- 1Department of Rheumatology, Allergy and Immunology, Tan Tock Seng Hospital, Singapore, Singapore
- 2Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, Singapore
- 3Clinical Research & Innovation Office, Tan Tock Seng Hospital, Singapore, Singapore
- 4Molecular Diagnostic Laboratory, Tan Tock Seng Hospital, Singapore, Singapore
- 5Department of Endocrinology, Tan Tock Seng Hospital, Singapore, Singapore
Introduction: Traditional risk factors do not fully explain the increased risk of cardiovascular disease (CVD) in patients with rheumatoid arthritis (RA). The Haptoglobin (Hp) 2-2 genotype confers a lower anti-oxidant and higher inflammatory effect on the vasculature compared to the non-Hp 2-2 genotype. This study investigates the association of the Hp genotype with CVD in patients with RA.
Methods: Data from 69 RA patients with CVD and 207 sex- and ethnicity-matched RA patients without CVD, collected from 1 January 2000 to 31 December 2020, were retrieved from the Tan Tock Seng Hospital RA Registry. CVD was examined against demographics, clinical and laboratory variables in univariate models. Associations between the Hp genotypes and CVD were analyzed using conditional logistic regression.
Results: We studied 276 patients (65.2% female, 82.6% Chinese, median age 60.9 years). Most participants were in low disease activity or remission (79.3%). The Hp 2-2 genotype was present in 49.6% (137/276). In the group with CVD, the prevalence of the Hp 2-2 genotype was 50.9% (29/57) in the Chinese, 100% (5/5) in the Indians, and 28.6% (2/7) in the Malays. In the non-CVD group, the respective prevalence was 46.8% (80/171), 66.7% (10/15), and 52.4% (11/21). In univariate analysis, the matched odds ratio (OR) of the Hp 2-2 genotype for CVD in RA was 1.34 [95% confidence interval (CI): 1.22–1.47; p < 0.001]. The Hp 2-2 genotype was significantly associated with CVD (adjusted matched OR: 1.13; 95% CI: 1.01–1.27; p = 0.033) in the multivariate logistic regression model after adjusting the confounding factors, including age, smoking, diabetes, hypertension, hyperlipidemia, anti-CCP autoantibodies, and disease activity.
Conclusion: The Hp 2-2 genotype is associated with an increased risk of CVD in patients with RA in this multi-ethnic cohort.
Introduction
Rheumatoid arthritis (RA) is an archetype of multi-systemic chronic inflammatory disease (1). Cardiovascular disease (CVD) is one of the major causes of mortality and morbidity of RA (1). The risk of CVD in RA is comparable to that conferred by diabetes mellitus (DM), after adjusting for the traditional risk factors (2). CVD disproportionately affects the young RA population (3). The European Alliance of Association for Rheumatology (EULAR) recommends raising the risk derived from standard algorithms by 1.5 for RA patients (4). However, it has been argued that this method does not reclassify into more appropriate risk categories since it does not address RA-specific risks.
Haptoglobin (Hp) is an acute phase reactant that prevents oxidative damage by binding oxygenated free hemoglobin (5). There are two alleles of the Hp gene, namely Hp1 and Hp2, and three genotypes, Hp 1-1, Hp 1-2 and Hp 2-2. The anti-oxidant effect of Hp 2-2 is inferior to the other two (5). Hp 2-2 is associated with an increased risk of CVD in patients with DM (6–9). Hp 2-2 is overexpressed in patients with a family history of RA (10) and systemic lupus erythematosus (SLE) (11, 12). High serum level of Hp is associated with inadequate response to methotrexate in RA (13). However, the role of Hp genotypes in reclassifying CVD risk in RA has not been investigated. This study aims to study the association of Hp genotypes with CVD in RA in a multi-ethnic Asian cohort.
Materials and methods
Patient’s clinical data and sample
The Tan Tock Seng Hospital (TTSH) RA Registry is a longitudinal multi-ethnic disease registry in Singapore inaugurated in 2001 (14, 15). RA patients fulfilled the 1987 American Rheumatism Association criteria or the 2010 American College of Rheumatology (ACR)/EULAR criteria (16, 17). The presence of CVD was reported by the attending physicians. We identified 69 patients with CVD in our Registry. We also selected sex- and ethnicity-matched RA patients without CVD in a ratio of one case to three controls. Biobanked DNA samples, serum samples, and matching clinical data were retrieved. The study was approved by the institutional review board (NHG DSRB reference number 2006/00011).
Haptoglobin genotype and protein measurement
The Hp genotyping was performed using TaqMan-based real-time polymerase chain reaction (PCR) as previously described (18). Plasma haptoglobin was measured using the immunoturbidimetric method on the Beckman Coulter AU system.
Statistics
The distribution of demographic and clinical characteristics was summarized using descriptive statistical methods. The normality of the data was assessed for continuous variables; mean (standard deviation, SD) or median (interquartile range, IQR) were used to summarise normally distributed or skewed data, respectively. Frequency and percentage were used to summarise the categorical variables.
Univariate and multivariate conditional logistic regression were performed to estimate the effect size of the covariate of interest, Hp 2-2 genotype, in the prediction of pre-specified clinical outcome (i.e., presence or absence of CVD event), because the study design was a gender and ethnicity matched case-control study (19, 20).
The pre-specified clinical outcome was the presence or absence of a CVD event (binary outcome), and the main covariate of interest was the Hp 2-2 genotype.
In our study, variables were initially selected for inclusion in the model if they were either theoretically relevant based on prior literature or demonstrated a bivariate association with the outcome at a significance level of p ≤ 0.10.
In the univariate variable selection stage, variables with a p-value ≤0.1 with odds ratios that exclude 1 were selected as preliminary predictors for inclusion in the multivariate model in order not to miss any potentially important clinical predictors. For the final multivariate conditional logistic regression model, statistical significance was defined by the conventional p ≤ 0.05, with odds ratios excluding 1.
In the multivariate conditional logistic regression model, a stepwise backward regression approach with robust variance estimation and frequency weighted analysis options were applied to account for the 1:3 matching (1 case:3 controls) in the study design.
During the backward stepwise process, covariates identified as potential confounders were included based on known associations with both the exposure and outcome variables with pre-determined p-value cutoff ≤0.10, as well as including variables exhibiting p-value ≤0.10 in the univariate variable selection stage. This approach was adopted to ensure that potentially significant clinical predictors were retained in the multivariate model.
Variables were then removed sequentially if they did not meet the final significance threshold of p < 0.05 with odds ratios excluding 1 after adjusting for other variables in the model.
To assess the model fit, the Hosmer–Lemeshow goodness-of-fit test was performed. The final model selection was based on the model with the lower deviance, defined by the −2×log-likelihood (−2LL) value, which is the better model. The final fitted model was chosen based on the −2×log-likelihood (−2LL) value with the number of significant clinically important variables in the model.
Effect sizes were presented as adjusted matched odds ratios (matched OR) with 95% CI.
Statistical significance was set at two-sided 5% level and all analyses were conducted using STATA 16.1.
Results
Clinical characteristics of patients with RA
This study included 276 patients, mostly female (65.2%) and of Chinese ethnicity (82.6%). There were 69 RA patients with CVD and 207 sex- and ethnicity-matched RA patients without CVD. The median age was 60.9 years [interquartile range (IQR): 53.8–68.0], and a quarter of the patients had a history of smoking (Table 1). The prevalences of diabetes, hypertension, and hyperlipidemia were 14.5, 46.4, and 57.2%, respectively (Table 1). The median RA duration was 119.2 (IQR: 32.8–215.2) months, with high positivity for rheumatoid factor (84.8%) and anti-cyclic citrullinated peptides (anti-CCP) autoantibodies (80.1%), and most were in remission or low disease activity (79.3%) (Table 1).
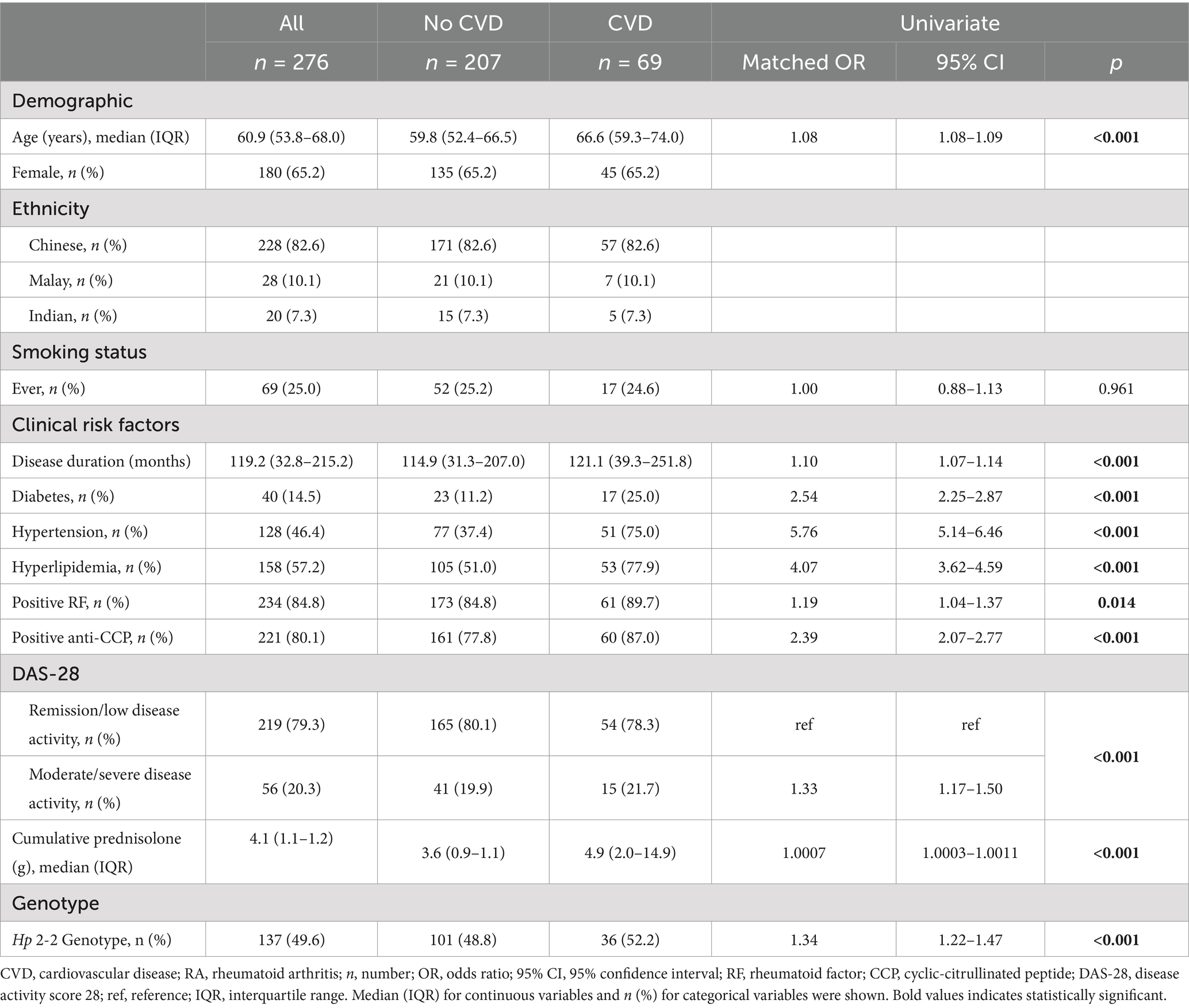
Table 1. Baseline characteristics and univariate analyses of the association between baseline characteristics and events of CVD in patients with RA.
Hp 2-2 genotype in different populations
The overall prevalence of the Hp 2-2 genotype was 49.6% (137/276). We found that 47.8% (109/228) of Chinese, 46.4% (13/28) of Malay, and 75% (15/20) of Indian patients carry the Hp 2-2 genotype (Table 2). Among patients with CVD classified into the three ethnicities, 50.9% (29/57) of the Chinese, 28.6% (2/7) of the Malays, and 100% (5/5) of the Indians carry Hp 2-2 genotype (Table 2). Among the patients without CVD, there were 46.8% (80/171) Chinese, 52.4% (11/21) Malay, and 66.7% (10/15) Indian patients carrying the Hp 2-2 genotype (Table 2). The haptoglobin protein level was significantly lower in patients with the Hp 2-2 genotype (mean ± SD: 130.0 ± 66.3 mg/dL) than those with the non Hp 2-2 genotype (mean ± SD: 161.2 ± 82.8 mg/dL) (p < 0.001) (Table 2).
Univariate analysis of risk factors for CVD
In univariate analysis, traditional clinical risk factors significantly associated with CVD events were age (66.6 vs. 59.8 years in CVD and control group respectively, p < 0.001), diabetes (25.0% vs. 11.2%, OR 2.54, 95% CI 2.25–2.87, p < 0.001), hypertension (75% vs. 37.4%, OR 5.76, 95% CI 5.14–6.46, p < 0.001), and hyperlipidemia (77.9% vs. 51.0%, OR 4.07, 95% CI 3.62–4.59, p < 0.001) (Table 1). RA-specific factors associated with CVD events include disease duration (median 118.6 vs. 100.3 months in CVD and control group respectively, p < 0.001), rheumatoid factor and anti-CCP autoantibodies positivity (OR 1.19, p = 0.0135 and OR 2.39, p < 0.001, respectively), moderate and severe disease activity (OR = 1.33, 95% CI 1.17–1.50, p < 0.001), and higher cumulative dose of prednisolone (4.9 g vs. 3.6 g in CVD and control groups respectively, p < 0.001) (Table 1).
The prevalence of the Hp 2-2 genotype was higher in the CVD group compared to controls (52.2% vs. 48.8%), with a matched odds ratio of 1.34 (95% CI 1.22–1.47, p < 0.001) (Table 1).
Multivariate analysis of risk factors for CVD
In multivariate analysis, after adjusting for age, smoking, diabetes, hypertension, hyperlipidemia, anti-CCP autoantibodies, and disease activity, the Hp 2-2 genotype remained independently associated with CVD (adjusted matched OR 1.13, 95% CI 1.01–1.27, p = 0.033) (Table 3). Other statistically significant associations with CVD events include age (adjusted matched OR 1.06, p < 0.001), smoking (adjusted matched OR 1.43, p < 0.001), diabetes (adjusted matched OR 1.21, p = 0.013), hypertension (adjusted matched OR 2.78, p < 0.001), hyperlipidemia (adjusted matched OR 2.77, p < 0.001), the presence of anti-CCP autoantibodies (adjusted matched OR 3.27, p < 0.001), and moderate/severe disease activity (adjusted matched OR 2.21, p < 0.001) (Table 3).

Table 3. Multivariate analyses of the association between Hp genotype and events of CVD in patients with RA.
Discussion
Our study shows that the Hp 2-2 genotype is significantly associated with the risk of CVD in RA patients in a Singaporean multi-ethnic cohort, independent of traditional CVD risk factors. This suggests that the Hp 2-2 genotype could be a potential biomarker for more accurate CVD risk predication in RA patients.
We found that the prevalence of the Hp 2-2 genotype varies among different ethnic groups. In decreasing order of frequency, they are Indians (75.0%), Chinese (47.8%), and Malays (46.4%), which aligns with previous studies on diabetes in Singapore (18) and other countries (21). The prevalence of RA also varies among different ethnicities, with a higher prevalence in India compared to other Asian countries (22). This could be due to genetic and environmental factors (22, 23). Previous studies reported that the prevalence of the Hp 2-2 genotype was higher in patients with a family history of RA (10) and SLE (11, 12). The over-representation of Indian ethnicity (12.1%) in our multi-ethnic RA cohort (14), compared to 7.5% in the population (24), and the increased risk of CVD in the Indian population (25), suggest that the Hp 2-2 genotype could play a role in these differences. Strikingly, all Indian patients with CVD were Hp 2-2 genotype carriers in our study, although the number was low.
In our study, the protein level of Hp was not different between non-CVD and CVD patients within the non Hp 2-2 subgroup, but higher in CVD patients than non-CVD patients within the Hp 2-2 subgroup. The antioxidant function might be impaired despite higher protein levels in patients with Hp 2-2. Serum haptoglobin α2 (expressed by Hp 2–1 or Hp 2-2 genotype), with lower antioxidant capacity than haptoglobin α1 (expressed by Hp 1–1 genotype), was found in higher concentration in patients with SLE (11). The high baseline haptoglobin protein level predicted poor response to MTX, independent of the DAS 28 score, and inflammatory markers (13). Our findings suggest the Hp 2-2 genotype may result in impaired antioxidant functions, potentially leading to enhanced inflammation and a diminished response to methotrexate, thereby increasing CVD risk.
The association of the Hp 2-2 genotype with DM and CVD complications in DM is well documented (6–9). Our study shows that the Hp 2-2 genotype is an independent risk factor for CVD in RA patients, even after adjusting traditional risk factors, including DM. The link between the Hp 2-2 genotype and increased CVD risk in our RA cohort is consistent with findings in DM populations. This parallel suggests a common pathogenic mechanism in these chronic inflammatory conditions (26). Inflammation is a stronger predictor for CVD than LDL in this era of statin therapy (27). The impaired antioxidant function of Hp 2-2 might lead to chronic inflammation. Furthermore, the Hp 2-2 genotype is associated with the disease severity (28), and survival (29) in CVD. Therefore, antioxidant therapies could be investigated as a potential intervention to mitigate CVD complications in the RA population, as demonstrated in DM (9).
The burden of CVD in RA is comparable to that in DM (2). The traditional risk prediction model is not accurate in RA, even with the 1.5-time multiplier recommended by EULAR (4). Hp polymorphism has been extensively studied in patients with DM, and the Hp 2-2 genotype has shown the potential for refining the cardiovascular risk assessment (30, 31). Moreover, the predictive value of traditional risk factors, i.e., elevated glycosylated hemoglobin (HbA1c), is more pronounced among patients with the Hp 2-2 genotype (7, 32). Elevated homocysteine levels (33) and increased Carotid Intima-Media Thickness (CIMT) (34) are associated with increased CVD risk. Incorporating risks such as Hp 2-2, homocysteine levels, and CIMT into risk algorithms could enhance their predictive accuracy and enable precise risk stratification in RA, leading to timely implementation of optimal therapy and ultimately improving outcomes.
This study has a few strengths. First, a multi-ethnic Asian cohort allows for examining genetic risk factors across diverse populations. This is particularly relevant given the variability in the prevalence of the Hp 2-2 genotype among different ethnicities. Second, the study has 20-year follow-up, providing a broad dataset for analysis. Third, by matching controls to cases on important variables such as sex and ethnicity, the study design controlled for confounding factors. Limitations include the relatively small sample size, the retrospective CVD diagnosis potentially introducing selection bias, and the high proportion of likely post-menopausal women, which may elevate baseline CVD risk and affect generalizability. Additionally, it is known the medications use may affect the risk of CVD in RA (35). Therefore, the potential for bias due to unmatched medication use between the two groups warrants cautious interpretation.
Our study provides evidence for the Hp 2-2 genotype as an independent risk factor for CVD in patients with RA. Future research should focus on prospective validation of these findings in larger cohorts and explore the mechanistic pathways linking the Hp 2-2 genotype to CVD in RA. Furthermore, clinical trials assessing the efficacy of antioxidant therapies in reducing CVD risk in RA patients with the Hp 2-2 genotype would be a logical extension of this work.
The TTSH Rheumatoid Arthritis Study Group
Andrea Ang, Angela Li-Huan Chan, Grace Yin Lai Chan, Madelynn Tsu-Li Chan, Faith Li-Ann Chia, Hiok Hee Chng, Choon Guan Chua, Hwee Siew Howe, Ee Tzun Koh, Li Wearn Koh, Kok Ooi Kong, Weng Giap Law, Samuel Lee Shang Ming, Khai Pang Leong, Tsui Yee Lian, Xin Rong Lim, Jess Mung Ee Loh, Mona Manghani, Justina Wei Lynn Tan, Sze-Chin Tan, Teck Choon Tan, Claire Teo Min-Li, Bernard Yu-Hor Thong, and Paula Permatasari Tjokrosaputro.
Data availability statement
The datasets used and/or analyzed for this study are available from the corresponding author upon reasonable request. Requests to access these datasets should be directed to Chuanhui Xu. E-mail: eHVjaHVhbmh1aTIwMDhAZ21haWwuY29t.
Ethics statement
The studies involving humans were approved by NHG DSRB reference number 2006/00011. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CX: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Validation, Writing – original draft. LK: Formal analysis, Investigation, Methodology, Software, Writing – review & editing. HT: Formal analysis, Investigation, Methodology, Software, Writing – review & editing. LG: Investigation, Methodology, Writing – review & editing. EK: Data curation, Investigation, Resources, Writing – review & editing. RD: Conceptualization, Formal analysis, Funding acquisition, Methodology, Supervision, Writing – review & editing. KL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work is supported by Tan Tock Seng Hospital FY2021 Pitch-for-Fund Program Grant (Grant ID: PFFP 21-08), NHG-LKC Medicine Clinician-Scientist Career Scheme (CSCS-22-02-01), and Ng Teng Fong Healthcare Innovation Program (NTF_SRP_P1).
Acknowledgments
The authors thank Ms. Amelia Lim Qiu Ru, Ms. Choong Ying Qi, and Mr. Shih-Huan Chou for their assistance with data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Smolen, JS, Aletaha, D, Barton, A, Burmester, GR, Emery, P, Firestein, GS, et al. Rheumatoid arthritis. Nat Rev Dis Primers. (2018) 4:18001. doi: 10.1038/nrdp.2018.1
2. van Halm, VP, Peters, MJL, Voskuyl, AE, Boers, M, Lems, WF, Visser, M, et al. Rheumatoid arthritis versus diabetes as a risk factor for cardiovascular disease: a cross-sectional study, the CARRE Investigation. Ann Rheum Dis. (2009) 68:1395–400. doi: 10.1136/ard.2008.094151
3. Conrad, N, Verbeke, G, Molenberghs, G, Goetschalckx, L, Callender, T, Cambridge, G, et al. Autoimmune diseases and cardiovascular risk: a population-based study on 19 autoimmune diseases and 12 cardiovascular diseases in 22 million individuals in the UK. Lancet. (2022) 400:733–43. doi: 10.1016/S0140-6736(22)01349-6
4. Agca, R, Heslinga, SC, Rollefstad, S, Heslinga, M, McInnes, IB, Peters, MJL, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. (2017) 76:17–28. doi: 10.1136/annrheumdis-2016-209775
5. Costacou, T, and Levy, AP. Haptoglobin genotype and its role in diabetic cardiovascular disease. J Cardiovasc Transl Res. (2012) 5:423–35. doi: 10.1007/s12265-012-9361-z
6. Adams, JN, Cox, AJ, Freedman, BI, Langefeld, CD, Carr, JJ, and Bowden, DW. Genetic analysis of haptoglobin polymorphisms with cardiovascular disease and type 2 diabetes in the diabetes heart study. Cardiovasc Diabetol. (2013) 12:31. doi: 10.1186/1475-2840-12-31
7. Cahill, LE, Levy, AP, Chiuve, SE, Jensen, MK, Wang, H, Shara, NM, et al. Haptoglobin genotype is a consistent marker of coronary heart disease risk among individuals with elevated glycosylated hemoglobin. J Am Coll Cardiol. (2013) 61:728–37. doi: 10.1016/j.jacc.2012.09.063
8. Levy, AP, Hochberg, I, Jablonski, K, Resnick, HE, Lee, ET, Best, L, et al. Haptoglobin phenotype is an independent risk factor for cardiovascular disease in individuals with diabetes: the strong heart study. J Am Coll Cardiol. (2002) 40:1984–90. doi: 10.1016/S0735-1097(02)02534-2
9. Dalan, R, and Liuh, LG. The protean role of haptoglobin and haptoglobin genotypes on vascular complications in diabetes mellitus. Eur J Prev Cardiol. (2018) 25:1502–19. doi: 10.1177/2047487318776829
10. Dahlqvist, SR, and Fröhlander, N. Haptoglobin groups and rheumatoid arthritis. Hum Hered. (1985) 35:207–11. doi: 10.1159/000153546
11. Pavón, EJ, Muñoz, P, Lario, A, Longobardo, V, Carrascal, M, Abián, J, et al. Proteomic analysis of plasma from patients with systemic lupus erythematosus: increased presence of haptoglobin alpha2 polypeptide chains over the alpha1 isoforms. Proteomics. (2006) 6:S282–92. doi: 10.1002/pmic.200500404
12. Rantapää Dahlqvist, S, Beckman, G, and Beckman, L. Serum protein markers in systemic lupus erythematosus. Hum Hered. (1988) 38:44–7. doi: 10.1159/000153753
13. Tan, W, Wang, F, Guo, D, Ke, Y, Shen, Y, Lv, C, et al. High serum level of haptoglobin is associated with the response of 12 weeks methotrexate therapy in recent-onset rheumatoid arthritis patients. Int J Rheum Dis. (2016) 19:482–9. doi: 10.1111/1756-185X.12380
14. Koh, ET, Tan, JWL, Thong, BY-H, Teh, CL, Lian, TY, Law, WG, et al. Major trends in the manifestations and treatment of rheumatoid arthritis in a multiethnic cohort in Singapore. Rheumatol Int. (2013) 33:1693–703. doi: 10.1007/s00296-012-2602-2
15. Xu, C, Yong, MY, Koh, ET, Dalan, R, and Leong, KP. The impact of diabetes mellitus on treatment and outcomes of rheumatoid arthritis at 5-year follow-up: results from a multi-ethnic Asian cohort. Rheumatol Adv Pract. (2021) 5:rkab077. doi: 10.1093/rap/rkab077
16. Aletaha, D, Neogi, T, Silman, AJ, Funovits, J, Felson, DT, Bingham, CO III, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. (2010) 62:2569–81. doi: 10.1002/art.27584
17. Arnett, FC, Edworthy, SM, Bloch, DA, Mcshane, DJ, Fries, JF, Cooper, NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. (1988) 31:315–24. doi: 10.1002/art.1780310302
18. Dalan, R, Liew, H, Goh, LL, Gao, X, Chew, DE, Boehm, BO, et al. The haptoglobin 2-2 genotype is associated with inflammation and carotid artery intima-media thickness. Diab Vasc Dis Res. (2016) 13:373–6. doi: 10.1177/1479164116645247
19. McFadden, D. Conditional logit analysis of qualitative choice behavior. New York: Academic Press (1972).
20. Breslow, NE, and Day, NE. Statistical methods in cancer research. Volume I—the analysis of case-control studies. Lyon: IARC Scientific Publication (1980). 5 p.
21. Langlois, MR, and Delanghe, JR. Biological and clinical significance of haptoglobin polymorphism in humans. Clin Chem. (1996) 42:1589–600. doi: 10.1093/clinchem/42.10.1589
22. Finckh, A, Gilbert, B, Hodkinson, B, Bae, S-C, Thomas, R, Deane, KD, et al. Global epidemiology of rheumatoid arthritis. Nat Rev Rheumatol. (2022) 18:591–602. doi: 10.1038/s41584-022-00827-y
23. Fujio, K. Rheumatology and functional genome analysis in East Asia. Rheumatol Autoimmun. (2022) 2:1–4. doi: 10.1002/rai2.12017
24. Singapore Department of Statistics. (2024). Singapore census of population 2020, release statistical release 1: demographic characteristics, education, language and religion. Available at: https://www.singstat.gov.sg/-/media/files/publications/cop2020/sr1/findings.pdf. (Accessed in March 2024)
25. Gupta, M, Singh, N, and Verma, S. South Asians and cardiovascular risk. Circulation. (2006) 113:e924–9. doi: 10.1161/CIRCULATIONAHA.105.583815
26. Furman, D, Campisi, J, Verdin, E, Carrera-Bastos, P, Targ, S, Franceschi, C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. (2019) 25:1822–32. doi: 10.1038/s41591-019-0675-0
27. Ridker, PM, Bhatt, DL, Pradhan, AD, Glynn, RJ, MacFadyen, JG, and Nissen, SE. Inflammation and cholesterol as predictors of cardiovascular events among patients receiving statin therapy: a collaborative analysis of three randomised trials. Lancet. (2023) 401:1293–301. doi: 10.1016/S0140-6736(23)00215-5
28. Chapelle, J-P, Albert, A, Smeets, J-P, Heusghem, C, and Kulbertus, HE. Effect of the haptoglobin phenotype on the size of a myocardial infarct. N Engl J Med. (1982) 307:457–63. doi: 10.1056/NEJM198208193070801
29. Van Vlierberghe, H, Langlois, M, and Delanghe, J. Haptoglobin polymorphisms and iron homeostasis in health and in disease. Clin Chim Acta. (2004) 345:35–42. doi: 10.1016/j.cccn.2004.03.016
30. Bale, BF, Doneen, AL, and Vigerust, DJ. Precision healthcare of type 2 diabetic patients through implementation of haptoglobin genotyping. Front Cardiovasc Med. (2018) 5:141. doi: 10.3389/fcvm.2018.00141
31. Horne, BD, and Anderson, JL. Haptoglobin 2-2 genotyping for refining standard cardiovascular risk assessment: a promising proposition in need of validation. J Am Coll Cardiol. (2015) 66:1800–2. doi: 10.1016/j.jacc.2015.08.020
32. Cahill, LE, Jensen, MK, Chiuve, SE, Shalom, H, Pai, JK, Flint, AJ, et al. The risk of coronary heart disease associated with glycosylated hemoglobin of 6.5% or greater is pronounced in the haptoglobin 2-2 genotype. J Am Coll Cardiol. (2015) 66:1791–9. doi: 10.1016/j.jacc.2015.07.076
33. Ganguly, P, and Alam, SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. (2015) 14:6. doi: 10.1186/1475-2891-14-6
34. Rajabzadeh, F, Akhlaghipour, I, Moosavi, SS, Nasimi Shad, A, Babazadeh Baghan, A, Shariati-Sarabi, Z, et al. Comparison of the intima-media thickness of the common carotid artery in patients with rheumatoid arthritis: a single-center cross-sectional case-control study, and a brief review of the literature. Health Sci Rep. (2023) 6:e1718. doi: 10.1002/hsr2.1718
Keywords: rheumatoid arthritis, cardiovascular disease, Haptoglobin genotypes, inflammation, personalized medicine
Citation: Xu C, Khin LW, Tam HZ, Goh LL, Koh ET, Dalan R and Leong KP (2024) Haptoglobin 2-2 genotype is associated with increased risk of cardiovascular disease in patients with rheumatoid arthritis: a matched case-control study. Front. Med. 11:1442858. doi: 10.3389/fmed.2024.1442858
Edited by:
Konstantinos Triantafyllias, Rheumatology Center Rhineland Palatinate, GermanyReviewed by:
Padmaja Mummaneni, United States Food and Drug Administration, United StatesMei Kun, Nanjing University of Chinese Medicine, China
Copyright © 2024 Xu, Khin, Tam, Goh, Koh, Dalan and Leong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuanhui Xu, eHVjaHVhbmh1aTIwMDhAZ21haWwuY29t
 Chuanhui Xu
Chuanhui Xu Lay Wai Khin3
Lay Wai Khin3 Liuh Ling Goh
Liuh Ling Goh Rinkoo Dalan
Rinkoo Dalan Khai Pang Leong
Khai Pang Leong