- 1Department of Pharmacology and Clinical Biochemistry, Ben-Gurion University of the Negev, Beer Sheva, Israel
- 2Department of Health and Exercise Science, University of Oklahoma, Norman, OK, United States
- 3Endocrinology Unit, Soroka University Medical Center, Beer Sheva, Israel
- 4Department of Family Medicine, Goldman Medical School, Ben-Gurion University of the Negev and Clalit Health Services, Beer Sheva, Israel
- 5Harold Hamm Diabetes Center, Oklahoma City, OK, United States
Ketogenic diet (KD) is a high-fat, low-carbohydrate (CHO) diet, designed to induce a metabolic state of ketosis in which the body metabolizes primarily lipids for energy production. Various forms of KD are being promoted as promising treatments for numerous health conditions from chronic headaches to weight-loss and even different forms of cancer and are becoming increasingly more popular. KD appears to be an efficacious approach for weight-loss, and maintenance, improved glycemia, cognitive function and cancer prognosis. However, there is a controversy regarding the safety of KD, and the potential health risks that might be associated with long-term exposure to KD. There is a gap between the acceptance and utilization of KD in individuals with health conditions and the criticism and negative attitudes toward KD by some clinicians. Many individuals choose to follow KD and are encouraged by the positive results they experience. Although the medical establishment does not endorse KD as a first line of treatment, clinicians need to be informed about KD, and offer support and medical supervision for patients who self-select to follow KD. This can ensure that within the boundaries of KD, patients will make good and healthy dietary choices and prevent clinical disengagement in extreme cases. To that end, there is an urgent need for good quality research to address the issues of long-term safety of KD in different clinical populations and for standardization of KD both in research and in the clinic.
1 Introduction
Ketogenic diet (KD) is a high-fat, low-carbohydrate (CHO) diet (1), in which the amount of daily CHO consumption is < 50 or even < 20 g/day—approximately 5–10% of total daily caloric intake (2–4). This is equivalent to 1–3 CHO exchange servings (e.g., 1–3 slices of bread) a day. Under fasting conditions or CHO restriction, metabolic products of lipid β-oxidation in the liver form ketone bodies [including acetoacetate and β-hydroxybutyrate (β-OHB)], which are also oxidized to produce energy. The accumulation of ketone bodies in the circulation is termed ketosis (serum β-OHB = 0.5–5 mmol/l) and represents a physiological response to low CHO availability. Various forms of KD are being promoted as promising treatments for numerous health conditions like chronic headaches (5), psychiatric diseases (6), hypertension (7), autoimmune diseases (8, 9), polycystic syndromes (10, 11), and even different forms of cancer (12). Many individuals utilize a KD for weight-loss, due to its contribution to increased appetite control, and reduced adiposity (13), and KDs are increasingly becoming more popular (14).
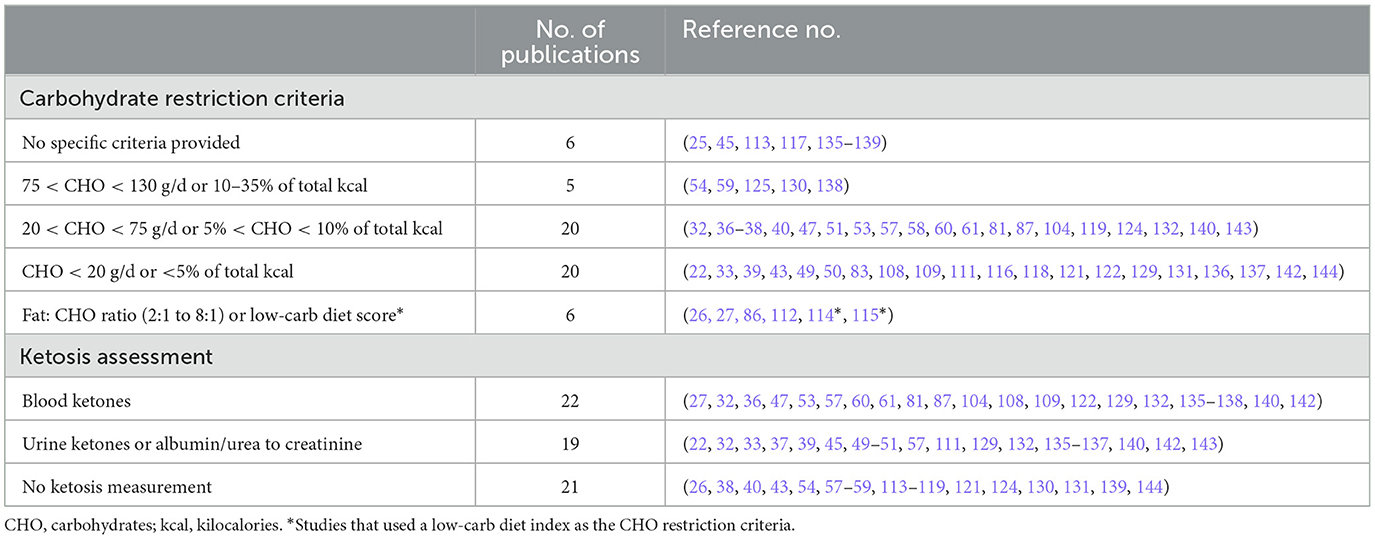
Nevertheless, there is a profound controversy regarding the safety of KD, and the potential health risks that might be associated with long-term exposure to KD. For example, one concern is the increased risk for cardiovascular disease (CVD) from chronically elevated levels of circulating lipids and lipoproteins—particularly LDL (15). However, conflicting evidence show both increases and decreases in LDL cholesterol following KD and several research findings suggest that KD may in fact be protective against CVD (see Potential Risks below). One of the contributing factors to the conflicting research evidence is the lack of standardization in the application of KD and the verification of metabolic ketosis in study participants. Some studies do not provide any criteria for KD while others use net carbohydrate content or percent of total caloric intake to classify KD and similarly, some studies do not evaluate metabolic ketosis while others measure ketone in the blood or urine or measure the ratio between albumin or urea to creatinine. Please refer to Table 1 for a summary of carbohydrate restriction criteria and ketosis assessment in all the references with human participants used in this review.
This review is written from a clinician perspective and is aimed to introduce KD to primary care practitioners and to summarize the benefits and challenges of KD. This is not meant to be a comprehensive review, and an in-depth description of the physiology of KD is beyond the scope of this paper. In this review, we will explore some of the different ways in which KD is being used in the treatment of several medical conditions, while addressing some potential risks of KD in clinical populations. We focus on pediatric epilepsy, weight-loss, diabetes management, neurodegenerative diseases and cancer.
2 Pediatric epilepsy
Epilepsy is characterized by recurrent seizures—brief episodes of involuntary movements, caused by excessive electrical activation in different neurons of the brain. Current prevalence is estimated to be 760 per 100,000 people worldwide, making it one of the most common neurological diseases globally (16). Although different dietary interventions were used to treat seizures since 500 BC, KD was introduced in the early 1920′s, as a novel way to treat pediatric epilepsy (17). Some mechanisms were suggested for the beneficial effects on epileptic seizures, from synaptic protection through activation of KATP channels (18), to anti-inflammatory (19), and antioxidant effects that were associated with ketosis (20, 21). The introduction of modern anti-epileptic drugs in the late 1930′s, resulted in near complete abandonment of KD as treatment for epilepsy. Moreover, the difficulty in meeting the strict dietary limitations associated with KD and as a result failing to maintain a proper state of ketosis made this treatment regime not sustainable for many, and also played a role in its neglect (17). Even though later work demonstrated that less drastic low-CHO diets also presented beneficial seizure-moderating effects (22), anticonvulsant drugs eventually replaced most of the dietary interventions for epilepsy (17).
Nowadays an estimated 20–30% of epileptic patients experience drug-resistance and do not benefit from the available antiepileptic medications (23, 24). For such patients, KD is still a viable and successful treatment option (25). The effects of KD on epilepsy appear to be age dependent, as several studies in drug-resistant pediatric epilepsy report higher rates of seizure cessation in infants compared to older kids. Although KD shows great promise, resulting in complete elimination of seizures in approximately one third of infants who were < 1.5 years old at initiation of KD, these rates were much lower in children who were >1.5 years old at initiation of KD (26). Similar results were reported when KD was compared to the less strict modified Atkins diet (MAD). KD was more favorable in seizure reduction in infants with refractory epilepsy under 2 years of age, these differences were not replicated in children 2–6 years and 6–18 years old. The proportion of subjects with >50% reduction in seizure prevalence at 6 months was 59, 38, and 22% vs. 45, 41, and 19% in KD vs. MAD in infants < 2, 2–6, and 6–18 years old, respectively (27). Other studies also report that the rates of seizure reduction in children with epilepsy on KD were not fully replicated in adults, and that the effects of KD intervention in epileptic adults are less conclusive (25).
Although some potential adverse effects might exist in long-term KD in young children, like bone fractures, kidney stones and delayed growth, these were reported in only one study and the research evidence is not strong (2). KD is still an efficacious treatment option, recommended by physicians in cases of medication-resistant epilepsy (28). Current (2018) guidelines of the International Ketogenic Diet Study Group, recommend to strongly consider KD in children who failed 2 antiseizure drugs, and that the specific KD used should be individualized for the child and their family—while being supported and guided by a KD team, including a neurologist and a nutritionist (2). The KD team should see the children every 3 months in the first year of KD initiation and every 6 months thereafter. In addition, it is recommended that children on a KD will be supplemented with multivitamins and calcium to prevent potential deficiencies. The group also states that discontinuation of KD is recommended after 3 months if not efficacious in reducing seizures, but if seizure control is nearly complete (>90% reduction), and no side effects are apparent, KD can be practiced for several years. In most cases KD is terminated after 2 years of initiation (2). Notably, very similar recommendations were made more recently (2023) by the Italian League Against Epilepsy Dietary Therapy Study Group (29).
3 Weight-loss
Obesity is a state of excess adipose tissue that adversely affects health. Clinically, obesity is determined as a body mass index (BMI) ≥ 30 kg/m2. The prevalence of adult obesity in the USA is 41.9% while 73.6% of Americans are overweight (BMI > 25), and rates are at a constant rise also in children (30).
The etiology of obesity is multifactorial, but excessive CHO consumption—particularly highly processed CHO is a major cause for obesity and its comorbidities (31). CHO comprises ~55% of the macronutrients in the Standard American Diet (SAD) (14). KD is very effective in inducing weight-loss, as early as 2 weeks (32) and even when compared to some weight-loss medications like Orlistat (33). This is attributed at least in part, to the low-CHO content which increases lipolysis and lipid oxidation and reduces adipose tissue mass (34). Many individuals living with obesity struggle with weight-loss and the promise of KD makes it very popular for weight-loss—even in individuals who are not clinically categorized as obese (35).
Several measures of adiposity were improved following 10 weeks of KD, compared to SAD in overweight individuals (36). Similarly, several studies report that 6 months of KD resulted in a significant weight-loss, in a quasi-experimental design (37) as well as in randomized controlled trials, when compared to a low-calorie, low-fat diet (38, 39). Moreover, the weight-loss and improved body composition are also associated with reduced blood glucose and lipids (38). Additionally, compared to low-fat control diet, KD also resulted in increased resting energy expenditure and thermic effect of feeding following weight-loss, which also makes KD a good strategy for weight maintenance (40).
These findings suggest that KD should be recommended as a potent and safe strategy for weight-loss and improved metabolism in individuals living with obesity. However, some publications suggest that these beneficial effects might not persist in the long term (>24 months). For example, a meta-analysis of 13 articles analyzing the effect of 12 months of KD in obesity concluded that although KD is a beneficial tool for weight-loss, the rate of weight-loss was lower during the 7–12 months compared to the first 6 months of the diet (41). Moreover, KD may result in a greater loss of muscle mass compared to low-fat diets (42). A comprehensive study testing changes in metabolic rate (RMR) and body composition found that 4 weeks of KD resulted in weight-loss and a slight increase in RMR, but lean mass declines were greater compared to high-CHO diet (32). Conversely, 52 weeks of calorie-restricted KD did not impair muscle strength or physical function and had similar changes to body composition compared to isocaloric high-fat diet (43). The deleterious effects of KD on muscle mass are yet to be corroborated, but the proposed mechanisms might be a combination of lower insulin and higher corticosterone in the blood, and decreased secretion of insulin-like growth factor (IGF-1) and inhibition of muscle protein synthesis (42). Maintaining adequate protein content in the KD might help to preserve muscle mass during weight-loss, but more research is needed to fully appreciate the effects of KD on muscle mass and protein synthetic activity, especially when physical activity is incorporated as a complimentary weight-loss strategy (42).
In summary, KD is a very efficacious approach for weight-loss, and maintenance, with some reported adverse effects, however with no strong evidence to determine that these adverse effects, if indeed truly exist, outweigh the potential benefits of KD. Additionally, although long-term use of KD (>1 year) for weight-loss was successful, it did not result in superior weight-loss rates compared to other dietary approaches.
4 Diabetes management
Diabetes affects millions of people worldwide and causes various health complications including heart disease, blindness, renal failure, and peripheral nerve damage. Type 1 diabetes (T1D) is characterized by a complete insulin deficiency and required exogenous insulin administration. Type 2 Diabetes (T2D) is typically characterized by insulin resistance, accompanied by hyperinsulinemia and hyperlipidemia and is highly associated with adiposity. There is no cure for diabetes (T1D or T2D), and current clinical goals are successful diabetes management using diet, physical activity and medications, aimed at controlling glycemia (HbA1C < 7%) (44). KD seems to be beneficial for controlling blood glucose primarily due to the restricted CHO and reliance on ketones for energy production. KD has also been found to decrease circulating markers of systemic inflammation, which can contribute to the improved glycemia (45). In fact, CHO restriction as a form of diabetes treatment is not a new concept and was the only available treatment in the pre-insulin era. In his textbook from 1892, William Osler describes a dietary treatment for diabetic patients, prescribing a diet with only 3% CHO but high in fat (65%)—very similar to modern day KD (46). The benefits of KD for individuals with T2D also include increased lipolysis, significant weight-loss and reduction of blood triglycerides (TG), and together with other changes these result in decreased insulin resistance (47). However, there is a scarcity of evidence as to the long-term efficacy of KD in maintaining improved glycemia in diabetes or whether the potential risks associated with KD might outweigh its potential metabolic benefits (48).
4.1 Type 2 diabetes
Several short-term studies (< 24 weeks) in individuals with T2D consistently show significant weight-loss (6–11% decrease), reduced HbA1C (16–17% reduction) and high rates of cessation of diabetes medications (80–95% of participants) following KD (49, 50), even if KD was administered as an online intervention (51); This is quite astonishing. However, there is limited research evidence that these beneficial outcomes persist in long-term KD treatment. For example, a meta-analysis of 36 trials (n = 2,161) comparing KD to low-fat diet reported that while HbA1C levels decreased more with KD in the short-term (< 12 months), by 1 year the mean difference between the diets was reduced by ~75% (52). Although other studies report significant weight-loss and improved glycemic control (decreased HbA1c and cessation of diabetes medications) following 12 months of KD (53), in their meta-analysis van Zuuren et al. report that difference in these beneficial effects between the diet groups completely disappeared at 2 years (52). One of the suggested reasons for the diminished effects of KD with time is the difficulty in long-term adherence to KD (52, 54), however adherence was not evaluated in this analysis (52).
An interesting alternative to KD, that is based on the metabolic principles of CHO-restriction was suggested by Chang et al. (55), using a very-low-CHO high fat breakfast with moderate CHO consumption during the rest of the day. The authors reported decreases in overall postprandial glycemia, glycemic variability and over-all hunger sensation following this ketogenic breakfast approach in 23 adults with T2D. This strategy might be sufficient to promote the metabolic benefits of KD in individuals with T2D but without the potential long-term risks of KD—but more research is required before this can be determined.
4.2 Type 1 diabetes
The use of KD in T1D as a complimentary strategy to insulin treatment is gaining popularity since optimal glycemic control (HbA1C < 7%) is only achieved by 15.8% of individuals with T1D and that dietary CHO has been identified as a major cause for glycemic excursions (56). Conversely, an observational study, albeit smaller in scale (n = 11 patients with T1D), on self-selected long-term KD (2.6 ± 3.3 years), showed remarkable HBA1c levels (5.3 ± 0.4%) and long duration of euglycemic time (74 ± 20% of the time). Nevertheless, 0.9 daily episodes of hypoglycemia were noted (3.6% of the time with blood glucose < 3.0 mmol/l), and dyslipidemia was prominent, as total cholesterol, LDL, and total-cholesterol/HDL-cholesterol ratio were above the recommended range in over 60% of participants. Notably, HDL levels remained within the recommended range (57). Similarly, an online survey of over 300 adults and children with T1D who followed KD for ~2 years on average, reported a mean HbA1C = 5.67 ± 0.66% with negligible number of adverse events (58). This again, is astonishing, considering the large sample size and that this HbA1C value is similar to that of individuals with no diabetes.
The benefits of restricting CHO in individuals with T1D are apparent in several other studies, showing stable 24-h glycemia, a blunted postprandial glucose response and lower insulin dosing (59, 60). A study using continuous glucose monitors in individuals with T1D, treated with an insulin pump reported that only 1 week of KD resulted in increased time in glucose target range (TIR) and decreased time below target range (TBR) and decreased glucose variability (61). However, a more moderate restriction of CHO consumption might be enough to achieve beneficial metabolic outcomes in individuals with T1D. Krebs et al. (62), utilized a moderate CHO-restriction strategy, with CHO consumption restricted only to < 75 g/day and not to the typical < 50 g/day commonly used in KD. This resulted in improved glycemic control and reduced insulin requirements without a significant weight-loss following 12 weeks on that diet. Additionally, no significant changes in blood glucose variability or blood lipid profile were found (62). The long-term effects of such approach, and how it compares to low-fat diet or KD remains to be examined.
When reviewing current literature, even with the increasing reports of beneficial outcomes of KD in individuals with T1D, there is still a considerable lack of adequate evidence, particularly long-term randomized controlled trials, to support a generalized clinical recommendation to use KD in individuals with T1D. In a recent review by Dr. David Ludwig—a longtime advocate for KD, several very compelling arguments were made in favor of using KD in T1D, primarily addressing the contribution of CHO load to glycemic excursions and the potential for KD to improve glycemia, minimize daily insulin requirements, and reduce glucose variability. Interestingly, the authors also describe some of the issues with current evidence and mention small sample size and selection bias, short term interventions, and flaws in dietary assessment in the studies they analyzed in that review (63).
Currently, the popularity of KD in individuals with T1D is increasing, particularly among highly educated individuals, in a middle to upper socioeconomic status. These individuals are highly motivated and involved in their medical care and have a good understanding of diabetes physiology and technology (56). Current clinical recommendations do not advocate for KD (64) and in the case of children and adolescents with T1D even recommend against it (65). This can lead to conflicts between the patients and the clinical team, which might be perceived by the patients as dismissive and condescending and this might lead to miscommunication and even clinical disengagement of the patients (56).
We believe that the metabolic benefits of KD and its efficacy in weight-loss and improving glycemia, particularly as they relate to individuals with diabetes cannot be ignored. Encouraged by the positive effects on glycemia and body weight, more patients adopt KD as a dietary approach. This means that clinicians cannot and should not dismiss KD as a dietary approach to treat diabetes. However, since some of the published work has different methodological issues that affect the robustness of the current evidence, they highlight the urgent and profound need for well-designed, adequately powered, long-term randomized controlled trials to assess its safety, potential risks, and health outcomes when applied as a clinically mandated and clinically supported treatment. Such research might help to make KD more acceptable in the medical establishment as an efficacious dietary approach for individuals living with obesity and diabetes.
5 Cancer
Cancer is the second leading cause of death in the United States, responsible for 22% of annual fatalities (66). Carcinogenesis results from mutations disrupting genes associated with cellular growth processes (67, 68), often caused by exposure to environmental mutagens combined with deficiencies in DNA repair mechanisms (69). Most tumor cells display a shift to aerobic glycolysis as their main energy pathway (70). Since ketone metabolism inhibits glycolysis, the prime bioenergetic pathway for many tumors (71, 72), cancer cells are vulnerable to CHO-restriction approaches such as KD (73).
In recent years, much focus has been directed to recognizing obesity as a major risk factor for many cancer types (74), prompting increased focus on lifestyle approaches. The potent weight-loss effect of KD makes it an attractive approach to reduce cancer risk by decreasing obesity. Consequently, several clinical investigations conclude that appropriate nutrition can significantly improve outcomes as a complementary therapy (75).
One proposed mechanism linking obesity and cancer involves elevated circulating insulin and insulin-like growth factor 1 (IGF-1) levels, which over-activate the IGF system (76). This may promote tumor proliferation and progression by interfering with mitogenic signaling pathways (12, 77–80). KD reduces blood glucose and lowers insulin and IGF-1 activity (81) and provides a metabolic strategy to potentially slow cancer cell expansion. KD also attenuate peritumoral inflammation and edema, key factors enabling invasion and metastasis (9). Hence, KD shows promising translational potential for integrated cancer treatment (82, 83).
It appears that KD may have a beneficial impact particularly in tumors of the central nervous system. Evidently, the most substantial evidence for KD suppressing tumor growth and progression comes from studies on glioblastoma (84). This is achieved by counteracting their metabolism, reducing inflammation, modulating pathological gene transcription, and influencing the tumor microenvironment (85).
Evidently, a recent meta-analysis showed that KD resulted in a significant reduction in tumor weight and volume as well as prolonged survival time in rodents (86). Similar results were reported in humans, showing a significant reduction in tumor growth and size (87–89), and improved survival (90).
In the longest study to date, that examined the clinical efficacy of KD in women with advanced breast cancer over 10-year, 12 months of KD resulted in markedly improved outcomes compared to those who did not use KD (83). However, a study in rats found that long-term KD led to enhanced growth of renal tumors, attributed to increased growth hormone and fluctuations in IGF-1 levels (91). This highlights the need for further research into the long-term impacts of KD on cancer development and progression across different organ systems.
While KDs show promise as an adjuvant cancer therapy, they may cause adverse effects in some patients. Multiple studies have reported fatigue, muscle cramps, hypotension, constipation, and unintentional weight-loss in patients on KD (5, 85). Current evidence suggests KD may work best in combination with conventional or targeted treatments, and may have the greatest benefit when initiated early in cancer progression (92). KD may improve outcomes and quality of life for cancer patients, but more research is needed to test KD efficacy in treating a wide variety of tumors, evaluate KD risk in cancer patients and define clinical guideline for KD use in cancer treatment for maximum therapeutic gain.
6 Neurodegenerative diseases
Neurodegeneration is a common characteristic of numerous debilitating, incurable diseases, and the prevalence of these conditions is increasing rapidly (93). A broad range of neurodegenerative disorders impacts the central nervous system (CNS), disrupting sensory, motor, and cognitive functions such as vision, hearing, movement, speech and language, and memory (94). Here, we will focus on the two most common neurodegenerative diseases -Dementia and Parkinson's Disease.
6.1 Dementia
Dementia is a general term for a decline in cognitive ability, severe enough to interfere with daily function. Alzheimer's disease (AD) is the most common type of dementia, accounting for at least two-thirds of dementia cases in people 65 and older (95). Despite extensive efforts toward prevention and remediation, dementia remains an urgent public health issue, affecting over 50 million people worldwide (96). The pathogenesis of AD involves brain anatomic changes, including impaired neuronal glucose metabolism and accumulations of amyloid-β plaques and neurofibrillary tangles (97). Impaired glucose metabolism and insulin resistance have been strongly associated with progressive cognitive deficiency, therefore, the beneficial effects of KD on glycemia and insulin resistance might be protective against AD progression (98–100).
Several studies in animal models of AD showed a positive effect of KD on age-related cognitive decline (101, 102). In humans, a pilot study in older adults at risk for AD showed that a modified Mediterranean-KD was well-tolerated and was associated with improved outcomes of AD compared to the American-Heart-Association diet (103). Moreover, KD also resulted in improved daily function and quality of life in older adults with AD (104, 105).
6.2 Parkinson's disease
Parkinson's disease (PD) is another prevalent neurodegenerative disorder, characterized by gradual degeneration of dopamine-producing neurons. Over 1% of people over 60 are impacted by PD. The neuronal damage leads to progressive motor dysfunction and cognitive difficulties (106, 107). People with PD have impaired neuronal glucose metabolism so reliance on ketone metabolism during KD may normalize neuronal metabolism and slow the deterioration in dopamine synthesis (106, 108). Evidence that KD significantly improved motor functions in PD exist from both animal models (107) and humans (108). Additionally, a significant improvement in Parkinson Anxiety Scale scores was also reported (109). KD in patients with PD was found to be both feasible and safe, and resulted in notable improvements in both motor symptoms (tremors, rigidity, balance) and non-motor symptoms (sleep, mood, cognition) (107–109).
Taken together, these studies suggest KD may be a practical complementary therapy that can enhance functional abilities and quality of life for those living with PD, possibly by optimizing neuronal energy metabolism or other protective mechanisms.
7 Potential risks
KD is not suitable for everyone, and although data regarding long-term complication is limited, some non-life-threating adverse effects, such as constipation or diarrhea, headaches, halitosis, muscle cramps, general weakness and micronutrients deficiencies were mentioned (110–112). Several studies reported increased all-cause mortality risk in individuals on a KD diet (113–115), but in the same cohort, mortality risk was comparable in individuals on a high-CHO diet (114). Interestingly, vegetable-based KD was associated with lower all-cause mortality risk compared to both high-CHO diet and animal-based KD (114, 115). One of the major concerns of KD, attributable to the high animal fat consumption, is its effect on the lipid profile and a possible increase of CVD risk over time. Evidently, Goldberg et al., reported a case series in which five patients on KD demonstrated extreme hypercholesterolemia (three of five patients with cholesterol >500 mg/dl) (116). Similarly, another case series reported three patients with a marked acute elevation of total and LDL-cholesterol in otherwise healthy patients on KD (117). Interestingly, cholesterol levels were lowered in all patients who stopped KD (116, 117) or remained on KD but initiated lipid-lowering medication treatment (116). Similar evidence of KD resulting in hyperlipidemia exist from randomized controlled studies (118, 119), and meta-analyses (41, 120), but other studies found minimal change in lipids (121) or even improved lipid profiles following KD in overweight and obese individuals (37–39).
A comprehensive study that examined the effects of KD on markers of CVD in individuals with T2D concluded that 2 years of KD has beneficial effects on lipidemia and did not increase CVD risk (122). The authors found that in addition to attenuation of atherosclerotic progression (measured as central intima-media thickness), there was a decrease in small LDL particles (which are associated with diabetic dyslipidemia and increased CVD risk), and an increase in the less deleterious larger LDL particles (which resulted in the increase in total LDL cholesterol) (122). Interestingly, meta-analyses summarizing intervention studies ranging from 6 to 24 months, report elevated LDL following KD, but an increase in HDL was also reported in these studies (41, 120, 123), which might offset the CVD risk.
The concept of Lean Mass Hyper-Responder (LMHR) was introduced in 2017, describing people who are consuming KD, with lean BMI (< 25 kg/m2), very high LDL (≥200 mg/dl) along with elevated HDL (≥80 mg/dl), and lower TG ( ≤ 70 mg/dl) (124). This phenotype of elevated LDL cholesterol in the absence of other atherosclerotic CVD risk factors (such as obesity, low HDL, high TG) was verified in several publications (124, 125) and might even be relevant in individuals with type 1 diabetes [see Table 1 in (57)]. LMHR phenotype has recently been shown not to be associated with increased coronary artery plaque (126). Budoff et al., compared markers of coronary plaque (Coronary artery calcium and coronary computed tomography angiography) between a sample of 80 individuals consuming KD for 4.7 ± 2.8 years and a matched cohort from the Miami Heart Study [MiHeart, a prospective study of subclinical CVD risk factors in asymptomatic adults (127)]. Budoff et al., report that while the participants on KD had elevated mean LDL (272 ± 91 vs. 123± 38 mg/dl in the KD vs. MiHeart, respectively), they also had elevated HDL (90 ± 20 mg/dl), and lower TG (64 ± 23 mg/dl), compared to HDL 63 ± 19 mg/dl and TG 96 ± 45 mg/dl in the MiHeart cohort. Despite this observation, measures of coronary plaque were not different between the groups which suggests that while on KD, high levels of LDL together with elevated HDL and low TG do not result in increased atherosclerotic CVD risk (126). Although Budoff et al., provide quite convincing evidence to support this conclusion, they also acknowledge some limitations of their work including relatively low sample size, a cross sectional design and not profiling LDL particle size distribution (126). Additionally, they (126), and others (128) warrant caution, recommend personalized CVD risk assessment and call for more research to test the effects of increased LDL in the absence of other CVD risk factors on atherosclerotic CVD progression.
Using flow mediated dilation (FMD, a measure of endothelial function) to evaluate CVD risk yielded conflicting results. Compared to high-CHO diet, 8 weeks of KD did not impair FMD and also resulted in improvements in several blood markers of CVD risk (129). Similarly, while in a 2016 study of 115 patients living with obesity and T2D, after consuming an energy-restricted-high-carbohydrate (53% of energy) or isocaloric KD (14% carbohydrate, < 50 g/day) for 52 weeks (~13 months), Wycherley et al. found no difference in FMD between diet groups (130); In an earlier study (2010), they reported that both diets resulted in improvements in pulse wave velocity (a measure of arterial function), and circulating markers of endothelial function but the KD resulted in impaired FMD (131). Notably, in both studies, the authors reported similar glycemic improvements and weight-loss between the two isocaloric diet groups (130, 131). Interestingly, a similar study using the same design (52 weeks of energy restricted isocaloric diets) reported that although the energy-restricted KD resulted in similar weight-loss and glycemic control as the isocaloric high-carbohydrate diet, it resulted in improved lipid profile (132).
Together, these findings suggest that in most individuals, KD is not a major CVD risk factor while in others, which we currently cannot predict, KD might be associated with increased CVD risk. It is important to acknowledge that by inducing significant weight-loss, KD might in fact reduce CVD risk, since obesity is recognized as a major CVD risk factor (133). Changes in lipidemia are common in KD and are dependent upon BMI and metabolic health. While in individuals with obesity or type 2 diabetes KD does not invoke hypercholesterolemia (125, 134), increased LDL and total cholesterol are more common in lean individuals on KD. However, since a concurrent decrease in the TG/HDL ratio is also observed, this increase is not associated with elevated atherosclerotic CVD risk, as evaluated in individuals following approximately 5 years on KD (126). Other possible reasons for the discrepancy in lipidemic responses and the degree of vascular function in response to KD might be related to study design and study duration, however, important factors related to diet composition and total caloric content might also play an important role. These include the total amount of CHO and the degree of ketosis, the source and quality of dietary fats (e.g., margarine vs. bacon vs. olive oil and salmon) and the inclusion of fruit and vegetables. In most studies—these factors are not evaluated or not reported (see Table 1). It seems that genetic predisposition for hyperlipidemia might play a lesser role (116), but it is yet to be systematically evaluated in a long-term study. Since its growing popularity and widespread use, particularly for weight-loss, it is imperative to understand the long-term effects of a KD on blood lipids and consequently its effects on CVD risk.
In addition to the CVD risk concern, when considering KD in individuals with T1D, some believe a potential concern is diabetic ketoacidosis (DKA), an acute metabolic complication which result in excessive ketone accumulation and acidosis (63, 135). A few case reports of DKA in the background of KD were published (136–138), but these reports include starvation (136), undiagnosed T1D (137), or misdiagnosed diabetes (138), which were likely the cause for the DKA, rather than KD. Moreover, no well-designed randomized controlled studies were found to suggest this is a widespread phenomenon and it seems that KD is not associated with DKA in individuals with T1D. Evidently, when measuring circulating β-OHB in a group of individuals with T1D, on a voluntary KD, Ozoran et al., found mean (±SD) β-OHB levels considerably lower (1.2 ± 0.14 mmol/l) than the DKA threshold of >5 mmol/l (139). Notably, several reports describe the increased risk for euglycemic DKA in individuals with diabetes treated with sodium-glucose cotransporter-2 (SGLT-2) inhibitors and KD (140, 141). Concurrent SGLT-2 treatment and KD should be avoided.
Another concern of KD in individuals with T1D is related to the high intake of protein, which may increase patients' risk for renal dysfunction (142). However, 12 months of KD did not have any adverse effects on renal function in individuals living with obesity (143) or T2D (144). KD was also well-tolerated and safe, with no change in renal outcomes in a group of individuals living with T2D with mild diabetic kidney disease, albeit the intervention was very short (~4 months) (145).
Based on the current evidence it seems that for most individuals with T1D, KD do not hold significant risks that outweigh the potential benefits, and it is important to acknowledge that the magnitude of benefits of KD as an adjunctive therapy in T1D cannot be overlooked. Moreover, the significant effects of KD on weight-loss and glycemia, and the relative low risk make KD an attractive therapeutic strategy, at least for periods of under 1 year. It is important to consider however, that a more moderate CHO restriction (< 75 g/day) also resulted in improved measures of glycemic control (62) and might be more appropriate for patients who cannot follow KD for various reasons.
8 Conclusions
KD seems to hold several benefits that can improve outcome in various clinical populations. It is becoming more and more popular but is still not endorsed by the medical establishment. In some cases, there is a gap between the widespread acceptance of KD and its utilization in individuals with health conditions and the criticism and negative attitudes toward KD by some clinicians. The metabolic benefits of KD, its efficacy in inducing weight-loss and its beneficial effects on some neurodegenerative diseases cannot be overlooked. KD appears to be well-tolerated and safe in most cases. However, there is a serious lack of well-designed, properly powered randomized controlled trials that explore KD long-term efficacy and safety—particularly the consequences of elevated LDL in the absence of other CVD risk factors.
With the exception of intractable pediatric epilepsy, KD is currently not recommended by the different clinical societies as an acceptable treatment for conditions like obesity, diabetes, cancer, and neurodegenerative diseases. However, many individuals choose to follow KD and are encouraged by the positive results they experience. We, as clinicians, need to find ways to contain our patients' choices, educate ourselves about KD, and offer our support and our medical supervision. This can ensure that within the boundaries of KD, our patients will make good and healthy dietary choices and include low-carb vegetables and healthy dietary fats and avoid highly processed products that are marketed as “Keto-friendly”. This is important both to ensure healthy choices and to prevent clinical disengagement in extreme cases where conflicting attitudes toward KD and miscommunication might lead to a breach of trust between the patient and the clinician.
There is an urgent need for good quality research to address the issues of long-term safety of KD in different clinical population and for standardization of KD both in research and in clinical applications. Based on such research, recommendations should be made to include or to avoid KD as a therapeutic strategy for different individuals, in different health conditions.
Author contributions
AZ: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. SS: Conceptualization, Data curation, Investigation, Writing – original draft, Writing – review & editing. UY: Writing – original draft, Writing – review & editing. AB: Writing – original draft, Writing – review & editing. YP: Conceptualization, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Paoli A, Rubini A, Volek JS, Grimaldi KA. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. (2013) 67:789–96. doi: 10.1038/ejcn.2013.116
2. Kossoff EH, Zupec-Kania BA, Auvin S, Ballaban-Gil KR, Christina Bergqvist AG, Blackford R, et al. Optimal clinical management of children receiving dietary therapies for epilepsy: updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open. (2018) 3:175–92. doi: 10.1002/epi4.12225
3. Dashti HM, Al-Zaid NS, Mathew TC, Al-Mousawi M, Talib H, Asfar SK, et al. Long term effects of ketogenic diet in obese subjects with high cholesterol level. Mol Cell Biochem. (2006) 286:1–9. doi: 10.1007/s11010-005-9001-x
4. Westman EC, Mavropoulos J, Yancy WS, Volek JS. A review of low-carbohydrate ketogenic diets. Curr Atheroscler Rep. (2003) 5:476–83. doi: 10.1007/s11883-003-0038-6
5. Di Lorenzo C, Ballerini G, Barbanti P, Bernardini A, D'Arrigo G, Egeo G, et al. Applications of ketogenic diets in patients with headache: clinical recommendations. Nutrients. (2021) 13:2307. doi: 10.3390/nu13072307
6. Sethi S, Wakeham D, Ketter T, Hooshmand F, Bjornstad J, Richards B, et al. Ketogenic diet intervention on metabolic and psychiatric health in bipolar and schizophrenia: a pilot trial. Psychiatry Res. (2024) 335:115866. doi: 10.1016/j.psychres.2024.115866
7. Saslow LR, Jones LM, Sen A, Wolfson JA, Diez HL, O'Brien A, et al. Comparing very low-carbohydrate vs DASH diets for overweight or obese adults with hypertension and prediabetes or type 2 diabetes: a randomized trial. Ann Fam Med. (2023) 21:256–63. doi: 10.1370/afm.2968
8. Lambadiari V, Katsimbri P, Kountouri A, Korakas E, Papathanasi A, Maratou E, et al. The effect of a ketogenic diet versus mediterranean diet on clinical and biochemical markers of inflammation in patients with obesity and psoriatic arthritis: a randomized crossover trial. Int J Mol Sci. (2024) 25:2475. doi: 10.3390/ijms25052475
9. Norwitz NG, Soto-Mota A. Case report: carnivore–ketogenic diet for the treatment of inflammatory bowel disease: a case series of 10 patients. Front Nutr. (2024) 11:1467475. doi: 10.3389/fnut.2024.1467475
10. Cukoski S, Lindemann CH, Arjune S, Todorova P, Brecht T, Kühn A, et al. Feasibility and impact of ketogenic dietary interventions in polycystic kidney disease: KETO-ADPKD—a randomized controlled trial. Cell Rep Med. (2023) 4:101283. doi: 10.1016/j.xcrm.2023.101283
11. Mavropoulos JC, Yancy WS, Hepburn J, Westman EC. The effects of a low-carbohydrate, ketogenic diet on the polycystic ovary syndrome: a pilot study. Nutr Metab. (2005) 2:35. doi: 10.1186/1743-7075-2-35
12. Bezerra LS, Santos-Veloso MAO. Ketogenic diet and metastasis: a critical review of the literature and possible mechanisms. Clin Nutr ESPEN. (2023) 57:207–12. doi: 10.1016/j.clnesp.2023.06.038
13. Kossoff EH, McGrogan JR. Worldwide use of the ketogenic diet. Epilepsia. (2005) 46:280–9. doi: 10.1111/j.0013-9580.2005.42704.x
14. Abbasi J. Interest in the ketogenic diet grows for weight loss and type 2 diabetes. Jama. (2018) 319:215–7. doi: 10.1001/jama.2017.20639
15. Surma S, Sahebkar A, Banach M. Low carbohydrate/ketogenic diet in the optimization of lipoprotein(a) levels: do we have sufficient evidence for any recommendation? Eur Heart J. (2023) 44:4904–6. doi: 10.1093/eurheartj/ehad635
16. Fiest KM, Sauro KM, Wiebe S, Patten SB, Kwon CS, Dykeman J, et al. Prevalence and incidence of epilepsy: a systematic review and meta-analysis of international studies. Neurology. (2017) 88:296–303. doi: 10.1212/WNL.0000000000003509
17. Wheless JW. History of the ketogenic diet. Epilepsia. (2008) 49(Suppl. 8):3–5. doi: 10.1111/j.1528-1167.2008.01821.x
18. Kim DY, Abdelwahab MG, Lee SH, O'Neill D, Thompson RJ, Duff HJ, et al. Ketones prevent oxidative impairment of hippocampal synaptic integrity through KATP channels. PLoS ONE. (2015) 10:e0119316. doi: 10.1371/journal.pone.0119316
19. Dupuis N, Curatolo N, Benoist JF, Auvin S. Ketogenic diet exhibits anti-inflammatory properties. Epilepsia. (2015) 56:e95–8. doi: 10.1111/epi.13038
20. Milder J, Patel M. Modulation of oxidative stress and mitochondrial function by the ketogenic diet. Epilepsy Res. (2012) 100:295–303. doi: 10.1016/j.eplepsyres.2011.09.021
21. Jarrett SG, Milder JB, Liang LP, Patel M. The ketogenic diet increases mitochondrial glutathione levels. J Neurochem. (2008) 106:1044–51. doi: 10.1111/j.1471-4159.2008.05460.x
22. Kossoff EH, McGrogan JR, Bluml RM, Pillas DJ, Rubenstein JE, Vining EP, et al. Modified atkins diet is effective for the treatment of intractable pediatric epilepsy. Epilepsia. (2006) 47:421–4. doi: 10.1111/j.1528-1167.2006.00438.x
23. Schmidt D, Schachter SC. Drug treatment of epilepsy in adults. Bmj. (2014) 348:g254. doi: 10.1136/bmj.g254
24. Kwan P, Schachter SC, Brodie MJ. Drug-resistant epilepsy. N Engl J Med. (2011) 365:919–26. doi: 10.1056/NEJMra1004418
25. Martin-McGill KJ, Bresnahan R, Levy RG, Cooper PN. Ketogenic diets for drug-resistant epilepsy. Cochr Database Syst Rev. (2020) 6:Cd001903. doi: 10.1002/14651858.CD001903.pub5
26. Dressler A, Trimmel-Schwahofer P, Reithofer E, Gröppel G, Mühlebner A, Samueli S, et al. The ketogenic diet in infants–Advantages of early use. Epilepsy Res. (2015) 116:53–8. doi: 10.1016/j.eplepsyres.2015.06.015
27. Kim JA, Yoon JR, Lee EJ, Lee JS, Kim JT, Kim HD, et al. Efficacy of the classic ketogenic and the modified Atkins diets in refractory childhood epilepsy. Epilepsia. (2016) 57:51–8. doi: 10.1111/epi.13256
28. Kossoff EH, Zupec-Kania BA, Amark PE, Ballaban-Gil KR, Christina Bergqvist AG, Blackford R, et al. Optimal clinical management of children receiving the ketogenic diet: recommendations of the International Ketogenic Diet Study Group. Epilepsia. (2009) 50:304–17. doi: 10.1111/j.1528-1167.2008.01765.x
29. De Giorgis V, Tagliabue A, Bisulli F, Brambilla I, Camerini A, Cusmai R, et al. Ketogenic dietary therapies in epilepsy: recommendations of the Italian League against Epilepsy Dietary Therapy Study Group. Front Neurol. (2023) 14:1215618. doi: 10.3389/fneur.2023.1215618
30. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief . (2020) 360:1–8.
31. Mann J, McLean R, Skeaff M, Morenga LT. Low carbohydrate diets: going against the grain. Lancet. (2014) 384:1479–80. doi: 10.1016/S0140-6736(14)61413-6
32. Hall KD, Chen KY, Guo J, Lam YY, Leibel RL, Mayer LE, et al. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am J Clin Nutr. (2016) 104:324–33. doi: 10.3945/ajcn.116.133561
33. Yancy WS Jr, Westman EC, McDuffie JR, Grambow SC, Jeffreys AS, Bolton J, et al. A randomized trial of a low-carbohydrate diet vs orlistat plus a low-fat diet for weight loss. Arch Intern Med. (2010) 170:136–45. doi: 10.1001/archinternmed.2009.492
34. Zhu H, Bi D, Zhang Y, Kong C, Du J, Wu X, et al. Ketogenic diet for human diseases: the underlying mechanisms and potential for clinical implementations. Signal Transd Target Therapy. (2022) 7:11. doi: 10.1038/s41392-021-00831-w
35. Kang J, Ratamess NA, Faigenbaum AD, Bush JA. Ergogenic properties of ketogenic diets in normal-weight individuals: a systematic review. J Am Coll Nutr. (2020) 39:665–75. doi: 10.1080/07315724.2020.1725686
36. Gibas MK, Gibas KJ. Induced and controlled dietary ketosis as a regulator of obesity and metabolic syndrome pathologies. Diabetes Metab Syndr. (2017) 11 Suppl 1:S385–s90. doi: 10.1016/j.dsx.2017.03.022
37. Westman EC, Yancy WS, Edman JS, Tomlin KF, Perkins CE. Effect of 6-month adherence to a very low carbohydrate diet program. Am J Med. (2002) 113:30–6. doi: 10.1016/S0002-9343(02)01129-4
38. Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. (2003) 348:2074–81. doi: 10.1056/NEJMoa022637
39. Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. (2003) 348:2082–90. doi: 10.1056/NEJMoa022207
40. Ebbeling CB, Swain JF, Feldman HA, Wong WW, Hachey DL, Garcia-Lago E, et al. Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA. (2012) 307:2627–34. doi: 10.1001/jama.2012.6607
41. Bueno NB, de Melo IS, de Oliveira SL, da Rocha Ataide T. Very-low-carbohydrate ketogenic diet vs. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. (2013) 110:1178–87. doi: 10.1017/S0007114513000548
42. Ashtary-Larky D, Bagheri R, Baker JS, Bavi H, Mancin L, Moro T, et al. Ketogenic diets, physical activity and body composition: a review. Br J Nutr. (2022) 127:1898–920. doi: 10.1017/S0007114521002609
43. Wycherley TP, Buckley JD, Noakes M, Clifton PM, Brinkworth GD. Long-term effects of a very low-carbohydrate weight loss diet on exercise capacity and tolerance in overweight and obese adults. J Am Coll Nutr. (2014) 33:267–73. doi: 10.1080/07315724.2014.911668
44. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 6. Glycemic targets: standards of care in diabetes-−2023. Diabetes Care. (2022) 46(Suppl_1):S97–110. doi: 10.2337/dc23-S006
45. Forsythe CE, Phinney SD, Fernandez ML, Quann EE, Wood RJ, Bibus DM, et al. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids. (2008) 43:65–77. doi: 10.1007/s11745-007-3132-7
46. Zinman B, Skyler JS, Riddle MC, Ferrannini E. Diabetes research and care through the ages. Diabetes Care. (2017) 40:1302–13. doi: 10.2337/dci17-0042
47. McKenzie AL, Hallberg SJ, Creighton BC, Volk BM, Link TM, Abner MK, et al. A novel intervention including individualized nutritional recommendations reduces hemoglobin A1c level, medication use, and weight in type 2 diabetes. JMIR Diabetes. (2017) 2:e5. doi: 10.2196/diabetes.6981
48. Firman CH, Mellor DD, Unwin D, Brown A. Does a ketogenic diet have a place within diabetes clinical practice? Review of current evidence and controversies. Diabetes Ther. (2024) 15:77–97. doi: 10.1007/s13300-023-01492-4
49. Yancy WS Jr, Foy M, Chalecki AM, Vernon MC, Westman EC. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr Metab. (2005) 2:34. doi: 10.1186/1743-7075-2-34
50. Westman EC, Yancy WS Jr, Mavropoulos JC, Marquart M, McDuffie JR. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr Metab. (2008) 5:36. doi: 10.1186/1743-7075-5-36
51. Saslow LR, Mason AE, Kim S, Goldman V, Ploutz-Snyder R, Bayandorian H, et al. An online intervention comparing a very low-carbohydrate ketogenic diet and lifestyle recommendations versus a plate method diet in overweight individuals with type 2 diabetes: a randomized controlled trial. J Med Int Res. (2017) 19:e36. doi: 10.2196/jmir.5806
52. van Zuuren EJ, Fedorowicz Z, Kuijpers T, Pijl H. Effects of low-carbohydrate- compared with low-fat-diet interventions on metabolic control in people with type 2 diabetes: a systematic review including GRADE assessments. Am J Clin Nutr. (2018) 108:300–31. doi: 10.1093/ajcn/nqy096
53. Saslow LR, Daubenmier JJ, Moskowitz JT, Kim S, Murphy EJ, Phinney SD, et al. Twelve-month outcomes of a randomized trial of a moderate-carbohydrate versus very low-carbohydrate diet in overweight adults with type 2 diabetes mellitus or prediabetes. Nutr Diabetes. (2017) 7:304. doi: 10.1038/s41387-017-0006-9
54. Keogh JB, Luscombe-Marsh ND, Noakes M, Wittert GA, Clifton PM. Long-term weight maintenance and cardiovascular risk factors are not different following weight loss on carbohydrate-restricted diets high in either monounsaturated fat or protein in obese hyperinsulinaemic men and women. Br J Nutr. (2007) 97:405–10. doi: 10.1017/S0007114507252687
55. Chang CR, Francois ME, Little JP. Restricting carbohydrates at breakfast is sufficient to reduce 24-hour exposure to postprandial hyperglycemia and improve glycemic variability. Am J Clin Nutr. (2019) 109:1302–9. doi: 10.1093/ajcn/nqy261
56. Hancock M, Burns K, Gan SK, Chew GT. Low-carbohydrate diets in type 1 diabetes: balancing benefits and risks. Curr Opin Endocrinol Diabetes Obes. (2023) 30:113–22. doi: 10.1097/MED.0000000000000797
57. Leow ZZX, Guelfi KJ, Davis EA, Jones TW, Fournier PA. The glycaemic benefits of a very-low-carbohydrate ketogenic diet in adults with Type 1 diabetes mellitus may be opposed by increased hypoglycaemia risk and dyslipidaemia. Diabet Med. (2018). doi: 10.1111/dme.13663
58. Lennerz BS, Barton A, Bernstein RK, Dikeman RD, Diulus C, Hallberg S, et al. Management of Type 1 Diabetes With a Very Low-Carbohydrate Diet. Pediatrics. (2018) 141(6). doi: 10.1542/peds.2017-3349
59. Bernstein RK. Virtually continuous euglycemia for 5 yr in a labile juvenile-onset diabetic patient under noninvasive closed-loop control. Diabetes Care. (1980) 3:140–3. doi: 10.2337/diacare.3.1.140
60. Turton JL, Brinkworth GD, Parker HM, Lim D, Lee K, Rush A, et al. Effects of a low-carbohydrate diet in adults with type 1 diabetes management: a single arm non-randomised clinical trial. PLoS ONE. (2023) 18:e0288440. doi: 10.1371/journal.pone.0288440
61. Ranjan A, Schmidt S, Damm-Frydenberg C, Holst JJ, Madsbad S, Nørgaard K. Short-term effects of a low carbohydrate diet on glycaemic variables and cardiovascular risk markers in patients with type 1 diabetes: A randomized open-label crossover trial. Diabetes Obes Metab. (2017) 19:1479–84. doi: 10.1111/dom.12953
62. Krebs JD, Parry Strong A, Cresswell P, Reynolds AN, Hanna A, Haeusler S, et al. Randomised trial of the feasibility of a low carbohydrate diet vs standard carbohydrate counting in adults with type 1 diabetes taking body weight into account. Asia Pac J Clin Nutr. (2016) 25:78–84. doi: 10.6133/apjcn.206.25.1.11
63. Lennerz BS, Koutnik AP, Azova S, Wolfsdorf JI, Ludwig DS. Carbohydrate restriction for diabetes: rediscovering centuries-old wisdom. J Clin Invest. (2021) 131e142246. doi: 10.1172/JCI142246
64. Evert AB, Dennison M, Gardner CD, Garvey WT, Lau KHK, MacLeod J, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. (2019) 42:731–54. doi: 10.2337/dci19-0014
65. Neyman A, Hannon TS. Low-carbohydrate diets in children and adolescents with or at risk for diabetes. Pediatrics. (2023) 152:e063755. doi: 10.1542/peds.2023-063755
66. Arends J, Baracos V, Bertz H, Bozzetti F, Calder PC, Deutz NEP, et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr. (2017) 36:1187–96. doi: 10.1016/j.clnu.2017.06.017
67. Font-Burgada J, Sun B, Karin M. Obesity and cancer: the oil that feeds the flame. Cell Metab. (2016) 23:48–62. doi: 10.1016/j.cmet.2015.12.015
68. Wu GY, Thompson JR. The effect of ketone bodies on alanine and glutamine metabolism in isolated skeletal muscle from the fasted chick. Biochem J. (1988) 255:139–44. doi: 10.1042/bj2550139
69. Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. (2014) 15:1243–53. doi: 10.15252/embr.201439246
70. Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. (2016) 41:211–8. doi: 10.1016/j.tibs.2015.12.001
71. Magee BA, Potezny N, Rofe AM, Conyers RA. The inhibition of malignant cell growth by ketone bodies. Aust J Exp Biol Med Sci. (1979) 57:529–39. doi: 10.1038/icb.1979.54
72. Seyfried TN, Mukherjee P, Iyikesici MS, Slocum A, Kalamian M, Spinosa JP, et al. Consideration of ketogenic metabolic therapy as a complementary or alternative approach for managing breast cancer. Front Nutr. (2020) 7:21. doi: 10.3389/fnut.2020.00021
73. Schugar RC, Crawford PA. Low-carbohydrate ketogenic diets, glucose homeostasis, and nonalcoholic fatty liver disease. Curr Opin Clin Nutr Metab Care. (2012) 15:374–80. doi: 10.1097/MCO.0b013e3283547157
74. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer–viewpoint of the IARC Working Group. N Engl J Med. (2016) 375:794–8. doi: 10.1056/NEJMsr1606602
75. Golemis EA, Scheet P, Beck TN, Scolnick EM, Hunter DJ, Hawk E, et al. Molecular mechanisms of the preventable causes of cancer in the United States. Genes Dev. (2018) 32:868–902. doi: 10.1101/gad.314849.118
76. Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles' heel? Nat Rev Cancer. (2014) 14:709–21. doi: 10.1038/nrc3803
77. Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. (2007) 28:20–47. doi: 10.1210/er.2006-0001
78. Malaguarnera R, Belfiore A. The insulin receptor: a new target for cancer therapy. Front Endocrinol. (2011) 2:93. doi: 10.3389/fendo.2011.00093
79. Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. (2008) 8:915–28. doi: 10.1038/nrc2536
80. Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. (2001) 131:3109s−20s. doi: 10.1093/jn/131.11.3109S
81. Meckling KA, O'Sullivan C, Saari D. Comparison of a low-fat diet to a low-carbohydrate diet on weight loss, body composition, and risk factors for diabetes and cardiovascular disease in free-living, overweight men and women. J Clin Endocrinol Metab. (2004) 89:2717–23. doi: 10.1210/jc.2003-031606
82. Mukherjee P, Augur ZM Li M, Hill C, Greenwood B, Domin MA, et al. Therapeutic benefit of combining calorie-restricted ketogenic diet and glutamine targeting in late-stage experimental glioblastoma. Commun Biol. (2019) 2:200. doi: 10.1038/s42003-019-0455-x
83. Egashira R, Matsunaga M, Miyake A, Hotta S, Nagai N, Yamaguchi C, et al. Long-term effects of a ketogenic diet for cancer. Nutrients. (2023) 15:2334. doi: 10.3390/nu15102334
84. Weber DD, Aminazdeh-Gohari S, Kofler B. Ketogenic diet in cancer therapy. Aging. (2018) 10:164–5. doi: 10.18632/aging.101382
85. Dal Bello S, Valdemarin F, Martinuzzi D, Filippi F, Gigli GL, Valente M. Ketogenic diet in the treatment of gliomas and glioblastomas. Nutrients. (2022) 14:3851. doi: 10.3390/nu14183851
86. Li J, Zhang H, Dai Z. Cancer treatment with the ketogenic diet: a systematic review and meta-analysis of animal studies. Front Nutr. (2021) 8:594408. doi: 10.3389/fnut.2021.594408
87. Khodabakhshi A, Akbari ME, Mirzaei HR, Seyfried TN, Kalamian M, Davoodi SH. Effects of ketogenic metabolic therapy on patients with breast cancer: a randomized controlled clinical trial. Clin Nutr. (2021) 40:751–8. doi: 10.1016/j.clnu.2020.06.028
88. Allen BG, Bhatia SK, Buatti JM, Brandt KE, Lindholm KE, Button AM, et al. Ketogenic diets enhance oxidative stress and radio-chemo-therapy responses in lung cancer xenografts. Clin Cancer Res. (2013) 19:3905–13. doi: 10.1158/1078-0432.CCR-12-0287
89. Weber DD, Aminzadeh-Gohari S, Thapa M, Redtenbacher AS, Catalano L, Capelôa T, et al. Ketogenic diets slow melanoma growth in vivo regardless of tumor genetics and metabolic plasticity. Cancer Metab. (2022) 10:12. doi: 10.1186/s40170-022-00288-7
90. Abdelwahab MG, Fenton KE, Preul MC, Rho JM, Lynch A, Stafford P, et al. The ketogenic diet is an effective adjuvant to radiation therapy for the treatment of malignant glioma. PLoS ONE. (2012) 7:e36197. doi: 10.1371/journal.pone.0036197
91. Liśkiewicz AD, Kasprowska D, Wojakowska A, Polański K, Lewin-Kowalik J, Kotulska K, et al. Long-term high fat ketogenic diet promotes renal tumor growth in a rat model of tuberous sclerosis. Sci Rep. (2016) 6:21807. doi: 10.1038/srep21807
92. Klement RJ. The emerging role of ketogenic diets in cancer treatment. Curr Opin Clin Nutr Metab Care. (2019) 22:129–34. doi: 10.1097/MCO.0000000000000540
93. Gitler AD, Dhillon P, Shorter J. Neurodegenerative disease: models, mechanisms, and a new hope. Dis Model Mech. (2017) 10:499–502. doi: 10.1242/dmm.030205
94. Wareham LK, Liddelow SA, Temple S, Benowitz LI, Di Polo A, Wellington C, et al. Solving neurodegeneration: common mechanisms and strategies for new treatments. Mol Neurodegener. (2022) 17:23. doi: 10.1186/s13024-022-00524-0
95. Kumar A, Sidhu J, Goyal A, Tsao JW. Alzheimer Disease. Treasure Island, FL: StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC. (2024).
96. Alzheimer's Disease International World Alzheimer Report 2019: Attitudes to dementias. London: Alzheimer's Disease International (2019).
97. Tiwari S, Atluri V, Kaushik A, Yndart A, Nair M. Alzheimer's disease: pathogenesis, diagnostics, and therapeutics. Int J Nanomed. (2019) 14:5541–54. doi: 10.2147/IJN.S200490
98. Rusek M, Pluta R, Ułamek-Kozioł M, Czuczwar SJ. Ketogenic diet in Alzheimer's disease. Int J Mol Sci. (2019) 20:3892. doi: 10.3390/ijms20163892
99. Castellano CA, Nugent S, Paquet N, Tremblay S, Bocti C, Lacombe G, et al. Lower brain 18F-fluorodeoxyglucose uptake but normal 11C-acetoacetate metabolism in mild Alzheimer's disease dementia. J Alzheimers Dis. (2015) 43:1343–53. doi: 10.3233/JAD-141074
100. Nichols E, Szoeke CEI, Vollset SE, Abbasi N, Abd-Allah F, Abdela J, et al. Global, regional, and national burden of Alzheimer's disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. (2019) 18:88–106. doi: 10.1016/S1474-4422(18)30403-4
101. Hernandez AR, Hernandez CM, Campos KT, Truckenbrod LM, Sakarya Y, McQuail JA, et al. The antiepileptic ketogenic diet alters hippocampal transporter levels and reduces adiposity in aged rats. J Gerontol A Biol Sci Med Sci. (2018) 73:450–8. doi: 10.1093/gerona/glx193
102. Newman JC, Covarrubias AJ, Zhao M, Yu X, Gut P, Ng CP, et al. Ketogenic diet reduces midlife mortality and improves memory in aging mice. Cell Metab. (2017) 26:547–57.e8. doi: 10.1016/j.cmet.2017.08.004
103. Neth BJ, Mintz A, Whitlow C, Jung Y, Solingapuram Sai K, Register TC, et al. Modified ketogenic diet is associated with improved cerebrospinal fluid biomarker profile, cerebral perfusion, and cerebral ketone body uptake in older adults at risk for Alzheimer's disease: a pilot study. Neurobiol Aging. (2020) 86:54–63. doi: 10.1016/j.neurobiolaging.2019.09.015
104. Phillips MCL, Deprez LM, Mortimer GMN, Murtagh DKJ, McCoy S, Mylchreest R, et al. Randomized crossover trial of a modified ketogenic diet in Alzheimer's disease. Alzheimers Res Ther. (2021) 13:51. doi: 10.1186/s13195-021-00783-x
105. Lilamand M, Mouton-Liger F, Di Valentin E, Sànchez Ortiz M, Paquet C. Efficacy and safety of ketone supplementation or ketogenic diets for Alzheimer's disease: a mini review. Front Nutr. (2021) 8:807970. doi: 10.3389/fnut.2021.807970
106. Grochowska K, Przeliorz A. The effect of the ketogenic diet on the therapy of neurodegenerative diseases and its impact on improving cognitive functions. Dement Geriatr Cogn Dis Extra. (2022) 12:100–6. doi: 10.1159/000524331
107. Shaafi S, Najmi S, Aliasgharpour H, Mahmoudi J, Sadigh-Etemad S, Farhoudi M, et al. The efficacy of the ketogenic diet on motor functions in Parkinson's disease: A rat model. Iran J Neurol. (2016) 15:63–9.
108. Phillips MCL, Murtagh DKJ, Gilbertson LJ, Asztely FJS, Lynch CDP. Low-fat versus ketogenic diet in Parkinson's disease: a pilot randomized controlled trial. Mov Disord. (2018) 33:1306–14. doi: 10.1002/mds.27390
109. Tidman MM, White D, White T. Effects of an low carbohydrate/healthy fat/ketogenic diet on biomarkers of health and symptoms, anxiety and depression in Parkinson's disease: a pilot study. Neurodegener Dis Manag. (2022) 12:57–66. doi: 10.2217/nmt-2021-0033
110. O'Neill B, Raggi P. The ketogenic diet: pros and cons. Atherosclerosis. (2020) 292:119–26. doi: 10.1016/j.atherosclerosis.2019.11.021
111. Yancy WS Jr, Olsen MK, Guyton JR, Bakst RP, Westman EC. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med. (2004) 140:769–77. doi: 10.7326/0003-4819-140-10-200405180-00006
112. Churuangsuk C, Griffiths D, Lean MEJ, Combet E. Impacts of carbohydrate-restricted diets on micronutrient intakes and status: a systematic review. Obes Rev. (2019) 20:1132–47. doi: 10.1111/obr.12857
113. Mazidi M, Katsiki N, Mikhailidis DP, Sattar N, Banach M. Lower carbohydrate diets and all-cause and cause-specific mortality: a population-based cohort study and pooling of prospective studies. Eur Heart J. (2019) 40:2870–9. doi: 10.1093/eurheartj/ehz174
114. Akter S, Mizoue T, Nanri A, Goto A, Noda M, Sawada N, et al. Low carbohydrate diet and all cause and cause-specific mortality. Clin Nutr. (2021) 40:2016–24. doi: 10.1016/j.clnu.2020.09.022
115. Sun C, Zhang WS, Jiang CQ, Jin YL, Deng XQ, Woo J, et al. Low-carbohydrate diets and mortality in older Asian people: a 15-year follow-up from a prospective cohort study. Nutrients. (2022) 14:71406. doi: 10.3390/nu14071406
116. Goldberg IJ, Ibrahim N, Bredefeld C, Foo S, Lim V, Gutman D, et al. Ketogenic diets, not for everyone. J Clin Lipidol. (2021) 15:61–7. doi: 10.1016/j.jacl.2020.10.005
117. Crosier R, McPherson R. Profound Elevation in LDL Cholesterol level following a ketogenic diet: a case series. CJC Open. (2022) 4:732–4. doi: 10.1016/j.cjco.2022.05.001
118. Retterstøl K, Svendsen M, Narverud I, Holven KB. Effect of low carbohydrate high fat diet on LDL cholesterol and gene expression in normal-weight, young adults: a randomized controlled study. Atherosclerosis. (2018) 279:52–61. doi: 10.1016/j.atherosclerosis.2018.10.013
119. Burén J, Ericsson M, Damasceno NRT, Sjödin A. A ketogenic low-carbohydrate high-fat diet increases LDL cholesterol in healthy, young, normal-weight women: a randomized controlled feeding trial. Nutrients. (2021) 13:814. doi: 10.3390/nu13030814
120. Mansoor N, Vinknes KJ, Veierød MB, Retterstøl K. Effects of low-carbohydrate diets v. low-fat diets on body weight and cardiovascular risk factors: a meta-analysis of randomised controlled trials. Br J Nutr. (2016) 115:466–79. doi: 10.1017/S0007114515004699
121. Tay J, Brinkworth GD, Noakes M, Keogh J, Clifton PM. Metabolic effects of weight loss on a very-low-carbohydrate diet compared with an isocaloric high-carbohydrate diet in abdominally obese subjects. J Am Coll Cardiol. (2008) 51:59–67. doi: 10.1016/j.jacc.2007.08.050
122. Athinarayanan SJ, Hallberg SJ, McKenzie AL, Lechner K, King S, McCarter JP, et al. Impact of a 2-year trial of nutritional ketosis on indices of cardiovascular disease risk in patients with type 2 diabetes. Cardiovasc Diabetol. (2020) 19:208. doi: 10.1186/s12933-020-01178-2
123. Luo W, Zhang J, Xu D, Zhou Y, Qu Z, Yang Q, et al. Low carbohydrate ketogenic diets reduce cardiovascular risk factor levels in obese or overweight patients with T2DM: a meta-analysis of randomized controlled trials. Front Nutr. (2022) 9:1092031. doi: 10.3389/fnut.2022.1092031
124. Norwitz NG, Feldman D, Soto-Mota A, Kalayjian T, Ludwig DS. Elevated LDL cholesterol with a carbohydrate-restricted diet: evidence for a “lean mass hyper-responder” phenotype. Curr Dev Nutr. (2022) 6:nzab144. doi: 10.1093/cdn/nzab144
125. Soto-Mota A, Flores-Jurado Y, Norwitz NG, Feldman D, Pereira MA, Danaei G, et al. Increased low-density lipoprotein cholesterol on a low-carbohydrate diet in adults with normal but not high body weight: a meta-analysis. Am J Clin Nutr. (2024) 119:740–7. doi: 10.1016/j.ajcnut.2024.01.009
126. Budoff M, Manubolu VS, Kinninger A, Norwitz NG, Feldman D, Wood TR, et al. Carbohydrate restriction-induced elevations in LDL-cholesterol and atherosclerosis: the KETO Trial. JACC Adv. (2024) 3:101109. doi: 10.1016/j.jacadv.2024.101109
127. Nasir K, Ziffer JA, Cainzos-Achirica M, Ali SS, Feldman DI, Arias L, et al. The Miami Heart Study (MiHeart) at Baptist Health South Florida, A prospective study of subclinical cardiovascular disease and emerging cardiovascular risk factors in asymptomatic young and middle-aged adults: The Miami Heart Study: Rationale and Design. Am J Prev Cardiol. (2021) 7:100202. doi: 10.1016/j.ajpc.2021.100202
128. Norwitz NG, Mindrum MR, Giral P, Kontush A, Soto-Mota A, Wood TR, et al. Elevated LDL-cholesterol levels among lean mass hyper-responders on low-carbohydrate ketogenic diets deserve urgent clinical attention and further research. J Clin Lipidol. (2022) 16:765–8. doi: 10.1016/j.jacl.2022.10.010
129. Keogh JB, Brinkworth GD, Noakes M, Belobrajdic DP, Buckley JD, Clifton PM. Effects of weight loss from a very-low-carbohydrate diet on endothelial function and markers of cardiovascular disease risk in subjects with abdominal obesity. Am J Clin Nutr. (2008) 87:567–76. doi: 10.1093/ajcn/87.3.567
130. Wycherley TP, Thompson CH, Buckley JD, Luscombe-Marsh ND, Noakes M, Wittert GA, et al. Long-term effects of weight loss with a very-low carbohydrate, low saturated fat diet on flow mediated dilatation in patients with type 2 diabetes: a randomised controlled trial. Atherosclerosis. (2016) 252:28–31. doi: 10.1016/j.atherosclerosis.2016.07.908
131. Wycherley TP, Brinkworth GD, Keogh JB, Noakes M, Buckley JD, Clifton PM. Long-term effects of weight loss with a very low carbohydrate and low fat diet on vascular function in overweight and obese patients. J Intern Med. (2010) 267:452–61. doi: 10.1111/j.1365-2796.2009.02174.x
132. Tay J, Luscombe-Marsh ND, Thompson CH, Noakes M, Buckley JD, Wittert GA, et al. Comparison of low- and high-carbohydrate diets for type 2 diabetes management: a randomized trial. Am J Clin Nutr. (2015) 102:780–90. doi: 10.3945/ajcn.115.112581
133. Powell-Wiley TM, Poirier P, Burke LE, Després J-P, Gordon-Larsen P, Lavie CJ, et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. (2021) 143:e984–e1010. doi: 10.1161/CIR.0000000000000973
134. Yuan X, Wang J, Yang S, Gao M, Cao L, Li X, et al. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: a systematic review and meta-analysis. Nutr Diabetes. (2020) 10:38. doi: 10.1038/s41387-020-00142-z
135. Chiasson JL, Aris-Jilwan N, Bélanger R, Bertrand S, Beauregard H, Ekoé JM, et al. Diagnosis and treatment of diabetic ketoacidosis and the hyperglycemic hyperosmolar state. CMAJ. (2003) 168:859–66.
136. Blanco JC, Khatri A, Kifayat A, Cho R, Aronow WS. Starvation ketoacidosis due to the ketogenic diet and prolonged fasting - a possibly dangerous diet trend. Am J Case Rep. (2019) 20:1728–31. doi: 10.12659/AJCR.917226
137. Charoensri S, Sothornwit J, Trirattanapikul A, Pongchaiyakul C. Ketogenic diet-induced diabetic ketoacidosis in a young adult with unrecognized type 1 diabetes. Case Rep Endocrinol. (2021) 2021:6620832. doi: 10.1155/2021/6620832
138. White-Cotsmire AJ, Healy AM. Ketogenic diet as a trigger for diabetic ketoacidosis in a misdiagnosis of diabetes: a case report. Clin Diabetes. (2020) 38:318–21. doi: 10.2337/cd20-0001
139. Ozoran H, Matheou M, Dyson P, Karpe F, Tan GD. Type 1 diabetes and low carbohydrate diets-Defining the degree of nutritional ketosis. Diabet Med. (2023) 40:e15178. doi: 10.1111/dme.15178
140. Mistry S, Eschler DC. Euglycemic diabetic ketoacidosis caused by SGLT2 inhibitors and a ketogenic diet: a case series and review of literature. AACE Clin Case Rep. (2021) 7:17–9. doi: 10.1016/j.aace.2020.11.009
141. Dorcely B, Nitis J, Schwartzbard A, Newman JD, Goldberg IJ, Sum M, et al. Case report: euglycemic diabetic ketoacidosis presenting as chest pain in a patient on a low carbohydrate diet. Curr Diabetes Rev. (2021) 17:243–6. doi: 10.2174/1573399816666200316112709
142. Ko GJ, Rhee CM, Kalantar-Zadeh K, Joshi S. The effects of high-protein diets on kidney health and longevity. J Am Soc Nephrol. (2020) 31:1667–79. doi: 10.1681/ASN.2020010028
143. Brinkworth GD, Buckley JD, Noakes M, Clifton PM. Renal function following long-term weight loss in individuals with abdominal obesity on a very-low-carbohydrate diet vs high-carbohydrate diet. J Am Diet Assoc. (2010) 110:633–8. doi: 10.1016/j.jada.2009.12.016
144. Tay J, Thompson CH, Luscombe-Marsh ND, Noakes M, Buckley JD, Wittert GA, et al. Long-term effects of a very low carbohydrate compared with a high carbohydrate diet on renal function in individuals with type 2 diabetes: a randomized trial. Medicine. (2015) 94:e2181. doi: 10.1097/MD.0000000000002181
Keywords: very-low-carbohydrate-diet, obesity, diabetes, weight-loss, cancer, intractable pediatric epilepsy, neurodegenerative disease
Citation: Zemer A, Samaei S, Yoel U, Biderman A and Pincu Y (2024) Ketogenic diet in clinical populations—a narrative review. Front. Med. 11:1432717. doi: 10.3389/fmed.2024.1432717
Received: 14 May 2024; Accepted: 14 October 2024;
Published: 29 October 2024.
Edited by:
Sergei V. Fedorovich, Belarusian State University, BelarusReviewed by:
Richard David Feinman, Downstate Health Sciences University, United StatesSviatlana Hrynevich, Belarusian State University, Belarus
Adrian Soto-Mota, National Institute of Medical Sciences and Nutrition Salvador Zubirán, Mexico
Zoltan Sarnyai, James Cook University, Australia
Copyright © 2024 Zemer, Samaei, Yoel, Biderman and Pincu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yair Pincu, eXBpbmN1JiN4MDAwNDA7b3UuZWR1
 Alon Zemer
Alon Zemer Shabnam Samaei
Shabnam Samaei Uri Yoel
Uri Yoel Aya Biderman
Aya Biderman Yair Pincu
Yair Pincu