- 1Central Hospital Affiliated to Shandong First Medical University, Jinan, China
- 2The First Affiliated Hospital of Shandong First Medical University (Shandong Qianfo Mountain Hospital), Jinan, China
Background: Postmenopausal osteoporosis (PMOP) increases fracture risk in women. Though traditional treatments are slow to act, combining romosozumab with conventional therapy shows promise. Despite its growing use, studies on effectiveness are limited. This study aims to systematically evaluate the combined therapy’s impact on pain relief, disease progression, and adverse reactions in PMOP patients.
Methods: Databases including PubMed, EMBASE, ScienceDirect, and the Cochrane Library were searched from their inception to September 2023 to identify randomized controlled trials (RCTs) evaluating the role of romosozumab in PMOP. Random or fixed effect models were employed for statistical analysis. Two reviewers independently assessed the quality of the included studies and extracted the data. The meta-analysis was conducted using RevMan 5.4 software.
Results: Six RCTs with a total sample size of 17,985 cases were included. The incidence of vertebral fractures was compared and analyzed after 12 and 24 months of treatment. Romosozumab significantly reduced the incidence of vertebral fractures at 24 months (OR = 0.36; 95% CI: 0.35–0.52) but not at 12 months (OR = 0.39; 95% CI: 0.14–1.05). It was also associated with a decreased incidence of nonvertebral fractures (OR = 0.79; 95% CI: 0.66–0.94) and clinical fractures at 24 months (OR = 0.70; 95% CI: 0.59–0.82) compared to standard therapy. Romosozumab demonstrated a significant improvement in percentage change in bone mineral density (BMD) [mean difference (MD) = 10.38; 95% CI: 4.62–16.14] and in hip joint BMD (MD = 4.24; 95% CI: 2.92–5.56). There was no notable difference in adverse reactions compared to standard care (p > 0.05). Funnel plots displayed a predominantly symmetrical pattern, suggesting no evidence of publication bias in the selected literature.
Conclusion: Combining romosozumab with conventional therapy effectively treats PMOP, significantly reducing vertebral, non-vertebral, and clinical fractures while increasing BMD in the hip, femoral neck, and lumbar spine. However, further high-quality studies are needed for validation.
1 Introduction
Postmenopausal osteoporosis (PMOP) is the most common form of primary osteoporosis, typically characterized by high bone turnover. PMOP is age-related and generally occurs within 5–10 years after menopause. The cumulative bone loss rate during early menopause is higher than that during late menopause. The occurrence of PMOP is mainly affected by the sharp decrease in estrogen levels in the postmenopausal body, which results in greater bone resorption than bone formation. This leads to reduced bone mass, changes in bone structure, bone pain, decreased height, brittle fractures, and other symptoms (1, 2). Osteoporosis (OP) is an asymptomatic or “silent” disease that represents a significant and growing economic burden on healthcare systems and societies worldwide. PMOM greatly increases the likelihood of fragility fractures in about 50% of women. These fractures can have severe and disabling effects, resulting in long-lasting discomfort and restrictions in physical abilities, ultimately leading to a reduced quality of life (3).
The China OP prevalence study revealed that the prevalence of OP among postmenopausal women was 32.1%, significantly higher than the prevalence among men (4). It is found that the prevalence level of OP in women over 50 years old in China is remarkably higher than that in the United States, Canada, and other countries (5, 6). OP patients experience varying degrees of decline in BMD and bone mass, decreased bone strength, and an increased risk of brittle fractures. According to relevant epidemiological data from the United States, the risk of fractures in female OP patients is even higher than the combined risk of breast, ovarian, and uterine cancers (7). Furthermore, osteoporotic fracture patients are more likely to experience recurrent fractures. Studies have shown that patients who have suffered hip fractures are at 2.5 times higher risk of recurrent fractures, while patients with vertebral fractures have a 4 times higher risk. Additionally, patients who have suffered other types of fractures also face a higher risk of recurrent fractures, estimated to be approximately 2–3 times higher (8, 9). Preventing, diagnosing, and treating OP is crucial as it has a remarkable impact on the life quality of patients and leads to a high incidence of osteoporotic fractures.
In addition to vitamin D and calcium as basic treatments, drugs to treat OP are classified into two main categories based on their mechanism of action. The first category includes drugs that inhibit bone resorption, such as calcitonin, estrogen receptor modulators, bisphosphonates, and receptor activator of NF-κB (RANKL) inhibitors. The second category includes drugs that promote bone formation, such as parathyroid hormone analogues and parathyroid hormone-related peptide analogues (10). In January 2018, the European Medicines Agency (EMA) approved the listing of romosozumab (EVENITY™), which was jointly developed by Amgen and UCB. The US Food and Drug Administration (FDA) approved the product for marketing in April 2019. Romosozumab is a monoclonal antibody that has been humanized, and it functions by inhibiting the activity of sclerostin, a protein that negatively regulates bone metabolism. This inhibition can promote both bone formation and bone resorption. The treatment is accessible to postmenopausal women at high risk of fractures, as well as patients who cannot tolerate other medications. Romosozumab holds the distinction of being the world’s first sclerostin inhibitor approved for marketing, and it is currently the sole OP drug with dual effects (11, 12).
Several clinical controlled studies have been conducted to explore the therapeutic effects of romosozumab on patients with PMOP. However, the conclusions of these studies vary, and significant differences exist in their designs, leading to poor applicability (13). The current literature on the clinical efficacy of romosozumab in treating PMOP provides inconclusive results. Therefore, romosozumab’s efficacy in treating PMOP should be evaluated through high-quality research. Additional research is needed to establish the efficacy of romosozumab in combination with conventional therapies. More high-quality scientific studies are necessary to provide reliable evidence on the feasibility of this approach. Hence, this study aims to conduct a meta-analysis to analyze the effectiveness of romosozumab in combination with conventional therapy for postmenopausal patients with OP. The study seeks to offer new insights for clinical advancements.
2 Methods
This systematic review and meta-analysis were performed in compliance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines.
2.1 Search strategy
We conducted a comprehensive search for relevant studies on PMOP across various databases, including PubMed, EMBASE, ScienceDirect, and the Cochrane Library. Relevant information regarding the treatment of PMOP with romosozumab was collected. A literature search was conducted using the combination of the following medical subject headings with the Boolean operators: romosozumab, menopause, OP, disease progression, and clinical prognosis.
2.2 Eligibility criteria
2.2.1 Literature inclusion criteria
Population/participants: Patients diagnosed with OP using dual-energy X-ray absorptiometry (DXA) measurements of bone mineral density (BMD) in one of three ways:
a. BMD T-score of ≤ −2.5 at the L1-4 lumbar spine, femoral neck, total hip, or distal radius 1/3.
b. History of brittle fracture occurring in the vertebral body or hip.
c. T-score between −2.5 and −1.0 (indicating low bone mass) accompanied by a history of brittle fracture in the proximal humerus, pelvis, or distal forearm.
Intervention: Romosozumab based routine treatment.
Comparison: Placebo.
Outcome: Studies that reported multiple outcomes, including vertebral fracture rate, non-vertebral fracture rate, clinical fracture rate, lumbar BMD, hip joint BMD, femoral neck BMD, and the incidence of adverse reactions.
Study design: Randomized controlled trials (RCT) evaluating the efficacy of romosozumab in combination with standard treatment for PMOP.
2.2.2 Exclusion criteria
Retrospective cohort studies, case-control studies, case series, reviews, case reports, meta-analyses, as well as in vitro and animal studies were not considered for inclusion.
2.3 Quality assessment and data extraction
The study included an evaluation of bias risk, which was performed using the bias risk assessment tool recommended by the Cochrane Systematic Review Manual 5.3.2. Two researchers independently screened the literature and extracted data, while also assessing the quality of the extracted data and cross-checking it. Any discrepancies were resolved through discussion or by consulting a third researcher. Express document management software and Excel office software were used to manage and extract research data. If the study provided incomplete data, we contacted the authors of articles. The extracted data included author name, publication date, number of cases, study methods, and outcome indicators such as vertebral fracture rate, non-vertebral fracture rate, clinical fracture rate, lumbar BMD, hip BMD, femoral neck BMD, and incidence of adverse events.
2.4 Data synthesis and statistical analysis
Meta-analysis was conducted using RevMan 5.4 software from the Cochrane Collaboration Network. A two-tailed p < 0.05 was considered statistically significant. Relative risk (RR) was employed as the effect index. The average and standard deviation values of lumbar BMD, hip joint BMD, and femoral neck BMD were entered into RevMan 5.4, with weighted mean difference (WMD) used as the effect index. A 95% confidence interval (CI) was calculated for all analyses. To assess the heterogeneity among studies, the χ2 test was performed initially. Studies with p-values greater than 0.05 and I2 values less than 50% were deemed homogeneous, prompting the adoption of a fixed-effect model for the meta-analysis. In cases where the p-value was less than 0.05 and the I2 value equaled or exceeded 50%, indicating a significant level of heterogeneity among studies, the random-effects model was utilized to ascertain the combined effect and evaluate the study’s homogeneity. When the p-value was less than 0.05 and the source of heterogeneity remained unclear, a descriptive analysis was employed instead of a meta-analysis.
3 Results
3.1 Literature search
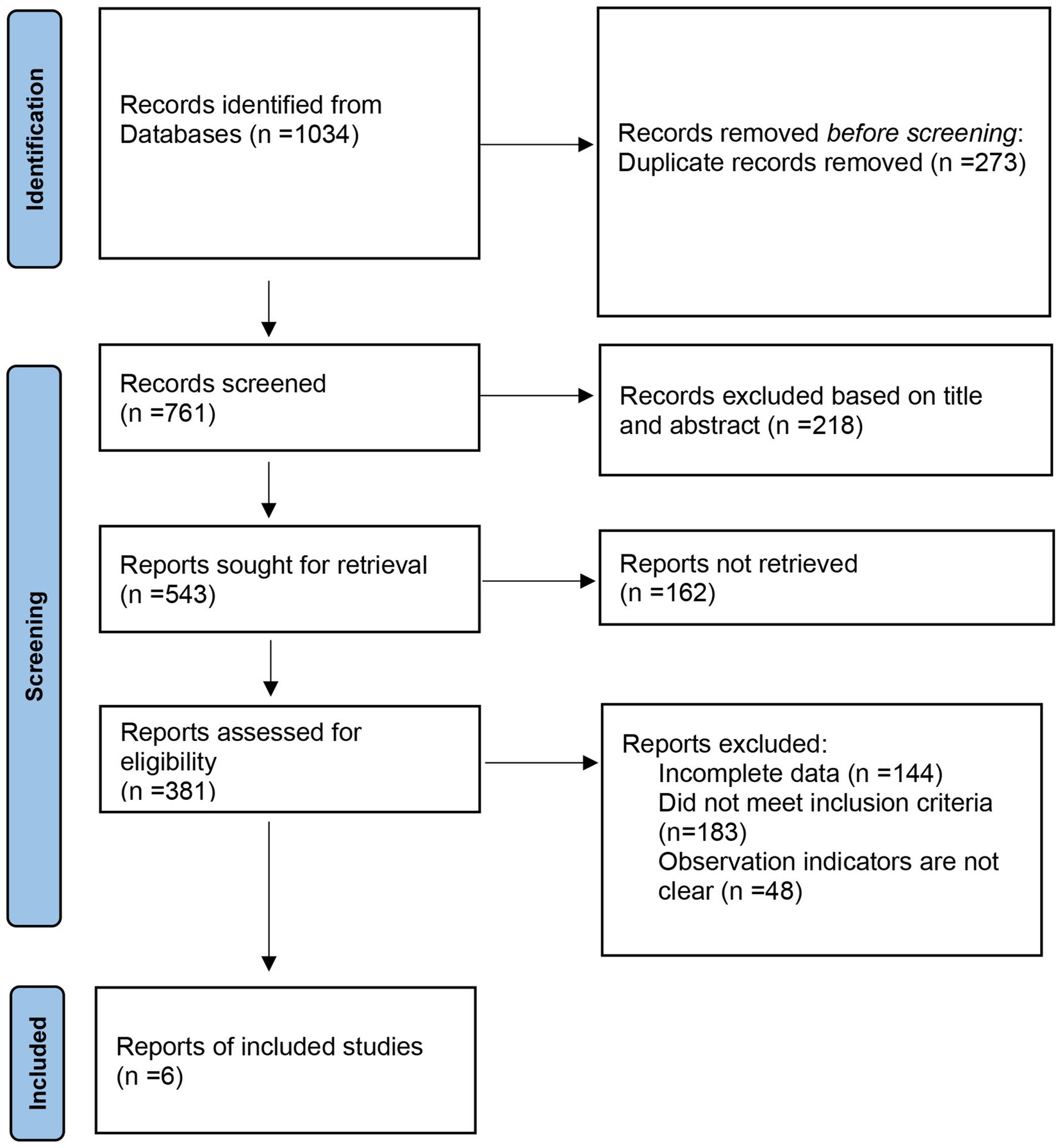
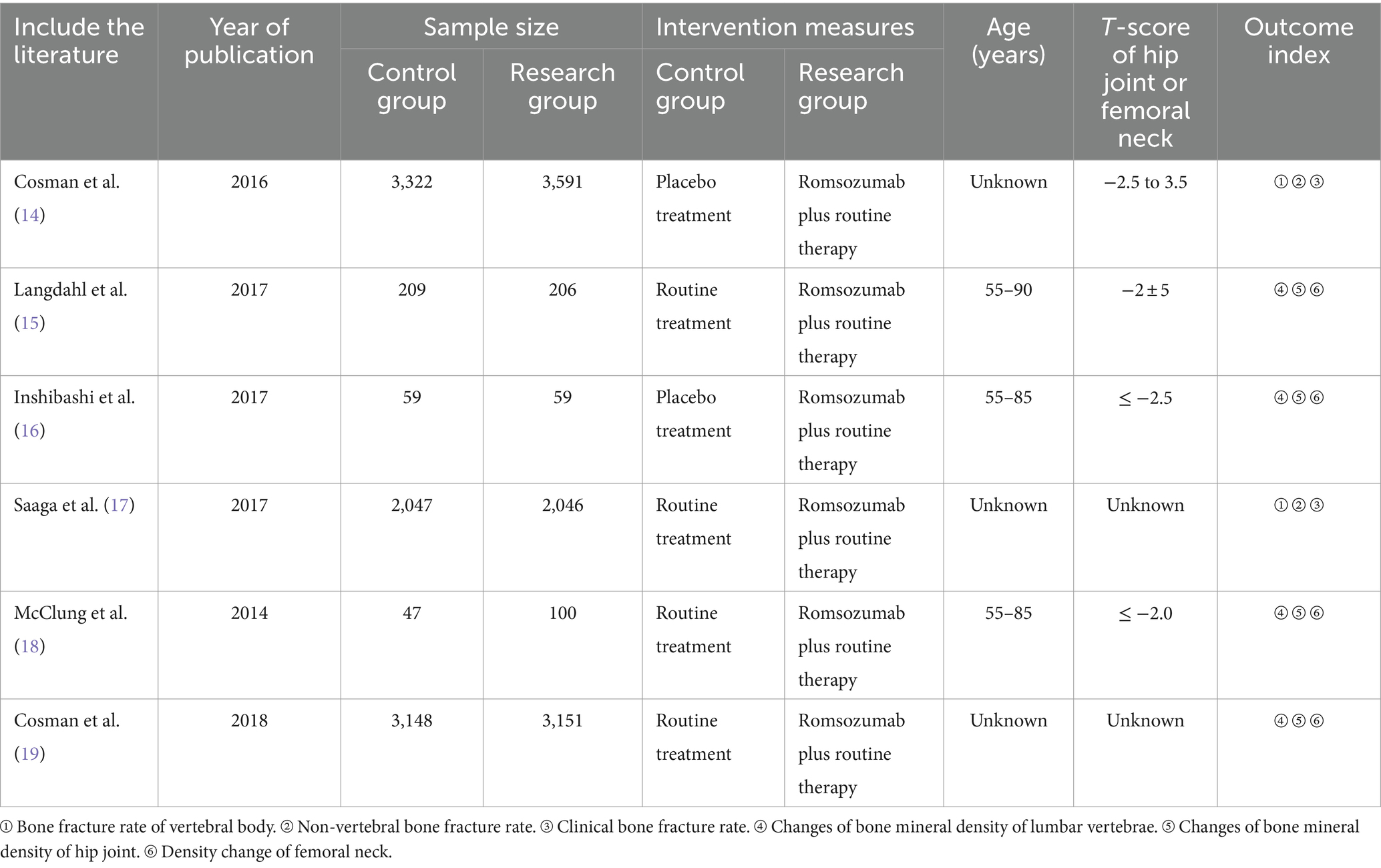
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, a literature study was conducted. The database search yielded 1,034 articles, and after removing duplicates, 761 articles remained. Upon reviewing titles and abstracts, 543 articles were identified, with 387 articles being excluded due to their irrelevance, being reviews, case reports, or lacking controlled literature. Subsequently, the full texts of the remaining articles were carefully reviewed, and incomplete data were excluded. Six randomized controlled trials (RCTs) were ultimately included, comprising a total of 17,985 participants for meta-analysis (14–19). The flowchart illustrating the literature screening process is displayed in Figure 1. Table 1 presents the characteristics of the included studies.
3.2 Quality assessment
In this meta-analysis, three out of the six studies reported detailed patient baseline characteristics. All literature included in this study provided comprehensive descriptions of the observational indicators and research methods employed, along with specific grouping methods. Additionally, they reported on the implementation of blinding, including the number and reasons for its use, as well as any loss of follow-up or withdrawal during the study period. Based on the analysis of the Jadad scale, studies with a score of ≥3 was considered high-quality, while that with a score of ≤2 was considered low-quality. Supplementary Figures S2, S3 display the risk bias analysis.
3.3 Meta analysis result
3.3.1 Bone fracture rate of vertebral body
Two studies reported vertebral fractures after 12 and 24 months of treatment. The pooled results indicated a significant decrease in the incidence of vertebral fractures at 24 months (OR = 0.36; 95% CI = 0.35–0.52, p < 0.00001; I2 = 87%) but not at 12 months (OR = 0.39; 95% CI: 0.14–1.05, p = 0.06; I2 = 90%) (Figure 2). According to the results of heterogeneity test, Chi2 = 17.87, df = 3, p = 0.0005, I2 = 83%, indicating that heterogeneity was evident in the research data, which was analyzed by random effect model (Figure 2).
3.3.2 Non-vertebral bone fracture rate
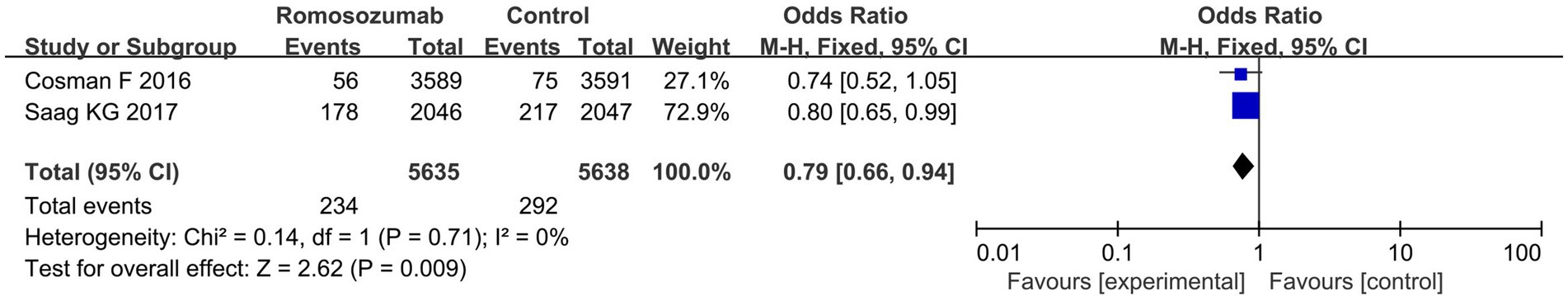
Two studies reported the incidence of non-vertebral fractures in patients after treatment. The meta-analysis results showed that romosozumab was associated with a decreased incidence of nonvertebral fractures (OR = 0.79; 95% CI = 0.66–0.94, p = 0.009; I2 = 0%) (Figure 3). According to the results of the heterogeneity test, Chi2 = 0.14, df = 1, p = 0.71, I2 = 0%, indicating that the included research data exhibited no significant heterogeneity (Figure 3).
3.3.3 Clinical fracture scale
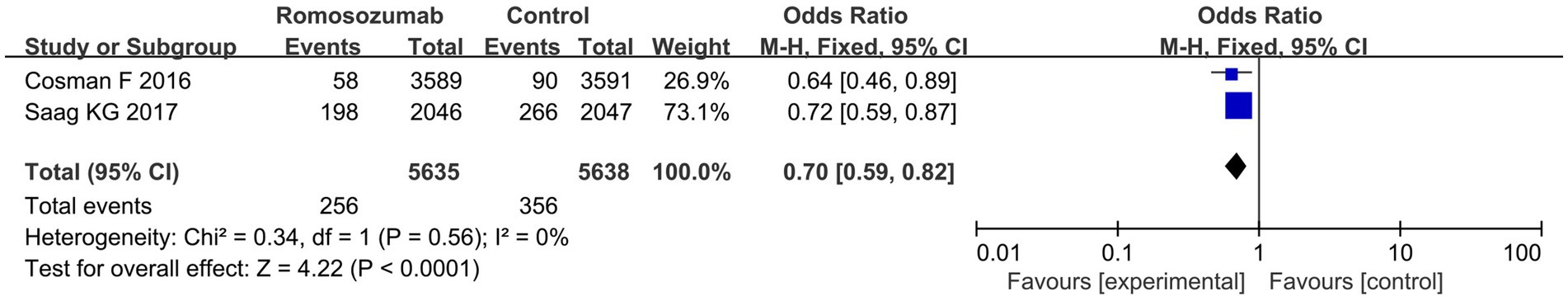
Two studies reported the incidence of clinical fractures in patients after treatment. Pooled results revealed that, compared to standard therapy, romosozumab decreased clinical fractures at 24 months (OR = 0.70; 95% CI = 0.59–0.82, p < 0.00001; I2 = 0%) (Figure 4). According to the results of the heterogeneity test, Chi2 = 0.34, df = 1, p = 0.56, I2 = 0%, indicating that the included research data showed no significant heterogeneity.
3.3.4 Percentage change in BMD of lumbar vertebra
Percentage BMD change at the lumbar spine in the romosozumab versus control group at 12 months was analyzed in four studies. Four RCTs, comprising a total of 3,516 patients in the romosozumab group and 3,463 patients in the standard care group, demonstrated significant improvement in percentage change BMD with romosozumab [mean difference (MD) = 10.38; 95% CI = 4.62–16.14, p = 0.0004; I2 = 100%] (Figure 5). Heterogeneity test results showed significant heterogeneity among the included studies, with Chi2 = 44538.17, df = 3, p < 0.00001, I2 = 100%. Therefore, a random-effects model was used for the analysis (Figure 5).
3.3.5 Percentage change in BMD of hip joint
Percentage BMD change in the romosozumab versus control group at 12 months was evaluated by four studies. The meta-analysis results showed a significant improvement in percentage change in hip joint BMD with romosozumab compared to standard care (MD = 4.46; 95% CI = 3.02–5.91, p < 0.00001; I2 = 97%) (Figure 6). The heterogeneity test showed that with Chi2 = 91.83, df = 3, p < 0.00001, I2 = 97%, the results indicated that heterogeneity was evident in the research data (Figure 6).
3.3.6 Percentage change in BMD of femoral neck
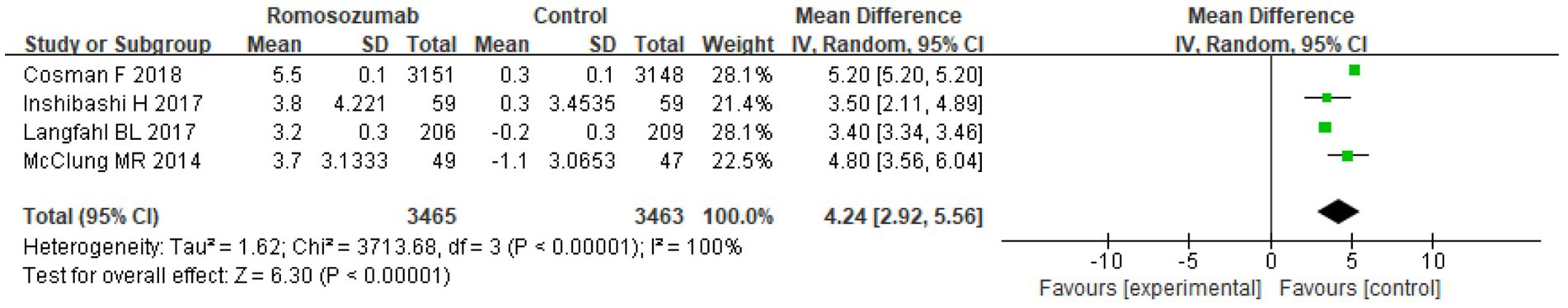
After 12 months of treatment, four studies compared the percentage change in the BMD of the femoral neck. The pooled results showed a significant improvement in hip BMD with romosozumab (MD = 4.24; 95% CI = 2.92–5.56, p < 0.00001; I2 = 100%) (Figure 7). The heterogeneity test results indicated significant heterogeneity among the included studies (Chi2 = 3713.68, df = 3, p < 0.00001, I2 = 100%).
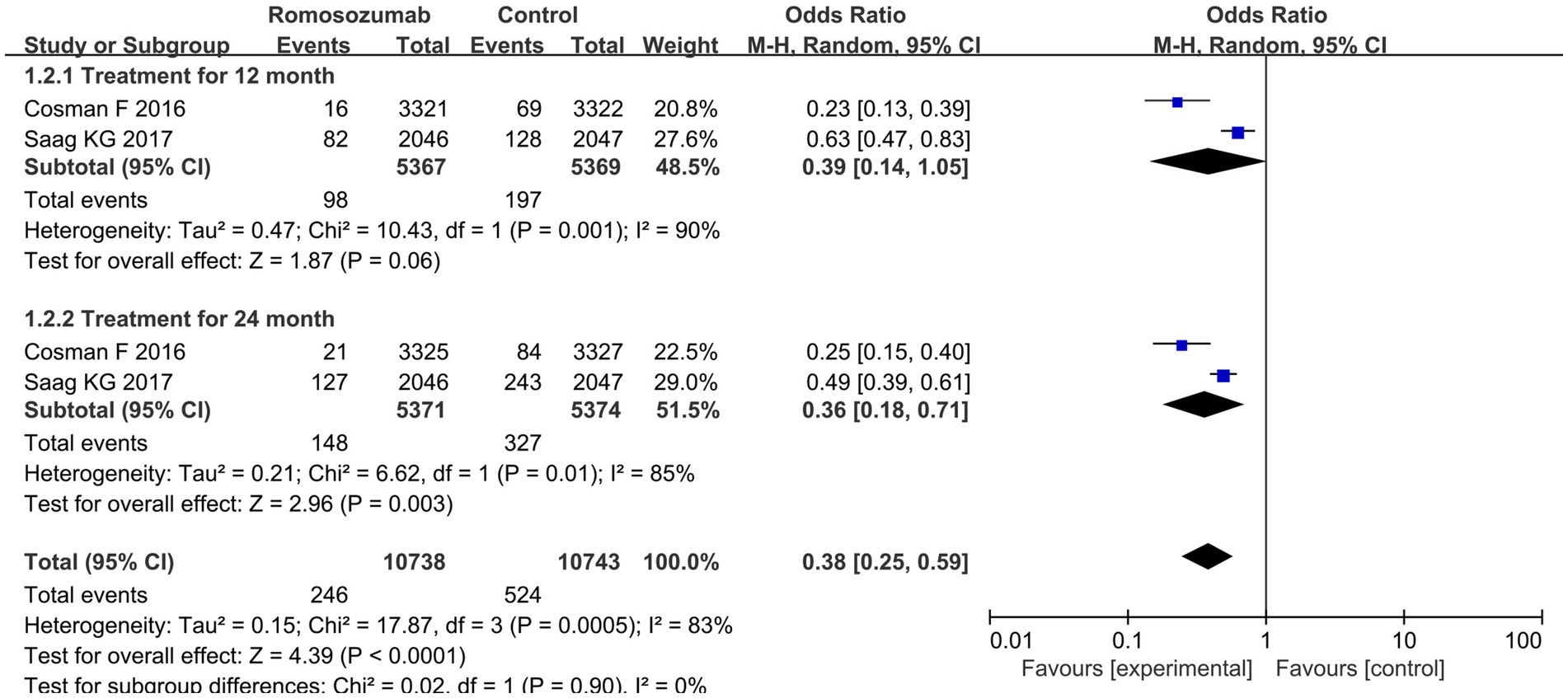
Figure 7. Forest plot comparing BMD of the femoral neck between two groups after 12 months of treatment.
3.3.7 Safety analysis
Five clinical controlled studies reported the incidence of adverse reactions (14–18). The study by Langdahl et al. (15) reported that nasopharyngitis (13% vs. 10%), hypercalcemia (1% vs. 10%), and arthralgia (10% vs. 6%) were common adverse reactions after treatment with romosozumab and standard care. In terms of adverse reactions, there was no notable difference (p > 0.05). Study by Ishibashi et al. (16) reported that patients treated with romosozumab had a higher risk of severe cardiovascular adverse reactions.
3.3.8 Publication bias analysis
Funnel plots were drawn based on vertebral fracture rate, non-vertebral fracture rate, and clinical fracture rate. Publication bias was analyzed (Supplementary Figures S3–S5). Upon analyzing the funnel plots, the majority showed symmetrical distribution.
4 Discussion
PMOP is a subtle disease that often presents no symptoms in the initial stages. It is characterized by a reduction in BMD and microstructural changes in the bone, which can result in decreased bone strength and a higher likelihood of fractures (20). The main factor influencing postmenopausal bone mass loss is estrogen deficiency. The decrease in estrogen during menopause can induce an increase in RANK receptor expression on the osteoclast membrane via the RANKL pathway during osteoclast differentiation, as well as a decrease in the secretion of osteoprotegerin (OPG). Within the RANKL pathway, RANKL is expressed by osteoblasts and bone marrow stromal cells, which then bind to RANK receptors on the surface of osteoclast precursors. This binding promotes osteoclast differentiation and increases bone resorption. This imbalance directly leads to rapid bone loss and an increased risk of osteoporotic fractures. Because of the high incidence of OP and the severe consequences of osteoporotic fractures, the WHO defines OP as a major public health problem of concern (21). It is estimated that 50-year-old women have a 50% chance of experiencing osteoporotic fractures. OP can lead to a significant reduction in their quality of life. Treating osteoporotic fractures can also place a huge burden on the entire healthcare system, and this burden will increase as the elderly population grows each year (22).
The current study demonstrated a significant reduction in the incidence of vertebral fractures at 12 and 24 months, as well as a decrease in nonvertebral and clinical fractures, with romosozumab compared to standard therapy. Supporting our findings, a meta-analysis by Liu et al. (23) also found that romosozumab was associated with a significantly reduced risk of new vertebral fracture, nonvertebral fractures and hip fractures at 24 months. Studies on the population of Latin American countries show that the incidence of osteoporosis (OP) varies from 22.2 to 33.8% due to differences in sample size, sample screening criteria, and research methods. The incidence of osteoporotic brittle fractures caused by frequent falls is as high as 11 to 23.8% (24). Among them, the mortality rate also increased remarkably with the increase of physical activity disorder and life quality degeneration, ranging from 21.5 to 30% (25). In Latin American countries, each post-menopausal patient with OP spends approximately US$775 per year on OP treatment, and the average cost of an 11-day conservative hospital stay for hip fracture in a Latin American public hospital is approximately US$394,000, with a mortality rate of 23.3% after 6 months (26). Currently, the representative drug for promoting bone resorption is tropism, but its widespread clinical use is limited by the increased risk of osteosarcoma (27). As a monoclonal antibody, romosozumab exerts its anti-osteoporotic effect by antagonizing the activation of the Wnt signaling pathway by osteosclerotic proteins and binding to RANKL (28). According to incomplete statistics, within 1 year after the occurrence of hip fracture, about 1 to 5 patients will die of various complications, and the overall disability rate of hip fracture is as high as 50% (29). Vertebral fracture is the most common fracture type in PMOP, and the probability of recurrent fracture after a vertebral fracture is relatively high. Combined with our results, the incidences of vertebral, non-vertebral, and clinical fractures after treatment were compared and analyzed. Study participants had a remarkably lower incidence of vertebral fractures, non-vertebral fractures, and clinical fractures. Our findings on BMD improvement at the lumbar spine, total hip, and femoral neck with romosozumab at 12 months corroborate the positive effects reported by Liu’s et al. (23) meta-analysis. Combining romosozumab therapy with conventional treatment can have a synergistic effect, significantly reducing the incidence of fractures and enhancing the clinical prognosis of patients with PMOP. Diagnostics of OP are based on BMD, which is the gold standard worldwide. This study compared and analyzed the changes in BMD of the lumbar spine, hip, and femoral neck bones after 12 months of treatment. Based on our analysis, the study group had higher BMD in the lumbar spine, hip, and femoral neck bones. It is revealed that the long-term effect of romosozumab combined with routine treatment is better and can successfully enhance the BMD of PMOP patients, promote bone strength, and significantly reduce the risk of fractures. The reason is that romosozumab, as a monoclonal antibody, can activate the Wnt pathway by antagonizing sclerostin, ensuring the normal transmission of the Wnt/β-catenin pathway, promoting bone formation, and inhibiting bone resorption (30, 31).
Cardiovascular events have been the focus of various drug studies, and romosozumab is no exception. The cardiovascular effects of sclerostin, the target of romosozumab, are complex, and changes in this index alone cannot be used to explain cardiovascular events. Additionally, the Wnt signaling pathway has both advantages and disadvantages regarding cardiovascular disease, with more evidence suggesting that this pathway has a protective effect. A black box warning has been issued for romosozumab in patients with high risk factors for cardiovascular disease or stroke. A study by Saag et al. (17) indicated that the rate of serious cardiovascular events was higher with romosozumab than with alendronate. The incidence of adverse reactions to romosozumab was similar to that of the control group. The mean age of the patients contained by McClung et al. (18) was higher; on the one hand, older patients have a poorer cardiovascular base; on the other hand, the effect of romosozumab on osteosclerotic proteins and the Wnt pathway may be influenced by age. Alendronate has a protective effect on the cardiovascular system, but this has not been confirmed by the meta-analysis. This study included a small number of studies and did not classify cardiovascular events. The number of one-off studies was low due to the lack of original reports. Additionally, there were ethnic and gender differences in the study’s population, which did not facilitate the generalization of the findings. Most of the study cycles focused on 12 months, and the evaluation of efficacy and safety was limited due to the small number of original studies and the lack of stratified analysis for different treatment cycles and long-term outcomes. This study has several limitations. Firstly, a small number of studies were included, and most of these were conducted in Western countries, which limits the generalizability of the findings to other races and regions. Secondly, the meta-analysis was limited to studies in English, which may lead to publication bias. Finally, due to the limited number of studies, we did not perform a sensitivity analysis, which may influence the quality of the results. Therefore, validation will require more high-quality RCTs.
5 Conclusion
In conclusion, the combination of romosozumab and conventional therapy emerges as a viable clinical treatment option for postmenopausal patients with OP. Our results demonstrated a significant reduction in fracture risk and improvement in BMD among postmenopausal women with OP who received this treatment, with no notable increase in the incidence of adverse effects.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
GG: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. JC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Writing – original draft, Writing – review & editing. YX: Conceptualization, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. JD: Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1440948/full#supplementary-material
References
1. Arceo-Mendoza, RM, and Camacho, PM. Postmenopausal osteoporosis: latest guidelines. Endocrinol Metab Clin N Am. (2021) 50:167–78. doi: 10.1016/j.ecl.2021.03.009
2. The North American Menopause Society. Management of osteoporosis in postmenopausal women: the 2021 position statement of The North American Menopause Society. Menopause. (2021) 28:973–97. doi: 10.1097/GME.0000000000001831
3. Lems, WF, and Raterman, HG. Critical issues and current challenges in osteoporosis and fracture prevention. An overview of unmet needs. Ther Adv Musculoskelet Dis. (2017) 9:299–316. doi: 10.1177/1759720X17732562
4. Wang, L, Yu, W, Yin, X, Cui, L, Tang, S, Jiang, N, et al. Prevalence of osteoporosis and fracture in China: the China osteoporosis prevalence study. JAMA Netw Open. (2021) 4:e2121106. doi: 10.1001/jamanetworkopen.2021.21106
5. Cosman, F, and Dempster, DW. Anabolic agents for postmenopausal osteoporosis: how do you choose? Curr Osteoporos Rep. (2021) 19:189–205. doi: 10.1007/s11914-021-00663-1
6. Chen, P, Li, Z, and Hu, Y. Prevalence of osteoporosis in China: a meta-analysis and systematic review. BMC Public Health. (2016) 16:1039. doi: 10.1186/s12889-016-3712-7
7. Ensrud, KE . Bisphosphonates for postmenopausal osteoporosis. JAMA. (2021) 325:96. doi: 10.1001/jama.2020.2923
8. Singh, S, Dutta, S, Khasbage, S, Kumar, T, Sachin, J, Sharma, J, et al. A systematic review and meta-analysis of efficacy and safety of romosozumab in postmenopausal osteoporosis. Osteoporos Int. (2022) 33:1–12. doi: 10.1007/s00198-021-06095-y
9. Kobayakawa, T, Miyazaki, A, Saito, M, Suzuki, T, Takahashi, J, and Nakamura, Y. Denosumab versus romosozumab for postmenopausal osteoporosis treatment. Sci Rep. (2021) 11:11801. doi: 10.1038/s41598-021-91248-6
10. Brown, JP . Long-term treatment of postmenopausal osteoporosis. Endocrinol Metab. (2021) 36:544–52. doi: 10.3803/EnM.2021.301
11. Migliorini, F, Maffulli, N, Colarossi, G, Eschweiler, J, Tingart, M, and Betsch, M. Effect of drugs on bone mineral density in postmenopausal osteoporosis: a Bayesian network meta-analysis. J Orthop Surg Res. (2021) 16:533. doi: 10.1186/s13018-021-02678-x
12. Wu, D, Li, L, Wen, Z, and Wang, G. Romosozumab in osteoporosis: yesterday, today and tomorrow. J Transl Med. (2023) 21:668. doi: 10.1186/s12967-023-04563-z
13. Lim, SY, and Bolster, MB. Clinical utility of romosozumab in the management of osteoporosis: focus on patient selection and perspectives. Int J Womens Health. (2022) 14:1733–47. doi: 10.2147/IJWH.S315184
14. Cosman, F, Crittenden, DB, Adachi, JD, Binkley, N, Czerwinski, E, Ferrari, S, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. (2016) 375:1532–43. doi: 10.1056/NEJMoa1607948
15. Langdahl, BL, Libanati, C, Crittenden, DB, Bolognese, MA, Brown, JP, Daizadeh, NS, et al. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial. Lancet. (2017) 390:1585–94. doi: 10.1016/S0140-6736(17)31613-6
16. Ishibashi, H, Crittenden, DB, Miyauchi, A, Libanati, C, Maddox, J, Fan, M, et al. Romosozumab increases bone mineral density in postmenopausal Japanese women with osteoporosis: a phase 2 study. Bone. (2017) 103:209–15. doi: 10.1016/j.bone.2017.07.005
17. Saag, KG, Petersen, J, Brandi, ML, Karaplis, AC, Lorentzon, M, Thomas, T, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. (2017) 377:1417–27. doi: 10.1056/NEJMoa1708322
18. McClung, MR, Grauer, A, Boonen, S, Bolognese, MA, Brown, JP, Diez-Perez, A, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. (2014) 370:412–20. doi: 10.1056/NEJMoa1305224
19. Cosman, F, Crittenden, DB, Ferrari, S, Khan, A, Lane, NE, Lippuner, K, et al. FRAME study: the foundation effect of building bone with 1 year of romosozumab leads to continued lower fracture risk after transition to denosumab. J Bone Miner Res. (2018) 33:1219–26. doi: 10.1002/jbmr.3427
20. Rozenberg, S, Al-Daghri, N, Aubertin-Leheudre, M, Brandi, M-L, Cano, A, Collins, P, et al. Is there a role for menopausal hormone therapy in the management of postmenopausal osteoporosis? Osteoporos Int. (2020) 31:2271–86. doi: 10.1007/s00198-020-05497-8
21. Barron, RL, Oster, G, Grauer, A, Crittenden, DB, and Weycker, D. Determinants of imminent fracture risk in postmenopausal women with osteoporosis. Osteoporos Int. (2020) 31:2103–11. doi: 10.1007/s00198-020-05294-3
22. Huidrom, S, Beg, MA, and Masood, T. Post-menopausal osteoporosis and probiotics. Curr Drug Targets. (2021) 22:816–22. doi: 10.2174/1389450121666201027124947
23. Liu, Y, Cao, Y, Zhang, S, Zhang, W, Zhang, B, Tang, Q, et al. Romosozumab treatment in postmenopausal women with osteoporosis: a meta-analysis of randomized controlled trials. Climacteric. (2018) 21:189–95. doi: 10.1080/13697137.2018.1433655
24. Tsai, JN, Lee, H, David, NL, Eastell, R, and Leder, BZ. Combination denosumab and high dose teriparatide for postmenopausal osteoporosis (DATA-HD): a randomised, controlled phase 4 trial. Lancet Diabetes Endocrinol. (2019) 7:767–75. doi: 10.1016/S2213-8587(19)30255-4
25. Gatti, D, and Fassio, A. Pharmacological management of osteoporosis in postmenopausal women: the current state of the art. J Popul Ther Clin Pharmacol. (2019) 26:e1–e17. doi: 10.15586/jptcp.v26.i4.646
26. Shojaa, M, von Stengel, S, Kohl, M, Schoene, D, and Kemmler, W. Effects of dynamic resistance exercise on bone mineral density in postmenopausal women: a systematic review and meta-analysis with special emphasis on exercise parameters. Osteoporos Int. (2020) 31:1427–44. doi: 10.1007/s00198-020-05441-w
27. Słupski, W, Jawień, P, and Nowak, B. Botanicals in postmenopausal osteoporosis. Nutrients. (2021) 13:1609. doi: 10.3390/nu13051609
28. Lim, SY, and Bolster, MB. Profile of romosozumab and its potential in the management of osteoporosis. Drug Des Devel Ther. (2017) 11:1221–31. doi: 10.2147/DDDT.S127568
29. Brown, JP, Engelke, K, Keaveny, TM, Chines, A, Chapurlat, R, Foldes, AJ, et al. Romosozumab improves lumbar spine bone mass and bone strength parameters relative to alendronate in postmenopausal women: results from the active-controlled fracture study in postmenopausal women with osteoporosis at high risk (ARCH) trial. J Bone Miner Res. (2021) 36:2139–52. doi: 10.1002/jbmr.4409
30. Slaton, RM, Boyd, K, and Iranikhah, M. Romosozumab and sequential therapy in postmenopausal osteoporosis. Sr care Pharm. (2020) 35:297–308. doi: 10.4140/TCP.n.2020.297
Keywords: romosozumab, menopause, osteoporosis, fracture, adverse reactions
Citation: Gao G, Cui J, Xie Y and Dong J (2024) Effects of romosozumab combined with routine therapy on pain relief, disease progression and adverse reactions in patients with postmenopausal osteoporosis: a systematic review and meta-analysis. Front. Med. 11:1440948. doi: 10.3389/fmed.2024.1440948
Edited by:
Mattia Dominoni, San Matteo Hospital Foundation (IRCCS), ItalyReviewed by:
Alan Lins Fernandes, University of São Paulo, BrazilPinar Yalcin Bahat, University of Health Sciences, Türkiye
Copyright © 2024 Gao, Cui, Xie and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Dong, MTg5Mjg4MTA1NTRAMTYzLmNvbQ==
 Ge Gao1
Ge Gao1 Jing Dong
Jing Dong