- 1Department of Rheumatology and Clinical Immunology, Faculty of Medicine, Medical Center–University of Freiburg, University of Freiburg, Freiburg im Breisgau, Germany
- 2Musculoskeletal Quantitative Imaging Research Group, University of California San Francisco, San Francisco, CA, United States
- 3Department of Anesthesiology, Krankenhaus Porz am Rhein, Cologne, Germany
- 4Department of Radiology and Nuclear Medicine, University of Basel, Basel, Switzerland
- 5Institute for Neuromodulation and Neurotechnology, University of Tübingen, Tübingen, Germany
- 6Department of Rheumatology and Immunology, University Hospital Bern, University of Bern, Bern, Switzerland
- 7Department of Rheumatology, University Hospital Basel, Basel, Switzerland
Background: Vasculopathy is a hallmark of systemic sclerosis (SSc) putting patients at an increased risk of cardiovascular disease. Approximately 20–25% of all SSc patients show prolonged elevated C-reactive protein (CRP) levels and thus signs of chronic low-grade inflammation. While CRP−positivity is an independent predictor of cardiovascular disease in non-SSc populations, the relationship between CRP−positivity and cardiovascular health/atherosclerosis in SSc patients is only incompletely understood. Here, we aimed to assess (1) which general, SSc disease-specific and cardiovascular parameters are associated with CRP−positivity in a cohort of SSc patients with prolonged CRP elevations (CRP+ SSc group) relative to SSc patients without CRP elevations (CRP− SSc group). In addition (2), we aimed to investigate whether prolonged CRP−positivity in SSc patients is associated with a higher cardiovascular risk and an increased atherosclerotic burden. We also aimed to (3) identify via random forest classification modeling which combined cardiovascular and/or SSc-specific parameters could differentiate best between SSc patients with elevated CRP levels (the so-called “inflammatory SSc subtype”) and SSc patients without increased CRP levels.
Methods: Sixty-five SSc patients were recruited and assigned to the CRP+ SSc group (n = 20) if their CRP levels were > 5 mg/L in at least three half-yearly visits within 2 years before enrolment or to the CRP− SSc group (n = 45), respectively. All patients underwent an anamnesis, physical examination, blood draw, and bilateral carotid ultrasound in order to assess arteriosclerotic burden including the presence, number and height of plaques, and carotid intima–media thickness (CIMT) as well as lipid profiles. 10-year ASCVD risk was estimated via the ASCVD risk estimator plus. Statistical evaluation included Spearman’s correlations, logistic regression and random forest modeling under 5-fold cross-validation, and permutation testing to determine combinations of cardiovascular variables highly discriminatory for CRP−positivity.
Results: SSc groups showed comparable mean age, height, and extent of SSc organ involvement. Regarding cardiovascular health, CRP+ SSc patients exhibited a significantly altered HDL-, LDL-, and triglyceride profile (0.001 ≤ p ≤ 0.017) and a significantly higher 10-year ASCVD risk (p = 0.047), relative to CRP− SSc patients. Additionally, within the subgroup of CRP+ SSc patients, positive correlations between CRP levels and CIMT right (ρ = 0.657, p = 0.002) and mean CIMT left and right (ρ = 0.497, p = 0.026) were seen. Combined ROC models identified the four lipid components (HDL, LDL, total cholesterol, and triglycerides) or the SSc duration and ASCVD category to differentiate with high cross-validated ROC-AUCs (AUC: 0.83 ± 0.15, and AUC: 0.86 ± 0.09, p < 0.001) for prolonged CRP−positivity among SSc patients.
Conclusion: Our data indicate that persistent CRP−positivity and thus chronic low-grade inflammation in SSc patients enhance the risk for arteriosclerotic-cardiovascular disease significantly beyond the ASCVD risk observed for our SSc patients without CRP elevations. It seems to be along with a disrupted lipid profile the hallmark of a distinct “inflammatory” subgroup of SSc patients. However, large population-based studies and clinical trials in patients with SSc are needed to validate our findings in a prospective or interventional setting.
1 Introduction
Systemic sclerosis (SSc) is an autoimmune disease characterized by excessive deposition of extracellular matrix resulting in distinct progressive fibrosis of the skin and of internal organs, such as the lungs, and in vasculopathy (1). SSc is linked to one of the highest mortality rates among all rheumatic diseases (2). Clinical diagnosis, treatment, and research are challenging due to the phenotypic heterogeneity of SSc. Efforts have been undertaken to identify biomarkers that can help predict SSc organ involvement and severity, SSc progression, and mortality and may therefore be utilized for risk stratification and personalized treatment.
C-reactive protein (CRP) is an easily accessible and cost-efficient serum marker of inflammation in daily clinical routine. To date, several studies have shown that approximately 23.2–41.5% of patients with SSc have elevated CRP levels (3, 4). In approximately 18% of SSc patients, CRP elevations are even persistent (5, 6). More importantly, prospective studies and a meta-analysis carried out in this particular “inflammatory subtype” of SSc patients have linked the presence of elevated CRP levels (cutoff >5 mg/L) to worse health outcomes such as an increased risk of pulmonary hypertension (7), progressive fibrosis, and higher mortality (8, 9). In addition, elevated CRP levels have been found associated with the increasing severity of skin and joint involvement (9, 10).
Microvascular and macrovascular diseases are additional hallmarks of SSc, putting SSc patients at a significantly increased risk of cardiovascular events compared to the general population (11, 12). In a recent meta-analysis, pooled hazard ratios in SSc patients ranged from 2.36 (95% CI 1.97–2.81) for cardiovascular disease (CVD) or myocardial infarction (95% CI 1.71–3.25) to a hazard ratio of HR 5.27 (95% CI 4.27–6.51) for peripheral vascular disease compared to the general population (12). For most inflammatory rheumatic diseases, an increased cardiovascular risk has been demonstrated with the most detailed data available for rheumatoid arthritis (RA). Analyzing carotid intima–media thickness (CIMT) and pulse wave velocity, Dimitroulas et al. (13) found comparable magnitudes of subclinical atherosclerosis in patients with SSc when compared with patients with RA, highlighting the importance and need for cardiovascular risk assessment and management also for SSc patients (13). While CRP elevation has long been established as a strong predictor and risk factor for carotid arteriosclerosis (14), future cardiovascular events (15), and poor prognosis after CV events (16, 17) in non-SSc patients, the relationship between CRP levels and cardiovascular health/arteriosclerosis in SSc patients has to date only been incompletely (18) and inconsistently (19–23) investigated as studies so far either assessed CRP–cardiovascular correlations only as a (bystander) secondary outcome (19) or their study design and sample size did not allow for or foresee more in-depth analyses (such as stratification by prolonged CRP status) (13, 22–25).
Therefore, the aims of this cross-sectional observational study were 3-fold: (1) to determine in a cohort of SSc patients with prolonged CRP elevations (CRP+ SSc group) relative to SSc patients without CRP elevations (CRP− SSc group) which general, and/or SSc disease-specific, and/or cardiovascular parameters are associated with a prolonged CRP elevation in systemic sclerosis. Furthermore, we aimed (2) to investigate whether prolonged CRP−positivity in SSc patients is associated with a higher cardiovascular risk as determined via the Framingham risk and arteriosclerotic cardiovascular disease (ASCVD) risk scores and with an increased atherosclerotic burden as determined by carotid ultrasound. We also aimed to (3) identify via random forest classification modeling, which combined cardiovascular and/or SSc-specific parameters could differentiate best between SSc patients with elevated CRP levels (the “inflammatory SSc subtype”) and SSc patients without increased CRP levels.
2 Materials and methods
2.1 Patient selection
In this cross-sectional, observational study, 65 patients with SSc from the outpatient clinic at the Department of Rheumatology and Clinical Immunology, University Medical Center, Freiburg, Germany, were enrolled. All patients fulfilled the ACR/EULAR 2013 classification criteria for SSc at the time of enrollment (26). Inclusion and exclusion criteria for this study as well as the group assignment into CRP−positive and CRP−negative groups have been published elsewhere (10). As described (10), 20 of the 65 patients exhibited elevated CRP levels >5 mg/L in at least three half-yearly visits within 2 years prior to enrolment and were assigned to the CRP−positive group (CRP+ SSc group), while 45 SSc patients exhibited normal CRP concentrations for at least 1.5 consecutive years before enrollment and were assigned to the CRP−negative group (CRP− SSc group). The Freiburg Institutional Review Board granted ethical approval for the study (386/17). Prior to any study-related measures, all patients provided written informed consent. The study was carried out in accordance with the principles of Good Clinical Practice (GCP) as developed by the International Conference of Harmonization (ICH) and set forth by the Declaration of Helsinki.
2.2 Study visits and clinical measurements
At the time of the study visit, all patients underwent a structured interview that assessed traditional risk factors for arteriosclerosis (such as the history of smoking, number of pack years, presence of arterial hypertension, type 2 diabetes mellitus, chronic kidney disease, and a positive family history with respect to the cardiovascular event [any cardiovascular event before the age of 65 years]). Information on the duration and extent of SSc and current medication use was extracted from the patient charts. In addition, all patients underwent a physical examination. This included apart from height, weight, heart rate, and blood pressure, the assessment of the acra for the presence of digital ulcerations, Raynaud’s syndrome, and a tender and swollen joint count, which were recorded in a standardized way.
2.3 Laboratory measurements
All patients underwent a blood draw at the time of the study visit, and serum antibody and CRP concentrations were measured at the Department of Rheumatology and Clinical Immunology University Medical Center Freiburg laboratory (10). In addition, data on fasting serum levels of total cholesterol, HDL cholesterol, LDL cholesterol, total triglycerides, and eGFR levels were retrieved from the patients’ laboratory charts, which all had been measured by the University Medical Center Freiburg’s Department of Clinical Chemistry and Laboratory Medicine laboratory according to the quality standards of routine clinical diagnostic laboratory measures.
2.4 Ultrasound evaluation of carotid arteries
At the study visit, bilateral carotid ultrasound was carried out at the common and external carotid arteries using an 18-MHz linear array transducer on the same Esaote MyLab Twice ultrasound scanner (Esaote, Genoa, Italy) by the same sonographer (SF) with 9-year experience in ultrasound at the time of examination. Carotid intima–media thickness (CIMT) was measured in millimeters as a well-established marker of arteriosclerosis and determinant of cardiovascular disease (27, 28). CIMT measurements were performed by the American Society of Echocardiography’s consensus scanning protocol (29). Common and external carotid arteries were scanned and evaluated in both the longitudinal and axial plane for the presence, height, and number of arteriosclerotic plaques at standardized locations as suggested by the American Society for Echocardiography. An arteriosclerotic plaque was therefore defined as a focal thickening of the arterial wall that was at least 50% thicker than the surrounding vessel walls and/or a focal thickening of the arterial wall protruding into the vessel lumen and being bigger than 1.5 mm (29). In order to not overlook cholesterol plaques, which are difficult to visualize in B-mode, color Doppler ultrasound scanning at 5.6 MHz was utilized. In total, the following arteriosclerotic ultrasound parameters were acquired for the left and right carotid arteries separately: carotid intima–media thickness (CIMT) in millimeters of the left and right common carotid arteries, the maximum plaque height in millimeters, and the number of plaques (n). In addition, the total number of plaques was calculated as a summary variable summarizing the number of all plaques of both sides. A mean CIMT of left and right carotid arteries was calculated as the sum of left and right CIMT divided by two and reported in millimeters.
2.5 Estimation of arteriosclerotic cardiovascular risk via Framingham, ASCVD, and SCORE 2019 risk scores
In order to estimate the arteriosclerotic cardiovascular risk in both SSc groups, three different risk calculators were utilized. For the individual 10-year risk of hard coronary heart disease (individual FRS 10-year risk of HCDH) and the average 10-year risk of hard coronary heart disease (average FRS 10-year risk of HCDH), we used the Framingham Risk Score for Hard Coronary Heart Disease risk calculator/outcome model, which is freely available online under the URLs https://www.mdcalc.com/calc/38/framingham-risk-score-hard-coronary-heart-disease and the https://www.framinghamheartstudy.org/fhs-risk-functions/hard-coronary-heart-disease-10-year-risk/ which is based on risk equations published by Wilson et al. (30). Outcome is the 10-year risk of hard coronary heart disease (=defined as any myocardial infarction or coronary death). The model is intended for use in patients aged 30–79 years with no prior history of coronary heart disease or intermittent claudication.
To estimate a patient’s 10-year risk for atherosclerotic cardiovascular disease (ASCVD), we utilized the ASCVD Risk Estimator Plus provided by the American Heart Association and the American College of Cardiology and freely accessible online under the URL https://tools.acc.org/ascvd-risk-estimator-plus/#!/calculate/estimate/. Atherosclerotic cardiovascular disease (ASCVD) is hereby defined as coronary death or non-fatal myocardial infarction, or fatal or non-fatal stroke, and the calculator is based on the pooled cohort equations and on the study published by Goff et al. (31) and others (32, 33). The input information needed to estimate ASCVD risk includes age, sex, race, total cholesterol, HDL cholesterol, systolic blood pressure, blood pressure-lowering medication use, diabetes status, and smoking status. Depending on their calculated ASCVD 10-year risk, all SSc patients were stratified in line with the recommendations of the American College of Cardiology into one of the following four ASCVD risk groups: low ASCVD risk group (containing SSc patients with <5% calculated ASCVD 10-year risk score), borderline ASCVD risk group (containing SSc patients with a 5–7.4% calculated ASCVD 10-year risk score), intermediate ASCVD risk group (containing SSc patients with a 7.5–19.9% calculated ASCVD 10-year risk score), and high ASCVD risk group (containing SSc patients with a ≥ 20% calculated ASCVD 10-year risk score).
To estimate a patient’s 10-year risk of fatal cardiovascular disease (CVD), we utilized the Systematic Coronary Risk Evaluation (SCORE) cardiovascular risk chart (SCORE 2019) as provided and developed by the European Society of Cardiology and as published and updated by Mach et al. (34). We employed the low-risk chart as Germany is considered a low-risk country (34). For the SCORE 2019 risk calculation, the input information encompassed the following risk factors: age, gender, smoking, systolic blood pressure, and total cholesterol levels.
2.6 Statistical evaluation
The normal distribution of data was visually verified via Q-Q plots and statistically tested using Kolmogorov–Smirnov and Shapiro–Wilk testing. For normally distributed data, differences between CRP+ and CRP− groups were assessed via independent t-tests, Pearson’s chi-squared tests, or Fisher’s exact tests, as appropriate. For non-normally distributed data, Mann–Whitney U-tests were utilized. Spearman’s rank order correlations were performed in order to assess the associations between CRP levels and cardiovascular risk factors, risk scores, and cardiovascular carotid ultrasound parameters in the overall cohort and the CRP+ and CRP− groups. Due to the relatively small patient sample, we could not test all variables in a common statistical model. Instead, we used binary logistic regression models to test the associations between the dependent variable (CRP positive yes/no) and the general, cardiovascular, and arteriosclerotic carotid ultrasound parameters. An odds ratio (OR) >1 indicates that a higher value in the respective candidate predictor variable (or response “yes” for categorical candidate predictor variables) is associated with a higher probability of belonging to the group CRP+. Subsequently, we used Random Forest classification along with a greedy feature selection procedure (35) with a 5-fold cross-validation and permutation test with 1,000 permutations in order to determine the optimal combination of cardiovascular variables associated with a positive CRP status. The binary classification area under the receiver operating curve (ROC-AUC) was employed as the scoring metric for feature selection. K-nearest neighbor imputation was performed ahead of any missing values. The AUCs between combined ROC models across the 5-fold cross-validation were compared using the Wilcoxon signed-rank test. Data analysis was carried out in Python using the scikit-learn 1.3.0 package and using SPSS statistics IBM, version 25. Statistical significance was defined as p < 0.05.
3 Results
3.1 Patients’ characteristics
Patient demographics, SSc disease characteristics, and information on SSc-related medication usage as well as laboratory measures are summarized in Tables 1A,B and have in part been previously published by Feldmann et al. (10). In brief, CRP+ and CRP− groups showed similar mean age and height and did not significantly differ with respect to their age of SSc onset and gender proportions and with respect to the extent of their SSc organ involvement. Particularly, the proportions of SSc patients suffering from SSc-related vascular complications such as digital ulcerations, Raynaud’s syndrome, or pulmonary arterial hypertension were comparable among both CRP− groups, as were the proportions of vasodilatory drug users (p > 0.05). However, CRP+ SSc patients had a significantly higher BMI (28.2 vs. 25.8 kg/m2, p = 0.037), used significantly more often glucocorticoids (p = 0.023), significantly less often hydroxychloroquine (p = 0.012) and showed a statistical trend toward having on average an almost 2-year shorter SSc disease duration. As outlined previously (10), CRP+ SSc patients had a higher frequency of being SCL70-antibody positive (p = 0.041), whereas the CRP− SSc patients showed significantly more often positive anti-nucleosome antibodies (p = 0.013).
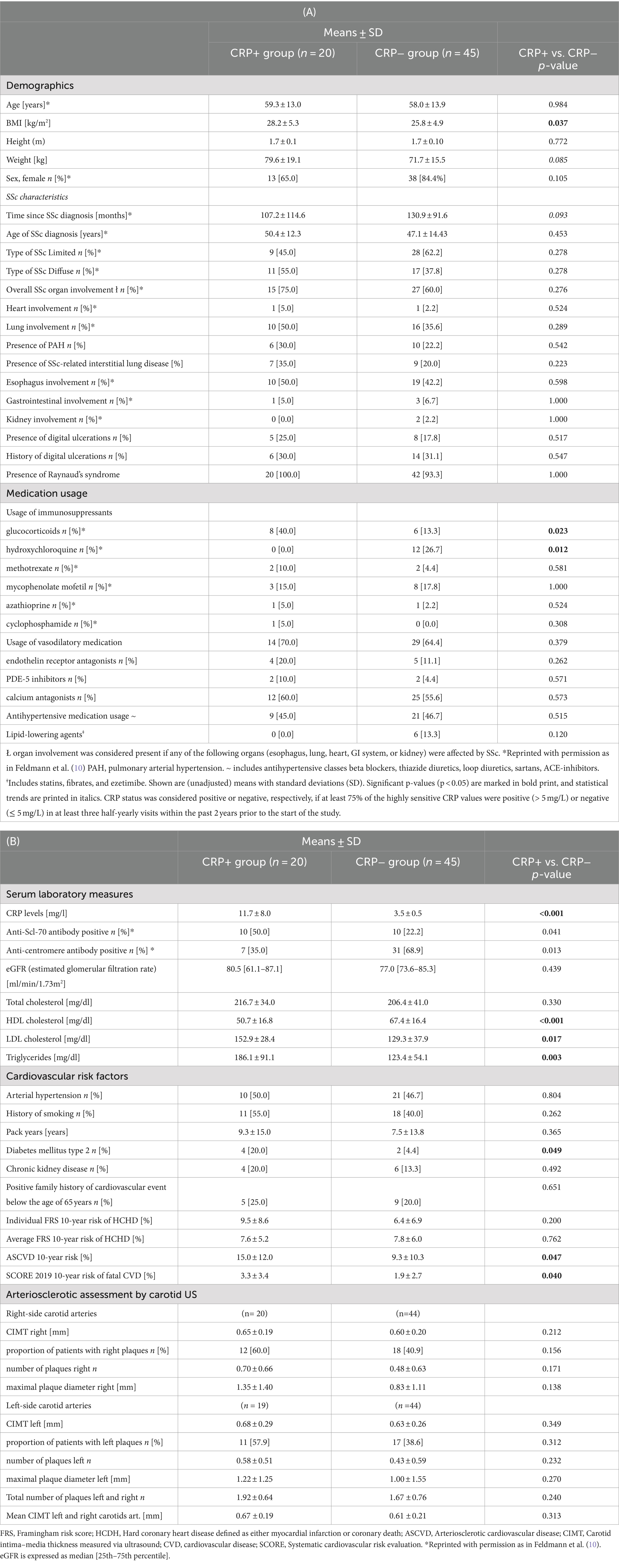
Table 1. Patient characteristics of all 65 study participants with systemic sclerosis (SSc), presented by CRP status.
With respect to cardiovascular health, CRP+ SSc patients showed a significantly altered HDL-, LDL, and triglyceride profile (Table 1B: 0.001 ≤ p ≤ 0.017) as well as a significantly higher proportion of type 2 diabetes compared to their CRP− SSc peers (p = 0.049). In addition, the estimation of cardiovascular risk scores revealed that CRP+ SSc patients had a significantly higher 10-year risk for ASCVD (p = 0.047) than the CRP− SSc patients. Risk stratification of SSc patients by ASCVD risk into a low-ASCVD SSc risk group (<5% ASCVD 10-year risk) and into a combined intermediate-to-high risk ASCVD risk group (IMH risk group, ≥7.5% or > 20% 10y-ASCVD risk estimated) confirmed significantly higher CRP levels in the IMH-risk SSc group compared to low-risk SSc individuals (p = 0.024) (Supplementary Table 1). Calculation and intergroup comparisons of SCORE 2019 risk scores demonstrated that the calculated 10-year risk for fatal cardiovascular disease was significantly higher in the CRP+ SSc group relative to the CRP− SSc group (p = 0.040). The individual Framingham risk scores for hard coronary heart disease were numerically higher in the CRP+ SSc group but did not reach statistical significance. Average Framingham risk scores for hard coronary heart disease and other cardiovascular risk factors such as the presence of arterial hypertension, chronic kidney disease, smoking history, and positive family history for a cardiovascular event below the age of 65 years were comparable between both groups, as were the atherosclerotic quantitative ultrasound measures of left and right carotid arteries and estimated glomerular filtration rates.
3.2 Associations between SSc-specific and demographic parameters, cardiovascular parameters, and CRP status
When assessing the associations between SSc disease-specific, demographic, and cardiovascular parameters with CRP positivity, we found that suffering from type 2 diabetes mellitus increased the risk of having a prolonged CRP−positive status by 5.3-fold (OR: 5.25 [0.87–31.52] p = 0.070) (Table 2). Moreover, a 10 mg/dL increase in HDL levels was associated with a 47% reduced risk of having a prolonged positive CRP status (OR: 0.53 [0.36, 0.79] p = 0.002) and a 10 mg/dL increase in LDL and triglyceride levels showed a significant 22% or 13% risk increase for being CRP−positive, respectively (p = 0.022, and p = 0.005). Moreover, a point increase in the 10-year ASCVD risk score resulted in a statistical trend for a 5% risk increase in experiencing sustained CRP−positivity. We also observed statistical trends for weight (OR: 1.03 [1.00–1.06] p = 0.089) and BMI (OR: 1.10 [0.99–1.22] p = 0.088) to be predictors for CRP−positivity, while other cardiovascular risk factors such as the presence of arterial hypertension or smoking, FRS risk scores and arteriosclerotic ultrasound parameters such as carotid intima–media thickness were not predictive of CRP−positive status (see Supplementary Table 2).
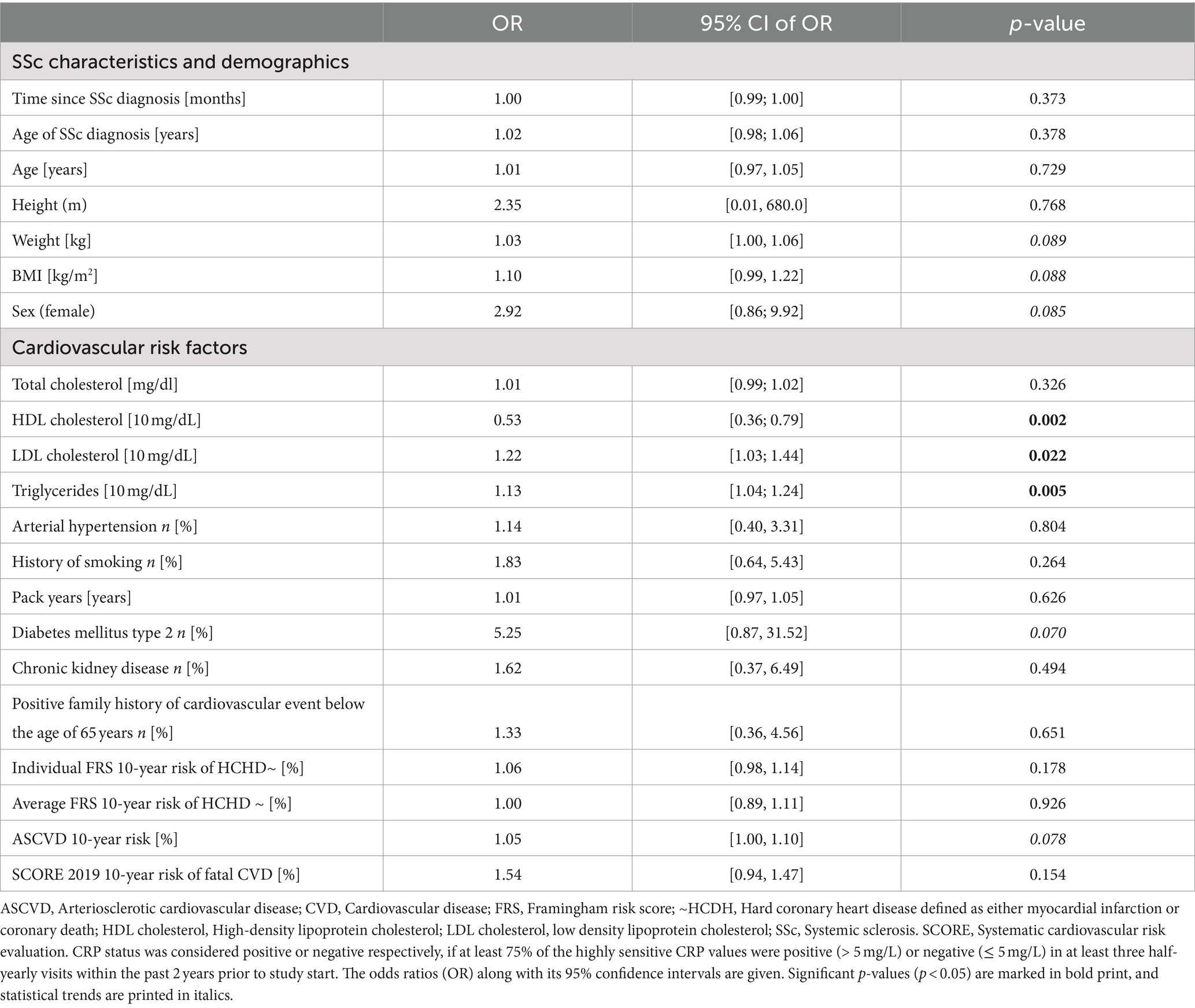
Table 2. Results of binary logistic regression analyses were carried out in all n = 65 SSc patients to assess associations between SSc disease-specific and demographic parameters, cardiovascular parameters, and CRP−positivity status.
3.3 Correlations between baseline CRP levels and cardiovascular risk factors, risk scores, and cardiovascular carotid ultrasound parameters
With respect to clinical and ultrasound correlations (Table 3), we found that in the overall SSc-cohort CRP levels were significant, although weakly, positively correlated with several indicators of cardiovascular health such as HDL cholesterol, LDL cholesterol, triglycerides, the 10-year risk of ASCVD, and the 10-year risks of fatal cardiovascular disease (SCORE 2019) (0.283 < ρ < 0.339, p ≤ 0.041). In addition, we noted in the overall SSc cohort a weak positive correlation between CRP levels and the right carotid intima–media thickness (CIMT right) (ρ = 0.283, p = 0.041) and an almost significant correlation between CRP levels and the mean CIMT left and right (ρ = 0.227, p = 0.071). When stratifying by the CRP group, both quantitative ultrasound parameters turned into moderate-to-strong positive correlations within the subgroup of CRP+ SSc patients (CIMT right ρ = 0.657, p = 0.002, mean CIMT left and right ρ = 0.497, p = 0.026). Additionally, moderate positive correlations for ultrasound markers of arteriosclerotic burden, such as the CIMT left and a number of plaques at the right carotid arteries, were found in the CRP+ SSc group, both approaching statistical significance (0.406 < ρ < 0.429, p ≤ 0.076). In the CRP− group, CRP levels were not correlated with any of the SSc or cardiovascular or ultrasound-specific parameters (data not shown).
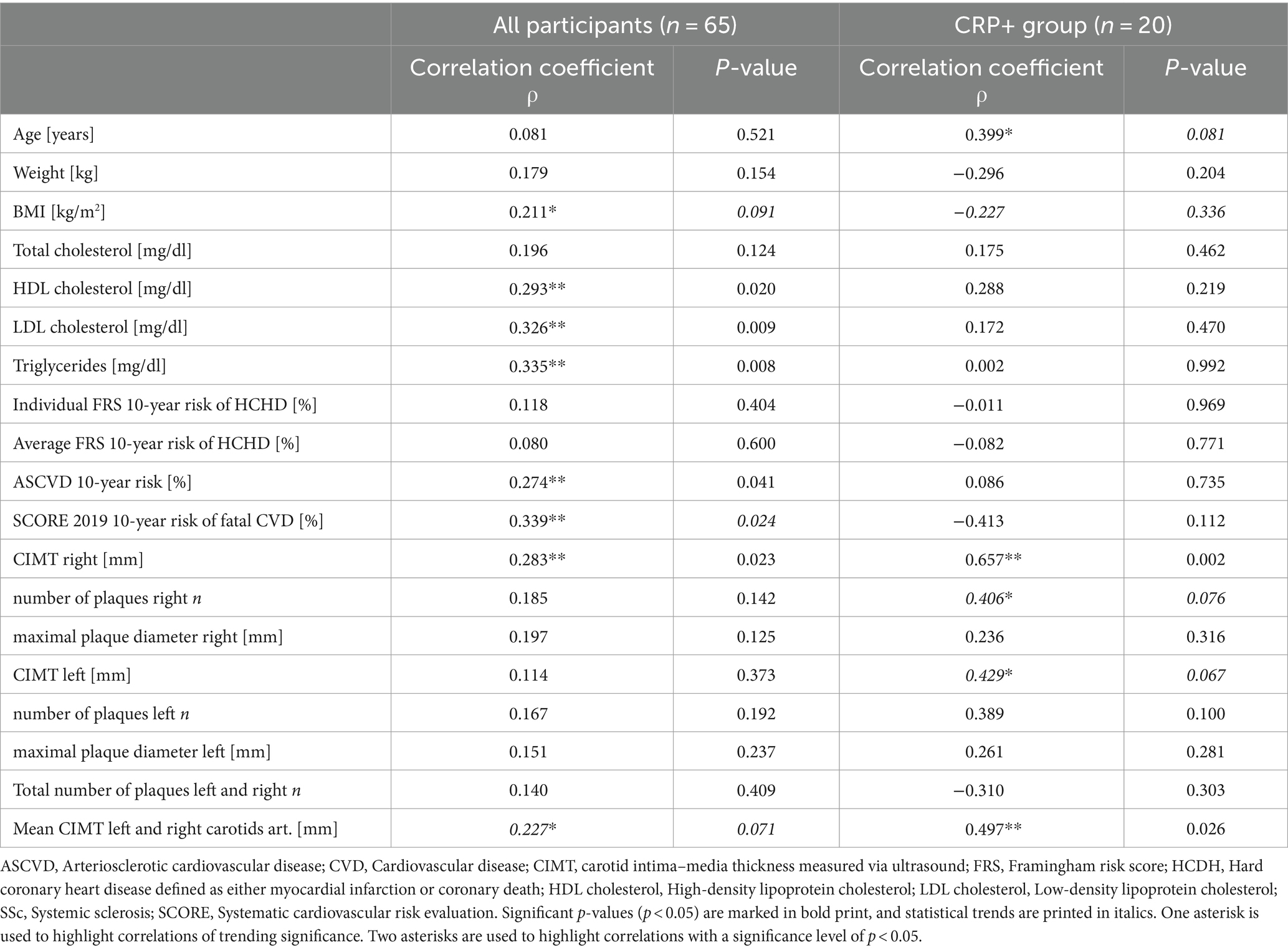
Table 3. Spearman’s rank-order correlations showing the associations between baseline CRP levels and cardiovascular risk factors, risk scores, and cardiovascular carotid ultrasound parameters in all SSc participants, and for the CRP+ SSc group, respectively.
3.4 Classification modeling
To assess the performance and diagnostic accuracy of clinical, cardiovascular, and ultrasound parameters and to identify interesting potential combinations of clinical, cardiovascular, and imaging parameters (ROC-models) with high diagnostic accuracy and discriminatory ability between CRP+ and CRP− groups, we next carried out random forest classification modeling with a greedy feature selection procedure under 5-fold cross-validation. We then performed permutation testing with 1,000 permutations to calculate the p-value of the ROC-AUC scores. Results are summarized in Table 4. As single ROC-models, SSc duration, total cholesterol, and HDL cholesterol demonstrated each a cross-validated AUC of 0.73, 0.75, and 0.76, respectively (0.005 ≤ p ≤ 0.011) and therefore showed good discriminatory ability to differentiate CRP−positivity in SSc patients. LDL cholesterol and ASCVD group assignment alone showed an AUC of 0.67 and 0.65, respectively, both approaching statistical significance (0.057 ≤ p ≤ 0.068). When looking at possible combinations of clinical and cardiovascular parameters, the greedy feature search procedure using the random forest classification model identified several combined ROC models with very high and significant cross-validated AUCs. These included a combined ROC model consisting of the four lipids: total cholesterol, HDL cholesterol, LDL cholesterol, and total triglycerides resulting in an AUC of 0.83 (model 1, p < 0.001). Moreover, combining SSc duration and ASCVD group into a combined ROC model yielded a significant AUC of 0.86 (model 2, p < 0.001). When the presence of type 2 diabetes mellitus was added, the significant cross-validated AUC of model 2 amounted to 0.85 (model 3, p < 0.001). All three combined ROC models showed a similarly high AUC. Combined ROC models using ultrasound parameters or the SCORE 2019 risk score did not reach comparably high AUC values (data not shown).
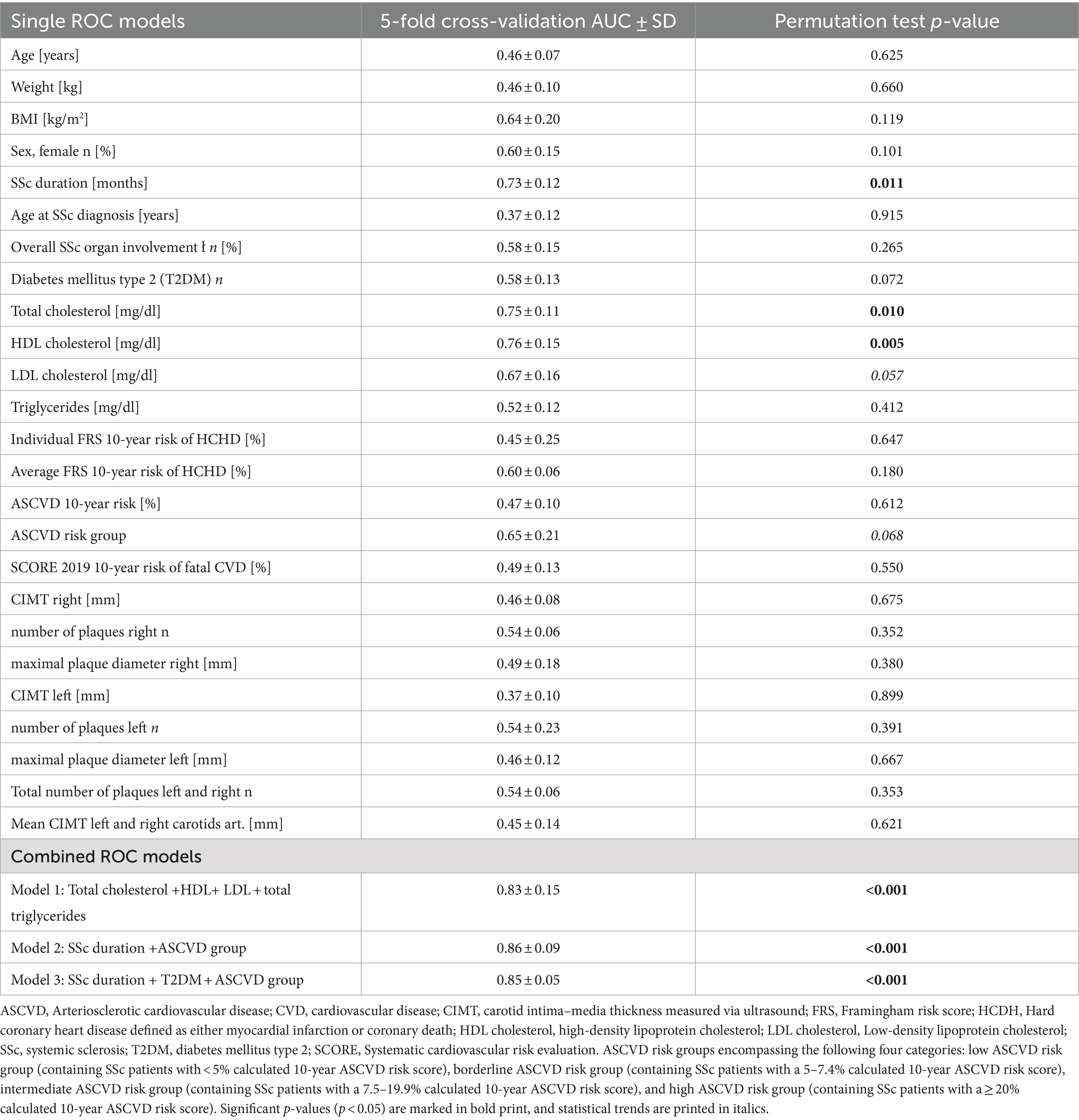
Table 4. Area under the ROC curve (AUC) values for different ROC models including combined ROC models.
4 Discussion
In this cross-sectional observational study, we aimed to explore and assess which general, SSc-specific, and cardiovascular parameters are associated with persistent CRP elevation and thus with chronic low-grade inflammation in SSc. In addition, we also investigated whether persistent CRP elevation in SSc patients is associated with a higher cardiovascular risk as determined using the Framingham risk and ASCVD risk scores and an increased atherosclerotic burden as determined via carotid ultrasound. Finally, we applied random forest classification modeling with cross-validation and permutation testing in order to identify combinations of cardiovascular and SSc-specific parameters that could best differentiate the two groups.
One of the main findings of our study is that a persistent CRP−positivity (of >5 mg/L for at least 1.5 consecutive years), and thus, chronic low-grade inflammation in SSc patients is associated with a significantly higher estimated 10-year risk for ASCVD risk than SSc patients without CRP elevations. The ASCVD risk estimation is based on sex- and age-specific Pooled Cohort Equations validated in Caucasian asymptomatic individuals (33, 36) and is therefore a rather conservative estimate. In a recent population-based cohort study, Kurmann et al. (37) demonstrated that for SSc patients in general (no stratification by CRP status yet), the real-world 10-year-risk for suffering a cardiovascular event was 5 times higher than what was predicted by their respective ASCVD risk scores (37), concluding that the currently recommended ASCVD risk calculator heavily underestimates the ASCVD risk in SSc. The mean ASCVD risk scores reported in Kurmann’s study was approximately 8.9% for all SSc patients (37), a magnitude comparable to the ASCVD-risk score magnitude reported in our study for our CRP− group (see Table 1). Given that Kurmann et al. (37) only looked at all SSc patients (without stratifying by CRP−positivity) and that our reported ASCVD risk for the CRP+ SSc patient group ranged with a mean risk of 15% even significantly above Kurmann’s SSc patient cohort ASCVD risk, it seems very likely that the significant relationship between prolonged CRP−positivity and 10-year arteriosclerotic-cardiovascular risk observed in our study may even be underestimated. Along these lines, Kurmann et al. (37) also found the FRS score to strongly underestimate CV risk in SSc patients by approximately 4-fold (37). Although we did not detect any significant association between FRS scores and CRP−positivity, it seems possible that our FRS-risk estimations for the CRP+ SSc group may have been overly conservative representations of hard coronary heart disease risk and thus may have obscured a potentially significant association with CRP levels in this specific patient group. Larger prospective population-based SSc studies stratified by persistent CRP positivity are therefore needed to validate and elucidate our findings further.
The mechanisms underlying the higher ASCVD risk in CRP+ SSc patients remain unclear but may be attributable in part to their chronic low-grade inflammatory state (38–40) and their disrupted lipid profile, higher BMI, and a more frequent glucocorticoid usage. Chronic inflammation (41) and particularly chronic-low-grade inflammation (42) has widely been accepted as important drivers of atherosclerosis in both non-SSc and SSc settings, leading to activation of leucocytes, oxidative stress, and endothelial dysfunction, triggering a cascade of events culminating in the disruption of atherosclerotic plaques (43–46). Particularly, CRP has been shown in a multitude of studies in non-SSc populations to predict the risk of (cardio)vascular disease independent of all traditional risk factors (47–49) and has emerged as a validated measure of cardiovascular inflammation of similar rank and importance as blood pressure or cholesterol (39). Despite this, CRP has been surprisingly underutilized in the field of primary care and cardiology as a determinant for vascular risk (39). In addition, in the rheumatologic field, SSc studies stratifying by elevated CRP status have been very scarce to date and have been limited so far to the analyses by Muangchan et al. (3) and Jha et al. (7)using data from the Canadian Scleroderma Research Group (3, 7). Although both authors did not focus on arteriosclerotic cardiovascular disease as the main outcome, Jha et al. (7) clearly demonstrated a significantly increased risk of death and pulmonary arterial hypertension in the presence of abnormally elevated CRP levels for SSc patients. Similar to our study, Muangchan et al. (3) observed a significantly higher BMI and more frequent corticosteroid usage in SSc patients with sustained CRP levels above 8 mg/L (3). Overall, both reports reinforce our current and previous (5) notion that chronic low-grade inflammation seems to be an important risk factor in SSc patients and that SSc patients with sustained CRP elevations should be classified as a separate “inflammatory” SSc phenotype.
Other conditions such as dyslipidemia, diabetes, and obesity have also been attributed to trigger and sustain inflammatory responses and thus contribute to atherosclerosis and ASCVD risk (41, 42). Interestingly, our CRP+ SSc group displayed a disrupted atherogenic lipid profile compared to their CRP− SSc peers and to recommended reference values. This altered lipid profile was characterized by increased borderline high to high levels of triglycerides, significantly reduced and borderline-low HDL cholesterol levels, and significantly increased borderline high to high LDL cholesterol levels. Moreover, combining all 4 lipids (HDL, LDL, total cholesterol, and triglycerides) into a classification ROC model was highly discriminatory to CRP−positivity. Abnormal lipid profiles have been described in SSc patients versus controls before (20, 50) and have been partly attributed to a reduced HDL cholesterol efflux capacity in SSc macrophages and thus an impaired ability of SSc patients to clear off cholesterol from the body (51). However, this is the first study to identify a strongly disrupted lipid profile as one of the main differentiating features of the inflammatory SSc phenotype. What drives the alteration in lipid profile in the CPR+ SSc group remains unknown. However, more and more experimental and clinical evidence in non-SSc patients hints toward a close interplay and a series of pathways linking inflammatory molecules such as CRP with lipid metabolism (41, 52, 53) which can both be pharmacologically targeted. Several non-SSc studies have demonstrated that, for example, statins are highly effective not only in lowering lipids but also in reducing CRP serum levels independently of effects on lipids (54, 55). As our patients in the CRP+ SSc group exhibited both elevated CRP levels and an abnormal lipid profile, one might speculate that this specific patient group may particularly benefit from the dual anti-inflammatory and lipid-lowering effects of lipid-lowering therapy. However, additional clinical trials of lipid-lowering agents in SSc patients are needed to prove the potential benefit of statins for this specific patient group.
Apart from the findings above, we also observed moderate-to-strong correlations between prolonged CRP−positivity in SSc patients with several carotid ultrasound parameters, especially with the left or/and right CIMT and with the number of arteriosclerotic plaques at the right carotid, suggesting an increasing subclinical atherosclerotic burden in inflammatory SSc patients. Our results extend to the exploratory observations by Gamal et al. (21) who reported CRP−positivity as a potential risk factor for atherosclerosis in SSc patients compared to controls (21). However, Gamal’s study neither reported the magnitude of CRP levels nor stratified by prolonged CRP status as CRP measurements were taken only at one time point (21). In line with our study, Schiopu et al. (19) and Sedky et al. (20) also observed in their SSc cohorts a significant association between CRP levels and carotid plaques number (19) and CIMT levels, respectively (20). However, unlike our study, both studies did not stratify their cohorts by CRP status due to limitations in the study design including the lack of serial CRP measurements (19).
Our study has several strengths such as a relatively large and well-characterized SSc patient cohort. In addition, our study design and patient selection were based on serial CRP measurements in order to identify an “inflammatory subtype” with a prolonged and thus chronic inflammation. During patient selection, we were also able to track patients for incurring infections and were able to exclude patients with elevated CRP levels due to concomitant infections.
Our study has also several limitations. The first limitation of our study pertains to the cross-sectional study design which only allowed for ASCVD risk estimation but did not provide any information on observed prospective ASCVD outcomes such as myocardial infarction. Second, a higher proportion of corticosteroid users and type 2 diabetics among the CRP+ SSc group may also have contributed to the higher amount of inflammation and subsequently the higher ASCVD risk noted in the CRP+ SSc group. However, due to the limited sample size further subgroup analyses, adjustments for confounding factors, especially further stratification by immunosuppressive medication usage, were not possible. To minimize these drawbacks, we tried to be as transparent as possible by calculating correlations separately for the groups, by reporting extensively group characteristics and group comparisons on all variables of interest, and by cautiously interpreting our findings. Third, we were only able to assess the atherosclerotic burden at the carotid artery level via ultrasound and did not have the means to quantify the extent of atherosclerosis at other ASCVD predilection sites such as the coronary arteries (via calcium-scoring). Moreover, unlike Pagkopoulou et al. (56), we did not have nail fold video capillaroscopy at disposition at our study visit and therefore could not assess morphological markers of microcirculatory dysregulation. Therefore, our study might have underestimated the true extent of microvascular damage present in our SSc patients. However, we collected information on the presence and history of digital ulcerations, PAH, and Raynaud’s syndrome through visual inspection and physical examination during our study visits and by chart review. As those standard vascular parameters showed comparable proportions across both CRP groups, we are confident that the significant association between ASCVD risk and CRP positivity is not mainly driven by a higher vascular dysfunction in the CRP+ group.
5 Conclusion
While having an autoimmune disease such as SSc alone increases the risk of experiencing a CV event (37) and for coronary heart disease by 3-fold (57) compared to the general population, our study provides first hints that suffering from SSc and having additionally a chronic inflammation as seen in our CRP+ SSc group may enhance the estimated risk for arteriosclerotic cardiovascular disease beyond the risk of CRP− SSc patients. Prolonged CRP−positivity may act as an additional important modifiable risk-enhancing factor for atherosclerotic-cardiovascular disease in patients with systemic sclerosis and seems to be along with a disrupted lipid profile the hallmark of a distinct “inflammatory” subgroup of SSc patients who are more prone to suffer from musculoskeletal involvement as well as to a higher cardiovascular risk. SSc patients might therefore benefit from serial tracking of inflammatory markers such as CRP and from lipid markers as part of their standard surveillance and follow-up regimen. Due to the dual anti-inflammatory and lipid-lowering effect of statins, those SSc patients may even benefit from aggressive lipid-lowering and anti-inflammatory pharmacological therapies. However, large population-based studies and clinical trials in patients with SSc are needed to validate our findings in a prospective or interventional setting.
Data availability statement
The datasets presented in this article are not readily available because of ethical and privacy restrictions. Requests to access the datasets should be directed to SF, c3RlcGhhbmllLmZpbnplbEB1bmlrbGluaWstZnJlaWJ1cmcuZGU=.
Ethics statement
The studies involving humans were approved by the Freiburg Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
UH: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. DF: Data curation, Investigation, Writing – original draft, Writing – review & editing. AL: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. MS: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. IJ: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. MK: Formal analysis, Methodology, Writing – review & editing. FK: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. RV: Conceptualization, Funding acquisition, Resources, Supervision, Writing – original draft, Writing – review & editing. SF: Conceptualization, Data curation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Bürgy Foundation (hosted by Sparkasse Pforzheim) and by the ECTS academy personal research grant (to UH).
Acknowledgments
The authors thank the study team of the Clinical Study Center, Department of Rheumatology and Clinical Immunology, University Hospital Freiburg for their help in recruiting and consenting the patients.
Conflict of interest
UH has received grant support through the ECTS Academy personal research grant. FK was employed by the F. Hoffmann-La Roche. RV received honoraria as a speaker for Novartis, Roche, Janssen, Galapagos, Pfizer, Astra Zeneca, Böhringer-Ingelheim, as well as grant support from Novartis, Pfizer and BMS. RV also is a consultant for Novartis, Roche, Janssen, Galapagos, and Astra Zeneca. SF has received consulting/speaker’s fees from Abbvie, Alphasigma/Galapagos, AstraZeneca, Biotest, Celltrion, Janssen/J&J, Novartis, NovoNordisk and UCB, all unrelated to this work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1446268/full#supplementary-material
References
1. Volkmann, ER, Andréasson, K, and Smith, V. Systemic sclerosis. Lancet. (2023) 401:304–18. doi: 10.1016/S0140-6736(22)01692-0
2. Bournia, VK, Fragoulis, GE, Mitrou, P, Mathioudakis, K, Tsolakidis, A, Konstantonis, G, et al. All-cause mortality in systemic rheumatic diseases under treatment compared with the general population, 2015-2019. RMD Open. (2021) 7:e001694. doi: 10.1136/rmdopen-2021-001694
3. Muangchan, C, Harding, S, Khimdas, S, Bonner, A, Canadian Scleroderma Research groupBaron, M, et al. Association of C-reactive protein with high disease activity in systemic sclerosis: results from the Canadian scleroderma research group. Arthritis Care Res. (2012) 64:1405–14. doi: 10.1002/acr.21716
4. Ohtsuka, T . Relation between elevated high-sensitivity C-reactive protein and anti-mitochondria antibody in patients with systemic sclerosis. J Dermatol. (2008) 35:70–5. doi: 10.1111/j.1346-8138.2008.00418.x
5. Mitev, A, Christ, L, Feldmann, D, Binder, M, Möller, K, Kanne, AM, et al. Inflammatory stays inflammatory: a subgroup of systemic sclerosis characterized by high morbidity and inflammatory resistance to cyclophosphamide. Arthritis Res Ther. (2019) 21:262. doi: 10.1186/s13075-019-2057-x
6. Mitev, A, Feldmann, D, Binder, M, Möller, K, Kanne, AM, Hügle, T, et al. (2023). Inflammatory stays inflammatory: a subgroup of systemic sclerosis characterized by high morbidity and inflammatory resistance to cyclophosphamide. ACR Meeting Abstracts. Available at: https://acrabstracts.org/abstract/inflammatory-stays-inflammatory-a-subgroup-of-systemic-sclerosis-characterized-by-high-morbidity-and-inflammatory-resistance-to-cyclophosphamide/ (Accessed November 22, 2023).
7. Jha, M, Wang, M, Steele, R, Baron, M, and Fritzler, MJCanadian Scleroderma Research Group, et al. NT-proBNP, hs-cTnT, and CRP predict the risk of cardiopulmonary outcomes in systemic sclerosis: findings from the Canadian scleroderma research group. J Scleroderma Relat Disord. (2022) 7:62–70. doi: 10.1177/23971983211040608
8. Pokeerbux, MR, Giovannelli, J, Dauchet, L, Mouthon, L, Agard, C, Lega, JC, et al. Survival and prognosis factors in systemic sclerosis: data of a French multicenter cohort, systematic review, and meta-analysis of the literature. Arthritis Res Ther. (2019) 21:86. doi: 10.1186/s13075-019-1867-1
9. Liu, X, Mayes, MD, Pedroza, C, Draeger, HT, Gonzalez, EB, Harper, BE, et al. Does C-reactive protein predict the long-term progression of interstitial lung disease and survival in patients with early systemic sclerosis? Arthritis Care Res. (2013) 65:1375–80. doi: 10.1002/acr.21968
10. Feldmann, D, Jandova, I, Heilmeier, U, Kollert, F, Voll, RE, and Finzel, S. Inflammatory arthritis in systemic sclerosis is associated with elevated C-reactive protein and requires musculoskeletal ultrasound for reliable detection. Front Med. (2022) 9:933809. doi: 10.3389/fmed.2022.933809
11. Aviña-Zubieta, JA, Man, A, Yurkovich, M, Huang, K, Sayre, EC, and Choi, HK. Early cardiovascular disease after the diagnosis of systemic sclerosis. Am J Med. (2016) 129:324–31. doi: 10.1016/j.amjmed.2015.10.037
12. Cen, X, Feng, S, Wei, S, Yan, L, and Sun, L. Systemic sclerosis and risk of cardiovascular disease. Medicine. (2020) 99:e23009. doi: 10.1097/MD.0000000000023009
13. Dimitroulas, T, Baniotopoulos, P, Pagkopoulou, E, Soulaidopoulos, S, Nightingale, P, Sandoo, A, et al. Subclinical atherosclerosis in systemic sclerosis and rheumatoid arthritis: a comparative matched-cohort study. Rheumatol Int. (2020) 40:1997–2004. doi: 10.1007/s00296-020-04677-3
14. Blackburn, R, Giral, P, Bruckert, E, André, JM, Gonbert, S, Bernard, M, et al. Elevated C-reactive protein constitutes an independent predictor of advanced carotid plaques in dyslipidemic subjects. Arterioscler Thromb Vasc Biol. (2001) 21:1962–8. doi: 10.1161/hq1201.099433
15. Ridker, PM . Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. (2003) 107:363–9. doi: 10.1161/01.CIR.0000053730.47739.3C
16. VanGilder, RL, Davidov, DM, Stinehart, KR, Huber, JD, Turner, RC, Wilson, KS, et al. C-reactive protein and long-term ischemic stroke prognosis. J Clin Neurosci. (2014) 21:547–53. doi: 10.1016/j.jocn.2013.06.015
17. Liu, S, Jiang, H, Dhuromsingh, M, Dai, L, Jiang, Y, and Zeng, H. Evaluation of C-reactive protein as predictor of adverse prognosis in acute myocardial infarction after percutaneous coronary intervention: a systematic review and meta-analysis from 18,715 individuals. Front Cardiovasc Med. (2022) 9:1013501. doi: 10.3389/fcvm.2022.1013501
18. Frerix, M, Stegbauer, J, Kreuter, A, and Weiner, SM. Atherosclerotic plaques occur in absence of intima-media thickening in both systemic sclerosis and systemic lupus erythematosus: a duplexsonography study of carotid and femoral arteries and follow-up for cardiovascular events. Arthritis Res Ther. (2014) 16:R54. doi: 10.1186/ar4489
19. Schiopu, E, Au, KM, McMahon, MA, Kaplan, MJ, Divekar, A, Singh, RR, et al. Prevalence of subclinical atherosclerosis is increased in systemic sclerosis and is associated with serum proteins: a cross-sectional, controlled study of carotid ultrasound. Rheumatology. (2014) 53:704–13. doi: 10.1093/rheumatology/ket411
20. Sedky Abdou, MM, El Desouky, SM, Helmy El Kaffas, KM, and Ahmed Hassan, AM. Premature atherosclerosis in systemic sclerosis patients: its relation to disease parameters and to traditional risk factors. Int J Rheum Dis. (2017) 20:383–9. doi: 10.1111/1756-185X.12987
21. Gamal, RM, Abozaid, HSM, Zidan, M, Abdelmegid, MAKF, Abdel-Razek, MR, Alsayed, SAR, et al. Study of MRI brain findings and carotid US features in systemic sclerosis patients, relationship with disease parameters. Arthritis Res Ther. (2019) 21:95. doi: 10.1186/s13075-019-1877-z
22. Mok, MY, Lau, CS, Chiu, SSH, Tso, AWK, Lo, Y, Law, LSC, et al. Systemic sclerosis is an independent risk factor for increased coronary artery calcium deposition. Arthritis Rheum. (2011) 63:1387–95. doi: 10.1002/art.30283
23. Hettema, ME, Zhang, D, de Leeuw, K, Stienstra, Y, Smit, AJ, Kallenberg, CGM, et al. Early atherosclerosis in systemic sclerosis and its relation to disease or traditional risk factors. Arthritis Res Ther. (2008) 10:R49. doi: 10.1186/ar2408
24. Bartoloni, E, Pucci, G, Cannarile, F, Battista, F, Alunno, A, Giuliani, M, et al. Central hemodynamics and arterial stiffness in systemic sclerosis. Hypertension. (2016) 68:1504–11. doi: 10.1161/HYPERTENSIONAHA.116.08345
25. Ozen, G, Inanc, N, Unal, AU, Korkmaz, F, Sunbul, M, Ozmen, M, et al. Subclinical atherosclerosis in systemic sclerosis: not less frequent than rheumatoid arthritis and not detected with cardiovascular risk indices. Arthritis Care Res. (2016) 68:1538–46. doi: 10.1002/acr.22852
26. van den Hoogen, F, Khanna, D, Fransen, J, Johnson, SR, Baron, M, Tyndall, A, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum. (2013) 65:2737–47. doi: 10.1002/art.38098
27. del Sol, AI, Moons, KG, Hollander, M, Hofman, A, Koudstaal, PJ, Grobbee, DE, et al. Is carotid intima-media thickness useful in cardiovascular disease risk assessment? Study Stroke. (2001) 32:1532–8. doi: 10.1161/01.STR.32.7.1532
28. Chambless, LE, Heiss, G, Folsom, AR, Rosamond, W, Szklo, M, Sharrett, AR, et al. Association of Coronary Heart Disease Incidence with carotid Arterial Wall thickness and major risk factors: the atherosclerosis risk in communities (ARIC) study, 1987–1993. Am J Epidemiol. (1997) 146:483–94. doi: 10.1093/oxfordjournals.aje.a009302
29. Stein, JH, Korcarz, CE, and Post, WS. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: summary and discussion of the American Society of Echocardiography consensus statement. Prev Cardiol. (2009) 12:34–8. doi: 10.1111/j.1751-7141.2008.00021.x
30. Wilson, PWF, D’Agostino, RB, Levy, D, Belanger, AM, Silbershatz, H, and Kannel, WB. Prediction of coronary heart disease using risk factor categories. Circulation. (1998) 97:1837–47. doi: 10.1161/01.CIR.97.18.1837
31. Goff, DC, Lloyd-Jones, DM, Bennett, G, Coady, S, D’Agostino, RB, Gibbons, R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. J Am Coll Cardiol. (2014) 63:2935–59. doi: 10.1016/j.jacc.2013.11.005
32. Lloyd-Jones, DM, Huffman, MD, Karmali, KN, Sanghavi, DM, Wright, JS, Pelser, C, et al. Estimating longitudinal risks and benefits from cardiovascular preventive therapies among Medicare patients. J Am Coll Cardiol. (2017) 69:1617–36. doi: 10.1016/j.jacc.2016.10.018
33. Arnett, DK, Blumenthal, RS, Albert, MA, Buroker, AB, Goldberger, ZD, Hahn, EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. (2019) 140:e596–646. doi: 10.1161/CIR.0000000000000678
34. Mach, F, Baigent, C, Catapano, AL, Koskinas, KC, Casula, M, Badimon, L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European atherosclerosis society (EAS). Eur Heart J. (2020) 41:111–88. doi: 10.1093/eurheartj/ehz455
35. Ferri, FJ, Pudil, P, Hatef, M, and Kittler, J. Comparative study of techniques for large-scale feature selection In: ES Gelsema and LS Kanal, editors. Machine Intelligence and Pattern Recognition. North-Holland: Elsevier (1994). 403–13.
36. Emdin, CA, Khera, AV, Natarajan, P, Klarin, D, Baber, U, Mehran, R, et al. Evaluation of the pooled cohort equations for prediction of cardiovascular risk in a contemporary prospective cohort. Am J Cardiol. (2017) 119:881–5. doi: 10.1016/j.amjcard.2016.11.042
37. Kurmann, RD, Sandhu, AS, Crowson, CS, Matteson, EL, Osborn, TG, Warrington, KJ, et al. Cardiovascular risk factors and atherosclerotic cardiovascular events among incident cases of systemic sclerosis: results from a population-based cohort (1980-2016). Mayo Clin Proc. (2020) 95:1369–78. doi: 10.1016/j.mayocp.2019.12.015
38. Ridker, PM . A test in context: high-sensitivity C-reactive protein. J Am Coll Cardiol. (2016) 67:712–23. doi: 10.1016/j.jacc.2015.11.037
39. Ridker, PM, Koenig, W, Kastelein, JJ, Mach, F, and Lüscher, TF. Has the time finally come to measure hsCRP universally in primary and secondary cardiovascular prevention? Eur Heart J. (2018) 39:4109–11. doi: 10.1093/eurheartj/ehy723
40. Emerging Risk Factors CollaborationKaptoge, S, Di Angelantonio, E, Lowe, G, Pepys, MB, Thompson, SG, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. (2010) 375:132–40. doi: 10.1016/S0140-6736(09)61717-7,
41. Libby, P . The changing landscape of atherosclerosis. Nature. (2021) 592:524–33. doi: 10.1038/s41586-021-03392-8
42. Amezcua-Castillo, E, González-Pacheco, H, Sáenz-San Martín, A, Méndez-Ocampo, P, Gutierrez-Moctezuma, I, Massó, F, et al. C-reactive protein: the quintessential marker of systemic inflammation in coronary artery disease-advancing toward precision medicine. Biomedicines. (2023) 11:2444. doi: 10.3390/biomedicines11092444
43. Tabas, I, and Bornfeldt, KE. Intracellular and intercellular aspects of macrophage Immunometabolism in atherosclerosis. Circ Res. (2020) 126:1209–27. doi: 10.1161/CIRCRESAHA.119.315939
44. Ketelhuth, DFJ, Lutgens, E, Bäck, M, Binder, CJ, Van den Bossche, J, Daniel, C, et al. Immunometabolism and atherosclerosis: perspectives and clinical significance: a position paper from the working group on atherosclerosis and vascular biology of the European society of cardiology. Cardiovasc Res. (2019) 115:1385–92. doi: 10.1093/cvr/cvz166
45. Frostegård, J . Atherosclerosis in patients with autoimmune disorders. Arterioscler Thromb Vasc Biol. (2005) 25:1776–85. doi: 10.1161/01.ATV.0000174800.78362.ec
46. Gerasimova, EV, Shayakhmetova, RU, Gerasimova, DA, Popkova, TV, and Ananyeva, LP. Systemic sclerosis and atherosclerosis: potential cellular biomarkers and mechanisms. Front Biosci. (2023) 15:16. doi: 10.31083/j.fbs1504016
47. Ridker, PM, Cushman, M, Stampfer, MJ, Tracy, RP, and Hennekens, CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. (1997) 336:973–9. doi: 10.1056/NEJM199704033361401
48. Koenig, W, Sund, M, Fröhlich, M, Fischer, HG, Löwel, H, Döring, A, et al. C-reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (monitoring trends and determinants in cardiovascular disease) Augsburg cohort study, 1984 to 1992. Circulation. (1999) 99:237–42. doi: 10.1161/01.CIR.99.2.237
49. Kaptoge, S, Di Angelantonio, E, Pennells, L, Wood, AM, and White, IR. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. (2012) 367:1310–20. doi: 10.1056/NEJMoa1107477
50. Tsifetaki, N, Georgiadis, AN, Alamanos, Y, Fanis, S, Argyropoulou, MI, and Drosos, AA. Subclinical atherosclerosis in scleroderma patients. Scand J Rheumatol. (2010) 39:326–9. doi: 10.3109/03009741003605648
51. Ferraz-Amaro, I, Delgado-Frías, E, Hernández-Hernández, V, Sánchez-Pérez, H, de Armas-Rillo, L, Armas-González, E, et al. HDL cholesterol efflux capacity and lipid profile in patients with systemic sclerosis. Arthritis Res Ther. (2021) 23:62. doi: 10.1186/s13075-021-02443-9
52. Pilely, K, Fumagalli, S, Rosbjerg, A, Genster, N, Skjoedt, MO, Perego, C, et al. C-reactive protein binds to cholesterol crystals and co-localizes with the terminal complement complex in human atherosclerotic plaques. Front Immunol. (2017) 8:1040. doi: 10.3389/fimmu.2017.01040
53. Hansen, SEJ, Madsen, CM, Varbo, A, and Nordestgaard, BG. Low-grade inflammation in the association between mild-to-moderate hypertriglyceridemia and risk of acute pancreatitis: a study of more than 115000 individuals from the general population. Clin Chem. (2019) 65:321–32. doi: 10.1373/clinchem.2018.294926
54. Albert, MA, Danielson, E, Rifai, N, Ridker, PM, and Investigators, P. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. (2001) 286:64–70. doi: 10.1001/jama.286.1.64
55. Ridker, PM, Rifai, N, Pfeffer, MA, Sacks, F, and Braunwald, E. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The cholesterol and recurrent events (CARE) Investigators. Circulation. (1999) 100:230–5. doi: 10.1161/01.CIR.100.3.230
56. Pagkopoulou, E, Soulaidopoulos, S, Triantafyllidou, E, Arvanitaki, A, Katsiki, N, Loutradis, C, et al. Peripheral microcirculatory abnormalities are associated with cardiovascular risk in systemic sclerosis: a nailfold video capillaroscopy study. Clin Rheumatol. (2021) 40:4957–68. doi: 10.1007/s10067-021-05795-4
Keywords: systemic sclerosis, CRP, arteriosclerotic cardiovascular risk, carotid ultorasonography, inflammatory systemic sclerosis, Framingham score, ASCVD risk score, SCORE (systemic coronary risk evaluation)
Citation: Heilmeier U, Feldmann D, Leynes A, Seng M, Jandova I, Keute M, Kollert F, Voll RE and Finzel S (2024) Chronic low-grade inflammation in patients with systemic sclerosis is associated with increased risk for arteriosclerotic cardiovascular disease. Front. Med. 11:1446268. doi: 10.3389/fmed.2024.1446268
Edited by:
Panagiota Anyfanti, Aristotle University of Thessaloniki, GreeceReviewed by:
Athanasia Dara, Hippokration General Hospital, GreeceTheodoros Dimitroulas, Aristotle University of Thessaloniki, Greece
Eleni Pagkopoulou, Hippokration Hospital of Thessaloniki, Greece
Copyright © 2024 Heilmeier, Feldmann, Leynes, Seng, Jandova, Keute, Kollert, Voll and Finzel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephanie Finzel, U3RlcGhhbmllLkZpbnplbEB1bmlrbGluaWstZnJlaWJ1cmcuZGU=
 Ursula Heilmeier
Ursula Heilmeier Daria Feldmann1,3
Daria Feldmann1,3 Magdalena Seng
Magdalena Seng Ilona Jandova
Ilona Jandova Florian Kollert
Florian Kollert Reinhard Edmund Voll
Reinhard Edmund Voll Stephanie Finzel
Stephanie Finzel