- 1Department of Hepatobiliary and Pancreatic Surgery, General Surgery Center, The First Hospital of Jilin University, Changchun, China
- 2Department of Abdominal Surgery, Guiqian International General Hospital, Guiyang, China
Intraductal papillary neoplasm of bile duct (IPNB), as a precancerous lesion of cholangiocarcinoma, is a rare biliary tract tumor. A 66-year-old female patient was found to have a bile duct mass by routine examination. The liver function tests and tumor markers were normal. Imaging findings revealed a 2.6 cm mass in the common hepatic duct, accompanied by dilatation of both intrahepatic and extrahepatic bile ducts. The patient underwent open extrahepatic bile duct resection, cholecystectomy and Roux-en-Y hepaticojejunostomy. We also conducted a literature review to summarize the clinicopathological features and surgical treatments of IPNB.
Introduction
Intraductal papillary neoplasm of bile duct (IPNB) is a rare bile duct tumor, which was first reported by Chen et al. (1). It is characterized by intraductal papillary or villous biliary neoplasms covering delicate fibrovascular stalks (2). According to the 2019 World Health Organization classification of tumors of the digestive system, IPNB is defined as an intraepithelial neoplasm based on its site of origin, excessive mucin secretion, prognosis, and histological features (3). Here, we report a case and summarize previous IPNB cases in the literature, in order to enhance understanding of the clinicopathological features and surgical methods of this rare disease and achieve the goal of early diagnosis and treatment.
Case presentation
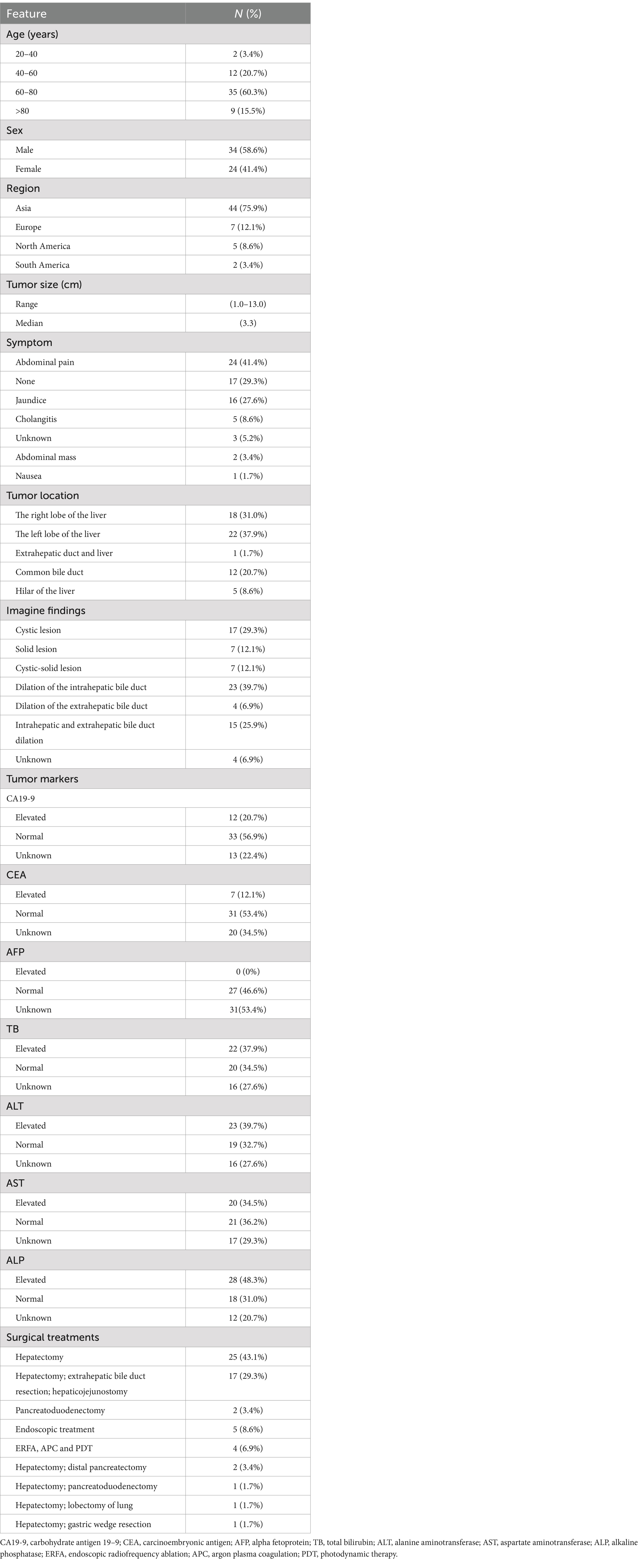
A 66-year-old female patient was admitted because of a mass discovered in the common hepatic duct during routine examination. The patient reported occasional abdominal distension but denied experiencing fever or abdominal pain. Physical examination had no remarkable findings. Enhanced computed tomography (CT) and magnetic resonance cholangiopancreatography (MRCP) showed obvious dilatation of the common bile duct, nodular soft tissue shadow with a diameter of 2.6 cm in the common hepatic duct, and dilatation of the intrahepatic and extrahepatic bile duct (Figure 1A and Supplementary Figures S1–S4). The tumor was located 1 cm below the bifurcation of the bile duct. Laboratory results indicated normal levels of liver enzymes, bilirubin, and tumor markers. Based on the results of laboratory tests and imaging, the preliminary diagnosis was IPNB. During exploration through a right upper abdominal rectus muscle incision, a lesion measuring 2.3 × 2 × 0.8 cm was identified in the common hepatic duct, accompanied by the observation of greenish-yellow mucus-like sludge (Figure 1B). No peripheral enlarged lymph nodes were found during the operation. The patient underwent open extrahepatic bile duct resection, cholecystectomy and Roux-en-Y hepaticojejunostomy. During the operation, negative margins were ensured through intraoperative frozen section diagnosis, and the left and right hepatic ducts were anastomosed to the jejunal loop separately. The operation took 115 minutes with an estimated blood loss of 30 mL. Microscopically, the common bile duct was lined by papillary growth neoplasia with high-grade intraepithelial neoplasia (Figures 1C,D).
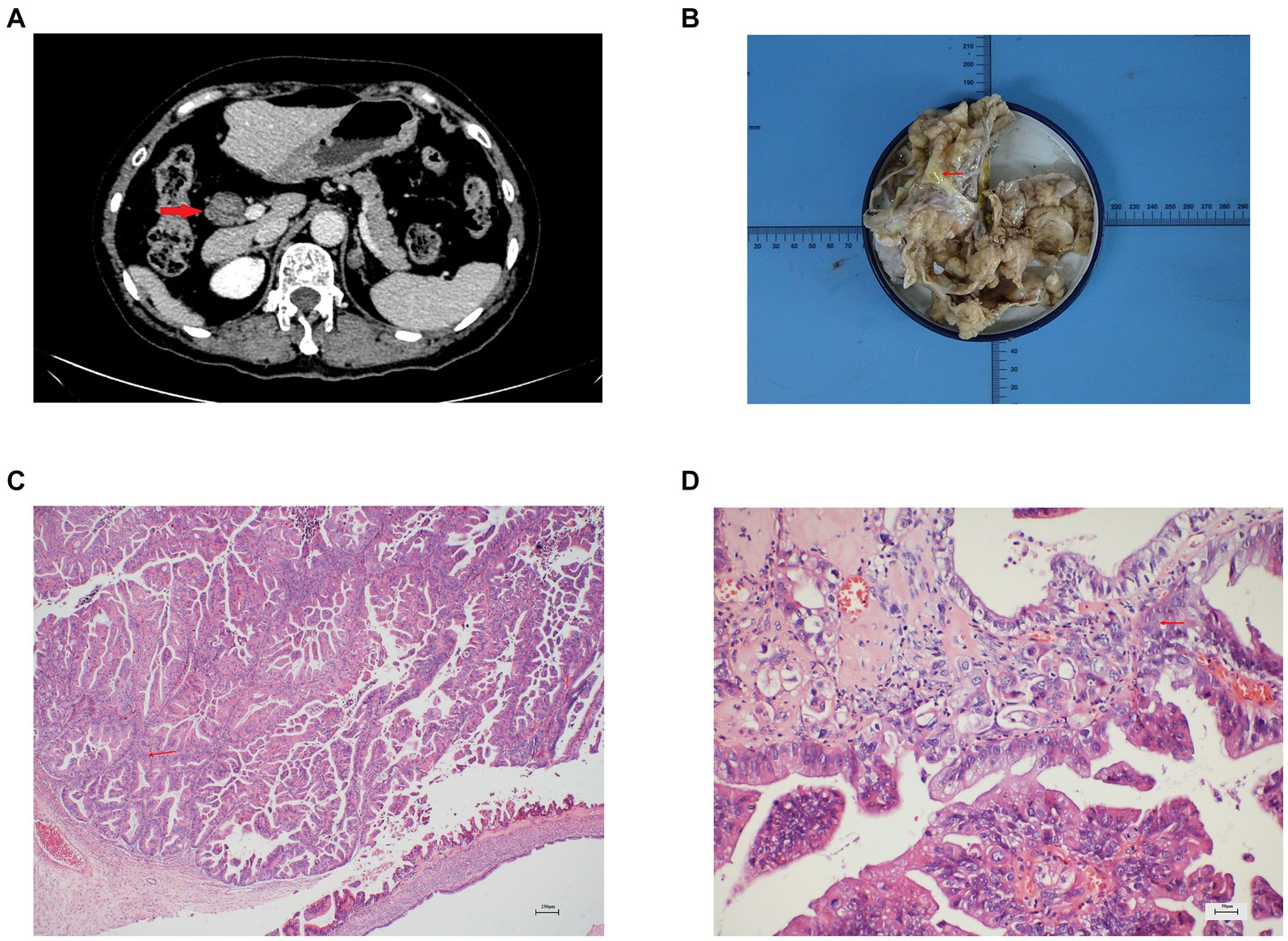
Figure 1. (A) Enhanced CT showed a round low-density mass in the hepatic hilar area and dilated bile duct, (B) Gross pathology showed the bile duct is filled with greenish-yellow mucus-like sludge, (C) Microscopic finding showed the characteristics of intraductal papillary growth (hematoxylin and eosin stain; ×40 original magnification), (D) Microscopic finding showed the papillary growth neoplasia with high-grade intraepithelial neoplasia (hematoxylin and eosin stain; ×200 original magnification).
Postoperative laboratory tests showed that liver enzymes and bilirubin were normal. The patient recovered well and was discharged after one week. After 18 months of follow-up, the patient had no tumor recurrence.
Discussion
IPNB, a rare intraductal preinvasive tumor, share characteristics akin to those of intraductal papillary mucinous neoplasm of the pancreas (IPMN). Arising from biliary tree stem cells in the peribiliary glands, IPNB encompasses gastric, oncocytic, pancreaticobiliary, and intestinal subtypes (4). The prevelent pancreaticobiliary subtype in Western population, characterized by the expression of the mucin core protein MUC1, bears a heightened risk of malignant progression. Conversely, the intestinal subtype, prevalent in Asian population and expressing MUC2 and MUC5AC, is more common. In the pancreaticobiliary histological type, the biliary type is more closely associated with malignant transformation, whereas the pancreatic subtype exhibits more mucin production (5). Remarkably, over 50% of IPNB cases coincide with invasive carcinoma (6, 7).
IPNB mostly occurs in East Asia, with some studies indicating its association with the high prevalence of hepatolithiasis and clonorchiasis (8, 9). Chronic biliary inflammation caused by hepatolithiasis and Clonorchis sinensis infection leads to the production of reactive oxygen or nitrogen species, which subsequently damage DNA and cause neoplastic changes in the biliary epithelium, ultimately resulting in the development of IPNB (10, 11). Additionally, IPNB exhibits significant early genetic alterations. Mutations in cancer-related genes TP53, KRAS, GNAS, and SMAD4 may lead to the inactivation of histone modifiers, activation of G protein signaling, and loss of genomic stability (12–14). Typically, IPNB is detected in individuals aged between 50 and 70 years, with a slightly higher incidence among males (15). It frequently presents with nonspecific clinical symptoms, and the most common symptoms are abdominal pain, fever and jaundice due to recurrent biliary obstruction. Abnormal liver enzyme and bilirubin levels may result from biliary obstruction. Some studies suggest that tumor markers are often deemed nonspecific for assessing IPNB malignancy, as their values mainly depend on the degree of biliary obstruction (16–18). Imaging examinations such as CT, magnetic resonance imaging (MRI) and ultrasound are commonly used for the diagnosis of IPNB. The typical imaging findings are bile duct dilatation and intraductal masses (5). In addition, the application of direct cholangiography, such as endoscopic retrograde cholangiopancreatography (ERCP), Spyglass, and percutaneous transhepatic cholangiogram (PTC), can improve the accuracy of diagnosis (19, 20).
Surgical resection is the first choice for the treatment of IPNB, which should be determined according to the location and extent of the tumor (21). Intraoperative frozen section is essential to ensure achieving R0 resection (22). For patients ineligible for surgery, endoscopic radiofrequency ablation (ERFA), argon plasma coagulation (APC) and photodynamic therapy (PDT) can be used for local treatment (23–27). Studies indicate that prognostic factors for IPNB include tumor invasiveness, margin status and lymph node metastasis (28–30). Gordon-Weeks et al. reported a 5-year overall survival rate of 65%, which was better than that of cholangiocarcinoma (7).
Due to the low incidence of IPNB and the limited number of large-sample studies, its clinicopathological features have not been fully elucidated. A multicenter retrospective study involving 85 IPNB patients showed a median age of 66 years, with 49.4% being female, and the overall 5-year postoperative survival rate was 63% (31). Kubota et al. focused on the differences in clinicopathological features and prognosis among different subtypes of IPNB (32). Another study conducted in Thailand found that lymph node metastasis and the completeness of resection are important factors affecting the prognosis of IPNB patients (33).
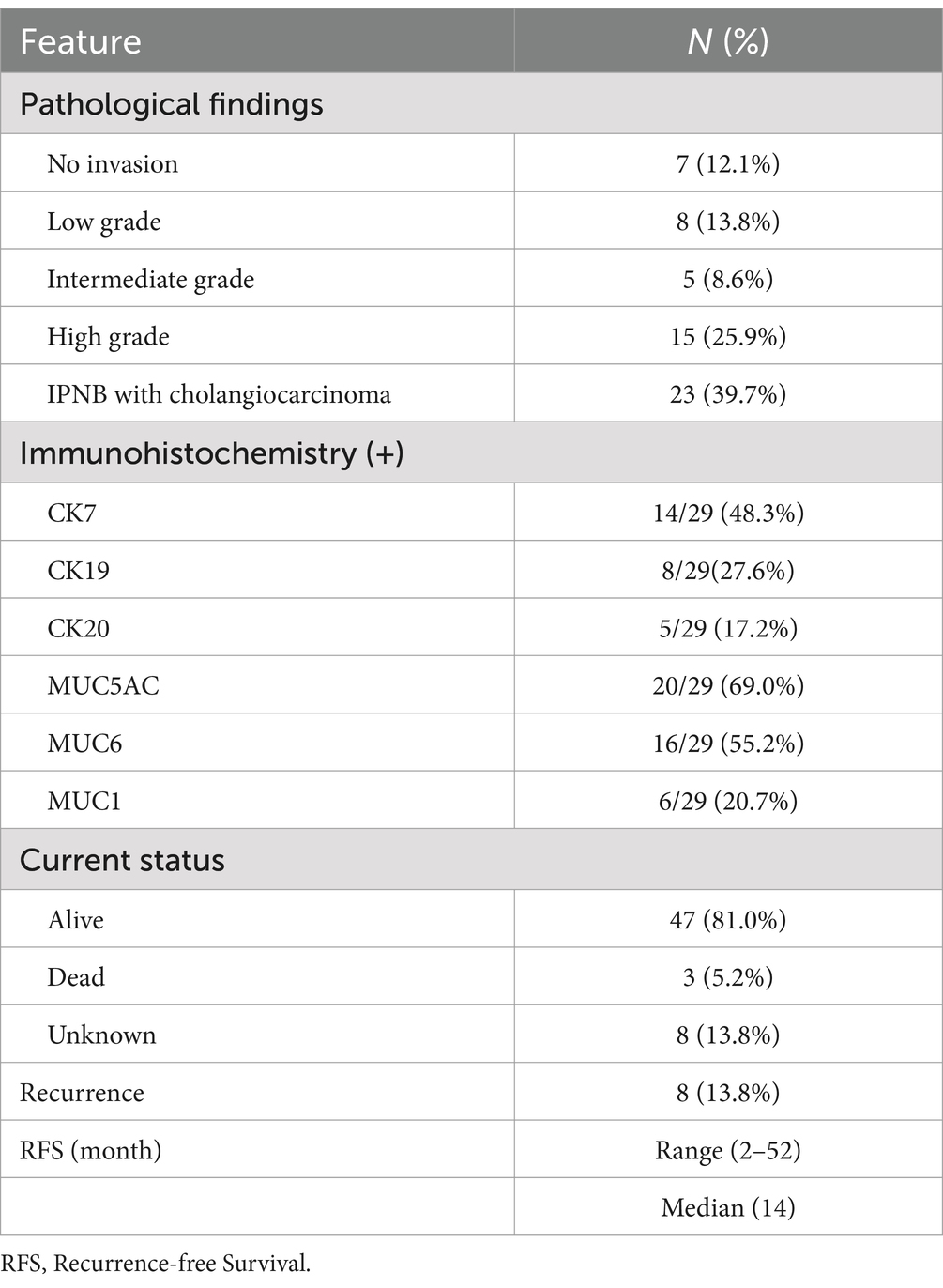
To comprehensively investigate the clinicopathological features and prognosis of IPNB, we conducted a review of case reports published in Pubmed over the past 22 years (Table 1). A total of 58 patients with ages ranging from 22 to 87 years (median age 68.5 years) were included, which 75.8% of the patients were over 60 years of age. There were more male patients than female patients in this literature review. Notably, IPNB demonstrated pronounced geographical distribution differences, with the majority of cases (75.9%) occurring in Asia. Abdominal pain (41.4%) is the most common symptoms, followed by jaundice (27.6%). The maximum diameter of the tumors ranged from 1 to 13 cm (median:3.3 cm). IPNB can occur in any part of the biliary tract, with 69.0% located in the liver, 20.7% in the common bile duct, 8.6% in the hilar of the liver and 1.7% in the extrahepatic duct and liver. Imaging findings often reveal lesions accompanied by bile duct dilatation. Approximately 1/3 of patients have elevated total bilirubin and liver enzymes due to biliary obstruction caused by IPNB. Tumor markers were normal in more than half of the patients. Among those undergoing radical tumor resection, 25 patients underwent hepatectomy, 17 patients underwent hepatectomy with extrahepatic bile duct resection and hepaticojejunostomy, and 2 patients underwent pancreaticoduodenectomy. Nine patients were treated with local treatments, including ERCP (n = 5), ERFA (n = 2), APC (n = 1), and PDT (n = 1), because the physical conditions were not suitable for surgery. Three patients underwent hepatectomy combined with distal pancreatectomy (n = 2) and hepatectomy combined with pancreaticoduodenectomy (n = 1) because of the presence of IPMN. Two patients underwent hepatectomy combined with gastric wedge resection (n = 1) and hepatectomy combined with lobectomy of lung (n = 1) because of the aggressive nature of IPNB (Table 1). Pathological results showed that IPNB was often aggressive, of which 65.5% showed high-grade intraepithelial neoplasia or cholangiocarcinoma. Immunohistochemistry results revealed positive expression of CK7 (48.3%), MUC5AC (69.0%) and MUC6 (55.2%) in IPNB. The median recurrence-free survival was 14 months (range, 2–52 months). Tumor recurrence or metastasis was found in 8 patients during the follow-up, whose IPNB pathology were high-grade intraepithelial neoplasia or cholangiocarcinoma (Table 2). Among them, three patients received adjuvant chemotherapy, one patient received metastasectomy combined with chemotherapy, one patient received metastasectomy combined with radiotherapy, one patient underwent radical resection, and two patients received symptomatic treatment (Table 2). For patients with recurrence and metastasis, obstruction complications should be actively treated. Due to the limited number of IPNB cases, there is currently no established treatment standard for patients with recurrent tumors. In conclusion, the treatment for malignant IPNB can be formulated by referring to cholangiocarcinoma, and the treatment for recurrent IPNB should be individualized according to the patient’s physical tolerance.
In conclusion, as a premalignant lesion of cholangiocarcinoma, IPNB mostly occurs in elderly male patients. The common symptoms of IPNB were abdominal pain and jaundice. Laboratory tests may reveal elevated bilirubin and liver enzymes. The imaging features were lesion and bile duct dilatation. Most IPNB cases are high-grade intraepithelial neoplasia or invasive carcinoma. Therefore, early diagnosis, prompt treatment and regular postoperative follow-up are of great significance for IPNB.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the First Hospital of Jilin University IRB. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
CF: Conceptualization, Writing – original draft. HJ: Data curation, Writing – original draft. YW: Data curation, Writing – original draft. HX: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1443599/full#supplementary-material
SUPPLEMENTARY FIGURES S1–S4 | Enhanced CT (axial, coronal, and sagittal views) and MRCP revealed significant dilation of the intrahepatic bile ducts.
References
1. Chen, TC, Nakanuma, Y, Zen, Y, Chen, MF, Jan, YY, Yeh, TS, et al. Intraductal papillary neoplasia of the liver associated with hepatolithiasis. Hepatology. (2001) 34:651–8. doi: 10.1053/jhep.2001.28199
2. Nakanuma, Y, Jang, KT, Fukushima, N, Furukawa, T, Hong, SM, Kim, H, et al. A statement by the Japan-Korea expert pathologists for future clinicopathological and molecular analyses toward consensus building of intraductal papillary neoplasm of the bile duct through several opinions at the present stage. J Hepatobiliary Pancreat Sci. (2018) 25:181–7. doi: 10.1002/jhbp.532
3. Nagtegaal, ID, Odze, RD, Klimstra, D, Paradis, V, Rugge, M, Schirmacher, P, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. (2020) 76:182–8. doi: 10.1111/his.13975
4. Bennett, S, Marginean, EC, Paquin-Gobeil, M, Wasserman, J, Weaver, J, Mimeault, R, et al. Clinical and pathological features of intraductal papillary neoplasm of the biliary tract and gallbladder. HPB (Oxford). (2015) 17:811–8. doi: 10.1111/hpb.12460
5. Chatterjee, A, Lopes Vendrami, C, Nikolaidis, P, Mittal, PK, Bandy, AJ, Menias, CO, et al. Uncommon intraluminal tumors of the gallbladder and biliary tract: Spectrum of imaging appearances. Radiographics. (2019) 39:388–412. doi: 10.1148/rg.2019180164
6. Rocha, FG, Lee, H, Katabi, N, DeMatteo, RP, Fong, Y, D'Angelica, MI, et al. Intraductal papillary neoplasm of the bile duct: a biliary equivalent to intraductal papillary mucinous neoplasm of the pancreas? Hepatology. (2012) 56:1352–60. doi: 10.1002/hep.25786
7. Gordon-Weeks, AN, Jones, K, Harriss, E, Smith, A, and Silva, M. Systematic review and meta-analysis of current experience in treating IPNB: clinical and pathological correlates. Ann Surg. (2016) 263:656–63. doi: 10.1097/SLA.0000000000001426
8. Onoe, S, Shimoyama, Y, Ebata, T, Yokoyama, Y, Igami, T, Sugawara, G, et al. Prognostic delineation of papillary cholangiocarcinoma based on the invasive proportion: a single-institution study with 184 patients. Surgery. (2014) 155:280–91. doi: 10.1016/j.surg.2013.08.011
9. Barton, JG, Barrett, DA, Maricevich, MA, Schnelldorfer, T, Wood, CM, Smyrk, TC, et al. Intraductal papillary mucinous neoplasm of the biliary tract: a real disease? HPB (Oxford). (2009) 11:684–91. doi: 10.1111/j.1477-2574.2009.00122.x
10. Cannito, S, Milani, C, Cappon, A, Parola, M, Strazzabosco, M, and Cadamuro, M. Fibroinflammatory liver injuries as preneoplastic condition in cholangiopathies. Int J Mol Sci. (2018) 19:3875. doi: 10.3390/ijms19123875
11. Jaiswal, M, LaRusso, NF, Shapiro, RA, Billiar, TR, and Gores, GJ. Nitric oxide-mediated inhibition of DNA repair potentiates oxidative DNA damage in cholangiocytes. Gastroenterology. (2001) 120:190–9. doi: 10.1053/gast.2001.20875
12. Aoki, Y, Mizuma, M, Hata, T, Aoki, T, Omori, Y, Ono, Y, et al. Intraductal papillary neoplasms of the bile duct consist of two distinct types specifically associated with clinicopathological features and molecular phenotypes. J Pathol. (2020) 251:38–48. doi: 10.1002/path.5398
13. Yang, CY, Huang, WJ, Tsai, JH, Cheng, A, Chen, CC, Hsu, HP, et al. Targeted next-generation sequencing identifies distinct clinicopathologic and molecular entities of intraductal papillary neoplasms of the bile duct. Mod Pathol. (2019) 32:1637–45. doi: 10.1038/s41379-019-0306-9
14. Xian, ZH, Qin, C, and Cong, WM. KRAS mutation and immunohistochemical profile in intraductal papillary neoplasm of the intrahepatic bile ducts. Pathol Res Pract. (2018) 214:105–11. doi: 10.1016/j.prp.2017.10.017
15. Wu, X, Li, B, Zheng, C, Chang, X, Zhang, T, He, X, et al. Intraductal papillary neoplasm of the bile duct: a single-center retrospective study. J Int Med Res. (2018) 46:4258–68. doi: 10.1177/0300060518792800
16. Minagawa, N, Sato, N, Mori, Y, Tamura, T, Higure, A, and Yamaguchi, K. A comparison between intraductal papillary neoplasms of the biliary tract (BT-IPMNs) and intraductal papillary mucinous neoplasms of the pancreas (P-IPMNs) reveals distinct clinical manifestations and outcomes. Eur J Surg Oncol. (2013) 39:554–8. doi: 10.1016/j.ejso.2013.02.016
17. Kubota, K, Nakanuma, Y, Kondo, F, Hachiya, H, Miyazaki, M, Nagino, M, et al. Clinicopathological features and prognosis of mucin-producing bile duct tumor and mucinous cystic tumor of the liver: a multi-institutional study by the Japan biliary association. J Hepatobiliary Pancreat Sci. (2014) 21:176–85. doi: 10.1002/jhbp.23
18. Li, T, Ji, Y, Zhi, XT, Wang, L, Yang, XR, Shi, GM, et al. A comparison of hepatic mucinous cystic neoplasms with biliary intraductal papillary neoplasms. Clin Gastroenterol Hepatol. (2009) 7:586–93. doi: 10.1016/j.cgh.2009.02.019
19. Ren, X, Zhu, CL, Qin, XF, Jiang, H, Xia, T, and Qu, YP. Co-occurrence of IPMN and malignant IPNB complicated by a pancreatobiliary fistula: a case report and review of the literature. World J Clin Cases. (2019) 7:102–8. doi: 10.12998/wjcc.v7.i1.102
20. Tsou, YK, Liu, NJ, Wu, RC, Lee, CS, Tang, JH, Hung, CF, et al. Endoscopic retrograde cholangiography in the diagnosis and treatment of mucobilia. Scand J Gastroenterol. (2008) 43:1137–44. doi: 10.1080/00365520802029856
21. Krawczyk, M, Ziarkiewicz-Wróblewska, B, Podgórska, J, Grzybowski, J, Gierej, B, Krawczyk, P, et al. Intraductal papillary neoplasm of the bile duct – a comprehensive review. Adv Med Sci. (2021) 66:138–47. doi: 10.1016/j.advms.2021.01.005
22. Ohtsuka, M, Kimura, F, Shimizu, H, Yoshidome, H, Kato, A, Yoshitomi, H, et al. Surgical strategy for mucin-producing bile duct tumor. J Hepatobiliary Pancreat Sci. (2010) 17:236–40. doi: 10.1007/s00534-009-0152-0
23. Zhang, YQ, Liang, Y, Liu, Y, and Feng, Y. Photodynamic therapy for hepatic hilar intraductal papillary neoplasm of the bile duct: a case report. VideoGIE. (2022) 7:178–81. doi: 10.1016/j.vgie.2022.01.016
24. Delaney, S, Zhou, Y, Pawa, S, and Pawa, R. Intraductal papillary neoplasm of the left hepatic duct treated with endoscopic retrograde cholangiopancreatography guided radiofrequency ablation. Clin J Gastroenterol. (2021) 14:346–50. doi: 10.1007/s12328-020-01284-4
25. Tang, W, Qiu, JG, Wei, XF, Xiao, H, Deng, X, Wang, SD, et al. Endoscopic Endoluminal radiofrequency ablation and single-operator Peroral Cholangioscopy system (SpyGlass) in the diagnosis and treatment of Intraductal papillary neoplasm of the bile duct: a case report and literature review. Front Med (Lausanne). (2021) 8:675720. doi: 10.3389/fmed.2021.675720
26. Natov, NS, Horton, LC, and Hegde, SR. Successful endoscopic treatment of an intraductal papillary neoplasm of the bile duct. World J Gastrointest Endosc. (2017) 9:238–42. doi: 10.4253/wjge.v9.i5.238
27. Arai, J, Kato, J, Toda, N, Kurokawa, K, Shibata, C, Kurosaki, S, et al. Long-term survival after palliative argon plasma coagulation for intraductal papillary mucinous neoplasm of the bile duct. Clin J Gastroenterol. (2021) 14:314–8. doi: 10.1007/s12328-020-01199-0
28. Kim, JR, Lee, KB, Kwon, W, Kim, E, Kim, SW, and Jang, JY. Comparison of the Clinicopathologic characteristics of Intraductal papillary neoplasm of the bile duct according to morphological and anatomical classifications. J Korean Med Sci. (2018) 33:e266. doi: 10.3346/jkms.2018.33.e266
29. Uemura, S, Higuchi, R, Yazawa, T, Izumo, W, Matsunaga, Y, Shiihara, M, et al. Prognostic factors for surgically resected intraductal papillary neoplasm of the bile duct: a retrospective cohort study. Ann Surg Oncol. (2021) 28:826–34. doi: 10.1245/s10434-020-08835-6
30. Kim, JR, Jang, KT, Jang, JY, Lee, K, Kim, JH, Kim, H, et al. Clinicopathologic analysis of intraductal papillary neoplasm of bile duct: Korean Multicenter Cohort Study. HPB (Oxford). (2020) 22:1139–48. doi: 10.1016/j.hpb.2019.11.007
31. Lluís, N, Serradilla-Martín, M, Achalandabaso, M, Jehaes, F, Dasari, BVM, Mambrilla-Herrero, S, et al. Intraductal papillary neoplasms of the bile duct: a European retrospective multicenter observational study (EUR-IPNB study). Int J Surg. (2023) 109:760–71. doi: 10.1097/JS9.0000000000000280
32. Kubota, K, Jang, JY, Nakanuma, Y, Jang, KT, Haruyama, Y, Fukushima, N, et al. Clinicopathological characteristics of intraductal papillary neoplasm of the bile duct: a Japan-Korea collaborative study. J Hepatobiliary Pancreat Sci. (2020) 27:581–97. doi: 10.1002/jhbp.785
Keywords: intraductal papillary neoplasm of the bile duct, cholangiocarcinoma, hepatobiliary disease, clinical features, prognosis
Citation: Fu C, Jin H, Wang Y and Xu H (2024) Clinicopathological features and surgical treatments of intraductal papillary neoplasm of the bile duct: a case report and literature review. Front. Med. 11:1443599. doi: 10.3389/fmed.2024.1443599
Edited by:
Mattia Garancini, San Gerardo Hospital, ItalyCopyright © 2024 Fu, Jin, Wang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongji Xu, eHVfaG9uZ2ppQDE2My5jb20=
 Chang Fu
Chang Fu Hengwei Jin1
Hengwei Jin1 Hongji Xu
Hongji Xu