- 1Department of Biology, College of Sciences, University of Hail, Hail, Saudi Arabia
- 2Medical and Diagnostic Research Center, University of Hail, Hail, Saudi Arabia
- 3Department of Chemistry, College of Sciences, University of Hail, Hail, Saudi Arabia
- 4Department of Biochemistry, Faculty of Life Sciences, Aligarh Muslim University, Aligarh, India
- 5Department of Clinical Biochemistry, College of Medicine, King Khalid University, Abha, Saudi Arabia
Endometriosis is a chronic, estrogen-dependent, proinflammatory disease that can cause various dysfunctions. The main clinical manifestations of endometriosis include chronic pelvic pain and impaired fertility. The disease is characterized by a spectrum of dysfunctions spanning hormonal signaling, inflammation, immune dysregulation, angiogenesis, neurogenic inflammation, epigenetic alterations, and tissue remodeling. Dysregulated hormonal signaling, particularly involving estrogen and progesterone, drives abnormal growth and survival of endometrial-like tissue outside the uterus. Chronic inflammation, marked by immune cell infiltration and inflammatory mediator secretion, perpetuates tissue damage and pain. Altered immune function, impaired ectopic tissue clearance, and dysregulated cytokine production contribute to immune dysregulation. Enhanced angiogenesis promotes lesion growth and survival. Epigenetic modifications influence gene expression patterns, e.g., HSD11B1 gene, affecting disease pathogenesis. Endometriosis related changes and infertility lead to depression in diagnosed women. Depression changes lifestyle and induces physiological and immunological changes. A higher rate of depression and anxiety has been reported in women diagnosed with endometriosis, unleashing physiological, clinical and immune imbalances which further accelerate chronic endometriosis or vice versa. Thus, both endometriosis and depression are concomitantly part of a vicious cycle that enhance disease complications. A multidimensional treatment strategy is needed which can cater for both endometrial disease and depression and anxiety disorders.
1 Introduction
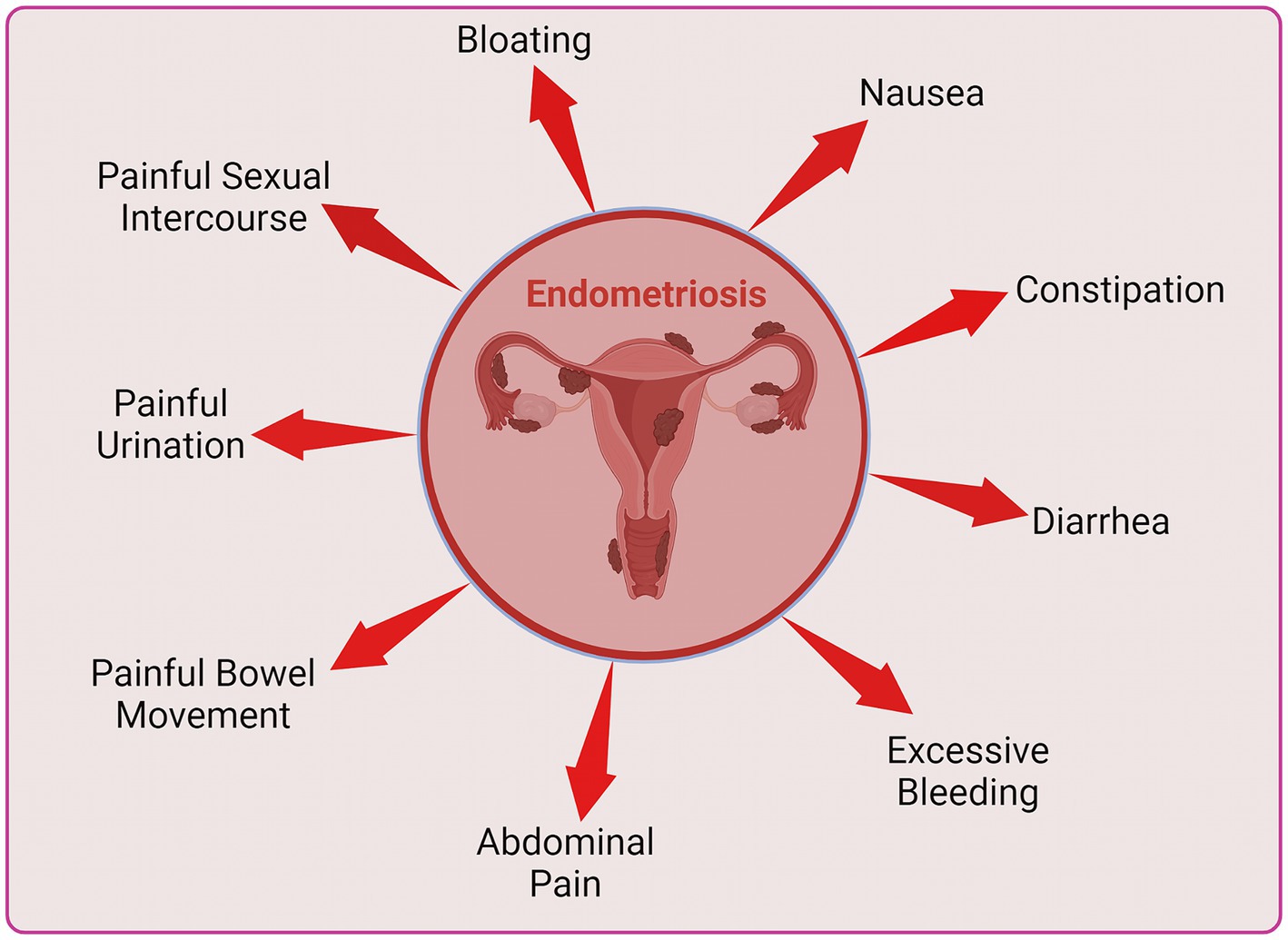
Endometriosis stands as one of the commonly encountered benign gynecological conditions in women, where endometrial glands and stroma exhibit extrauterine location, with a prevalence ranging from 6 to 10% among those of reproductive age (1, 2). Aberrant endometrial cells, characterized by genetic polymorphisms and proliferation rather than apoptosis, in response to local signals, lead to disease progression. Additionally, these cells when anomalously displaced into the peritoneal cavity, not only evade peritoneal destruction but also exploit the immediate environment to sustain proliferation in a clonal manner, while normal cells of the individual are systematically removed. Despite its non-malignant character, the inflammatory and erosive nature of the disease contributes to enduring alterations in a woman’s life, manifesting as persistent pelvic pain, dysmenorrhea, dyspareunia, and infertility (3). The disease can lead to additional symptoms such as painful bowel movements or urination, excessive bleeding, fatigue, diarrhea, constipation, bloating and nausea (4, 5). The challenge is exacerbated by the recurrent delay in diagnosis following the manifestation of symptoms and the restricted scope of available intervention strategies. Despite the potential existence of endometriotic lesions in asymptomatic women, a conclusive diagnosis of endometriosis is typically established when the presence of endometrial tissue or lesions is established beyond the confines of the uterus, frequently through surgical means (6). Endometriosis exhibits diverse classifications based on its anatomical location, including superficial peritoneal lesions which is the most common, ovarian endometrioma, deep sub-peritoneal infiltrating endometriosis and adenomyoma, which represents internal endometriosis within the myometrium (7). Endometriotic lesions have been identified in extra-pelvic locations, such as upper abdominal visceral organs, abdominal wall, diaphragm, and pleura, as well as within the nervous system (8). Patients may exhibit various forms concurrently.
The predominant classification method in use is an updated scoring system established by the American Society for Reproductive Medicine. This system is employed to ascertain the stage of endometriosis, denoted by Roman numerals I to IV, which represent the spectrum from ‘minimal’ to ‘severe’. It involves an assessment of type, location, appearance, depth of lesions, and an evaluation of overall extent of disease as well as presence of adhesions (9). However, grading using the ASRM criteria often demonstrates weak correlations of the abundance and location of lesions with the type of lesions, and symptoms of pain reported by patients, when compared to the disease stage. The occurrence of endometriosis in asymptomatic women, along with ambiguous reasons for its manifestation, contributes to varying perspectives on considering endometriosis as a ‘syndrome’ (10). Diagnosis is typically established only when a patient presents with both observable lesions and symptomatic manifestations.
An in-depth understanding of immune imbalance in endometriosis related depression and vice versa may include enhanced immune cell function, altered cytokine and chemokine levels and malfunctioning of regulatory proteins such as growth factors. Major immune cells such as macrophages, neutrophils, dendritic cells (DCs), natural killer cells (NK cells), T cells, and B cells exhibit great importance in the pathogenesis of endometriosis and depression. Increased levels of macrophages were observed in the peritoneal fluid of endometriotic patients (11). Neutrophil to lymphocyte ratio (NLR) was shown to be a clinically relevant indicator of endometriosis and associated outcomes. Increased NLR was also observed in a recent study showing higher numbers of neutrophils in endometriosis subjects (12, 13). Dendritic cells are important antigen presenting cells and in endometriotic patients, peritoneal DCs are found to increase. Furthermore, numbers of immature DCs are found to be greater as compared to mature DCs (14).
Endometriosis is associated with dysfunction in NK cell cytotoxicity and immunomodulation, by tolerating or inhibiting implantation, proliferation, and survival of endometrial cells, impairing their ability to eliminate these cells at ectopic sites (15). This review also sheds light on the role of the adaptive immune response in endometriosis, including helper T and B cells, whose roles remain incompletely understood. Several serum cytokines such as interleukins (IL) IL1β, IL-5, IL-6, IL-7 and IL-12 are involved, and their levels were found to be altered in endometriosis as compared to in healthy women (16). Cytokines IL-6, IL-8, IL-10, TNF-α also have important roles in the development of VEGF, which is involved in the pathogenesis of the disease (17–23).
Chronic stress or chronic depression events can modulate innate and adaptive immune responses with the involvement of enhanced inflammation and lowering the activity of immune protective cells (24). Inflammatory responses can be increased in stress (25). Furthermore, animal studies, showed that administration of proinflammatory cytokines (TNF or IL-1β) affect the central nervous system through decreased motor activity as well as increased social alienation, disturbed sleep patterns, altered appetite, reduced water intake and greater sensitivity to pain (26–28). Immune dysregulation and associated outcomes are the hallmarks of endometriosis. Immune dysregulation has also been shown to cause depression in susceptible individuals and hence may be the primary cause of depression in women with endometriosis.
Women diagnosed with endometriosis exhibit imbalanced immunological states often because of which major lifestyle changes are inevitable (29–31). Endometriosis patients may undergo mental health issues such as depression, physiological stress and anxiety (32). These women may bear day-to-day abdominal pain, painful bowel movements or urination, excessive bleeding, fatigue, diarrhea, constipation, bloating, nausea, fatigue and painful intercourse (4, 5), leading to a stressful life. In chronic cases infertility is very common (3, 33). This review aims to explore the potential links between depression, immunological factors and endometriosis.
1.1 Literature search for the review article
An electronic literature search was meticulously carried out by the authors S.S., M.W.A.K, S.R., K.M., Q.H., and W.A.K., as published by Centini et al. (34). The search team evaluated the existing literature on endometriosis, which included disease identification, symptoms, diagnosis, pathogenesis and immune dysregulations. The search was performed using the online medical MEDLINE database (accessed via PubMed). Terminologies included endometriosis, biomarkers, endometriotic symptoms and diagnosis, gynecological issues in endometriosis, pathogenesis in endometriosis, endometriotic depression. This review includes the most updated published articles as well as original articles which include randomized and non-randomized clinical trials, prospective observational studies, retrospective cohort studies, and case–control studies, review articles, and case reports. The selected articles were further checked for relevance with the aim and objective of the review. The bibliography of the selected articles was thoroughly checked for additional relevant articles. This procedure effectively helped in compiling more relevant, updated and high-quality peer-reviewed articles, providing a nuanced understanding of the specified topics “endometriosis, depression, and their associated immune imbalances.”
1.2 Etiology and incidence
Various physiological factors, including hormonal, metabolic, neurological, and immunological elements, play a role in the processes leading to the manifestation of symptoms. Epidemiological investigations reveal an increased susceptibility to various cancers (ovarian, breast and melanoma), rheumatoid arthritis, asthma and cardiovascular disease among women with endometriosis lesions (10). Endometriosis has familial incidence with heritability of up to 50% (35). It has been reported that having a first degree relative with a severe form of endometriosis raises the risk by up to seven times (36). A study focusing solely on relatives of individuals with endometriosis revealed that 16% of mothers and 22% of sisters of reproductive age had received a surgical diagnosis of endometriosis (36). Genome-wide association studies have found overrepresented single nucleotide polymorphisms (SNPs) in cases of severe disease. Gynecological disorders such as infertility, fibroids, and cancer were found to have overlaps with common SNPs associated with endometriosis, the etiology of which all involve steroid hormones (34, 37–40).
Additionally, five loci significantly associated with endometriosis risk were identified through a meta-analysis of 11 GWAS datasets, which genetically involved sex steroid hormone pathways (41). Irregularities in the role of extracellular matrix protein signaling such as fibronectin (42), laminin (43) and collagen (44) are implicated in abnormal cell migration and adhesion, contributing to fibrosis. Genomic studies have revealed associations between endometriosis and various biological pathways and cellular regulators. Notably, vezatin, a transmembrane adherens junctions’ protein, has been implicated (45), along with vascular endothelial growth factor receptor 2 (VEGFR-2) (46), the mitogen activated protein (MAP) kinase signaling cascade (47), IL1A (48), wingless related integration site (WNT) signaling (49), and steroid metabolism (50). Meta-analysis has highlighted common genetic signatures between migraine and depression in endometriosis, of which depression underscores an association with changes in gut mucosa (51). Additional determinants for endometriosis are low BMI, low birth weight, lower parity, Mullerian abnormalities, early menarche, short menstrual cycles or heavy and prolonged menstrual flow. Scientific evidence indicates variations in prevalence of endometriosis diagnosis across racial and ethnic groups. A systematic review revealed that Asian women exhibited an elevated risk, while Black women demonstrated a reduced risk compared to White women. However, it is plausible that these estimates may be influenced by biases linked to diagnosis and healthcare accessibility (52). The prevalence of endometriosis amongst Asian women of reproductive age is reported to range from 6.8% to as high as 16% (53) (see Table 1).
2 Clinical symptoms and diagnosis
Medical diagnosis of endometriosis is often difficult and delayed due to a lack of awareness and knowledge of the condition among healthcare professionals and limited understanding of its pathogenesis (35). Further, the complex nature of the disease as well as its manifestations, varying from asymptomatic to its evident phenotypes, add to a complicated diagnosis (3). Pelvic pain stands out as the primary indicator of endometriosis, manifesting in various forms such as dysmenorrhea, dyspareunia, or chronic pelvic pain (54). The intensity of pelvic pain is correlated with type of lesion classification and disease progression (55). Additional symptoms which are commonly found in individuals with the disease include abdominal discomfort, bloating, menometrorrhagia, lower back pain, and fatigue (3). Surgery remains the main method of obtaining a conclusive histopathological diagnosis, with Laparoscopy considered the gold standard diagnostic test. However, prevailing guidelines advocate for a non-surgical diagnostic approach reliant upon symptomatology, physical examination outcomes, and imaging findings. This strategy aims to mitigate delays in commencing treatment. In female patients undergoing surgical interventions, more than 50% will necessitate subsequent surgical interventions within a five-year timeframe (1). Numerous hormonal medical interventions are associated with adverse effects (56).
Research indicates that the greatest prevalence of endometriosis is observed between 25 and 29 years of age (57). However, there is often a significant diagnostic delay, with the average time from the onset of first symptoms to final diagnosis ranging from 4.4 years in the United States to 10.4 years in Germany (58, 59). The primary reasons for this delay may include intermittent use of contraceptives, misdiagnosis, and self-treatment of pain with over-the-counter painkillers. These findings align with the presented study’s results, which report a mean age of 26.9 years at the time of disease recognition and symptom onset ranging from 18.8 years for dysmenorrhea to 24.0 years for dyspareunia. This underscores the importance of early and accurate diagnosis to mitigate prolonged suffering and improve patient outcomes.
Central sensitization (CS) is a type of nociplastic pain characterized by a central nervous system response to peripheral nociceptive or neuropathic triggers, often seen in patients with chronic pains (60). Symptoms of CS include chronic pain, allodynia (pain from stimuli that do not usually provoke pain), hypersensitivity, hyperalgesia (increased sensitivity to painful stimuli), and mood changes (anxiety, panic attacks, and depression) (61–63). A Central Sensitization Inventory (CSI) score of 40 or higher has been effective in identifying CS in women with chronic pelvic pain, including those with endometriosis (62, 64). In a recent study, it has been showed that in endometriosis patients, CS can significantly worsen pain symptoms and is prevalent particularly among those with moderate to severe chronic pelvic pain, involvement of the posterolateral parametrium, high tone pelvic floor (HTF), and comorbid with central sensitivity syndromes like irritable bowel syndrome, anxiety, migraines or severe headaches (65). Therefore, recognizing and addressing CS is crucial for early and accurate diagnosis to mitigate prolonged suffering and improve endometriosis patient outcomes.
The need for reliable noninvasive biomarkers for the diagnosis, potential treatment response and disease prognosis persists as a significant unaddressed requirement. While certain types of endometriosis diagnosis can be expedited through imaging modalities, progress towards validating a dependable noninvasive blood test has been sluggish thus far (66). Other non-surgical diagnostic methods such as transvaginal ultrasonography and magnetic resonance imaging (MRI) have enabled identification of deep endometriosis types (67).
3 Hormones
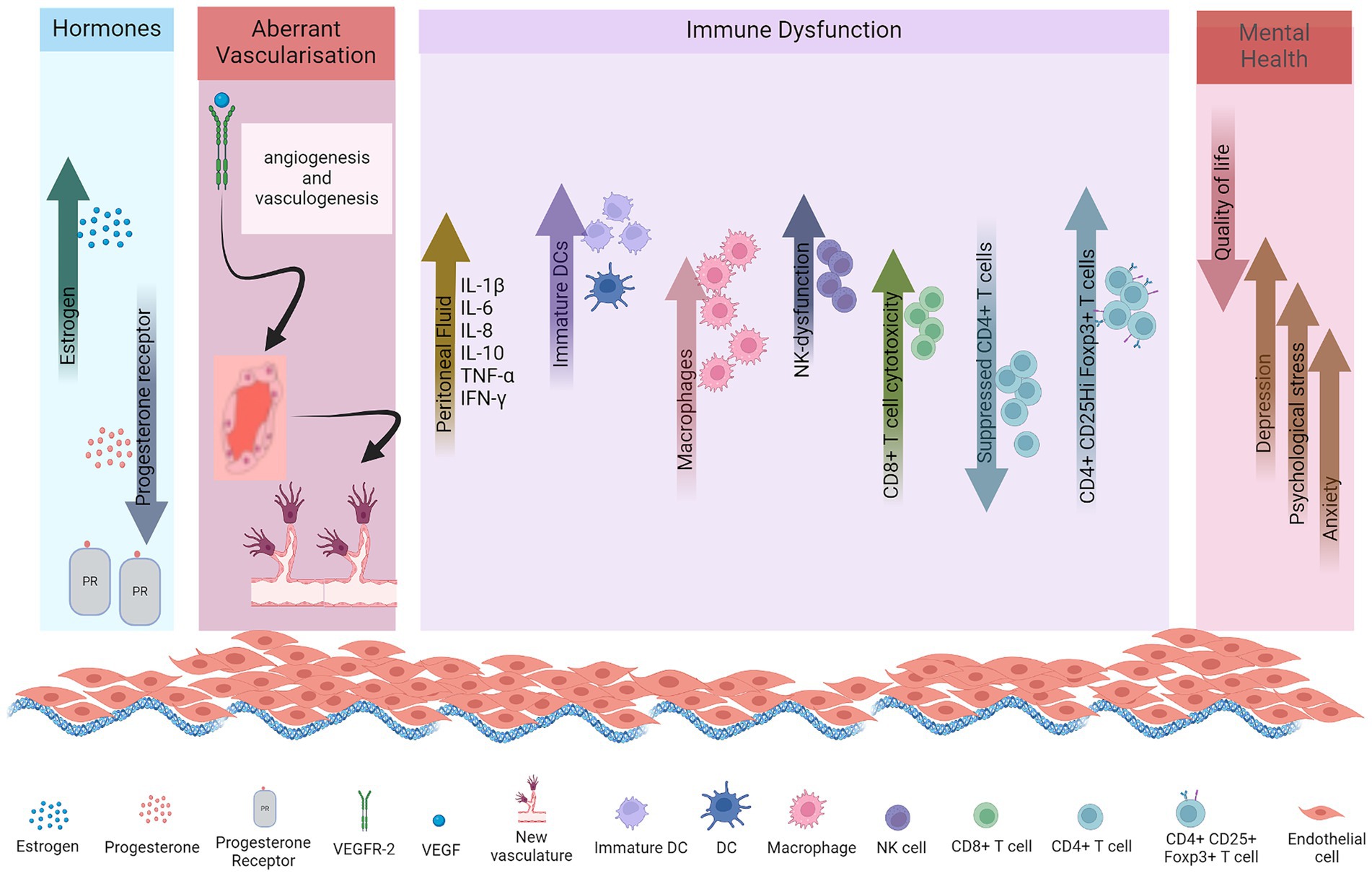
Female sex hormones, estrogen and progesterone, play critical roles in the pathogenesis of endometriosis. Increased levels of estrogen with decreased progesterone receptor pathway signaling are implicated in disease pathogenesis (Figure 1).
3.1 Enhanced estrogen production
Elevated estrogen production consistently emerges as a dysregulated endocrine characteristic in eutopic endometrium and ectopic endometriotic lesions. The predominant estrogen, estradiol (E2), has a pivotal role in the post-menstrual endometrial regeneration (7). Both proliferation of endothelial cells and the re-establishment of microvasculature in this layer are orchestrated by E2, through interactions with its estrogen receptors (ERs), ERα and ERβ (68). Distinct intracellular localizations of the ERs lead to intricately coordinated and precisely regulated estrogen (E2) signaling pathways, which govern cellular proliferation, differentiation, and apoptosis. Endometrial E2 predominantly originates from the ovaries and, to a lesser extent, from adipocytes and the adrenal gland, transported to tissues through the circulatory system (69). Aromatase P450 (aromP450) is a rate limiting hormone in estrogen biosynthesis that catalyzes the conversion of androgens to estrogen, with subsequent transformation into E2 facilitated by 17β-hydroxysteroid dehydrogenase type 1 (17βHSDT1) (70). Prostaglandin E2 (PGE2) synthesis step is initiated by the rate-limiting cyclooxygenase-2 (COX-2) enzyme, acting on arachidonic acid, inducing dose-dependent aromP450 synthesis in endometriotic lesions (69). In healthy women’s endometrium, aromP450 activity is negligible (71). Cell-specific and menstrual cycle phase-dependent expression of receptors that bind to estrogens (ERα, ERβ and GPER1), androgens, progestins and glucocorticoids are observed in the healthy endometrium (72). However, both the endometrium and ectopic endometriotic lesions in women with endometriosis exhibit significantly elevated levels of aromP450, facilitating local E2 production. The capacity of the lesion to independently generate E2, coupled with the synthesis of the necessary enzymes, may enhance intraperitoneal endometriotic tissue implantation (56). It has been observed that the expression of ERβ is extraordinarily higher in stromal cells of women with endometriosis as compared to ERα. It is suggested that rather than just estrogen dependent, endometriosis should be considered steroid-dependent. Thus, the abnormal functioning of estrogen, its receptors, and estradiol synthesis-related enzymes is closely associated with endometriosis.
3.2 Progesterone resistance
Progesterone is the dominant hormone in the secretory phase of the menstrual cycle, where it counteracts effects of estrogen and prepares the uterus for supporting an embryo. It plays a decisive role in facilitating the differentiation of endometrial epithelial and stromal cells. Suppressed progesterone receptor (PR) expression, a characteristic feature of endometriosis, leads to resistance to progesterone and contributes to the development of severe endometriosis conditions (Figure 1). Endometriotic stromal cells demonstrate resistance to progesterone with reduced responsiveness to hormone (73). This diminished communication between stromal and epithelial cells leads to a subsequent elevation in the expression of ERβ within endometriotic lesions and stromal cells (1). PR-A and PR-B are the two functionally distinct receptor isoforms which interact with progesterone. In mice, the absence of PR-A results in abnormalities in the ovary and uterus, while the lack of PR-B has negligible impact on their function (74). Notably, the transcript for both receptor isoforms originate from the same gene, with PR-A having a shorter transcript than PR-B. This structure allows transrepression of PR-B and other nuclear receptors (75). Lesions in endometriosis exhibit a deficiency in PR-B expression, with minimal expression of the transrepressor PR-A, offering molecular substantiation for progesterone resistance. Subsequently, this leads to elevated local levels of estrogen (E2) as progesterone fails to stimulate 17β-hydroxysteroid dehydrogenase type 2 (17β-HSDT2) (69).
4 Aberrant vascularisation
The normal endometrium constitutes a steroid responsive tissue comprising richly vascularized epithelial and stromal cells as well as a diverse range of immune cells. Cells released from this tissue during menstruation encompass epithelial cells, stromal fibroblasts, vascular cells, and immune cells (neutrophils, monocytes, macrophages and uterine natural killer cells) (76). In retrograde menstruation, these cell types can potentially lead to lesions provided they maintain viability and evade the innate immune response and clearance within the intraperitoneal space. The three most implicated cells in peritoneal lesions are stem/progenitor cells, stromal fibroblasts, and immune cells, particularly stromal and immune cells, which play pivotal roles.
Endometriosis is postulated to originate due to endometrial fragment implantation within the peritoneal space. It potentially employs angiogenesis and vasculogenesis mechanisms to develop vascularization, essential for its sustenance (77, 78). The viability of endometriotic implants within the peritoneal cavity relies on establishing a blood supply to deliver oxygen and nutrients to the developing lesions. Concurrent with endometrial growth, the endometrial vasculature undergoes cyclical proliferation and regeneration orchestrated by ovarian steroids, particularly E2. Vascular endothelial growth factor (VEGF) serves a pivotal function in initiating angiogenesis in endometriosis, particularly in ectopic lesions (69, 79). As a vasoactive agent, it participates in numerous physiological functions, such reestablishment of a vascular network and subsequent healing of the uterus, by modulating proliferation and migration of endothelial cells. Heightened expression of VEGF mRNA in the superficial endometrial layer was reported during both the two phases of the uterine cycle, i.e., proliferative and secretory, suggesting ongoing angiogenesis (80). Furthermore, it was also demonstrated that estradiol was responsible for stimulating expression of VEGF in endometrial cells. Administration of E2 resulted in elevated levels of VEGF mRNA expression compared to endometrial cells not exposed to E2 stimulation. Given the intrinsic angiogenic capacity of healthy endometrium regulated by estradiol, it becomes apparent that dysregulated VEGF expression and E2 levels promote neovascularization in lesions, facilitating their establishment in ectopic sites. Studies indicate that peritoneal fluid (PF) from subjects with advanced endometriosis harbors elevated VEGF concentrations versus those with mild disease or healthy individuals (81). Various immune cells participate in angiogenesis by generating and subsequently increasing levels of proinflammatory and angiogenic cytokines, as well as cellular adhesion factors within the PF, surrounding endometriotic lesions. Secretion of VEGF by neutrophils and macrophages within intraperitoneal lesions facilitates angiogenesis (82). Disruptions in peritoneal homeostasis, coupled with the induction of proinflammatory and proangiogenic cytokine production in endometriosis, collectively contribute to modified innervation and the modulation of pain pathways in affected individuals (54). DCs have also been linked to angiogenesis (83). This was evidenced by a study revealing heightened perivascular localization of VEGFR-2 secreting immature dendritic cells within such lesions. These DCs exhibited the ability to stimulate endothelial cell migration in vitro. Intraperitoneal DCs in the peritoneal cavity led to the development of endometriotic lesions in the murine model (84). An investigation employing a transgenic murine model featuring diphtheria toxin mediated conditional depletion of DCs, scientists observed that endometriotic lesions in DC-depleted mice exhibited notable increased size versus control counterparts, along with reduced CD69 expression, indicative of antigen stimulated T and natural killer cell activation. These results underscore the direct involvement of DCs in regulating the angiogenic process and modulating immune activation subsets during the development of lesions (85). Endometrial cells exhibit enhanced resistance to cell mediated immunity, alongside enhanced proliferation and heightened aromatase expression, culminating in elevated estrogen levels (69, 70, 86).
Comparative studies investigating stromal fibroblast phenotypes in women with endometriosis have revealed behavioral disparities, notably epigenetic alterations leading to aberrant responses to estrogen (87). It is plausible that cell plasticity evolved to expedite endometrial repair post-menstruation, leading to multicellular lesion formation in extrauterine locations. Mechanistic similarities between menstrual regulation and lesion formation encompass transient hypoxia (88), iron release, and platelet activation (89, 90).
5 Immune dysfunction
Endometrial lesions adhere to the peritoneum or are closely associated with the ovaries, exposing them to an altered peritoneal environment comprising immune cells, cytokines, and regulatory proteins such as growth factors, with a high potential for anomalous behavior of these entities. Endometriosis animal model studies are suggestive of the fact that immune cells within lesions consist of a combination of cells from endometrial shedding as well as cells from peritoneal microenvironment (91). Fragments of endometrial tissue elicit intraperitoneal inflammation, which results in activation and recruitment of neutrophils and macrophages to the area. Hence, women with the disease often exhibit elevated concentrations of activated macrophages secreting proinflammatory and chemotactic cytokines in the peritoneal fluid (92). Given that various estrogen receptors are expressed on both macrophages and nerve fibers, estrogen is postulated to modulate macrophage and nerve fibers behavior. Thus, estrogen regulation encompasses macrophage recruitment, atypical neurogenesis atypical inflammation observed in endometriosis (93).
5.1 Cytokines
Several studies were conducted for the involvement of cytokines in the pathogenesis of endometriosis (Figure 1) (16–23). Multan et al., found that serum cytokines IL1β, IL-5, IL-6, IL-7 and IL-12 levels were elevated in serum samples of endometriosis patients compared to normal women (16). Cytokines (IL-6, IL-8, IL-10, TNF-α) and chemokines (CCL-2) as well as growth factor VEGF increased in peritoneal fluid of patients (18–23).
Nerve fibers demonstrate an exceptional capacity to recruit macrophages to the injury site. Numerous mediators identified in this process including leukemia inhibitory factor, IL1α, IL1β (94) and pancreatitis-associated protein 3 (PAP3) (95). Estrogen has also been shown to promote colony-stimulating factor 1 and C-C motif ligand 2 (CCL2) secretions from PNS, thereby amplifying macrophage movement towards lesions (96). Additionally, macrophages contribute to the proliferation of peritoneal implants and act as significant sources of angiogenic factors like TNF-α and IL-8. They also contribute to hypoxia-induced angiogenesis (92).
Endometriosis, like cancer, can be categorized as a metabolic disorder. Under the influence of transforming TGF-β1, tumor cells adopt aerobic glycolytic phenotype, leading to enhanced lactate secretion and accumulation (97). Elevated levels of TGF-β1 and lactate are observed in endometriotic PF. Concurrently, there is a shift from typical mitochondrial phosphorylation to glycolysis in the mesothelial cells lining the peritoneum to support cell survival in a tumor like microenvironment (98). Like in tumorigenesis, endometrial cells also exhibit the Warburg effect, where cells adjacent to tumors exhibit a programmed utilization of aerobic glycolysis induced by TGF-β1, leading to lactate production. This lactate serves as a nutrient source for neighboring tumor cells, thereby establishing a cohesive metabolic microenvironment conducive to tumor progression (99). Lactate induces lactylation or the covalent modification of lysine residues on histones and other proteins. Research findings indicate that elevated levels of lactate and lactate dehydrogenase-A, contribute to enhanced lactylation of histone H3 lysine 18 in ectopic endometrial tissues and ectopic endometrial stromal cells, compared to normal cells (100). Furthermore, lactate promotes cell proliferation, migration, and invasion in endometriosis progression, further linked to immune suppression and possible transformation to a malignant form.
5.2 Macrophages
Macrophages represent the predominant immune cell population in the peritoneum. Alterations in macrophage phenotype, or polarization, are linked to significant metabolic shifts. The peritoneal fluid of patients with endometriosis exhibits increased levels of macrophages (11), as shown in Figure 1. These macrophages do not effectively clear endometrial tissue; instead, they significantly contribute to high levels of cytokines (95). Proinflammatory macrophages primarily rely on glycolysis, whereas anti-inflammatory M2 macrophages exhibit a greater dependence on oxidative phosphorylation (101). Moreover, macrophages produce angiogenic mediators, such as TNF-α and IL-8, thereby promoting the growth of lesions (102). While macrophages appear to play a role in the growth and development of endometriotic tissue, depletion of macrophages does not prevent the implantation of endometrial cells in the peritoneum.
5.3 Neutrophils
Neutrophils are postulated to essay a pivotal role in endometriosis pathogenesis. Neutrophils significantly contribute to the resolution of inflammatory responses. A study found that when neutrophils from healthy women were exposed to endometrial plasma or PF, reduced neutrophil apoptosis was observed versus controls, elucidating the presence of antiapoptotic factors in the plasma and PF (12). Interleukin-8 stood out in the study due to its proinflammatory nature and its involvement in neutrophil chemotaxis during inflammation (12).
5.4 Dendritic cells
Dendritic cells are antigen-presenting cells which initiate and modulate adaptive immune responses. DCs additionally serve a crucial function in the prevention of autoimmunity by functioning as mobile sentinels. They transport self-antigens to naïve T cells residing in lymphoid organs, thereby facilitating the induction of self-tolerance (103). In healthy women, immature dendritic cells are absent from the peritoneal membrane. In endometriosis they are present within endometriotic lesions and adjacent to peritoneum. Additionally, the numbers of mature DCs are significantly reduced in the endometrium throughout the menstrual cycle in women with endometriosis compared to those with healthy endometrium (Figure 1). Endometriotic conditions may impede the maturation of immature DCs and prompt their transition into a macrophage phenotype. Moreover, the progression and vascularization of lesions necessitate the presence of endogenous DCs, which infiltrate these lesions and augment endothelial cell migration through the secretion of proangiogenic factors (104). In murine models, the cell density of peritoneal dendritic cells increased promptly following the injection of endometrial tissues, peaking at 14 days. The proportion of mature DCs within peritoneal DCs initially decreased post-injection, then gradually rose over time, although remaining lower than the control group at 42 days. Conversely, the proportion of immature DCs exhibited contrasting changes (14). The administration of lipopolysaccharide resulted in a significant increase in mature DCs proportion, consequently leading to reduced volume and weight of endometriosis lesions. While DC maturation suppresses the angiogenic response, immature DCs actively promote angiogenesis and lesion growth, thus undergoing a shift in their immunological function from antigen presentation to supporting angiogenesis and the progression of the disease.
5.5 Natural killer
NK cells are cytotoxic effector lymphocytes of the innate immune response characterized by their capacity to induce lysis of target cells independent of prior antigen exposure. Endometriosis is associated with a dysfunction in NK cell cytotoxicity and immunomodulation, by tolerating or inhibiting implantation, proliferation, and survival of endometrial cells, impairing their ability to eliminate these cells at ectopic sites (15). A study identified soluble immunosuppressive factors present in the media of both normal endometrial cells and endometriotic stromal cells. Healthy endometrium possesses immunosuppressive capabilities against NK cell cytotoxicity, potentially facilitating embryo implantation (Figure 1). However, in endometriosis, the immunosuppression is more pronounced, potentially allowing retrogradely displaced endometrial tissue to develop into lesions within the peritoneal environment (105). Functional defects and dysregulation of NK cell cytotoxicity are attributed to various cytokines and inhibitory factors present in both serum and PF. The reduction in NK cytotoxicity appears to result from functional defects. The dysregulated cytotoxicity of peritoneal NK cells in endometriosis can be attributed to various cytokines (IL-6, IL-8, IL-1β, IFN-γ, and TNF-α) and inhibitory factors present in both serum and peritoneal fluid. Also, for such patients, there is a notable reduction in the populations of mature NK cells (CD32CD56+), while immature NK cells are elevated in the PF, leading to apoptosis (106). The observed abnormalities in NK cells among women with endometriosis may indeed be outcomes resulting from the local regulation of microenvironment due to the pathology itself.
Treatment modalities such as inhibition of receptor-ligand interactions involving KIR2DL1, NKG2A, LILRB1/2, and PD-1/PD-L1, TGF-β; stimulation of NK cells via IL-2; and mycobacterial therapy utilizing Bacillus Calmette-Guérin (BCG) (82, 107–109). Moreover, ongoing research is exploring the potential of adoptive NK cell therapy for managing endometriosis. Endometriosis holds promise as a candidate for immunotherapy aimed at blocking negative regulatory checkpoints of NK cells, such as inhibitory NK cell receptors. Attenuating the cellular cytotoxicity of NK cells could potentially mitigate the progression of pelvic pain in individuals affected by the disease. The principal inhibitory receptors on NK cells, which are potential checkpoints for eradication of ectopic endometrial tissue, are leukocyte immunoglobulin-like receptors (LILRs).
5.6 T and B cells
Adaptive immune response entails helper T and B cells, in endometriosis, which remains incompletely understood. A study showed that a higher number of CD8 T cells are present in endometriotic lesions compared to eutopic endometrium (110). However, in blood circulation the CD8 T cell populations show no difference between patients and healthy women. It has been noted that CD8 T cell cytotoxicity is enhanced in menstrual effluent of patients, specifically CD8 T effector memory cells are enriched in eutopic endometrium of patients (Figure 1) (110).
Suppressed CD4 T cells have been reported in endometriosis due to the systemic and local alterations in immune responses (Figure 1). These impaired CD4 T cells potentially contribute to the pathogenesis of endometriosis disease through cytokines, which are important for implantation and proliferation of ectopic endometrial cells, inflammation and angiogenesis (111). In women with this condition, there appears to be a bias towards Th2 cell polarization, as evidenced by robust intracellular IL-4 expression and the absence of IL-2 in ectopic lesion derived lymphocytes (82). The equilibrium of CD4 cells in endometriosis remains contentious, with studies indicating reduced activation of both Th1 and Th2 cells in the peritoneal fluid of affected individuals (110).
Regulatory T (Treg) cells constitute a distinct subset within the T cell population, balancing immunological self-tolerance and homeostasis, thus modulating the immune system’s response to prevent excessive reactions against the host (97). Nevertheless, the precise involvement and significance of Treg cells in the context of endometriosis remain inadequately elucidated. The Forkhead box 3 protein (Foxp3), identified as a pivotal transcriptional factor, serves as a master regulator gene governing the differentiation of CD4+ Treg cells (112). Berbic et al. (113), demonstrated heightened expression levels of Foxp3 within both eutopic and ectopic endometrial tissues during the secretory phase of the menstrual cycle in patients afflicted with endometriosis. Furthermore, elevated Foxp3 expression at the messenger RNA level within ovarian endometrioma tissue (114), along with a relatively higher ratio of CD4 + Foxp3+ cells within the CD4+ cell population (115).
Additionally, recent studies have shown a significant increase in the proportion of CD4 + CD25hiFoxp3+ cells within the PF, but not in peripheral blood, of endometriosis patients, as opposed to those without the disease (Figure 1) (116, 117). These collective findings proved the abundance of Treg cells within localized endometrial lesions, implicating their potential involvement in the pathophysiology of endometriosis.
Additionally, heightened activation of B cells has been observed in both eutopic endometrium and lesions compared to healthy endometrium. Notably, the presence of anti-endometrial antibodies in the serum of endometriosis subjects has led to its occasional classification as an autoimmune disease (118).
5.7 Stem cells
Traditional hypotheses concerning the development of endometriotic lesions have lacked detailed mechanistic explanations for their proliferation and survival until recent studies revealed the involvement of mesenchymal stem cells (MSCs) and myeloid-derived suppressor cells (MDSCs) within a complex network of immune-endocrine signaling. MDSCs typically have strong immunosuppressive and angiogenic characteristics and are found in low numbers in healthy tissue. However, their accumulation is linked to interactions with inflammatory cytokines and has been implicated in several inflammatory diseases. Increased levels of these pro-inflammatory cytokines within the PF of individuals with endometriosis-associated pain may influence the differentiation of monocytes into MDSCs (119).
6 Immunological pathogenesis of endometriosis
Estrogen dominance fosters immune dysregulation, whereby many features observed in endometriosis mirror immune processes observed in various cancers, including heightened somatic mutations in endometrial epithelial cells. This elevated mutational burden contributes to the development of endometriosis-specific neoantigens, potentially altering the immune microenvironment of the lesions. Additionally, endometriosis often coexists with several chronic inflammatory conditions, characterized by shared dysregulation of the IL-23/IL-17 pathway, as evidenced in inflammatory bowel disease, psoriasis, and rheumatoid arthritis (120).
The crosstalk between immune cells, nerves, and central pain pathways plays a significant role in the pathophysiology of endometriosis. Endometrium is unique among mucosal tissues in the body in that it typically lacks innervation under normal physiological conditions. Nerve fibers are rare within the functional layer of the endometrium in women without any pathology (121). Sensory nerves surrounding endometriotic lesions drive the chronic pain associated with the condition and contribute to a pro-growth phenotype (122). Substantial alterations in nerve activity occur both within endometriotic lesions and the nervous system. Studies indicate that women experiencing pain symptoms associated with endometriosis exhibit notably higher nerve fiber density within the endometrium, myometrium and lesions as compared to those without the condition (123). Nerve fibers within endometriotic lesions consist of a combination of sensory, sympathetic, and parasympathetic fibers, collectively contributing to pain and inflammatory processes (124). The pain associated with endometriosis implies neuronal mechanisms that culminate in CS.
The interplay between macrophages and nerve fibers fosters inflammation and pain manifestations in endometriosis. Given their abundance within endometriotic lesions, macrophages stimulate sensory innervation and sensitization, thereby contributing to lesion proliferation and the prevalent pain experienced in endometriosis (19, 23). Moreover, immune cells release pro-nociceptive and pro-inflammatory mediators that can sensitize nerve fibers, leading to neurogenic inflammation (125). This communication between immune cells and nerves presents promising avenues for therapeutic interventions in endometriosis.
Prostaglandins, particularly prostaglandin E2 (PGE2), also play a significant role in the pathophysiology of endometriosis, contributing to pain and inflammation. Women with endometriosis produce an excess of PGE2, which is responsible for uterine contractions, pain, and inflammation (126). PGE2 is upregulated in the peritoneal cavity in endometriosis and is produced by macrophages and ectopic endometrial cells (127). It is involved in the development and continued growth of endometriosis, as it increases estrogen synthesis, inhibits apoptosis, promotes cell proliferation, affects leukocyte populations, and promotes angiogenesis (127). The presence of endometriosis lesions can trigger inflammation, which further promotes PGE2 activity (128). The release of PGE2 is associated with the development of symptoms and the progression of endometriosis, making it a potential target for therapeutic interventions. These changes in nerve activity contribute to the complex and debilitating pain experienced by individuals with endometriosis. However, the use of painkillers or non-steroidal anti-inflammatory drugs (NSAIDs) alone is not always ideal for managing endometriosis pain, as they may have limited efficacy and potential adverse effects (1). Therefore, understanding the role of PGE2 in endometriosis is important for developing targeted treatment strategies to address the associated pain and inflammation.
7 Clinical consequences of depression in endometriosis
Endometriosis changes the lifestyle of women and may lead to mental health issues such as depression, physiological stress and anxiety as depicted in Figure 1. Endometriosis is linked to psychological disorders in several ways. The disease in chronic stage can cause life impacting abdominal pain during periods, painful bowel movements or urination, chronic pelvic pain, excessive bleeding, fatigue, diarrhea, constipation, bloating, nausea, fatigue and painful intercourse (Figure 2), leading to a compromised quality of life and in several cases infertility. These co-occurring conditions may cause stress, anxiety and psychological disorders (4, 5).
A study conducted by Pope et al. (129) highlighted the correlation between endometriosis and a diverse array of psychiatric symptoms, notably depression, anxiety, psychosocial stress, and diminished quality of life. Recent literature further substantiates the prevalence of depression and anxiety as the predominant psychiatric comorbidities in individuals with endometriosis (129–136). In an investigation by Low et al. (137), for the potential role of a distinct psychological profile associated with endometriosis, the author included 81 women participants in the study who were experiencing pelvic pain. Of these, 40 were diagnosed with endometriosis disease and 41 presenting with alternative gynecological issues. All the subjects underwent evaluation through six standardized psychometric assessments, including the Eysenck Personality Questionnaire (EPQ), Beck Depression Inventory (BDI), General Health Questionnaire, State–Trait Anxiety Inventory (STAI), The Golombok Rust Inventory of Marital State, and The Short-Form McGill Pain Questionnaire. In assessments using these criteria, the endometriosis patients exhibited increased level of psychoticism, introversion and anxiety scores than women with other gynecological issues (138).
In a recent study conducted by Warzecha et al. (139), 15.1% of women with endometriosis were diagnosed with depression which aligns with findings by Fried et al., who reported a 14.5% incidence of depressive symptoms. In another study the incidence of symptoms of anxiety were estimated to be 29% among Austrian women with endometriosis (130). A meta-analysis by Gambadauro et al. (140), encompassing 24 studies and 99,614 women, confirmed higher levels of depression in such subjects. Researchers indicate that women with endometriosis accompanied by pelvic pain, the rate of depressive symptoms is significantly higher than in cases of endometriosis without pain. This evidence suggests that endometriosis associated complications such as pain may be a more critical factor in the development of depressive symptoms than the presence of endometriosis alone (140). Furthermore, Warzecha et al. (140), revealed that the mean age at the onset of depressive symptoms among women with endometriosis was 22.2 years, which is closely aligned with the age range for the onset of endometriosis symptoms between 18.8 and 24 years. Additionally, the study found that certain types of pain, specifically chronic pelvic pain and painful defecation, significantly increased the incidence of depressive symptoms (140). These findings underscore the profound impact that specific pain manifestations can have on the mental health of women suffering from endometriosis. In these studies, clinicians caring for women with chronic pelvic pain, particularly when coexisting with endometriosis, should be cognizant of the elevated risk of depressive disorders in this population. Understanding the strong correlation between chronic pain and mental health is essential for providing holistic care. Early recognition and intervention for depressive symptoms in these patients can significantly improve their overall quality of life and treatment outcomes.
Another study based on meta-analysis included 18 relevant quantitative studies (129). Out of the 18 studies, 17 included clinical patients’ samples. Fourteen out of eighteen studies indicated that endometriosis or chronic pelvic pain significantly impaired at least some aspects of psychological functioning, mental health, elevated risk for depression, hypomanic, or anxiety symptoms among affected women.
From the 18 studies, 4 studies (137, 141–143) used clinical diagnostic criteria to assess psychiatric diagnosis. Out of these 4 studies, 3 were used as comparator group (137, 141, 142). From the clinical samples of women (age from late teens to mid-40s), 37% of participants showed endometriosis and 50% exhibited pelvic pain with a reported family history of mood disorders. From the 3 comparator group studies, 2 showed higher risk of psychiatric disorders in women with endometriosis (137, 141). Data from these three studies exhibited that 44 (56%) of the 79 women with endometriosis met the criteria for at least one psychiatric disorder.
Another study was conducted on a Brazilian population including 103 women with an age range of 15 to 49 years (average age 33.4 years) (144). Out of 103 patients, 53 (51.5%) were diagnosed with endometriosis and 50 (48.5%) without endometriosis (control). Subjects were evaluated using a questionnaire (Beck Depression Inventory) providing different levels of depression (mild, moderate, moderate to severe, and severe). Based on the questionnaire, symptoms for depression were observed in 35 (66%) women with endometriosis. Out of these, 20 (37.7%) women showed mild depression, 4 women (7.5%) exhibited mild to moderate, 6 women (11.3%) were found to have moderate to severe depression, and 5 (9.4%) women had severe depression. However, according to the Fisher’s exact test, there was no relationship between endometriosis and depressive symptoms (p = 0.423) (144).
Traumatic stress is very likely in endometriosis diagnosed women compared with the women without endometriosis (145, 146). Harris et al. concluded in a study that children who experienced physical or sexual abuse were likely to develop endometriosis in later stages of life (147). Post-traumatic stress disorder and childhood trauma can impact individuals and may contribute to the development of depression at an early stage (148). Furthermore, a study conducted by Reis et al., showed that depression or stress in the early stages of life may be considered an important factor for the development of endometriosis (149). The persistence of such conditions over a long period of time may lead to hormonal imbalance, neuroendocrine dysfunction, chronic inflammation which are leading factors in the development of depression and endometriosis (146, 150).
8 Immunological aspects of depression in endometriosis
Depression is very much associated with the secretion or formation of proinflammatory molecules such as IL-6, IFN-γ, TNF-α, and IL1β (151–153). Additionally, depression also enhances oxidative stress and increases oxidative molecules such as protein bound carbonyl content and methylglyoxal (151, 152, 154). A study revealed that methylglyoxal, which is a well-known reactive metabolite, plays a vital role in various central nervous system associated cognitive functions and can be linked to stress, depression, anxiety, and neurodegenerative diseases (154, 155). Numerous studies conducted in this area have proven that endometriosis is strongly linked with the increased risk of psychological depression, anxiety, and eating disorders (156).
Studies indicated that endometriosis patients have an increased incidence of autoimmune diseases and cancer (157–159). Women diagnosed with endometriosis are more prone to several autoimmune diseases such as multiple sclerosis, rheumatoid arthritis, inflammatory bowel disease, systemic lupus erythematosus, and Sjogren’s Syndrome (159, 160). Higher estrogen levels in women during endometriosis lead to the modification of macromolecules like insulin, serum albumin etc. (161–163). These modifications not only compromise the functioning of these macromolecules but also lead to the formation of neo-antigens on these molecules that activate a cascade of the reactions causing production of autoantibodies (161–163). Higher levels of autoantibodies were detected in patients with depression (161–163). These elevated levels of autoantibodies, together with several pathological complications in endometriosis as discussed above, further aggravate the disease to extremely severe levels.
T cell involvement in depression has not been investigated in detail. Some studies conducted in this area revealed that T cell responses decrease in depression (164–166). T cell responses were found to decrease against antigens encountered in the skin of depressed individuals (164, 167). In a meta-analysis conducted by Zorrilla et al. (166), depression was associated with a decreased percentage of T cells. CD4+ T cells in depressed individuals exhibited increased expression of Fas (CD95) which is known as death receptor as it triggers apoptosis when it interacts with its ligand (168, 169).
T cell function can be inhibited by glucocorticoid pathways in major depression. Increased levels of glucocorticoids in circulatory blood are hallmark of depression (170). Glucocorticoids mediate cell migration and induce apoptosis of immune cells including T cells (166, 171). Endometriosis is characterized by elevated expression of the HSD11B1 gene, which converts inactive cortisone to cortisol, a biologically potent glucocorticoid in peripheral tissues. Receptor for glucocorticoid expression increases up to 3.5-fold in endometriosis. The interaction of higher levels of glucocorticoids with increased level of receptors in endometriosis may increase the proinflammatory environment surrounding the endometriotic lesion and enhance the activity that supports endometriotic cell survival (172). Higher levels of glucocorticoids also induce infertility in women. Infertility treatment, which are often long and painful processes, as well as the condition itself induce depression and compromises in quality of life.
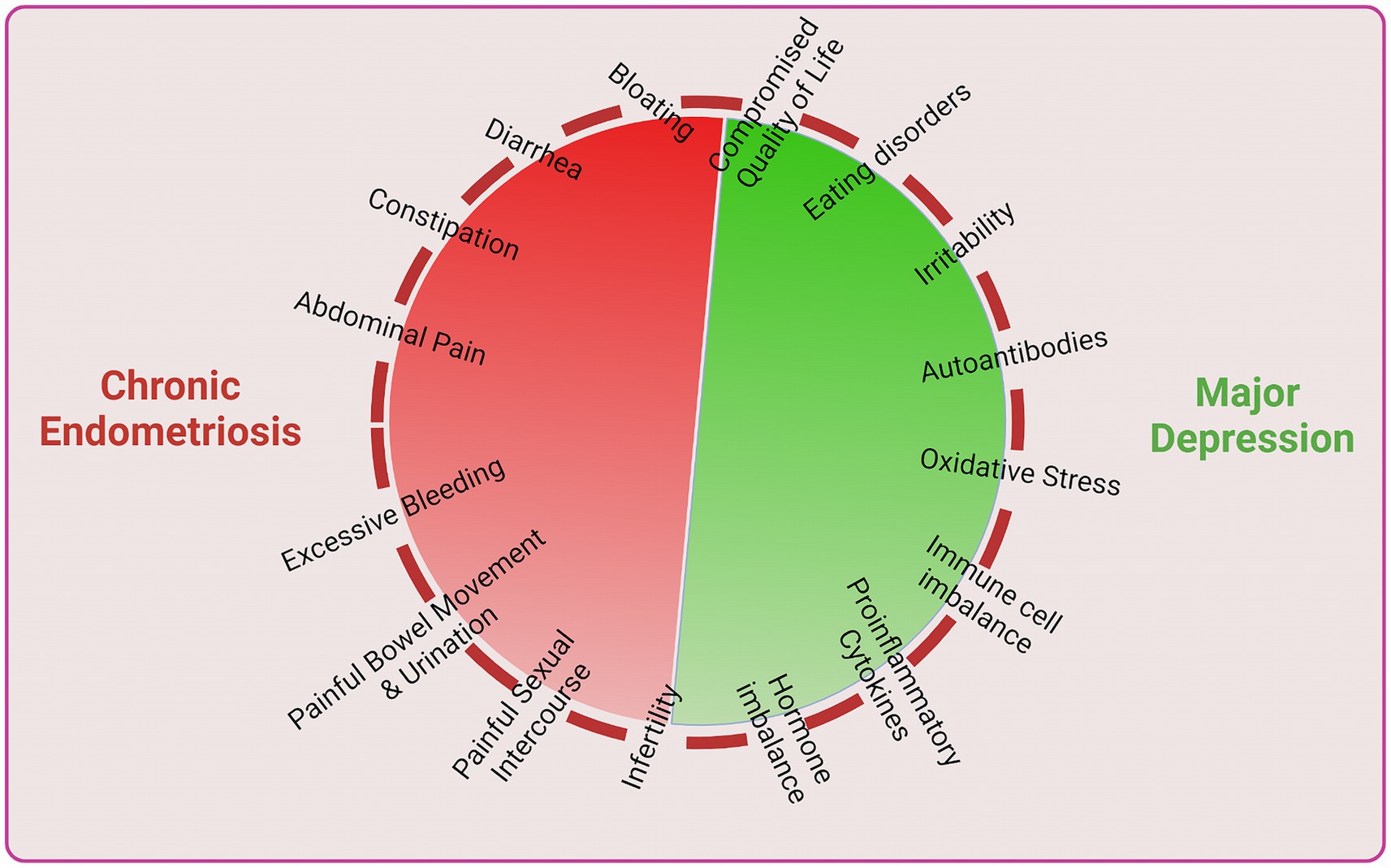
Finally, it has been suggested that chronic endometriosis arises due to various dysfunctions imbalances and may lead to infertility which may cause women to develop depression and subsequently unleash further physiological, clinical and immune imbalances which further accelerate chronic endometriosis or vice versa (Figure 3). Thus, both endometriosis and depression concomitantly develop a vicious cycle which enhance and exacerbate disease complications.
9 Links between depression and immunological factors for potential malignant transformation of endometriosis
Although endometriosis is classified as a benign disease, it has the potential to transform into malignancy, which occurs in about 1% of endometriosis patients (173, 174). This malignant transformation most frequently affects the ovaries, with ovarian endometrioid carcinoma and ovarian clear cell carcinoma being the most common types. These two malignancies account for 76% of all endometriosis-related ovarian cancers (174, 175).
Recently, several carcinogenic pathways have been identified for endometriosis-related malignant transformation. Uncontrolled cell division, tissue infiltration, neoangiogenesis, and apoptosis evasion may result from oncogene demethylation and tumor suppressor gene hypermethylation (173, 174). Key events include hypermethylation of the hMLH1 gene promoter, reducing DNA mismatch repair gene expression, and hypomethylation of LINE-1. Tumor suppressor genes RUNX3 and RASSF2 are inactivated by promoter hypermethylation (173). In endometrioid cancer, KRAS oncogene activation and PTEN tumor suppressor gene inactivation is significant (175, 176). Loss of PTEN activity, an early event in malignant transformation, is linked to PTEN gene mutations (177). Additionally, somatic mutations in cancer driver genes ARID1A, PIK3CA, KRAS, and PPP2R1A are found in deep infiltrating endometriosis (178).
A recent meta-analysis study conducted by Centini et al. (34), focusses on atypical endometriosis, which is present in 12–35% of ovarian endometriosis cases and 60–80% of endometriosis associated ovarian cancers. The SWItch/Sucrose Non-Fermentable (SWI/SNF) complex and ARID1A gene alterations offer valuable insights into the pathogenesis of endometriosis and endometriosis-associated ovarian cancer. Also, the use of potential therapeutics based on inhibitors and suggested the use of PARP inhibitors in treating ovarian cancer which may potentially improve outcomes for these conditions.
Retrograde menstruation, where menstrual blood containing erythrocytes, macrophages, and endometrial tissue travels through the fallopian tubes to the peritoneal cavity, is crucial for understanding endometriosis pathogenesis (179, 180). Periodic hemorrhage from ectopic endometriotic lesions causes iron overload, with erythrocyte-derived iron being a well-known inducer of oxidative stress (180). This altered iron metabolism can contribute to endometriosis development and progression (181). At moderate levels, iron-induced reactive oxygen species (ROS) stimulate ectopic endometrial cell proliferation, angiogenesis, and adhesion. Animal models show that iron treatment increases the number and size of endometriotic lesions compared to controls, suggesting that imbalances in iron homeostasis regulate endometriotic cell proliferation (182). These finding suggest that alterations in iron hemostasis may promote endometriotic cell proliferation. Iron overload intensifies intracellular oxidative stress through the Fenton reaction (Fe2+ + H₂O₂ → Fe3+ + OH− + OH), leading to DNA, lipid, and protein damage, and resulting in cytotoxic effects on cells (183). This reaction generates reactive hydroxyl radicals that contribute to cellular injury and dysfunction. Furthermore, excess iron can decrease transferrin concentration in follicular fluid due to increased transferrin saturation. This iron overload and transferrin insufficiency lead to elevated ROS levels, compromising mitotic spindle integrity and promoting chromosome instability (184, 185). Consequently, this may affect the number and maturation of oocytes retrieved from women with endometriosis (184, 185). High content of iron in ovarian endometriomas exert negative effect on granulosa cells via increased level of ROS cause decrease in the number and quality of oocytes leading to impaired fertility (186–188). The increased levels of free radical generation in physiological stress concomitant with impaired fertility in endometriosis may be due to an imbalance in ROS homeostasis.
Furthermore, there is a persistent production of antioxidants, where endometriotic cells adapt to oxidative stress with the support of macrophages. This adaptation enhances antioxidative defenses and influences redox signaling, energy metabolism, and the tumor immune microenvironment, potentially leading to malignant transformation. Moreover, specific molecular alterations, including mutations in ARIDA1/BAF250a, PIK3CA, CTNNB1, and PTEN, as well as microsatellite instability and loss of heterozygosity, have been reported (189–193).
10 Conclusion
Endometriosis is a chronic, estrogen-dependent, proinflammatory disease that can cause various dysfunctions. Hormonal imbalance, inflammation, immune dysregulation, angiogenesis, neurogenic inflammation, epigenetic alterations, and tissue remodeling are common in the pathogenesis of endometriosis. Higher numbers of women diagnosed with endometriosis showed increased levels of depression which can potentially further aggravate the disease. According to published literature strong synergisms were observed in endometriosis patients with depression or vice versa. This review article focuses on the immunological aspects of depression in endometriosis patients by looking at the links between depression and immunological factors responsible for potential malignant transformation of endometriosis. There is a huge gap in the awareness of endometriosis and proper counselling and treatment, especially in underdeveloped and developing countries due to continued reluctance of open discussion of female gynecological issues. Clinicians, academicians, and scientists should reach out to these communities and provide vital information promoting regular screening, early detection of the disease and counselling to prevent further complications. Importantly, increased funding is critical for investigation and identification of factors against which multifactorial drug development is critical to alleviate the pain and suffering of women diagnosed with endometriosis and depression.
Author contributions
SS: Conceptualization, Data curation, Supervision, Writing – original draft, Writing – review & editing. MK: Data curation, Writing – original draft, Writing – review & editing. SR: Data curation, Writing – original draft. KA-M: Data curation, Writing – review & editing. QH: Data curation, Writing – review & editing. WK: Data curation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research has been funded by the Scientific Research Deanship at the University of Ha’il-Saudi Arabia through project number MDR-22017.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Saunders, PTK, and Horne, AW. Endometriosis: etiology, pathobiology, and therapeutic prospects. Cell. (2021) 184:2807–24. doi: 10.1016/j.cell.2021.04.041
2. Zondervan, KT, Becker, CM, and Missmer, SA. Endometriosis. N Engl J Med. (2020) 382:1244–56. doi: 10.1056/NEJMra1810764
3. Allaire, C, Bedaiwy, MA, and Yong, PJ. Diagnosis and management of endometriosis. CMAJ. (2023) 195:E363–71. doi: 10.1503/cmaj.220637
4. Maroun, P, Cooper, MJ, Reid, GD, and Keirse, MJ. Relevance of gastrointestinal symptoms in endometriosis. Aust N Z J Obstet Gynaecol. (2009) 49:411–4. doi: 10.1111/j.1479-828X.2009.01030.x
5. Velho, RV, Werner, F, and Mechsner, S. Endo belly: what is it and why does it happen?—a narrative review. J Clin Med. (2023) 12:7176. doi: 10.3390/jcm12227176
6. Parasar, P, Ozcan, P, and Terry, KL. Endometriosis: epidemiology, diagnosis and clinical management. Curr Obstet Gynecol Rep. (2017) 6:34–41. doi: 10.1007/s13669-017-0187-1
7. Chantalat, E, Valera, MC, Vaysse, C, Noirrit, E, Rusidze, M, Weyl, A, et al. Estrogen receptors and endometriosis. Int J Mol Sci. (2020) 21:2815. doi: 10.3390/ijms21082815
8. Andres, MP, Arcoverde, FVL, Souza, CCC, Fernandes, LFC, Abrão, MS, and Kho, RM. Extrapelvic endometriosis: a systematic review. J Minim Invasive Gynecol. (2020) 27:373–89. doi: 10.1016/j.jmig.2019.10.004
9. Lee, SY, Koo, YJ, and Lee, DH. Classification of endometriosis. Yeungnam Univ J Med. (2021) 38:10–8. doi: 10.12701/yujm.2020.00444
10. Kvaskoff, M, Mu, F, Terry, KL, Harris, HR, Poole, EM, Farland, L, et al. Endometriosis: a high-risk population for major chronic diseases? Hum Reprod Update. (2015) 21:500–16. doi: 10.1093/humupd/dmv013
11. Hogg, C, Horne, AW, and Greaves, E. Endometriosis-associated macrophages: origin, phenotype, and function. Front Endocrinol. (2020) 11:7. doi: 10.3389/fendo.2020.00007
12. Kwak, JY, Park, SW, Kim, KH, Na, YJ, and Lee, KS. Modulation of neutrophil apoptosis by plasma and peritoneal fluid from patients with advanced endometriosis. Hum Reprod. (2002) 17:595–600. doi: 10.1093/humrep/17.3.595
13. Tabatabaei, F, Tahernia, H, Ghaedi, A, Bazrgar, A, and Khanzadeh, S. Diagnostic significance of neutrophil to lymphocyte ratio in endometriosis: a systematic review and meta-analysis. BMC Womens Health. (2023) 23:576. doi: 10.1186/s12905-023-02692-7
14. Qiaomei, Z, Ping, W, Yanjing, Z, Jinhua, W, Shaozhan, C, and Lihong, C. Features of peritoneal dendritic cells in the development of endometriosis. Reprod Biol Endocrinol. (2023) 21:4. doi: 10.1186/s12958-023-01058-w
15. Sikora, J, Mielczarek-Palacz, A, and Kondera-Anasz, Z. Role of natural killer cell activity in the pathogenesis of endometriosis. Curr Med Chem. (2011) 18:200–8. doi: 10.2174/092986711794088416
16. Malutan, AM, Drugan, T, Costin, N, Ciortea, R, Bucuri, C, Rada, MP, et al. Pro-inflammatory cytokines for evaluation of inflammatory status in endometriosis. Cent Eur J Immunol. (2015) 40:96–102. doi: 10.5114/ceji.2015.50840
17. Luckow Invitti, A, Schor, E, Martins Parreira, R, Kopelman, A, Kamergorodsky, G, Gonalves, G, et al. Inflammatory cytokine profile of co-cultivated primary cells from the endometrium of women with and without endometriosis. Mol Med Reports. (2018) 18:1287–96. doi: 10.3892/mmr.2018.9137
18. Lie, I, and Ilie, R. Cytokines and endometriosis-the role of immunological alterations. Biotechnol Mol Biol Nanomed. (2013) 1:8–19.
19. Sikora, J, Smycz-Kubańska, M, Mielczarek-Palacz, A, and Kondera-Anasz, Z. Abnormal peritoneal regulation of chemokine activation-the role of IL-8 in pathogenesis of endometriosis. Am J Reprod Immunol. (2017) 77:e12622. doi: 10.1111/aji.12622
20. Sikora, J, Mielczarek-Palacz, A, and Kondera-Anasz, Z. Association of the precursor of interleukin-1β and peritoneal inflammation-role in pathogenesis of endometriosis. J Clin Lab Anal. (2016) 30:831–7. doi: 10.1002/jcla.21944
21. May, CD, Sphyris, N, Evans, KW, Werden, SJ, Guo, W, and Mani, SA. Epithelial-mesenchymal transition and cancer stem cells: a dangerously dynamic duo in breast cancer progression. Breast Cancer Res. (2011) 13:1–10. doi: 10.1186/bcr2789
22. Fan, YY, Chen, HY, Chen, W, Liu, YN, Fu, Y, and Wang, LN. Expression of inflammatory cytokines in serum and peritoneal fluid from patients with different stages of endometriosis. Gynecol Endocrinol. (2018) 34:507–12. doi: 10.1080/09513590.2017.1409717
23. Jørgensen, H, Hill, AS, Beste, MT, Kumar, MP, Chiswick, E, Fedorcsak, P, et al. Peritoneal fluid cytokines related to endometriosis in patients evaluated for infertility. Fertil Steril. (2017) 107:1191–1199.e2. doi: 10.1016/j.fertnstert.2017.03.013
24. Dhabhar, FS. Effects of stress on immune function: the good, the bad, and the beautiful. Immunol Res. (2014) 58:193–210. doi: 10.1007/s12026-014-8517-0
25. Bartolomucci, A, Palanza, P, Parmigiani, S, Pederzani, T, Merlot, E, Neveu, PJ, et al. Chronic psychosocial stress down-regulates central cytokines mRNA. Brain Res Bull. (2003) 62:173–8. doi: 10.1016/j.brainresbull.2003.09.009
26. Bluthe, RM, Laye, S, Michaud, B, Combe, C, Dantzer, R, and Parnet, P. Role of interleukin-1beta and tumour necrosis factor-alpha in lipopolysaccharide-induced sickness behaviour: a study with interleukin-1 type I receptor-deficient mice. Eur J Neurosci. (2000) 12:4447–56. doi: 10.1046/j.1460-9568.2000.01348.x
27. Dantzer, R. Cytokine, sickness behavior, and depression. Immunol Allergy Clin N Am. (2009) 29:247–64. doi: 10.1016/j.iac.2009.02.002
28. Seiler, A, Fagundes, CP, and Christian, LM. The impact of everyday stressors on the immune system and health In: A Chouker, editor. Stress challenges and immunity in space. Cham: Springer (2020). 71–92.
29. Culley, L, Law, C, Hudson, N, Denny, E, Mitchell, H, Baumgarten, M, et al. The social and psychological impact of endometriosis on women’s lives: a critical narrative review. Hum Reprod Update. (2013) 19:625–39. doi: 10.1093/humupd/dmt027
30. De Graaff, AA, D’hooghe, TM, Dunselman, GA, Dirksen, CD, and Hummelshoj, L WERF EndoCost Consortium, et al. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Hum Reprod. (2013) 28:2677–85. doi: 10.1093/humrep/det284
31. Della Corte, L, Di Filippo, C, Gabrielli, O, Reppuccia, S, La Rosa, VL, Ragusa, R, et al. The burden of endometriosis on women’s lifespan: a narrative overview on quality of life and psychosocial wellbeing. Int J Environ Res Public Health. (2020) 17:4683. doi: 10.3390/ijerph17134683
32. Szypłowska, M, Tarkowski, R, and Kułak, K. The impact of endometriosis on depressive and anxiety symptoms and quality of life: a systematic review. Front Public Health. (2023) 11:1230303. doi: 10.3389/fpubh.2023.1230303
33. Bonavina, G, and Taylor, HS. Endometriosis-associated infertility: from pathophysiology to tailored treatment. Front Endocrinol. (2022) 13:1020827. doi: 10.3389/fendo.2022.1020827
34. Centini, G, Schettini, G, Pieri, E, Giorgi, M, Lazzeri, L, Martire, FG, et al. Endometriosis-related ovarian Cancer: where are we now? A narrative review towards a pragmatic approach. J Clin Med. (2024) 13:1933. doi: 10.3390/jcm13071933
35. Lalami, I, Abo, C, Borghese, B, Chapron, C, and Vaiman, D. Genomics of endometriosis: from genome wide association studies to exome sequencing. Int J Mol Sci. (2021) 22:7297. doi: 10.3390/ijms22147297
36. Nouri, K, Ott, J, Krupitz, B, Huber, JC, and Wenzl, R. Family incidence of endometriosis in first, second-, and third-degree relatives: case-control study. Reprod Biol Endocrinol. (2010) 8:85. doi: 10.1186/1477-7827-8-85
37. Gallagher, CS, Mäkinen, N, Harris, HR, Rahmioglu, N, Uimari, O, Cook, JP, et al. Genome-wide association and epidemiological analyses reveal common genetic origins between uterine leiomyomata and endometriosis. Nat Commun. (2019) 10:4857. doi: 10.1038/s41467-019-12536-4
38. Masuda, T, Low, SK, Akiyama, M, Harita, M, Ueda, Y, Matsuda, K, et al. GWAS of five gynecologic diseases and cross-trait analysis in Japanese. Eur J Hum Genet. (2020) 28:95–107. doi: 10.1038/s41431-019-0495-1
39. Saunders, PTK. Insights from genomic studies on the role of sex steroids in the aetiology of endometriosis. Reprod Fertil. (2022) 3:R51–65. doi: 10.1530/RAF-21-0078
40. Zhang, Z, Suo, L, Chen, Y, Zhu, L, Wan, G, and Han, X. Endometriotic peritoneal fluid promotes Myofibroblast differentiation of endometrial mesenchymal stem cells. Stem Cells Int. (2019) 2019:1–13. doi: 10.1155/2019/6183796
41. Sapkota, Y, Steinthorsdottir, V, Morris, AP, Fassbender, A, Rahmioglu, N, De Vivo, I, et al. Meta-analysis identifies five novel loci associated with endometriosis highlighting key genes involved in hormone metabolism. Nat Commun. (2017) 8:15539. doi: 10.1038/ncomms15539
42. Warzecha, D, Załęcka, J, Mańka, G, Kieka, M, Lipa, M, Spaczynski, R, et al. Plasma and peritoneal fluid fibronectin and collagen IV levels as potential biomarkers of endometriosis. Int J Mol Sci. (2022) 23:15669. doi: 10.3390/ijms232415669
43. Béliard, A, Donnez, J, Nisolle, M, and Foidart, JM. Localization of laminin, fibronectin, E-cadherin, and integrins in endometrium and endometriosis. Fertil Steril. (1997) 67:266–72. doi: 10.1016/S0015-0282(97)81909-7
44. Yuge, A, Nasu, K, Matsumoto, H, Nishida, M, and Narahara, H. Collagen gel contractility is enhanced in human endometriotic stromal cells: a possible mechanism underlying the pathogenesis of endometriosis-associated fibrosis. Hum Reprod. (2007) 22:938–44. doi: 10.1093/humrep/del485
45. Holdsworth-Carson, SJ, Fung, JN, Luong, HT, Sapkota, Y, Bowdler, LM, Wallace, L, et al. Endometrial vezatin and its association with endometriosis risk. Hum Reprod. (2016) 31:999–1013. doi: 10.1093/humrep/dew047
46. Machado, DE, Berardo, PT, Palmero, CY, and Nasciutti, LE. Higher expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 (Flk-1) and metalloproteinase-9 (MMP-9) in a rat model of peritoneal endometriosis is similar to cancer diseases. J Exp Clin Cancer Res. (2010) 29:4. doi: 10.1186/1756-9966-29-4
47. Bora, G, and Yaba, A. The role of mitogen-activated protein kinase signaling pathway in endometriosis. J Obstet Gynaecol Res. (2021) 47:1610–23. doi: 10.1111/jog.14710
48. Sikora, J, Ferrero, S, Mielczarek-Palacz, A, and Kondera-Anasz, Z. The delicate balance between the good and the bad IL-1 Proinflammatory effects in endometriosis. Curr Med Chem. (2018) 25:2105–21. doi: 10.2174/0929867325666180111093547
49. Matsuzaki, S, and Darcha, C. Involvement of the Wnt/β-catenin signaling pathway in the cellular and molecular mechanisms of fibrosis in endometriosis. PLoS One. (2013) 8:e76808. doi: 10.1371/journal.pone.0076808
50. Sapkota, Y, Fassbender, A, Bowdler, L, Fung, JN, Peterse, D, Dorien, O, et al. Independent replication and Meta-analysis for endometriosis risk loci. Twin Res Hum Genet. (2015) 18:518–25. doi: 10.1017/thg.2015.61
51. Adewuyi, EO, Mehta, D, Sapkota, Y, Auta, A, Yoshihara, K, Nyegaard, M, et al. Genetic analysis of endometriosis and depression identifies shared loci and implicates causal links with gastric mucosa abnormality. Hum Genet. (2021) 140:529–52. doi: 10.1007/s00439-020-02223-6
52. Bougie, O, Yap, MI, Sikora, L, Flaxman, T, and Singh, S. Influence of race/ethnicity on prevalence and presentation of endometriosis: a systematic review and meta-analysis. BJOG. (2019) 126:1104–15. doi: 10.1111/1471-0528.15692
53. Yen, CF, Kim, MR, and Lee, CL. Epidemiologic factors associated with endometriosis in East Asia. Gynecol Minim Invasive Ther. (2019) 8:4–11. doi: 10.4103/GMIT.GMIT_83_18
54. Triolo, O, Laganà, AS, and Sturlese, E. Chronic pelvic pain in endometriosis: an overview. J Clin Med Res. (2013) 5:153–63. doi: 10.4021/jocmr1288w
55. Vercellini, P, Fedele, L, Aimi, G, Pietropaolo, G, Consonni, D, and Crosignani, PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. (2007) 22:266–71. doi: 10.1093/humrep/del339
56. Vannuccini, S, Clemenza, S, Rossi, M, and Petraglia, F. Hormonal treatments for endometriosis: the endocrine background. Rev Endocr Metab Disord. (2022) 23:333–55. doi: 10.1007/s11154-021-09666-w
57. Missmer, SA, Hankinson, SE, Spiegelman, D, Barbieri, RL, Marshall, LM, and Hunter, DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. (2004) 160:784–96. doi: 10.1093/aje/kwh275
58. Hudelist, G, Fritzer, N, Thomas, A, Niehues, C, Oppelt, P, Haas, D, et al. Diagnostic delay for endometriosis in Austria and Germany: causes and possible consequences. Hum Reprod. (2012) 27:3412–6. doi: 10.1093/humrep/des316
59. Soliman, AM, Fuldeore, M, and Snabes, MC. Factors associated with time to endometriosis diagnosis in the United States. J Women’s Health. (2017) 26:788–97. doi: 10.1089/jwh.2016.6003
60. Littlejohn, G. Neuroinflammation in fibromyalgia and CRPS: top-down or bottom-up? Nat Rev Rheumatol. (2016) 12:242–2. doi: 10.1038/nrrheum.2016.26
61. Orr, NL, Wahl, KJ, Noga, H, Allaire, C, Williams, C, Bedaiwy, MA, et al. Phenotyping sexual pain in endometriosis using the central sensitization inventory. J Sex Med. (2020) 17:761–70. doi: 10.1016/j.jsxm.2019.12.019
62. Orr, NL, Wahl, KJ, Lisonek, M, Joannou, A, Noga, H, Albert, A, et al. Central sensitization inventory in endometriosis. Pain. (2022) 163:e234–45. doi: 10.1097/j.pain.0000000000002351
63. Chiarotto, A, Viti, C, Sulli, A, Cutolo, M, Testa, M, and Piscitelli, D. Cross-cultural adaptation and validity of the Italian version of the central sensitization inventory. Musculoskeletal Sci Pract. (2018) 37:20–8. doi: 10.1016/j.msksp.2018.06.005
64. Mayer, TG, Neblett, R, Cohen, H, Howard, KJ, Choi, YH, Williams, MJ, et al. The development and psychometric validation of the central sensitization inventory. Pain Pract. (2012) 12:276–85. doi: 10.1111/j.1533-2500.2011.00493.x
65. Raimondo, D, Raffone, A, Renzulli, F, Sanna, G, Raspollini, A, Bertoldo, L, et al. Prevalence and risk factors of central sensitization in women with endometriosis. J Min Invas Gynecol. (2023) 30:73–80.e1. doi: 10.1016/j.jmig.2022.10.007
66. Nisenblat, V, Bossuyt, PM, Shaikh, R, Farquhar, C, Jordan, V, Scheffers, CS, et al. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. (2016) 2016:CD012179. doi: 10.1002/14651858.CD012179
67. Horne, AW. Saunders PTK. SnapShot: endometriosis. Cell. (2019) 179:1677–7. doi: 10.1016/j.cell.2019.11.033
68. Yaşar, P, Ayaz, G, User, SD, Güpür, G, and Muyan, M. Molecular mechanism of estrogen-estrogen receptor signaling. Reprod Med Biol. (2016) 16:4–20. doi: 10.1002/rmb2.12006
69. Ahn, SH, Monsanto, SP, Miller, C, Singh, SS, Thomas, R, and Tayade, C. Pathophysiology and immune dysfunction in endometriosis. Biomed Res Int. (2015) 2015:795976:1–12. doi: 10.1155/2015/795976
70. Zhao, H, Zhou, L, Shangguan, AJ, and Bulun, SE. Aromatase expression and regulation in breast and endometrial cancer. J Mol Endocrinol. (2016) 57:R19–33. doi: 10.1530/JME-15-0310
71. Mettler, L, Schmidt, D, and Maher, P. The impact of endometriosis on the health of women 2016. Biomed Res Int. (2016) 2016:1747280. doi: 10.1155/2016/1747280
72. Yilmaz, BD, and Bulun, SE. Endometriosis and nuclear receptors. Hum Reprod Update. (2019) 25:473–85. doi: 10.1093/humupd/dmz005
73. Zhang, P, and Wang, G. Progesterone resistance in endometriosis: current evidence and putative mechanisms. Int J Mol Sci. (2023) 24:6992. doi: 10.3390/ijms24086992
74. Azeez, JM, Susmi, TR, Remadevi, V, Ravindran, V, Sujatha, AS, and Sreeja, S. New insights into the functions of progesterone receptor (PR) isoforms and progesterone signaling. Am J Cancer Res. (2021) 11:5214–32.
75. Attia, GR, Zeitoun, K, Edwards, D, Johns, A, Carr, BR, and Bulun, SE. Progesterone receptor isoform a but not B is expressed in endometriosis. J Clin Endocrinol Metab. (2000) 85:2897–902. doi: 10.1210/jcem.85.8.6739
76. Armstrong, GM, Maybin, JA, Murray, AA, Nicol, M, Walker, C, Saunders, PTK, et al. Endometrial apoptosis and neutrophil infiltration during menstruation exhibits spatial and temporal dynamics that are recapitulated in a mouse model. Sci Rep. (2017) 7:17416. doi: 10.1038/s41598-017-17565-x
77. Rocha, AL, Reis, FM, and Taylor, RN. Angiogenesis and endometriosis. Obstet Gynecol Int. (2013) 2013:859619:1–8. doi: 10.1155/2013/859619
78. Laschke, MW, Giebels, C, and Menger, MD. Vasculogenesis: a new piece of the endometriosis puzzle. Hum Reprod Update. (2011) 17:628–36. doi: 10.1093/humupd/dmr023
79. Donnez, J, Smoes, P, Gillerot, S, Casanas-Roux, F, and Nisolle, M. Vascular endothelial growth factor (VEGF) in endometriosis. Hum Reprod. (1998) 13:1686–90. doi: 10.1093/humrep/13.6.1686
80. Shifren, JL, Tseng, JF, Zaloudek, CJ, Ryan, IP, Meng, YG, Ferrara, N, et al. Ovarian steroid regulation of vascular endothelial growth factor in the human endometrium: implications for angiogenesis during the menstrual cycle and in the pathogenesis of endometriosis. J Clin Endocrinol Metab. (1996) 81:3112–8. doi: 10.1210/jcem.81.8.8768883
81. Wang, H, Gorpudolo, N, Li, Y, Feng, D, Wang, Z, and Zhang, Y. Elevated vascular endothelia growth factor-a in the serum and peritoneal fluid of patients with endometriosis. J Huazhong Univ Sci Technolog Med Sci. (2009) 29:637–41. doi: 10.1007/s11596-009-0520-7
82. Abramiuk, M, Grywalska, E, Małkowska, P, Sierawska, O, Hrynkiewicz, R, and Niedźwiedzka-Rystwej, P. The role of the immune system in the development of endometriosis. Cells. (2022) 11:2028. doi: 10.3390/cells11132028
83. Sozzani, S, Rusnati, M, Riboldi, E, Mitola, S, and Presta, M. Dendritic cell-endothelial cell cross-talk in angiogenesis. Trends Immunol. (2007) 28:385–92. doi: 10.1016/j.it.2007.07.006
84. Fainaru, O, Adini, A, Benny, O, Adini, I, Short, S, Bazinet, L, et al. Dendritic cells support angiogenesis and promote lesion growth in a murine model of endometriosis. FASEB J. (2008) 22:522–9. doi: 10.1096/fj.07-9034com
85. Stanic, AK, Kim, M, Styer, AK, and Rueda, BR. Dendritic cells attenuate the early establishment of endometriosis-like lesions in a murine model. Reprod Sci. (2014) 21:1228–36. doi: 10.1177/1933719114525267
86. Liu, H, and Lang, JH. Is abnormal eutopic endometrium the cause of endometriosis? The role of eutopic endometrium in pathogenesis of endometriosis. Med Sci Monit. (2011) 17:RA92-99:CR542–6.
87. Houshdaran, S, Oke, AB, Fung, JC, Vo, KC, Nezhat, C, and Giudice, LC. Steroid hormones regulate genome-wide epigenetic programming and gene transcription in human endometrial cells with marked aberrancies in endometriosis. PLoS Genet. (2020) 16:e1008601. doi: 10.1371/journal.pgen.1008601
88. Li, Y, Zhao, L, and Li, XF. Hypoxia and the tumor microenvironment. Technol Cancer Res Treat. (2021) 20:153303382110363. doi: 10.1177/15330338211036304
89. Zhang, Y, Liu, X, Deng, M, Xu, C, Zhang, Y, Wu, D, et al. Ferroptosis induced by iron overload promotes fibrosis in ovarian endometriosis and is related to subpopulations of endometrial stromal cells. Front Pharmacol. (2022) 13:930614. doi: 10.3389/fphar.2022.930614
90. Ding, D, Liu, X, Duan, J, and Guo, SW. Platelets are an unindicted culprit in the development of endometriosis: clinical and experimental evidence. Hum Reprod. (2015) 30:812–32. doi: 10.1093/humrep/dev025
91. Bruner-Tran, KL, Mokshagundam, S, Herington, JL, Ding, T, and Osteen, KG. Rodent models of experimental endometriosis: identifying mechanisms of disease and therapeutic targets. Curr Womens Health Rev. (2018) 14:173–88. doi: 10.2174/1573404813666170921162041
92. Chen, S, Liu, Y, Zhong, Z, Wei, C, Liu, Y, and Zhu, X. Peritoneal immune microenvironment of endometriosis: role and therapeutic perspectives. Front Immunol. (2023) 14:1134663. doi: 10.3389/fimmu.2023.1134663
93. Liang, Y, Xie, H, Wu, J, Liu, D, and Yao, S. Villainous role of estrogen in macrophage-nerve interaction in endometriosis. Reprod Biol Endocrinol. (2018) 16:122. doi: 10.1186/s12958-018-0441-z
94. Zigmond, RE, and Echevarria, FD. Macrophage biology in the peripheral nervous system after injury. Prog Neurobiol. (2019) 173:102–21. doi: 10.1016/j.pneurobio.2018.12.001
95. Wu, J, Xie, H, Yao, S, and Liang, Y. Macrophage and nerve interaction in endometriosis. J Neuroinflammation. (2017) 14:53. doi: 10.1186/s12974-017-0828-3
96. Greaves, E, Temp, J, Esnal-Zufiurre, A, Mechsner, S, Horne, AW, and Saunders, PT. Estradiol is a critical mediator of macrophage-nerve cross talk in peritoneal endometriosis. Am J Pathol. (2015) 185:2286–97. doi: 10.1016/j.ajpath.2015.04.012
97. Sakaguchi, S, Yamaguchi, T, Nomura, T, and Ono, M. Regulatory T cells and immune tolerance. Cell. (2008) 133:775–87. doi: 10.1016/j.cell.2008.05.009
98. Young, VJ, Brown, JK, Maybin, J, Saunders, PT, Duncan, WC, and Horne, AW. Transforming growth factor-β induced Warburg-like metabolic reprogramming may underpin the development of peritoneal endometriosis. J Clin Endocrinol Metab. (2014) 99:3450–9. doi: 10.1210/jc.2014-1026
99. Hirschhaeuser, F, Sattler, UG, and Mueller-Klieser, W. Lactate: a metabolic key player in cancer. Cancer Res. (2011) 71:6921–5. doi: 10.1158/0008-5472.CAN-11-1457
100. Chen, J, Qin, P, Sun, Y, and Han, S. Histone lactylation promotes cell proliferation, migration and invasion through targeting HMGB1 in endometriosis. J Biomed Res. (2023) 37:470–8. doi: 10.7555/JBR.37.20230095
101. Viola, A, Munari, F, Sánchez-Rodríguez, R, Scolaro, T, and Castegna, A. The metabolic signature of macrophage responses. Front Immunol. (2019) 10:1462. doi: 10.3389/fimmu.2019.01462
102. Ramírez-Pavez, TN, Martínez-Esparza, M, Ruiz-Alcaraz, AJ, Marín-Sánchez, P, Machado-Linde, F, and García-Peñarrubia, P. The role of peritoneal macrophages in endometriosis. Int J Mol Sci. (2021) 22:10792. doi: 10.3390/ijms221910792
103. Zanna, MY, Yasmin, AR, Omar, AR, Arshad, SS, Mariatulqabtiah, AR, Nur-Fazila, SH, et al. Review of dendritic cells, their role in clinical immunology, and distribution in various animal species. Int J Mol Sci. (2021) 22:8044. doi: 10.3390/ijms22158044
104. Laginha, PA, Arcoverde, FVL, Riccio, LGC, Andres, MP, and Abrão, MS. The role of dendritic cells in endometriosis: a systematic review. J Reprod Immunol. (2022) 149:103462. doi: 10.1016/j.jri.2021.103462
105. Somigliana, E, Viganò, P, Gaffuri, B, Candiani, M, Busacca, M, Blasio, AM, et al. Modulation of NK cell lytic function by endometrial secretory factors: potential role in endometriosis. Am J Reprod Immunol. (1996) 36:295–300. doi: 10.1111/j.1600-0897.1996.tb00179.x
106. Jeung, I, Cheon, K, and Kim, MR. Decreased cytotoxicity of peripheral and peritoneal natural killer cell in endometriosis. Biomed Res Int. (2016) 2016:1–6. doi: 10.1155/2016/2916070
107. Hoogstad-van Evert, J, Paap, R, Nap, A, and van der Molen, R. The promises of natural killer cell therapy in endometriosis. Int J Mol Sci. (2022) 23:5539. doi: 10.3390/ijms23105539
108. Thiruchelvam, U, Wingfield, M, and O’Farrelly, C. Natural killer cells: key players in endometriosis. Am J Reprod Immunol. (2015) 74:291–301. doi: 10.1111/aji.12408
109. Maruyama, T, and Yoshimura, Y. Molecular and cellular mechanisms for differentiation and regeneration of the uterine endometrium. Endocr J. (2008) 55:795–810. doi: 10.1507/endocrj.K08E-067
110. Maksym, RB, Hoffmann-Młodzianowska, M, Skibińska, M, Rabijewski, M, Mackiewicz, A, and Kieda, C. Immunology and immunotherapy of endometriosis. J Clin Med. (2021) 10:5879. doi: 10.3390/jcm10245879
111. Pashizeh, F, Mansouri, R, Davari-Tanha, F, Hosseini, R, Asgari, Z, Aghaei, H, et al. Alterations of CD4+ T cell subsets in blood and peritoneal fluid in different stages of endometriosis. Int J fertile Steril. (2020) 14:201. doi: 10.22074/ijfs.2020.6127
112. Hori, S, Nomura, T, and Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science. (2003) 299:1057–61. doi: 10.1126/science.1079490
113. Berbic, M, Hey-Cunningham, AJ, Ng, C, Tokushige, N, Ganewatta, S, Markham, R, et al. The role of Foxp3+ regulatory T-cells in endometriosis: a potential controlling mechanism for a complex, chronic immunological condition. Hum Reprod. (2010) 25:900–7. doi: 10.1093/humrep/deq020
114. Budiu, RA, Diaconu, I, Chrissluis, R, Dricu, A, Edwards, RP, and Vlad, AM. A conditional mouse model for human MUC1-positive endometriosis shows the presence of anti-MUC1 antibodies and Foxp3+ regulatory T cells. Dis Model Mec. (2009) 2:593–603. doi: 10.1242/dmm.002535
115. Basta, P, Majka, M, Jozwicki, W, Lukaszewska, E, Knafel, A, Grabiec, M, et al. The frequency of CD25+ CD4+ and FOXP3+ regulatory T cells in ectopic endometrium and ectopic decidua. Reprod Biol Endocrinol. (2010) 8:1–7. doi: 10.1186/1477-7827-8-116
116. Olkowska-Truchanowicz, J, Bocian, K, Maksym, RB, Białoszewska, A, Włodarczyk, D, Baranowski, W, et al. CD4+ CD25+ FOXP3+ regulatory T cells in peripheral blood and peritoneal fluid of patients with endometriosis. Hum Reprod. (2013) 28:119–24. doi: 10.1093/humrep/des346
117. Tanaka, Y, Mori, T, Ito, F, Koshiba, A, Takaoka, O, Kataoka, H, et al. Exacerbation of endometriosis due to regulatory T-cell dysfunction. J Clin Endocrinol Metab. (2017) 102:3206–17. doi: 10.1210/jc.2017-00052
118. Vanni, VS, Villanacci, R, Salmeri, N, Papaleo, E, Delprato, D, Ottolina, J, et al. Publisher correction: concomitant autoimmunity may be a predictor of more severe stages of endometriosis. Sci Rep. (2021) 11:17715. doi: 10.1038/s41598-021-97506-x
119. Kapor, S, and Santibanez, JF. Myeloid-derived suppressor cells and mesenchymal stem/stromal cells in myeloid malignancies. J Clin Med. (2021) 10:2788. doi: 10.3390/jcm10132788
120. McCallion, A, Sisnett, DJ, Zutautas, KB, Hayati, D, Spiess, KG, Aleksieva, S, et al. Endometriosis through an immunological lens: a pathophysiology based in immune dysregulation. Explor Immunol. (2022) 2:454–83. doi: 10.37349/ei.2022.00062
121. Tokushige, N, Markham, R, Russell, P, and Fraser, IS. Nerve fibres in peritoneal endometriosis. Hum Reprod. (2006) 21:3001–7. doi: 10.1093/humrep/del260
122. Velho, RV, Taube, E, Sehouli, J, and Mechsner, S. Neurogenic inflammation in the context of endometriosis-what do we know? Int J Mol Sci. (2021) 22:13102. doi: 10.3390/ijms222313102
123. Morotti, M, Vincent, K, Brawn, J, Zondervan, KT, and Becker, CM. Peripheral changes in endometriosis-associated pain. Hum Reprod Update. (2014) 20:717–36. doi: 10.1093/humupd/dmu021
124. Miller, EJ, and Fraser, IS. The importance of pelvic nerve fibers in endometriosis. Womens Health. (2015) 11:611–8. doi: 10.2217/whe.15.47
125. Domoto, R, Sekiguchi, F, Tsubota, M, and Kawabata, A. Macrophage as a peripheral pain regulator. Cells. (2021) 10:1881. doi: 10.3390/cells10081881
126. García-Gómez, E, Vázquez-Martínez, ER, Reyes-Mayoral, C, Cruz-Orozco, OP, Camacho-Arroyo, I, and Cerbón, M. Regulation of inflammation pathways and Inflammasome by sex steroid hormones in endometriosis. Front Endocrinol. (2020) 10:935. doi: 10.3389/fendo.2019.00935
127. Sacco, K, Portelli, M, Pollacco, J, Schembri-Wismayer, P, and Calleja-Agius, J. The role of prostaglandin E2 in endometriosis. Gynecol Endocrinol. (2012) 28:134–8. doi: 10.3109/09513590.2011.588753
128. Lai, ZZ, Yang, HL, Ha, SY, Chang, KK, Mei, J, Zhou, WJ, et al. Cyclooxygenase-2 in endometriosis. Int J Biol Sci. (2019) 15:2783–97. doi: 10.7150/ijbs.35128
129. Pope, CJ, Sharma, V, Sharma, S, and Mazmanian, D. A systematic review of the association between psychiatric disturbances and endometriosis. J Obstet Gynaecol Can. (2015) 37:1006–15. doi: 10.1016/S1701-2163(16)30050-0
130. Friedl, F, Riedl, D, Fessler, S, Wildt, L, Richter, R, Schubler, G, et al. Impact of endometriosis on quality of life, anxiety, and depression: an Austrian perspective. Arch Gynecol Obstet. (2015) 292:1393–9. doi: 10.1007/s00404-015-3789-8
131. Vitale, SG, La Rosa, VL, Rapisarda, AM, and Laganà, AS. Impact of endometriosis on quality of life and psychological well-being. J Psychosom Obstet Gynaecol. (2017) 38:317–9. doi: 10.1080/0167482X.2016.1244185
132. De Graaff, AA, Van Lankveld, J, Smits, LJ, Van Beek, JJ, and Dunselman, GA. Dyspareunia and depressive symptoms are associated with impaired sexual functioning in women with endometriosis, whereas sexual functioning in their male partners is not affected. Hum Reprod. (2016) 31:2577–86. doi: 10.1093/humrep/dew215
133. Chen, LC, Hsu, JW, Huang, KL, Bai, YM, Su, TP, Li, CT, et al. Risk of developing major depression and anxiety disorders among women with endometriosis: a longitudinal follow-up study. J Affect Disord. (2016) 190:282–5. doi: 10.1016/j.jad.2015.10.030
134. Laganà, AS, La Rosa, V, Petrosino, B, and Vitale, SG. Comment on “risk of developing major depression and anxiety disorders among women with endometriosis: a longitudinal follow-up study”. J Affect Disord. (2017) 208:672–3. doi: 10.1016/j.jad.2016.07.016
135. Laganà, AS, Condemi, I, Retto, G, Muscatello, MR, Bruno, A, Zoccali, RA, et al. Analysis of psychopathological comorbidity behind the common symptoms and signs of endometriosis. Eur J Obstet Gynecol Reprod Biol. (2015) 194:30–3. doi: 10.1016/j.ejogrb.2015.08.015
136. Cavaggioni, G, Lia, C, Resta, S, Antonielli, T, Benedetti Panici, P, Megiorni, F, et al. Are mood and anxiety disorders and alexithymia associated with endometriosis? A preliminary study. Biomed Res Int. (2014) 2014:1–5. doi: 10.1155/2014/786830
137. Low, WY, Edelmann, RJ, and Sutton, C. A psychological profile of endometriosis patients in comparison to patients with pelvic pain of other origins. J Psychosom Res. (1993) 37:111–6. doi: 10.1016/0022-3999(93)90077-S
138. Laganà, AS, La Rosa, VL, Rapisarda, AM, Valenti, G, Sapia, F, Chiofalo, B, et al. Anxiety and depression in patients with endometriosis: impact and management challenges. Int J Women’s Health. (2017) 9:323–30. doi: 10.2147/IJWH.S119729
139. Warzecha, D, Szymusik, I, Wielgos, M, and Pietrzak, B. The impact of endometriosis on the quality of life and the incidence of depression—a cohort study. Int J Environ Res Public Health. (2020) 17:3641. doi: 10.3390/ijerph17103641
140. Gambadauro, P, Carli, V, and Hadlaczky, G. Depressive symptoms among women with endometriosis: a systematic review and meta-analysis. Am J Obstet Gynecol. (2019) 220:230–41. doi: 10.1016/j.ajog.2018.11.123
141. Kumar, V, Khan, M, Vilos, GA, and Sharma, V. Revisiting the association between endometriosis and bipolar disorder. J Obstet Gynaecol Can. (2011) 33:1141–5. doi: 10.1016/S1701-2163(16)35082-4
142. Walker, E, Katon, W, Jones, LM, and Russo, J. Relationship between endometriosis and affective disorder. Am J Psychiatry. (1989) 146:380–1. doi: 10.1176/ajp.146.3.380
143. Lewis, DO, Comite, F, Mallouh, C, Zadunaisky, L, Hutchinson-Williams, K, Cherksey, BD, et al. Bipolar mood disorder and endometriosis: preliminary findings. Am J Psychiatry. (1987) 144:1588–91. doi: 10.1176/ajp.144.12.1588
144. Novais, RF, da Câmara-França, BE, Lasmar, RB, and Lasmar, BP. Endometriosis and its relationship with depression. Int J Clin Med. (2018) 9:71–8. doi: 10.4236/ijcm.2018.92008
145. Carbone, MG, Campo, G, Papaleo, E, Marazziti, D, and Maremmani, I. The importance of a multi-disciplinary approach to the Endometriotic patients: the relationship between endometriosis and psychic vulnerability. J Clin Med. (2021) 10:1616. doi: 10.3390/jcm10081616
146. Van Stein, K, Schubert, K, Ditzen, B, and Weise, C. Understanding psychological symptoms of endometriosis from a research domain criteria perspective. J Clin Med. (2023) 12:4056. doi: 10.3390/jcm12124056
147. Harris, HR, Wieser, F, Vitonis, AF, Rich-Edwards, J, Boynton-Jarrett, R, Bertone-Johnson, ER, et al. Early life abuse and risk of endometriosis. Hum Reprod. (2018) 33:1657–68. doi: 10.1093/humrep/dey248
148. Harricharan, S, McKinnon, MC, and Lanius, RA. How processing of sensory information from the internal and external worlds shape the perception and engagement with the world in the aftermath of trauma: implications for PTSD. Front Neurosci. (2021) 15:360. doi: 10.3389/fnins.2021.625490
149. Reis, FM, Coutinho, LM, Vannuccini, S, Luisi, S, and Petraglia, F. Is stress a cause or a consequence of endometriosis? Reprod Sci. (2020) 27:39–45. doi: 10.1007/s43032-019-00053-0
150. Liebermann, C, Schwartz, ASK, Charpidou, T, Geraedts, K, Rauchfuss, M, Wölfler, M, et al. Maltreatment during childhood: a risk factor for the development of endometriosis? Hum Reprod. (2018) 33:1449–58. doi: 10.1093/humrep/dey111
151. Sherwani, S, and Khan, MW. Autoantibodies against ROS-human serum albumin-a potent immunological marker in depressed individuals with smoking history. Rev Romana Med Lab. (2022) 30:399–411. doi: 10.2478/rrlm-2022-0039
152. Sherwani, S, Raafat, M, Rajendrasozhan, S, Khan, M, Saleem, M, Husain, Q, et al. Increased levels of autoantibodies against ROS-modified proteins in depressed individuals with decrease in antibodies against SARS-CoV-2 antigen (S1-RBD). Curr Issues Mol Biol. (2022) 44:5260–76. doi: 10.3390/cimb44110358
153. Farooq, RK, Asghar, K, Kanwal, S, and Zulqernain, A. Role of inflammatory cytokines in depression: focus on interleukin-1β. Biomed Rep. (2017) 6:15–20. doi: 10.3892/br.2016.807
154. Patil, M, Kulsange, S, Kazi, R, Chirmade, T, Kale, V, Mote, C, et al. Behavioral and proteomic studies reveal methylglyoxal activate pathways associated with Alzheimer’s disease. ACS Pharmacol Transl Sci. (2022) 6:65–75. doi: 10.1021/acsptsci.2c00143
155. Ramasamy, R, Vannucci, SJ, Yan, SS, Herold, K, Yan, SF, and Schmidt, A. Advanced glycation end-products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology. (2005) 15:16R–28R. doi: 10.1093/glycob/cwi053
156. Koller, D, Pathak, GA, Wendt, FR, Tylee, DS, Levey, DF, Overstreet, C, et al. Epidemiologic and genetic associations of endometriosis with depression, anxiety, and eating disorders. JAMA Netw Open. (2023) 6:e2251214. doi: 10.1001/jamanetworkopen.2022.51214
157. Kajiyama, H, Suzuki, S, Yoshihara, M, Tamauchi, S, Yoshikawa, N, Niimi, K, et al. Endometriosis and cancer. Free Rad Biol Med. (2019) 133:186–92. doi: 10.1016/j.freeradbiomed.2018.12.015
158. Bulun, SE, Yilmaz, BD, Sison, C, Miyazaki, K, Bernardi, L, Liu, S, et al. Endometriosis. Endocr Rev. (2019) 40:1048–79. doi: 10.1210/er.2018-00242
159. Szukiewicz, D. Epigenetic regulation and T-cell responses in endometriosis–something other than autoimmunity. Front Immunol. (2022) 13:943839. doi: 10.3389/fimmu.2022.943839
160. Shigesi, N, Kvaskoff, M, Kirtley, S, Feng, Q, Fang, H, Knight, JC, et al. The association between endometriosis and autoimmune diseases: a systematic review and meta-analysis. Hum Reprod Update. (2019) 25:486–503. doi: 10.1093/humupd/dmz014
161. Khan, WA, Malik, A, and Khan, MW. Depression linked to higher antibodies production against estrogenized insulin in type 1 diabetes. Int Immunopharmacol. (2020) 86:106712. doi: 10.1016/j.intimp.2020.106712
162. Khan, WA, Malik, A, and Khan, MW. Estrogenization of insulin by catecholestrogen produced high affinity autoantibodies and altered the normal function of insulin in type 1 diabetes. Life Sci. (2020) 256:117910. doi: 10.1016/j.lfs.2020.117910
163. Khan, WA, Alsamghan, AS, and Khan, MW. Endometrial cancer patients have high affinity antibodies for estrogen metabolite–receptor aggregate: a potential biomarker for EC. J Obstet Gynaecol Res. (2020) 46:2115–25. doi: 10.1111/jog.14413
164. Miller, AH. Depression and immunity: a role for T cells? Brain Behav Immun. (2010) 24:1–8. doi: 10.1016/j.bbi.2009.09.009
165. Irwin, MR, and Miller, AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. (2007) 21:374–83. doi: 10.1016/j.bbi.2007.01.010
166. Zorrilla, EP, Luborsky, L, McKay, JR, Rosenthal, R, Houldin, A, Tax, A, et al. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun. (2001) 15:199–226. doi: 10.1006/brbi.2000.0597
167. Sephton, SE, Dhabhar, FS, Keuroghlian, AS, Giese-Davis, J, McEwen, BS, Ionan, AC, et al. Depression, cortisol, and suppressed cell-mediated immunity in metastatic breast cancer. Brain Behav Immun. (2009) 23:1148–55. doi: 10.1016/j.bbi.2009.07.007
168. Ivanova, SA, Semke, VY, Vetlugina, TP, Rakitina, NM, Kudyakova, TA, and Simutkin, GG. Signs of apoptosis of immunocompetent cells in patients with depression. Neurosci Behav Physiol. (2007) 37:527–30. doi: 10.1007/s11055-007-0047-y
169. Szuster-Ciesielska, A, Słotwińska, M, Stachura, A, Marmurowska-Michałowska, H, Dubas-Ślemp, H, Bojarska-Junak, A, et al. Accelerated apoptosis of blood leukocytes and oxidative stress in blood of patients with major depression. Prog Neuro-Psychopharmacol Biol Psychiatry. (2008) 32:686–94. doi: 10.1016/j.pnpbp.2007.11.012
170. Pariante, CM, and Miller, AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. (2001) 49:391–404. doi: 10.1016/S0006-3223(00)01088-X
171. McEwen, BS, Biron, CA, Brunson, KW, Bulloch, K, Chambers, WH, Dhabhar, FS, et al. The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine and immune interactions. Brain Res Brain Res Rev. (1997) 23:79–133. doi: 10.1016/S0165-0173(96)00012-4
172. Monsivais, D, Bray, JD, Su, E, Pavone, ME, Dyson, MT, Navarro, A, et al. Activated glucocorticoid and eicosanoid pathways in endometriosis. Fertil Steril. (2012) 98:117–25. doi: 10.1016/j.fertnstert.2012.03.030
173. Koukoura, O, Sifakis, S, and Spandidos, DA. DNA methylation in endometriosis. Mol Med Rep. (2016) 13:2939–48. doi: 10.3892/mmr.2016.4925
174. Lamceva, J, Uljanovs, R, and Strumfa, I. The main theories on the pathogenesis of endometriosis. Int J Mol Sci. (2023) 24:4254. doi: 10.3390/ijms24054254
175. Králíčková, M, Losan, P, and Vetvicka, V. Endometriosis and cancer. Womens Health. (2014) 10:591–7. doi: 10.2217/WHE.14.43
176. Esfandiari, F, Favaedi, R, Heidari-Khoei, H, Chitsazian, F, Yari, S, Piryaei, A, et al. Insight into epigenetics of human endometriosis organoids: DNA methylation analysis of HOX genes and their cofactors. Fertil Steril. (2021) 115:125–37. doi: 10.1016/j.fertnstert.2020.08.1398
177. Teague, EM, Print, CG, and Hull, ML. The role of microRNAs in endometriosis and associated reproductive conditions. Hum Reprod Update. (2010) 16:142–65. doi: 10.1093/humupd/dmp034
178. Anglesio, MS, Papadopoulos, N, Ayhan, A, Nazeran, TM, Noë, M, Horlings, HM, et al. Cancer-associated mutations in endometriosis without cancer. N Engl J Med. (2017) 376:1835–48. doi: 10.1056/NEJMoa1614814
179. Van Langendonckt, A, Casanas-Roux, F, and Donnez, J. Oxidative stress and peritoneal endometriosis. Fertil Steril. (2002) 77:861–70. doi: 10.1016/S0015-0282(02)02959-X
180. Scutiero, G, Iannone, P, Bernardi, G, Bonaccorsi, G, Spadaro, S, Volta, CA, et al. Oxidative stress and endometriosis: a systematic review of the literature. Oxidative Med Cell Longev. (2017) 2017:7265238. doi: 10.1155/2017/7265238
181. Kobayashi, H, Yamada, Y, Kanayama, S, Furukawa, N, Noguchi, T, Haruta, S, et al. The role of iron in the pathogenesis of endometriosis. Gynecol Endocrinol. (2009) 25:39–52. doi: 10.1080/09513590802366204
182. Defrère, S, Van Langendonckt, A, Vaesen, S, Jouret, M, González Ramos, R, Gonzalez, D, et al. Iron overload enhances epithelial cell proliferation in endometriotic lesions induced in a murine model. Hum Reprod. (2006) 21:2810–6. doi: 10.1093/humrep/del261
183. Iwabuchi, T, Yoshimoto, C, Shigetomi, H, and Kobayashi, H. Cyst fluid hemoglobin species in endometriosis and its malignant transformation: the role of metallobiology. Oncol Lett. (2016) 11:3384–8. doi: 10.3892/ol.2016.4383
184. Ni, Z, Li, Y, Song, D, Ding, J, Mei, S, Sun, S, et al. Iron-overloaded follicular fluid increases the risk of endometriosis-related infertility by triggering granulosa cell ferroptosis and oocyte dysmaturity. Cell Death Dis. (2022) 13:579. doi: 10.1038/s41419-022-05037-8
185. Li, A, Ni, Z, Zhang, J, Cai, Z, Kuang, Y, and Yu, C. Transferrin insufficiency and iron overload in follicular fluid contribute to oocyte dysmaturity in infertile women with advanced endometriosis. Front Endocrinol. (2020) 11:391. doi: 10.3389/fendo.2020.00391
186. Xu, B, Guo, N, Zhang, XM, Shi, W, Tong, XH, Iqbal, F, et al. Oocyte quality is decreased in women with minimal or mild endometriosis. Sci Rep. (2015) 5:10779. doi: 10.1038/srep10779
187. Sanchez, AM, Papaleo, E, Corti, L, Santambrogio, P, Levi, S, Vigano, P, et al. Iron availability is increased in individual human ovarian follicles in close proximity to an endometrioma compared with distal ones. Hum Reprod. (2014) 29:577–83. doi: 10.1093/humrep/det466
188. Giacomini, E, Sanchez, AM, Sarais, V, Al Beitawi, S, Candiani, M, and Viganò, P. Characteristics of follicular fluid in ovaries with endometriomas. Eur J Obstet Gynecol Reprod Biol. (2017) 209:34–8. doi: 10.1016/j.ejogrb.2016.01.032
189. Wiegand, KC, Shah, SP, Al-Agha, OM, Zhao, Y, Tse, K, Zeng, T, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. (2010) 363:1532–43. doi: 10.1056/NEJMoa1008433
190. McConechy, MK, Ding, J, Senz, J, Yang, W, Melnyk, N, Tone, AA, et al. Ovarian and endometrial endometrioid carcinomas have distinct CTNNB1 and PTEN mutation profiles. Mod Pathol. (2014) 27:128–34. doi: 10.1038/modpathol.2013.107
191. Matsumoto, T, Yamazaki, M, Takahashi, H, Kajita, S, Suzuki, E, Tsuruta, T, et al. Distinct β-catenin and PIK3CA mutation profiles in endometriosis-associated ovarian endometrioid and clear cell carcinomas. Am J Clin Pathol. (2015) 144:452–63. doi: 10.1309/AJCPZ5T2POOFMQVN
192. Catasús, L, Bussaglia, E, Rodrı́guez, I, Gallardo, A, Pons, C, Irving, JA, et al. Molecular genetic alterations in endometrioid carcinomas of the ovary: similar frequency of beta-catenin abnormalities but lower rate of microsatellite instability and PTEN alterations than in uterine endometrioid carcinomas. Hum Pathol. (2004) 35:1360–8. doi: 10.1016/j.humpath.2004.07.019
193. Sato, N, Tsunoda, H, Nishida, M, Morishita, Y, Takimoto, Y, Kubo, T, et al. Loss of heterozygosity on 10q23. 3 and mutation of the tumor suppressor gene PTEN in benign endometrial cyst of the ovary: possible sequence progression from benign endometrial cyst to endometrioid carcinoma and clear cell carcinoma of the ovary. Cancer Res. (2000) 60:7052–6.
Keywords: estrogen, endometriosis, estrogen receptor, inflammation, depression, immune imbalance
Citation: Sherwani S, Khan MWA, Rajendrasozhan S, Al-Motair K, Husain Q and Khan WA (2024) The vicious cycle of chronic endometriosis and depression—an immunological and physiological perspective. Front. Med. 11:1425691. doi: 10.3389/fmed.2024.1425691
Edited by:
Lan Zheng, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Diana Buzinskiene, Vilnius University, LithuaniaMatteo Giorgi, University of Siena, Italy
Copyright © 2024 Sherwani, Khan, Rajendrasozhan, Al-Motair, Husain and Khan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Subuhi Sherwani, cy5zaGVyd2FuaUB1b2guZWR1LnNh; Mohd Wajid Ali Khan, bXcua2hhbkB1b2guZWR1LnNh
 Subuhi Sherwani
Subuhi Sherwani Mohd Wajid Ali Khan
Mohd Wajid Ali Khan Saravanan Rajendrasozhan
Saravanan Rajendrasozhan Khalid Al-Motair2
Khalid Al-Motair2 Wahid Ali Khan
Wahid Ali Khan