
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 03 October 2024
Sec. Hepatobiliary Diseases
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1444473
Objective: The impact of primary biliary cholangitis (PBC) on sleep disturbance is relevant to treatment decision-making processes. Studies on sleep disturbance in Chinese patients with PBC are still lacking.
Methods: We analyzed and compared the health-related quality of life (HRQoL) of 107 PBC patients by using the Pittsburgh Sleep Quality Index (PSQI) questionnaire, Generalized Anxiety Disorder Scale (GAD-7), Patient Health Questionnaire 9 (PHQ-9), Short Form (36) Health Survey Questionnaire (SF-36), Fatigue Visual Analog Scale (F-VAS). Patients’ biochemical markers were also collected for correlation analysis with HRQoL. Receiver operating characteristic (ROC) curves and area under the curve (AUCs) were used to determine the diagnostic performance of PSQI, GAD-7, and biochemical markers for assessing the impaired liver function (Child–Pugh B–C) of PBC diagnosis.
Results: Sixty-two (57.9%) PBC patients suffered from poor sleep quality (PSQI >5). The global PSQI score was positively correlated with GAD-7 (r = 0.561, p < 0.001), and PHQ-9 scores (r = 0.652, p < 0.001). There was a negative correlation (r = −0.216, p = 0.025) between sleep quality and red blood cell (RBC) count. PBC patients with poor sleep quality had significantly higher GAD-7 scores (5 vs. 0, p < 0.001), PHQ-9 scores (5.5 vs. 0, p < 0.001), and lower albumin levels (39.6 vs. 37.6 g/L, p = 0.040) than those with good sleep quality. Based on the SF-36 scores, PBC patients with poor sleep quality had lower physical functioning scores (85 vs. 80, p = 0.022), role physical scores (100 vs. 75, p = 0.007), and worse mental health (60 vs. 56, p = 0.002) than those with good sleep quality. ROC analyses showed that the AUC and optimal cut-off values of the combination of PSQI, GAD-7, and RBC for assessing the impaired liver function in PBC diagnosis were 0.771 and 0.193, respectively.
Conclusion: The sleep disturbance was strongly correlated with the severity of anxiety, depression, and RBC count in PBC patients. Meanwhile, PBC patients with poor sleep had poor HRQoL and lower albumin levels. It is feasible to use the combination of PSQI, GAD-7, and RBC for initial screening of the impaired liver function in PBC. Besides routine blood biochemical and imaging indicators, evaluating mental health-related indicators in PBC patients is imperative.
The exact cause of PBC, a chronic liver disease characterized by cholestasis, is still unknown. Seventy years ago, the diagnosis of PBC was often associated with the stage of cirrhosis (1, 2). Early diagnosis of PBC has improved significantly with more accurate cholestasis measurement and anti-mitochondrial antibody (AMA) detection. In 2015, primary biliary cirrhosis was renamed as primary biliary cholangitis to describe the natural history of the disease more accurately and reduce the mental stress of PBC patients (3).
The prevalence of sleep disturbance among PBC patients reported in different articles varies, ranging from approximately 29 to 88%, due to ethnic, geographic, cultural, and social backgrounds (4–6). Generally, sleep regulates adaptive and innate immune responses by influencing the hypothalamus–pituitary–adrenal (HPA) axis and the sympathetic nervous system (SNS) (7). Sleep disturbance leads to activation of the HPA and SNS pathways, which contribute to an increased proinflammatory of the basal gene expression profile (8). Although a large number of studies have been conducted on the inflammatory cell pathways in PBC patients, the interplay between biochemical indicators, sleep, and psychological factors remains unknown, which needs to be explored. To measure the sleep and psychological factors of PBC patients, various non-invasive psychometric tools such as PSQI, GAD-7, PHQ-9, SF-36, and PBC-40 are widely used, and their reliabilities have been verified through practice (5, 9–11).
The purpose of the present study was to analyze the influencing factors of sleep disturbance and HRQoL in Chinese PBC patients, which were evaluated by PSQI, GAD-7, PHQ-9, and SF-36 and investigated their links with the severity of disease.
Newly diagnosed PBC patients (aged ≥18 years) were hospitalized and prospectively enrolled between January 2021 and June 2023. The diagnosis of PBC was based on the 2018 Practice Guidance from the American Association for the Study of Liver Diseases (12). The exclusion criteria included patients who (a) exhibited other clearly diagnosed comorbid liver diseases, for instance, viral hepatitis, drug-related liver diseases, or fatty liver disease of alcoholic or non-alcoholic origin; (b) presented with obstructive cholestasis; and (c) were affected by other systemic diseases including autoimmune hepatitis, primary sclerosing cholangitis, and sarcoidosis. PBC patients received a standardized, self-completed questionnaire, and detailed biochemical indicators were collected during their visit to the Affiliated Hospital of Qingdao University. Only one staff member was responsible for collecting questionnaire answers and matching clinical information, and the patient’s personal information was anonymized in the subsequent analysis of the data. Written informed consent was obtained from all participants. The procedures followed in this study were approved by the Ethics Committee of the Affiliated Hospital of Qingdao University (approval no. QYFYWZLL 27020) and conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
The laboratory investigation of red blood cell (RBC) count, white blood cell (WBC) count, and platelet (PLT) count was performed using automated hematology analyzers (Sangon Biotech Co., Ltd., Shanghai).
C-reactive protein (CRP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin(ALB), alkaline phosphatase (ALP), γ-glutamyl transpeptidase (γ-GT), total bilirubin (TBil), direct bilirubin (DBIL), and indirect bilirubin (IBIL) were measured using the automatic biochemical analyzer (Sangon Biotech Co., Ltd., Shanghai) and related reagents.
By following the manufacturer’s ELISA protocols (Sangon Biotech Co., Ltd., Shanghai), general laboratory testing for serological assays was performed, including assessing the values of immunoglobulin M (IgM), immunoglobulin G (IgG), immunoglobulin A (IgA), and immunoglobulin E (IgE).
Anti-nuclear antibody (ANA), anti-ribonucleoprotein (RNP), anti-Smith (anti-Sm), anti-Sjögren’s syndrome-related antigen A (SSA), anti-Sjögren’s syndrome-related antigen B (SSB), anti-double-stranded deoxyribonucleic acid (ds-DNA), anti-scleroderma-polymyositis (SCL-PM), anti-histidyl-tRNA synthetase (HRS = Jo-1), anti-centromere antibody (ACA), anti-proliferating cell nuclear antigen (PCNA), anti-nucleosome antibody (ANuA), anti-histone antibody (AHA), anti-ribosomal P protein antibody (anti-Rib-P), anti-mitochondrial antibody (AMA), M2 subtype of anti-mitochondrial antibody (AMA-M2) chemiluminescent immunoassay assay kits were obtained from HOB Biotech Group (BioCLIA Autoimmune, China), according to the manufacturer’s instructions described in the assay procedure. The assay was performed on the SMART 6500 instrument (Keysmile Biological Technology Co., Ltd., Chongqing).
The Chinese version of the PSQI was used to assess sleep quality and screen for sleep disturbance. It consisted of 19 items divided into 7 components: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, sleep medication use, and daytime dysfunction. The total score for the seven components was the global PSQI score, ranging from 0 to 21. Higher PSQI scores indicated poorer sleep quality. A global PSQI score of >5 indicated poor sleep quality (13).
The Chinese versions of the GAD-7 and PHQ-9 were used to assess anxiety and depression, respectively, with scores ranging from 0 (none at all) to 3 (almost every day) over the past 2 weeks (14, 15). An overall GAD-7 or PHQ-9 score of ≥10 indicated the prevalence of anxiety symptoms and depressive symptoms. Any participant who scored above the therapeutic threshold (>10) on the PHQ-9 and GAD-7 was referred to the department of psychology for clinical evaluation.
HRQoL was measured by the Chinese version of SF-36, which was a widely used survey of self-reported physical and mental health with 36 questions that measure 9 dimensions of health: health transition (HT), physical functioning (PF), role physical (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role emotional (RE), and mental health (MH) (16). The aggregated scores for all dimensions range from 0 to 100, with higher scores indicating better HRQoL in PBC patients.
F-VAS was typically comprised a 100-mm horizontal line, anchored by two statements representing extreme ends of a single fatigue continuum (“Not at all tired” to “Very tired”). Respondents were instructed to make a mark across or on the F-VAS line to describe the point between the two anchors that best-reflected fatigue status. A higher score on a scale of 0 to 10 represented a greater severity of fatigue. F-VAS greater than 2 was considered fatigue (17).
The data were analyzed using SPSS v 25.0 (Chicago, IL, United States). The data were presented as the mean ± standard deviation (SD) and analyzed using implementing Student’s t-tests, Mann–Whitney U-tests, 2 × 2 χ2 tests, or Fisher’s exact assessments as appropriate based on the data type and distribution. The correlation coefficient between PSQI and HRQoL was determined using Spearman’s rank correlation test. ROC curves and AUCs were used to determine the diagnostic performance of the PSQI, GAD-7, RBC, and the combination of PSQI, GAD-7, and RBC in assessing the impaired liver function (Child–Pugh B–C) of PBC. The optimal cutoffs were determined based on the sensitivity and specificity. Normally distributed quantitative variables were expressed as mean ± SD, and non-normally distributed data were expressed as median (interquartile range). Descriptive statistics were presented as frequencies (n [%]). Statistical significance was defined at the 95% confidence level.
A total of 107 PBC patients were recruited, whose clinical information is shown in Table 1. There were 8 (7.4%) men and 99 (92.3%) women. Their ages ranged from 24 to 89 years old, with a median age of 62 years old.
Among the patients, 45 reported good sleep, while 62 reported poor sleep. It was observed that the patients with poor sleep quality and PBC had significantly lower albumin levels compared to those who reported good sleep (39.6 vs. 37.6 g/L, p = 0.040). However, there were no significant differences in other biochemical markers, including anti-ENA antibody profiles (Tables 1, 2). There was a negative correlation between sleep quality and RBC count (r = −0.216, p = 0.025; Figure 1). In addition, no correlation was found between sleep quality and other biochemical indicators (Table 3).
The prevalence of anxiety and depressive symptoms was 9.3 and 18.7%, respectively. As shown in Tables 4, 5, PBC patients with poor sleep tended to have higher GAD-7 and PHQ-9 scores, as well as lower PF, RP, and MH scores (all p < 0.05). The severity of sleep disturbance in PBC patients was significantly associated with the level of anxiety and depression (Figure 1).
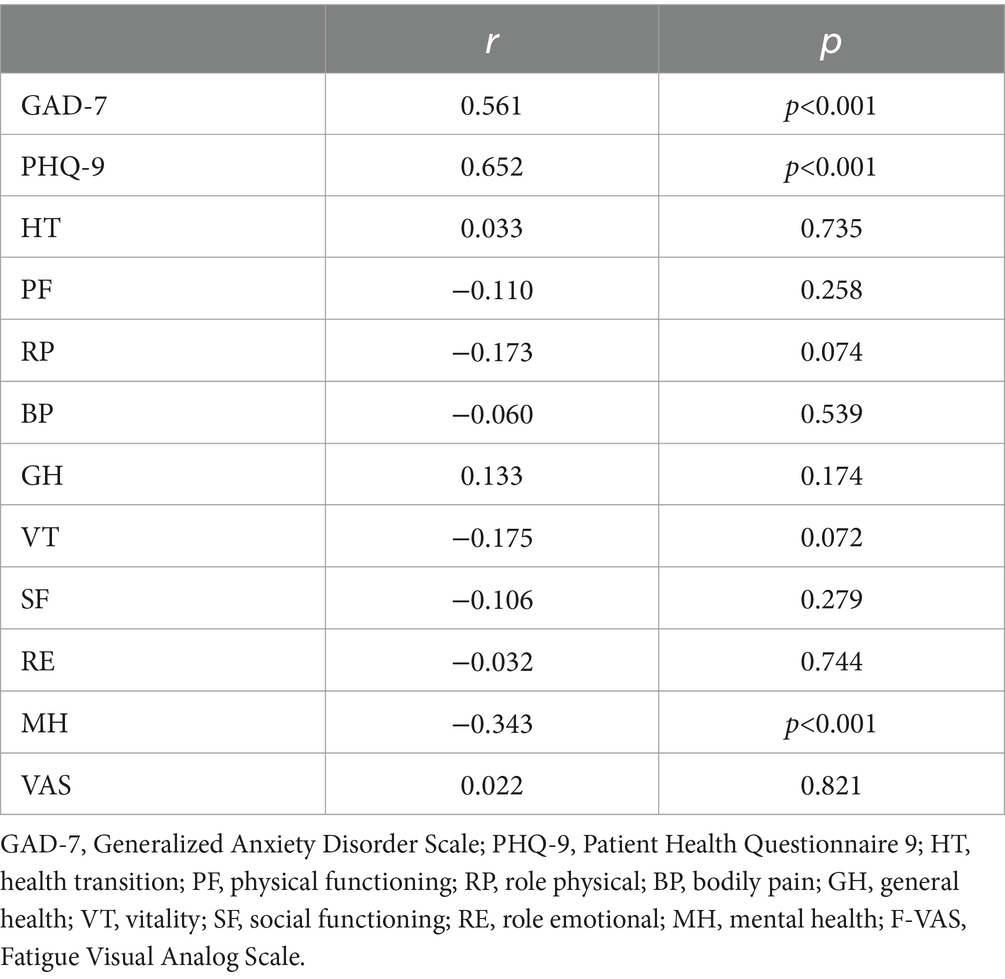
Table 5. Correlation between sleep state and GAD-7, PHQ-9, SF-36, and F-VAS scores for patients with PBC.
Compared with the combination of PSQI, GAD-7, and RBC, the diagnostic capabilities of the PSQI, GAD-7, and RBC had fewer values for assessing the impaired liver function (Child–Pugh B–C) of PBC (Table 6; Figure 2).
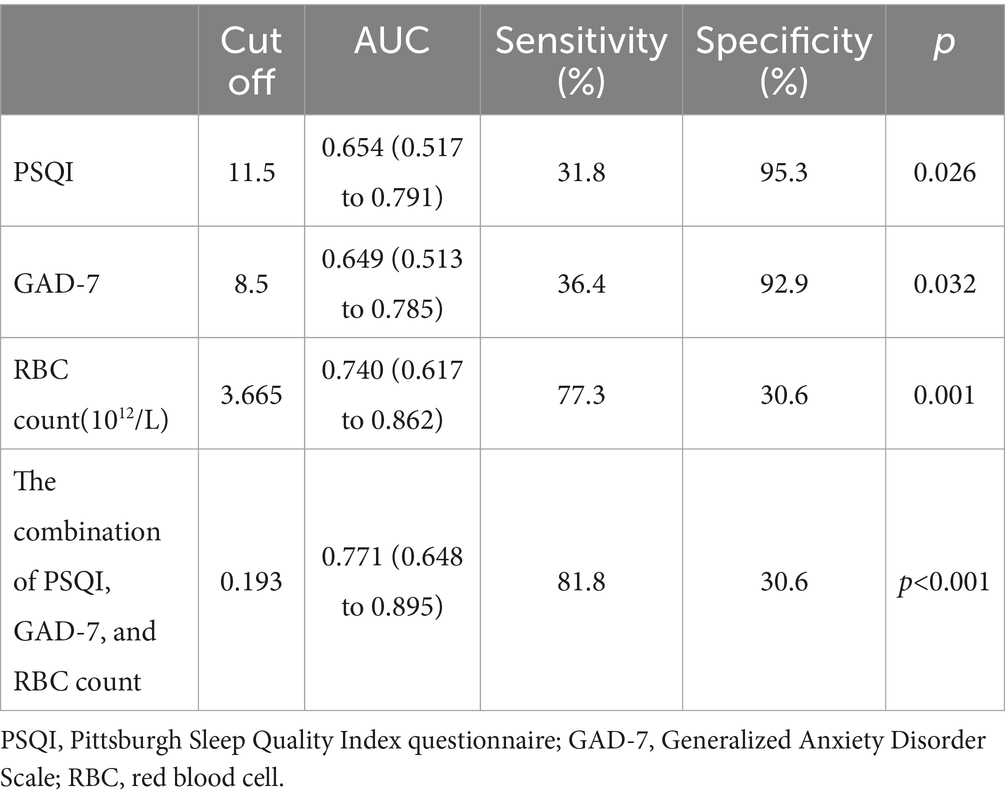
Table 6. Diagnostic performance of the PSQI, GAD-7, RBC, and the combination of PSQI, GAD-7, and RBC in assessing the impaired liver function (Child–Pugh B–C) of PBC.
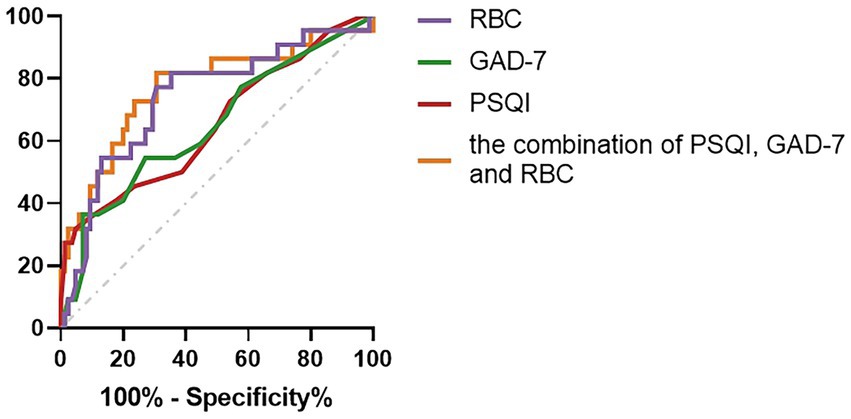
Figure 2. Compared with the combination of PSQI, GAD-7, and RBC, the diagnostic capabilities of the PSQI, GAD-7, and RBC values for assessing the impaired liver function (Child–Pugh B–C) of PBC.
PBC is a chronic liver disease that can progress to cirrhosis, whose symptoms of depression and anxiety can interfere with sleep (18). As the most important sleep disturbance, the incidence of insomnia varies from 5.8 to 20% among the adult population in wider prospects (19). However, the incidence of PBC-related insomnia is much higher than that of primary insomnia, revealing that PBC has a unique pathogenesis (4–6).
There were copious findings that sleep disturbance appeared to be prevalent in PBC patients (20, 21). Basically, sleep disturbances had a negative efficaciousness to psychological, HRQoL, and biochemical markers (18, 22). Patients with chronic liver disease exhibited psychological impairments through extrahepatic pathways. The trajectory of chronic disease and the patient’s ability to cope with the disease are exceedingly determined by them, which reduces perceived HRQoL (23). The patient’s HRQoL can exert influence on the patient’s biochemical parameters in turn (9). It is imperative to analyze the interaction between this sleep disturbance and other psychological factors as well as serology so as to render the theoretical basis for ameliorating PBC patients with sleep disturbance.
A pivotal finding of the current investigation was that PBC patients with poor sleep quality had lower albumin levels. Meanwhile, the sleep disturbance was strongly correlated with the severity of anxiety, depression, and RBC count in PBC patients. Our study revealed that the combination of PSQI, GAD-7, and RBC was effective in assessing impaired liver function (Child–Pugh B–C) in PBC. Some aspects of HRQoL have been shown to be significantly correlated with age and albumin levels (6). Recent research suggested that sleep disturbance was closely associated with a high risk of malnutrition in patients with liver cirrhosis, and albumin was a crucial factor in the assessment of malnutrition (22). Several feasible mechanisms had been suggested that high malnutrition risk resulted in impaired sleep quality. At first, chronic inflammation had been widely demonstrated to be associated with sleep disturbances, and elevated inflammatory cytokines might modulate sleep behavior in patients with cirrhosis (24). On the other hand, liver diseases were orchestrated by a labyrinthine network of cytokine-mediated signaling pathways (25). Aberrant expression of inflammatory cytokines augmented the time of non-rapid eye movement (NREM) sleep, while peculiar antagonists of these cytokines alleviated the time of NREM sleep (26). In addition, the rampant and incessant inflammatory response also predisposed individuals to malnutrition by increasing muscle catabolism and resting energy expenditure (27). Finally, it had been proposed that cirrhosis had increased the risk of daytime melatonin levels and delayed melatonin peak onset during the night (28, 29). Circadian rhythms were synchronized by melatonin, a hormone secreted by the pineal gland in the dark, which was considerably higher at night than during the day (30). The disturbance of the circadian rhythm involved in melatonin increases the risk of PBC patients to have sleep disturbance. In this context, albumin and RBC levels were remarkably correlated with PBC patients’ sleep disturbance. Rising albumin and RBC levels in PBC patients might give assistance to ameliorate their sleep quality, and it is possible that this could further enhance HRQoL.
Examining the relationship between objective serological indicators and sleep disturbance might boost the augmentation of sleep through the rectification of objective conditions in the future. However, the study did not find dissimilarity in the anti-ENA antibody profiles of PBC patients with good and poor sleep quality. This indicated that the mechanism of sleep disturbance in PBC was not caused by autoantibodies.
There were several methods to measure the symptom burden and HRQoL in PBC patients, such as GAD-7, PHQ-9, PBC-40, and SF-36 (18). They had the dual character in gaging HRQoL of PBC patients (18). A European study illustrated that a large proportion of PBC patients had abnormal sleep, mainly manifested as daytime sleepiness (5). PBC patients had severely impaired HRQoL, which was substantially associated not only with fatigue, anxiety, and depression but also with gender, age, and body mass index (9, 31). The prevalence of sleep disturbances in this study was analogous to other studies, but the prevalence of anxiety and depression was lower than in other studies. This might be attributed to the different quantification methods. At the same time, sleep disturbance was profoundly connected to anxiety and depression in PBC patients in this study. Depression and anxiety in PBC might incorporate a multifactorial rationalization. The recent study hypothesized that the disruption of neurotransmitter synthesis and the modification in the brain structure seen on imaging were directly caused by the symptom burden of fatigue and itching (18). In fact, antidepressants and anxiolytics were indispensable to improve the psychological symptoms of PBC patients (32). A structural management to address these additional symptoms would represent a potential treatment for sleep disturbance.
HRQoL was significantly impaired in PBC patients, as measured by SF-36, regardless of sex or disease severity (10). Further research suggested that HRQoL was impaired in both Physical and social role (6). In this circumstance, there might be common characteristics of HRQoL injury in PBC patients. Sleep disturbance caused drastic impairment of the patient’s physiology and physiological functions, which might be related to complications such as bone disease, fat-soluble vitamin deficiency, hyperlipidemia, fatigue, and pruritus in PBC (18). Morning bright light therapy was beneficial to the amelioration of sleep disturbance, which had also been confirmed in some studies (20). Raising sleep disturbance in PBC patients provided potential value for boosting HRQoL. However, further large population follow-up and controlled studies are warranted.
In this study, differences in fatigue levels were not found among PBC patients with good and bad sleep. The interdependence between daytime sleepiness and fatigue in PBC patients had been observed in previous studies (5), and demographic background might be accountable for this corollary. Fatigue in PBC was independent of histopathological stage, degree of liver dysfunction, or autoantibody level (4, 33). The pathogenesis of fatigue in PBC remains poorly understood because of the multi-factor involved. Central nervous system abnormalities, autonomic nervous system abnormalities, abnormal levels of cytokines, fat, or progesterone metabolites, and peripheral muscle motor dysfunction might be interconnected with the pathogenesis of PBC fatigue (34).
The ultimate goal of this work was to ameliorate sleep and HRQoL in PBC patients. The effect of sleep quality on PBC patients’ lives was itself tricky. Although the presence of poor sleep quality showed a sizable association with HRQoL, patients with poor sleep quality rated their HRQoL as poor, which was associated with notable symptoms of social functioning. It demonstrated that sleep quality might eventually be altered by the extent to which they maintained social connections. This observation built on previous determinations revealed that coping strategies were predominant for perceptions of HRQoL in PBC (35). In effect, strengthening their social support might be a worthwhile way to help them augment PBC patients’ HRQoL and sleep in situations where medication was not appropriate. PBC patients also had plentiful selections of using natural remedies to diminish the burden of symptoms, such as yoga and meditation. They also had general health benefits, such as minimizing the risk of heart disease and stroke (36, 37).
However, the study had some unavoidable limitations: (i) This study was a single-center and cross-sectional study with a few patients. (ii) This study only incorporated the influence of some psychological variables on sleep quality. Psychological variables of self-efficacy, stress, and autonomic symptoms were not contemplated. (iii) This study used subjective rating scales to evaluate relevant data and lacked objective sleep and psychological assessment data, such as polysomnography or actigraphy. (iv) Multidimensional social environmental features, such as neighborhood deprivation and social cohesion, could also affect sleep quality, which was classified as a privacy issue, and it was difficult to obtain relevant information within local legal restrictions. Future studies should use objective tools to measure to obtain more accurate and rigorous psychological data on sleep. Multicenter, large sample, and follow-up cohort studies were needed to corroborate our conclusions, and more psychological variables should be included in these studies.
Impaired HRQoL was remarkably associated with PBC patients with poor sleep. The combination of PSQI, GAD-7, and RBC was beneficial for preliminary screening impaired liver function. Based on the results of our research, ameliorating the depression and anxiety of patients and strengthening social support might be beneficial to the enhancement of patients’ sleep status. PBC patients with poor sleep had lower albumin levels, which might indicate a higher nutritional risk and a potential entry point for future treatment. However, it is necessary to further study the psycho-neuro-immunological pathway between PBC and sleep.
The datasets presented in this article are not readily available because the data are not publicly available due to them containing information that could compromise research participant privacy. Requests to access the datasets should be directed to BL, YmlubGl1NzIzMTRAMTYzLmNvbQ==.
The studies involving humans were approved by the institutional review board and the ethics committee of the Affiliated Hospital of Qingdao University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
CZ: Formal analysis, Investigation, Methodology, Software, Writing – original draft. BZ: Conceptualization, Data curation, Formal analysis, Investigation, Writing – review & editing. QL: Data curation, Formal analysis, Software, Writing – review & editing. BqL: Data curation, Investigation, Writing – review & editing. YuY: Data curation, Writing – review & editing. HL: Software, Writing – review & editing. YiY: Data curation, Investigation, Software, Writing – review & editing. BL: Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by National Natural Science Foundation of China [grant numbers 8167060249 and 81241094]; the Natural Science Foundation of Shandong Province, China [grant numbers ZR2016HM13 and ZR2023MH066] and the Science and Technology Project of Qingdao West Coast, China [grant number 2020–50, 2022–49].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Tanaka, A. Current understanding of primary biliary cholangitis. Clin Mol Hepatol. (2021) 27:1–21. doi: 10.3350/cmh.2020.0028
3. Beuers, U, Gershwin, ME, Gish, RG, Invernizzi, P, Jones, DE, Lindor, K, et al. Changing nomenclature for PBC: from 'Cirrhosis' to 'Cholangitis'. Am J Gastroenterol. (2015) 110:1536–8. doi: 10.1038/ajg.2015.312
4. Cauch-Dudek, K, Abbey, S, Stewart, DE, and Heathcote, EJ. Fatigue in primary biliary cirrhosis. Gut. (1998) 43:705–10. doi: 10.1136/gut.43.5.705
5. Newton, JL, Gibson, GJ, Tomlinson, M, Wilton, K, and Jones, D. Fatigue in primary biliary cirrhosis is associated with excessive daytime somnolence. Hepatology. (2006) 44:91–8. doi: 10.1002/hep.21230
6. Yagi, M, Tanaka, A, Abe, M, Namisaki, T, Yoshiji, H, Takahashi, A, et al. Symptoms and health-related quality of life in Japanese patients with primary biliary cholangitis. Sci Rep. (2018) 8:12542. doi: 10.1038/s41598-018-31063-8
7. Besedovsky, L, Lange, T, and Born, J. Sleep and immune function. Pflugers Arch. (2012) 463:121–37. doi: 10.1007/s00424-011-1044-0
8. Vgontzas, AN, Fernandez-Mendoza, J, Liao, D, and Bixler, EO. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev. (2013) 17:241–54. doi: 10.1016/j.smrv.2012.09.005
9. Liu, Y, Tian, S, Jia, G, Han, Z, Guo, C, Shang, Y, et al. Symptoms burden and health-related quality of life in Chinese patients with primary biliary cholangitis. J Clin Transl Hepatol. (2021) 1:001–7. doi: 10.14218/JCTH.2020.00119
10. Raszeja-Wyszomirska, J, Wunsch, E, Krawczyk, M, Rigopoulou, EI, Kostrzewa, K, Norman, GL, et al. Assessment of health related quality of life in polish patients with primary biliary cirrhosis. Clin Res Hepatol Gastroenterol. (2016) 40:471–9. doi: 10.1016/j.clinre.2015.10.006
11. Kempinska-Podhorodecka, A, Adamowicz, M, Chmielarz, M, Janik, MK, Milkiewicz, P, and Milkiewicz, M. Vitamin-D receptor-gene polymorphisms affect quality of life in patients with autoimmune liver diseases. Nutrients. (2020) 12:2244. doi: 10.3390/nu12082244
12. Lindor, KD, Bowlus, CL, Boyer, J, Levy, C, and Mayo, M. Primary biliary cholangitis: 2018 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. (2019) 69:394–419. doi: 10.1002/hep.30145
13. Buysse, DJ, Reynolds, CF 3rd, Monk, TH, Berman, SR, and Kupfer, DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
14. Wang, W, Bian, Q, Zhao, Y, Li, X, Wang, W, Du, J, et al. Reliability and validity of the Chinese version of the patient health questionnaire (PHQ-9) in the general population. Gen Hosp Psychiatry. (2014) 36:539–44. doi: 10.1016/j.genhosppsych.2014.05.021
15. Spitzer, RL, Kroenke, K, Williams, JB, and Lowe, B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. (2006) 166:1092–7. doi: 10.1001/archinte.166.10.1092
16. Li, L, Wang, HM, and Shen, Y. Chinese SF-36 health survey: translation, cultural adaptation, validation, and normalisation. J Epidemiol Community Health. (2003) 57:259–63. doi: 10.1136/jech.57.4.259
17. Hewlett, S, Dures, E, and Almeida, C. Measures of fatigue: Bristol rheumatoid arthritis fatigue multi-dimensional questionnaire (BRAF MDQ), Bristol rheumatoid arthritis fatigue numerical rating scales (BRAF NRS) for severity, effect, and coping, Chalder fatigue questionnaire (CFQ), checklist individual strength (CIS20R and CIS8R), fatigue severity scale (FSS), functional assessment chronic illness therapy (fatigue) (FACIT-F), multi-dimensional assessment of fatigue (MAF), multi-dimensional fatigue inventory (MFI), pediatric quality of life (PedsQL) multi-dimensional fatigue scale, profile of fatigue (ProF), short form 36 vitality subscale (SF-36 VT), and visual analog scales (VAS). Arthritis Care Res. (2011) 63:S263–86. doi: 10.1002/acr.20579
18. Sivakumar, T, and Kowdley, KV. Anxiety and depression in patients with primary biliary cholangitis: current insights and impact on quality of life. Hepat Med. (2021) 13:83–92. doi: 10.2147/HMER.S256692
19. Bjoroy, I, Jorgensen, VA, Pallesen, S, and Bjorvatn, B. The prevalence of insomnia subtypes in relation to demographic characteristics, anxiety, depression, alcohol consumption and use of hypnotics. Front Psychol. (2020) 11:527. doi: 10.3389/fpsyg.2020.00527
20. Shah, NM, Malhotra, AM, and Kaltsakas, G. Sleep disorder in patients with chronic liver disease: a narrative review. J Thorac Dis. (2020) 12:S248–60. doi: 10.21037/jtd-cus-2020-012
21. Montagnese, S, Nsemi, LM, Cazzagon, N, Facchini, S, Costa, L, Bergasa, NV, et al. Sleep-wake profiles in patients with primary biliary cirrhosis. Liver Int. (2013) 33:203–9. doi: 10.1111/liv.12026
22. Hui, Y, Wang, X, Yu, Z, Feng, H, Li, C, Mao, L, et al. Relationship between sleep-wake disturbance and risk of malnutrition in hospitalized patients with cirrhosis. Front Nutr. (2021) 8:719176. doi: 10.3389/fnut.2021.719176
23. Huang, X, Liu, X, and Yu, Y. Depression and chronic liver diseases: are there shared underlying mechanisms? Front Mol Neurosci. (2017) 10:134. doi: 10.3389/fnmol.2017.00134
24. Entzian, P, Linnemann, K, Schlaak, M, and Zabel, P. Obstructive sleep apnea syndrome and circadian rhythms of hormones and cytokines. Am J Respir Crit Care Med. (1996) 153:1080–6. doi: 10.1164/ajrccm.153.3.8630548
25. Zhou, WC, Zhang, QB, and Qiao, L. Pathogenesis of liver cirrhosis. World J Gastroenterol. (2014) 20:7312–24. doi: 10.3748/wjg.v20.i23.7312
26. Imeri, L, and Opp, MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci. (2009) 10:199–210. doi: 10.1038/nrn2576
27. Jensen, GL. Malnutrition and inflammation-"burning down the house": inflammation as an adaptive physiologic response versus self-destruction? JPEN J Parenter Enteral Nutr. (2015) 39:56–62. doi: 10.1177/0148607114529597
28. Steindl, PE, Finn, B, Bendok, B, Rothke, S, Zee, PC, and Blei, AT. Disruption of the diurnal rhythm of plasma melatonin in cirrhosis. Ann Intern Med. (1995) 123:274–7. doi: 10.7326/0003-4819-123-4-199508150-00005
29. Iguchi, H, Kato, KI, and Ibayashi, H. Melatonin serum levels and metabolic clearance rate in patients with liver cirrhosis. J Clin Endocrinol Metab. (1982) 54:1025–7. doi: 10.1210/jcem-54-5-1025
30. Quay, WB. Circadian and estrous rhythms in pineal melatonin and 5-Hydroxy Indole-3-acetic acid. Proc Soc Exp Biol Med. (1964) 115:710–3. doi: 10.3181/00379727-115-29014
31. Castellanos-Fernández, MI, Borges-González, SA, Stepanova, M, Infante-Velázquez, ME, Ruenes-Domech, C, González-Suero, SM, et al. Health-related quality of life in Cuban patients with chronic liver disease: a real-world experience. Ann Hepatol. (2021) 22:100277. doi: 10.1016/j.aohep.2020.10.005
32. Shaheen, AA, Kaplan, GG, Almishri, W, Vallerand, I, Frolkis, AD, Patten, S, et al. The impact of depression and antidepressant usage on primary biliary cholangitis clinical outcomes. PLoS One. (2018) 13:e0194839. doi: 10.1371/journal.pone.0194839
33. Huet, PM, Deslauriers, J, Tran, A, Faucher, C, and Charbonneau, J. Impact of fatigue on the quality of life of patients with primary biliary cirrhosis. Am J Gastroenterol. (2000) 95:760–7. doi: 10.1111/j.1572-0241.2000.01857.x
34. Abbas, G, Jorgensen, RA, and Lindor, KD. Fatigue in primary biliary cirrhosis. Nat Rev Gastroenterol Hepatol. (2010) 7:313–9. doi: 10.1038/nrgastro.2010.62
35. Blackburn, P, Freeston, M, Baker, CR, Jones, DE, and Newton, JL. The role of psychological factors in the fatigue of primary biliary cirrhosis. Liver Int. (2007) 27:654–61. doi: 10.1111/j.1478-3231.2007.01500.x
36. Hallsworth, K, Jopson, L, Jones, DE, and Trenell, MI. Exercise therapy in primary biliary cirrhosis: the importance of moving while sitting on a surgical waiting list-a case study. Frontline Gastroenterol. (2016) 7:167–9. doi: 10.1136/flgastro-2015-100672
Keywords: primary biliary cholangitis, sleep disturbance, Pittsburgh sleep quality index, generalized anxiety disorder scale, patient health questionnaire
Citation: Zhao C, Zang B, Liu Q, Liu B, Yao Y, Li H, Yang Y and Liu B (2024) Psychological factors and biochemical indicators influencing sleep disturbance of patients with primary biliary cholangitis in China: a cross-sectional survey analysis. Front. Med. 11:1444473. doi: 10.3389/fmed.2024.1444473
Received: 14 June 2024; Accepted: 05 September 2024;
Published: 03 October 2024.
Edited by:
Yong-Bo Zheng, Peking University Sixth Hospital, ChinaReviewed by:
Zhoulong Yu, Peking University, ChinaCopyright © 2024 Zhao, Zang, Liu, Liu, Yao, Li, Yang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Liu, YmlubGl1NzIzMTRAMTYzLmNvbQ==
†Present address: Qixuan Liu, Graduate group of Epidemiology, School of Veterinary Medicine, University of California, Davis, Davis, CA, United States
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.