- 1Physiology Research Center, Iran University of Medical Sciences, Tehran, Iran
- 2Lung Diseases Research Center, Ardabil University of Medical Sciences, Ardabil, Iran
Objective: The current study was designed with the aim of conducting a systematic review and meta-analysis to determine the circulating levels of visfatin in patients with chronic obstructive pulmonary disease (COPD) compared to healthy individuals.
Methods: Until March 2024, we searched the Web of Science, PubMed/Medline, and Scopus databases. The analysis included case–control studies assessing the association between circulating visfatin and COPD. The random effects model was utilized to analyse the results with the help of Standard Mean of Differences (SMD) and 95% confidence interval (CI). The heterogeneity of the data was assessed using Cochrane Q and I2 values.
Results: Seven studies were eligible to be included in the meta-analysis, with the COPD and healthy (control) groups having 265 and 244 subjects, respectively. The pooled results showed that although the circulating concentration of visfatin was lower in patients with COPD, no significant difference was observed (SMD: −0.48 mg/L; 95% CI: −1.67 to 0.70; p = 0.43). Subgroup analysis revealed that visfatin levels were significantly reduced in FEV1 less than 50% (p < 0.001) and in GOLD grade I-II (p < 0.05). Visfatin was shown to be significantly associated with IL-6 (p < 0.001) and TNF-α (p < 0.01) in the correlation meta-analysis. Meta-regression analysis revealed a significant correlation between the pooled SMD visfatin and pooled SMD age (p < 0.01), BMI (p < 0.001), FEV1 (p < 0.001), and IL-6 (p < 0.001).
Conclusion: The findings showed an insignificant decline in visfatin level among COPD patients, but additional research is necessary due to the heterogeneity in study results.
Systematic review registration: PROSPERO (CRD42023450851), https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023450851.
1 Introduction
Airflow limitation and chronic respiratory symptoms are distinctive of chronic obstructive pulmonary disease (COPD). COPD patients can suffer from restricted airflow due to the destruction of lung parenchyma or airway disease (1–3). COPD, a complex disorder, is caused by a range of factors including genetic predisposition, cigarette smoke exposure, dietary habits, occupational hazards, physical activity, lifestyle, and air pollution both indoors and outdoors (4, 5). The pathophysiology of COPD is affected by a variety of factors, such as neutrophilic airway inflammation, an imbalance of proteases and antiproteases, oxidative stress, endoplasmic reticulum stress, tissue hypoxia, lung hyperinflation, and apoptosis (6–8). Elevated levels of cytokines and inflammatory markers such as interleukin-6 (IL-6), tumour necrosis factor-alpha (TNF-α), IL-8, and C-reactive protein (CRP) have been observed in the airways of COPD patients during exacerbation (9, 10). In addition, results from recent investigations have revealed the key contribution of adipokines [such as leptin, adiponectin, visfatin, adipolin, and fatty acid binding protein 4 (FABP4)] released from adipose tissue in the pathogenesis of chronic lung diseases (11–13).
Visfatin is an adipokine formerly known as B-cell colony-enhancing factor (PBEF) and nicotinamide phosphoribosyltransferase (NAMPT) (14). The majority of studies have posited visfatin as pro-inflammatory factor, due to the significant positive correlation between visfatin levels and inflammatory markers (15, 16). Moreover, inflammatory markers are able to prompt the secretion of visfatin, and visfatin itself can also evoke the secretion of inflammatory components (14). Despite numerous studies exploring the connection between visfatin and COPD, the outcomes have been profoundly conflicting. Consequently, this systematic review and meta-analysis study was aimed at assessing the circulating levels of visfatin among COPD patients and determining its potential association with inflammatory factors.
2 Methods
The present systematic review and meta-analysis were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) strategies (17). In addition, the study protocol was registered in PROSPERO (CRD42023450851).
2.1 Search strategy
From inception to March 2024, the electronic databases of PubMed / Medline, the Web of Sciences, the Scopus, as well as manual search in article references and Google Scholar were searched for studies examining the circulating level of visfain in COPD patients. The mesh and non-mesh terms utilized in the searching were summarized in Supplementary Table 1. The language requirement for articles was English, but no time limits were imposed. Moreover, a manual search was done of all applicable article reference lists to recognize possibly applicable studies.
2.2 Eligibility criteria
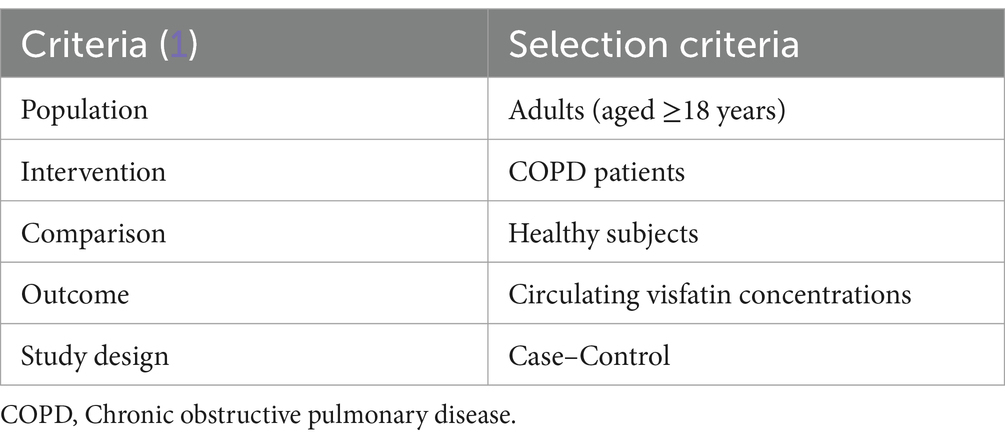
The criterion for the current meta-analysis study was designed based on population, intervention, comparison, and outcome (PICO) (Table 1). No correspondence was entered into with the authors.
To be included, the criteria were: (1) observational studies, (2) COPD patients in accordance with the American or European Pulmonary Association guidelines, (3) with a sample size of more than 10 subjects, and (4) healthy participants with no evidence of disease or infection. The exclusion criteria, if mentioned in the articles, were: (1) patients with a history of a lung disease outside of COPD; (2) patients receiving a supportive nutritional treatment; (3) patients receiving specific drugs; (4) comorbidity that could affect circulating visfatin levels.
2.3 Data extraction
Human studies in which visfatin serum/plasma levels in patients with COPD compared to healthy subjects were included in the meta-analysis. Two researchers (A.M. and N.A.) were responsible for data extraction, independently, with the final referee (MR.A.) intervening in the event of a disagreement. The information taken from each article included the authors name, date of publication, country of investigation, age of those involved, sample size of participants, sex, visfatin serum/plasma level, body mass index (BMI), CRP level, IL-6 level, TNF-α level, and forced expiratory volume in 1 s (FEV1). All parameters values were reported with mean and standard deviation.
2.4 Study quality assessment
The Newcastle-Ottawa Scale (NOS) was used to assess risk of bias for cohort studies. The NOS includes criteria such as selection, comparison, and outcome or exposure depending on the type of study (cohort or case–control series) (18). A star system is used, ranging from zero to nine stars. Thresholds were set based on the overall score, with 7 to 9 stars considered “low risk of bias,” 4 to 6 stars “unclear risk of bias,” and 3 stars or less “high risk of bias” (19).
2.5 Statistical analysis
The results were expressed in the form of mean ± SD. In order to determine the standard mean difference (SMD), transform the reported results to SD by converting them into a confidence interval and standard error (SE). The results were analyzed utilizing Comprehensive Meta-Analysis (CMA) software version 2 and the random-effects model, with p < 0.05 defining statistical significance, and MedCalc was used for meta-regression. The pooled SMD of BMI, age, FEV1, and IL-6 were calculated and the correlation of the above values with pooled SMD visfatin were reported using meta-regression analysis.
The Q-test and the I2 index were employed to assess heterogeneity, with a significant level of heterogeneity set at p < 0.10 (I2 < 25%, no heterogeneity; I2 between 25 and 50%, moderate heterogeneity; I2 between 50 and 75%, large heterogeneity; and I2 > 75%, extreme heterogeneity). An examination of sensitivity was undertaken to measure the effect of each study on the pooled effect size. The funnel plot examination and Egger’s regression test were utilized to analyze publication bias.
3 Result
3.1 Search results
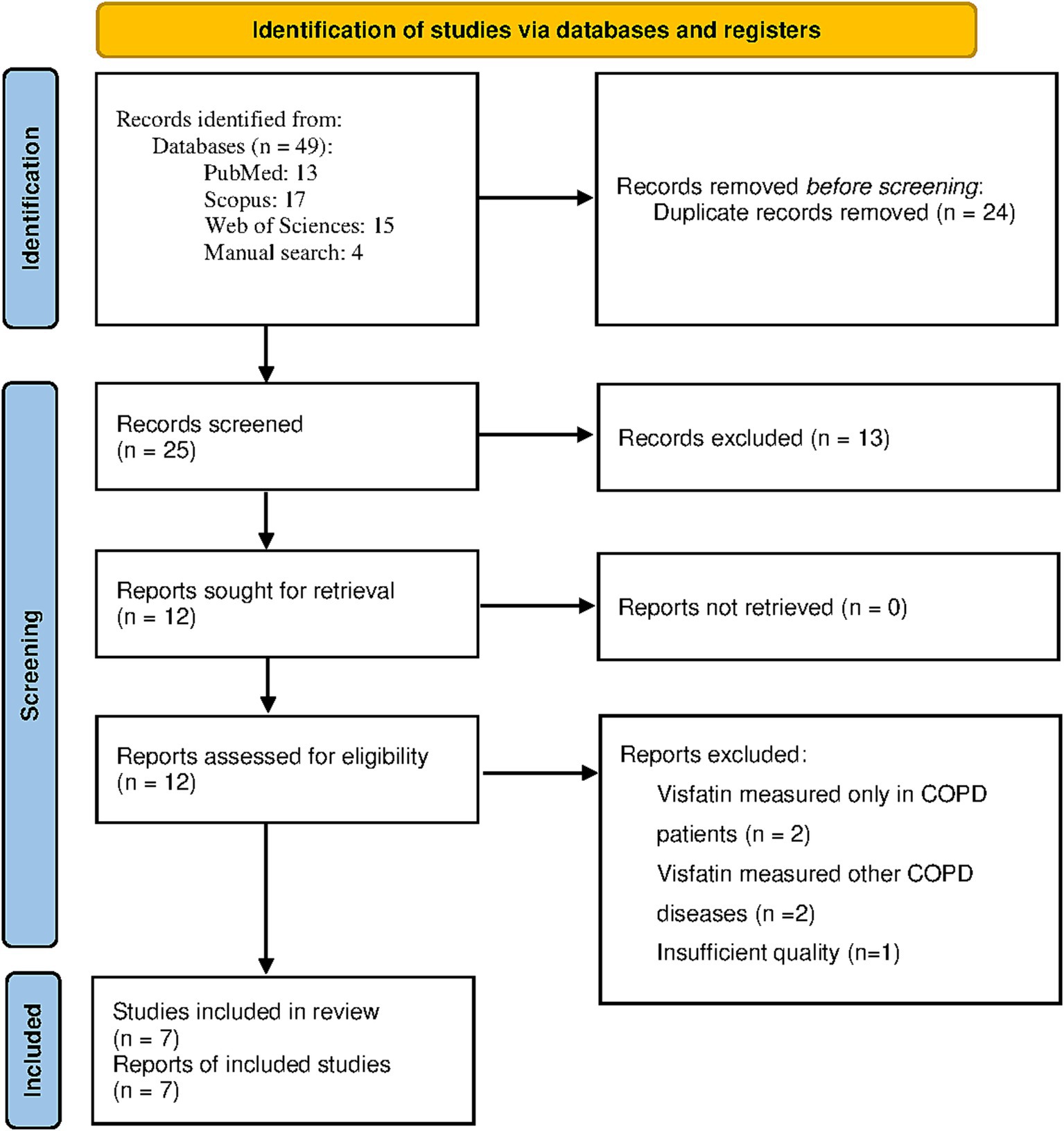
The flowchart in Figure 1 displays the screening process of the current study. Initially, a total of 49 studies were identified. Twenty four studies were excluded as they were either duplicated or irrelevant. After examining the entirety of 25 studies, 13 were removed for not adhering to the criteria for inclusion. In addition, we removed four articles as the level of visfatin was measured only in COPD patients or other than COPD respiratory disorder (20–23). Also, one study was not included in the study due to insufficient quality (24). Finally, meta-analysis was performed with 7 studies (14, 25–30).
The case–control studies which were retrieved were published from 2009 up to 2022, with their aspects detailed in Table 2. Investigations were carried out in Turkey, Iran, Finland, China, and Mexico. The mean age of those identified with COPD was 63.59, compared to 61.71 in those without COPD. All research indicated a considerable variance in visfatin circulating concentration between COPD patients and control subjects, with 4 study results indicating a notable decrease for COPD patients and 2 study results a prominent increase (Table 2).
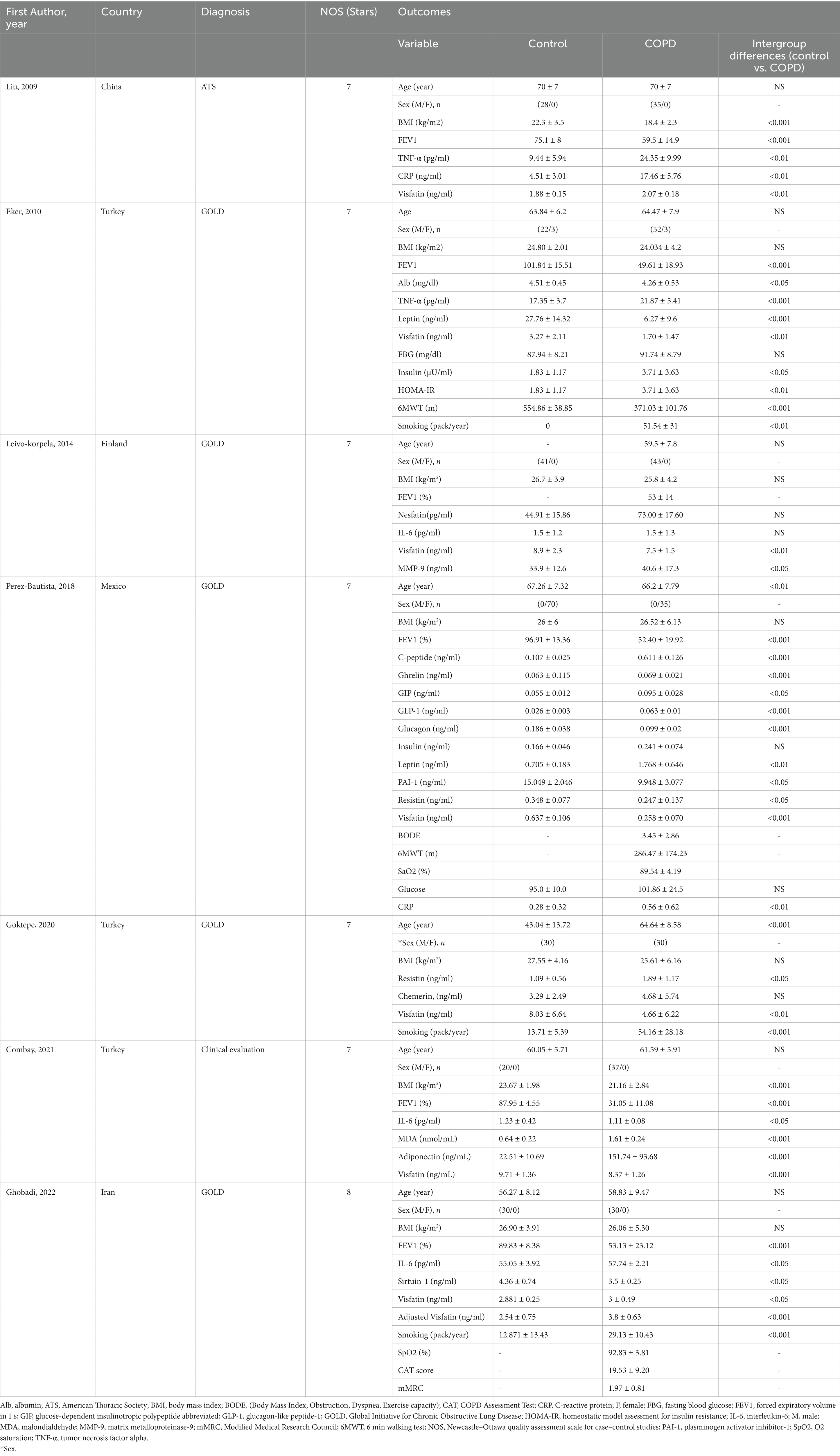
Table 2. Characteristics of included studies investigating the visfatin circulating level in COPD patients.
3.2 Quality assessment
The Newcastle-Ottawa scale was used to evaluate the quality of the studies. Evaluation was based on criteria such as the selection of the cohort, ensuring group comparability through design or analysis, the determination of exposure, and the assessment of outcomes of interest. The entry requirement for the study was a score of 6 or higher (Supplementary Table 2). The two researchers assessed the criteria for each paper in the study (A.M. and N.A.), independently. The results showed that one of the articles did not have the necessary quality to enter the analysis (24).
3.3 Study characteristics
Table 2 shows a summary of the characteristics of the included studies. In 7 included studies, 244 subjects were in the control group and 265 subjects were in the COPD group. All studies were in English and published between 2009 and 2022. The diagnosis of COPD was based on GOLD in 5 studies (14, 26–28, 30), ATS in one study (29), and clinical evaluation in one study (25). In 4 studies only male gender (14, 25, 27, 29), in one study only female gender (30), and in two studies both genders were selected (26, 28). Age and BMI values were reported in all studies. In one study, the FEV1 (%) value was reported only in the COPD group (14), while in 5 studies, it was reported in both the COPD and control groups (25–27, 29, 30). TNF-α values were reported in two studies (26, 29), IL-6 in three studies (14, 27, 28), CRP in two studies (29, 30), fasting blood glucose (FBG) in two studies (26, 30), and insulin in two studies (30). Visfatin values based on the severity of COPD disease (GOLD grade) were reported in 3 studies (26–28), in one study, the number of samples was mentioned in GOLD grade IV, and the other GOLD grades did not have sample size (28). In 5 studies, the participants were under 65 years old (14, 25–28) and in two studies, they were over 65 years old (29, 30). In 4 studies, subjects had a BMI over than 25 kg/m2 (14, 27, 28, 30) and in 3 studies under 25 kg/m2 (25, 26, 29). In 4 studies, FEV1 (%) value was over than 50 (14, 27, 29, 30) and in 2 studies it was under 50 (25, 26) (Table 2).
3.4 Effectiveness
3.4.1 Visfatin circulating change level between COPD patients and control subjects
Figure 2 displays the forest plot of visfatin concentration. Given the heterogeneity between studies, random effects models were used to analyze the results (I2 = 97%, p < 0.001). The pooled results showed that although the circulating concentration of visfatin was lower in patients with COPD, no significant difference was observed (SMD: −0.48 mg/L; 95% CI: −1.67 to 0.70; p = 0.43).
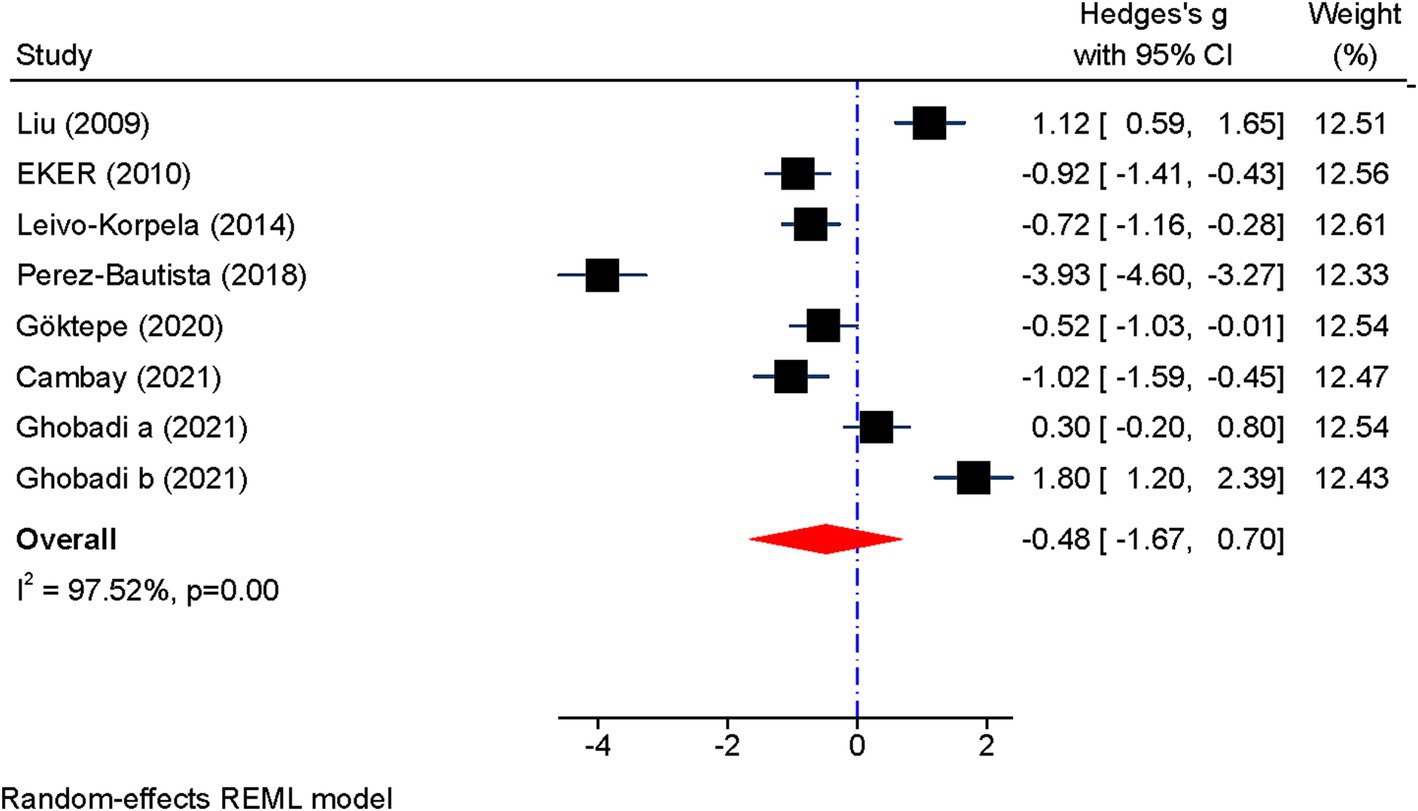
Figure 2. Forest plot showing the summary effect size for visfatin levels between COPD patients and control group.
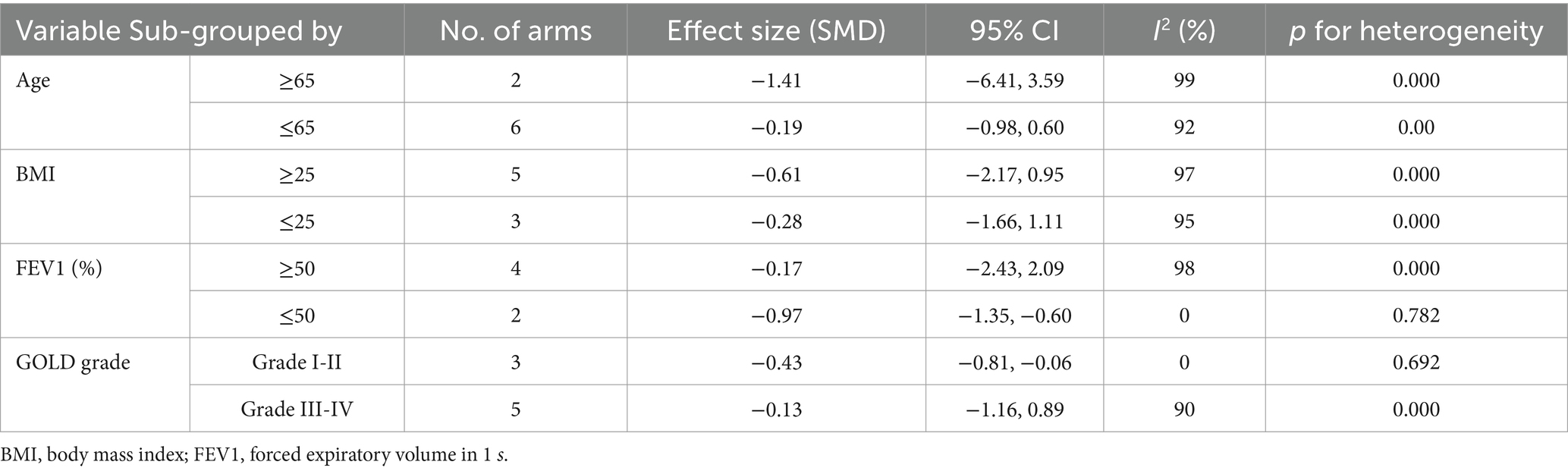
Subgroup analysis revealed that visfatin levels were significantly reduced in FEV1 less than 50% (SMD: −0.97, 95% CI: −1.35 to −0.60, p = 0.000); I2: 0%, p = 0.78). In addition, a significant decrease in visfatin levels was observed in GOLD grade I-II [(SMD: −0.43, 95% CI: −0.81 to-0.06, p = 0.022); I2 = 0%, p = 0.69]. However, subgroup analysis for BMI above and below 25 kg/m2 as well as age above and below 65 year had no effect on heterogeneity (Table 3).
3.4.2 Sensitivity analysis
The sensitivity analysis further demonstrated that omitting of studies had no impact on effect size of visfatin (Figure 3).
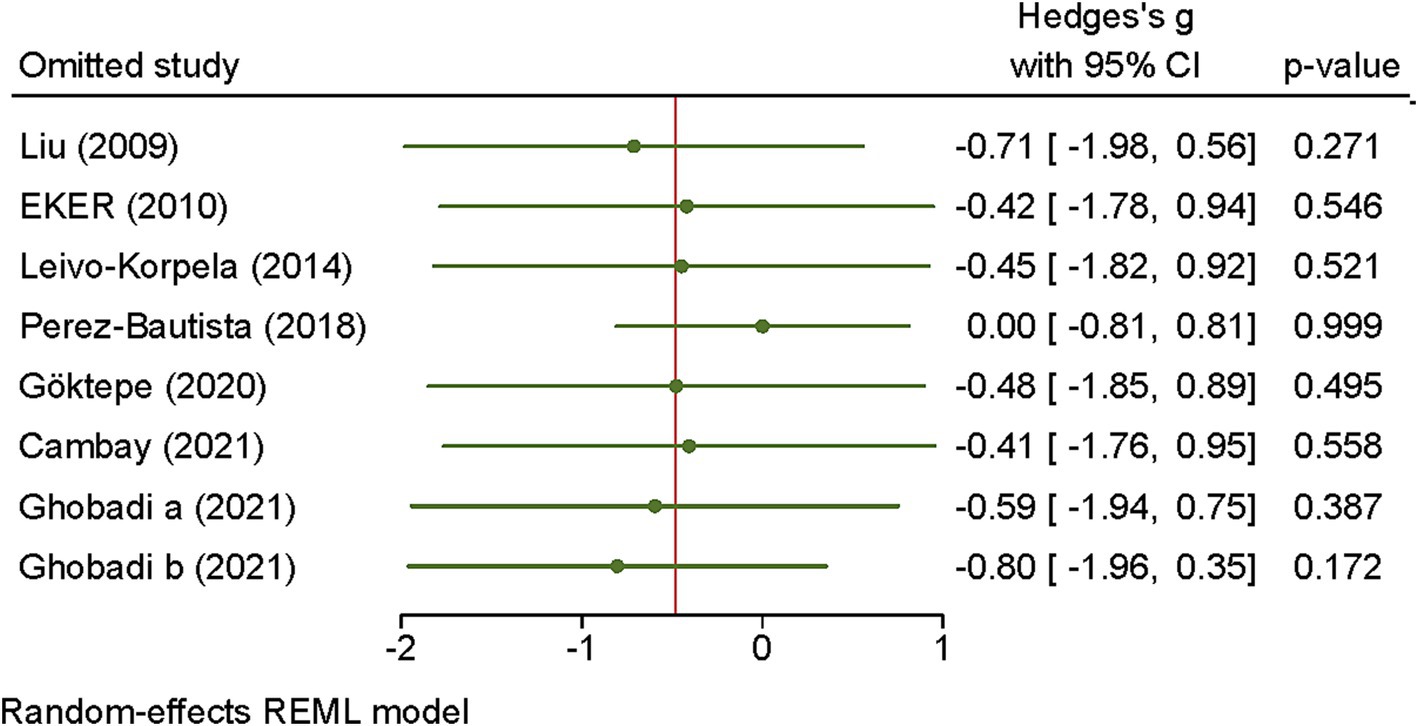
Figure 3. Sensitivity analysis of the association between visfatin and COPD. The influence of individual studies on the overall standardized mean difference (SMD) is shown. The middle vertical axis indicates the overall SMD. The circles represent the pooled SMD when the remaining study is omitted from the meta-analysis.
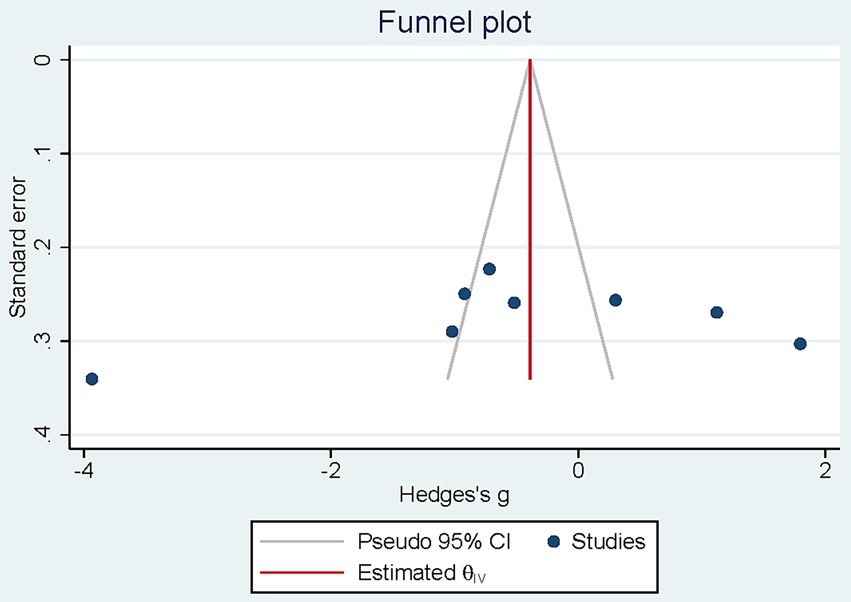
3.4.3 Publication bias
The visual examination of the funnel plots demonstrate any asymmetry. Results from Egger test (p = 0.308) revealed no considerable publication bias (Figure 4).
3.4.4 Meta-analysis of correlations between visfatin levels and inflammatory parameters
This study utilized correlational meta-analysis to examine the association between visfatin levels and inflammatory parameters. The findings of the meta-analysis demonstrated a notable positive association between visfatin concentrations and IL-6 (r = 0.46, 95% CI = 0.22 to 0.65, p < 0.001) and TNF-α (r = 0.34, 95% CI = 0.13 to 0.53, p < 0.01). No significant relationship was observed between visfatin levels and FEV1 (r = −0.13, 95% CI = −0.58 to 0.37) (Figure 5).
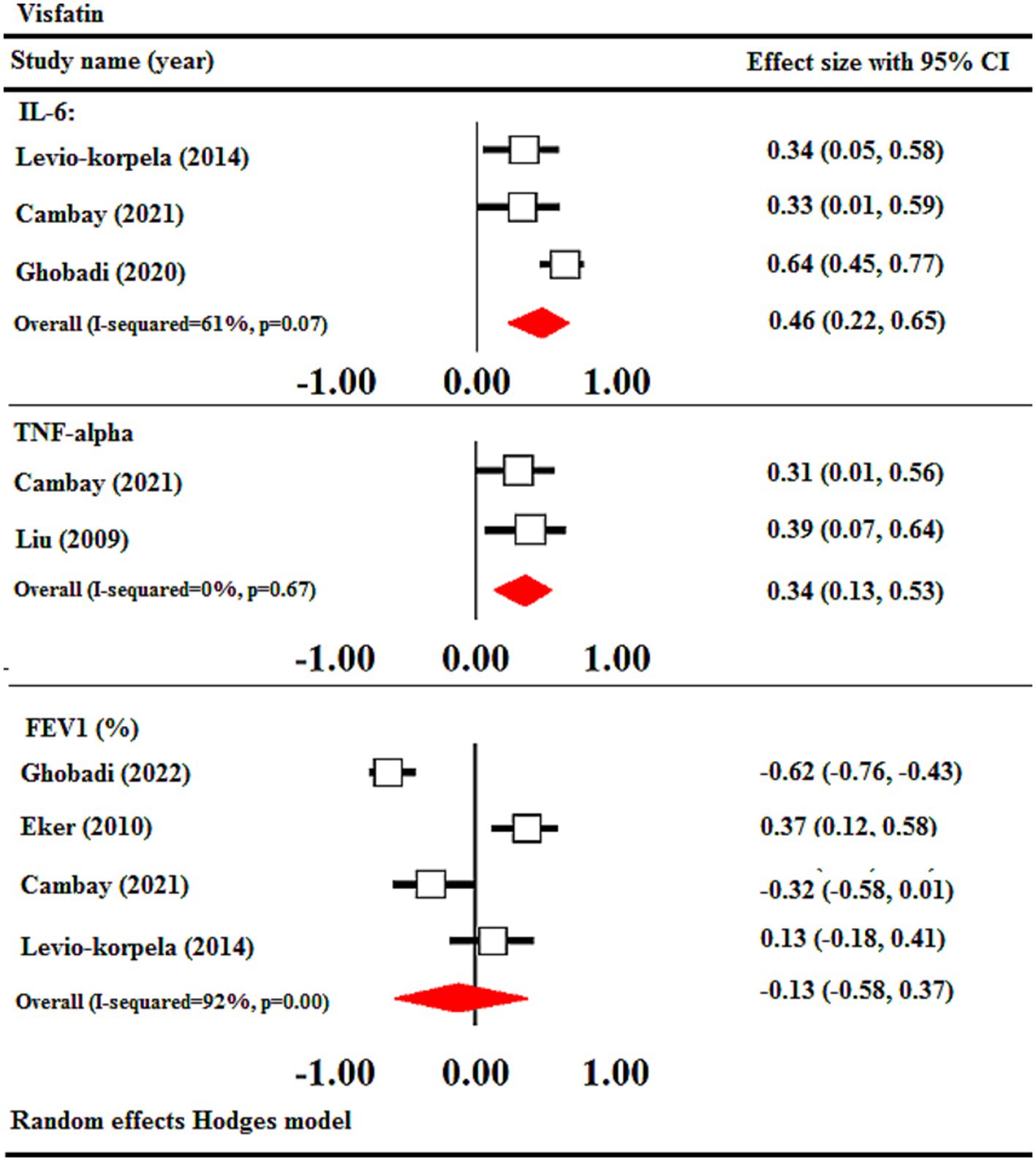
Figure 5. Meta-analysis of correlation coefficient between serum visfatin levels of COPD, IL-6, TNF-α, and FEV1. CI, confidence interval; IL-6, interleukin-6; TNF-α, tumor necrosis factor alpha; FEV1: forced expiratory volume in 1 s.
3.4.5 Meta-regression
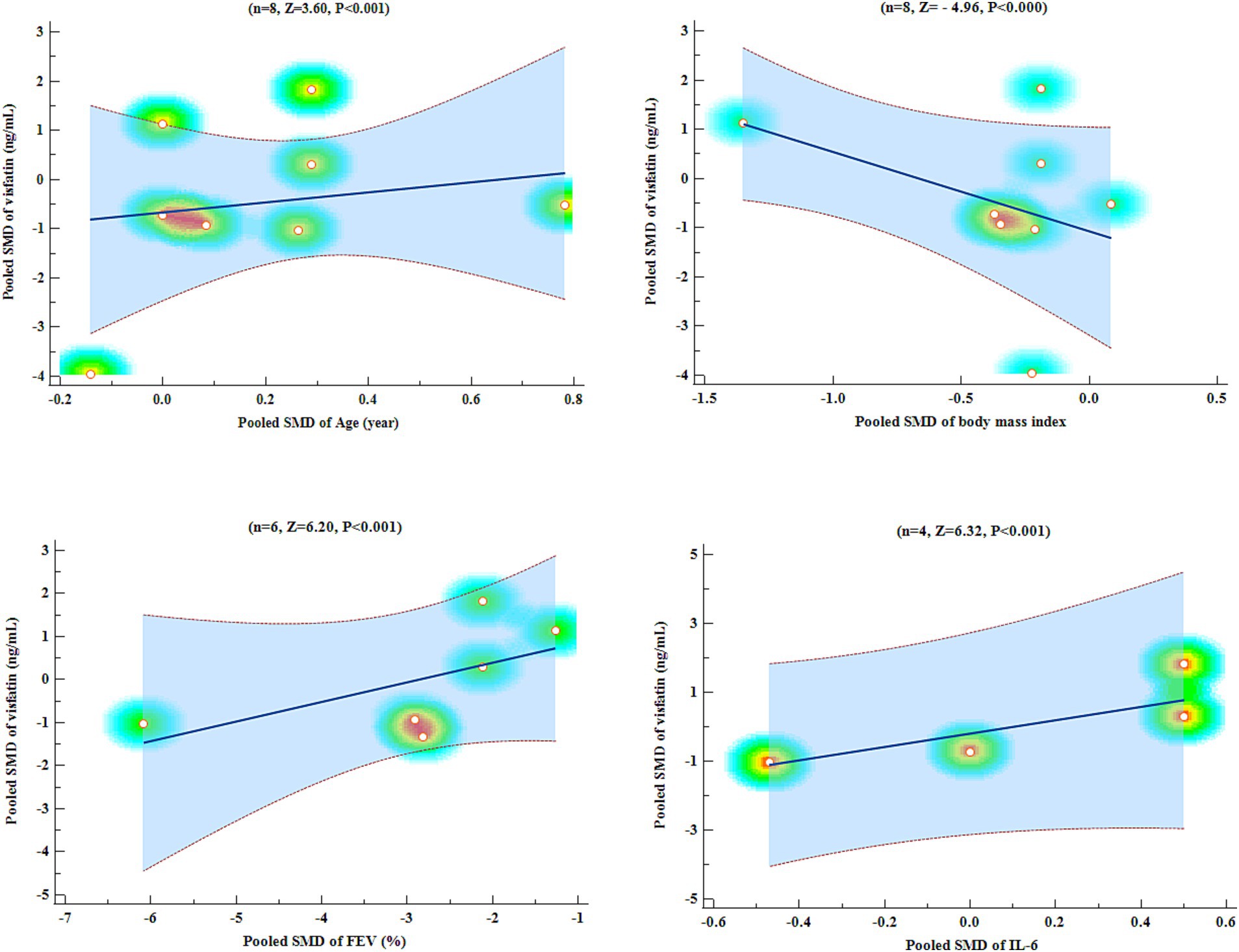
An analysis of meta-regression was carried out to compare the pooled SMD of visfatin with pooled SMD of variables such as age, BMI, FEV1, and IL-6. Meta-regression analysis showed that pooled SMD visfatin was significantly associated with pooled SMD of age (Z = 3.60, p < 0.001), BMI (Z = −4.96, p < 0.001), FEV1 (Z = 6.20, p < 0.001), and IL-6 (Z = 6.32, p < 0.001) (Figure 6).
4 Discussion
The aim of this meta-analysis was to explore the potential association of circulating visfatin levels and COPD. From 7 relevant case–control studies, the following results were obtained: (1) visfatin level in COPD patients compared to healthy subjects did not show any significant difference based on meta-analysis, (2) correlation meta-analysis results indicated that there was a significant positive correlation between visfatin levels and IL-6 and TNF-α, and (3) results of the meta-regression analysis indicated that there was a direct correlation between pooled SMD visfatin and pooled SMD in age, BMI, FEV1, and IL-6 levels.
It has been noted through research that adipokines are agents related to chronic low-grade inflammation in metabolic disorders and inflammatory diseases (31). Numerous studies on humans and animals have revealed modified adipokine levels in inflammatory lung diseases such as asthma and COPD (31). Moreover, in critically ill patients, elevated adipokine levels are typically linked to organ malfunction and tissue inflammation (32). It has been established that visfatin plays a role in certain chronic inflammatory diseases (14), however, its association with COPD is not clear. As with those with obesity and insulin resistance (33, 34), COPD patients show both elevated and reduced visfatin levels when compared to healthy individuals (25, 27).
Despite the findings of the recent meta-analysis showing no significant difference in visfatin levels between COPD patients and the control group, it is possible that the heterogeneity among the studies impacted the outcome. The findings of the subgroup analysis indicated that as FEV1 levels decreased and disease severity reached GOLD grade I-II, the heterogeneity within the study decreased. The findings demonstrated that different variables, like the diseases severity, influence visfatin outcomes in COPD patients and must be taken into account during patient selection. Visfatin is predominantly expressed in visceral adipose tissue. Results on the relationship between visfatin level and BMI are conflicting, as obese people have been observed to have higher, lower, or unchanged visfatin levels. It has been documented in some studies that underweight COPD patients had higher visfatin levels (35, 36). The cause of elevated visfatin levels in COPD patients with reduced BMI is not fully understood, yet it is speculated that severe systemic inflammation is likely to be a significant contributor (37).
A correlation meta-analysis demonstrated a significant positive association between visfatin levels and inflammatory factors such as IL-6 and TNF-α. Furthermore, meta-regression results indicated a significant correlation between visfatin level and IL-6 levels. While visfatin expression is mainly derived from visceral adipose tissue, macrophages, and dendritic cells are also able to produce visfatin (38). On the other hand, among the cells that play a key role in the pathophysiology of COPD are neutrophils and macrophages (7). Macrophages are considered as the main source of cytokines such as IL-6 and TNF-α. It is believed that macrophage activation causes the release of adipokines in patients with COPD (36). In addition, it has been observed that inflammatory cytokines such as IL-6, TNF-α, and IL-1β can stimulate visfatin expression (39). Interestingly, it has been revealed that visfatin itself induces the production of inflammatory factors (IL-6 and TNF-α) (36).
Overall, it appears that visfatin is pro-inflammatory factor in chronic diseases. An ongoing debate exists regarding visfatin’s pro-inflammatory nature and its decreased concentrations in COPD patients. This controversy could be explained by a number of factors. First, high and low levels of adipokines in chronic inflammatory diseases have been acknowledged to have an impact on the immune system (40). Lower concentrations of some adipokines have been linked to recurrent lung infections due to compromised immune function (14). Using GOLD criteria to evaluate visfatin levels in COPD patients could have been more precise as a study found heightened visfatin levels in severe GOLD grade (27). Finally, the meta-analysis investigated visfatin levels in serum/plasma samples of COPD patients. Previous research has demonstrated that adipokines can produce systemic and local effects. Investigating visfatin levels in the lung tissue appears to provide more precise assessments of alterations in its levels.
5 Limitations
This study had some limitations. At first, there were a limited number of studies included in the meta-analysis, therefore more studies are required for an in-depth analysis. Because only articles published in English were selected for the study, the small number of articles included may have an impact, which could be considered a limitation of the study. Second, there might be bias in the current results because some studies were not included or some data was inaccessible. The connection between visfatin levels and disease severity in COPD patients was particularly evident in this case. Within the meta-analysis, research on visfatin outcomes were analyzed with reference to GOLD grade in certain studies. Moreover, the studies failed to address whether COPD patients were hospitalized during their stable or exacerbation phases, possibly impacting the integrity of the results. The study was limited by the absence of details on patient’s treatment conditions, such as their current medication, duration of illness, and etc. Previous research has demonstrated that visfatin plays a pivotal role as a pro-inflammatory factor. Therapeutic interventions in patients with COPD may have been effective in modulating the results of visfatin levels, which should be considered as an influential factor. Additionally, changes in visfatin levels may be influenced by both comorbidities and metabolic conditions within COPD patients, variables that were not taken into consideration in the included studies.
6 Conclusion
The pooled analysis of visfatin levels in COPD patients versus healthy subjects indicated no notable variance between the groups. The high levels of heterogeneity among the studies may have played a role in this lack of differentiation. The subgroup analysis conducted using FEV1 and disease severity (GOLD grade) revealed them as primary sources of heterogeneity in the studies, while acknowledging the potential influence of other variables like BMI and age. It is suggested that additional studies be conducted in order to better interpret the results and identify the sources of heterogeneity. In the present study, a correlation meta-analysis indicated a positive association between visfatin levels and inflammatory factors including IL-6 and TNF-α. In future research studies, it may be beneficial to closely analyze visfatin levels in patients with COPD while taking into account their inflammatory conditions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This study was conducted after approved by the ethics committee of the Iran University of Medical Sciences (IR.IUMS.REC.1402.239).
Author contributions
NA: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing. AM: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Resources, Software, Writing – original draft. HM: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft. MA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research funded by Iran University of Medical Sciences, Tehran, Iran.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1432025/full#supplementary-material
References
1. Singh, D, Agusti, A, Anzueto, A, Barnes, PJ, Bourbeau, J, Celli, BR, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. (2019) 53:1900164. doi: 10.1183/13993003.00164-2019
2. Amani, M, Ghadimi, N, Aslani, MR, and Ghobadi, H. Correlation of serum vascular adhesion protein-1 with airflow limitation and quality of life in stable chronic obstructive pulmonary disease. Respir Med. (2017) 132:149–53. doi: 10.1016/j.rmed.2017.10.011
3. Hosseninia, S, Ghobadi, H, Garjani, K, Hosseini, SAH, and Aslani, MR. Aggregate index of systemic inflammation (AISI) in admission as a reliable predictor of mortality in COPD patients with COVID-19. BMC Pulm Med. (2023) 23:107. doi: 10.1186/s12890-023-02397-5
4. Wang, C, Xu, J, Yang, L, Xu, Y, Zhang, X, Bai, C, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. (2018) 391:1706–17. doi: 10.1016/S0140-6736(18)30841-9
5. Ghobadi, H, Aslani, MR, Hosseinian, A, and Farzaneh, E. The correlation of serum brain natriuretic peptide and Interleukin-6 with quality of life using the chronic obstructive pulmonary disease assessment test. Med Princ Pract. (2017) 26:509–15. doi: 10.1159/000484900
6. Halpin, DMG, Criner, GJ, Papi, A, Singh, D, Anzueto, A, Martinez, FJ, et al. Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. The 2020 GOLD science committee report on COVID-19 and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2021) 203:24–36. doi: 10.1164/rccm.202009-3533SO
7. Barnes, PJ. Cellular and molecular mechanisms of asthma and COPD. Clin Sci (Lond). (2017) 131:1541–58. doi: 10.1042/CS20160487
8. Ghobadi, H, Abdollahi, N, Madani, H, and Aslani, MR. Effect of Crocin from saffron (Crocus sativus L.) supplementation on oxidant/antioxidant markers, exercise capacity, and pulmonary function tests in COPD patients: a randomized, double-blind, placebo-controlled trial. Front Pharmacol. (2022) 13:884710. doi: 10.3389/fphar.2022.884710
9. Ghobadi, H, Hosseini, N, and Aslani, MR. Correlations between serum decoy receptor 3 and airflow limitation and quality of life in male patients with stable stage and acute exacerbation of COPD. Lung. (2020) 198:515–23. doi: 10.1007/s00408-020-00348-z
10. Aslani, MR, Abdollahi, N, Matin, S, Zakeri, A, and Ghobadi, H. Effect of crocin of Crocus sativus L. on serum inflammatory markers (IL-6 and TNF-α) in chronic obstructive pulmonary disease patients: a randomised, double-blind, placebo-controlled trial. Br J Nutr. (2023) 130:446–53. doi: 10.1017/S0007114522003397
11. Aslani, MR, Ghazaei, Z, and Ghobadi, H. Correlation of serum fatty acid binding protein-4 and interleukin-6 with airflow limitation and quality of life in stable and acute exacerbation of COPD. Turk J Med Sci. (2020) 50:337–45. doi: 10.3906/sag-1909-9
12. Ghobadi, H, Alipour, MR, Keyhanmanesh, R, Boskabady, MH, and Aslani, MR. Effect of high-fat diet on tracheal responsiveness to methacholine and insulin resistance index in ovalbumin-sensitized male and female rats. Iran J Allergy Asthma Immunol. (2019) 18:48–61. doi: 10.18502/ijaai.v18i1.630
13. Aslani, MR, Keyhanmanesh, R, and Alipour, MR. Increased Visfatin expression is associated with nuclear factor-κB in obese ovalbumin-sensitized male Wistar rat tracheae. Med Princ Pract. (2017) 26:351–8. doi: 10.1159/000475772
14. Leivo-Korpela, S, Lehtimäki, L, Hämälainen, M, Vuolteenaho, K, Kööbi, L, Järvenpää, R, et al. Adipokines NUCB2/nesfatin-1 and visfatin as novel inflammatory factors in chronic obstructive pulmonary disease. Mediat Inflamm. (2014) 2014:232167:1–6. doi: 10.1155/2014/232167
15. Avesta, L, Doustkami, H, Zamani, B, Nejati, A, Mousavy, S, and Aslani, MR. Association of plasma visfatin with epicardial fat thickness and severity of coronary artery diseases in patients with acute myocardial infarction and stable angina pectoris. ARYA Atheroscler. (2022) 18:1–10. doi: 10.48305/arya.v18i0.2262
16. Lynn, H, Sun, X, Casanova, NG, Bime, C, Reyes Hernon, V, Lanham, C, et al. Linkage of NAMPT promoter variants to eNAMPT secretion, plasma eNAMPT levels, and ARDS severity. Ther Adv Respir Dis. (2023) 17:17534666231181262. doi: 10.1177/17534666231181262
17. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
18. Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
19. Noll, L, Mitham, K, Moran, J, and Mallows, A. Identifying current uses of return to work screening tests and their effectiveness of reducing the risk of reinjury in athletic occupations – a systematic review. Phys Ther Sport. (2022) 58:141–50. doi: 10.1016/j.ptsp.2022.10.010
20. Ayada, C, Toru, Ü, Genc, O, Sahin, S, Arik, Ö, and Bulut, I. Serum levels of leptin and visfatin in chronic obstructive pulmonary disease. Eur Respiratory Soc. (2015):PA3669. doi: 10.1183/13993003.congress-2015.PA3669
21. Ochman, M, Stanjek-Cichoracka, A, Latos, M, Wojarski, J, Kukla, M, Woźniak-Grygiel, E, et al. Serum Adipokine levels in patients considered for lung transplantation. Transplant Proc. (2018) 50:2039–43. doi: 10.1016/j.transproceed.2018.02.169
22. Dimov, D, Tacheva, T, Zhelyazkova, Y, O’Donoghue, N, Vlaykova, D, and Vlaykova, T. Visfatin as a possible serum biomarker in COPD. Eur Respiratory Soc. (2019). doi: 10.1183/13993003.congress-2019.PA5408
23. Ochman, M, Maruszewski, M, Wojarski, J, Żegleń, S, Karolak, W, Stanjek-Cichoracka, A, et al. Serum levels of Visfatin, Omentin and Irisin in patients with end-stage lung disease before and after lung transplantation. Ann Transplant. (2017) 22:761–8. doi: 10.12659/AOT.904994
24. Salman, HH, Shaker Al-joda, BM, and Abdulridh Alshok, MM. Assessment of serum Visfatin levels in patients with chronic obstructive pulmonary diseases in Babylon-Iraq. Indian J Forensic Med Toxicol. (2020) 14:2366–72. doi: 10.37506/ijfmt.v14i3.10789
25. Cambay, Z, Ilhan, N, Susam, S, and Muz, MH. BMI and adipocytokine changes in COPD exacerbation and stable COPD. Indian J Biochem Biophys. (2021) 58:472–7. doi: 10.56042/ijbb.v58i5.25958
26. Eker, S, Ayaz, L, Tamer, L, and Ulubas, B. Leptin, visfatin, insulin resistance, and body composition change in chronic obstructive pulmonary disease. Scand J Clin Lab Invest. (2010) 70:40–4. doi: 10.3109/00365510903484063
27. Ghobadi, H, Mokhtari, S, and Aslani, MR. Serum levels of visfatin, sirtuin-1, and interleukin-6 in stable and acute exacerbation of chronic obstructive pulmonary disease. J Res Med Sci. (2021) 26:17. doi: 10.4103/wjrms.JRMS_626_19
28. Göktepe, M, Korkmaz, C, Zamani, A, Demirbaş, S, and Kılınç, İ. Evaluation of serum resistin, visfatin, and chemerin levels in patients with lung cancer and chronic obstructive pulmonary disease. Turk Thorac J. (2020) 21:169–73. doi: 10.5152/TurkThoracJ.2019.19001
29. Liu, X, Ji, Y, Chen, J, Li, S, and Luo, F. Circulating visfatin in chronic obstructive pulmonary disease. Nutrition. (2009) 25:373–8. doi: 10.1016/j.nut.2008.09.008
30. Pérez-Bautista, O, Montaño, M, Pérez-Padilla, R, Zúñiga-Ramos, J, Camacho-Priego, M, Barrientos-Gutiérrez, T, et al. Women with COPD by biomass show different serum profile of adipokines, incretins, and peptide hormones than smokers. Respir Res. (2018) 19:239. doi: 10.1186/s12931-018-0943-4
31. Keyhanmanesh, R, Alipour, MR, Ebrahimi, H, and Aslani, MR. Effects of diet-induced obesity on tracheal responsiveness to methacholine, tracheal Visfatin level, and lung histological changes in ovalbumin-sensitized female Wistar rats. Inflammation. (2018) 41:846–58. doi: 10.1007/s10753-018-0738-2
32. Hajri, T, Gharib, M, Kaul, S, and Karpeh, MS Jr. Association between adipokines and critical illness outcomes. J Trauma Acute Care Surg. (2017) 83:507–19. doi: 10.1097/TA.0000000000001610
33. Filippatos, TD, Derdemezis, CS, Kiortsis, DN, Tselepis, AD, and Elisaf, MS. Increased plasma levels of visfatin/pre-B cell colony-enhancing factor in obese and overweight patients with metabolic syndrome. J Endocrinol Investig. (2007) 30:323–6. doi: 10.1007/BF03346300
34. Zahorska-Markiewicz, B, Olszanecka-Glinianowicz, M, Janowska, J, Kocełak, P, Semik-Grabarczyk, E, Holecki, M, et al. Serum concentration of visfatin in obese women. Metabolism. (2007) 56:1131–4. doi: 10.1016/j.metabol.2007.04.007
35. Makki, K, Froguel, P, and Wolowczuk, I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. (2013) 2013:139239:1–12. doi: 10.1155/2013/139239
36. Moschen, AR, Kaser, A, Enrich, B, Mosheimer, B, Theurl, M, Niederegger, H, et al. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol. (2007) 178:1748–58. doi: 10.4049/jimmunol.178.3.1748
37. Koehler, F, Doehner, W, Hoernig, S, Witt, C, Anker, SD, and John, M. Anorexia in chronic obstructive pulmonary disease--association to cachexia and hormonal derangement. Int J Cardiol. (2007) 119:83–9. doi: 10.1016/j.ijcard.2006.07.088
38. Fantuzzi, G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. (2005) 115:911–9. doi: 10.1016/j.jaci.2005.02.023
39. Ognjanovic, S, Bao, S, Yamamoto, SY, Garibay-Tupas, J, Samal, B, and Bryant-Greenwood, GD. Genomic organization of the gene coding for human pre-B-cell colony enhancing factor and expression in human fetal membranes. J Mol Endocrinol. (2001) 26:107–17. doi: 10.1677/jme.0.0260107
Keywords: visfatin, meta-analysis, COPD, IL-6, BMI
Citation: Aboutaleb N, Moradi A, Mirshekari Jahangiri H and Aslani MR (2025) Circulating visfatin concentrations in patients with chronic obstructive pulmonary disease: systematic review and meta-analysis. Front. Med. 11:1432025. doi: 10.3389/fmed.2024.1432025
Edited by:
Xiaoguang Sun, University of Arizona, United StatesReviewed by:
Valentina Petkova, Medical University of Sofia, BulgariaMohamed Chahboune, Hassan First University of Settat, Morocco
Copyright © 2025 Aboutaleb, Moradi, Mirshekari Jahangiri and Aslani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Reza Aslani, bXJhc2xhbmkxMDVAeWFob28uY29t; bXIuYXNsYW5pQGFydW1zLmFjLmly
†ORCID: Nahid Aboutaleb, orcid.org/0000-0002-7514-5939
Alireza Moradi, orcid.org/0000-0002-2792-513x
Hamzeh Mirshekari Jahangiri, orcid.org/0000-0002-7186-467x
Mohammad Reza Aslani, orcid.org/0000-0003-1519-7611
 Nahid Aboutaleb
Nahid Aboutaleb Alireza Moradi1†
Alireza Moradi1† Mohammad Reza Aslani
Mohammad Reza Aslani