- 1Department of Anesthesiology, Peking University First Hospital, Beijing, China
- 2Department of Anesthesiology, The University of Hong Kong Shen Zhen Hospital, Shen Zhen, China
- 3Department of Obstetrics and Gynecology, Peking University First Hospital, Beijing, China
Purpose: Gynecologic oncology laparotomy leads to severe postoperative pain. We aimed to evaluate the effects of preemptive multimodal analgesic regimen on postoperative opioid consumption for patients undergoing gynecologic oncology laparotomy.
Methods: In this prospective, randomized clinical trial, 80 female patients scheduled for gynecologic oncology laparotomy were randomized to receive preemptive multimodal analgesia consisted of transversus abdominis plane (TAP) block, cyclooxygenase−2 inhibitors, acetaminophen and intravenous morphine patient-controlled analgesia (PCA) (Study group) or conventional analgesia with cyclooxygenase−2 inhibitors and morphine PCA (Control group). The primary outcome was morphine consumption in the first 24 h after surgery. Secondary outcomes were pain scores, nausea, vomiting, time to ambulation and flatus, length of hospital stay, satisfaction score, the 40-item Quality of Recovery score (QoR-40) and the Short-Form Health Survey (SF-36) scale.
Results: Morphine consumption in the first 24 h was 6 (3–9.8) mg in the Study group and 7 (3.5–12.5) mg in the Control group (p = 0.222). The Study group showed lower morphine consumption up to 6 h, lower pain scores up to 48 h, and earlier time to ambulation and flatus. The global QoR-40 score at 48 h [182 (173–195) vs. 173.5 (154–185.5), p = 0.024], subdimension scores of physical dependence at 24 h, physical comfort and pain at 48 h were significantly improved in the Study group.
Conclusion: Preemptive multimodal analgesia was not superior to conventional analgesia in reducing 24 h morphine consumption; however, it showed a significantly improved pain control and early quality of recovery thus can be recommended for gynecologic oncology patients undergoing laparotomy.
Introduction
Gynecologic malignancy is one of the most common tumors affecting women throughout the world. Gynecologic oncology surgery can vary from minimally invasive laparoscopic surgery to major debulking procedures. Midline laparotomy often results in more severe postoperative pain than minimally invasive surgery. Since insufficient pain control may lead to chronic postsurgical pain, and the incidence and severity of chronic pain could be reduced or prevented by well-managed perioperative pain, adequate pain control is critical to patient recovery and often more challenging in the perioperative period (1, 2).
Neuraxial block with superior analgesia and better recovery of gastrointestinal function has once been considered as the gold standard of pain control after major abdominal surgery (3–5). However, perioperative venous thromboembolism prophylaxis and fast track protocols have questioned the position of epidural analgesia as a preferred analgesic technique, thus less invasive techniques including continuous wound infiltration, paravertebral and transversus abdominis plane (TAP) blocks are now widely used for abdominal surgery. Recent studies confirmed that TAP block has decreased incidence of hypotension when compared to epidural analgesia in major abdominal surgery (6, 7).
As a key component of Enhanced Recovery After Surgery (ERAS) pathway, multimodal analgesia using multiple pharmacologic agents with different analgesic mechanisms of action is recommended to improve patient’s recovery. Multimodal analgesia has been demonstrated to result in less opioid consumption and reduced length of hospital stay for different types of surgery (8–10).
TAP block resulted in reduced pain scores and opioid consumption when used as part of multimodal pain protocol in abdominal procedures (11–15). To our knowledge, there is limited evidence for the effect of preemptive TAP block-based multimodal analgesia on gynecologic oncology laparotomy (16–19). We hypothesized that preemptive TAP block-based multimodal analgesia would reduce total opioid consumption in the first 24 h compared to conventional analgesia. The primary aim of this study was to compare the total morphine consumption in the first 24 h when patient was randomized to either preemptive TAP block-based multimodal analgesia or conventional analgesia. Secondary aims included pain scores, nausea and vomiting, function recovery, length of hospital stay, satisfaction score, patient-reported quality of recovery measured using the 40-item Quality of Recovery score (QoR-40) and the Short-Form Health Survey (SF-36).
Materials and methods
Study design
This single-center, prospective randomized controlled trial was conducted at Peking University First Hospital, with ethical approval (No. 2019–199) provided by the Ethics Committee of Peking University First Hospital, Peking, China on 11 September 2019. The study was registered prior to patient enrollment at chictr.org.cn (ChiCTR 2,000,029,903; Principal investigator: Bojie Wang; Date of registration: February 16, 2020). Written informed consent was obtained from all patients participating in the trial. This manuscript adheres to the applicable Consolidated Standards of Reporting Trials (CONSORT) guidelines.
Inclusion and exclusion criteria
From August 2021 to August 2022, all female patients scheduled for elective gynecologic oncology laparotomy were screened for eligibility. Eligible criteria included 18–65 years of age, and American Society of Anesthesiologists (ASA) physical status I-III. Patients with significant liver or renal disease, history of peptic ulcer or gastrointestinal bleeding, contraindications or sensitivities to any medication used in the study, recent use of analgesic drug, known history of chronic pain disorders, inability to understand how to use the patient-controlled analgesia (PCA) device or communicate with research personnel were excluded.
Randomization and blinding
The enrolled patients were assigned to the Study group or Control group with a ratio of 1:1. Randomization was performed using a random number generated by the computer and each number sealed in an opaque envelope by a research nurse who was not involved in this study. On the day of surgery, the envelope with allocation information was delivered to the anesthetic provider. The patients and investigators involved in outcome assessing and data collection were blinded to the group allocation.
Anesthesia technique
Following standard monitoring, general anesthesia was induced with midazolam 0.03 mg/kg, propofol 1.5–2 mg/kg, and sufentanil 0.2 μg/kg. Rocuronium 0.6 mg/kg was given to facilitate tracheal intubation. Anesthesia was maintained with continuous infusion of propofol, target-controlled infusion of remifentanil, and intermittent bolus of sufentanil and rocuronium. Mean blood pressure and heart rate were kept within 20% of the baseline values, and the bispectral index was maintained between 40 and 60. Antiemetic therapy comprised of dexamethasone 5 mg before induction and tropisetron 5 mg during wound closure. After completion of the surgery, continuous infusion of remifentanil and propofol was ceased. Residual curarization was reversed with neostigmine and atropine. After extubation, the patient was transferred to the post-anesthesia care unit (PACU) for recovery.
Intervention
The Study group received preemptive multimodal analgesia consisted of TAP block and parecoxib before incision, acetaminophen, celecoxib, and morphine PCA after procedure. After induction, ultrasound-guided posterior TAP block was performed before incision. After negative aspiration of blood, the correct position of the needle tip was confirmed by observed distending when 1 mL of normal saline was injected. 20ml of ropivacaine 0.375% was injected on each side between the internal oblique and transversus abdominis plane under direct visualization. Intravenous (IV) parecoxib sodium 40 mg was administered before incision. Another dose of parecoxib sodium 40 mg was administered 6 h after surgery in the surgical ward. Oral acetaminophen 650 mg every 8 h and celecoxib 200 mg every 12 h were administered on postoperative day (POD) 1 and 2. The Control group received conventional analgesic protocol including parecoxib sodium 40 mg before the end of procedure and morphine PCA.
In the PACU, all patients were given a morphine IV PCA pump which was programmed to deliver 1 mg bolus on demand with 6-min lockout interval without continuous dose as part of multimodal analgesia. Pain intensity was measured with an 11-point numerical rating scale (NRS; 0 = no pain and 10 = worst imaginable pain). Additional morphine 1–2 mg bolus was administered as rescue analgesia when breakthrough pain (NRS ≥ 4) appeared despite IV PCA administration. Nausea intensity was assessed with a verbal rating scale (0 = none, 1 = mild, 2 = moderate, 3 = severe). IV metoclopramide 5 mg was administered as rescue antiemetics for severe degree of nausea or any vomiting that patients were unable to tolerate. In the surgical ward, deep vein thrombosis prophylaxis was administered with lower extremity sequential compression devices and subcutaneous low molecular heparin. At 48 h, the doses of morphine PCA and rescued morphine in PACU were recorded as the total morphine consumption.
Patient demographics included age, weight, height, type of the surgical incision, duration of surgery, and anesthetic doses were recorded. The observation period started from the end of surgery. A blinded researcher assessed the patients at PACU, 2, 6, 24 and 48 h after surgery. After discharge, patients were followed up on POD 30 to complete the SF-36 Health Survey Questionnaire. An investigator blinded to the group allocation collected all perioperative and outcome data.
Outcomes
The primary outcome was the total morphine consumption within the first 24 h after surgery. The secondary outcomes included: (1): NRS scores at rest and during coughing; (2) Morphine consumption at other time points; (3) Nausea, vomiting, and rescued antiemetics within the first 48 h; (4) Time to first ambulation and flatus; (5) Quality of recovery assessed with QoR-40 score at 24 and 48 h; (6) Patient satisfaction (0 = totally unsatisfied, 10 = total satisfied) assessed at 48 h; (7) Quality of life measured with the SF-36 questionnaire on POD 30; (8) length of hospital stay; (9) Major postoperative complications including surgical site infection, urinary tract infection, sepsis, pneumonia, myocardial infarction, renal insufficiency, red blood transfusion, thromboembolic events, intensive care unit (ICU) admission, and requiring repeat surgery.
Sample size calculation
Sample size calculation was based on previous studies involving TAP block-based analgesia after gynecologic laparotomy (13, 17, 18). To detect a 30% reduction in postoperative morphine consumption, the sample size was 36 patients in each group with a power of 0.80 and a 2-tailed alpha of 0.05. Considering a 10% possible dropout, we planned to recruit a total of 80 patients in the study.
Statistical analysis
The data were analyzed with SPSS version 22.0 (IBM, United States). Continuous variables were reported as means ± standard deviation (SD) or medians [interquartile ranges (IQRs)] according to the normality of data distribution checked by the Shapiro–Wilk test. The significance of differences between groups was compared using 2 independent sample Student’s t test or Mann–Whitney U test. The differences of the medians and 95% confidence interval (CI) were estimated using the Hodges–Lehman method. Bonferroni correction was used and the p value was adjusted for repeated measurements. Categorical variables were described as number (percentage), and compared using Pearson’s χ2 test or Fisher’s exact test as appropriate. All p values were 2-sided, and p < 0.05 was deemed statistically significant.
Results
From August 2021 to August 2022, 92 patients were assessed for eligibility. 12 patients were excluded for not meeting the inclusion criteria (n = 10) or declining to participate (n = 2). Thus, 80 patients were randomized to either the Study group or the Control group. All patients received analgesia in compliance with the protocol and were included in the intention-to-treat analysis of primary outcome. The CONSORT flow diagram is presented in Figure 1.
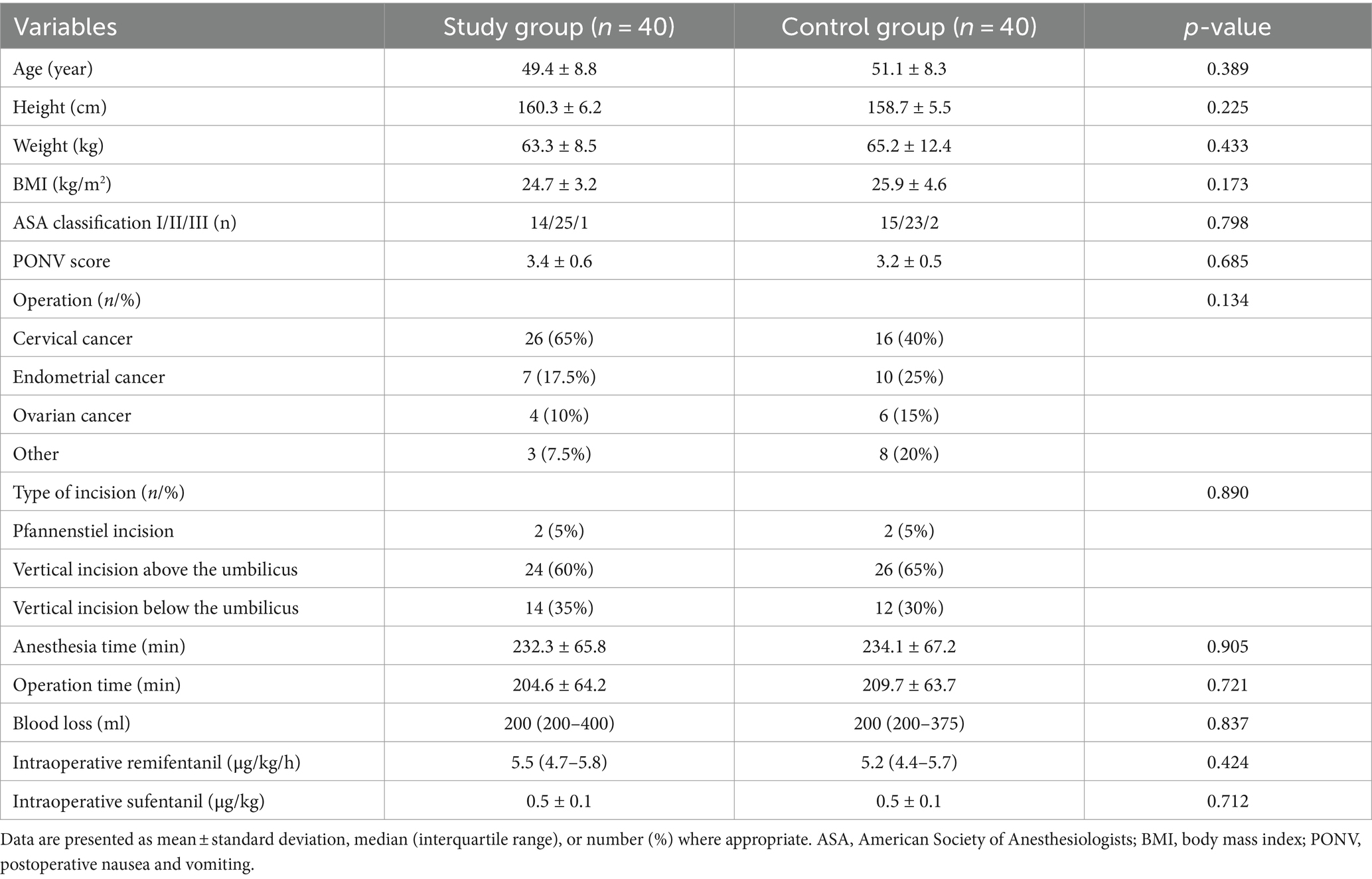
The two groups were similar in baseline demographics and operation characteristics. There were no significant differences in intraoperative propofol, remifentanil, or sufentanil doses between two groups (Table 1).
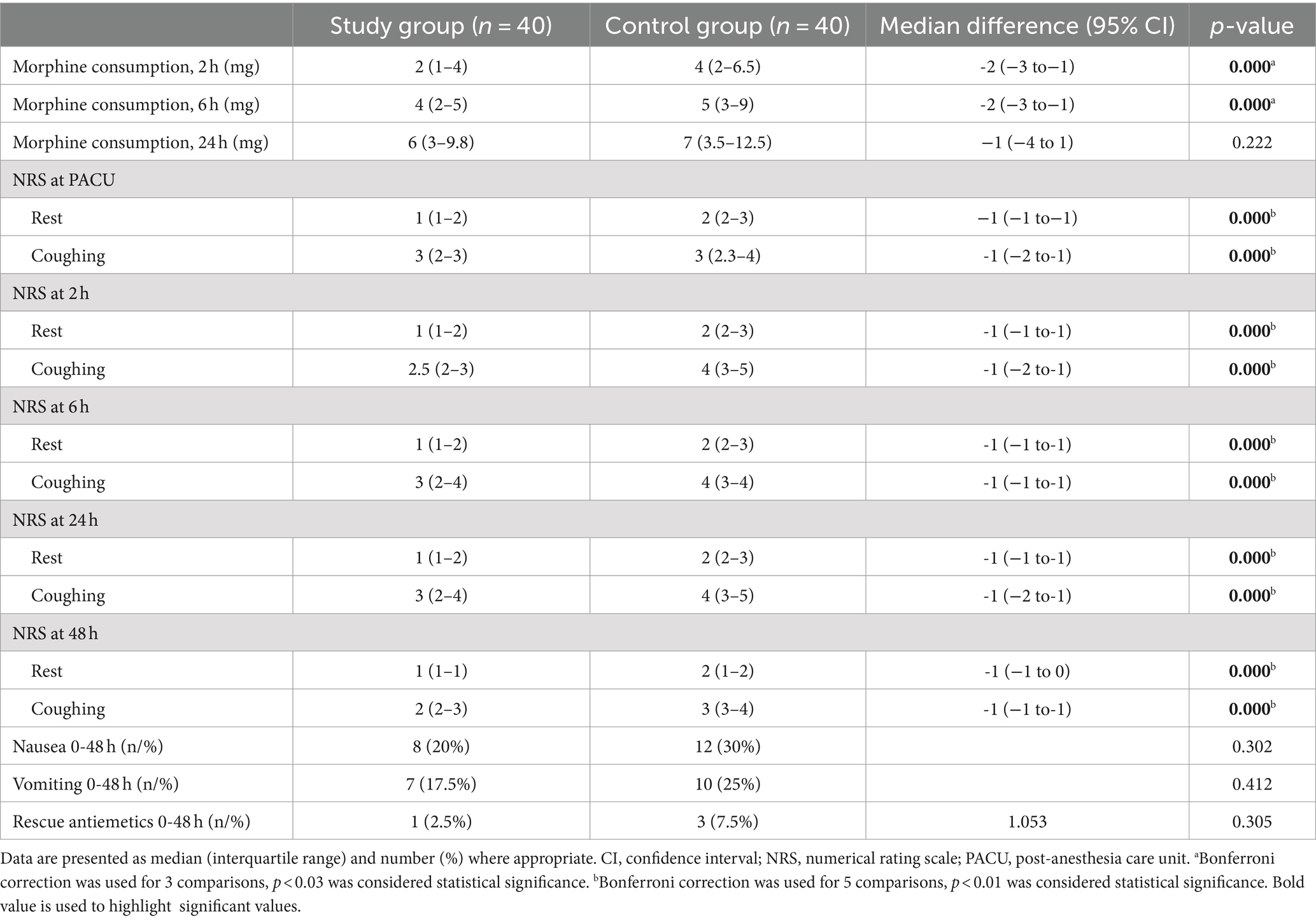
Morphine consumption within the first 24 h was similar between the two groups [Study group: 6 (3–9) mg vs. Control group: 7 (3.5–12.5) mg, mean difference, −1 (95% confidence interval CI, −4 to 1) mg, p = 0.222]. For secondary outcomes, the Study group had significantly less morphine consumption up to 6 h [4 (2–5) mg vs. 5 (3–9) mg, mean difference, −2 (95% CI, −3 to−1) mg, p = 0.000], lower pain scores at rest and during coughing at any time point up to 48 h than the Control group (Table 2).
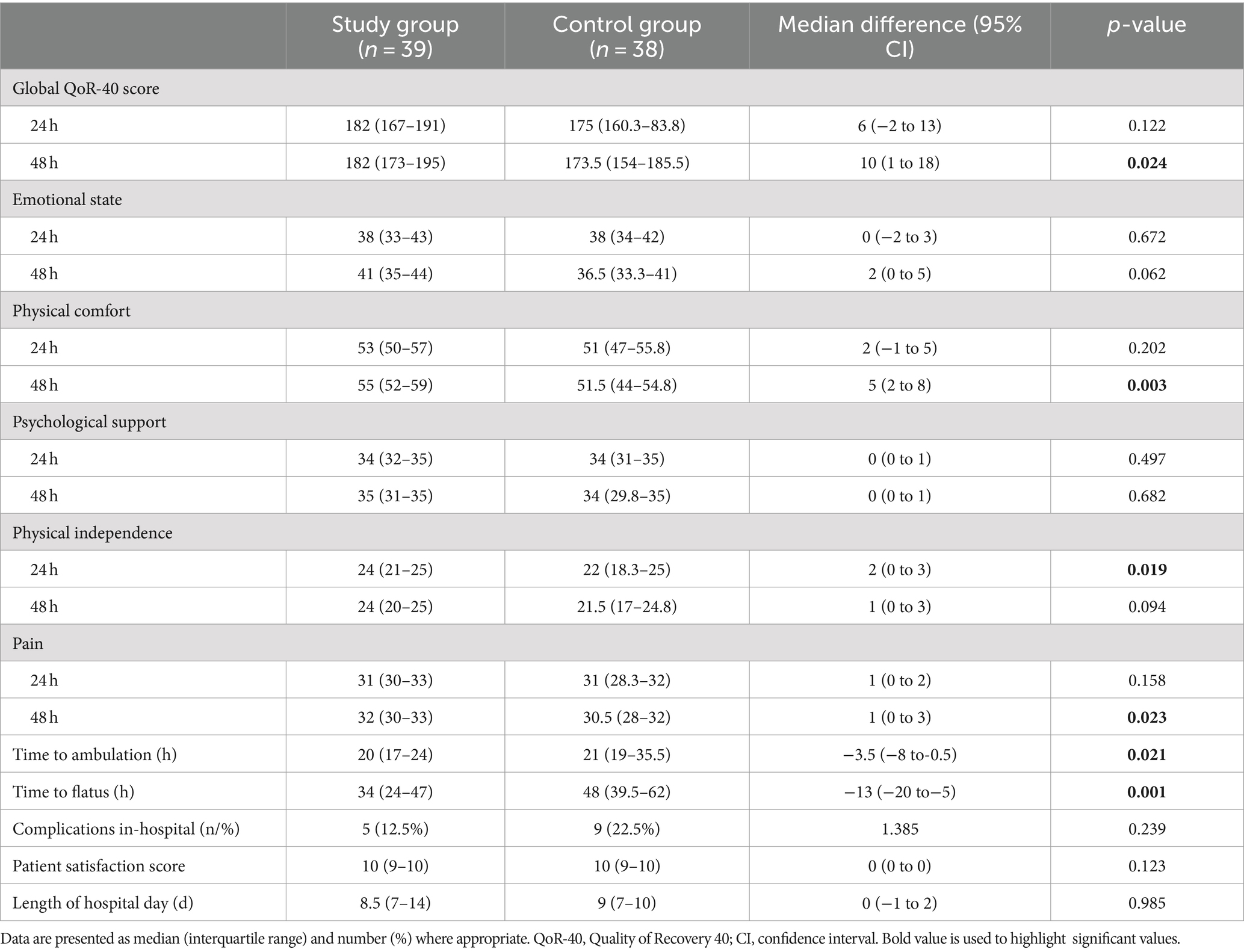
The global QoR-40 score at 48 h was significantly higher in the Study group compared to the Control group [182 (173–195) vs. 174 (154–186), p = 0.024]. Among five dimensions of QoR-40, physical dependence at 24 h [24 (21–25) vs. 22 (18–25), p = 0.019], physical comfort [55 (52–59) vs. 51 (44–55), p = 0.003] and pain at 48 h [32 (30–33) vs. 31 (28–32), p = 0.023] were significantly improved in the Study group (Table 3).
Patients in the Study group had shorter time to ambulation [20 (17–24) h vs. 21 (19–35.5) h, p = 0.021] and flatus [34 (24–47) h vs. 48 (39.5–62) h, p = 0.001] than the Control group.
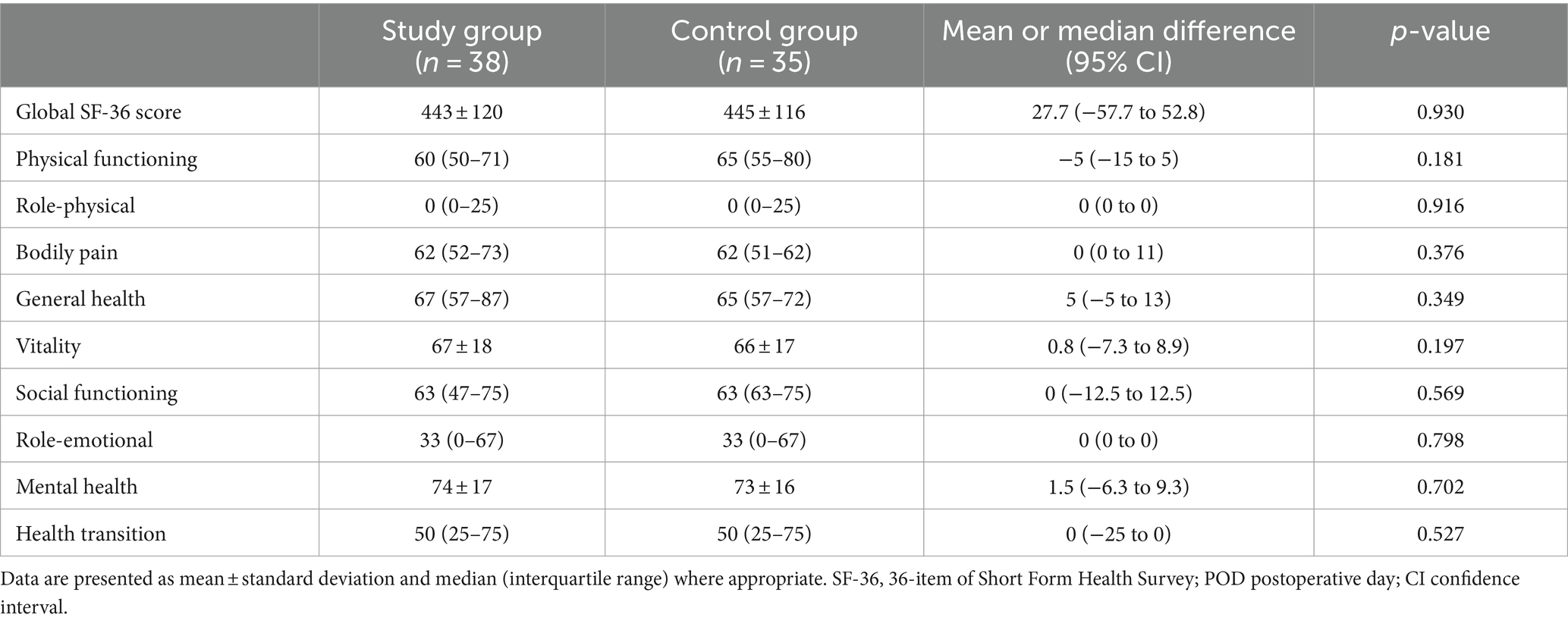
No difference in global QoR-40 score at 24 h and SF-36 scores on POD 30 was observed (Tables 3, 4). Nausea, vomiting, need for rescue antiemetic, complications in-hospital, and length of hospital stay were similar between groups. Patient satisfaction was equally high in both groups.
Discussion
In this randomized controlled trial, patients who received preemptive TAP block-based multimodal analgesia did not have lower 24-h morphine consumption than the patients who received conventional analgesia. However, morphine consumption in the first 6 h, pain scores up to 48 h, and in-hospital functional recovery were significantly improved in the preemptive TAP block-based multimodal analgesic group.
Pain after abdominal surgery is caused by the incision (somatic pain) and trauma to intra-abdominal structures (visceral pain). TAP block is performed to anaesthetize the branches of T6 to L1 sensory nerve roots that innervate the anterior abdominal wall (20). Abdominal wall pain from laparotomy incision can be effectively prevented by the TAP block, and visceral pain can be treated by systemic analgesia consisted of acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), and opioids.
For patients undergoing hysterectomy for benign disease, TAP block has shown significant reduction in pain scores and opioid consumption, and its effect is even more profound if administered before rather than after surgical incision (21). Gasanova et al. (13) showed that for patients undergoing total abdominal hysterectomy, pain on coughing was less variability in TAP block with acetaminophen and NSAID group compared to only TAP block or only acetaminophen and NSAID group. Røjskjaer et al. (22) demonstrated lower pain scores in the early postoperatively period when adding TAP block to a multimodal analgesic regimen consisted of acetaminophen, ibuprofen, dexamethasone, and celecoxib.
Gynecologic oncology laparotomy often involves a large midline vertical incision and considerable visceral disruption thus could induce more complex surgical stress response and severe postoperative pain. However, previous studies investigating TAP block on opioid requirement or pain intensity failed to show any benefit effects in this setting.
In a retrospective study of 120 patients who underwent extensive surgical resection for ovarian cancer, there was no significant difference in opioid consumption within the first 24 h between TAP block group (15.83 mg of morphine equivalent daily dose, IQR 10–34) and no TAP block group (18.75 mg of morphine equivalent daily dose, IQR 7.5–31) (23). Griffiths et al. (18) demonstrated that TAP block performed at the end of gynecologic cancer surgery conferred no benefit in addition to multimodal analgesia. Postoperative morphine consumption at 2 h or 24 h were similar between the placebo and TAP groups. Hotujec et al. (19) showed that preoperative TAP block did not reduce opioid use or postoperative pain scores for gynecologic malignancy patients underwent robotic-assisted laparoscopic surgery. The TAP block group used a mean of 64.9 mg morphine in the first 24 h compared to 69.3 mg for controls.
Preemptive analgesia is an analgesia intervention given before noxious stimulus arises to prevent peripheral and central sensitization caused by incisional and inflammatory injuries. Preemptive analgesia is thought to reduce postoperative pain and hyperalgesia by decreasing production of proinflammatory cytokines (24). A recent meta-analysis indicated that preemptive analgesia reduced postoperative pain, opioid consumption, postoperative nausea or vomiting, and delayed rescue analgesia (25). NSAIDs and cyclooxygenase-2 (COX-2) inhibitors were recommended as preemptive pain medication prior to abdominal hysterectomy to decrease narcotic requirements and improve patient pain assessment and satisfaction scores (26).
In our present study, TAP block combined with intravenous parecoxib was given prior to incision as preemptive multimodal analgesic regimen. Although morphine consumption was only decreased up to 6 h, pain scores at rest and with coughing were significantly improved throughout the 48 h postoperatively in the Study group.
As an essential component of ERAS pathway, multimodal analgesic regimen aims to facilitate patient’s recovery and return to baseline function other than provide adequate pain relief. The QoR-40 score is a patient-reported global measure of quality of recovery and is recommended to be used as standardized endpoints in perioperative clinical trials (27, 28). The SF-36 health survey is a multidimensional measure used to assess health-related quality of life after surgery. The high score indicates a more favorable health state (29). A poor-quality recovery measured by lower QoR-40 score in the early postoperative period can predict a poor quality of life measured by the SF-36 at 3 months after surgery (30).
Our result showed that patients in the Study group had higher global QoR-40 score at 48 h, subdimension score of physical dependence at 24 h, physical comfort and pain at 48 h, confirming a higher recovery quality in the postoperative period. Adequate pain controls up to postoperative 48 h may contribute to improved functional recovery and patients in the Study group had earlier mobilization and shorter time to flatus in the early postoperative period.
Our study has several limitations. First, the sensory blockade level of TAP block was not assessed before incision since it was performed after induction. Nevertheless, we performed the block under real-time ultrasound guidance to obtain proper spread of the solution in the target plane and adequate analgesia was provided in the Study group. Second, the study size was relatively small and there may be other factors that cannot be controlled adequately, such as patient frailty and surgical complexity score, which are important factors associated with postoperative complications (31, 32). Finally, we did not design this study to analyze the chronic postsurgical pain. Further study is needed to investigate whether multimodal analgesia could reduce or prevent chronic postsurgical pain after gynecologic oncology laparotomy.
Conclusion
Our results demonstrated preemptive multimodal analgesia was not superior to conventional multimodal analgesia in reducing 24-h morphine consumption; however, it showed a significantly improved pain control and early quality of recovery thus can be recommended for gynecologic oncology patients undergoing laparotomy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Peking University First Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ZG: Conceptualization, Formal analysis, Writing – original draft. BW: Conceptualization, Data curation, Funding acquisition, Writing – review & editing. YZ: Investigation, Project administration, Resources, Writing – review & editing. XY: Project administration, Resources, Writing – review & editing. JH: Project administration, Resources, Writing – review & editing. RC: Supervision, Visualization, Writing – review & editing. LS: Software, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by Peking University Medicine Fund of Fostering Young Scholars (BMU2020PYB010).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Patel, K, Shergill, S, Vadivelu, N, and Rajput, K. Analgesia for gynecologic oncologic surgeries: a narrative review. Curr Pain Headache Rep. (2020) 26:1–13. doi: 10.1007/s11916-022-00998-z
2. Steyaert, A, and Lavand’homme, P. Prevention and treatment of chronic postsurgical pain: a narrative review. Drugs. (2018) 78:339–54. doi: 10.1007/s40265-018-0866-x
3. Hemmerling, TM. Pain management in abdominal surgery. Langenbeck’s Arch Surg. (2018) 403:791–803. doi: 10.1007/s00423-018-1705-y
4. Ferguson, SE, Malhotra, T, Seshan, VE, Levine, DA, Sonoda, Y, Chi, DS, et al. A prospective randomized trial comparing patient-controlled epidural analgesia to patient-controlled intravenous analgesia on postoperative pain control and recovery after major open gynecologic cancer surgery. Gynecol Oncol. (2009) 114:111–6. doi: 10.1016/j.ygyno.2009.03.014
5. Rivard, C, Dickson, EL, Vogel, RI, Argenta, PA, and Teoh, D. The effect of anesthesia choice on post-operative outcomes in women undergoing exploratory laparotomy for a suspected gynecologic malignancy. Gynecol Oncol. (2014) 133:278–82. doi: 10.1016/j.ygyno.2014.02.027
6. Turan, A, Cohen, B, Elsharkawy, H, Maheshwari, K, Soliman, LM, Babazade, R, et al. Transversus abdominis plane block with liposomal bupivacaine versus continuous epidural analgesia for major abdominal surgery: the EXPLANE randomized trial. J Clin Anesth. (2022) 77:110640. doi: 10.1016/j.jclinane.2021.110640
7. Shaker, TM, Carroll, JT, Chung, MH, Koehler, TJ, Lane, BR, Wolf, AM, et al. Efficacy and safety of transversus abdominis plane blocks versus thoracic epidural anesthesia in patients undergoing major abdominal oncologic resections: a prospective, randomized controlled trial. Am J Surg. (2018) 215:498–501. doi: 10.1016/j.amjsurg.2017.10.055
8. Munro, A, Sjaus, A, and George, RB. Anesthesia and analgesia for gynecological surgery. Curr Opin Anaesthesiol. (2018) 31:274–9. doi: 10.1097/ACO.0000000000000584
9. Wick, EC, Grant, MC, and Wu, CL. Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: a review. JAMA Surg. (2017) 152:691–7. doi: 10.1001/jamasurg.2017.0898
10. Mattson, J, Thayer, M, Mott, SL, Lyons, YA, Hardy-Fairbanks, A, and Hill, EK. Multimodal perioperative pain protocol for gynecologic laparotomy is associated with reduced hospital length of stay. J Obstet Gynaecol Res. (2021) 47:1082–9. doi: 10.1111/jog.14640
11. Jadon, A, Jain, P, Chakraborty, S, Motaka, M, Parida, SS, Sinha, N, et al. Role of ultrasound guided transversus abdominis plane block as a component of multimodal analgesic regimen for lower segment caesarean section: a randomized double blind clinical study. BMC Anesthesiol. (2018) 18:53. doi: 10.1186/s12871-018-0512-x
12. Oksar, M, Koyuncu, O, Turhanoglu, S, Temiz, M, and Oran, MC. Transversus abdominis plane block as a component of multimodal analgesia for laparoscopic cholecystectomy. J Clin Anesth. (2016) 34:72–8. doi: 10.1016/j.jclinane.2016.03.033
13. Gasanova, I, Grant, E, Way, M, Rosero, EB, and Joshi, GP. Ultrasound-guided transversus abdominal plane block with multimodal analgesia for pain management after total abdominal hysterectomy. Arch Gynecol Obstet. (2013) 288:105–11. doi: 10.1007/s00404-012-2698-3
14. Mittal, T, Dey, A, Siddhartha, R, Nali, A, Sharma, B, and Malik, V. Efficacy of ultrasound-guided transversus abdominis plane (TAP) block for postoperative analgesia in laparoscopic gastric sleeve resection: a randomized single blinded case control study. Surg Endosc. (2018) 32:4985–9. doi: 10.1007/s00464-018-6261-6
15. Torup, H, Hansen, EG, Bøgeskov, M, Rosenberg, J, Mitchell, AU, Petersen, PL, et al. Transversus abdominis plane block after laparoscopic colonic resection in cancer patients: a randomised clinical trial. Eur J Anaesthesiol. (2016) 33:725–30. doi: 10.1097/EJA.0000000000000510
16. McDonald, V, Wang, Y, Patel, A, Betcher, R, Fontenot, AC, Scoggin, S, et al. Laparoscopic guided liposomal bupivacaine injection compared to transversus abdominus plane block for postoperative pain after robotic gynecologic oncology surgery. Gynecol Oncol. (2022) 166:432–7. doi: 10.1016/j.ygyno.2022.06.006
17. Hotta, K, Inoue, S, Taira, K, Sata, N, Tamai, K, and Takeuchi, M. Comparison of the analgesic effect between continuous wound infiltration and single-injection transversus abdominis plane block after gynecologic laparotomy. J Anesth. (2016) 30:31–8. doi: 10.1007/s00540-015-2083-z
18. Griffiths, JD, Middle, JV, Barron, FA, Grant, SJ, Popham, PA, and Royse, CF. Transversus abdominis plane block does not provide additional benefit to multimodal analgesia in gynecological cancer surgery. Anesth Analg. (2010) 111:797–801. doi: 10.1213/ANE.0b013e3181e53517
19. Hotujec, BT, Spencer, RJ, Donnelly, MJ, Bruggink, SM, Rose, SL, Al-Niaimi, A, et al. Transversus abdominis plane block in robotic gynecologic oncology: a randomized, placebo-controlled trial. Gynecol Oncol. (2015) 136:460–5. doi: 10.1016/j.ygyno.2014.11.013
20. Tran, DQ, Bravo, D, Leurcharusmee, P, and Neal, JM. Transversus abdominis plane block: a narrative review. Anesthesiology. (2019) 131:1166–90. doi: 10.1097/ALN.0000000000002842
21. Bacal, V, Rana, U, McIsaac, DI, and Chen, I. Transversus abdominis plane block for post hysterectomy pain: a systematic review and Meta-analysis. J Minim Invasive Gynecol. (2019) 26:40–52. doi: 10.1016/j.jmig.2018.04.020
22. Røjskjaer, JO, Gade, E, Kiel, LB, Lind, MN, Pedersen, LM, Kristensen, BB, et al. Analgesic effect of ultrasound-guided transversus abdominis plane block after total abdominal hysterectomy: a randomized, double-blind, placebo-controlled trial. Acta Obstet Gynecol Scand. (2015) 94:274–8. doi: 10.1111/aogs.12567
23. Bisch, SP, Kooy, J, Glaze, S, Cameron, A, Chu, P, Ghatage, P, et al. Impact of transversus abdominis plane blocks versus non-steroidal anti-inflammatory on post-operative opioid use in ERAS ovarian cancer surgery. Int J Gynecol Cancer. (2019) 29:1372–6. doi: 10.1136/ijgc-2019-000724
24. Beilin, B, Bessler, H, Mayburd, E, Smirnov, G, Dekel, A, Yardeni, I, et al. Effects of preemptive analgesia on pain and cytokine production in the postoperative period. Anesthesiology. (2003) 98:151–5. doi: 10.1097/00000542-200301000-00024
25. Xuan, C, Yan, W, Wang, D, Li, C, Ma, H, Mueller, A, et al. Efficacy of preemptive analgesia treatments for the management of postoperative pain: a network meta-analysis. Br J Anaesth. (2022) 129:946–58. doi: 10.1016/j.bja.2022.08.038
26. Steinberg, AC, Schimpf, MO, White, AB, Mathews, C, Ellington, DR, Jeppson, P, et al. Preemptive analgesia for postoperative hysterectomy pain control: systematic review and clinical practice guidelines. Am J Obstet Gynecol. (2017) 217:303–313.e6. doi: 10.1016/j.ajog.2017.03.013
27. Wessels, E, Perrie, H, Scribante, J, and Jooma, Z. Quality of recovery in the perioperative setting: a narrative review. J Clin Anesth. (2022) 78:110685. doi: 10.1016/j.jclinane.2022.110685
28. Myles, PS, Boney, O, Botti, M, Cyna, AM, Gan, TJ, Jensen, MP, et al. Systematic review and consensus definitions for the standardised endpoints in perioperative medicine (StEP) initiative: patient comfort. Br J Anaesth. (2018) 120:705–11. doi: 10.1016/j.bja.2017.12.037
29. Aaronson, NK, Muller, M, Cohen, PD, Essink-Bot, ML, Fekkes, M, Sanderman, R, et al. Translation, validation, and norming of the Dutch language version of the SF-36 health survey in community and chronic disease populations. J Clin Epidemiol. (1998) 51:1055–68. doi: 10.1016/s0895-4356(98)00097-3
30. Myles, PS, Hunt, JO, Fletcher, H, Solly, R, Woodward, D, and Kelly, S. Relation between quality of recovery in hospital and quality of life at 3 months after cardiac surgery. Anesthesiology. (2001) 95:862–7. doi: 10.1097/00000542-200110000-00013
31. Di Donato, V, Di Pinto, A, Giannini, A, Caruso, G, D’Oria, O, Tomao, F, et al. Modified fragility index and surgical complexity score are able to predict postoperative morbidity and mortality after cytoreductive surgery for advanced ovarian cancer. Gynecol Oncol. (2021) 161:4–10. doi: 10.1016/j.ygyno.2020.08.022
Keywords: gynecologic oncology, laparotomy, multimodal analgesia, postoperative pain, recovery
Citation: Geng Z, Wang B, Zhang Y, Yan X, Hu J, Cui R and Song L (2024) Preemptive multimodal analgesia for gynecologic oncology patients undergoing laparotomy: a randomized controlled trial. Front. Med. 11:1427548. doi: 10.3389/fmed.2024.1427548
Edited by:
Cristina Secosan, Victor Babes University of Medicine and Pharmacy, RomaniaReviewed by:
Yong tao Sun, The First Affiliated Hospital of Shandong First Medical University and Shandong Provincial Qianfoshan Hospital, ChinaIlaria Cuccu, Sapienza University of Rome, Italy
Stefania Tudorache, University of Medicine and Pharmacy of Craiova, Romania
Copyright © 2024 Geng, Wang, Zhang, Yan, Hu, Cui and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiyu Geng, Z2VuZ3poaXl1MjAxM0AxNjMuY29t
 Zhiyu Geng
Zhiyu Geng Bojie Wang2
Bojie Wang2 Linlin Song
Linlin Song