- 1Key Laboratory of Biotherapy and Regenerative Medicine of Gansu Province, First Hospital of Lanzhou University, Lanzhou, China
- 2Department of Epidemiology and Biostatistics, Indiana University School of Public Health, Bloomington, IN, United States
- 3Department of Medicine, Duke University School of Medicine, Durham, NC, United States
- 4Division of Nephrology, Department of Medicine, University of Washington, Seattle, WA, United States
- 5Justice, Equity, Diversity, and Inclusion Center for Transformational Research, Office of Healthcare Equity, University of Washington, Seattle, WA, United States
Background: New Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations without a race adjustment were developed to estimate the glomerular filtration rate (eGFR). We aimed to compare the performance of five CKD-EPI eGFR equations, with or without race, in predicting cardiovascular disease (CVD) events and all-cause mortality in Black Americans from the Jackson Heart Study.
Methods: JHS is an ongoing population-based prospective cohort study of African Americans in the Jackson, Mississippi, metropolitan area. Five CKD-EPI equations were used to estimate GFR at baseline using serum creatinine (Cr) or cystatin C (cys), including 2009 eGFRcr(ASR [age, sex, race]), 2021 eGFRcr(AS [age and sex]), 2012 eGFRcr-cys(ASR), 2021 eGFRcr-cys(AS), 2012 eGFRcys(AS). Endpoints were incident CVD events and all-cause mortality. Cox proportional hazards regression was used to assess the associations between different eGFRs and outcomes adjusting for atherosclerotic risk factors. Harrell’s C-statistics and Net Reclassification Index (NRI) were used to assess the predictive utility.
Results: Among 5,129 participants (average age 54.8 ± 12.8 yrs), 1898 were male (37.0%). eGFRcr(AS) provided lower estimates and resulting in a greater proportion of participants categorized as CKD than eGFRcr(ASR), eGFRcr-cys(ASR), eGFRcr-cys(AS) and eGFRcys(AS). A median follow-up of 13.7 and 14.3 years revealed 411 (9.3%) CVD incidents and 1,207 (23.5%) deaths. Lower eGFRs were associated with CVD incidents and all-cause mortality. eGFRcr-cys(ASR), eGFRcr-cys(AS) and eGFRcys(AS) were strongly associated with incident CVD events and all-cause mortality than eGFRcr(ASR) and eGFRcr(AS). A significant discrimination improvement was found in C-statistics for predicting incident CVD events and all-cause mortality after adding each eGFR measure to the basic model including atherosclerotic risk factors. Across a 7.5% 10-year risk threshold, eGFRcys(AS) improved net classification of all-cause mortality (NRI: 2.19, 95%CI: 0.08, 4.65%).
Conclusion: eGFR based on creatinine omit race has the lowest mean and detects more CKD patients in Black population. The eGFRs incorporating cystatin C strengthens the association between the eGFR and the risks of incident CVD and all-cause mortality. Cystatin C-based eGFR equations might be more appropriate for predicting CVD and mortality among Black population.
Introduction
Chronic kidney disease (CKD) has been recognized as a major public health problem that is an important risk factor for cardiovascular disease (CVD) and all-cause mortality (1). CKD affects more than 10% of the general population around the world, accounting for more than 800 million (2). In the United States, 15% of adults were estimated to have CKD, and CKD is more common in non-Hispanic Black adults than in non-Hispanic White or Asian adults (3).
Glomerular filtration rate (GFR) is generally accepted as the best overall index of kidney function in disease and health and is recommended as a principal measure to define CKD and categorize the damage stage of CKD in guidelines (4). The equations commonly used for eGFR are the serum creatinine (cr) based Modification of Diet in Renal Disease (MDRD) study equation (5) and the Chronic Kidney Disease Epidemiology Collaboration (2009 CKD-EPI eGFRcr) equation (6), the serum cystatin C (cys) based CKD-EPI (2012 CKD-EPI eGFRcys) and the combination of creatinine and cystatin C based CKD-EPI (2012 CKD-EPI eGFRcr-cys) (7). 2009 CKD-EPI eGFRcr and 2012 CKD-EPI eGFRcr-cys incorporate race in calculation. However, recognizing the limitations of race-based medicine and an evolved consideration of race as a social, not biological construct, new non-race-based equations were derived, including 2021 CKD-EPI eGFRcr and 2021 CKD-EPI eGFRcr-cys (8). The National Kidney Foundation (NKF) and the American Society of Nephrology (ASN) convened a Task Force to review the inclusion of race in eGFR and recommended immediate implementation of CKD-EPI new race-free eGFRcr or eGFRcr-cys equations for kidney function estimation (9).
The Jackson Heart Study (JHS) is a population-based cohort study of African Americans, with a high prevalence of diabetes, CKD, and cardiovascular diseases (10). Studies have reported that lower eGFR is significantly associated with a higher risk of all-cause mortality and cardiovascular diseases independently of other traditional risk factors (11–16), and lower eGFR has been demonstrated as a significant risk factor for CVD and mortality in JHS (17). Recently, a new Predicting Risk of Cardiovascular Disease Events (PREVENT) equation was released by the American Heart Association (AHA) including eGFR as well as traditional risk factors, the eGFR was calculated using 2021 CKD-EPI eGFRcr (18). Accordingly, we aimed to compare the performance of different CKD-EPI eGFR equations with and without race in predicting CVD events and all-cause mortality in JHS participants.
Methods
Study sample
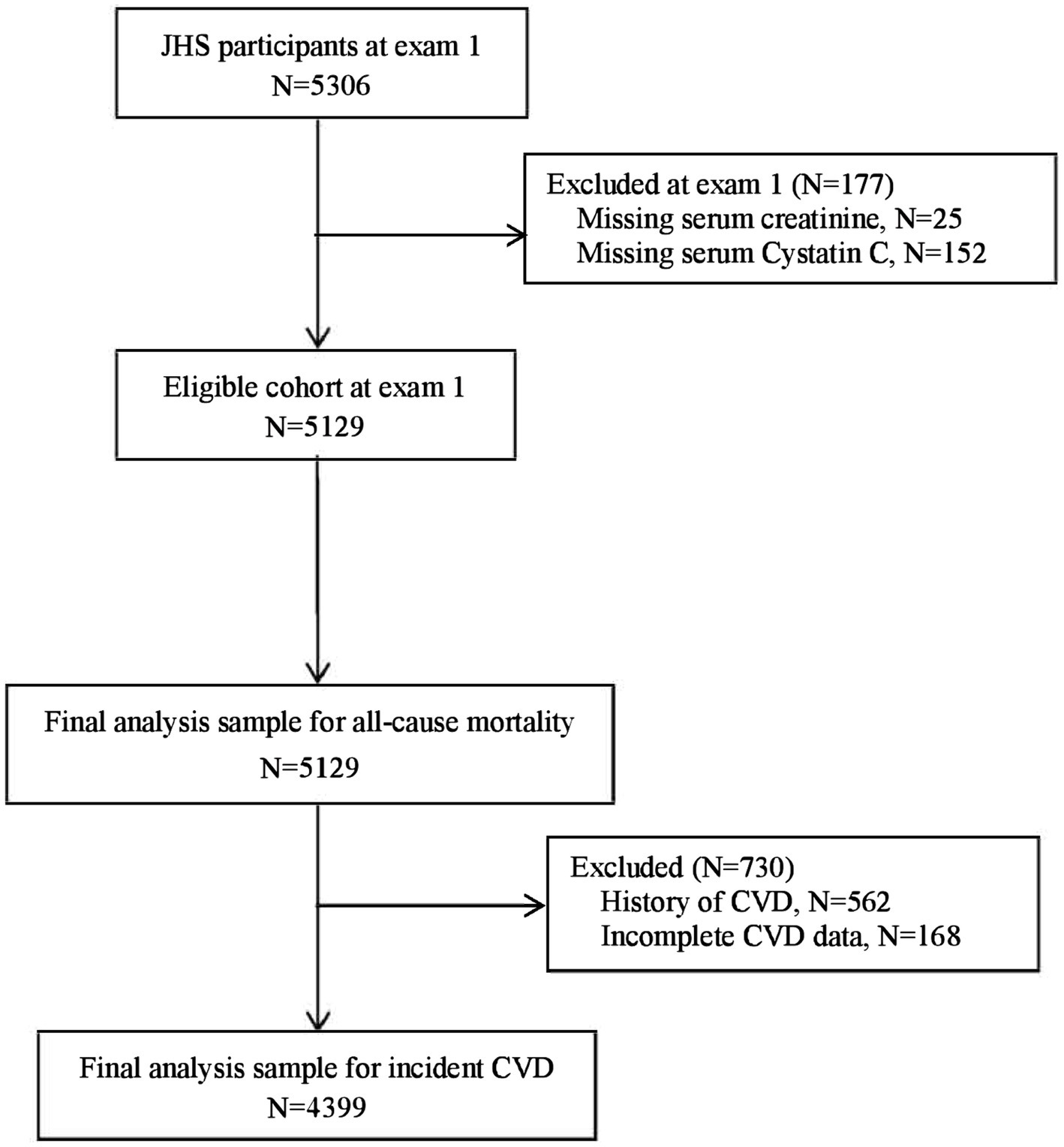
The JHS is an ongoing community-based, prospective cohort study intended to evaluate CVD risk among self-reported African Americans. The details of the study have been published previously (19, 20). The original JHS cohort enrolled participants from September 2000 to March 2004 (exam 1) and comprised 5,306 participants with 20–95 years. For the current analysis, participants with complete serum creatinine and cystatin C at exam 1 were included, n = 5,129 (excluding 25 participants with missing serum creatinine, and 152 participants missing cystatin C). For incident CVD, an additional 730 participants were excluded (168 participants had incomplete CHD and stroke information and 562 participants had CHD or stroke at baseline). Finally, 4,399 participants and 5,129 participants were included in the incident CVD and all-cause mortality analyses (Figure 1), respectively. The study was approved by the institutional review board of the participating institutions (University of Mississippi Medical Center, Jackson State University, and Tugaloo College). All of the participants provided informed consent.
eGFR
Serum creatinine (mg/dl) and cystatin C (mg/L) were collected at exam 1. Serum creatinine was assessed using an enzymatic method on a Vitros 950 (21) and calibrated to the isotope-dilution mass spectrometry (IDMS) traceable method (22). Serum cystatin C was measured by a particle-enhanced immunonephelometric assay. Five CKD-EPI equations were considered in this study (Supplementary Table S1), for calculating eGFRs based on serum creatinine or cystatin C with and without race (8), including 2009 CKD-EPI eGFRcr(ASR [age, sex, and race]), 2021 CKD-EPI eGFRcr(AS [age and sex]), 2012 CKD-EPI eGFRcr-cys(ASR), 2021 CKD-EPI eGFRcr-cys(AS), 2012 CKD-EPI eGFRcys(AS).
Outcomes
The outcomes were incident CVD and all-cause mortality. Incident CVD was defined as the first occurrence of coronary heart disease (CHD) or stroke among participants without CVD at baseline. Annual follow-up interviews and medical record reviews were used to adjudicate CHD, stroke, and all-cause mortality from 2000, whereas the adjudication of heart failure (HF) which was excluded from our measure of incident CVD began in 2005. The detailed adjudication description has been published previously (23). At the time of analyses for this study, incident CVD events were adjudicated through December 31, 2016, and all-cause mortality was assessed through December 31, 2018.
Covariate assessments
All basic characteristics and clinical covariates were measured at baseline, including the following: age (years); sex; body mass index (BMI) in kg/m2; current smoker; alcohol; income; education; systolic blood pressure (SBP); diastolic blood pressure (DBP); hypertension; diabetes; history of CVD (coronary heart disease, stroke or both); self-reported history of CKD; medication status of hypertension, diabetes and statin; fasting plasma glucose (mg/dl); total cholesterol (mg/dl); high-density lipoproteins (HDL) cholesterol (mg/dl); low-density lipoproteins (LDL) cholesterol (mg/dl); triglycerides (mg/dl); high-sensitivity C-reactive protein (hsCRP, mg/dl). Sitting blood pressure was measured twice at 1-min intervals after 5-min resting and the average of two measurements was used for analysis. Hypertension was defined as blood pressure ≥ 140/90 mmHg or the use of antihypertension medications. Diabetes was defined as fasting glucose ≥126 mg/dl or hemoglobin A1c ≥ 6.5% or the use of diabetic medications. CKD was defined as the eGFR less than 60 ml/min/1.73 m2 (4).
Statistical analysis
Participants were classified according to 2009 eGFRcr(ASR) categories (≥90, 60–90, <60 ml/min/1.73m2). Continuous variables were expressed as the mean (standard deviation, SD) or median (interquartile range, IQR), and categorical variables were expressed as numbers (percentages). Tests for trends across categories were conducted using linear regression, the Jonckheere-Terpstra test, or the Cochran-Armitage trend test, as appropriate.
A kernel density plot was conducted to display the distribution of different eGFRs. Event-free survival curves for each outcome were plotted using the Kaplan–Meier method. To explore the relationship between each eGFR and outcome, restricted cubic splines, with 8 knots at eGFR of 20, 30, 45, 60, 75, 90, 105, and 115 ml/min/1.73m2, were conducted using Cox proportional hazard regression adjusted for atherosclerotic risk factors (age, sex, current smoking, SBP, DBP, diabetes, total cholesterol, HDL cholesterol, antihypertensive medication, and statin medication) for incident CVD, and additionally CVD history for all-cause mortality. Atherosclerotic risk factors were from the American Heart Association/American College of Cardiology (AHA/ACC) guidelines (24). An eGFR of 90 ml/min/1.73m2 was considered the reference. Ethnicity was not included as our participants are Black.
Cox regression was used to assess hazard ratios (HRs) and 95% confidence intervals (CIs) for incident CVD events and all-cause mortality, and different eGFRcr categories as independent variables. Two models were generated using the following stages: Model 1 adjusted for age and sex; Model 2 adjusted for atherosclerotic risk factors (age, sex, current smoking, SBP, DBP, diabetes, total and HDL cholesterol, antihypertensive, and statin medication) for incident CVD, and additionally CVD history for all-cause mortality. Harrell’s C-statistics was calculated to compare the predictive value after the addition of eGFRcr(ASR), eGFRcr(AS), eGFRcr-cys(ASR), eGFRcr-cys(AS), eGFRcys(AS) to base multivariable model which included atherosclerotic risk factors for incident CVD, and additionally CVD history for all-cause mortality. Net Reclassification Index (NRI) was calculated for the reclassification of participants from “low” to “intermediate” risk, across the threshold 7.5% 10-year risk of CVD (24). The 95% CIs were obtained from 1,000 bootstrap replicates. Analyses were performed using SAS 9.4 (SAS Institute) and the nricens package in R studio (version 4.3.2) for NRI. A 2-sided p-value of 0.05 was considered statistically significant.
Results
Demographics of characteristics
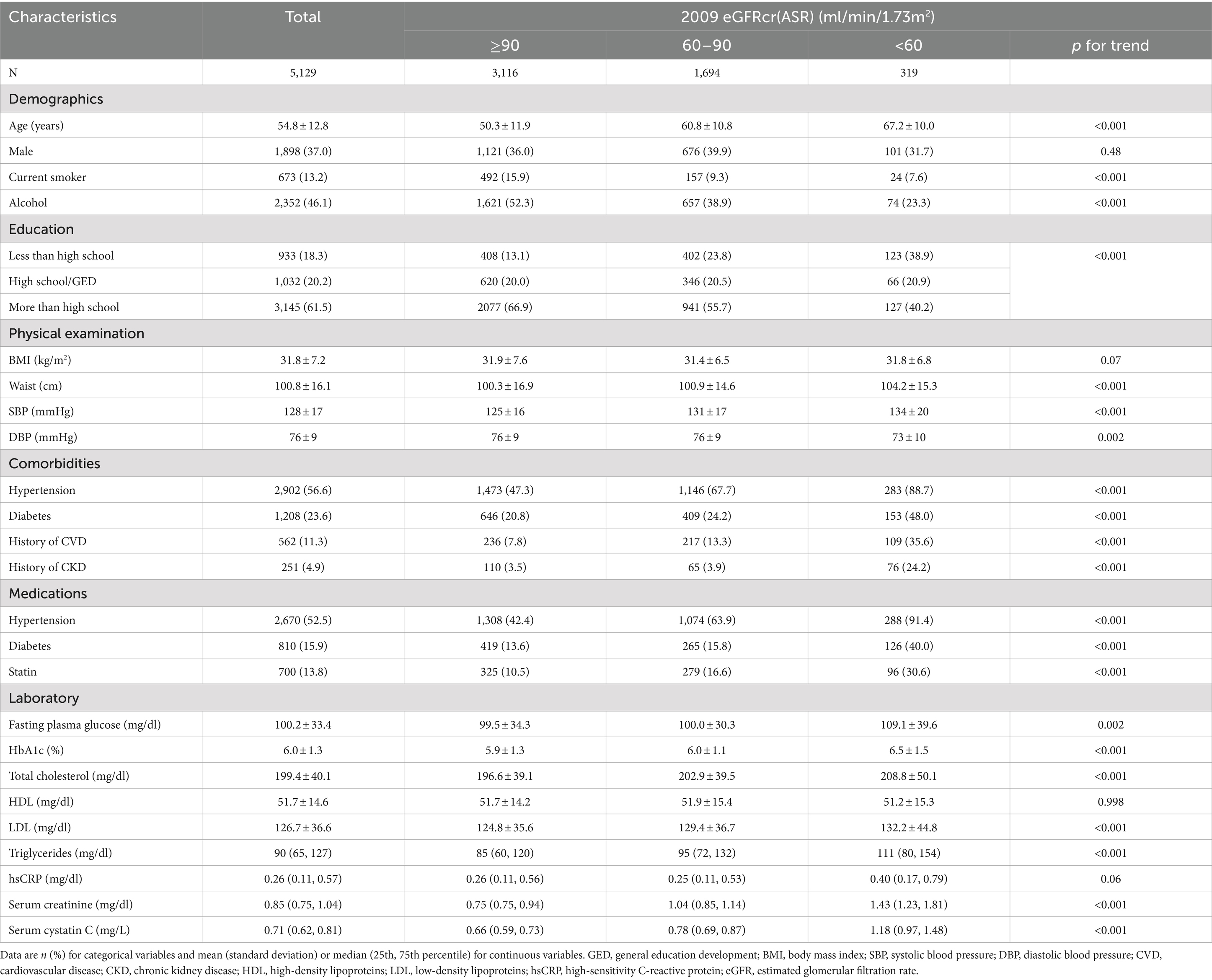
A total of 5,129 JHS participants were included in the current study presented in Table 1. The average age was 54.8 ± 12.8 years and 1898 (37.0%) were male. The details about baseline and clinical characteristics of participants were compared according to the 2009 eGFRcr(ASR) categories. Participants with lower eGFR had higher age, waist, SBP, fasting plasma glucose, HbA1c, total cholesterol, LDL, triglycerides and lower DBP (P for trend over eGFR categories <0.01). Non-current smokers, lower alcohol intake and education attainment were more common in those participants with lower eGFR (P for trend <0.001). Lower eGFR participants were more likely to have hypertension, diabetes, CVD history, CKD history, and were more likely to take statins and medications for hypertension and diabetes (P for trend <0.001).
Among our participants, eGFRcr(AS) provided lower estimates than eGFRcr(ASR), eGFRcr-cys(ASR), eGFRcr-cys(AS) and eGFRcys(AS), and resulting in a greater proportion of participants categorized as CKD. Mean eGFR ± standard deviations were 94.4 ± 22.1, 84.9 ± 19.1, 104.1 ± 22.4, 100.4 ± 20.2, 106.6 ± 21.4 ml/min/1.73 m2 of eGFRcr(ASR), eGFRcr(AS), eGFRcr-cys(ASR), eGFRcr-cys(AS), eGFRcys(AS), respectively. The proportion of participants categorized as CKD was 6.2% for eGFRcr(ASR), 9.0% for eGFRcr(AS), 4.3% for eGFRcr-cys(ASR), 4.4% for eGFRcr-cys(AS) and 3.9% for eGFRcys(AS). The distribution of eGFR values was displayed in Figure 2A and the frequency of participants in each category was shown in Figure 2B.
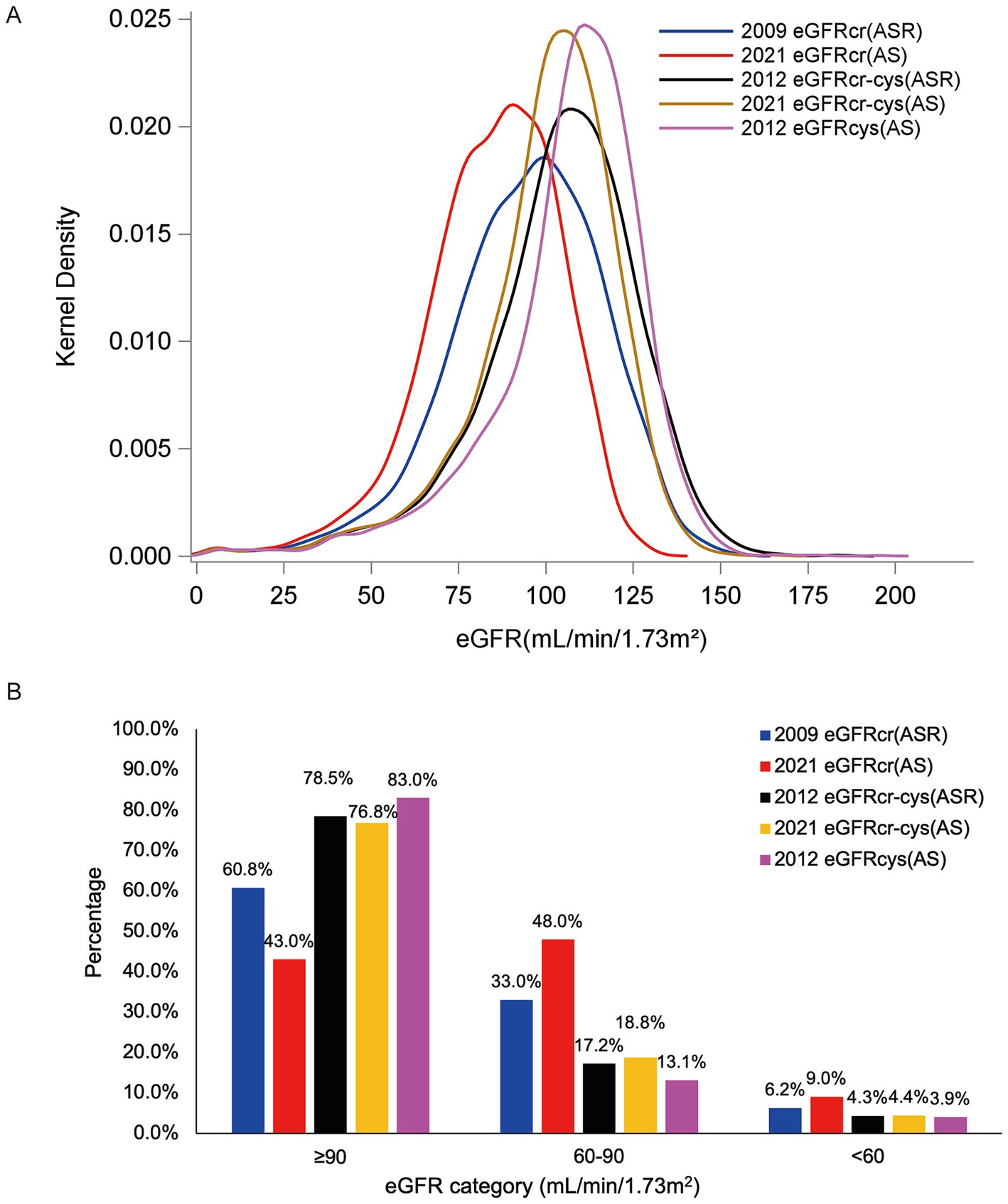
Figure 2. Distribution of eGFRs [(A) Kernel density of eGFR among our JHS participants. (B) Frequency of our participants in each eGFR category by eGFR estimating equations].
Associations between eGFR measures and incident CVD, all-cause mortality
During a median follow-up period of 13.7 years, 9.3% (411/4399) of participants had incident CVD. During a median follow-up for 14.3 years, 23.5% (1,207/5129) observed deaths were observed. The unadjusted cumulative incidence of incident CVD and all-cause mortality were shown in Figure 3, and both incidences gradually increased with decreasing eGFRs. The restricted cubic spline showed relationships between all eGFR measures and incident CVD (Figures 4A–E), which were negative and then relatively flat. The adjusted shape of the associations between eGFR measures and all-cause mortality displayed an initially negative association and then flat except for eGFRcr(ASR) and eGFRcr(AS), which increased mortality after 105 ml/min/1.73m2 (Figures 4H–J).
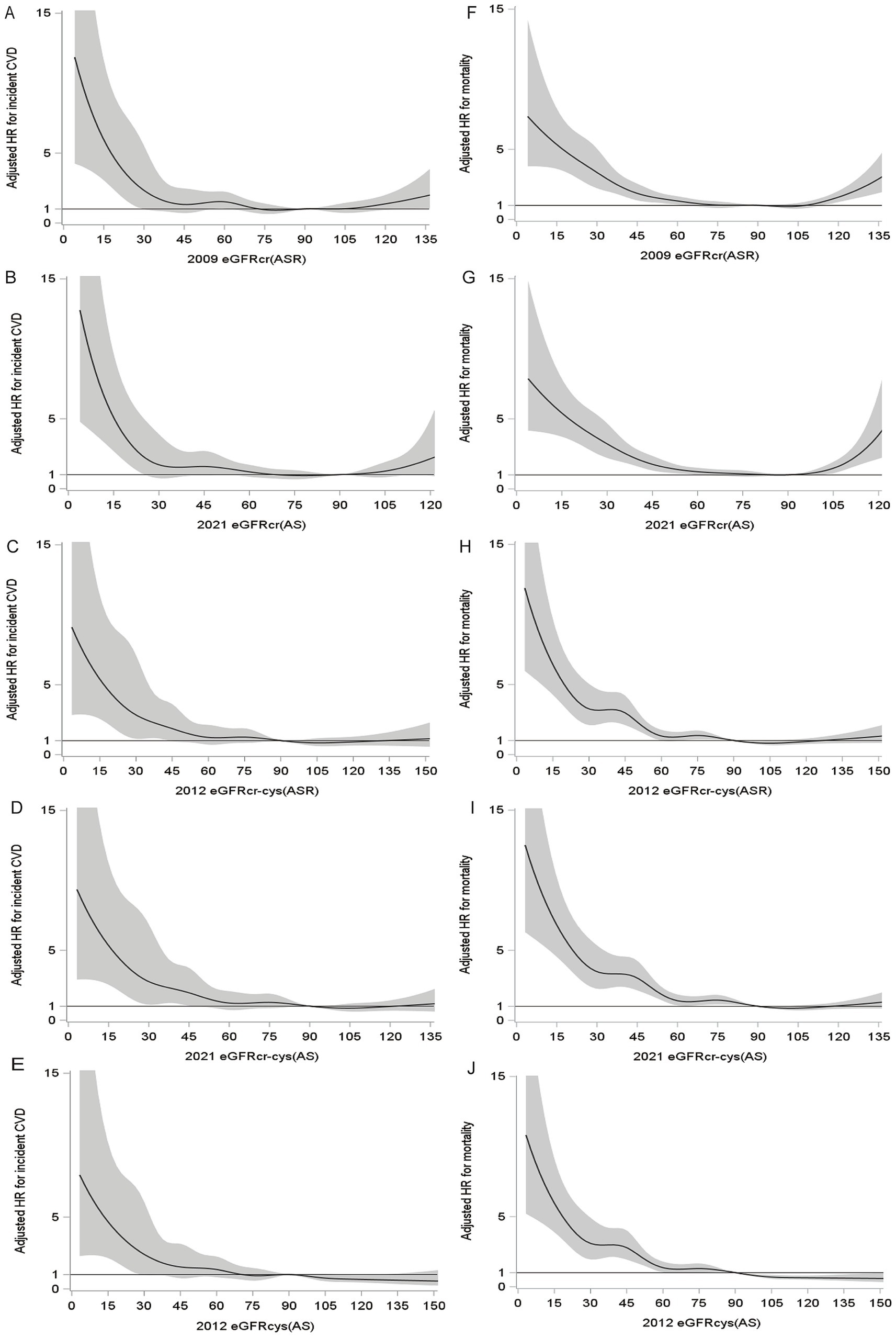
Figure 4. Fully adjusted splines of eGFR against adjusted hazard ratio (with 95% CI) for incident CVD and all-cause mortality. For incident CVD (A–E), adjusted for atherosclerotic risk factors (age, sex, smoking, diabetes, SBP, DBP, hypertension medications, statin medications, total cholesterol, HDL). For all-cause mortality (F–J), adjusted for atherosclerotic risk factors and CVD history.
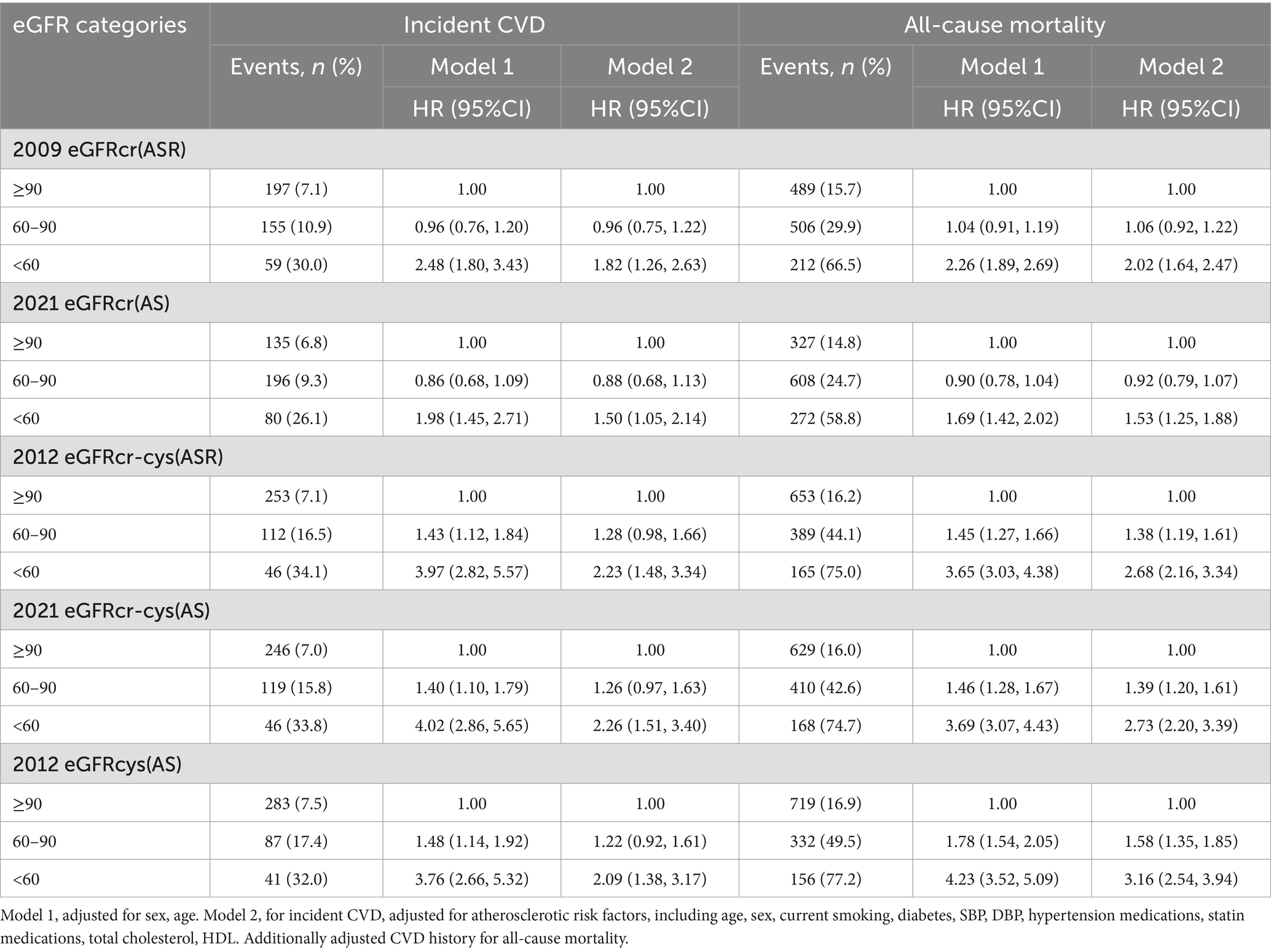
Adjusted HRs for the associations between eGFR measurements and incident CVD and all-cause mortality were consistent in that lower eGFR has been associated with higher risk (Table 2). For both outcomes, there was a trend that the HRs for eGFR measurements incorporating cystatin C were stronger than eGFRs using creatinine alone (Table 2). For incident CVD, corresponding multivariable-adjusted HRs (95% CIs) of eGFRcr-cys(ASR), eGFRcr-cys(AS) and eGFRcys(AS) were 2.23 (1.48, 3.34), 2.26 (1.51, 3.40) and 2.09 (1.38, 3.17), and HRs (95% CIs) of eGFRcr(ASR) and eGFRcr(AS) were 1.82 (1.26, 2.63) and 1.50 (1.05, 2.14), compared group of eGFR<60 with ≥90 ml/min/1.73 m2. For all-cause mortality, multivariable-adjusted HRs (95% CIs) of eGFRcr-cys(ASR), eGFRcr-cys(AS) and eGFRcys(AS) were 2.68 (2.16, 3.34), 2.73 (2.20, 3.39), 3.16 (2.54, 3.94), and HRs (95% CIs) of eGFRcr(ASR) and eGFRcr(AS) were 2.02 (1.64, 2.47) and 1.53 (1.25, 1.88), compared group of eGFR<60 with ≥90 ml/min/1.73 m2.
Prediction of incident CVD and all-cause mortality by eGFR measures
The C-statistics of atherosclerotic risk factors (age, sex, current smoking, SBP, DBP, diabetes, total cholesterol, HDL cholesterol, antihypertensive medication, and statin medication) for incident CVDwas 0.7791, and C-statistics of atherosclerotic risk factors and CVD history for all-cause mortality was 0.7811. There was a significant discrimination improvement in C-statistics for the predictive ability of incident CVD events and all-cause mortality after adding each eGFR measure to the basic model including atherosclerotic risk factors (Table 3).
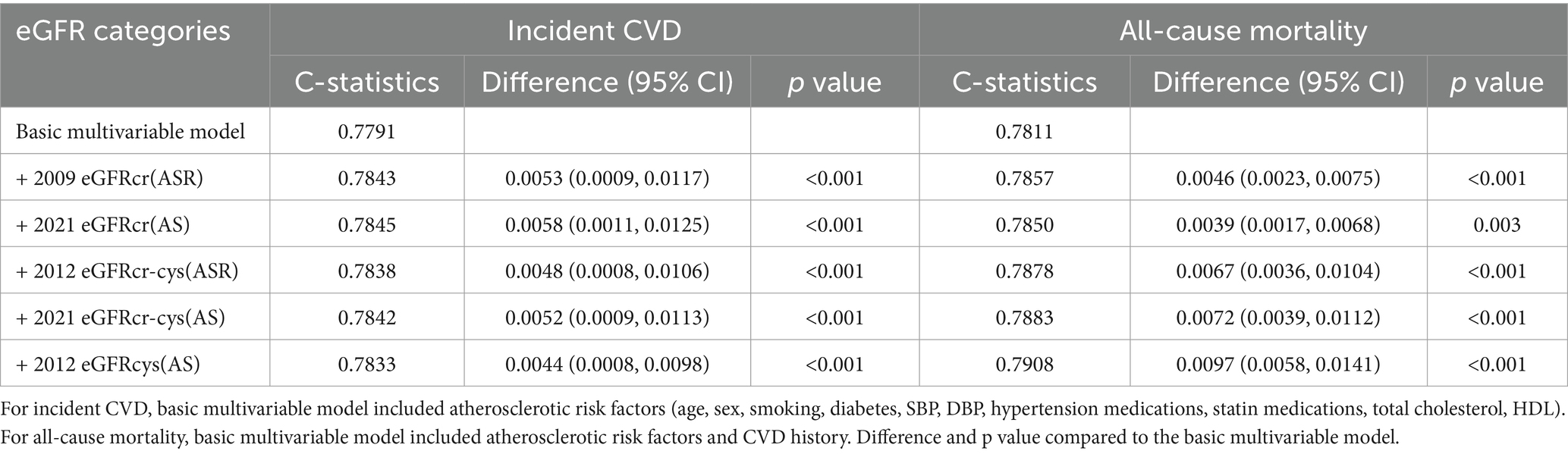
Table 3. C-statistics and differences of C-statistics for the prediction of incident CVD and all-cause mortality by 5 eGFR measures.
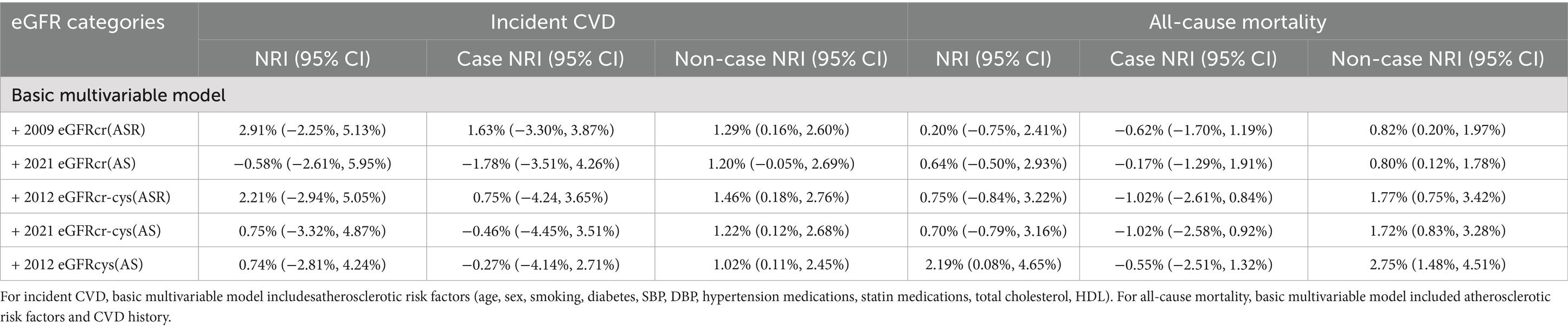
We tested the improvement of risk classification across the 7.5% 10-year risk threshold for statin therapy used in AHA/ACC guidelines (24). For incident CVD events, the addition of any eGFR measures to atherosclerotic risk factors did not improve NRI (Table 4). For mortality, race and race-free eGFRs including creatinine or both creatinine and cystatin C did not improve risk classification, but eGFRcys(AS) did (NRI: 2.19, 95%CI: 0.08, 4.65%).
Discussion
This study represents the largest prospective cohort analysis, comparing the predictive performance of five CKD-EPI eGFR equations, both with and without race adjustments, in predicting the incident CVD and all-cause mortality within a general African American community population. Results showed that the race-free eGFRcr and eGFRcr-cys were lower than the previous eGFRcr and eGFRcr-cys included a race correction term, respectively, and a similar trend was found among Black participants in CRIC (Chronic Renal Insufficiency Cohort) study (25). Lower eGFR was associated consistently with a higher risk of incident CVD events and all-cause mortality regardless of which race or race-free CKD-EPI equations were used. The eGFRs including cystatin C were strongly associated with incident CVD and all-cause mortality than the eGFRs using creatinine alone. A significant improvement in c-statistics was observed when all eGFR measures were added to the atherosclerotic risk factors model.
The new race-free eGFR equations represent a major advance towards eliminating racial biases. In the present study, 2021 eGFRcr(AS) had the lowest eGFR and classified more people to the group of less than 60 ml/min/1.73 m2 compared with the other equations. Timely care is of particular importance for CKD patients. The previous race-based eGFRcr had higher eGFR and underestimated the proportion of CKD patients in Black general population, which may lead to delayed timely care, inadequate drug dosing, and less access to dialysis and kidney transplantation. Meanwhile, the eGFRcr-cys(AS) was slightly lower than eGFRcr-cys(ASR), which also had small but potentially meaningful effects on CKD events. The comparison between eGFRcr(ASR) and eGFRcr(AS) from our study is consistent with the results from other Black population studies. In a study of 2,225 African American patients, up to one in three patients would be reclassified to a more severe CKD stage using eGFRcr(AS) instead of eGFRcr(ASR) (26). Among the 2,521 Black patients in Butt’s study, the mean eGFR reduced from 75 ± 25 to 68 ± 22 ml/min/1.73m2, and the proportion of <60 ml/min/1.73m2 increased from 29.8 to 39.1% using the eGFRcr(ASR) and eGFRcr(AS) (27). In the CREDENCE (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation) trial, eGFRcr(AS) was lower than eGFRcr(ASR) (52 ± 15 vs. 56 ± 16 ml/min/1.73 m2) in 223 Black participants (28). However, different comparison results were observed in other studies. eGFRcr(AS) is higher than eGFRcr(ASR) in Thai patients (29). eGFRcr(AS) has a higher estimation of GFR than eGFRcr(ASR) among Asian cohorts and the European population (30–32). A study indicates that among these five eGFR measures, eGFRcys(AR) has the lowest estimate in the Chinese population (33).
We have demonstrated that eGFR is independently associated with incident CVD and all-cause mortality, regardless of the equations. The adjusted shape revealed that the relationship between eGFR measures and incident CVD was monotonic. Concerning mortality, the shape exhibited an initially negative association and followed by a flat trend, except for eGFRcr(ASR) and eGFRcr(AS), which showed increased mortality after reaching 105 ml/min/1.73m2. Other studies showed a similar trend. A U-shaped association was found between eGFRcr(ASR) and mortality, while a monotonic association in eGFRcys(AS) and eGFRcr-cys(ASR) (34, 35). For CVD, an inflection point at approximately 90 ml/min/1.73m2 was found for eGFRcr(AS), below and above which there were increasing hazards (36). Compared with normal eGFR, high eGFRcys was associated with a lower risk of CVD (37), and eGFRcr(ASR) ≥105 ml/min/1.73m2 was associated with a 2-fold increased mortality (38). In the meantime, a negative linear relationship between eGFR measures and CVD events and mortality was observed in other studies (15, 39).
Our analysis showed that significant renal dysfunction (any eGFR<60 ml/min/1.73 m2) is associated with incident CVD events, supporting the published results from other studies (13, 14). However, mild renal dysfunction (60–90 ml/min/1.73 m2) did not prove to be an independent risk factor for incident CVD in our study, which is similar to other studies. The prospective Reykjavik study did not show an association between eGFR of 60–90 ml/min/1.73 m2 and risk of coronary heart disease (40). A large-scale retrospective study based on 10,909 subjects with normal to mildly reduced renal function found that the association between lower eGFR and new onset of CVD is no longer significant after adjusting other cardiovascular risk factors (41). Whereas, a different result was reported in ARIC (Atherosclerosis Risk in Communities) study (42), FHS (The Framingham Heart Study) (43), suggesting that a lower eGFR even in the normal or mildly impaired range is associated with a higher incidence of CVD. These controversial results may be related to dissimilar populations.
Keeping with other published data from large cohort studies (11, 12, 44–46), we have shown that lower eGFR was associated with a high risk of all-cause mortality. Even milder impairments in renal function (60–90 ml/min/1.73 m2) based on eGFRcr-cys(ASR) or eGFRcr-cys(AS) and eGFRcys(AS) have also been shown to increase the risk of all-cause mortality, while eGFRcr(ASR) and eGFRcr(AS) are not. Controversial findings have been reported in other studies. In the AusDiab (Austrialian Diabetes, Obesity and Lifestyle) study, eGFR of 60–90 ml/min/1.73 m2 using eGFRcr(ASR), eGFRcys(AS) and eGFRcr-cys(ASR) was not associated with all-cause mortality (47). In older adults of Good Aging in Skåne study, no significant association was observed between moderate renal dysfunction and mortality [adjusted HR (95% CI): 0.90 (0.67–1.21) for eGFRcr-cys(ASR) (>90 vs. 60–89 ml/min/1.73 m2)] (46).
The eGFRs based on both creatinine and cystatin or cystatin C alone had a higher association with risks for incident CVD and all-cause mortality than GFR estimates using creatinine alone. Compared group of eGFR<60 with ≥90 ml/min/1.73m2, multivariable-adjusted HRs of eGFRcr-cys(ASR), eGFRcr-cys(AS) and eGFRcys(AS) were mostly 2.09 or higher, HRs of eGFRcr(ASR) and eGFRcr(AS) were 1.82 and 1.50 for incident CVD, and HRs of eGFRcr-cys(ASR), eGFRcr-cys(AS) and eGFRcys(AS) were 2.68 to 3.16, and eGFRcr(ASR) and eGFRcr(AS) were 2.02 and 1.53 for all-cause mortality. This occurred in studies reported by Shlipak et al. (39) and Barr et al. (47), which compared eGFRcr(ASR), eGFRcr-cys(ASR), eGFRcys(AS). A significant improvement in C-statistics when any eGFR measures were added to a base risk factors model in this study. Only eGFRcys(AS) improved the net reclassification of all-cause mortality in our study. Previous studies have shown a similar result that eGFRCys(AS) has more predictive utility for all-cause mortality than eGFRCr(ASR) and eGFRcr-cys(ASR) (15, 36, 47, 48). In the meantime, cystatin C is considered a more sensitive marker of kidney function and is less influenced by muscle mass, age, gender and protein diet compared with creatinine (49, 50). eGFRcys(AS) indicated a more significant association with ischaemic stroke compared to eGFRCr(ASR). This effect was more pronounced in women than men. The measurement of cystatin C may enhance risk stratification for ischaemic stroke and improve clinical treatment in a general population, especially for women (51). NKF and ASN recommended nationwide efforts to widely measure and use cystatin C, especially for adults at risk for or have CKD (9). Therefore, the measure of cystatin C may be necessary and cystatin C-based eGFRcys might be more appropriate for predicting CVD and mortality in Black population.
Strengths and limitations
Our study has strengths and limitations. The JHS is a large community-based sample of African Americans, with a high prevalence of CKD and CVD. The new CKD-EPI eGFR without race was developed to address racial bias. Therefore, JHS is one of the best populations to evaluate the performance of the new eGFRs in African Americans. Furthermore, serum creatinine and cystatin C were collected at baseline and GFR can be evaluated with five CKD-EPI eGFRs in the JHS. Some limitations need to be mentioned. First, there was no available data on gold standard measured GFR, which limited our ability to validate the race and race-free eGFRs against measured GFR and make inferences on which one was most appropriate for our study. In addition, our results were obtained from an African American cohort in Mississippi and may not be generalizable to Black individuals from other communities or countries.
Conclusion
In summary, Reducing eGFR was related to a higher incidence of CVD events and mortality, and e GFRcr-cys(ASR), eGFRcr-cys(AS) and eGFRcys(AS) strengthened the association. Cystatin C-based eGFR equations might be more appropriate for predicting CVD and mortality in Black population.
Data availability statement
The datasets presented in this article are not readily available because the data are from the JHS. The JHS data are available to researchers with approved manuscript proposals. Requests to access the datasets should be directed to the JHS Committee at https://www.jacksonheartstudy.org/Research/Study-Data/Data-Access.
Ethics statement
The studies involving human participants were approved by University of Mississippi Medical Center, Jackson State University, Tugaloo College. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HW: Writing – original draft, Conceptualization, Formal analysis, Methodology, Data curation, Software. JC: Writing – review & editing, Conceptualization, Data curation, Formal analysis, Methodology, Software. HF: Conceptualization, Software, Writing – review & editing. CD: Conceptualization, Writing – review & editing, Supervision. BY: Supervision, Writing – review & editing, Conceptualization. AB: Conceptualization, Supervision, Writing – review & editing, Data curation.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The Jackson Heart Study (JHS) is supported and conducted in collaboration with Jackson State University (HHSN268201800013I), Tougaloo College (HHSN268201800014I), the Mississippi State Department of Health (HHSN268201800015I) and the University of Mississippi Medical Center (HHSN268201800010I, HHSN268201800011I, and HHSN268201800012I) contracts from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute for Minority Health and Health Disparities (NIMHD).
Acknowledgments
The authors wish to thank the staff and participants of the JHS.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1432965/full#supplementary-material
References
1. Webster, AC, Nagler, EV, Morton, RL, and Masson, P. Chronic kidney disease. Lancet. (2017) 389:1238–52. doi: 10.1016/S0140-6736(16)32064-5
2. Kovesdy, CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl. (2022) 12:7–11. doi: 10.1016/j.kisu.2021.11.003
3. Prevention CfDCa. Chronic kidney disease in the United States, 2021. US Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA (2021).
4. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Inter. (2013) 3:1–150.
5. Levey, AS, Coresh, J, Greene, T, Marsh, J, Stevens, LA, Kusek, JW, et al. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. (2007) 53:766–72. doi: 10.1373/clinchem.2006.077180
6. Levey, AS, Stevens, LA, Schmid, CH, Zhang, YL, Castro, AF 3rd, Feldman, HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
7. Inker, LA, Schmid, CH, Tighiouart, H, Eckfeldt, JH, Feldman, HI, Greene, T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. (2012) 367:20–9. doi: 10.1056/NEJMoa1114248
8. Inker, LA, Eneanya, ND, Coresh, J, Tighiouart, H, Wang, D, Sang, Y, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. (2021) 385:1737–49. doi: 10.1056/NEJMoa2102953
9. Delgado, C, Baweja, M, Crews, DC, Eneanya, ND, Gadegbeku, CA, Inker, LA, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. Am J Kidney Dis. (2022) 79:268–88.e1. doi: 10.1053/j.ajkd.2021.08.003
10. Flessner, MF, Wyatt, SB, Akylbekova, EL, Coady, S, Fulop, T, Lee, F, et al. Prevalence and awareness of CKD among African Americans: the Jackson Heart Study. Am J Kidney Dis. (2009) 53:238–47. doi: 10.1053/j.ajkd.2008.08.035
11. Matsushita, K, van der Velde, M, Astor, BC, Woodward, M, Levey, AS, de Jong, PE, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. (2010) 375:2073–81. doi: 10.1016/S0140-6736(10)60674-5
12. van der Velde, M, Matsushita, K, Coresh, J, Astor, BC, Woodward, M, Levey, A, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. (2011) 79:1341–52. doi: 10.1038/ki.2010.536
13. Coyle, M, Flaherty, G, and Jennings, C. A critical review of chronic kidney disease as a risk factor for coronary artery disease. Int J Cardiol Heart Vasc. (2021) 35:100822. doi: 10.1016/j.ijcha.2021.100822
14. Attar, A, and Sayadi, M. Effect of chronic kidney disease on cardiovascular events: an epidemiological aspect from SPRINT trial. Iran J Kidney Dis. (2019) 13:328–36.
15. Lees, JS, Welsh, CE, Celis-Morales, CA, Mackay, D, Lewsey, J, Gray, SR, et al. Glomerular filtration rate by differing measures, albuminuria and prediction of cardiovascular disease, mortality and end-stage kidney disease. Nat Med. (2019) 25:1753–60. doi: 10.1038/s41591-019-0627-8
16. Zhang, Y, Yu, Y, Zhu, J, Zhao, Q, Qiu, Y, Cui, S, et al. Association between estimated glomerular filtration rate and 10-year atherosclerotic cardiovascular disease risk among community residents in Shanghai, China. Nutr Metab Cardiovasc Dis. (2022) 32:948–56. doi: 10.1016/j.numecd.2021.11.007
17. Afkarian, M, Katz, R, Bansal, N, Correa, A, Kestenbaum, B, Himmelfarb, J, et al. Diabetes, kidney disease, and cardiovascular outcomes in the Jackson Heart Study. Clin J Am Soc Nephrol. (2016) 11:1384–91. doi: 10.2215/CJN.13111215
18. Khan, SS, Matsushita, K, Sang, Y, Ballew, SH, Grams, ME, Surapaneni, A, et al. Development and validation of the American Heart Association's PREVENT equations. Circulation. (2024) 149:430–49. doi: 10.1161/CIRCULATIONAHA.123.067626
19. Fuqua, SR, Wyatt, SB, Andrew, ME, Sarpong, DF, Henderson, FR, Cunningham, MF, et al. Recruiting African-American research participation in the Jackson Heart Study: methods, response rates, and sample description. Ethn Dis. (2005) 15:S6-18-29.
20. Taylor, HA Jr, Wilson, JG, Jones, DW, Sarpong, DF, Srinivasan, A, Garrison, RJ, et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson heart study. Ethn Dis. (2005) 15:S6-4-17.
21. Carpenter, MA, Crow, R, Steffes, M, Rock, W, Heilbraun, J, Evans, G, et al. Laboratory, reading center, and coordinating center data management methods in the Jackson heart study. Am J Med Sci. (2004) 328:131–44. doi: 10.1097/00000441-200409000-00001
22. Wang, W, Young, BA, Fulop, T, de Boer, IH, Boulware, LE, Katz, R, et al. Effects of serum creatinine calibration on estimated renal function in african americans: the Jackson heart study. Am J Med Sci. (2015) 349:379–84. doi: 10.1097/MAJ.0000000000000446
23. Keku, E, Rosamond, W, Taylor, HA Jr, Garrison, R, Wyatt, SB, Richard, M, et al. Cardiovascular disease event classification in the Jackson heart study: methods and procedures. Ethn Dis. (2005) 15:S6-62-70.
24. Goff, DC Jr, Lloyd-Jones, DM, Bennett, G, Coady, S, D'Agostino, RB Sr, Gibbons, R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. (2014) 63:2935–59. doi: 10.1016/j.jacc.2013.11.005
25. Bundy, JD, Mills, KT, Anderson, AH, Yang, W, Chen, J, and He, J. Prediction of end-stage kidney disease using estimated glomerular filtration rate with and without race: a prospective Cohort study. Ann Intern Med. (2022) 175:305–13. doi: 10.7326/M21-2928
26. Ahmed, S, Nutt, CT, Eneanya, ND, Reese, PP, Sivashanker, K, Morse, M, et al. Examining the potential impact of race multiplier utilization in estimated glomerular filtration rate calculation on African-American care outcomes. J Gen Intern Med. (2021) 36:464–71. doi: 10.1007/s11606-020-06280-5
27. Butt, JH, Adamson, C, Docherty, KF, Vaduganathan, M, Solomon, SD, Anand, IS, et al. Eligibility for pharmacological therapies in heart failure with reduced ejection fraction: implications of the new chronic kidney disease epidemiology collaboration creatinine equation for estimating glomerular filtration rate. Eur J Heart Fail. (2022) 24:861–6. doi: 10.1002/ejhf.2460
28. Charytan, DM, Yu, J, Jardine, MJ, Cannon, CP, Agarwal, R, Bakris, G, et al. Potential effects of elimination of the black race coefficient in eGFR calculations in the CREDENCE trial. Clin J Am Soc Nephrol. (2022) 17:361–73. doi: 10.2215/CJN.08980621
29. Sitaruno, S, Santimaleeworagun, W, Pattharachayakul, S, DeBacker, KC, Vattanavanit, V, Binyala, W, et al. Comparison of race-based and non-race-based equations for kidney function estimation in critically ill Thai patients for vancomycin dosing. J Clin Pharmacol. (2022) 62:1215–26. doi: 10.1002/jcph.2070
30. Betzler, BK, Sultana, R, He, F, Tham, YC, Lim, CC, Wang, YX, et al. Impact of chronic kidney disease epidemiology collaboration (CKD-EPI) GFR estimating equations on CKD prevalence and classification among Asians. Front Med (Lausanne). (2022) 9:957437. doi: 10.3389/fmed.2022.957437
31. Kim, H, Hur, M, Lee, S, Lee, GH, Moon, HW, and Yun, YM. European kidney function consortium equation vs. chronic kidney disease epidemiology collaboration (CKD-EPI) refit equations for estimating glomerular filtration rate: comparison with CKD-EPI equations in the Korean population. J Clin Med. (2022) 11:4323. doi: 10.3390/jcm11154323
32. Fu, EL, Coresh, J, Grams, ME, Clase, CM, Elinder, CG, Paik, J, et al. Removing race from the CKD-EPI equation and its impact on prognosis in a predominantly white European population. Nephrol Dial Transplant. (2023) 38:119–28. doi: 10.1093/ndt/gfac197
33. Zhu, C, Zhang, H, Shen, Z, Chen, J, Gu, Y, Lv, S, et al. Cystatin C-based estimated GFR performs best in identifying individuals with poorer survival in an unselected Chinese population: results from the China health and retirement longitudinal study (CHARLS). Clin Kidney J. (2022) 15:1322–32. doi: 10.1093/ckj/sfac070
34. Astor, BC, Levey, AS, Stevens, LA, Van Lente, F, Selvin, E, and Coresh, J. Method of glomerular filtration rate estimation affects prediction of mortality risk. J Am Soc Nephrol. (2009) 20:2214–22. doi: 10.1681/ASN.2008090980
35. Chen, S, Zhou, Y, Liang, G, Wu, W, Huang, Z, Shi, L, et al. Predictive effect of estimated glomerular filtrate rate by creatinine or cystatin C on mortality in patients with coronary artery disease. Ren Fail. (2024) 46:2327494. doi: 10.1080/0886022X.2024.2327494
36. Lees, JS, Rutherford, E, Stevens, KI, Chen, DC, Scherzer, R, Estrella, MM, et al. Assessment of cystatin C level for risk stratification in adults with chronic kidney disease. JAMA Netw Open. (2022) 5:e2238300. doi: 10.1001/jamanetworkopen.2022.38300
37. Liu, M, Ye, Z, He, P, Wu, Q, Yang, S, Zhang, Y, et al. Different cardiovascular risks associated with elevated creatinine-based eGFR and cystatin C-based eGFR. NPJ Cardiovasc Health. (2024) 1:3. doi: 10.1038/s44325-024-00005-x
38. Korhonen, PE, Kiiski, S, Kautiainen, H, Ojanen, S, and Tertti, R. The relationship of kidney function, cardiovascular morbidity, and all-cause mortality: a prospective primary care Cohort study. J Gen Intern Med. (2023) 38:1834–42. doi: 10.1007/s11606-022-07885-8
39. Shlipak, MG, Matsushita, K, Arnlov, J, Inker, LA, Katz, R, Polkinghorne, KR, et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. (2013) 369:932–43. doi: 10.1056/NEJMoa1214234
40. Di Angelantonio, E, Danesh, J, Eiriksdottir, G, and Gudnason, V. Renal function and risk of coronary heart disease in general populations: new prospective study and systematic review. PLoS Med. (2007) 4:e270. doi: 10.1371/journal.pmed.0040270
41. Yahalom, G, Kivity, S, Segev, S, Sidi, Y, and Kurnik, D. Estimated glomerular filtration rate in a population with normal to mildly reduced renal function as predictor of cardiovascular disease. Eur J Prev Cardiol. (2014) 21:941–8. doi: 10.1177/2047487313476963
42. Manjunath, G, Tighiouart, H, Ibrahim, H, MacLeod, B, Salem, DN, Griffith, JL, et al. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. (2003) 41:47–55. doi: 10.1016/s0735-1097(02)02663-3
43. Ataklte, F, Song, RJ, Upadhyay, A, Musa Yola, I, Vasan, RS, and Xanthakis, V. Association of Mildly Reduced Kidney Function with Cardiovascular Disease: the Framingham heart study. J Am Heart Assoc. (2021) 10:e020301. doi: 10.1161/JAHA.120.020301
44. Coresh, J, Turin, TC, Matsushita, K, Sang, Y, Ballew, SH, Appel, LJ, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. (2014) 311:2518–31. doi: 10.1001/jama.2014.6634
45. Kim, H, Wang, D, Chalmers, J, Jun, M, Zoungas, S, Marre, M, et al. Alternative kidney filtration markers and the risk of major macrovascular and microvascular events, and all-cause mortality in individuals with type 2 diabetes in the ADVANCE trial. J Diabetes. (2020) 12:929–41. doi: 10.1111/1753-0407.13083
46. Werner, K, Christensson, A, Legrand, H, Pihlsgård, M, Sterner, G, and Elmståhl, S. Cystatin C and creatinine-based eGFR levels and their correlation to long-term morbidity and mortality in older adults. Aging Clin Exp Res. (2019) 31:1461–9. doi: 10.1007/s40520-018-1091-x
47. Barr, EL, Reutens, A, Magliano, DJ, Wolfe, R, Lu, ZX, Sikaris, KA, et al. Cystatin C estimated glomerular filtration rate and all-cause and cardiovascular disease mortality risk in the general population: AusDiab study. Nephrology (Carlton). (2017) 22:243–50. doi: 10.1111/nep.12759
48. Kabasawa, A, Konta, T, Suzuki, N, Kamei, K, Watanabe, S, Araumi, A, et al. The association between glomerular filtration rate estimated using different equations and mortality in the Japanese community-based population: the Yamagata (Takahata) study. Dis Markers. (2018) 2018:1–7. doi: 10.1155/2018/9191832
49. Vinge, E, Lindergård, B, Nilsson-Ehle, P, and Grubb, A. Relationships among serum cystatin C, serum creatinine, lean tissue mass and glomerular filtration rate in healthy adults. Scand J Clin Lab Invest. (1999) 59:587–92. doi: 10.1080/00365519950185076
50. Tangri, N, Stevens, LA, Schmid, CH, Zhang, YL, Beck, GJ, Greene, T, et al. Changes in dietary protein intake has no effect on serum cystatin C levels independent of the glomerular filtration rate. Kidney Int. (2011) 79:471–7. doi: 10.1038/ki.2010.431
51. Lees, JS, De La Mata, NL, Sullivan, MK, Wyld, ML, Rosales, BM, Cutting, R, et al. Sex differences in associations between creatinine and cystatin C-based kidney function measures with stroke and major bleeding. Eur Stroke J. (2023) 8:756–68. doi: 10.1177/23969873231173282
Glossary
Keywords: estimate glomerular filtration rate, creatinine, cystatin C, cardiovascular disease, all-cause mortality, African Americans
Citation: Wang H, Cai J, Fan H, Diamantidis CJ, Young BA and Bidulescu A (2024) Prediction of cardiovascular events and all-cause mortality using race and race-free estimated glomerular filtration rate in African Americans: the Jackson Heart Study. Front. Med. 11:1432965. doi: 10.3389/fmed.2024.1432965
Edited by:
Gregory Braden, University of Massachusetts Medical School, United StatesReviewed by:
Byung Sik Kim, Hanyang University Guri Hospital, Republic of KoreaFlavia Prodam, University of Eastern Piedmont, Italy
Copyright © 2024 Wang, Cai, Fan, Diamantidis, Young and Bidulescu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aurelian Bidulescu, YWJpZHVsZXNAaXUuZWR1
 Haiping Wang
Haiping Wang Jiahui Cai2
Jiahui Cai2 Hao Fan
Hao Fan Aurelian Bidulescu
Aurelian Bidulescu