- 1Department of Gynecology and Obstetrics, Shijiazhuang People's Hospital, Shijiazhuang, China
- 2Transfusion Department, Shijiazhuang People's Hospital, Shijiazhuang, China
Introduction: Intrauterine adhesion (IUA), a common gynecological disease, is mainly caused by traumatic or infectious factors that lead to basal endometrial layer physiological repair disorders. IUA is mostly treated via hysteroscopic transcervical resection of adhesion and although it can restore uterine cavity shape, its endometrial repair effectiveness is limited. The figures showed that after surgery, patients with IUA have a high recurrence rate. Therefore, quick endometrial damage repair is key to successful treatment.
Case presentation: A 34-year-old patient visited our hospital after experiencing amenorrhea for 4 months following an induced abortion and had a fertility requirement. Based on the American Fertility Society intrauterine scores, the patient was diagnosed with moderate IUA. She underwent transcervical resection of adhesion, followed by autologous platelet-rich gel intrauterine perfusion and periodic estrogen–progesterone treatment for three menstrual cycles. No complications developed during treatment and the patient’s endometrium was significantly repaired, with successful pregnancy being achieved.
Conclusion: Autologous platelet-rich gel promoted endometrial repair and acted as a mechanical barrier to prevent intrauterine adhesion. This approach May offer new insights into IUA treatment.
1 Introduction
Intrauterine adhesion (IUA), which is also known as Asherman’s syndrome, is a common gynecological disease. Its major causes include multiple intrauterine operations, such as induced abortion and dilatation and curettage, as well as infection, which can damage the endometrium’s basal layer. Failure of normal endometrial regeneration and damage repair leads to fibrosis and adhesion formation, eventually leading to a partial or complete closure of the uterine cavity (1). The main IUA symptoms include reduced menstrual volume and even amenorrhea, secondary infertility, and miscarriage (2). Severe IUA recurrence rates after hysteroscopic transcervical resection of adhesion (TCRA), the preferred IUA treatment method, are as high as 62.5% (3). This is because although surgery can improve uterine cavity morphosis, it May aggravate endometrial damage. Currently, postoperative IUA recurrence is often prevented using drug therapy, mechanical barriers, or anti-adhesion agents. However, these strategies are associated with long treatment cycles, limited efficacy for moderate-to-severe IUA, and the risk of complications (1). Platelet-rich plasma (PRP) is a platelet concentrate extracted from fresh autologous blood, and after its activation, platelet α-granules release various growth factors, including vascular endothelial growth factor and platelet-derived growth factor, and cytokines, which promote cell proliferation, angiogenesis, and matrix remodeling (4). Moreover, PRP activation converts soluble fibrinogen into insoluble fibrin, thereby forming an autologous platelet-rich gel (APG), which prolongs the retention and action time of active substances (5). APG May prevent intrauterine adhesion by a offering mechanical barrier function. Calcium gluconate-activated PRP has been shown to accelerate diabetic wound healing and to possess neuroprotective and neuroregenerative effects (6, 7). This report describes for the first time that the postoperative application of calcium gluconate-activated APG in patients with IUA. This patient’s menses has recovered and then she is pregnant.
2 Case presentation
In February 2023, a 34-year-old patient visited our hospital after experiencing amenorrhea for four months following an induced abortion and had a fertility requirement. The patient had previously undergone two natural births and three induced abortions. Transvaginal ultrasound revealed that the endometrium had grown unevenly, at a thickness of 0.1–0.29 cm (average: 0.20 cm). However, a hormone test did not reveal significant abnormalities. Hysteroscopy examination indicated intrauterine adhesions immediately (Figures 1A–C) and based on American Fertility Society intrauterine scores, the patient was diagnosed with moderate IUA (8).
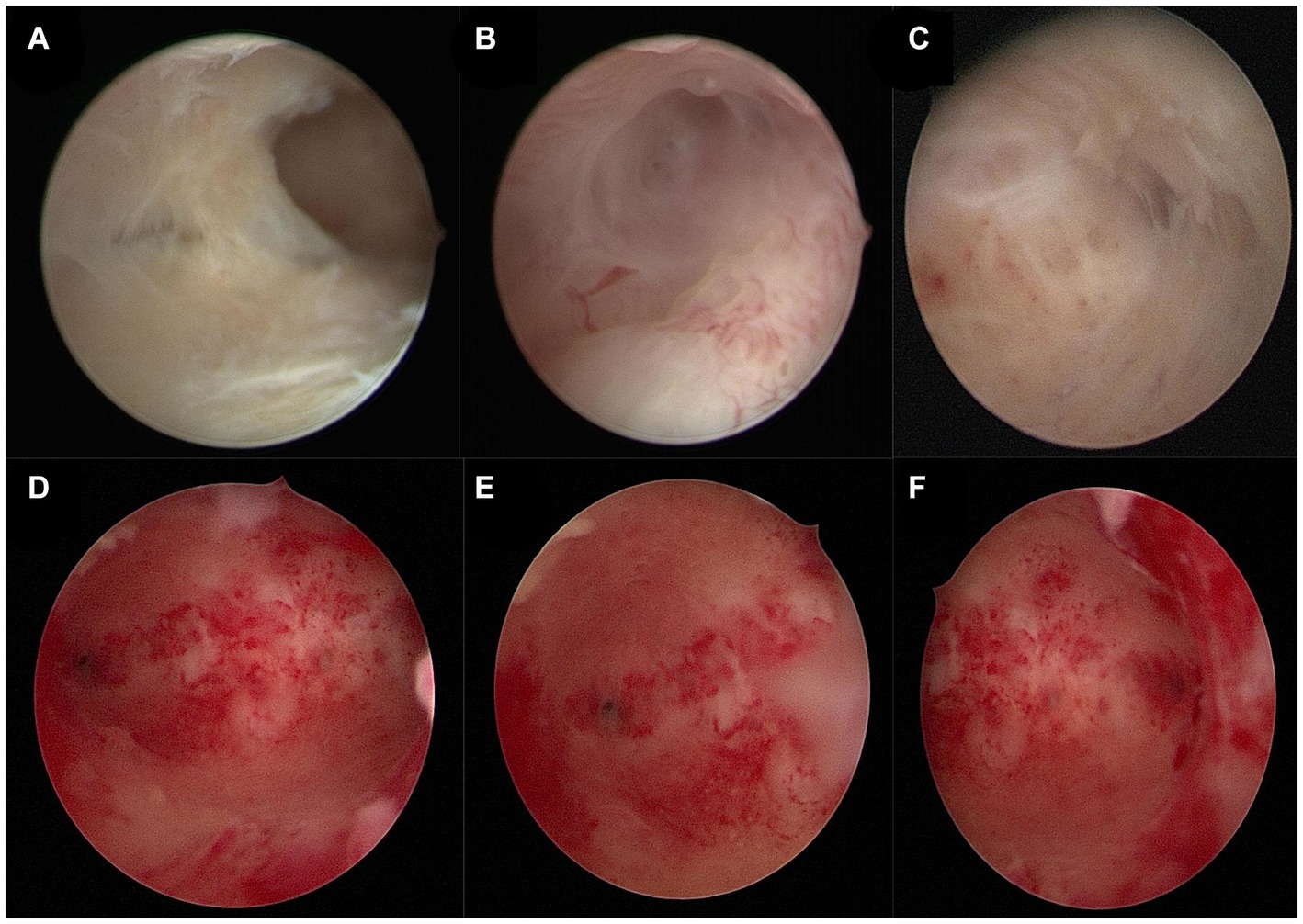
Figure 1. (A–C) Pre-surgery hysteroscopic patient examination images. (A) Adhesion of the uterine cavity’s right wall. (B) Right fallopian tube opening. (C) Left fallopian tube opening. (D–F) Post-treatment hysteroscopic patient examination images. (D) Normal uterine cavity. (E) Right fallopian tube opening. (F) Left fallopian tube opening.
3 Surgical procedure
PRP was extracted using a blood cell separator (NGLXCF3000, Nigale Biomedical, Sichuan, China), followed by hysteroscopic TCRA the next day. To prepare APG, 10% calcium gluconate was added to the PRP at a 1:9 ratio, mixed, and immediately administered into the uterine cavity via perfusion (9), and the patient was asked to remain in the perfusion position for 15–20 min. After the surgery, the patient took 4 mg of estradiol valerate daily and underwent APG intrauterine perfusion every 7–10 days. The patient started menstruating in April 2023 at about 1/3 to 1/2 of her normal menstrual volume. Hysteroscopy examination was performed on the first day after menstruation. The images revealed improved uterine cavity morphology without adhesion and that the endometrium was uniform (Figures 1D–F). Intrauterine APG perfusion was done on the first and eighth days after menstruation, with periodic treatment for three menstrual cycles using estrogen (4 mg of estradiol valerate daily for 21 days) and progesterone (20 mg of dydrogesterone daily for the last 10 days). During the mid-luteal phase, endometrial thickness increased gradually to 0.6 cm (Figure 2). No complications were observed during the treatment and the patient got pregnant in November 2023.
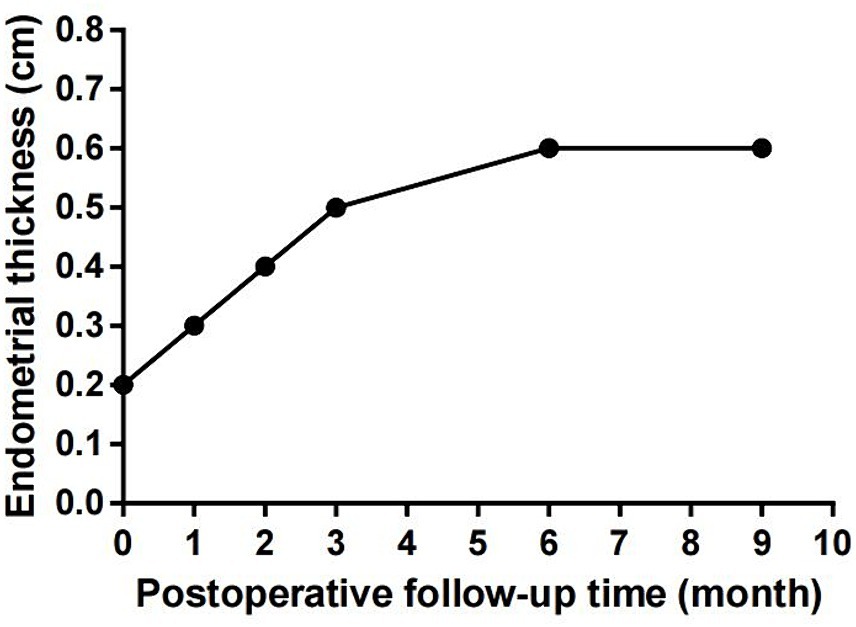
Figure 2. The patient’s endometrial thickness increased gradually during the treatment, and there was no significant change during post-treatment follow-up.
4 Discussion
IUA is one of the common gynecological diseases. The main reason for its formation is the repair dysfunction of endometrial cells after injury. IUA patients often seek medical advice due to hypomenorrhoea, amenorrhea and infertility. Hysteroscopic TCRA, as the preferred surgery for IUA patients, can improve the morphosis of uterine cavity. But the recurrence rate of postoperative adhesion is high. Therefore, adopting effective methods to repair the endometrium and prevent recurrence after surgery is also crucial in the treatment of IUA. In recent years, the role of PRP cell therapy in regenerative medicine has been gradually explored. Autologous PRP is easily obtainable and it does not carry disease transmission or immune rejection risks. Through growth factor and cytokine release, PRP promotes cell proliferation and anti-inflammation (10, 11). In the clinical application of obstetrics and gynecology, PRP can relieve vulvovaginal atrophy symptoms and urinary incontinence stress in genitourinary syndrome during menopause, as well as prevent uterine niche formation after cesarean section (12–14). PRP improves endometrial thickness and receptivity by increasing angiogenesis-related markers and modulating immune cells and the microbiome. Consequently, PRP improves menstruation and pregnancy outcomes in patients with thin endometria and endometritis (15–17). A study on an IUA mouse model revealed that intrauterine PRP injection improves endometrial stromal cell migration, embryo implantation, and live birth rates. Currently, there are few clinical data on the application of PRP in postoperative patients with IUA. Javaheri et al. (18) demonstrated that there was no significant difference in menstrual improvement and prevention of adhesion recurrence between IUA patients who received 1 mL PRP single intrauterine injection after surgery and those who did not receive it. Aghajanova et al. (19) also confirmed that estrogen therapy combined with 0.5-1 mL PRP single intrauterine injection after IUA surgery showed no changes in endometrial thickness and pregnancy rate of patients compared to the estrogen only treatment group. The emergence of these results May be related to the liquid properties of PRP, which cannot stay in the uterine cavity for a long time to fully exert its effect.
It is reported that after PRP activation, platelets are degranulated, thereby releasing several growth factors (5). Fibrinogen activation can form a gel that provides a temporary cell proliferation and migration scaffold. At the same time, bioactive factors can be protected from proteolytic enzyme degradation and be released slowly (20, 21). Common PRP agonists include calcium gluconate, calcium chloride, and thrombin. Considering its heterogeneity, thrombin use as a PRP activator May pose certain clinical application risks (22). Growth factor and cytokine release in 10% calcium gluconate-activated PRP gels is similar to thrombin’s (23, 24) and is associated with a higher cell proliferation rate when compared with inactive PRP (25). When compared with calcium chloride, calcium deposition is rare in gels formed by calcium gluconate-activated PRP (23). Calcium gluconate-activated PRP gels have been used as diabetes skin ulcer dressing to promote wound healing (6). Moreover, it is reported that calcium gluconate-activated PRP gels can promote spiral ganglion neuron neuroregeneration and neuroprotection in vitro (7). Injecting calcium gluconate-activated PRP gels into the ovaries of patients with premature ovarian insufficiency is reported to improve ovarian function (26). Additionally, the gel formed by calcium salt retracted more slowly, which is beneficial for barrier properties and adhesion prevention (23).
In this report, we describe the therapeutic effects of a calcium gluconate-activated autologous platelet-rich gel in combination with hormone therapy in patients with IUA after adhesiolysis. First, to ensure cellular component stability, we used a blood cell separator to extract PRP. Second, because calcium gluconate-activated PRP gels at 37°C after about 10 min (9), the patient was required to maintain the perfusion position for 15–20 min. Furthermore, for unresistant perfusion without cervical dilatation, a tube was used for hydrotubation to reduce patient discomfort. The time interval for APG perfusion was determined based on the platelet metabolic cycle. There were no complications during the treatment process. Moreover, the patient’s endometrial thickness increased steadily, and she became pregnant. Because endometrial damage-associated risks, such as miscarriage and premature birth remain, close follow-up is required. Because there is no consensus about the protocol for APG use for endometrial repair, this case report May provide some ideas. However, randomized controlled trials are needed to validate the effectiveness and safety of this case’s protocol, as well as to evaluate long-term APG effects. Moreover, simultaneous cell and animal experiments are needed to determine the underlying mechanisms.
5 Conclusion
This case indicates that in patients with moderate IUA, autologous platelet-rich gels have the potential to prevent postoperative recurrence, repair the endometrium, and increase the probability of pregnancy. It is important to provide timely and effective treatment measures for patients with intrauterine adhesions after surgery for preventing recurrence. However, larger clinical datasets are needed to confirm this method’s safety and effectiveness. Nonetheless, this approach May offer new IUA treatment ideas.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committees of Shijiazhuang People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. The manuscript presents research on animals that do not require ethical approval for their study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YL: Data curation, Funding acquisition, Investigation, Writing – original draft. YH: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing. XS: Investigation, Software, Writing – original draft. JC: Conceptualization, Funding acquisition, Investigation, Writing – original draft. JL: Investigation, Software, Writing – original draft. WZ: Investigation, Software, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the Medical Science Research Project of Hebei Province in China (grant nos. 20240726, 20231593).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ma, J, Zhan, H, Li, W, Zhang, L, Yun, F, Wu, R, et al. Recent trends in therapeutic strategies for repairing endometrial tissue in intrauterine adhesion. Biomater Res. (2021) 25:40. doi: 10.1186/s40824-021-00242-6
2. Zhang, H, Xu, D, Li, Y, Lan, J, Zhu, Y, Cao, J, et al. Organoid transplantation can improve reproductive prognosis by promoting endometrial repair in mice. Int J Biol Sci. (2022) 18:2627–38. doi: 10.7150/ijbs.69410
3. Li, C, Cai, A, Sun, C, Wu, B, Chen, X, Mao, Y, et al. The study on the safety and efficacy of amnion graft for preventing the recurrence of moderate to severe intrauterine adhesions. Genes Dis. (2020) 7:266–71. doi: 10.1016/j.gendis.2019.03.003
4. Mastrogiacomo, M, Nardini, M, Collina, MC, Di Campli, CC, Filaci, G, Cancedda, R, et al. Innovative cell and platelet rich plasma therapies for diabetic foot ulcer treatment: the allogeneic approach. Front Bioeng Biotechnol. (2022) 10:869408. doi: 10.3389/fbioe.2022.869408
5. Bielecki, T, Gazdzik, TS, and Szczepanski, T. Re: “the effects of local platelet rich plasma delivery on diabetic fracture healing”. What do we use: platelet-rich plasma or platelet-rich gel? Bone. (2006) 39:1388; author reply 1389. doi: 10.1016/j.bone.2006.06.015
6. Zhou, J, Liu, Y, Liu, X, Wan, J, Zuo, S, Pan, T, et al. Hyaluronic acid-based dual network hydrogel with sustained release of platelet-rich plasma as a diabetic wound dressing. Carbohydr Polym. (2023) 314:120924. doi: 10.1016/j.carbpol.2023.120924
7. Stolle, M, Schulze, J, Roemer, A, Lenarz, T, Durisin, M, and Warnecke, A. Human plasma rich in growth factors improves survival and neurite outgrowth of spiral ganglion neurons in vitro. Tissue Eng Part A. (2018) 24:493–501. doi: 10.1089/ten.TEA.2017.0120
8. The American Fertility Society Classifications of Adnexal Adhesions . Distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, Müllerian anomalies and intrauterine adhesions. Fertil Steril. (1988) 49:944–55. doi: 10.1016/s0015-0282(16)59942-7
9. Yang, Y, Zhang, W, and Cheng, B. Experimental research on the effects of different activators on the formation of platelet-rich gel and the release of bioactive substances in human platelet-rich plasma. Zhonghua Shao Shang Za Zhi. (2017) 33:12–7. doi: 10.3760/cma.j.issn.1009-2587.2017.01.004
10. Chen, LS, Chen, CK, Pang, JS, Lin, LP, Yu, TY, and Tsai, WC. Leukocyte-poor platelet-rich plasma and leukocyte-rich platelet-rich plasma promote myoblast proliferation through the upregulation of cyclin a, cdk1, and cdk2. J Orthop Res. (2024) 42:32–42. doi: 10.1002/jor.25666
11. Blanchard, E, Harvi, J, Vasudevan, J, and Swanson, RL. Platelet-rich plasma for adhesive capsulitis: a systematic review. Cureus. (2023) 15:e46580. doi: 10.7759/cureus.46580
12. Maris, E, Salerno, J, Hédon, B, and Mares, P. Management of vulvovaginal atrophy: physical therapies. Postmenopausal women management: CNGOF and GEMVi clinical practice guidelines. Gynecol Obstet Fertil Senol. (2021) 49:414–9. doi: 10.1016/j.gofs.2021.03.021
13. Grigoriadis, T, Kalantzis, C, Zacharakis, D, Kathopoulis, N, Prodromidou, A, Xadzilia, S, et al. Platelet-rich plasma for the treatment of stress urinary incontinence-a randomized trial. Urogynecology (Phila). (2024) 30:42–9. doi: 10.1097/SPV.0000000000001378
14. Backer, S, Khanna, D, Sadr, S, and Khatibi, A. Intra-operative guidelines for the prevention of uterine niche formation in cesarean sections: a review. Cureus. (2023) 15:e44521. doi: 10.7759/cureus.44521
15. Agarwal, M, Mettler, L, Jain, S, Meshram, S, Günther, V, and Alkatout, I. Management of a thin endometrium by hysteroscopic instillation of platelet-rich plasma into the endomyometrial junction: a pilot study. J Clin Med. (2020) 9:2795. doi: 10.3390/jcm9092795
16. Nazari, L, Salehpour, S, Hoseini, S, Zadehmodarres, S, and Azargashb, E. Effects of autologous platelet-rich plasma on endometrial expansion in patients undergoing frozen-thawed embryo transfer: a double-blind RCT. Int J Reprod Biomed. (2019) 17:443–8. doi: 10.18502/ijrm.v17i6.4816
17. Sfakianoudis, K, Simopoulou, M, Nitsos, N, Lazaros, L, Rapani, A, Pantou, A, et al. Successful implantation and live birth following autologous platelet-rich plasma treatment for a patient with recurrent implantation failure and chronic endometritis. In Vivo. (2019) 33:515–21. doi: 10.21873/invivo.11504
18. Javaheri, A, Kianfar, K, Pourmasumi, S, and Eftekhar, M. Platelet-rich plasma in the management of Asherman's syndrome: an RCT. Int J Reprod Biomed. (2020) 18:113–20. doi: 10.18502/ijrm.v18i2.6423
19. Aghajanova, L, Sundaram, V, Kao, CN, Letourneau, JM, Manvelyan, E, Cedars, MI, et al. Autologous platelet-rich plasma treatment for moderate-severe Asherman syndrome: the first experience. J Assist Reprod Gen. (2021) 38:2955–63. doi: 10.1007/s10815-021-02328-5
20. Mendes, BB, Gómez-Florit, M, Babo, PS, Domingues, RM, Reis, RL, and Gomes, ME. Blood derivatives awaken in regenerative medicine strategies to modulate wound healing. Adv Drug Deliv Rev. (2018) 129:376–93. doi: 10.1016/j.addr.2017.12.018
21. Chen, D, Wang, C, Cui, L, and Ran, X. Autologous platelet-rich gel treatment of chronic nonhealing ulcerated tophaceous gout. Indian J Dermatol. (2020) 65:141–4. doi: 10.4103/ijd.IJD_157_18
22. Aydin, O, Karaca, G, Pehlivanli, F, Altunkaya, C, Uzun, H, Özden, H, et al. Platelet-rich plasma may offer a new Hope in suppressed wound healing when compared to mesenchymal stem cells. J Clin Med. (2018) 7:143. doi: 10.3390/jcm7060143
23. Giraldo, CE, Álvarez, ME, and Carmona, JU. Influence of calcium salts and bovine thrombin on growth factor release from equine platelet-rich gel supernatants. Vet Comp Orthop Traumatol. (2017) 30:1–7. doi: 10.3415/VCOT-16-02-0026
24. Carmona, JU, López, C, and Ceballos-Márquez, A. Temporal release and denature of several mediators in pure platelet-rich plasma and temperature-induced platelet lysates derived from a similar bovine platelet concentrate. Vet Med Int. (2022) 2022:2609508. doi: 10.1155/2022/2609508
25. Vahabi, S, Yadegari, Z, and Mohammad-Rahimi, H. Comparison of the effect of activated or non-activated PRP in various concentrations on osteoblast and fibroblast cell line proliferation. Cell Tissue Bank. (2017) 18:347–53. doi: 10.1007/s10561-017-9640-7
Keywords: intrauterine adhesion, autologous platelet-rich gel, calcium gluconate, endometrial repair, pregnancy, case report
Citation: Li Y, Han Y, Su X, Cao J, Liu J and Zhang W (2024) Application of autologous platelet-rich gel formed by calcium gluconate combined with hormone therapy for endometrial repair after hysteroscopic transcervical resection of adhesion surgery and successful pregnancy: case report and literature review. Front. Med. 11:1436089. doi: 10.3389/fmed.2024.1436089
Edited by:
Erol Tavmergen, Ege University, TürkiyeReviewed by:
Zhaoxian Ni, Fudan University, ChinaThierry Vancaillie, University of New South Wales, Australia
Copyright © 2024 Li, Han, Su, Cao, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingxue Han, eWluZ3h1ZWhhbjIwMjIwODI4QDEyNi5jb20=
 Yunying Li1
Yunying Li1 Yingxue Han
Yingxue Han