were both rated as low risk if consistent with our meta-analysis definitions. Detail information was provided in the Supplementary Table 3.
Data extraction
Two researchers independently conducted data extraction for all included papers. The extracted data covered the following categories: (1) author and year of publication, (2) radiotracer used, (3) study characteristics, including country, study design, reference standard, and study period, and (4) patient characteristics, including the number of patients, PSA level, mean or median age, Gleason score, and clinical indication. For the extraction of true positive (TP), true negative (TN), false positive (FP), and false negative (FN) values, if these were not directly provided in the articles, we utilized the calculator tool in RevMan 5.4 to back-calculate these values based on reported sensitivity and specificity.
In instances of disagreement, the researchers deliberated on the matter until they reached a consensus, ensuring the accuracy of the extracted data.
Statistical analysis
The bivariate random-effects model was employed to jointly assess sensitivity and specificity, providing a more accurate estimation of diagnostic performance. This method accounts for the correlation between sensitivity and specificity across studies. Confidence intervals were calculated based on the bivariate model, ensuring robust and reliable results. The degree of heterogeneity within and between groups was evaluated using the Cochrane Q and I2 statistics (19). Significant heterogeneity, defined as P < 0.05 or I2 > 50%, prompted further meta-regression and leave-one-out sensitivity analysis to identify its source.
Publication bias was assessed utilizing funnel plot analysis and Egger’s test (20). A P-value of less than 0.05 was deemed statistically significant for all tests. All statistical analyses were conducted using R software version 4.3.3 and Stata 15.1.
Results
Study selection
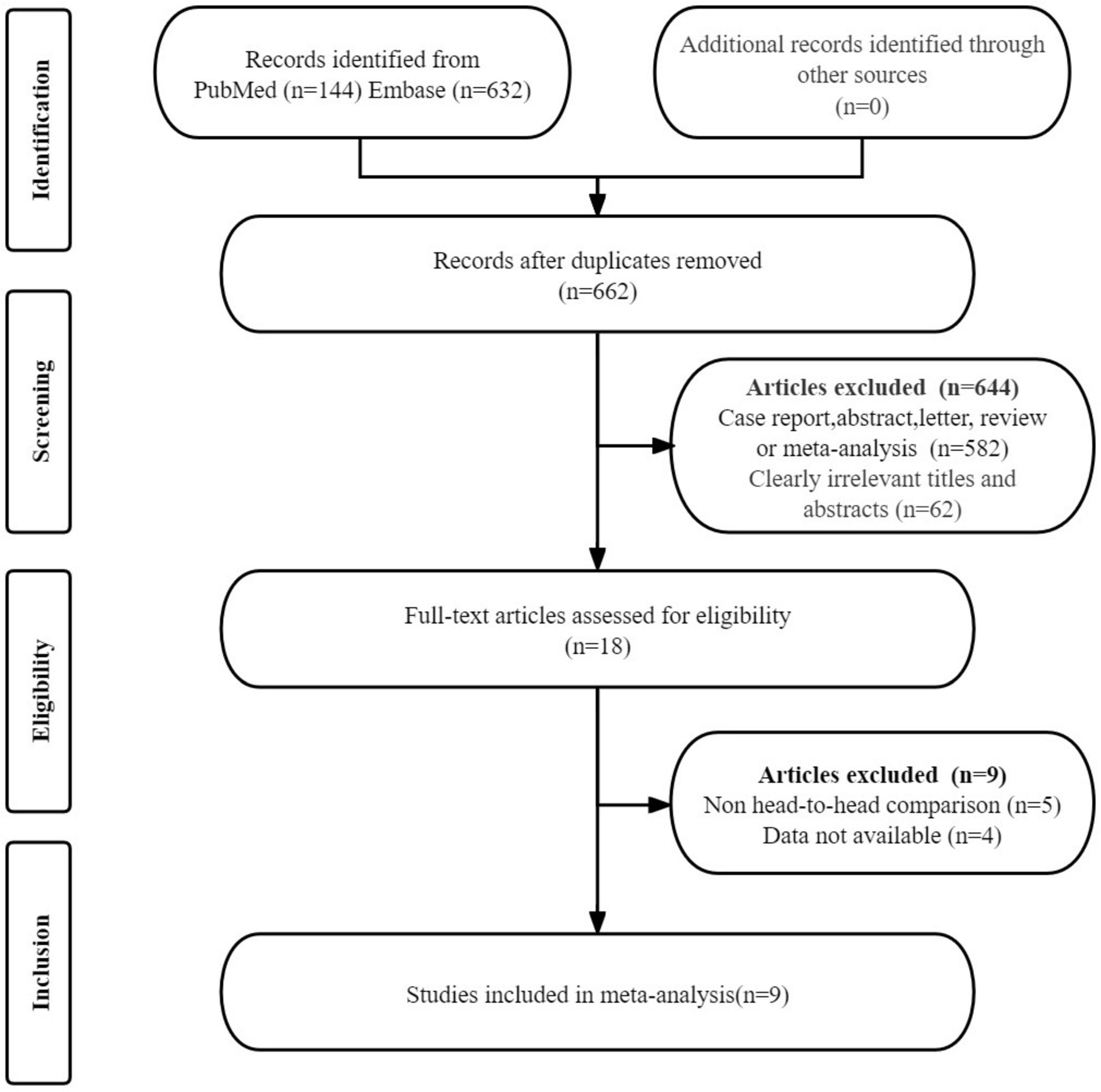
The initial search yielded 776 publications. After removing 114 duplicate studies, 644 studies were excluded on the basis that they did not meet the eligibility criteria. A detailed review of the full texts of the remaining 18 articles led to the exclusion of an additional nine studies: four studies were excluded due to the unavailability of data (TP, FP, FN, and TN), and five studies were excluded because they were not head-to-head comparison articles. Ultimately, nine articles that evaluated the diagnostic efficacy of PSMA PET/CT (including [68Ga]Ga-PSMA-11 and [18F]DCFPyL) and [99mTc]Tc-MDP BS were included in the meta-analysis (21–29). The article selection process is illustrated using the PRISMA flow diagram shown in Figure 1.
Study description and quality assessment
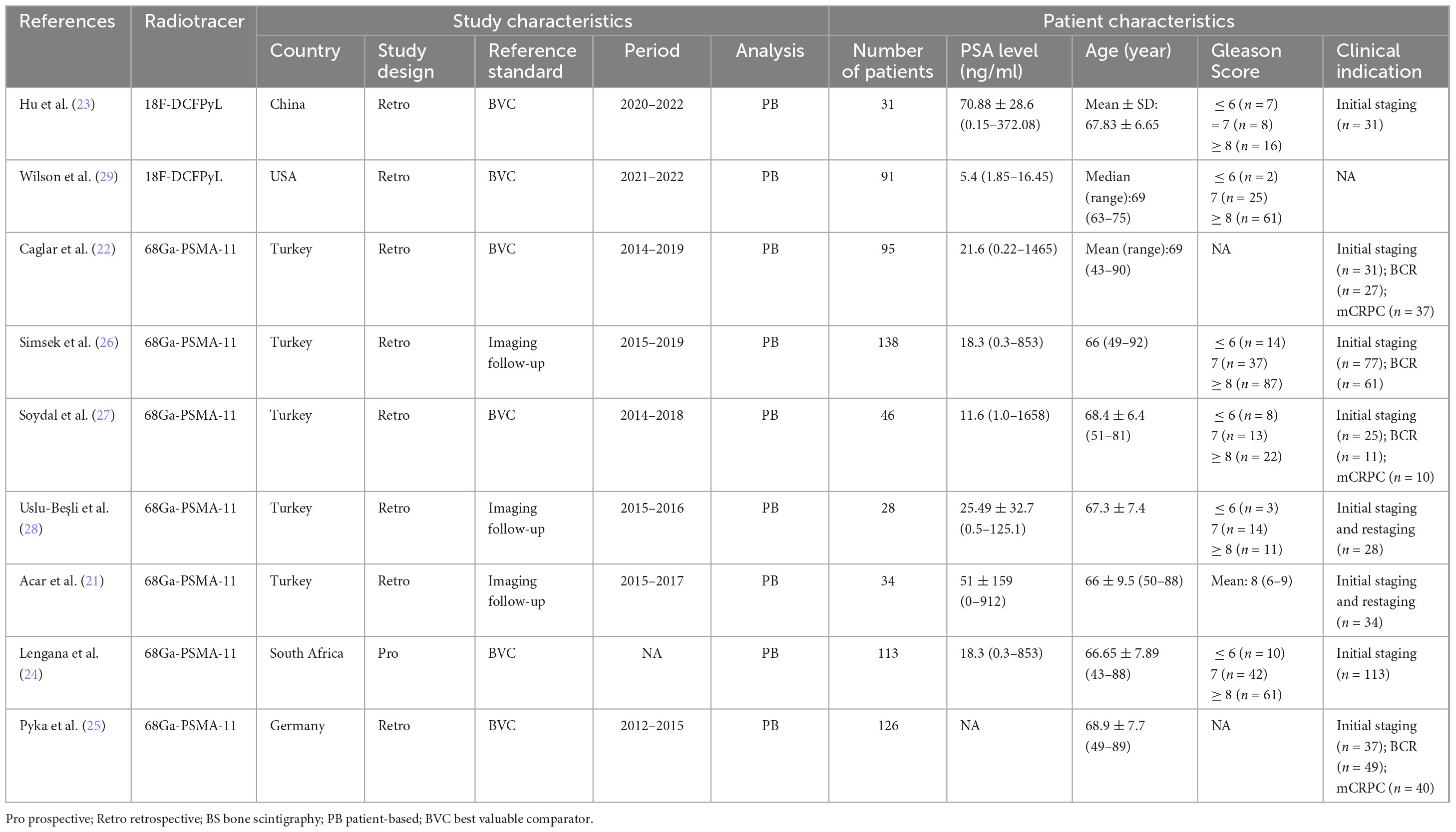
The nine eligible studies included 702 PCa patients (range from 28 to 138). Of these studies, eight were retrospective studies (21–23, 25–29), while one article was prospective study (24). Six articles used best valuable comparator (BVC) as the reference standard (22–25, 27, 29), while three relied on imaging follow-up (21, 26, 28). Regarding the radiotracer of PSMA PET/CT, 7 articles used [68Ga]Ga-PSMA-11 (21, 22, 24–28),while the remaining two articles used [18F]DCFPyL (23, 29). Among the included studies, four utilized planar BS (21, 22, 25, 27), three employed single photon emission computed tomography (SPECT) imaging (23, 24, 29), and two studies incorporated both planar BS and SPECT imaging (26, 28). A summary of the study and patient characteristics of the included studies is presented in Table 1.
The risk of bias for each study, as assessed utilizing the QUADAS-2 tool, is shown in Figure 2. For patient selection, three studies were rated as “unclear risk” due to a lack of information on whether consecutive patients were included (26–28). With regard to the index test, four studies were assigned an “unclear risk” rating due to a lack of information regarding the pre-determined cut-off values applied (21, 22, 25, 26). In terms of the reference standard, eight studies were rated as having an “unclear risk” due to the fact that the final diagnosis was not independently determined by two or more physicians (21, 22, 24–29). Overall, the quality assessment revealed no major concerns about the quality of the included studies.
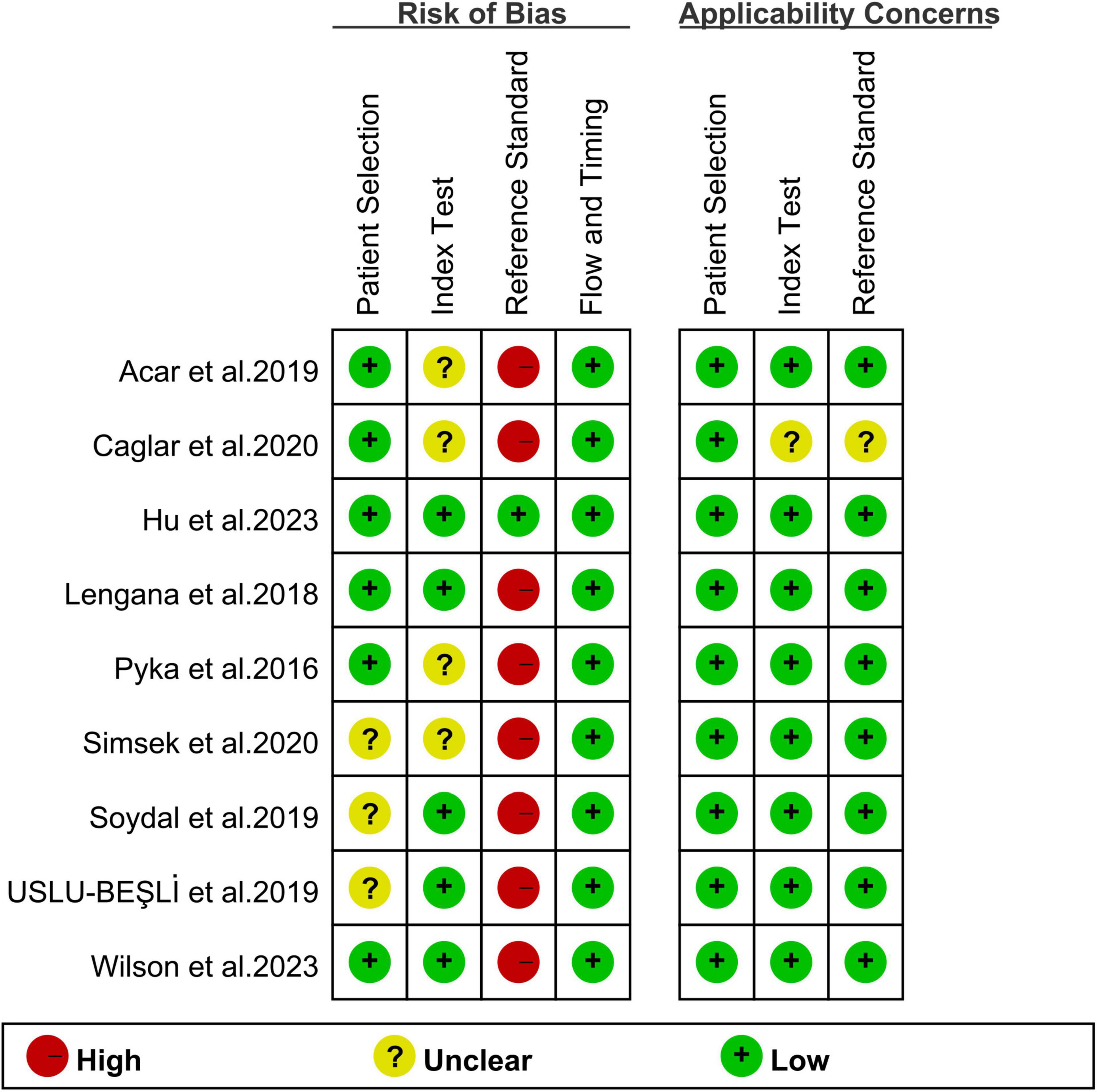
Figure 2. Risk of bias and applicability concerns of the included studies using the Quality Assessment of Diagnostic Performance Studies QUADAS-2 tool.
Comparing the sensitivity and specificity of PSMA PET/CT to [99mTc]Tc-MDP BS for detecting bone metastases in PCa
Nine studies were included in the analysis (21–29). The sensitivity for detecting bone metastases in PCa in patient-based analysis was significantly higher for PSMA PET/CT at 0.98 (95% CI: 0.94–0.99) compared to [99mTc]Tc-MDP BS, which had a sensitivity of 0.85 (95% CI: 0.75–0.92) (Figures 3, 4). This difference in sensitivity between PSMA PET/CT and [99mTc]Tc-MDP BS was statistically significant (P < 0.01). Similarly, the specificity of PSMA PET/CT was 0.97 (95% CI: 0.93–0.99), markedly higher than the specificity of [99mTc]Tc-MDP BS at 0.70 (95% CI: 0.49–0.85) (Figures 3, 4). This difference in specificity was also statistically significant (P < 0.01).
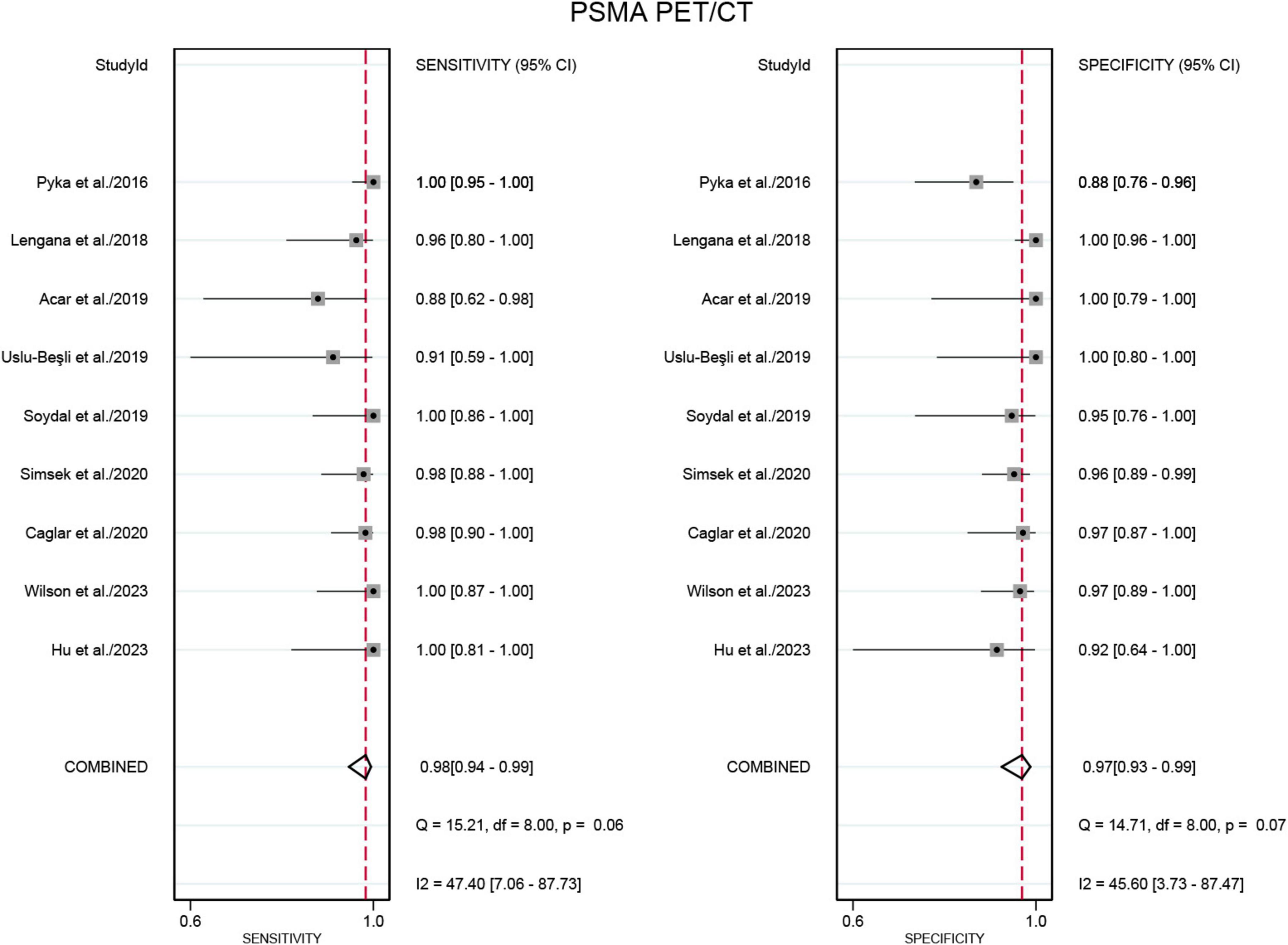
Figure 3. Forest plot for the sensitivity and specificity of PSMA PET/CT in detecting bone metastases in prostate cancer. The plot displays individual study estimates (squares) with corresponding 95% confidence intervals (horizontal lines) and the pooled sensitivity estimate (diamond) for both modalities. The size of the squares represents the relative weight of each study in the meta-analysis.
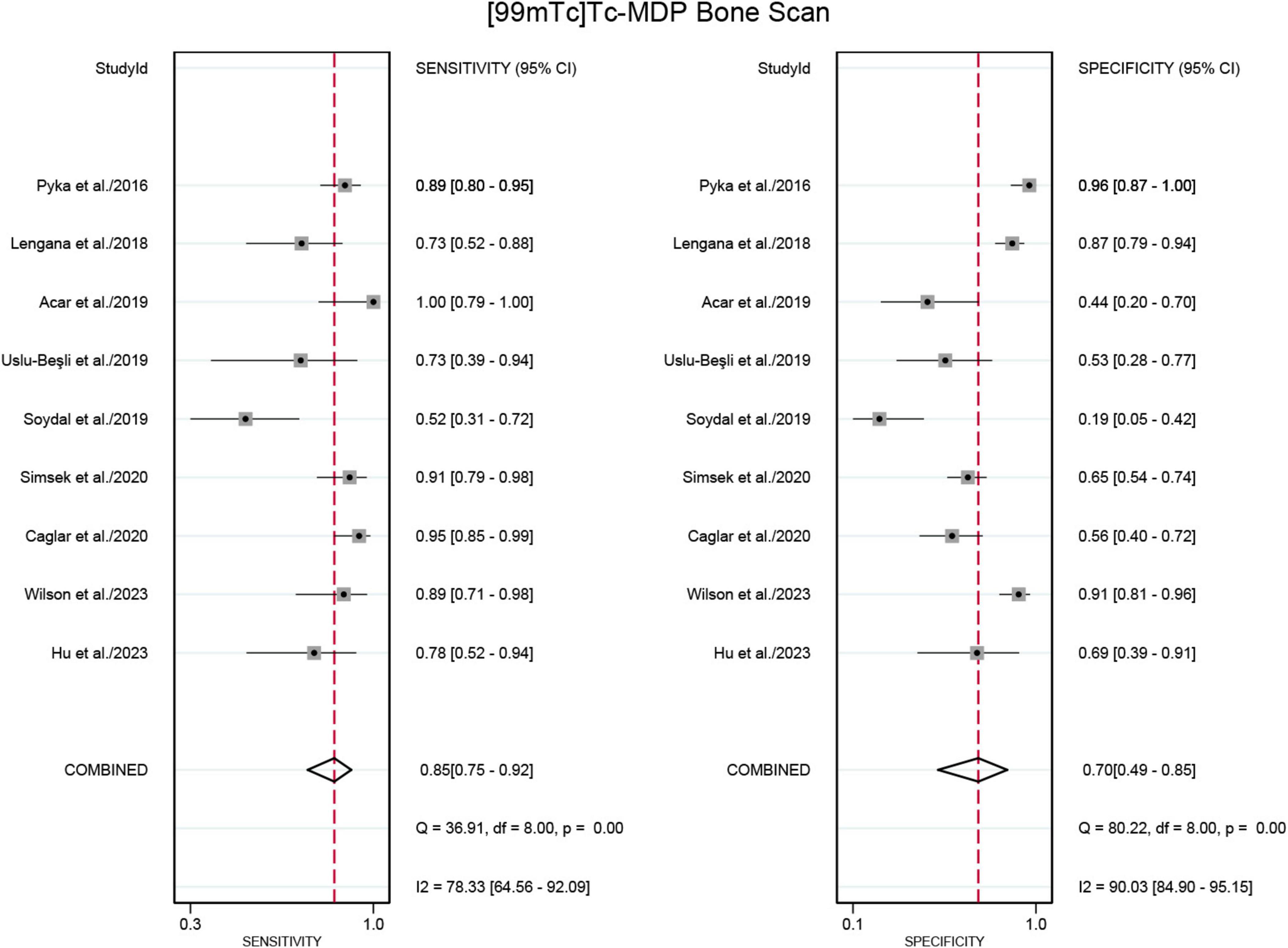
Figure 4. Forest plot for sensitivity and specificity of [99mTc]Tc-MDP BS in detecting bone metastases in prostate cancer. The plot displays individual study estimates (squares) with corresponding 95% confidence intervals (horizontal lines) and the pooled sensitivity estimate (diamond) for both modalities. The size of the squares represents the relative weight of each study in the meta-analysis.
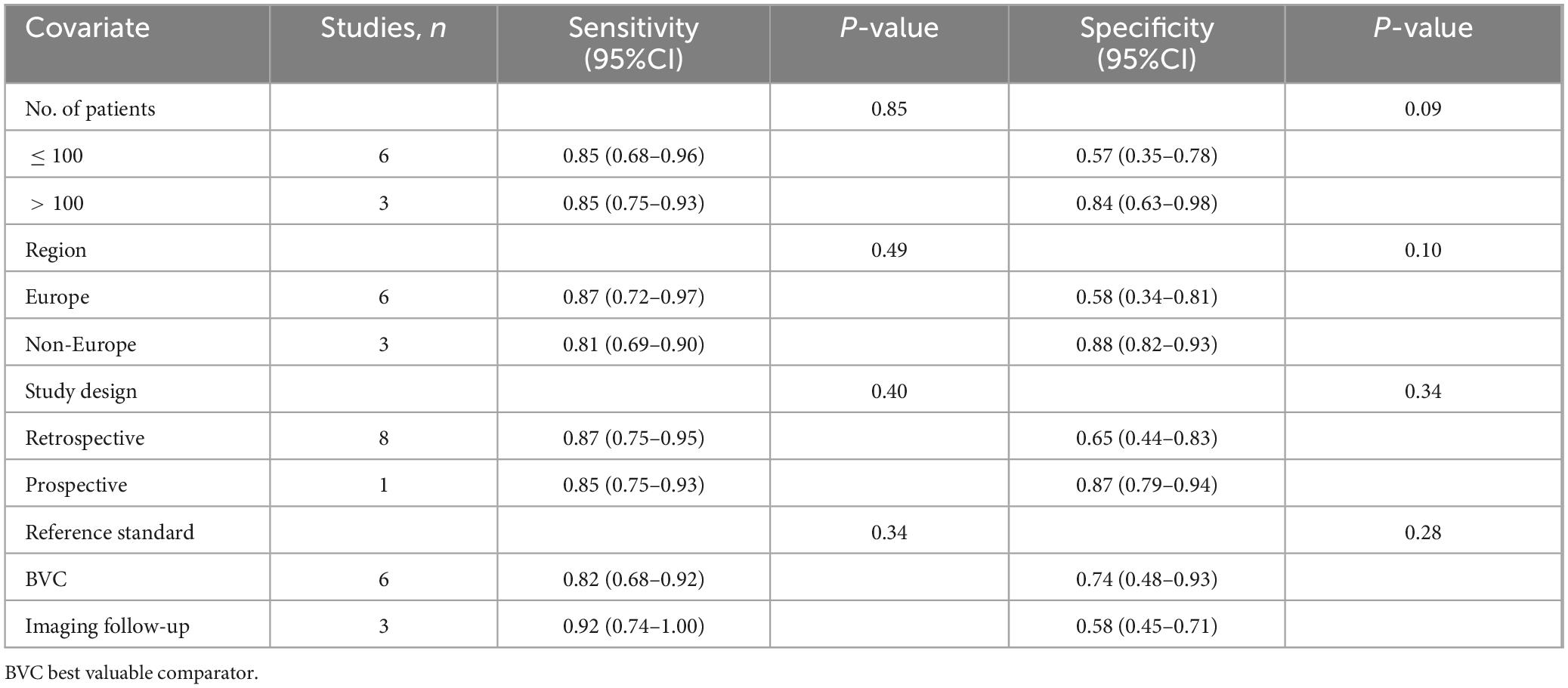
The sensitivity and specificity of [99mTc]Tc-MDP BS exhibited I2 values of 78.33% and 90.03%,respectively. The meta-regression analysis for sensitivity revealed no source of heterogeneity (Table 2). However, a leave-one-out sensitivity analysis revealed that omitting the study by Soydal et al. resulted in a reduction of the I2 to 51%, indicating that this study may be a significant source of heterogeneity (Supplementary Figure 1) (27). With regard to specificity, the leave-one-out sensitivity analysis identified no source of heterogeneity (Supplementary Figure 2).
Comparing the sensitivity and specificity of [68Ga]Ga-PSMA-11 PET/CT to [99mTc]Tc-MDP BS for detecting bone metastases in PCa
Seven studies were included in the analysis (21, 22, 24–28). The pooled sensitivity for detecting bone metastases in PCa in patient-based analysis was 0.98 (95% CI: 0.93–0.99) for [68Ga]Ga-PSMA-11 PET/CT, compared to 0.86 (95% CI: 0.72–0.93) for [99mTc]Tc-MDP BS, with this difference in sensitivity being statistically significant (P < 0.01) (Figures 5, 6). Additionally, the pooled specificity was 0.98 (95% CI: 0.92–0.99) for [68Ga]Ga-PSMA-11 PET/CT, significantly higher than the specificity of 0.65 (95% CI: 0.40–0.84) for [99mTc]Tc-MDP BS (P < 0.01) (Figures 5, 6).
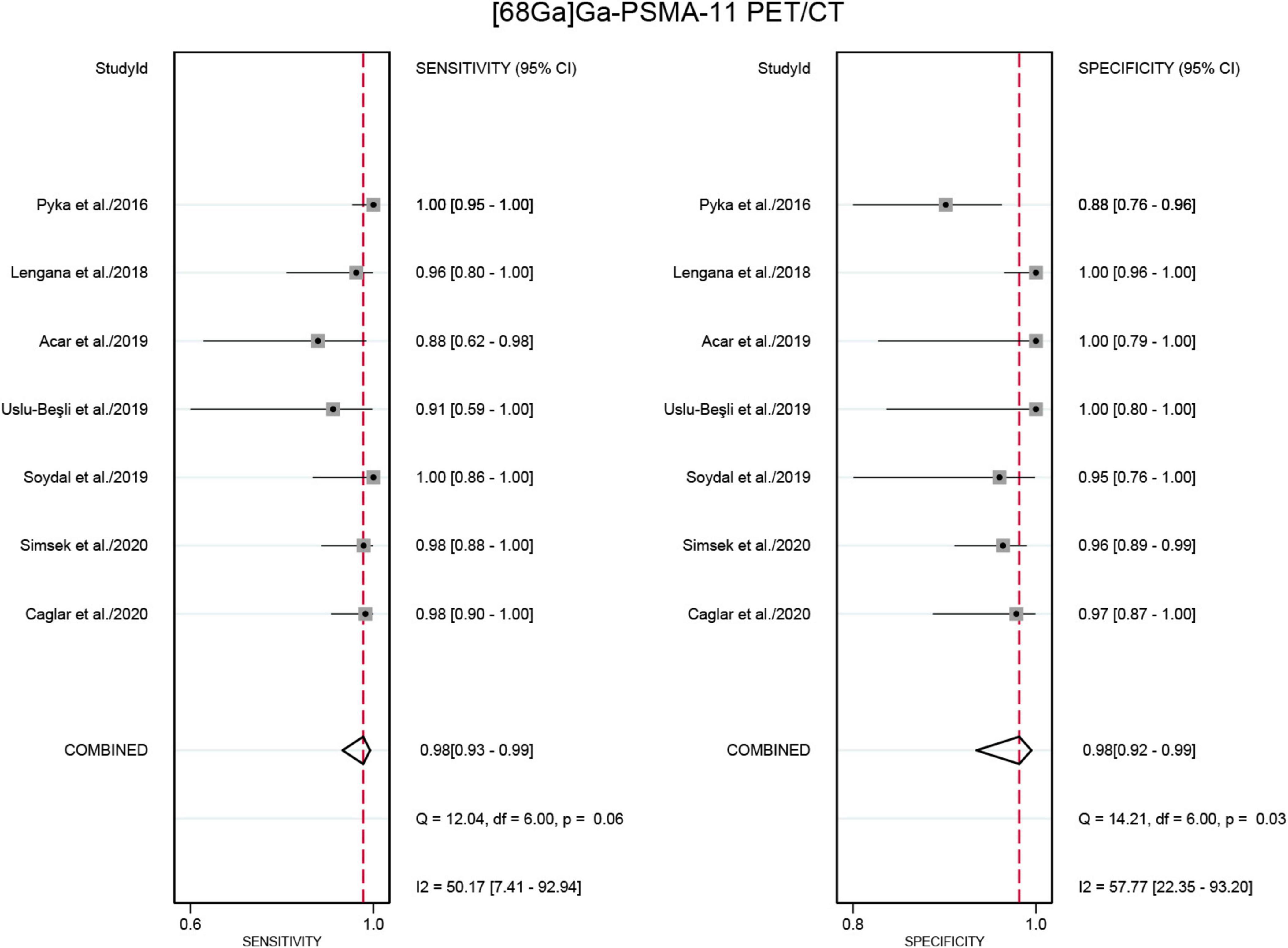
Figure 5. Forest plot for the sensitivity and specificity of [68Ga]Ga-PSMA-11 PET/CT in detecting bone metastases in prostate cancer. The plot displays individual study estimates (squares) with corresponding 95% confidence intervals (horizontal lines) and the pooled sensitivity estimate (diamond) for both modalities. The size of the squares represents the relative weight of each study in the meta-analysis.
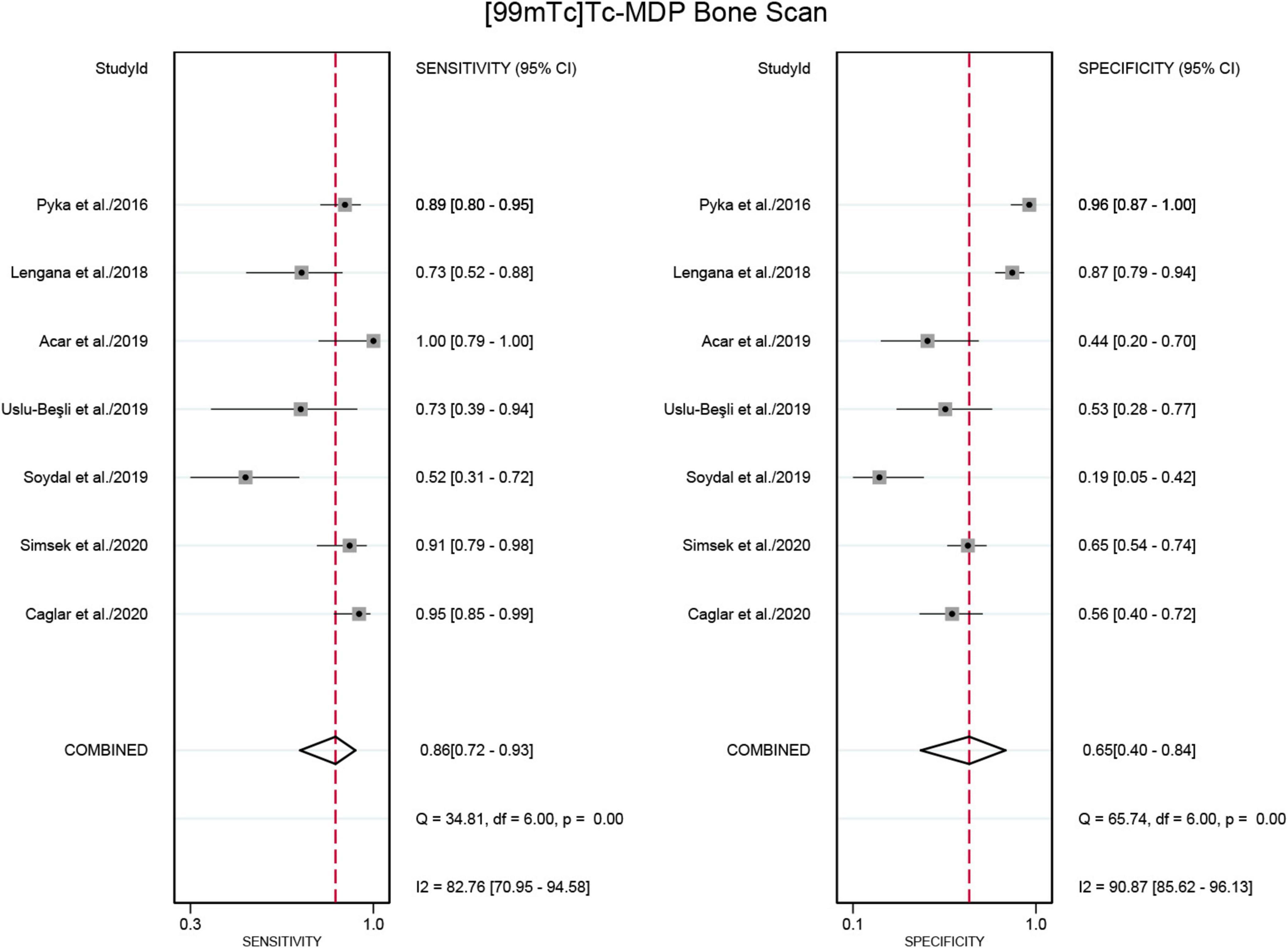
Figure 6. Forest plot showing the sensitivity and specificity of [99mTc]Tc-MDP BS in detecting bone metastases in prostate cancer (including only studies that used [68Ga]Ga-PSMA-11 PET/CT for comparison). The plot displays individual study estimates (squares) with corresponding 95% confidence intervals (horizontal lines) and the pooled specificity estimate (diamond) for both modalities. The size of the squares represents the relative weight of each study in the meta-analysis.
The rates of therapeutic management changes after PSMA PET/CT
Four studies were included in the analysis (22, 26–28). The pooled rates of management changes in PCa cancer patients after PSMA PET/CT was 0.25 (95% CI: 0.06–0.51) (Figure 7).
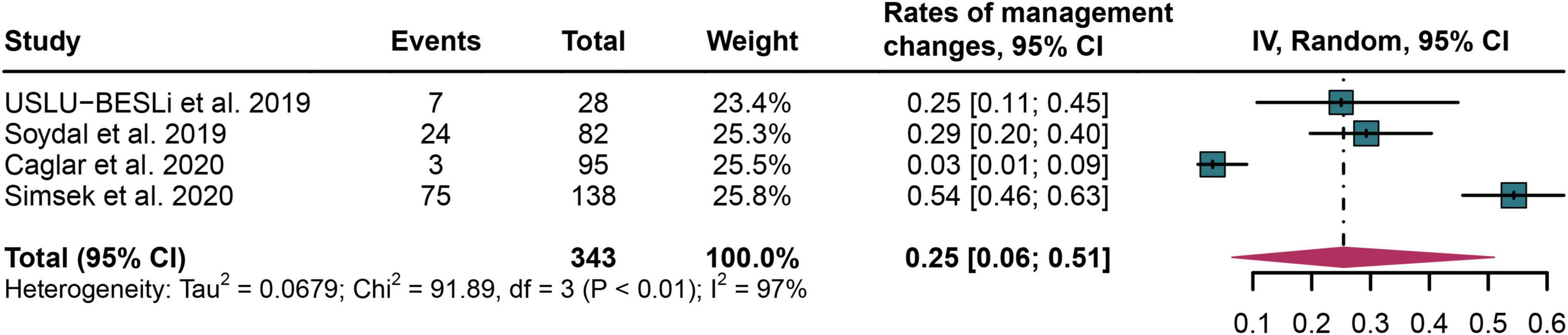
Figure 7. Forest plot of therapeutic management change rates following PSMA PET/CT in prostate cancer bone metastasis.
Publication bias
The funnel plot asymmetry test revealed no evidence of significant publication bias for any of the outcomes (Egger’s test: all P > 0.05) (Supplementary Figures 3–6).
Discussion
A number of studies have recently demonstrated that both PSMA PET/CT and [99mTc]Tc-MDP BS possess strong diagnostic capabilities for detecting bone metastasis in PCa (8, 16, 23). However, a systematic comparison between these two diagnostic tools to determine which has superior diagnostic accuracy remains uncertain (30). The head-to-head comparative meta-analysis presented here aims to address this gap. Our analysis indicates that PSMA PET/CT, with its higher sensitivity and specificity, shows greater promise in identifying bone metastasis in PCa compared to [99mTc]Tc-MDP BS. The data suggests that PSMA PET/CT could potentially replace [99mTc]Tc-MDP BS as the preferred diagnostic method, provided its accessibility and cost-effectiveness are addressed.
The main findings of this meta-analysis reveal that PSMA PET/CT significantly outperforms [99mTc]Tc-MDP BS in diagnosing bone metastasis in PCa. Specifically, PSMA PET/CT demonstrated higher sensitivity (0.98 vs. 0.85) and specificity (0.97 vs. 0.70) in comparison to 99mTc-MDP BS. Subgroup analysis further showed that 68Ga-PSMA-11 PET/CT had higher sensitivity (0.98 vs. 0.86) and specificity (0.98 vs. 0.65) than [99mTc]Tc-MDP BS. The enhanced sensitivity and specificity of PSMA PET/CT are attributed to its ability to target the PSMA protein, which is highly expressed on PCa cells, allowing for more accurate detection of metastatic sites (31, 32). This high affinity and specific binding result in clearer imaging and better differentiation between malignant and benign lesions (33). Overall, these findings suggest that PSMA PET/CT, particularly with [68Ga]Ga-PSMA-11, provides a more reliable diagnostic tool for detecting bone metastasis in PCa, potentially offering significant improvements in patient management and treatment planning.
Comparing our study with previous meta-analyses, we provide significant advancements and address the limitations noted in earlier researches. Ji et al. (16) conducted the first systematic evaluation of [68Ga]Ga-PSMA-11 PET/CT versus [99mTc]Tc-MDP BS for diagnosing bone metastasis in PCa (16). Their results indicated higher sensitivity (98% vs. 83%) and specificity (97% vs. 61%) for [68Ga]Ga-PSMA-11 PET/CT. However, the study had limitations, including a small number of included articles (only six) and the absence of standardized statistical tests to compare sensitivity and specificity between the diagnostic tools. Additionally, the diagnostic performance of the key radiotracer [18F]DCFPyL was not fully explored, indicating a need for further investigation in this area.
In a comprehensive meta-analysis, Chow et al. (34) compared PSMA PET/CT with conventional tools, including BS and MRI, for initial PCa diagnosis, lymph node metastasis, and bone metastasis (34). Despite its thorough approach, the study has limitations such as not employing leave-one-out sensitivity analyses or meta-regression analysis to explore the sources of high heterogeneity in the PET vs. BS comparison subgroup. Furthermore, our study incorporated a larger pool of literature and included the analysis of [18F]DCFPyL, enhancing the robustness and comprehensiveness of our findings. We performed subgroup analyses based on different PET/CT radiotracers, providing a detailed comparison of diagnostic performance between specific radiotracers and BS. This methodological rigor allowed us to deliver more precise insights into the diagnostic performance of various PSMA PET/CT radiotracers in comparison to BS.
In the other meta-analysis, Shen et al. (35) compared choline-PET/CT, MRI, SPECT, and BS in the diagnosis of bone metastases in patients with PCa and concluded that SPECT imaging has demonstrated its potential to significantly improve diagnostic accuracy compared to traditional conventional imaging techniques including planar BS. The current study is constrained by the limited number of available SPECT studies, which restricts our ability to conduct a comprehensive comparison between PSMA PET/CT and SPECT. This limitation highlights the need for future research that rigorously compares PSMA PET with SPECT, a topic that holds considerable potential for advancing diagnostic methodologies.
In our meta-analysis, we chose to exclude studies utilizing [18F]PSMA-1007 as a radiotracer. This decision was primarily driven by the higher rate of false-positive findings associated with [18F]PSMA-1007, particularly in bone imaging (36). Furthermore, there is a notable lack of studies that directly compare [18F]PSMA-1007 PET imaging with conventional BS in a head-to-head manner, limiting our ability to perform a comprehensive meta-analysis on this particular radiotracer. However, [18F]PSMA-1007 remains a significant PSMA radiotracer, widely used in clinical practice due to its high sensitivity in detecting PCa lesions. Future research should focus on better understanding its diagnostic performance, particularly in comparison to other imaging modalities.
Since PSMA PET/CT exhibits higher sensitivity and specificity in comparison with [99mTc]Tc-MDP BS in detecting bone metastasis in PCa, making it a seemingly better choice when only diagnostic performance is considered. The advantages of PSMA PET/CT include its superior diagnostic accuracy and ability to provide more detailed imaging, which is crucial for early detection and treatment planning (37, 38). However, PSMA PET/CT is less widely available and typically more expensive than BS, which might limit its accessibility for some patients (39). Moreover, although both imaging modalities are generally safe, the choice between them should be guided by the patient’s individual condition and the specific clinical context (40). A combined diagnostic model utilizing both PSMA PET/CT and BS could potentially enhance diagnostic performance by leveraging the complementary strengths of each modality (41). Clinicians should carefully evaluate the benefits and limitations of each tool to make informed decisions tailored to patient-specific circumstances (42).
Interpreting the findings of this meta-analysis, several limitations should be considered. Firstly, the heterogeneity among the included studies might have influenced the pooled sensitivities and specificities of PSMA PET/CT and [99mTc]Tc-MDP BS. To address this, we performed leave-one-out sensitivity analyses, which identified Soydal et al. (27) as a potential source of heterogeneity. Secondly, the number of studies in the head-to-head comparison of [18F]DCFPyL PET/CT versus [99mTc]Tc-MDP BS was relatively small, indicating the need for well-designed prospective head-to-head studies to confirm our findings. Thirdly, not all patients underwent pathological biopsy as the gold standard, which may introduce bias into the results. Future research should include more studies where pathological biopsy is used as the gold standard.
Conclusion
Based on our findings, the meta-analysis illustrates that PSMA PET/CT (including [68Ga]Ga-PSMA-11 and [18F]DCFPyL) demonstrates higher sensitivity and comparable specificity to [99mTc]Tc-MDP BS in the identification of bone metastases in PCa patients. Additional research with head-to-head design and extensive pathological data is necessary to validate these observations and to assess the clinical utility of these imaging techniques.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YW: Data curation, Formal analysis, Methodology, Software, Writing – original draft. YQ: Data curation, Formal analysis, Methodology, Software, Writing – original draft. XY: Conceptualization, Supervision, Validation, Visualization, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1451565/full#supplementary-material
References
1. Garcia-Ruiz A, Macarro C, Zacchi F, Morales-Barrera R, Grussu F, Casanova-Salas I, et al. Whole-body magnetic resonance imaging as a treatment response biomarker in castration-resistant prostate cancer with bone metastases: The iPROMET clinical trial. Eur Urol. (2024): doi: 10.1016/j.eururo.2024.02.016 [Epub ahead of print].
2. Ottosson F, Baco E, Lauritzen P, Rud E. The prevalence and locations of bone metastases using whole-body MRI in treatment-naïve intermediate- and high-risk prostate cancer. Eur Radiol. (2021) 31:2747–53. doi: 10.1007/s00330-020-07363-x
3. Beheshti M, Langsteger W, Fogelman I. Prostate cancer: Role of SPECT and PET in imaging bone metastases. Semin Nucl Med. (2009) 39:396–407. doi: 10.1053/j.semnuclmed.2009.05.003
4. Groot M, Boeken Kruger C, Pelger R, Uyl-de Groot C. Costs of prostate cancer, metastatic to the bone, in the Netherlands. Eur Urol. (2003) 43:226–32. doi: 10.1016/s0302-2838(03)00007-1
5. Saad F, Karakiewicz P, Perrotte P. The role of bisphosphonates in hormone-refractory prostate cancer. World J Urol. (2005) 23:14–8. doi: 10.1007/s00345-004-0472-2
6. Lin J, Hearn C, Getzen E, Long Q, Lee D, Keaveny T, et al. Validation of biomechanical computed tomography for fracture risk classification in metastatic hormone-sensitive prostate cancer. Eur Urol Oncol. (2023) 7:794–803. doi: 10.1016/j.euo.2023.10.016
7. Cook G, Goh V. Functional and hybrid imaging of bone metastases. J Bone Miner Res. (2018) 33:961–72. doi: 10.1002/jbmr.3444
8. Radzina M, Tirane M, Roznere L, Zemniece L, Dronka L, Kalnina M, et al. Accuracy of (68)Ga-PSMA-11 PET/CT and multiparametric MRI for the detection of local tumor and lymph node metastases in early biochemical recurrence of prostate cancer. Am J Nucl Med Mol Imaging. (2020) 10:106–18.
9. Lother D, Robert M, Elwood E, Smith S, Tunariu N, Johnston S, et al. Imaging in metastatic breast cancer, CT, PET/CT, MRI, WB-DWI, CCA: Review and new perspectives. Cancer Imaging. (2023) 23:53. doi: 10.1186/s40644-023-00557-8
10. Büttner T, Dietrich D, Zarbl R, Klümper N, Ellinger J, Krausewitz P, et al. Feasibility of monitoring response to metastatic prostate cancer treatment with a methylation-based circulating tumor DNA approach. Cancers (Basel). (2024) 16:482. doi: 10.3390/cancers16030482
11. Schiavina R, Scattoni V, Castellucci P, Picchio M, Corti B, Briganti A, et al. 11C-choline positron emission tomography/computerized tomography for preoperative lymph-node staging in intermediate-risk and high-risk prostate cancer: Comparison with clinical staging nomograms. Eur Urol. (2008) 54:392–401. doi: 10.1016/j.eururo.2008.04.030
12. Chaussé G, Niazi T, Abikhzer G, Probst S. Biopsy-proven diffuse mediastinal prostate cancer metastases negative on 18f-fluorocholine, diagnosed on 68Ga-PSMA and 18F-PSMA PET/CT. Clin Nucl Med. (2017) 42:801–2. doi: 10.1097/rlu.0000000000001766
13. Budäus L, Leyh-Bannurah S, Salomon G, Michl U, Heinzer H, Huland H, et al. Initial experience of (68)Ga-PSMA PET/CT imaging in high-risk prostate cancer patients prior to radical prostatectomy. Eur Urol. (2016) 69:393–6. doi: 10.1016/j.eururo.2015.06.010
14. Mei R, Farolfi A, Castellucci P, Nanni C, Zanoni L, Fanti S. PET/CT variants and pitfalls in prostate cancer: What you might see on PET and should never forget. Semin Nucl Med. (2021) 51:621–32. doi: 10.1053/j.semnuclmed.2021.06.016
15. Zaman M, Fatima N, Sajjad Z, Hashmi I, Khan K. Higher scrotal uptake ratio of (99m)Tc-MDP on bone scans in newly diagnosed prostate cancer: A reliable indicator of pelvic node metastasis. Ann Nucl Med. (2012) 26:676–80. doi: 10.1007/s12149-012-0626-z
16. Zhao G, Ji B. Head-to-head comparison of (68)Ga-PSMA-11 PET/CT and (99m)Tc-MDP bone scintigraphy for the detection of bone metastases in patients with prostate cancer: A meta-analysis. AJR Am J Roentgenol. (2022) 219:386–95. doi: 10.2214/ajr.21.27323
17. McInnes M, Moher D, Thombs B, McGrath T, Bossuyt P, Clifford T, et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: The PRISMA-DTA statement. JAMA. (2018) 319:388–96. doi: 10.1001/jama.2017.19163
18. Whiting P, Rutjes A, Westwood M, Mallett S, Deeks J, Reitsma J, et al. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
19. Bossuyt P, Deeks J, Leeflang M, Takwoingi Y, Flemyng E. Evaluating medical tests: Introducing the cochrane handbook for systematic reviews of diagnostic test accuracy. Cochrane Database Syst Rev. (2023) 7:Ed000163. doi: 10.1002/14651858.Ed000163
20. Baek M, Park E, Ha H, Park S, Lim M, Fotopoulou C, et al. Secondary cytoreductive surgery in platinum-sensitive recurrent ovarian cancer: A meta-analysis. J Clin Oncol. (2022) 40:1659–70. doi: 10.1200/jco.21.02085
21. Acar E, Bekiş R, Polack B. Comparison of bone uptake in bone scan and Ga-68 PSMA PET/CT images in patients with prostate cancer. Curr Med Imaging Rev. (2019) 15:589–94. doi: 10.2174/1573405615666190225155254
22. Caglar M, Tuncel M, Yildiz E, Karabulut E. Bone scintigraphy as a gatekeeper for the detection of bone metastases in patients with prostate cancer: Comparison with Ga-68 PSMA PET/CT. Ann Nucl Med. (2020) 34:932–41. doi: 10.1007/s12149-020-01529-9
23. Hu X, Cao Y, Ji B, Zhao M, Wen Q, Chen B. Comparative study of (18)F-DCFPyL PET/CT and (99m)Tc-MDP SPECT/CT bone imaging for the detection of bone metastases in prostate cancer. Front Med (Lausanne). (2023) 10:1201977. doi: 10.3389/fmed.2023.1201977
24. Lengana T, Lawal I, Boshomane T, Popoola G, Mokoala K, Moshokoa E, et al. (68)Ga-PSMA PET/CT replacing bone scan in the initial staging of skeletal metastasis in prostate cancer: A fait accompli? Clin Genitourin Cancer. (2018) 16:392–401. doi: 10.1016/j.clgc.2018.07.009
25. Pyka T, Okamoto S, Dahlbender M, Tauber R, Retz M, Tamaki N, et al. Comparison between 68Ga-PSMA PET and bone scintigraphy for skeletal staging in prostate cancer. J Nuclear Med. (2016) 57:2114–21.
26. Simsek D, Sanli Y, Civan C, Engin M, Isik E, Ozkan Z, et al. Does bone scintigraphy still have a role in the era of 68 Ga-PSMA PET/CT in prostate cancer? Ann Nucl Med. (2020) 34:476–85. doi: 10.1007/s12149-020-01474-7
27. Soydal C, Nak D, Araz M, Akkus P, Urun Y, Ozkan E, et al. Comparison of bone scintigraphy and Ga-68 prostate-specific membrane antigen positron emission tomography/computed tomography in the detection of bone metastases of prostate carcinoma. Nucl Med Commun. (2019) 40:1243–9. doi: 10.1097/mnm.0000000000001106
28. Uslu-Beşli L, Sağer S, Akgün E, Asa S, Şahin O, Demirdağ Ç, et al. Comparison of Ga-68 PSMA positron emission tomography/computerized tomography with Tc-99m MDP bone scan in prostate cancer patients. Turk J Med Sci. (2019) 49:301–10. doi: 10.3906/sag-1807-4
29. Wilson Z, Xu G, Tewari S, Lu Y. Comparison of PSMA-based (18)F-DCFPyL PET/CT and Tc-99m MDP bone scan in detection of bone metastasis in prostate cancer. Am J Nucl Med Mol Imaging. (2023) 13:1–10.
30. Hammes J, Hohberg M, Täger P, Wild M, Zlatopolskiy B, Krapf P, et al. Uptake in non-affected bone tissue does not differ between [18F]-DCFPyL and [68Ga]-HBED-CC PSMA PET/CT. PLoS One. (2018) 13:e0209613. doi: 10.1371/journal.pone.0209613
31. Yu W, Zhao M, Deng Y, Liu S, Du G, Yan B, et al. Meta-analysis of (18) F-PSMA-1007 PET/CT, (18) F-FDG PET/CT, and (68)Ga-PSMA PET/CT in diagnostic efficacy of prostate Cancer. Cancer Imaging. (2023) 23:77. doi: 10.1186/s40644-023-00599-y
32. Yang J, Li J, Xiao L, Zhou M, Fang Z, Cai Y, et al. 68Ga-PSMA PET/CT-based multivariate model for highly accurate and noninvasive diagnosis of clinically significant prostate cancer in the PSA gray zone. Cancer Imaging (2023) 23:81. doi: 10.1186/s40644-023-00562-x
33. Lenzo N, Meyrick D, Turner J. Review of gallium-68 PSMA PET/CT imaging in the management of prostate cancer. Diagnostics (Basel). (2018) 8:16. doi: 10.3390/diagnostics8010016
34. Chow K, So W, Lee H, Lee A, Yap D, Takwoingi Y, et al. Head-to-head comparison of the diagnostic accuracy of prostate-specific membrane antigen positron emission tomography and conventional imaging modalities for initial staging of intermediate- to high-risk prostate cancer: A systematic review and meta-analysis. Eur Urol. (2023) 84:36–48. doi: 10.1016/j.eururo.2023.03.001
35. Shen G, Deng H, Hu S, Jia Z. Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: A meta-analysis. Skeletal Radiol. (2014) 43:1503–13. doi: 10.1007/s00256-014-1903-9
36. Rizzo A, Morbelli S, Albano D, Fornarini G, Cioffi M, Laudicella R, et al. The homunculus of unspecific bone uptakes associated with PSMA-targeted tracers: A systematic review-based definition. Eur J Nucl Med Mol Imaging. (2024). doi: 10.1007/s00259-024-06797-5 [Epub ahead of print].
37. Hofman M, Lawrentschuk N, Francis R, Tang C, Vela I, Thomas P, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet. (2020) 395:1208–16. doi: 10.1016/s0140-6736(20)30314-7
38. Karagiannis V, Wichmann V, Saarinen J, Eigeliene N, Andersen H, Jekunen A. Radiotherapy treatment modification for prostate cancer patients based on PSMA-PET/CT. Radiat Oncol. (2022) 17:19. doi: 10.1186/s13014-022-01989-5
39. Szczesniewski J, Tellez Fouz C, García Tello A, de la Rubia Marcos M, García Alonso MP, Llanes González L. Cost analysis of next-generation imaging in high-risk prostate cancer staging. Actas Urol Esp. (2024) 48:328–34. doi: 10.1016/j.acuroe.2023.12.003
40. Dondi F, Albano D, Bertagna F, Treglia G. Bone scintigraphy versus PSMA-targeted PET/CT or PET/MRI in prostate cancer: Lessons learned from recent systematic reviews and meta-analyses. Cancers (Basel). (2022) 14:4470. doi: 10.3390/cancers14184470
41. Zhou J, Gou Z, Wu R, Yuan Y, Yu G, Zhao Y. Comparison of PSMA-PET/CT, choline-PET/CT, NaF-PET/CT, MRI, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: A systematic review and meta-analysis. Skeletal Radiol. (2019) 48:1915–24. doi: 10.1007/s00256-019-03230-z
Keywords: [68Ga]Ga-PSMA-11, [18F]DCFPyL, [99mTc]Tc-MDP bone scan, prostate cancer, bone metastases
Citation: Wang Y, Qiu Y and Yan X (2024) Prostate-specific membrane antigen PET versus [99mTc]Tc-MDP bone scan for diagnosing bone metastasis in prostate cancer: a head-to-head comparative meta-analysis. Front. Med. 11:1451565. doi: 10.3389/fmed.2024.1451565
Received: 19 June 2024; Accepted: 30 August 2024;
Published: 25 September 2024.
Edited by:
Giorgio Treglia, Ente Ospedaliero Cantonale (EOC), SwitzerlandReviewed by:
Maryam Zamanian, Isfahan University of Medical Sciences, IranAlessio Rizzo, IRCCS Candiolo Cancer Institute, Italy
Copyright © 2024 Wang, Qiu and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingjian Yan, eWFueGluZ2ppYW5Aamx1LmVkdS5jbg==
†These authors have contributed equally to this work
 Yiming Wang
Yiming Wang Yiran Qiu
Yiran Qiu Xingjian Yan
Xingjian Yan