- 1Department of Internal Medicine and Pediatrics, Ghent University, Ghent, Belgium
- 2Department of Gastroenterology, University Hospital Ghent, Ghent, Belgium
- 3VIB Center for Inflammation Research (IRC), Ghent University, Ghent, Belgium
- 4Ghent Gut Inflammation Group (GGIG), Ghent University, Ghent, Belgium
A significant percentage of patients with an inflammatory bowel disease (IBD) encounter fatigue which can profoundly diminish patients’ quality of life, particularly during periods of disease remission when gastrointestinal symptoms have receded. Various contributing risk factors have been identified including active inflammation, anemia, psychological, lifestyle and drug-related factors. While addressing these risk factors has been suggested as the initial approach to managing fatigue, a considerable number of patients still experience persisting symptoms, the primary causes of which remain incompletely understood. Recent insights suggest that dysfunction of the gut-brain axis may play a pathogenic role. This review provides an overview of established risk factors for fatigue, alongside emerging perspectives on the role of the gut-brain axis, and potential treatment strategies.
1 Introduction
In recent years, there have been significant advances in the understanding of inflammatory pathways involved in inflammatory bowel diseases (IBD), leading to a steep increase in the number of available treatments for IBD (1). With the expanding treatment options and advancements in diagnostic tools, the aspirations in treatment goals have shifted and a treat to target strategy is proposed, which includes long-term treatment goals such as a normalized quality of life (QoL) (2). The latter has gained more importance in recent decades, since it has become clear that IBD symptoms are not limited to the gastrointestinal tract. Patients with IBD often encounter disabling fatigue which can severely influence their social and interpersonal functioning and significantly reduce their QoL (3). During stages of active disease up to 72% of patients with IBD report fatigue, but even when disease remission is reached, and intestinal symptoms become less dominant over daily life, up to 42% of patients remain fatigued (4). This prevalence is remarkably higher than the 7% rate of fatigue observed in the general population (4, 5).
Psychological comorbidities of IBD not only reduce QoL (6, 7), but they can also impact the course of IBD itself, implying bidirectional gut-brain interactions in IBD. On the one hand, active IBD is associated with higher rates of psychological symptoms (4, 8). On the other hand, under-recognized and/or suboptimal treated mental health problems are associated with worse IBD-related outcomes including an increased risk of flare-ups, hospitalizations, surgery and corticosteroid use (9–13). The incidence of IBD is the highest in the 15–29-year-old age group (14), and impairment due to IBD has been shown to affect educational and employment prospects, implying an increased socioeconomic burden (15, 16). Moreover, fatigue in patients with IBD is associated with adverse outcomes and increased IBD-related and all-cause healthcare costs (13).
This review covers the definition and assessment of IBD-related fatigue, followed by an account of factors contributing to this condition. Next, an overview on recent insights into the pathophysiology will be provided, with an emphasis on the gut-brain axis. Finally, potential management strategies are discussed.
2 Definition of IBD-related fatigue and its assessment
Despite the high prevalence of fatigue, there is still a lack of clarity in the terminology, definition, and conceptualization of IBD-related fatigue, with different definitions being used throughout the current literature (17). Fatigue can be defined as an ongoing exhaustion or tiredness that is disproportionate or unrelated to activity or exertion and not alleviated by rest (18, 19). Several authors have suggested a multidimensional concept of fatigue including a physical, cognitive, and affective dimension (17, 20). The physical aspect of fatigue consists of the subjective feeling of weakness and the objective measurable decrease in physical activity and/or performance (20). Secondly, the cognitive aspect can manifest itself as a subjective difficulty in concentrating and the objective decrease in cognitive function or performance. Lastly, the affective component includes a decrease in motivation and mood (20).
The same unclarity that exists in the definition of IBD-related fatigue continues in the lack of consensus on how to assess fatigue (21). Over 250 different scales have been reported, of which at least nine have been used for research on IBD-related fatigue and five have been validated for this purpose. These include the functional assessment of chronic illness therapy fatigue (FACIT-F), the fatigue questionnaire (FQ), multidimensional assessment fatigue (MAF), the multidimensional fatigue inventory (MFI), and the inflammatory bowel disease fatigue (IBD-F); of which the latter is recommended for research and clinical use (22, 23).
3 Factors associated with IBD-related fatigue
3.1 Active disease correlates strongly with fatigue burden
Disease activity influences fatigue through various mechanisms. Activation of the systemic immune system increases central cytokine release, inducing sickness behavior similar to SARS-CoV-2 infection (24, 25). Additionally, the symptom burden, including frequent stools and nocturnal diarrhea, reduces energy levels (26). The important role of disease activity is clearly reflected by the significant difference in fatigue prevalence in patients with active disease compared to those in remission (4) and by the similar fatigue rates in other immune mediated inflammatory disorders (IMIDs) (27, 28). In multiple IBD-studies, clinical disease activity scores have been linked to increased fatigue burden (26, 29–31) and long-term persistence of disease activity has been associated with higher fatigue levels (32). The same trend was seen when assessing the correlation between fatigue and biochemical indices of inflammation such as C-reactive protein (CRP) (31, 33), erythrocyte sedimentation rate (34) and fecal calprotectin (35). Up to date, endoscopic disease activity has only been linked to fatigue in patients with ulcerative colitis (UC) (35, 36).
The relationship between fatigue and IBD-treatments is rather complex. Fatigue is a reported side effect of treatments such as immunomodulators and TNF-α inhibitors (26, 37–41). However, the necessity for these treatments suggests more severe disease, indicating that disease severity may have a greater impact on fatigue than the medication itself (26). Continued use of TNF-α inhibitors for 1 year has been associated with lower fatigue levels, likely due to achieving disease remission (41). Even though an improvement of disease activity does parallel amelioration of fatigue, fatigue often persists in patients in remission (30, 42–45), indicating that disease activity is not the sole contributor to fatigue (Figure 1).
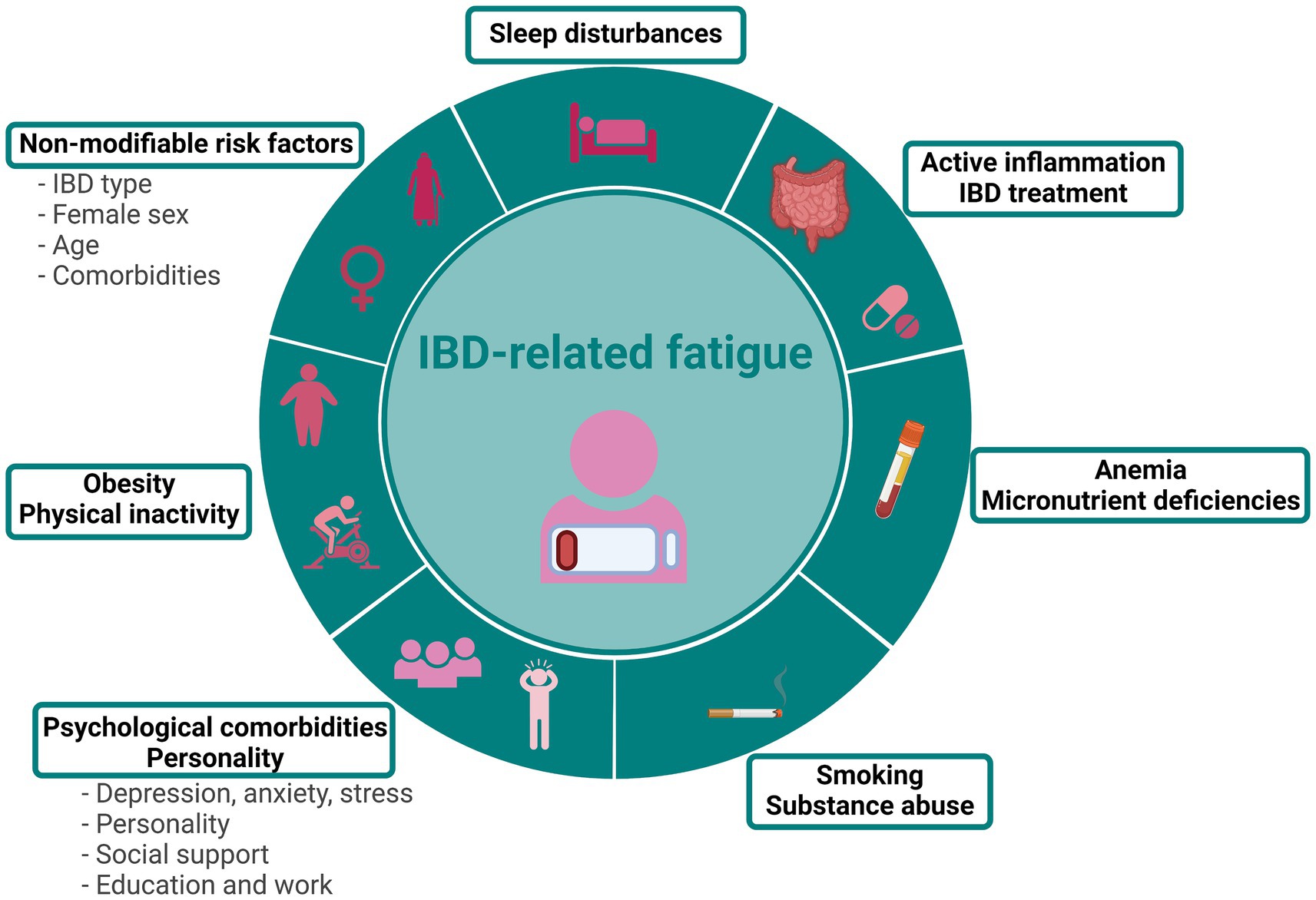
Figure 1. Risk factors associated with IBD-related fatigue. Figure created with BioRender.com.
3.2 The persistence of fatigue in patients with IBD in remission: a role for subclinical inflammation?
Typically, “sickness behavior” subsides once the initial acute illness ends. However, prolonged cytokine activation and dysregulation have been identified in chronic conditions such as long COVID, which can be associated with severe fatigue (25). When assessing cytokine patterns in fatigued patients with IBD in remission, conflicting results have been reported. In the study of Vogelaar et al., fatigue was associated with increased TNF-α and IFN-γ levels, and the immune profiles of these patients suggested a chronically active and T-helper cell 1 (Th1)-skewed immune system, despite clinical disease remission (46). Nevertheless, several other studies found no relationship between interleukin levels and fatigue in quiescent IBD (35, 42, 43). Thus, at present, insufficient evidence exists for a role of subclinical inflammation in fatigue pathogenesis during disease remission.
3.3 Micronutrient deficiencies and anemia
Anemia is a frequent complication of IBD and is mainly caused by chronic iron deficiency, anemia of chronic disease or a combination of both (47). In general, fatigue has been described as a symptom of anemia and this relationship was confirmed in several (18, 48) but not all studies (49–51). Iron plays a critical role in normal brain function and iron deficiency can negatively impact behavioral and mental health (52). Whereas some studies identified iron deficiency (in the absence of anemia) as a risk factor for fatigue (35, 53), this effect was not uniformly reported (30, 50, 51, 54).
Besides iron deficiency, patients with IBD are also at risk for deficiencies in other micronutrients but the role of these deficiencies in IBD-related fatigue is even more equivocal. Vitamin D deficiency is highly prevalent in IBD, ranging from 22 to 100% (55–57) and lower vitamin D levels has been linked to IBD-related fatigue (31) and muscle fatigue in patients with Crohn’s disease (CD) (58). Patients with IBD are also at higher risk for deficiencies in thiamine, vitamin B12 and folate, which in theory could lead to fatigue (59, 60) but studies supporting this association are lacking.
3.4 Psychological comorbidities and personality
Patients with IBD not only struggle with fatigue but also report high levels of depression (25%) and anxiety (35%), both of which are major risk factors for fatigue during active disease and remission (4, 8, 61, 62). Additionally, up to 51% of patients with IBD experience significant life stress (63), further increasing fatigue (30). Chronic diseases such as IBD can be associated with a significant burden on patients and not all patients have the same ability to cope with the disease and its consequences (64). Patients who experience increased disease-related worrying, negative perceptions about fatigue, feel a lack of control over their symptoms or engage in maladaptive behavioral coping strategies will experience higher levels of fatigue (65, 66). In addition, fatigue interference with daily life was negatively associated with a person’s sense of coherence, which can be defined as someone’s capacity to respond to stressful situations (64). On the other hand, patients with a self-directness and persistence dimension of personality experience lower fatigue levels (67). Stronger social support is associated with fewer psychological symptoms, yet nearly 70% of IBD patients face social constraints, and their fatigue burden is often underestimated by family and friends (68–70). Lower education levels (26), unemployment (40) and lower income are also significantly associated with higher fatigue and its interference with daily life (71).
3.5 Sleep disturbances
Up to 82% of patients with active IBD and 50% with inactive disease report disrupted sleep, compared to 32% of the general population (30). There is a bidirectional relationship between sleep and IBD: active disease leads to worse sleep quality due to higher symptom burden (72), and sleep disturbances in patients with CD in remission increase the risk of future flares (30, 72–74). Poor sleep quality is linked to increased fatigue, with associations noted with daytime sleepiness, prolonged sleep latency, sleep disturbances, and insomnia (67, 75). Furthermore, a recent study identified disturbed sleep in non-fatigued patients as a strong predictor of fatigue 6 months later (76). Importantly, sleep disturbances are also associated with other risk factors for fatigue such as disease activity and mood disorders and poor sleep quality is negatively correlated with vigorous physical activity, highlighting the complex interplay in fatigue etiology (72, 77).
3.6 Sarcopenia and obesity
High rates of sarcopenia are seen in patients with IBD (78), which has been related to severe fatigue (33). On the other hand, there is an ongoing global obesity epidemic, and up to 15–40% of adult patients with IBD are obese (79). Patients with obesity are not only less likely to reach clinical remission, but they also score worse on domains such as anxiety, depression, and fatigue (40, 79–81). Furthermore, individuals who follow an unhealthy diet, characterized by frequent consumption of foods like fatty red meat, sugary beverages, and fried dishes, reported heightened levels of fatigue (82).
3.7 Physical activity
Physical activity is increasingly being recognized for its beneficial effects on overall health, well-being, and QoL of patients with IBD (83). Conversely, nearly 30% of patients with non-severe IBD expressed concerns that physical activity might reactivate or exacerbate their disease status, a belief strongly linked to decreased physical activity levels (84). Patients with IBD are often more physically inactive (85–87), have higher rates of muscle fatigue (58), reduced hand grip strength (88) and lower quadriceps strength (86) as compared to their healthy peers. Importantly, patients feel that their disease imposes barriers on regular exercise, in part due to abdominal pain and urge, but mainly due to fatigue (89, 90). Patients with IBD-related fatigue showed significantly lower intensity of daily physical activity and reduced cardiorespiratory fitness (26, 91) and hand grip strength correlated significantly with fatigue levels (92). Both during active and quiescent stages of IBD, fatigue can restrict exercise capability, initiating a vicious cycle of physical inactivity, impaired physical fitness and worsening fatigue (91, 93).
3.8 Smoking and substance abuse
Nicotine use significantly impacts the risk of developing IBD and the disease course (94), and certain studies have linked smoking to higher rates of fatigue (31, 80). In addition to smoking, the use of psychotropics and narcotics is also higher in patients with IBD reporting fatigue compared to those that did not (74).
3.9 Non-modifiable risk factors
3.9.1 IBD type and disease duration
Differences in fatigue levels exist between IBD entities, with a recent meta-analysis reporting a general fatigue prevalence of 51% in CD and 45% in UC (95). This may be attributed to variations in disease behavior and severity between the two entities. For instance, factors indicating more complicated disease such as penetrating phenotype, previous biological use, and intestinal surgery have also been associated with higher fatigue burden (26, 31, 96). Additionally, malnutrition and sarcopenia rates are higher in CD compared to UC which could also contribute to fatigue (78).
The association between disease duration and fatigue remains ambiguous. While one study identified disease chronicity as a factor contributing to fatigue (31) another study observed lower fatigue levels in patients with longer disease duration (97). Finally, one study showed no significant relationship between disease duration and fatigue (44).
3.9.2 Sex and age
Several studies have identified a connection between the female sex and IBD-related fatigue, yet the reasons for the higher rates of fatigue in women remain uncertain (32, 35, 40, 42, 80, 98). Possibly, women do experience more severe fatigue compared to men, and additionally, women may have a greater willingness to report their (mental) health problems (71). Differential hormonal responses and their effects on the immune function might also explain part of the sex differences in fatigue (98).
In general, increasing age has been associated with lower levels of fatigue as compared to younger patients with IBD, potentially due to differences in coping strategies, responsibilities or adaptation (54, 97, 99, 100).
3.9.3 Comorbidities of persistent fatigue
Up to 35% of patients with IBD in remission have symptoms compatible with irritable bowel syndrome (IBS) which can lead to a higher symptom burden, increased (unjustified) disease-related worrying and reduced QoL (66, 101, 102). Moreover, several studies have confirmed the important correlation between IBS and IBD-related fatigue (66, 99, 103).
Fatigue can be associated with endocrinopathies such as adrenal insufficiency, hypopituitarism, hypogonadism, and thyroid diseases and patients with IBD are known to be at risk for autoimmune disorders (104, 105). Patients with IBD also have a higher risk of EIMs that can involve several organ systems such as skin, joints, hepatobiliary tract, or eyes (106). The presence of an EIM correlated significantly with the severity of fatigue (49) and patients with fatigue had a higher prevalence of EIMs compared to patients without fatigue (26). Joint EIMs are the most common in IBD, and in the subanalysis, these were the EIMs significantly linked to fatigue (49).
Patients with IBD are at higher risk for infections, especially during immunosuppressive treatment (107). While some infections may manifest with tiredness, evidence supporting their role in fatigue pathogenesis is limited. Recently, systemic antibody repertoires were assessed in patients with quiescent IBD, and increased antibody responses toward viral (including Epstein–Barr virus) and bacterial antigens were seen in patients with higher fatigue levels (108). Moreover, in patients with an IMID a history of SARS-CoV-2 infection was associated with elevated fatigue levels (109).
Even though IBD is typically diagnosed in patients aged 20–40 years, a second rise in incidence is reported in 60–70-year-old patients (110). Additionally, as the population exhibits aging globally, an increase in IBD among elderly patients is expected (110). This leads to higher rates of comorbidities and polypharmacy, both of which have been linked with fatigue (111). Moreover, aging is associated with longer exposure to chronic inflammation and/or immunosuppression, increasing the risk of cancer development, again potentially contributing to fatigue (112). Thus, screening for endocrinopathies or other comorbidities can be advised in patients with IBD presenting with fatigue.
4 New insights into the pathophysiology of IBD-related fatigue: the gut-brain axis
It is increasingly recognized that bidirectional communication exists between the gastro-intestinal tract and the central nervous system (CNS, Figure 2) (113). Different regions in the CNS such as the amygdala, hippocampus, and prefrontal cortex are involved both in the modulation of gut function as well as in the regulation of emotional and cognitive behaviors (114). Different anatomical sites determine gut-brain interaction including the CNS, the autonomic nervous system (ANS), the enteric nervous system (ENS), and the hypothalamus-pituitary–adrenal (HPA axis); while immune, endocrine, and neuronal mediators control these interactions (115). Another key player in the gut-brain axis is the gut microbiome which can influence gut-brain communication in different ways, interacting with all pathways implied in the gut-brain axis, including local interaction with the ENS and direct communication with the CNS through neuroendocrine and metabolic pathways (115, 116). Importantly, the CNS is an immunologically privileged site, maintained by three different barriers: the blood–brain barrier (BBB), the blood cerebrospinal fluid barrier (BCSFB), and the arachnoid barrier, which in physiological conditions limits the entrance of immune cells into the brain (117). Whereas the BBB is an endothelial barrier that strictly blocks the entrance of leukocytes into the brain (117), the BCSFB is formed by the choroid plexus which is a highly vascularized structure present in all brain ventricles that plays an important role in immune surveillance and regulation of immune cell trafficking (118).
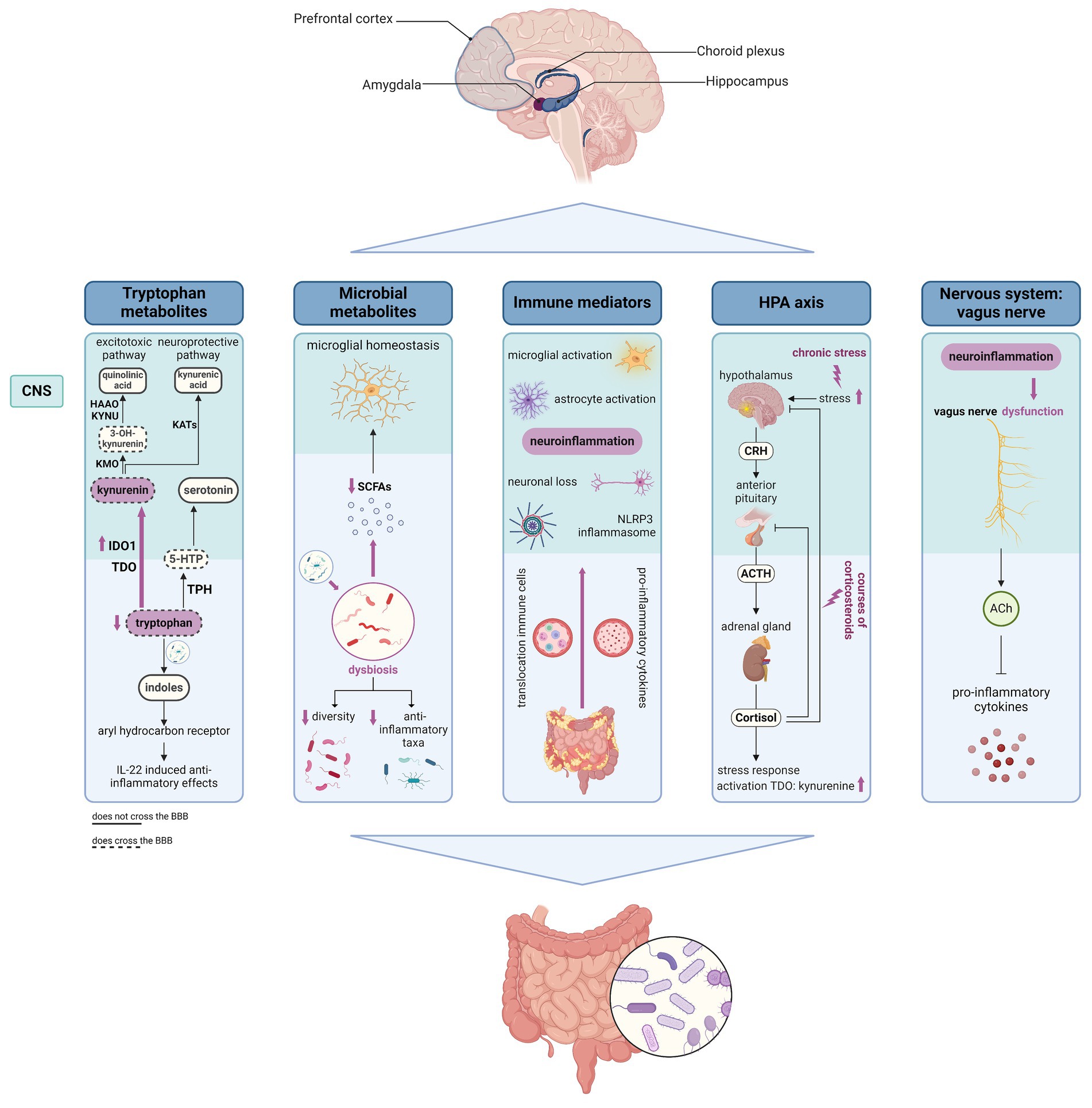
Figure 2. Schematic overview of different gut-brain communication pathways that could contribute to the pathogenesis of IBD-related fatigue. Figure created with BioRender.com; the HPA axis and potential impacts of IBD on the axis was created based on the Biorender.com template from Camilla Maria Fontana, University of Padova. 5-HTP, 5-hydroxytrypthophan; ACh, acetylcholine; ACTH, adrenocorticotropic hormone; BBB, blood–brain barrier; CNS, central nervous system; CRH, corticotropin releasing hormone; IBD, inflammatory bowel disease; HAAO, 3-hydroxyanthranillic acid 3,4-dioxygenase; KATs, kynurenine aminotransferases; KMO, kynurenine 3-monooxygenase; KYNU, kynureninase; IDO1, indoleamine 2,3-dioxygenase 1; SCFAs, short chain fatty-acids; TDO, tryptophan 2,3-dioxygenase.
4.1 Metabolic and microbial pathways
4.1.1 Tryptophan metabolism and fatigue
Tryptophan (Trp) is an essential amino acid that is metabolized along three different pathways (119). The kynurenine pathway represents the main Trp degradation route, in which Trp is metabolized to kynurenine by indoleamine 2,3-dioxygenase 1 (IDO1) or tryptophan 2,3-dioxygenase (TDO) (120). Whereas TDO predominantly mediates the basal metabolism of kynurenine, IDO1 is induced by inflammatory cytokines at the transcriptional level (121), which is also observed in patients with active IBD, thus shifting Trp metabolism toward the kynurenine pathway (120, 121). Even when disease remission is reached, Ido1 expression has been shown to remain high (122). Both Trp and kynurenine can pass the BBB, after which enzymes expressed in astrocytes and microglia can lead to the formation of neuroactive kynurenine metabolites in the CNS. These are categorized as excitotoxic (including 3-hydroxy-kynurenine and quinolinic acid) and neuroprotective (including kynurenic acid) (121, 123). The kynurenine 3-monooxygenase enzyme (KMO), leading to formation of 3-hydroxy-kynurenine, is mainly expressed in immunocompetent cells and the excitotoxic kynurenine pathway which is only weakly active under normal circumstances, is strongly induced during inflammatory conditions (123). Kynurenic acid is generated from kynurenine through the action of various kynurenine aminotransferases. These enzymes exhibit a lower affinity for kynurenine compared to KMO and increased activity of KMO further shifts the metabolic pathway toward the production of neurotoxic metabolites (124). In patients with depression, without antidepressant treatment, lower levels of kynurenic acid and higher levels of quinolinic acid were seen in the blood (125).
The second Trp pathway is the serotonin pathway, in which tryptophan hydroxylase (TPH) leads to the conversion of Trp to 5-hydroxytryptophan (5-HTP), the direct precursor of serotonin, a well-known neurotransmitter associated with the regulation of mood, behavior, and cognitive function (126–128). Both Trp as well as 5-HTP can cross the BBB, in contrast to serotonin itself (126).
In a third Trp pathway, intestinal bacteria process Trp to indoles, some of which can act as ligands for the aryl hydrocarbon receptor (AhR) (120). The latter plays an important role in the intestinal immune system, and mainly exerts anti-inflammatory effects in the gut through IL-22 (120). Again, inflammation induced upregulation of Ido1 can impede the formation of AhR ligands, and an inflammation-induced downregulation of AhR has been described in patients with CD (120, 121). Besides the metabolization of Trp, the microbiome can also influence the kynurenine and serotonin pathway by increasing IDO1 and TPH1 activity (120, 126). Interestingly, a variety of bacteria express tryptophanases and other enzymes in the Trp pathway that will theoretically further influence the fate of dietary Trp in the colon (129), but detailed studies in that area are lacking.
Reduced serum Trp levels have been associated with fatigue in patients with cancer (130), chronic renal insufficiency (131), and in stroke survivors (132). In patients with IBS, fatigue was significantly associated with a polymorphism in Tph2, supporting a role for dysregulated serotonin synthesis in fatigue pathophysiology (133). In patients with IBD, reduced serum Trp levels have consistently been linked to active disease (134, 135). The evolution of serum Trp levels during stages of disease remission is more ambiguous. In the study of Gupta et al. in four patients with CD in remission, Trp levels were comparable to the healthy controls (135). In a larger study cohort of Nikolaus et al., inactive CD was associated with lower Trp levels compared to the healthy controls (134). Recently, Tran et al. followed a cohort of 76 patients throughout the induction phase of biological treatment and found a strong correlation between Trp levels and FACIT-F scores (29). Noteworthy, the FACIT-F correlated strongly with the disease activity, and an influence of disease activity both on fatigue as well as on Trp levels could also impact this relationship. Nevertheless, in a study assessing metabolite profiles in IBD in remission, patients with fatigue had lower serum Trp levels compared with their non-fatigued counterparts (43).
4.1.2 The microbiome composition
The microbiome continuously interacts with the host’s epithelial and immune cells, ensuring homeostasis (136). Intestinal bacteria actively metabolize unabsorbed carbohydrates, exfoliated epithelial cells, and mucus, which in turn produces multiple metabolites such as short chain fatty-acids (SCFAs), that influences the host’s energy balance, immune system, and intestinal epithelial function (120, 136). The microbiome also regulates microglial homeostasis by the production of SCFAs (137) and these SCFAs have shown the potential to reduce lipopolysaccharide (LPS)-induced microglial activation in vitro (138).
Multiple studies in germ-free mice have confirmed the importance of the microbiome in different aspects of physiology, including normal functioning of the gut-brain axis (139, 140). Disruptions in the microbial equilibrium, termed dysbiosis, can render the gut vulnerable to pathogenic insults (141). In case of IBD, several studies have identified an overall reduction in the diversity of the microbiome, a reduction in the abundance of anti-inflammatory taxa, and a decrease in beneficial SCFA-producing bacteria (136, 142). Both depression and IBD separately have been linked to dysbiosis, but recently evidence also suggested differences in bacterial communities in patients with IBD and depressive symptoms compared to IBD patients without those symptoms (143). Additionally, fecal microbiome transplant from patients with IBD and depression to healthy mice induces not only colitis, but also depression-like behaviors, an effect that was not seen when transplanting the microbiome of patients with IBD without depression (144).
Previously, markers of gut dysbiosis, including circulating levels of LPS and antibodies against bacterial endotoxins, were associated with the severity of the chronic fatigue syndrome (145). Interestingly, the microbiome also has a crucial role in the motivation for exercise, as mice treated with broad-spectrum antibiotics show a decreased exercise performance (146). One study in patients with IBD showed that both fatigue and depression were associated with a distinct microbiota composition including lower abundances of SCFA-producing bacteria (147). Additionally, fatigue in patients with IBD in clinical and endoscopical remission was linked to a decrease in the diversity of the gut microbiome and reductions in butyrate-producing bacteria (43).
4.2 Immune pathways and neuroinflammation
Increasing evidence suggests that damage to the gut barrier integrity can lead to translocation of bacterial compounds across the gut epithelium, affecting the function of the BBB (148). Acute colitis in mice can lead to a decrease in the expression of tight junction proteins at the BBB, persisting up to 21 days after DSS initiation, suggesting a sustained effect of colitis (149, 150). Besides the BBB, the BCSFB barrier can also be severely affected by systemic inflammatory diseases, leading to increased permeability or alterations in the transcriptome of the choroid plexus (151–153).
The relationship between gut inflammation and neuroinflammation was shown in different animal models of acute and chronic colitis. In the brain of mice with acute colitis, RNA sequencing data revealed enriched inflammation-related pathways including the IL-17 signaling pathway, regulation of inflammatory responses, and antimicrobial peptides pathway (154). Differentially expressed genes included lipocalin-2 (Lcn2), S100a8, and S100a9 (154). The latter two genes encode for proteins that form the heterodimer calprotectin (S100A8/A9), a well-known disease marker in IBD (155). Lipocalin-2 has a critical involvement in the maintenance of intestinal homeostasis, and both serum and fecal Lcn2 levels correlate with disease activity in patients with IBD (156–158). Acute colitis, induced by DSS or DNBS, was also associated with increased expression of inflammatory genes, such as Tnf-α and Il-6, in the hippocampus (154, 159, 160). Soon after the induction of colitis through DSS administration, an effect on the microglial phenotype has been noted, including a shortening of branch length and decrease in the number of junctions (152). Moreover, acute DSS-induced colitis has been associated with increased Iba1 immunoreactivity (154), a marker of both resting as well as activated microglia. In mice, acute colitis has also been associated with behavioral alterations including increased anxiety-like behavior and a decrease in general locomotion (161). Moreover, acute colitis resulted in a reduction of voluntary wheel running behavior, which is a measure of general activity, potentially indicating fatigue-like behavior (162).
Studies in mice with active chronic colitis confirmed that chronic gut inflammation is associated with sustained neuroinflammatory responses. Mice with chronic colitis showed reduced hippocampal brain activity, astrocytosis and astrocyte activation, and an upregulation of inflammatory cytokines, such as IL-6, TNF-α, and IL-1β, in the hippocampus (163–165). This increase in inflammatory mediators in the hippocampus during chronic DSS-colitis was associated with reduced long-term memory (163). Additionally, chronic DSS-induced colitis has been linked to an increase in microglial and astrocytic density, both in the hippocampus as well as in the cortex (166). Salvo et al. found an increase in hippocampal microglia following low-dose DSS in early life, leading to decreased neurogenesis in the hippocampus, ultimately resulting in behavioral deficits (167). Chronic experimental colitis has also been linked to significant neuronal loss and reduced neurogenesis in the hippocampus, suggesting a role for colitis in neurodegeneration (164, 166).
He et al. found that chronic colitis leads to a significant upregulation of the NLR domain-containing protein 3 (NLRP3) inflammasome, both in microglia as in astrocytes and was associated with enhanced anxiety-like behavior and signs of cognitive dysfunction (166). Recent evidence suggests an interaction between the NLRP3 inflammasome and the gut microbiome, which shapes the peripheral and central immune responses (168). Dysbiosis and changes in the enteric, peripheral, and central activation of the NLRP3 inflammasome were previously identified in patients with Parkinson’s disease, Alzheimer’s disease, and depression (168). In these diseases, overactivation of the NLRP3 inflammasome is thought to impair the BBB and contribute to the central inflammatory response, contributing to CNS pathology (168).
Different pathways can convey the inflammatory response from gut to the brain, including an increase in circulating levels of inflammatory mediators such as IL-6, CXCL-1, and HMGB1 as identified in different animal studies (149, 150, 154, 163). These inflammatory mediators can signal to the brain either through neuronal pathways or through direct access into the CNS (149). Additionally, the increased permeability of the CNS barriers can lead to translocation of immune cells into the brain further propagating neuroinflammation (155). In mice with acute colitis, an increase in the rolling and adherent leukocytes on cerebral endothelial cells was seen, which can direct recruitment of neutrophils into the brain vasculature (169). Likewise, acute colitis was associated with an increase in monocytes and neutrophils in the brain of mice (154). In a model of chronic colitis, the adoptive T-cell transfer model, an increase in BBB permeability and brain-infiltrating T-cells was noted (165).
4.3 Neuronal pathways
The ANS consists of parasympathetic (including the vagus nerve) and sympathetic nervous systems, which relays information from the ENS to the CNS (170). The vagal nerve has anti-inflammatory effects in the gut by the release of acetylcholine, which inhibits pro-inflammatory cytokine secretion by macrophages (171). This cholinergic anti-inflammatory pathway (CAIP) can be stimulated or inhibited by vagal nerve stimulation (VNS) or vagotomy, respectively. Vagotomy in mice with experimental colitis worsens disease activity (172), while VNS improved stool quality and reduced inflammation in rats with colitis (173). Moreover, both the prefrontal cortex and hippocampus hold modulatory roles over vagal nerve functioning, and it is hypothesized colitis-induced neuroinflammation might trigger vagus nerve dysfunction (155). Interestingly, patients with IBD and positive affective adjustment (lower scores of anxiety and depression) had more beneficial coping strategies and an adapted ANS activity (114). In patients with CD and positive affectivity, higher sympathetic activity was observed, probably indicating an adaptation mechanism since the sympathetic nervous system is known to exert anti-inflammatory effects in CD (114). In patients with UC, negative affectivity was associated with a parasympathetic blunt (114). Thus, ANS dysfunction could also play a role in IBD-related fatigue, but further research into this topic is required.
4.4 Endocrine pathways
Stress is a universal aspect of life, and the HPA axis plays a crucial role in our ability to adapt and respond to stressors, including the increase in blood sugar and suppression of the immune function (174).
Activation of the HPA axis plays a crucial role in the communication between immune and neuroendocrine systems, which is important in regaining homeostasis after immune activation (151). In patients with IBD, chronic exposure to stress and inflammation can result in dysregulation of the HPA axis (155, 175). This is supported by evidence correlating serum cortisol levels with psychological stress in CD (175). In healthy adults, the ANS and HPA axis cooperate in the anti-inflammatory response to stress, whereas these appeared to be uncoupled in patients with IBD (176, 177). In mice, acute DSS-induced colitis was linked to alterations in the HPA axis activity resulting in an altered behavioral response to stress (178). Moreover, induction of stress in mice led to activation of IL-6 signaling which was reported to play an important role in the changes occurring in the HPA axis in response to repeated stress (179). Chronic stress also induces visceral pain in mice, a process mediated by microglia activity in the central nucleus of the amygdala (180). Potentially, inflammation in the gut feeds forward to the CNS, leading to neuroinflammation-induced damage to brain regions implied in HPA axis regulation (155). In addition, the HPA axis can also influence behavior through modulation of the Trp metabolism since cortisol is known to activate TDO, leading to increased kynurenine production, and increased serotonin re-uptake, resulting in lower availability of serotonin which could enhance depressive symptoms (155). Patients with IBD often require courses of corticosteroids due to disease flare-ups, which can also impact the function of the HPA axis and lead to adrenal insufficiency, which can be quite prolonged (181, 182). Symptoms of adrenal insufficiency are rather non-specific but can include fatigue (181). Interestingly, patients with multiple sclerosis and fatigue had a higher activity of the HPA axis, with elevated plasma levels of ACTH, compared to their non-fatigued counterparts (183). On the contrary, in patients with IBD in remission without steroid use in the last year, no correlation was found between cortisol levels and fatigue (184). Thus, despite the apparent dysregulation of the HPA-axis in IBD, evidence on a role in fatigue pathogenesis remains limited.
4.5 Structural and functional alterations in the CNS of patients with IBD
As seen in other neurodegenerative diseases, several MRI studies have shown both structural and functional alterations in the CNS of patients with CD (185–187). Patients with CD had lower glutamate and glutamine concentrations in the brain, which was previously seen in Alzheimer’s disease (188). Additionally, in patients with CD, the volume of the choroid plexus was negatively correlated with biochemical markers of disease activity (CRP and fecal calprotectin) (185). These findings support the potential impact of systemic inflammation leading to alterations in the CNS, which could contribute to fatigue pathogenesis (188).
As mentioned earlier, IBS is linked to tiredness associated with IBD and among patients with CD in remission, whether experiencing abdominal pain or not, variations were observed in the resting-state brain activity within specific brain regions (189). Moreover, patients with CD exhibit changes in gray matter volume and cortical thickness compared to healthy controls, although when adjusting for anxiety and depression, the alterations in various brain regions implicated in emotional processing lose significance (187). Fatigue in patients with CD correlates with diminished gray matter volume in the precentral gyrus and other sensorimotor areas, particularly evident during periods of disease remission (190). Furthermore, significant disparities in cognitive functioning, cerebral perfusion, and neurochemistry are observed in patients with quiescent CD experiencing fatigue, compared to their healthy counterparts (188).
5 The management of IBD-related fatigue
Despite the high prevalence of fatigue, effective evidence-based treatment options remain limited. In general, health-care professionals’ experience multiple barriers in assessing and treating fatigue due to difficulties in conceptualizing this subjective complaint, the limited understanding of the impact of fatigue on the patients’ lives and/or gaps in their knowledge of the pathogenesis (191). Furthermore, patients sometimes feel their fatigue complaint is either ignored or they themselves do not report it to their physician since they are unaware it can be a manifestation of IBD (191). Therefore, the first step in the management should be to raise awareness, screen, and acknowledge patients’ fatigue levels (Figure 3). Additionally, general strategies can be proposed (193). This includes day planning, structured rests and breaks, distribution of their energy throughout the day and prioritization of important events (193). Maladaptive coping strategies such as daytime naps and all-or-nothing behavior should be discouraged (65, 194) and support from relatives can be advantageous in accepting and dealing with fatigue (193). To optimize communication with the physician during consultations patients can be encouraged to keep a fatigue diary, as this was proven beneficial in cancer-related fatigue (195, 196). In the future, there might also be a place for the use of telemedicine tools to offer patients continuous and personalized monitoring of the disease and its complications (including fatigue), such as the recently developed tool myIBDcoach® (197).
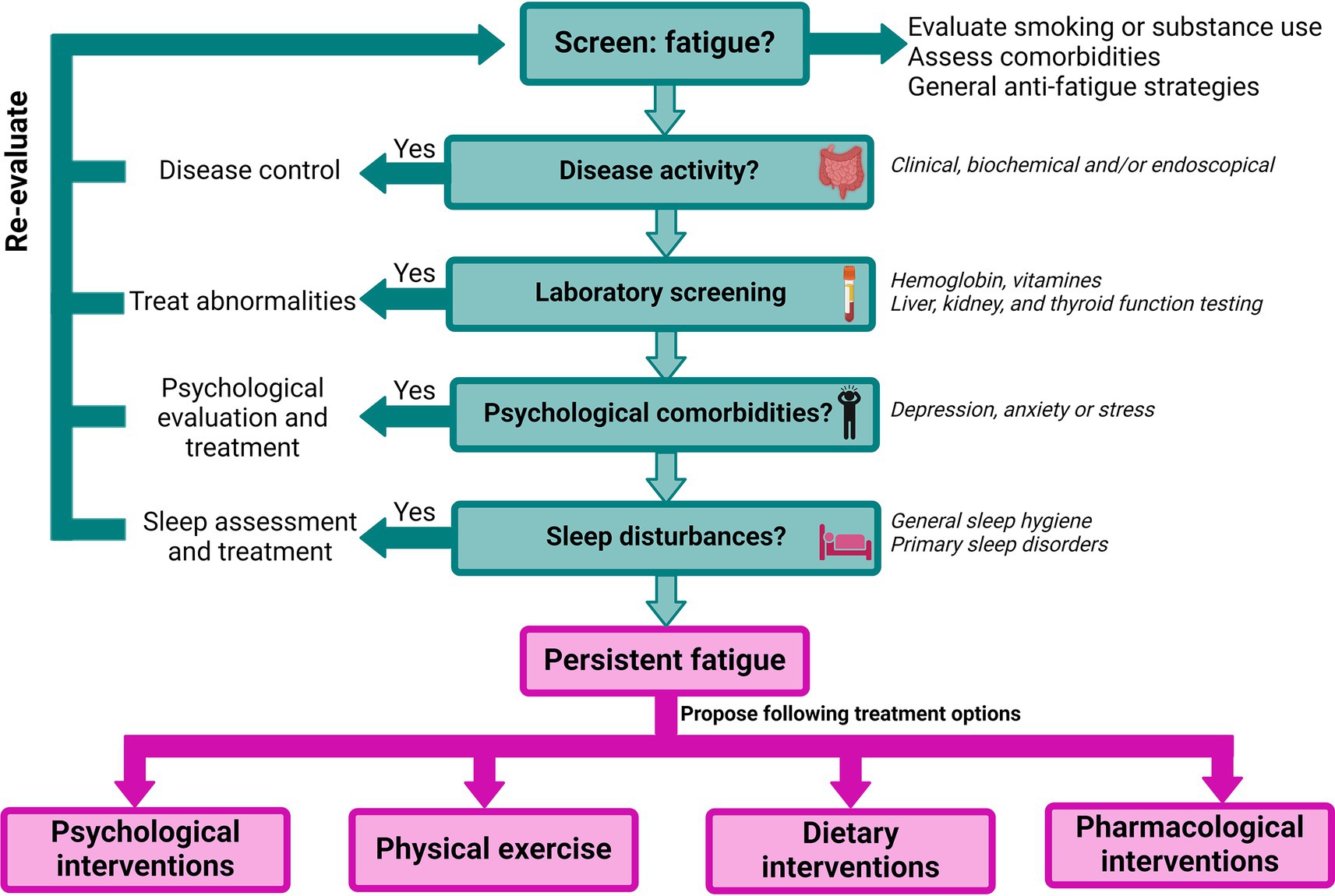
Figure 3. Proposed flowchart for the management of fatigue in IBD, adapted from Hindryckx et al. (21), Nocerino et al. (22), and Borren et al. (192). Figure created with BioRender.com.
5.1 Treating active inflammation
Given the well-known association between fatigue and disease activity management of IBD activity is essential. Part of this treatment optimization is the assessment of treatment adherence as psychiatric comorbidities have been associated with noncompliance (198). Several studies have shown a beneficial effect of biologicals on fatigue and QoL and increasingly, fatigue is being incorporated as a formal outcome in randomized controlled trials (RCTs) assessing newer treatments for IBD. For example, TNF-inhibitors (199, 200), JAK-inhibitors (201, 202), and IL23-inhibitors (203–205) have shown beneficial effects on fatigue in patients with IBD. Moreover, some head-to-head trials have assessed advanced therapies for addressing fatigue associated with IBD. In patients with CD, ustekinumab demonstrated superior efficacy in reducing fatigue compared to adalimumab (206), while vedolizumab and tofacitinib showed similar effectiveness in managing fatigue among patients with UC (207). Despite these promising results, fatigue often persists during disease remission (208) and no data exist on the efficacy of biologicals for the treatment of fatigue in patients with clinically inactive IBD (192).
5.2 Correction of anemia and nutritional deficiencies
In patients with IBD and low hemoglobin levels, further anemia workup should be initiated, including iron and vitamin studies and substitution is recommended in case of deficiencies (209). In patients with IBD and iron-deficiency anemia, both ferric carboxymaltose (FCM) and ferric derisomaltose (FDI) led to a significant improvement in FACIT-F scores, with the greatest improvement in the FDI group (210). Treatment with FCM led to higher rates of hypophosphatemia and the severity of post-infusion hypophosphatemia was associated with slower improvement in fatigue (210). Evidence is less clear when assessing the need for iron supplementation in non-anemic patients with IBD-related fatigue. On the one hand, in the study of Ҫekiç et al., patients with IBD, all non-anemic with iron deficiency, showed a significant increase in QoL after an intravenous (IV) infusion with iron sucrose (211). On the other hand, in the RCT performed by Fiorino et al., IV FCM did not improve chronic fatigue in IBD patients with non-anemic iron deficiency (212).
There is even less evidence for a beneficial effect of vitamin substitution on IBD-related fatigue. In an RCT including 214 patients with IBD (without vitamin deficiencies), supplementation with an over-the-counter multivitamin and mineral supplement led to some alleviation of fatigue, particularly noticeable in patients with UC (213). Another RCT in 95 fatigued patients with IBD or IBS, and normal vitamin B12 levels, could not identify a beneficial effect of additional vitamin B12 supplementation (1,000 μg per day) on fatigue levels (214). Vitamin D substitution has been shown to be effective to reduce fatigue in patients with other IMIDs such as systemic lupus erythematosus (215) and to reduce health-care utilization (56) and improve health-related QoL (216) in patients with IBD. Nonetheless, there is insufficient evidence supporting vitamin D treatment for IBD-related fatigue.
5.3 Identification and treatment of mood and sleep disorders
In a next step, psychological comorbidities should be assessed since these are not only strongly related to fatigue but also to other adverse IBD-related outcomes (9, 10). In case of clinical suspicion, patients should either be treated by their IBD specialist or be referred to mental health services for further evaluation and treatment (217).
A routine evaluation of sleep quality should be performed in patients with IBD, especially in case of fatigue (72). When poor sleep quality is suspected, known risk factors for sleep disturbances such as mood disorders, medication use or smoking should first be identified and treated (72). Patients should be counseled on the importance of adequate sleep hygiene and aim to reach at least 7 h of sleep per night (218). Up to date, melatonin supplements, to improve the circadian function, cannot be recommended as insufficient evidence is available on their safety and effectiveness in patients with IBD (218). In case of unexplained poor sleep quality, a thorough assessment by a sleep professional may be recommended to exclude primary sleep disorders (72).
5.4 Treatment of persisting fatigue: non-pharmacological interventions
When other potential causes of fatigue are excluded and/or treated, re-evaluation should be performed, and potentially modifiable risk factors should be treated. In case of persisting, unexplained fatigue, psychosocial, physical or pharmacological interventions can be considered.
5.4.1 Psychosocial interventions
Several trials have assessed psychological interventions in IBD, with fatigue either as a primary or secondary outcome. Different psychological interventions were tested including stress management techniques (219), solution-focused therapy (220, 221), cognitive behavioral therapy (222), mindfulness-based cognitive therapy (223), and psychoeducational sessions (224). Globally, all trials showed a beneficial effect on fatigue, even though this could not always be confirmed statistically, supporting a promising role for psychological therapies in the management of fatigue in quiescent disease (225). In contrast with the other trials, the study of Hashash et al. specifically included patients with reduced sleep quality, and in a first phase, behavioral therapy showed beneficial effects on both sleep and fatigue (226). Whereas the majority of other trials included patients in remission, a recent study assessed the effect of a cognitive behavioral and mindfulness-based stress reduction (COBMINDEX) program in patients with mild to moderately active CD (Harvey Bradshaw Index of 5–16) (227). After 3 months of intervention, patients in the COBMINDEX group experienced a significant reduction of their fatigue levels compared to baseline (227).
Notwithstanding, due to the scarce number of trials, heterogeneous study designs, underpowering, and small effect sizes, it is difficult to draw definite conclusions as regards to the most recommendable intervention in patients with IBD-related fatigue (225). In addition, little is known about the long-term impact of these interventions. For example, in the study of Vogelaar et al. the effect of solution-focused therapy disappeared at 9 months of follow-up (221). Some patients with IBD also experience barriers to engage in psychotherapy, which can severely impact the success of these interventions (228). For example, in adolescents and young adults with IBD and depression, patients identified fatigue as an important barrier to participate in mindfulness (229).
5.4.2 Physical activity
Physical activity has numerous beneficial effects such as increasing anti-inflammatory adipokines, increasing bacterial diversity, and decreasing visceral adiposity (230). In addition, exercise is thought to improve well-being, stress, and QoL of IBD patients and might play a role in disease management (83). There is some evidence for a positive effect of physical exercise on fatigue levels, although fatigue was rarely the primary outcome measure, and several studies did not include a control group. In a pilot trial including 52 patients with CD individual exercise advice significantly improved fatigue, as measured with the IBD-F, one of the secondary outcomes, as compared to placebo (231). Fagan et al. evaluated the use of an unsupervised exercise program (an individualized instruction booklet) in patients that were either in remission or had mild to moderate disease activity, which led to a significant improvement in fatigue and QoL (232). In another cohort of 20 patients with CD, low intensity exercise improved QoL and even though fatigue was not an outcome measure in this study, patients did report increased energy levels and complained less about fatigue (233). The PROTECT trial assessed the effect of a 6-month exercise program, consisting of both impact as well as resistance training. Whereas fatigue was not a primary outcome of this trial, patients in the exercise group did show significantly lower fatigue scores compared to the controls (234). In a small case series of Nathan et al., patients with IBD who performed regular exercise were asked about benefits and difficulties they experienced, and four out of 11 patients felt that exercise helped them with fatigue (235). Another pilot trial in 32 patients with IBD found that a 16-week exercise program (IBD-Fit), significantly improved patients’ physical fitness and fatigue scores (236). In a larger survey assessing exercise habits of 918 patients with IBD, up to 12% felt physical activity helped them boost their energy levels (90). On the other hand, in a pilot study assessing the effect of high intensity interval training and moderate intensity continuous training, no clear effect was seen of these exercise regimens on fatigue levels (237). A potential benefit of yoga-based interventions can also be expected as these have previously been shown to improve QoL of patients with IBD and fatigue in patients with cancer and multiple sclerosis. However, trials in patients with IBD-related fatigue are currently lacking (238).
More research is required with fatigue as a primary outcome measure in physical activity intervention studies. Nevertheless, a beneficial trend is seen, and exercise interventions were generally well-tolerated and safe. Thus, for patients medically fit to do so, increasing rather than reducing exercise should be considered to address fatigue. During IBD consultations the international Physical Activity Questionnaire (IPAQ) can be used to assess patients current physical activity levels and discuss potential for improvement (84, 239).
5.4.3 Diet
The high prevalence of IBD in Western countries underscores the importance of diet and its impact on the development of IBD (240). Given the associations between vitamin deficiencies, sarcopenia, and an unhealthy diet with fatigue, dietary interventions also hold promise for managing IBD-related fatigue, although up to date studies are still limited and often uncontrolled.
In a study with 26 patients with mildly active IBD or in IBD remission, diet quality was improved by increasing the intake of vegetables, fruits, whole grains, legumes, nuts, dairy, and fish and decreasing consumption of red and processed meat, sweetened beverages, alcohol, and unhealthy choices. This improved diet was significantly associated with reduced fatigue (241). Additionally, another intervention program consisting of one-on-one counseling sessions with a dietitian, resulted in improvement of fatigue in up to 94% of patients (242). Strobel et al. examined a holistic functional medicine approach encompassing sessions on nutrition, lifestyle and sleep. Additionally, patients were started on a six-week elimination diet, avoiding dairy, eggs, gluten, peanuts, shellfish, beef/red meat, soy, corn, refined sugar, caffeine and alcohol, followed by a calculated food reintroduction. In this first pilot trial, a significant improvement of fatigue was noted (243). A diet low in Fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs) is currently often used in patients with IBS, in whom it has been shown to ameliorate gastrointestinal symptoms, fatigue and QoL (244, 245). A recent systematic review assessed the effectiveness of a diet low in FODMAPs in patients with IBD and even though the diet was not effective in reducing stool consistency or mucosal inflammation, a significant reduction in fatigue levels was seen (245).
To conclude, dietary interventions can improve symptoms of fatigue, but further confirmation is required through larger randomized controlled trials. Of note, caution is needed when imposing a restrictive diet, such as the FODMAP diet, on patients with IBD due to the high prevalence of malnutrition. These diets should be supervised by a dietitian (246).
5.4.4 Other non-pharmacological interventions
Some anecdotical evidence exists for other interventions such as (electro)acupuncture (247), a mushroom supplement (AndoSan™) (248), and aromatherapy (249) in the treatment of IBD-related fatigue. Other natural supplements such as omega-3 showed no effect on fatigue (231). Since the evidence from these smaller, single-center studies is rather limited, further studies are required to confirm the effectiveness of these interventions for the treatment of IBD-related fatigue.
5.5 Treatment of persisting fatigue: pharmacological interventions
5.5.1 Thiamine
In an initial open label trial high-dose thiamine led to complete regression of IBD related fatigue (250), which was subsequently confirmed in a cross-over RCT assessing the effectiveness of high-dose oral thiamine (dose depending on the sex and body weight) for 4 weeks (251). Treatment with thiamine led to a significant reduction of fatigue as measured with the IBD-F compared to placebo after which an increase of fatigue burden was seen (251). Noteworthy, thiamine treatment had no impact on handgrip strength, one of the objective markers of fatigue. Next, a long-term extension trial was performed to assess the effect of continued treatment with low dose thiamine (300 mg for 12 weeks), however, this trial could not show a beneficial effect over placebo (252). Therefore, even though a short thiamine course can be proposed, no evidence exists on how to proceed after this treatment.
5.5.2 Antidepressants
Some studies have also assessed the effect of antidepressants on patients with IBD without any evidence for an uncontrolled depressive or anxiety disorder. In this setting, the effect of antidepressants on QoL is rather conflicting, with a beneficial effect of duloxetine (253) over placebo, but not with fluoxetine (254). A recent trial investigated the effect of brief behavioral therapy for sleep in IBD (BBTS-I) with or without the antidepressant bupropion on fatigue in patients with IBD with poor sleep quality (226). Even though in both groups a significant improvement of fatigue was seen, bupropion + BBTS-I was not better than BBTS-I alone (226).
5.5.3 Other pharmacological interventions
Methylphenidate is a psychostimulant that increases dopamine levels in the CNS and is mainly used in the treatment of attention deficit disorder (255). Some data suggest a therapeutic effect of methylphenidate on cancer related fatigue, yet information on the effectiveness, safety and long-term risks is still limited (255). Additionally, methylphenidate has not been studied in the context of IBD-related fatigue, thus cannot be recommended in this setting.
5.6 Modulation of the gut-brain axis
5.6.1 Targeting metabolomic pathways
Based on the evidence of reduced serum Trp levels related to fatigue (29, 43), in theory, Trp substitution can be considered for the treatment of fatigue. A recent crossover RCT compared the effectiveness of 5-HTP to placebo for the treatment of fatigue in patients with IBD in remission (256). Despite a significant increase in serum 5-HTP and serotonin levels, oral 5-HTP did not modulate IBD-related fatigue better than placebo.
Another strategy aimed at modulating the gut-brain axis is the use of probiotics, which have shown to improve intestinal barrier function, increase bacterial diversity, and inhibit the growth of potentially pathogenic bacteria (142, 192). Modest evidence suggests a beneficial effect of probiotics on fatigue levels in patients with post-COVID fatigue (257) and patients with chronic fatigue syndrome (258). In mice with colitis, probiotics have shown efficacy in the treatment of depressive-like behaviors and neuroinflammation (259). The effect of probiotics on IBD-related fatigue has not yet been tested, however, currently a trial is ongoing that compares the effect of a probiotic mix with placebo on IBD-related fatigue (260).
5.6.2 Treatment of neuroinflammation
Some studies have attempted to diminish colitis-induced neuroinflammation. For example, prophylactic treatment with an S100A9 inhibitor has been shown to reduce colitis-induced neuroinflammation (154) and NLRP3−/− mice showed attenuated neuroinflammation and neurological dysfunction following DSS (166). Although treating neuroinflammation associated with IBD could be a highly specific and novel approach to targeting fatigue, identifying the precise nature of these interactions requires carefully considered experimental designs, since such treatments often also mitigate the severity of the experimental colitis itself (154, 166).
5.6.3 Modulation of neuronal pathways
Vagal nerve stimulation has previously been shown to induce both anti-inflammatory and anti-depressive effects and some evidence exists for a beneficial effect of vagal nerve stimulation on fatigue associated with IMIDs (155, 261). In a small cohort of female patients with Sjögren’s syndrome, vagal nerve stimulation was effective in improving fatigue scores, however, no sham control group was included (262). Another study in patients with systemic lupus erythematosus, non-invasive vagal nerve stimulation was significantly better than the sham procedure in improving fatigue (263). A successful pilot trial was performed with 9 patients with CD where chronic vagal nerve stimulation resulted in decreased alpha frequency bands as measured by electroencephalography. This decrease in alpha power was correlated with decreased anxiety and clinical improvement, although it should be noted that causality could not be inferred (264). It remains to be elucidated if vagus nerve stimulation could be effective in IBD-related fatigue.
6 Conclusion
Patients with IBD often encounter persisting fatigue, impacting their QoL both during active and remission stages of the disease. While the pathogenesis of IBD-related fatigue remains incompletely unraveled, various risk factors have been identified, offering opportunities for targeted management strategies. In case of persisting, unexplained fatigue several therapeutic options are available, including pharmacological and non-pharmacological approaches. In recent years, emerging evidence highlights the role of the gut-brain axis in fatigue pathogenesis, yet further research is needed to elucidate the precise interplay between chronic gut inflammation and CNS dysfunction. Increased understanding in this area is crucial for the development of novel therapeutic interventions aimed at alleviating IBD-related fatigue.
Author contributions
MT: Writing – review & editing, Writing – original draft, Visualization, Conceptualization. HL: Writing – review & editing, Writing – original draft, Visualization. MD: Writing – review & editing, Supervision. DL: Writing – review & editing, Supervision. TL: Writing – review & editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Katsanos, KH, Papamichael, K, Feuerstein, JD, Christodoulou, DK, and Cheifetz, AS. Biological therapies in inflammatory bowel disease: beyond anti-TNF therapies. Clin Immunol. (2019) 206:9–14. doi: 10.1016/j.clim.2018.03.004
2. Turner, D, Ricciuto, A, Lewis, A, D’amico, F, Dhaliwal, J, Griffiths, AM, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. (2021) 160:1570–83. doi: 10.1053/j.gastro.2020.12.031
3. Knowles, SR, Graff, LA, Wilding, H, Hewitt, C, Keefer, L, and Mikocka-Walus, A. Quality of life in inflammatory bowel disease: a systematic review and meta-analyses—part I. Inflamm Bowel Dis. (2018) 24:742–51. doi: 10.1093/ibd/izx100
4. D'Silva, A, Fox, DE, Nasser, Y, Vallance, JK, Quinn, RR, Ronksley, PE, et al. Prevalence and risk factors for fatigue in adults with inflammatory bowel disease: a systematic review with Meta-analysis. Clin Gastroenterol Hepatol. (2021) 20:995–1009. doi: 10.1016/j.cgh.2021.06.034
5. Walker, EA, Katon, WJ, and Jemelka, RP. Psychiatric disorders and medical care utilization among people in the general population who report fatigue. J Gen Intern Med. (1993) 8:436–40. doi: 10.1007/BF02599621
6. Mussell, M, Böcker, U, Nagel, N, and Singer, MV. Predictors of disease-related concerns and other aspects of health-related quality of life in outpatients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. (2004) 16:1273–80. doi: 10.1097/00042737-200412000-00007
7. Guthrie, E, Jackson, J, Shaffer, J, Thompson, D, Tomenson, B, and Creed, F. Psychological disorder and severity of inflammatory bowel disease predict health-related quality of life in ulcerative colitis and Crohn’s disease. Am J Gastroenterol. (2002) 97:1994–9. doi: 10.1111/j.1572-0241.2002.05842.x
8. Barberio, B, Zamani, M, Black, CJ, Savarino, EV, and Ford, AC. Prevalence of symptoms of anxiety and depression in patients with inflammatory bowel disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2021) 6:359–70. doi: 10.1016/S2468-1253(21)00014-5
9. Kredentser, MS, Graff, LA, and Bernstein, CN. Psychological comorbidity and intervention in inflammatory bowel disease. J Clin Gastroenterol. (2021) 55:30–5. doi: 10.1097/MCG.0000000000001463
10. Mikocka-Walus, A, Massuger, W, Knowles, SR, Moore, GT, Buckton, S, Connell, W, et al. Psychological distress is highly prevalent in inflammatory bowel disease: a survey of psychological needs and attitudes. JGH Open. (2020) 4:166–71. doi: 10.1002/jgh3.12236
11. Ananthakrishnan, AN, Gainer, VS, Perez, RG, Cai, T, Cheng, SC, Savova, G, et al. Psychiatric co-morbidity is associated with increased risk of surgery in Crohn's disease. Aliment Pharmacol Ther. (2013) 37:445–54. doi: 10.1111/apt.12195
12. Van Langenberg, DR, Lange, K, Hetzel, DJ, Holtmann, GJ, and Andrews, JM. Adverse clinical phenotype in inflammatory bowel disease: a cross sectional study identifying factors potentially amenable to change. J Gastroenterol Hepatol. (2010) 25:1250–8. doi: 10.1111/j.1440-1746.2010.06302.x
13. Ananthakrishnan, AN, Desai, R, Lee, WJ, Griffith, J, Chen, N, and Loftus, EV Jr. Economic burden of fatigue in inflammatory bowel disease. Crohns Colitis. (2023) 5:otad020.
14. Johnston, RD, and Logan, RFA. What is the peak age for onset of IBD? Inflamm Bowel Dis. (2008) 14:S4–5. doi: 10.1002/ibd.20545
15. Zand, A, van Deen, WK, Inserra, EK, Hall, L, Kane, E, Centeno, A, et al. Presenteeism in inflammatory bowel diseases: a hidden problem with significant economic impact. Inflamm Bowel Dis. (2015) 21:1623–30. doi: 10.1097/MIB.0000000000000399
16. Thomas, PW, den Broeder, N, Derikx, M, Kievit, W, West, RL, Russel, MG, et al. Impact of biological therapies and Tofacitinib on real-world work impairment in inflammatory bowel disease patients: a prospective study. Inflamm Bowel Dis. (2022) 28:1813–20. doi: 10.1093/ibd/izac002
17. Czuber-Dochan, W, Ream, E, and Norton, C. Review article: description and management of fatigue in inflammatory bowel disease. Aliment Pharmacol Ther. (2013) 37:505–16. doi: 10.1111/apt.12205
18. Romberg-Camps, MJ, Bol, Y, Dagnelie, PC, Hesselink-van de Kruijs, MA, Kester, AD, Engels, LG, et al. Fatigue and health-related quality of life in inflammatory bowel disease: results from a population-based study in the Netherlands: the IBD-South Limburg cohort. Inflamm Bowel Dis. (2010) 16:2137–47. doi: 10.1002/ibd.21285
19. Romkens, TE, Vugt-van Pinxteren, MW, Nagengast, FM, van Oijen, MG, and de Jong, DJ. High prevalence of fatigue in inflammatory bowel disease: a case control study. J Crohns Colitis (2011) 5, 332–337. doi: 10.1016/j.crohns.2011.02.008
20. van Langenberg, DR, and Gibson, PR. Systematic review: fatigue in inflammatory bowel disease. Aliment Pharmacol Ther. (2010) 32:131–43. doi: 10.1111/j.1365-2036.2010.04347.x
21. Hindryckx, P, Laukens, D, D'Amico, F, and Danese, S. Unmet needs in IBD: the case of fatigue. Clin Rev Allergy Immunol. (2017) 55:368–78. doi: 10.1007/s12016-017-8641-4
22. Nocerino, A, Nguyen, A, Agrawal, M, Mone, A, Lakhani, K, and Swaminath, A. Fatigue in inflammatory bowel diseases: etiologies and management. Adv Ther. (2020) 37:97–112. doi: 10.1007/s12325-019-01151-w
23. Fan, VSK, Mehdipour, A, O’Grady, HK, Richardson, J, Griffith, LE, Campbell, KL, et al. Systematic review: patient-reported outcome measures of fatigue in inflammatory bowel disease. Fatigue. (2022) 10:60–82. doi: 10.1186/s41687-023-00642-3
24. Dantzer, R . Cytokine-induced sickness behavior: mechanisms and implications. Ann N Y Acad Sci. (2001) 933:222–34. doi: 10.1111/j.1749-6632.2001.tb05827.x
25. Low, RN, Low, RJ, and Akrami, A. A review of cytokine-based pathophysiology of Long COVID symptoms. Front Med. (2023) 10:1011936. doi: 10.3389/fmed.2023.1011936
26. Schreiner, P, Rossel, JB, Biedermann, L, Valko, PO, Baumann, CR, Greuter, T, et al. Fatigue in inflammatory bowel disease and its impact on daily activities. Aliment Pharmacol Ther. (2021) 53:138–49. doi: 10.1111/apt.16145
27. López-Medina, C, Schiotis, RE, Font-Ugalde, P, Castro-Villegas, MC, Calvo-Gutiérrez, J, Ortega-Castro, R, et al. Assessment of fatigue in spondyloarthritis and its association with disease activity. J Rheumatol. (2016) 43:751–7. doi: 10.3899/jrheum.150832
28. Skoie, I, Dalen, I, Ternowitz, T, Jonsson, G, Kvivik, I, Norheim, K, et al. Fatigue in psoriasis: a controlled study. Br J Dermatol. (2017) 177:505–12. doi: 10.1111/bjd.15375
29. Tran, F, Schrinner, F, Nikolaus, S, Kümpers, J, Sievers, L, Lessing, A, et al. P277 assessment of fatigue as a patient-reported outcome: correlation with baseline disease activity and therapy response in inflammatory bowel disease. J Crohn's Colitis. (2022) 16:i312–3. doi: 10.1093/ecco-jcc/jjab232.404
30. Graff, LA, Vincent, N, Walker, JR, Clara, I, Carr, R, Ediger, J, et al. A population-based study of fatigue and sleep difficulties in inflammatory bowel disease. Inflamm Bowel Dis. (2011) 17:1882–9. doi: 10.1002/ibd.21580
31. Christensen, KR, Ainsworth, MA, Steenholdt, C, Buhl, S, Skougaard, M, Brynskov, J, et al. Fatigue is a systemic extraintestinal disease manifestation largely independent of disease activity, chronicity, and nutritional deficiencies in inflammatory bowel disease on biologics. Scand J Gastroenterol. (2022) 57:1051–7. doi: 10.1080/00365521.2022.2060049
32. Klusmann, B, Fleer, J, Tovote, KA, Weersma, RK, van Dullemen, HM, Dijkstra, G, et al. Trajectories of fatigue in inflammatory bowel disease. Inflamm Bowel Dis. (2021) 27:1919–30. doi: 10.1093/ibd/izab007
33. Tasson, L, Zingone, F, Barberio, B, Valentini, R, Ballotta, P, Ford, AC, et al. Sarcopenia, severe anxiety and increased C-reactive protein are associated with severe fatigue in patients with inflammatory bowel diseases. Sci Rep. (2021) 11:15251. doi: 10.1038/s41598-021-94685-5
34. Yoo, S, Jung, YS, Park, JH, Kim, HJ, Cho, YK, Sohn, CI, et al. Fatigue severity and factors associated with high fatigue levels in Korean patients with inflammatory bowel disease. Gut Liver. (2014) 8:148–53. doi: 10.5009/gnl.2014.8.2.148
35. Jonefjäll, B, Simrén, M, Lasson, A, Öhman, L, and Strid, H. Psychological distress, iron deficiency, active disease and female gender are independent risk factors for fatigue in patients with ulcerative colitis. United European Gastroenterol J. (2018) 6:148–58. doi: 10.1177/2050640617703868
36. Holten, KA, Bernklev, T, Opheim, R, Johansen, I, Olsen, BC, Lund, C, et al. Fatigue in patients with newly diagnosed inflammatory bowel disease: results from a prospective inception cohort, the IBSEN III study. J Crohns Colitis. (2023) 17:1781–90. doi: 10.1093/ecco-jcc/jjad094
37. Lee, TW, Iser, JH, Sparrow, MP, Newnham, ED, Headon, BJ, and Gibson, PR. Thiopurines, a previously unrecognised cause for fatigue in patients with inflammatory bowel disease. J Crohn's Colitis. (2009) 3:196–9. doi: 10.1016/j.crohns.2009.03.004
38. Jelsness-Jorgensen, LP, Bernklev, T, Henriksen, M, Torp, R, and Moum, BA. Chronic fatigue is associated with impaired health-related quality of life in inflammatory bowel disease. Aliment Pharmacol Ther. (2011) 33:106–14. doi: 10.1111/j.1365-2036.2010.04498.x
39. van Langenberg, DR, and Gibson, PR. Factors associated with physical and cognitive fatigue in patients with Crohn's disease: a cross-sectional and longitudinal study. Inflamm Bowel Dis. (2014) 20:115–25. doi: 10.1097/01.MIB.0000437614.91258.70
40. Williet, N, Sarter, H, Gower-Rousseau, C, Adrianjafy, C, Olympie, A, Buisson, A, et al. Patient-reported outcomes in a French Nationwide survey of inflammatory bowel disease patients. J Crohns Colitis. (2017) 11:165–74. doi: 10.1093/ecco-jcc/jjw145
41. Vogelaar, L, van’t Spijker, A, van Tilburg, AJP, Kuipers, EJ, Timman, R, and van der Woude, CJ. Determinants of fatigue in Crohn's disease patients. Eur J Gastroenterol Hepatol. (2013) 25:246–51. doi: 10.1097/MEG.0b013e32835aba83
42. Villoria, A, Garcia, V, Dosal, A, Moreno, L, Montserrat, A, Figuerola, A, et al. Fatigue in out-patients with inflammatory bowel disease: prevalence and predictive factors. PLoS One. (2017) 12:e0181435. doi: 10.1371/journal.pone.0181435
43. Borren, NZ, Plichta, D, Joshi, AD, Bonilla, G, Peng, V, Colizzo, FP, et al. Alterations in fecal microbiomes and serum metabolomes of fatigued patients with quiescent inflammatory bowel diseases. Clin Gastroenterol Hepatol. (2021) 19:519–527.e5. doi: 10.1016/j.cgh.2020.03.013
44. Truyens, M, De Ruyck, E, Gonzales, GB, Bos, S, Laukens, D, and De Vos, M. Prevalence of fatigue and unrecognized depression in patients with inflammatory bowel disease in remission under Immunosuppressants and biologicals. J Clin Med. (2021) 10:4107. doi: 10.3390/jcm10184107
45. Yzet, C, Brazier, F, Meudjo, E, Robert, C, Moreau, C, Derval, E, et al. Prevalence and risk factors for fatigue in patients with inflammatory bowel disease and endoscopic healing. Oxford: Oxford Univ Press (2024).
46. Vogelaar, L, de Haar, C, Aerts, BR, Peppelenbosch, MP, Timman, R, Hanssen, BE, et al. Fatigue in patients with inflammatory bowel disease is associated with distinct differences in immune parameters. Clin Exp Gastroenterol. (2017) 10:83–90. doi: 10.2147/CEG.S123942
47. Gordon, H, Burisch, J, Ellul, P, Karmiris, K, Katsanos, K, Allocca, M, et al. ECCO guidelines on Extraintestinal manifestations in inflammatory bowel disease. J Crohns Colitis. (2024) 18:1–37. doi: 10.1093/ecco-jcc/jjad108
48. Jelsness-Jorgensen, LP, Bernklev, T, Henriksen, M, Torp, R, and Moum, BA. Chronic fatigue is more prevalent in patients with inflammatory bowel disease than in healthy controls. Inflamm Bowel Dis. (2011) 17:1564–72. doi: 10.1002/ibd.21530
49. Chavarria, C, Casanova, MJ, Chaparro, M, Barreiro-de Acosta, M, Ezquiaga, E, Bujanda, L, et al. Prevalence and factors associated with fatigue in patients with inflammatory bowel disease: a multicentre study. J Crohns Colitis. (2019) 13:996–1002. doi: 10.1093/ecco-jcc/jjz024
50. Goldenberg, BA, Graff, LA, Clara, I, Zarychanski, R, Walker, JR, Carr, R, et al. Is iron deficiency in the absence of anemia associated with fatigue in inflammatory bowel disease? Am J Gastroenterol. (2013) 108:1392–7. doi: 10.1038/ajg.2013.14
51. König, P, Jimenez, K, Saletu-Zyhlarz, G, Mittlböck, M, and Gasche, C. Iron deficiency, depression, and fatigue in inflammatory bowel diseases. Z Gastroenterol. (2020) 58:1191–200. doi: 10.1055/a-1283-6832
52. Kim, J, and Wessling-Resnick, M. Iron and mechanisms of emotional behavior. J Nutr Biochem. (2014) 25:1101–7. doi: 10.1016/j.jnutbio.2014.07.003
53. Alayón, CG, Crespo, CP, Pedrosa, SM, Benítez, JM, Flores, EI, Rodríguez, IS, et al. Prevalence of iron deficiency without anaemia in inflammatory bowel disease and impact on health-related quality of life. Gastroenterol Hepatol. (2018) 41:22–9. doi: 10.1016/j.gastre.2017.07.014
54. Bager, P, Befrits, R, Wikman, O, Lindgren, S, Moum, B, Hjortswang, H, et al. Fatigue in out-patients with inflammatory bowel disease is common and multifactorial. Aliment Pharmacol Ther. (2012) 35:133–41. doi: 10.1111/j.1365-2036.2011.04914.x
55. Fletcher, J, Cooper, SC, Ghosh, S, and Hewison, M. The role of vitamin D in inflammatory bowel disease: mechanism to management. Nutrients. (2019) 11:1019. doi: 10.3390/nu11051019
56. Kabbani, TA, Koutroubakis, IE, Schoen, RE, Ramos-Rivers, C, Shah, N, Swoger, J, et al. Association of vitamin D level with clinical status in inflammatory bowel disease: a 5-year longitudinal study. J Am College Gastroenterol. (2016) 111:712–9. doi: 10.1038/ajg.2016.53
57. Gubatan, J, Chou, ND, Nielsen, OH, and Moss, AC. Systematic review with meta-analysis: association of vitamin D status with clinical outcomes in adult patients with inflammatory bowel disease. Aliment Pharmacol Ther. (2019) 50:1146–58. doi: 10.1111/apt.15506
58. Van Langenberg, D, Della Gatta, P, Warmington, SA, Kidgell, D, Gibson, PR, and Russell, AP. Objectively measured muscle fatigue in Crohn's disease: correlation with self-reported fatigue and associated factors for clinical application. J Crohn's Colitis. (2014) 8:137–46. doi: 10.1016/j.crohns.2013.07.006
59. Tardy, A-L, Pouteau, E, Marquez, D, Yilmaz, C, and Scholey, A. Vitamins and minerals for energy, fatigue and cognition: a narrative review of the biochemical and clinical evidence. Nutrients. (2020) 12:228. doi: 10.3390/nu12010228
60. Pan, Y, Liu, Y, Guo, H, Jabir, MS, Liu, X, Cui, W, et al. Associations between folate and vitamin B12 levels and inflammatory bowel disease: a Meta-analysis. Nutrients. (2017) 9:382. doi: 10.3390/nu9040382
61. Neuendorf, R, Harding, A, Stello, N, Hanes, D, and Wahbeh, H. Depression and anxiety in patients with inflammatory bowel disease: a systematic review. J Psychosom Res. (2016) 87:70–80. doi: 10.1016/j.jpsychores.2016.06.001
62. Stroie, T, Preda, C, Istratescu, D, Ciora, C, Croitoru, A, and Diculescu, M. Anxiety and depression in patients with inactive inflammatory bowel disease: The role of fatigue and health-related quality of life. Medicine. (2023) 102:e33713. doi: 10.1097/MD.0000000000033713
63. Singh, S, Blanchard, A, Walker, JR, Graff, LA, Miller, N, and Bernstein, CN. Common symptoms and stressors among individuals with inflammatory bowel diseases. Clin Gastroenterol Hepatol. (2011) 9:769–75. doi: 10.1016/j.cgh.2011.05.016
64. Opheim, R, Fagermoen, MS, Jelsness-Jørgensen, L-P, Bernklev, T, and Moum, B. Sense of coherence in patients with inflammatory bowel disease. Gastroenterol Res Pract. (2014) 2014:1–9. doi: 10.1155/2014/989038
65. Artom, M, Czuber-Dochan, W, Sturt, J, Murrells, T, and Norton, C. The contribution of clinical and psychosocial factors to fatigue in 182 patients with inflammatory bowel disease: a cross-sectional study. Aliment Pharmacol Ther. (2017) 45:403–16. doi: 10.1111/apt.13870
66. Jelsness-Jørgensen, L-P, Bernklev, T, and Moum, B. Fatigue and disease-related worries among inflammatory bowel disease patients in remission; is it a reflection of coexisting IBS-like symptoms? A short report. J Psychosom Res. (2012) 73:469–72. doi: 10.1016/j.jpsychores.2012.08.009
67. Banovic, I, Gilibert, D, Jebrane, A, and Cosnes, J. Personality and fatigue perception in a sample of IBD outpatients in remission: a preliminary study. J Crohns Colitis. (2012) 6:571–7. doi: 10.1016/j.crohns.2011.11.006
68. Kamp, KJ, West, P, Holmstrom, A, Luo, Z, Wyatt, G, and Given, B. Systematic review of social support on psychological symptoms and self-management behaviors among adults with inflammatory bowel disease. J Nurs Scholarsh. (2019) 51:380–9. doi: 10.1111/jnu.12487
69. Lesage, A-C, Hagege, H, Tucat, G, and Gendre, J-P. Results of a national survey on quality of life in inflammatory bowel diseases. Clin Res Hepatol Gastroenterol. (2011) 35:117–24. doi: 10.1016/j.gcb.2009.08.015
70. Radford, SJ, McGing, J, Czuber-Dochan, W, and Moran, G. Systematic review: the impact of inflammatory bowel disease-related fatigue on health-related quality of life. Frontline Gastroenterol. (2021) 12:11–21. doi: 10.1136/flgastro-2019-101355
71. Opheim, R, Fagermoen, MS, Bernklev, T, Jelsness-Jorgensen, LP, and Moum, B. Fatigue interference with daily living among patients with inflammatory bowel disease. Qual Life Res. (2014) 23:707–17. doi: 10.1007/s11136-013-0508-4
72. Qazi, T, and Farraye, FA. Sleep and inflammatory bowel disease: an important bi-directional relationship. Inflamm Bowel Dis. (2018) 25:843–52. doi: 10.1093/ibd/izy334
73. Ananthakrishnan, AN, Long, MD, Martin, CF, Sandler, RS, and Kappelman, MD. Sleep disturbance and risk of active disease in patients with Crohn's disease and ulcerative colitis. Clin Gastroenterol Hepatol. (2013) 11:965–71. doi: 10.1016/j.cgh.2013.01.021
74. Hashash, JG, Ramos-Rivers, C, Youk, A, Chiu, WK, Duff, K, Regueiro, M, et al. Quality of sleep and coexistent psychopathology have significant impact on fatigue burden in patients with inflammatory bowel disease. J Clin Gastroenterol. (2018) 52:423–30. doi: 10.1097/MCG.0000000000000729
75. Orellana, AF, Holguín, NNP, and Yamamoto-Furusho, JK. Mental health factors associated with fatigue in Mexican patients with inflammatory bowel disease. J Clin Gastroenterol. (2021) 55:609–14. doi: 10.1097/MCG.0000000000001397
76. Borren, NZ, Long, MD, Sandler, RS, and Ananthakrishnan, AN. Longitudinal trajectory of fatigue in patients with inflammatory bowel disease: a prospective study. Inflamm Bowel Dis. (2021) 27:1740–6. doi: 10.1093/ibd/izaa338
77. Calvo, EG, Gil, MD, Jiménez, BV, Salazar, LIF, Elisa, GC, Miguel, DG, et al. Prevalence and factors associated with poor sleep quality in inflammatory bowel disease outpatients. Revista Espanola Enfermedades Digestivas. (2021) 113:512–8. doi: 10.17235/reed.2020.7202/2020
78. Dhaliwal, A, Quinlan, JI, Overthrow, K, Greig, C, Lord, JM, Armstrong, MJ, et al. Sarcopenia in inflammatory bowel disease: a narrative overview. Nutrients. (2021) 13:656. doi: 10.3390/nu13020656
79. Jain, A, Nguyen, NH, Proudfoot, JA, Martin, CF, Sandborn, WJ, Kappelman, MD, et al. Impact of obesity on disease activity and patient-reported outcomes measurement information system (PROMIS) in inflammatory bowel diseases. Am J Gastroenterol. (2019) 114:630–9. doi: 10.14309/ajg.0000000000000197
80. Derikx, L, Siakavellas, S, Derr, L, Williams, L, Nikolas, P, Jenkinson, P, et al. OP22 factors independently associated with fatigue in IBD: results from the baseline dataset of the PREdiCCt study. J Crohns Colitis. (2021) 15:S021. doi: 10.1093/ecco-jcc/jjab075.021
81. Bacha, RA, Bouhnik, Y, Serrero, M, Filippi, J, Roblin, X, Bourrier, A, et al. Obesity in adult patients with inflammatory bowel disease: clinical features and impact on disability. A cross-sectional survey from the GETAID. Dig Liver Dis. (2023) 55:1632–9. doi: 10.1016/j.dld.2023.05.008
82. Stroie, T, Preda, C, Istratescu, D, Nitescu, M, Manuc, T, Manuc, M, et al. P1003 dietary pattern can influence the level of fatigue in patients with inflammatory bowel disease in remission. J Crohns Colitis. (2024) 18:i1816. doi: 10.1093/ecco-jcc/jjad212.1133
83. Torres, J, Ellul, P, Langhorst, J, Mikocka-Walus, A, Barreiro-de Acosta, M, Basnayake, C, et al. European Crohn’s and colitis organisation topical review on complementary medicine and psychotherapy in inflammatory bowel disease. J Crohns Colitis. (2019) 13:673–685e. doi: 10.1093/ecco-jcc/jjz051
84. Gravina, AG, Pellegrino, R, Durante, T, Palladino, G, D’Onofrio, R, Mammone, S, et al. Inflammatory bowel diseases patients suffer from significant low levels and barriers to physical activity: The “BE-FIT-IBD” study. World J Gastroenterol. (2023) 29:5668–82. doi: 10.3748/wjg.v29.i41.5668
85. Mack, DE, Wilson, PM, Gilmore, JC, and Gunnell, KE. Leisure-time physical activity in Canadians living with Crohn disease and ulcerative colitis: population-based estimates. Gastroenterol Nurs. (2011) 34:288–94. doi: 10.1097/SGA.0b013e3182248732
86. Zaltman, C, Braulio, VB, Outeiral, R, Nunes, T, and Natividade de Castro, CL. Lower extremity mobility limitation and impaired muscle function in women with ulcerative colitis. J Crohns Colitis. (2014) 8:529–35. doi: 10.1016/j.crohns.2013.11.006
87. Gatt, K, Schembri, J, Katsanos, KH, Christodoulou, D, Karmiris, K, Kopylov, U, et al. Inflammatory bowel disease [IBD] and physical activity: a study on the impact of diagnosis on the level of exercise amongst patients with IBD. J Crohns Colitis. (2019) 13:686–92. doi: 10.1093/ecco-jcc/jjy214
88. Mcging, J, Nicholas, R, Serres, S, Greenhaff, P, Francis, S, and Moran, GW. P062 31P MRS and MRI phenotyping of muscle metabolic quality in IBD fatigue. J Crohn's Colitis. (2022) 16:i171–2. doi: 10.1093/ecco-jcc/jjab232.191
89. Fagan, G, Osborne, H, and Schultz, M. Physical activity in patients with inflammatory bowel disease: a cross-sectional study. Inflamm Intest Dis. (2021) 6:61–9. doi: 10.1159/000511212
90. Chan, D, Robbins, H, Rogers, S, Clark, S, and Poullis, A. Inflammatory bowel disease and exercise: results of a Crohn's and Colitis UK survey. Frontline Gastroenterol. (2014) 5:44–8. doi: 10.1136/flgastro-2013-100339
91. Vogelaar, L, van den Berg-Emons, R, Bussmann, H, Rozenberg, R, Timman, R, and van der Woude, CJ. Physical fitness and physical activity in fatigued and non-fatigued inflammatory bowel disease patients. Scand J Gastroenterol. (2015) 50:1357–67. doi: 10.3109/00365521.2015.1046135
92. Stack, R, Ní Chonnacháin, C, Doherty, J, O'Moran, N, Girod, P, Noronha, N, et al. P948 hand grip strength is a useful objective measure associated with fatigue and impaired quality of life in patients with inflammatory bowel disease. J Crohns Colitis. (2024) 18:i1724. doi: 10.1093/ecco-jcc/jjad212.1078
93. DeFilippis, EM, Tabani, S, Warren, RU, Christos, PJ, Bosworth, BP, and Scherl, EJ. Exercise and self-reported limitations in patients with inflammatory bowel disease. Dig Dis Sci. (2016) 61:215–20. doi: 10.1007/s10620-015-3832-4
94. Cosnes, J . Smoking, physical activity, nutrition and lifestyle: environmental factors and their impact on IBD. Dig Dis. (2010) 28:411–7. doi: 10.1159/000320395
95. Kim, Y-J, Lee, S-G, Lee, J-S, Choi, Y-J, and Son, C-G. Comparative characteristics of fatigue in irritable bowel syndrome and inflammatory bowel disease: a systematic review and meta-analysis. J Psychosom Res. (2024) 177:111589. doi: 10.1016/j.jpsychores.2024.111589
96. Gong, S-S, Fan, Y-H, Lv, B, Zhang, M-Q, Xu, Y, and Zhao, J. Fatigue in patients with inflammatory bowel disease in eastern China. World J Gastroenterol. (2021) 27:1076–89. doi: 10.3748/wjg.v27.i11.1076
97. Aluzaite, K, Al-Mandhari, R, Osborne, H, Ho, C, Williams, M, Sullivan, MM, et al. Detailed multi-dimensional assessment of fatigue in inflammatory bowel disease. Inflamm Intest Dis. (2019) 3:192–202. doi: 10.1159/000496054
98. Varbobitis, I, Kokkotis, G, Gizis, M, Perlepe, N, Laoudi, E, Bletsa, M, et al. The IBD-F patient self-assessment scale accurately depicts the level of fatigue and predicts a negative effect on the quality of life of patients with IBD in clinical remission. Inflamm Bowel Dis. (2021) 27:826–35. doi: 10.1093/ibd/izaa201
99. Davis, SP, Chen, D-G, Crane, PB, Bolin, LP, Johnson, LA, and Long, MD. Influencing factors of inflammatory bowel disease–fatigue: a path analysis model. Nurs Res. (2021) 70:256–65. doi: 10.1097/NNR.0000000000000517
100. Fons, AB, Asscher, VER, Stuyt, RJL, Baven-Pronk, AMC, van der Marel, S, Jacobs, RJ, et al. Deficits in geriatric assessment are important in relation to fatigue in older patients with inflammatory bowel disease. Dig Liver Dis. (2024). doi: 10.1016/j.dld.2024.01.196
101. Halpin, SJ, and Ford, AC. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: systematic review and meta-analysis. J Am College Gastroenterol. (2012) 107:1474–82. doi: 10.1038/ajg.2012.260
102. Zeitz, J, Ak, M, Müller-Mottet, S, Scharl, S, Biedermann, L, Fournier, N, et al. Pain in IBD patients: very frequent and frequently insufficiently taken into account. PLoS One. (2016) 11:e0156666. doi: 10.1371/journal.pone.0156666
103. Janssen, LM, Rezazadeh Ardabili, A, Romberg-Camps, MJL, Winkens, B, van den Broek, RJ, Hulst, J, et al. Abdominal pain in patients with inflammatory bowel disease in remission: a prospective study on contributing factors. Aliment Pharmacol Ther. (2023) 58:1041–51. doi: 10.1111/apt.17718
104. Kaltsas, G, Vgontzas, A, and Chrousos, G. Fatigue, Endocrinopathies, and metabolic disorders. PM&R. (2010) 2:393–8. doi: 10.1016/j.pmrj.2010.04.011
105. Shizuma, T . Concomitant thyroid disorders and inflammatory bowel disease: a literature review. Biomed Res Int. (2016) 2016:1–12. doi: 10.1155/2016/5187061
106. Vavricka, SR, Schoepfer, A, Scharl, M, Lakatos, PL, Navarini, A, and Rogler, G. Extraintestinal manifestations of inflammatory bowel disease. Inflamm Bowel Dis. (2015) 21:1982–92. doi: 10.1097/MIB.0000000000000392
107. Kucharzik, T, Ellul, P, Greuter, T, Rahier, JF, Verstockt, B, Abreu, C, et al. ECCO guidelines on the prevention, diagnosis, and Management of Infections in inflammatory bowel disease. J Crohn's Colitis. (2021) 15:879–913. doi: 10.1093/ecco-jcc/jjab052
108. Griesbaum, M, Vogl, T, Andreu-Sánchez, S, Klompus, S, Kalka, I, Leviatan, S, et al. P193 fatigued patients with inflammatory bowel disease exhibit distinct systemic antibody epitope repertoires. J Crohn's Colitis. (2024) 18:i507–8. doi: 10.1093/ecco-jcc/jjad212.0323
109. Van De Pol, N, van der Woude, C, Vis, M, van Doorn, M, Schrauwen, S, Cetinözman-Teunissen, F, et al. P325 post-COVID general health symptoms in patients with immune mediated inflammatory diseases. J Crohn's Colitis. (2024) 18:i711–3. doi: 10.1093/ecco-jcc/jjad212.0455
110. Ananthakrishnan, AN, Donaldson, T, Lasch, K, and Yajnik, V. Management of inflammatory bowel disease in the elderly patient: challenges and opportunities. Inflamm Bowel Dis. (2017) 23:882–93. doi: 10.1097/MIB.0000000000001099
111. Qazi, T . Fatigue in inflammatory bowel disease: a problematic ailment. Curr Opin Gastroenterol. (2020) 36:284–94. doi: 10.1097/MOG.0000000000000644
112. Axelrad, JE, Lichtiger, S, and Yajnik, V. Inflammatory bowel disease and cancer: the role of inflammation, immunosuppression, and cancer treatment. World J Gastroenterol. (2016) 22:4794. doi: 10.3748/wjg.v22.i20.4794
113. Mayer, EA . Gut feelings: the emerging biology of gut–brain communication. Nat Rev Neurosci. (2011) 12:453–66. doi: 10.1038/nrn3071
114. Pellissier, S, Dantzer, C, Canini, F, Mathieu, N, and Bonaz, B. Psychological adjustment and autonomic disturbances in inflammatory bowel diseases and irritable bowel syndrome. Psychoneuroendocrinology. (2010) 35:653–62. doi: 10.1016/j.psyneuen.2009.10.004
115. Carabotti, M, Scirocco, A, Maselli, MA, and Severi, C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. (2015) 28:203.
116. Foster, JA, and Neufeld, K-AM. Gut–brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. (2013) 36:305–12. doi: 10.1016/j.tins.2013.01.005
117. Demeestere, D, Libert, C, and Vandenbroucke, RE. Clinical implications of leukocyte infiltration at the choroid plexus in (neuro) inflammatory disorders. Drug Discov Today. (2015) 20:928–41. doi: 10.1016/j.drudis.2015.05.003
118. Vandenbroucke, RE . A hidden epithelial barrier in the brain with a central role in regulating brain homeostasis. Implications for aging. Ann Am Thorac Soc. (2016) 13:S407–10. doi: 10.1513/AnnalsATS.201609-676AW
119. Sun, M, Ma, N, He, T, Johnstou, LJ, and Ma, X. Tryptophan (Trp) modulates gut homeostasis via aryl hydrocarbon receptor (AhR). Crit Rev Food Sci Nutr.. (2020) 60:1760–68.
120. Lavelle, A, and Sokol, H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. (2020) 17:223–37. doi: 10.1038/s41575-019-0258-z
121. Chen, L-M, Bao, C-H, Wu, Y, Liang, S-H, Wang, D, Wu, L-Y, et al. Tryptophan-kynurenine metabolism: a link between the gut and brain for depression in inflammatory bowel disease. J Neuroinflammation. (2021) 18:1–13. doi: 10.1186/s12950-020-00266-0
122. Arijs, I, Wollants, W-J, Clarke, G, Persoons, P, Vanhove, W, Van Lommel, L, et al. P096 analysis of the tryptophan metabolism and indoleamine 2, 3-dioxygenase expression (IDO) in patients with inflammatory bowel disease before and after infliximab treatment. J Crohn's Colitis. (2014) 8:S102. doi: 10.1016/S1873-9946(14)60218-X
123. Troubat, R, Barone, P, Leman, S, Desmidt, T, Cressant, A, Atanasova, B, et al. Neuroinflammation and depression: a review. Eur J Neurosci. (2021) 53:151–71. doi: 10.1111/ejn.14720
124. Ostapiuk, A, and Urbanska, EM. Kynurenic acid in neurodegenerative disorders—unique neuroprotection or double-edged sword? CNS Neurosci Therapeutics. (2022) 28:19–35. doi: 10.1111/cns.13768
125. Ogyu, K, Kubo, K, Noda, Y, Iwata, Y, Tsugawa, S, Omura, Y, et al. Kynurenine pathway in depression: a systematic review and meta-analysis. Neurosci Biobehav Rev. (2018) 90:16–25. doi: 10.1016/j.neubiorev.2018.03.023
126. Agus, A, Planchais, J, and Sokol, H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. (2018) 23:716–24. doi: 10.1016/j.chom.2018.05.003
127. Iskandar, HN, Linan, EE, Patel, A, Moore, R, Lasanajak, Y, Gyawali, CP, et al. Self-reported sleep disturbance in Crohn's disease is not confirmed by objective sleep measures. Sci Rep. (2020) 10:1980. doi: 10.1038/s41598-020-58807-9
128. Bosi, A, Banfi, D, Bistoletti, M, Giaroni, C, and Baj, A. Tryptophan metabolites along the microbiota-gut-brain axis: an interkingdom communication system influencing the gut in health and disease. Int J Tryptophan Res. (2020) 13:117864692092898. doi: 10.1177/1178646920928984
129. Kaur, H, Bose, C, and Mande, SS. Tryptophan Metabolism by Gut Microbiome and Gut-Brain-Axis: An in silico analysis. Front Neurosci. (2019) 13:1365. doi: 10.3389/fnins.2019.01365
130. Kurz, K, Fiegl, M, Holzner, B, Giesinger, J, Pircher, M, Weiss, G, et al. Fatigue in patients with lung cancer is related with accelerated tryptophan breakdown. PLoS One. (2012) 7:e36956. doi: 10.1371/journal.pone.0036956
131. Mutsaers, HA, Masereeuw, R, and Olinga, P. Altered tryptophan metabolism and CKD-associated fatigue. Kidney Int. (2014) 86:1061–2. doi: 10.1038/ki.2014.237
132. Ormstad, H, Verkerk, R, Amthor, KF, and Sandvik, L. Activation of the kynurenine pathway in the acute phase of stroke and its role in fatigue and depression following stroke. J Mol Neurosci. (2014) 54:181–7. doi: 10.1007/s12031-014-0272-0
133. Han, CJ, Jarrett, ME, Cain, KC, Jun, S, and Heitkemper, MM. Association of fatigue with TPH2 genetic polymorphisms in women with irritable bowel syndrome. Biol Res Nurs. (2019) 21:72–9. doi: 10.1177/1099800418806055
134. Nikolaus, S, Schulte, B, Al-Massad, N, Thieme, F, Schulte, DM, Bethge, J, et al. Increased tryptophan metabolism is associated with activity of inflammatory bowel diseases. Gastroenterology. (2017) 153:1504–16.e2. doi: 10.1053/j.gastro.2017.08.028
135. Gupta, NK, Thaker, AI, Kanuri, N, Riehl, TE, Rowley, CW, Stenson, WF, et al. Serum analysis of tryptophan catabolism pathway: correlation with Crohn's disease activity. Inflamm Bowel Dis. (2012) 18:1214–20. doi: 10.1002/ibd.21849
136. Sartor, RB . Microbial influences in inflammatory bowel diseases. Gastroenterology. (2008) 134:577–94. doi: 10.1053/j.gastro.2007.11.059
137. Erny, D, Hrabě de Angelis, AL, Jaitin, D, Wieghofer, P, Staszewski, O, David, E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. (2015) 18:965–77. doi: 10.1038/nn.4030
138. Caetano-Silva, ME, Rund, L, Hutchinson, NT, Woods, JA, Steelman, AJ, and Johnson, RW. Inhibition of inflammatory microglia by dietary fiber and short-chain fatty acids. Sci Rep. (2023) 13:2819. doi: 10.1038/s41598-022-27086-x
139. Cryan, JF, and Dinan, TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. (2012) 13:701–12. doi: 10.1038/nrn3346
140. Sudo, N, Chida, Y, Aiba, Y, Sonoda, J, Oyama, N, Yu, XN, et al. Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J Physiol. (2004) 558:263–75. doi: 10.1113/jphysiol.2004.063388
141. DeGruttola, AK, Low, D, Mizoguchi, A, and Mizoguchi, E. Current understanding of dysbiosis in disease in human and animal models. Inflamm Bowel Dis. (2016) 22:1137–50. doi: 10.1097/MIB.0000000000000750
142. Glassner, KL, Abraham, BP, and Quigley, EM. The microbiome and inflammatory bowel disease. J Allergy Clin Immunol. (2020) 145:16–27. doi: 10.1016/j.jaci.2019.11.003
143. Morell Miranda, P, Bertolini, F, and Kadarmideen, H. Investigation of gut microbiome association with inflammatory bowel disease and depression: a machine learning approach [version 2; peer review: 2 approved with reservations]. F1000Research. (2019) 7:702. doi: 10.12688/f1000research.15091.2
144. Jang, H-M, Kim, J-K, Joo, M-K, Shin, Y-J, Lee, CK, Kim, H-J, et al. Transplantation of fecal microbiota from patients with inflammatory bowel disease and depression alters immune response and behavior in recipient mice. Sci Rep. (2021) 11:20406. doi: 10.1038/s41598-021-00088-x
145. Safadi, JM, Quinton, AMG, Lennox, BR, Burnet, PWJ, and Minichino, A. Gut dysbiosis in severe mental illness and chronic fatigue: a novel trans-diagnostic construct? A systematic review and meta-analysis. Mol Psychiatry. (2022) 27:141–53. doi: 10.1038/s41380-021-01032-1
146. Dohnalová, L, Lundgren, P, Carty, JRE, Goldstein, N, Wenski, SL, Nanudorn, P, et al. A microbiome-dependent gut–brain pathway regulates motivation for exercise. Nature. (2022) 612:739–47. doi: 10.1038/s41586-022-05525-z
147. Thomann, A, Wuestenberg, T, Wirbel, J, Zeller, G, Thomann, P, Knoedler, L, et al. P232 depression and fatigue in active IBD from a microbiome perspective-a bayesian approach to faecal metagenomics. J Crohn's Colitis. (2022) 16:i280–2. doi: 10.1093/ecco-jcc/jjab232.359
148. Laman, JD, Bert, A, Power, C, and Dziarski, R. Bacterial peptidoglycan as a driver of chronic brain inflammation. Trends Mol Med. (2020) 26:670–82. doi: 10.1016/j.molmed.2019.11.006
149. Han, Y, Zhao, T, Cheng, X, Zhao, M, Gong, S-H, Zhao, Y-Q, et al. Cortical inflammation is increased in a DSS-induced colitis mouse model. Neurosci Bull. (2018) 34:1058–66. doi: 10.1007/s12264-018-0288-5
150. Barnes, SE, Zera, KA, Ivison, GT, Buckwalter, MS, and Engleman, EG. Brain profiling in murine colitis and human epilepsy reveals neutrophils and TNFα as mediators of neuronal hyperexcitability. J Neuroinflammation. (2021) 18:1–13. doi: 10.1186/s12974-021-02262-4
151. Vandenbroucke, RE, Dejonckheere, E, Van Lint, P, Demeestere, D, Van Wonterghem, E, Vanlaere, I, et al. Matrix metalloprotease 8-dependent extracellular matrix cleavage at the blood–CSF barrier contributes to lethality during systemic inflammatory diseases. J Neurosci. (2012) 32:9805–16. doi: 10.1523/JNEUROSCI.0967-12.2012
152. Carloni, S, Bertocchi, A, Mancinelli, S, Bellini, M, Erreni, M, Borreca, A, et al. Identification of a choroid plexus vascular barrier closing during intestinal inflammation. Science. (2021) 374:439–48. doi: 10.1126/science.abc6108
153. Marques, F, Sousa, JC, Coppola, G, Geschwind, DH, Sousa, N, Palha, JA, et al. The choroid plexus response to a repeated peripheral inflammatory stimulus. BMC Neurosci. (2009) 10:1–10. doi: 10.1186/1471-2202-10-135
154. Talley, S, Valiauga, R, Anderson, L, Cannon, AR, Choudhry, MA, and Campbell, EM. DSS-induced inflammation in the colon drives a proinflammatory signature in the brain that is ameliorated by prophylactic treatment with the S100A9 inhibitor paquinimod. J Neuroinflammation. (2021) 18:1–14. doi: 10.1186/s12974-021-02317-6
155. Craig, CF, Filippone, RT, Stavely, R, Bornstein, JC, Apostolopoulos, V, and Nurgali, K. Neuroinflammation as an etiological trigger for depression comorbid with inflammatory bowel disease. J Neuroinflammation. (2022) 19:1–30. doi: 10.1186/s12974-021-02354-1
156. Oikonomou, KA, Kapsoritakis, AN, Theodoridou, C, Karangelis, D, Germenis, A, Stefanidis, I, et al. Neutrophil gelatinase-associated lipocalin (NGAL) in inflammatory bowel disease: association with pathophysiology of inflammation, established markers, and disease activity. J Gastroenterol. (2012) 47:519–30. doi: 10.1007/s00535-011-0516-5
157. Thorsvik, S, Damås, JK, Granlund, AV, Flo, TH, Bergh, K, Østvik, AE, et al. Fecal neutrophil gelatinase-associated lipocalin as a biomarker for inflammatory bowel disease. J Gastroenterol Hepatol. (2017) 32:128–35. doi: 10.1111/jgh.13598
158. Moschen, AR, Adolph, TE, Gerner, RR, Wieser, V, and Tilg, H. Lipocalin-2: a master mediator of intestinal and metabolic inflammation. Trends Endocrinol Metab. (2017) 28:388–97. doi: 10.1016/j.tem.2017.01.003
159. Do, J, and Woo, J. From gut to brain: alteration in inflammation markers in the brain of dextran sodium sulfate-induced colitis model mice. Clin Psychopharmacol Neurosci. (2018) 16:422–33. doi: 10.9758/cpn.2018.16.4.422
160. Haj-Mirzaian, A, Amiri, S, Amini-Khoei, H, Hosseini, M-J, Haj-Mirzaian, A, Momeny, M, et al. Anxiety-and depressive-like behaviors are associated with altered hippocampal energy and inflammatory status in a mouse model of Crohn’s disease. Neuroscience. (2017) 366:124–37. doi: 10.1016/j.neuroscience.2017.10.023
161. Vicentini, FA, Szamosi, JC, Rossi, L, Griffin, L, Nieves, K, Bihan, D, et al. Colitis-associated microbiota drives changes in behaviour in male mice in the absence of inflammation. Brain Behav Immun. (2022) 102:266–78. doi: 10.1016/j.bbi.2022.03.001
162. Weegh, N, Füner, J, Janke, O, Winter, Y, Jung, C, Struve, B, et al. Wheel running behaviour in group-housed female mice indicates disturbed wellbeing due to DSS colitis. Lab Anim. (2020) 54:63–72. doi: 10.1177/0023677219879455
163. Mitchell, J, Kim, SJ, Howe, C, Lee, S, Her, JY, Patel, M, et al. Chronic intestinal inflammation suppresses brain activity by inducing neuroinflammation in mice. Am J Pathol. (2022) 192:72–86. doi: 10.1016/j.ajpath.2021.09.006
164. Zonis, S, Pechnick, RN, Ljubimov, VA, Mahgerefteh, M, Wawrowsky, K, Michelsen, KS, et al. Chronic intestinal inflammation alters hippocampal neurogenesis. J Neuroinflammation. (2015) 12:1–12. doi: 10.1186/s12974-015-0281-0
165. Mickael, ME, Bhaumik, S, Chakraborti, A, Umfress, AA, van Groen, T, Macaluso, M, et al. RORγt-expressing pathogenic CD4+ T cells cause brain inflammation during chronic colitis. J Immunol. (2022) 208:2054–66. doi: 10.4049/jimmunol.2100869
166. He, X-f, Li, L-l, Xian, W-b, Li, M-y, Zhang, L-y, Xu, J-h, et al. Chronic colitis exacerbates NLRP3-dependent neuroinflammation and cognitive impairment in middle-aged brain. J Neuroinflammation. (2021) 18:1–17. doi: 10.1186/s12974-021-02199-8
167. Salvo, E, Stokes, P, Keogh, CE, Brust-Mascher, I, Hennessey, C, Knotts, TA, et al. A murine model of pediatric inflammatory bowel disease causes microbiota-gut-brain axis deficits in adulthood. Am J Physiol Gastrointestinal Liver Physiol. (2020) 319:G361–74. doi: 10.1152/ajpgi.00177.2020
168. Pellegrini, C, Antonioli, L, Calderone, V, Colucci, R, Fornai, M, and Blandizzi, C. Microbiota-gut-brain axis in health and disease: is NLRP3 inflammasome at the crossroads of microbiota-gut-brain communications? Prog Neurobiol. (2020) 191:101806. doi: 10.1016/j.pneurobio.2020.101806
169. Cluny, NL, Nyuyki, KD, Almishri, W, Griffin, L, Lee, BH, Hirota, SA, et al. Recruitment of α4β7 monocytes and neutrophils to the brain in experimental colitis is associated with elevated cytokines and anxiety-like behavior. J Neuroinflammation. (2022) 19:1–17. doi: 10.1186/s12974-022-02431-z
170. Taylor, CT, and Keely, SJ. The autonomic nervous system and inflammatory bowel disease. Auton Neurosci. (2007) 133:104–14. doi: 10.1016/j.autneu.2006.11.005
171. De Jonge, WJ, Van Der Zanden, EP, The, FO, Bijlsma, MF, Van Westerloo, DJ, Bennink, RJ, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. (2005) 6:844–51. doi: 10.1038/ni1229
172. Ghia, JE, Blennerhassett, P, Kumar-Ondiveeran, H, Verdu, EF, and Collins, SM. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology. (2006) 131:1122–30. doi: 10.1053/j.gastro.2006.08.016
173. Payne, SC, Furness, JB, Burns, O, Sedo, A, Hyakumura, T, Shepherd, RK, et al. Anti-inflammatory effects of abdominal Vagus nerve stimulation on experimental intestinal inflammation. Front Neurosci. (2019) 13:418. doi: 10.3389/fnins.2019.00418
174. Frankiensztajn, LM, Elliott, E, and Koren, O. The microbiota and the hypothalamus-pituitary-adrenocortical (HPA) axis, implications for anxiety and stress disorders. Curr Opin Neurobiol. (2020) 62:76–82. doi: 10.1016/j.conb.2019.12.003
175. Nemirovsky, A, Ilan, K, Lerner, L, Cohen-Lavi, L, Schwartz, D, Goren, G, et al. Brain-immune axis regulation is responsive to cognitive behavioral therapy and mindfulness intervention: observations from a randomized controlled trial in patients with Crohn's disease. Brain Behav Immun Health. (2022) 19:100407. doi: 10.1016/j.bbih.2021.100407
176. Pellissier, S, Dantzer, C, Mondillon, L, Trocme, C, Gauchez, A-S, Ducros, V, et al. Relationship between vagal tone, cortisol, TNF-alpha, epinephrine and negative affects in Crohn’s disease and irritable bowel syndrome. PLoS One. (2014) 9:e105328. doi: 10.1371/journal.pone.0105328
177. Straub, RH, Herfarth, H, Falk, W, Andus, T, and Schölmerich, J. Uncoupling of the sympathetic nervous system and the hypothalamic–pituitary–adrenal axis in inflammatory bowel disease? J Neuroimmunol. (2002) 126:116–25. doi: 10.1016/s0165-5728(02)00047-4
178. Reichmann, F, Hassan, AM, Farzi, A, Jain, P, Schuligoi, R, and Holzer, P. Dextran sulfate sodium-induced colitis alters stress-associated behaviour and neuropeptide gene expression in the amygdala-hippocampus network of mice. Sci Rep. (2015) 5:1–13. doi: 10.1038/srep09970
179. Girotti, M, Donegan, JJ, and Morilak, DA. Influence of hypothalamic IL-6/gp130 receptor signaling on the HPA axis response to chronic stress. Psychoneuroendocrinology. (2013) 38:1158–69. doi: 10.1016/j.psyneuen.2012.11.004
180. Yuan, T, Orock, A, and Greenwood-VanMeerveld, B. An enriched environment reduces chronic stress-induced visceral pain through modulating microglial activity in the central nucleus of the amygdala. American journal of physiology-gastrointestinal and liver. Physiology. (2022) 322:G223–33. doi: 10.1152/ajpgi.00307.2021
181. Broersen, LH, Pereira, AM, Jørgensen, JOL, and Dekkers, OM. Adrenal insufficiency in corticosteroids use: systematic review and meta-analysis. J Clin Endocrinol Metabol. (2015) 100:2171–80. doi: 10.1210/jc.2015-1218
182. Ibrahim, A, Dahlqvist, P, Olsson, T, Lundgren, D, Werner, M, Suhr, OB, et al. The clinical course after glucocorticoid treatment in patients with inflammatory bowel disease is linked to suppression of the hypothalamic–pituitary–adrenal axis: a retrospective observational study. Ther Adv Gastroenterol. (2017) 10:829–36. doi: 10.1177/1756283X17730748
183. Gottschalk, M, Kümpfel, T, Flachenecker, P, Uhr, M, Trenkwalder, C, Holsboer, F, et al. Fatigue and regulation of the hypothalamo-pituitary-adrenal axis in multiple sclerosis. Arch Neurol. (2005) 62:277–80. doi: 10.1001/archneur.62.2.277
184. Minderhoud, IM, Oldenburg, B, van Dam, PS, and van Berge Henegouwen, GP. High prevalence of fatigue in quiescent inflammatory bowel disease is not related to adrenocortical insufficiency. Am J Gastroenterol. (2003) 98:1088–93. doi: 10.1111/j.1572-0241.2003.07414.x
185. Bonifacio, C, Savini, G, Reca, C, Garoli, F, Levi, R, Vatteroni, G, et al. The gut-brain axis: correlation of choroid plexus volume and permeability with inflammatory biomarkers in Crohn's disease. Neurobiol Dis. (2024) 192:106416. doi: 10.1016/j.nbd.2024.106416
186. Agostini, A, Benuzzi, F, Ballotta, D, Rizzello, F, Gionchetti, P, and Filippini, N. Differential brain structural and functional patterns in Crohn’s disease patients are associated with different disease stages. Inflamm Bowel Dis. (2023) 29:1297–305. doi: 10.1093/ibd/izad029
187. Bao, CH, Liu, P, Liu, HR, Wu, LY, Shi, Y, Chen, WF, et al. Alterations in brain Grey matter structures in patients with Crohn’s disease and their correlation with psychological distress☆. J Crohn's Colitis. (2015) 9:532–40. doi: 10.1093/ecco-jcc/jjv057
188. van Erp, S, Ercan, E, Breedveld, P, Brakenhoff, L, Ghariq, E, Schmid, S, et al. Cerebral magnetic resonance imaging in quiescent Crohn’s disease patients with fatigue. World J Gastroenterol. (2017) 23:1018. doi: 10.3748/wjg.v23.i6.1018
189. Bao, C-H, Liu, P, Liu, H-R, Wu, L-Y, Jin, X-M, Wang, S-Y, et al. Differences in regional homogeneity between patients with Crohn's disease with and without abdominal pain revealed by resting-state functional magnetic resonance imaging. Pain. (2016) 157:1037–44. doi: 10.1097/j.pain.0000000000000479
190. Thomann, A, Schmitgen, M, Szabo, K, Ebert, M, Reindl, W, and Wolf, C. P483 fatigue and brain morphology in active and remitted Crohn’s disease. J Crohn's Colitis. (2024) 18:i967–8. doi: 10.1093/ecco-jcc/jjad212.0613
191. Czuber-Dochan, W, Norton, C, Bredin, F, Darvell, M, Nathan, I, and Terry, H. Healthcare professionals' perceptions of fatigue experienced by people with IBD. J Crohns Colitis. (2014) 8:835–44. doi: 10.1016/j.crohns.2014.01.004
192. Borren, NZ, van der Woude, CJ, and Ananthakrishnan, AN. Fatigue in IBD: epidemiology, pathophysiology and management. Nat Rev Gastroenterol Hepatol. (2019) 16:247–59. doi: 10.1038/s41575-018-0091-9
193. Kreijne, J, Lie, M, Vogelaar, L, and Van Der Woude, C. Practical guideline for fatigue management in inflammatory bowel disease. J Crohn's Colitis. (2016) 10:105–11. doi: 10.1093/ecco-jcc/jjv168
194. Moulton, CD, Jordan, C, Hayee, B, and Chalder, T. All-or-nothing behavior and catastrophic thinking predict fatigue in inflammatory bowel disease: a prospective cohort study. Inflamm Bowel Dis. (2023). doi: 10.1093/ibd/izad193
195. Milzer, M, Steindorf, K, Reinke, P, and Schmidt, ME. The cancer patients’ perspective on feasibility of using a fatigue diary and the benefits on self-management: results from a longitudinal study. Support Care Cancer. (2022) 30:10213–21. doi: 10.1007/s00520-022-07397-5
196. Coolbrandt, A, Van den Heede, K, Vanhove, E, De Bom, A, Milisen, K, and Wildiers, H. Immediate versus delayed self-reporting of symptoms and side effects during chemotherapy: does timing matter? Eur J Oncol Nurs. (2011) 15:130–6. doi: 10.1016/j.ejon.2010.06.010
197. de Jong, M, van der Meulen-de, JA, Romberg-Camps, M, Degens, J, Becx, M, Markus, T, et al. Development and feasibility study of a telemedicine tool for all patients with IBD: MyIBDcoach. Inflamm Bowel Dis. (2017) 23:485–93. doi: 10.1097/MIB.0000000000001034
198. Nigro, G, Angelini, G, Grosso, SB, Caula, G, and Sategna-Guidetti, C. Psychiatric predictors of noncompliance in inflammatory bowel disease: psychiatry and compliance. J Clin Gastroenterol. (2001) 32:66–8. doi: 10.1097/00004836-200101000-00015
199. Loftus, EV, Feagan, BG, Colombel, J-F, Rubin, DT, Wu, EQ, Andrew, PY, et al. Effects of adalimumab maintenance therapy on health-related quality of life of patients with Crohn's disease: patient-reported outcomes of the CHARM trial. J Am College Gastroenterol. (2008) 103:3132–41. doi: 10.1111/j.1572-0241.2008.02175.x
200. Lichtenstein, GR, Bala, M, Han, C, DeWoody, K, and Schaible, T. Infliximab improves quality of life in patients with Crohn's disease. Inflamm Bowel Dis. (2002) 8:237–43. doi: 10.1097/00054725-200207000-00001
201. Danese, S, Tran, J, D'Haens, G, Rubin, DT, Aoyama, N, Zhou, W, et al. Upadacitinib induction and maintenance therapy improves abdominal pain, bowel urgency, and fatigue in patients with ulcerative colitis: a post hoc analysis of phase 3 data. Inflamm Bowel Dis. (2023) 29:1723–9. doi: 10.1093/ibd/izad016
202. Armuzzi, A, Hart, A, Cappelleri, JC, Mammar, N, Hur, P, Hoskin, B, et al. Characteristics, clinical outcomes and patient-reported outcomes of patients with ulcerative colitis receiving tofacitinib: a real-world survey in the United States and five European countries. BMC Gastroenterol. (2023) 23:17. doi: 10.1186/s12876-023-02640-7
203. Travis, S, Hisamatsu, T, Fischer, M, Dubinsky, M, Jairath, V, Chen, M, et al. OP12 Mirikizumab improves fatigue, bowel urgency, and quality of life in patients with moderately to severely active Crohn’s disease: results from a phase 3 clinical trial. J Crohn's Colitis. (2024) 18:i21–3. doi: 10.1093/ecco-jcc/jjad212.0012
204. Peyrin-Biroulet, L, Ghosh, S, Lee, SD, Lee, W-J, Griffith, J, Wallace, K, et al. Effect of risankizumab on health-related quality of life in patients with Crohn's disease: results from phase 3 MOTIVATE, ADVANCE and FORTIFY clinical trials. Aliment Pharmacol Ther. (2023) 57:496–508. doi: 10.1111/apt.17242
205. Dignass, A, Panés, J, Rubin, D, Huang, K, Germinaro, M, Pandya, D, et al. DOP52 Guselkumab improves symptoms of fatigue in patients with moderately to severely active ulcerative colitis: phase 3 QUASAR induction study results at week 12. J Crohn's Colitis. (2024) 18:i166–7. doi: 10.1093/ecco-jcc/jjad212.0092
206. Lewis, JD, Allez, M, Irving, PM, Hoops, T, Izanec, JL, Ma, T, et al. Health-related quality of life with ustekinumab vs adalimumab for induction and maintenance therapy in biologic-naïve patients with moderate-to-severe Crohn’s disease: PROMIS-29 in the SEAVUE study. J Am College Gastroenterol. (2021) 116:S395. doi: 10.1016/S0140-6736(22)00688-2
207. Kappelman, MD, Long, MD, Zhang, X, Lin, FC, Weisbein, L, Chen, W, et al. Comparing patient-reported outcomes among anti-TNF experienced patients with ulcerative colitis initiating vedolizumab versus tofacitinib. Crohns Colitis. (2023) 5:otad031. doi: 10.1093/crocol/otad031
208. Borren, NZ, Tan, W, Colizzo, FP, Luther, J, Garber, JJ, Khalili, H, et al. Longitudinal trajectory of fatigue with initiation of biologic therapy in inflammatory bowel diseases: a prospective cohort study. J Crohns Colitis. (2019) 14:309–15. doi: 10.1093/ecco-jcc/jjz148
209. Dignass, AU, Gasche, C, Bettenworth, D, Birgegård, G, Danese, S, Gisbert, JP, et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohn's Colitis. (2015) 9:211–22. doi: 10.1093/ecco-jcc/jju009
210. Zoller, H, Wolf, M, Blumenstein, I, Primas, C, Lindgren, S, Thomsen, LL, et al. Hypophosphataemia following ferric derisomaltose and ferric carboxymaltose in patients with iron deficiency anaemia due to inflammatory bowel disease (PHOSPHARE-IBD): a randomised clinical trial. Gut. (2023) 72:644–53. doi: 10.1136/gutjnl-2022-327897
211. Çekiç, C, Ipek, S, Aslan, F, Akpınar, Z, Arabul, M, Topal, F, et al. The effect of intravenous iron treatment on quality of life in inflammatory bowel disease patients with nonanemic iron deficiency. Gastroenterol Res Pract. (2015) 2015:1–5. doi: 10.1155/2015/582163
212. Fiorino, G, Allocca, M, Gilardi, D, Alfieri, MF, Danieli, P, Furfaro, F, et al. Sa1793 efficacy and safety of iron carboxymaltose on chronic fatigue in patients with inflammatory bowel disease: a randomized controlled trial. Gastroenterology. (2020) 158:–426. doi: 10.1016/S0016-5085(20)31753-4
213. Laheij, R, Bierens-Peters, D, Bruin, K, Lutgens, M, Sikkema, M, de Wit, U, et al. P742 The efficacy of comprehensive multivitamin and mineral supplement to treat symptoms of fatigue in patients with inflammatory bowel disease in remission with immunomodulators and or biological agents. J Crohn's Colitis. (2024) 18:i1390–1. doi: 10.1093/ecco-jcc/jjad212.0872
214. Scholten, A-M, Vermeulen, E, Dhonukshe-Rutten, RAM, Verhagen, T, Visscher, A, Olivier, A, et al. Surplus vitamin B12 use does not reduce fatigue in patients with irritable bowel syndrome or inflammatory bowel disease: a randomized double-blind placebo-controlled trial. Clinical Nutrition ESPEN. (2018) 23:48–53. doi: 10.1016/j.clnesp.2017.10.004
215. Lima, GL, Paupitz, J, Aikawa, NE, Takayama, L, Bonfa, E, and Pereira, RM. Vitamin D supplementation in adolescents and young adults with juvenile systemic lupus erythematosus for improvement in disease activity and fatigue scores: a randomized, double-blind, placebo-controlled trial. Arthritis Care Res. (2016) 68:91–8. doi: 10.1002/acr.22621
216. Yang, L, Weaver, V, Smith, JP, Bingaman, S, Hartman, TJ, and Cantorna, MT. Therapeutic effect of vitamin d supplementation in a pilot study of Crohn's patients. Clin Transl Gastroenterol. (2013) 4:e33. doi: 10.1038/ctg.2013.1
217. Keefer, L, Bedell, A, Norton, C, and Hart, AL. How should pain, fatigue and emotional wellness be incorporated into treatment goals for optimal management of IBD? Gastroenterology. (2022) 162:1439–51. doi: 10.1053/j.gastro.2021.08.060
218. Swanson, GR, and Burgess, HJ. Sleep and circadian hygiene and inflammatory bowel disease. Gastroenterol Clin N Am. (2017) 46:881–93. doi: 10.1016/j.gtc.2017.08.014
219. Garcia-Vega, E, and Fernandez-Rodriguez, C. A stress management programme for Crohn’s disease. Behav Res Ther. (2004) 42:367–83. doi: 10.1016/S0005-7967(03)00146-3
220. Vogelaar, L, Van't Spijker, A, Vogelaar, T, van Busschbach, JJ, Visser, MS, Kuipers, EJ, et al. Solution focused therapy: a promising new tool in the management of fatigue in Crohn's disease patients: psychological interventions for the management of fatigue in Crohn's disease. J Crohn's Colitis. (2011) 5:585–91. doi: 10.1016/j.crohns.2011.06.001
221. Vogelaar, L, vant Spijker, A, Timman, R, van Tilburg, AJ, Bac, D, Vogelaar, T, et al. Fatigue management in patients with IBD: a randomised controlled trial. Gut. (2014) 63:911–8. doi: 10.1136/gutjnl-2013-305191
222. Artom, M, Czuber-Dochan, W, Sturt, J, Proudfoot, H, Roberts, D, and Norton, C. Cognitive-behavioural therapy for the management of inflammatory bowel disease-fatigue: a feasibility randomised controlled trial. Pilot Feasibility Stud. (2019) 5:145. doi: 10.1186/s40814-019-0538-y
223. Ter Avest, M, Speckens, A, Dijkstra, G, Dresler, M, Bosman, S, Horjus, C, et al. P1079 mindfulness-based cognitive therapy to reduce psychological distress in patients with inflammatory bowel disease: first results of a multicentre randomised controlled trial (MindIBD). J Crohn's Colitis. (2024) 18:i1941. doi: 10.1093/ecco-jcc/jjad212.1209
224. O'Connor, A, Ratnakumaran, R, Warren, L, Pullen, D, Errington, A, Gracie, DJ, et al. Randomized controlled trial: a pilot study of a psychoeducational intervention for fatigue in patients with quiescent inflammatory bowel disease. Ther Adv Chronic Dis. (2019) 10:204062231983843. doi: 10.1177/2040622319838439
225. Emerson, C, Barhoun, P, Olive, L, Fuller-Tyszkiewicz, M, Gibson, PR, Skvarc, D, et al. A systematic review of psychological treatments to manage fatigue in patients with inflammatory bowel disease. J Psychosom Res. (2021) 147:110524. doi: 10.1016/j.jpsychores.2021.110524
226. Hashash, JG, Knisely, MR, Germain, A, McAuliff, K, Strassburger, M, Vachon, A, et al. Brief behavioral therapy and bupropion for sleep and fatigue in young adults with Crohn’s disease: an exploratory open trial study. Clin Gastroenterol Hepatol. (2022) 20:96–104. doi: 10.1016/j.cgh.2020.09.047
227. Goren, G, Schwartz, D, Friger, M, Banai, H, Sergienko, R, Regev, S, et al. Randomized controlled trial of cognitive-behavioral and mindfulness-based stress reduction on the quality of life of patients with Crohn disease. Inflamm Bowel Dis. (2022) 28:393–408. doi: 10.1093/ibd/izab083
228. Emerson, C, Skvarc, D, Fuller-Tyszkiewicz, M, Olive, L, Gibson, PR, and Mikocka-Walus, A. Self-worth beliefs predict willingness to engage in psychotherapy for fatigue in inflammatory bowel disease. Dig Dis Sci. (2022) 67:5472–82. doi: 10.1007/s10620-022-07476-x
229. Ewais, T, Begun, J, Kenny, M, Headey, A, Tefay, M, and Kisely, S. Mindfulness-based cognitive therapy experiences in youth with inflammatory bowel disease and depression: findings from a mixed methods qualitative study. BMJ Open. (2020) 10:e041140. doi: 10.1136/bmjopen-2020-041140
230. Raman, M, Rajagopalan, V, Kaur, S, Reimer, RA, Ma, C, Ghosh, S, et al. Physical activity in patients with inflammatory bowel disease: a narrative review. Inflamm Bowel Dis. (2021) 28:1100–11. doi: 10.1093/ibd/izab218
231. McNelly, AS, Nathan, I, Monti, M, Grimble, GK, Norton, C, Bredin, F, et al. The effect of increasing physical activity and/or omega-3 supplementation on fatigue in inflammatory bowel disease. Gastrointest Nurs. (2016) 14:39–50. doi: 10.12968/gasn.2016.14.8.39
232. Fagan, G, Schultz, M, and Osborne, H. Sa1877 – individualised, unsupervised exercise program achieves high levels of compliance and improvements in patient reported outcomes - a prospective cohort study in patients with Ibd. Gastroenterology. (2019) 156:S-438. doi: 10.1016/S0016-5085(19)37943-0
233. Ng, V, Millard, W, Lebrun, C, and Howard, J. Low-intensity exercise improves quality of life in patients with Crohn's disease. Clin J Sport Med. (2007) 17:384–8. doi: 10.1097/JSM.0b013e31802b4fda
234. Jones, K, Baker, K, Speight, RA, Thompson, NP, and Tew, GA. Randomised clinical trial: combined impact and resistance training in adults with stable Crohn's disease. Aliment Pharmacol Ther. (2020) 52:964–75. doi: 10.1111/apt.16002
235. Nathan, I, Norton, C, Czuber-Dochan, W, and Forbes, A. Exercise in individuals with inflammatory bowel disease. Gastroenterol Nurs. (2013) 36:437–42. doi: 10.1097/SGA.0000000000000005
236. Van De Pol, N, Visser, E, van der Woude, C, de Vries, A, de Jonge, V, and West, R. P744 evaluation of a lifestyle program based on physical activity on quality of life and fatigue in patients with inflammatory bowel disease: a pilot study. J Crohn's Colitis. (2024) 18:i1393–4. doi: 10.1093/ecco-jcc/jjad212.0874
237. Tew, GA, Leighton, D, Carpenter, R, Anderson, S, Langmead, L, Ramage, J, et al. High-intensity interval training and moderate-intensity continuous training in adults with Crohn’s disease: a pilot randomised controlled trial. BMC Gastroenterol. (2019) 19:1–11. doi: 10.1186/s12876-019-0936-x
238. Wilke, E, Reindl, W, Thomann, PA, Ebert, MP, Wuestenberg, T, and Thomann, AK. Effects of yoga in inflammatory bowel diseases and on frequent IBD-associated extraintestinal symptoms like fatigue and depression. Complement Ther Clin Pract. (2021) 45:101465. doi: 10.1016/j.ctcp.2021.101465
239. Craig, CL, Marshall, AL, Sjöström, M, Bauman, AE, Booth, ML, Ainsworth, BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exercise. (2003) 35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB
240. Reznikov, EA, and Suskind, DL. Current nutritional therapies in inflammatory bowel disease: improving clinical remission rates and sustainability of Long-term dietary therapies. Nutrients. (2023) 15:668. doi: 10.3390/nu15030668
241. Lamers, CR, de Roos, NM, Heerink, HH, van de Worp-Kalter, LA, and Witteman, BJM. Lower impact of disease on daily life and less fatigue in patients with inflammatory bowel disease following a lifestyle intervention. Inflamm Bowel Dis. (2022) 28:1791–9. doi: 10.1093/ibd/izac027
242. Rogers, J, and Rogers, B. A diet and lifestyle-factor intervention program to improve nutritional status, symptoms, and quality of life in patients with inflammatory bowel disease. Inflamm Bowel Dis. (2023) 29:S15. doi: 10.1093/ibd/izac247.030
243. Strobel, TM, Nguyen, C, Riggs, T, Horst, SN, Motley, A, Upadhyaya, S, et al. Functional medicine approach to patient care improves sleep, fatigue, and quality of life in patients with inflammatory bowel disease. Crohns Colitis. (2022) 360, 4:otac032. doi: 10.1093/crocol/otac032
244. Kortlever, TL, Ten Bokkel, HS, Offereins, M, Hebblethwaite, C, O'Brien, L, Leeper, J, et al. Low-FODMAP diet is associated with improved quality of life in IBS patients—a prospective observational study. Nutr Clin Pract. (2019) 34:623–30. doi: 10.1002/ncp.10233
245. Peng, Z, Yi, J, and Liu, X. A low-FODMAP diet provides benefits for functional gastrointestinal symptoms but not for improving stool consistency and mucosal inflammation in IBD: a systematic review and meta-analysis. Nutrients. (2022) 14:2072. doi: 10.3390/nu14102072
246. Gibson, PR . Use of the low-FODMAP diet in inflammatory bowel disease. J Gastroenterol Hepatol. (2017) 32:40–2. doi: 10.1111/jgh.13695
247. Horta, D, Lira, A, Sanchez-Lloansi, M, Villoria, A, Teggiachi, M, García-Rojo, D, et al. A prospective pilot randomized study: electroacupuncture vs. sham procedure for the treatment of fatigue in patients with quiescent inflammatory bowel disease. Inflamm Bowel Dis. (2020) 26:484–92. doi: 10.1093/ibd/izz091
248. Therkelsen, SP, Hetland, G, Lyberg, T, Lygren, I, and Johnson, E. Effect of a medicinal Agaricus blazei Murill-based mushroom extract, AndoSan™, on symptoms, fatigue and quality of life in patients with ulcerative colitis in a randomized single-blinded placebo controlled study. PLoS One. (2016) 11:e0150191. doi: 10.1371/journal.pone.0150191
249. You, L, Guo, N, Wang, T, Yu, X, Kang, X, Guan, Y, et al. Effects of aromatherapy on fatigue, quality of sleep and quality of life in patients with inflammatory bowel disease: a feasibility study. Complement Ther Clin Pract. (2022) 49:101648. doi: 10.1016/j.ctcp.2022.101648
250. Costantini, A, and Pala, MI. Thiamine and fatigue in inflammatory bowel diseases: an open-label pilot study. J Altern Complement Med. (2013) 19:704–8. doi: 10.1089/acm.2011.0840
251. Bager, P, Hvas, CL, Rud, CL, and Dahlerup, JF. Randomised clinical trial: high-dose oral thiamine versus placebo for chronic fatigue in patients with quiescent inflammatory bowel disease. Aliment Pharmacol Ther. (2021) 53:79–86. doi: 10.1111/apt.16166
252. Bager, P, Hvas, CL, Rud, CL, and Dahlerup, JF. Long-term maintenance treatment with 300 mg thiamine for fatigue in patients with inflammatory bowel disease: results from an open-label extension of the TARIF study. Scand J Gastroenterol. (2021) 57:37–43. doi: 10.1080/00365521.2021.1983640
253. Daghaghzadeh, H, Naji, F, Afshar, H, Sharbafchi, MR, Feizi, A, Maroufi, M, et al. Efficacy of duloxetine add on in treatment of inflammatory bowel disease patients: a double-blind controlled study. J Res Med Sci. (2015) 20:595. doi: 10.4103/1735-1995.165969
254. Mikocka-Walus, A, Hughes, PA, Bampton, P, Gordon, A, Campaniello, MA, Mavrangelos, C, et al. Fluoxetine for maintenance of remission and to improve quality of life in patients with Crohn’s disease: a pilot randomized placebo-controlled trial. J Crohn's Colitis. (2017) 11:509–14. doi: 10.1093/ecco-jcc/jjw165
255. Gong, S, Sheng, P, Jin, H, He, H, Qi, E, Chen, W, et al. Effect of methylphenidate in patients with cancer-related fatigue: a systematic review and meta-analysis. PLoS One. (2014) 9:e84391. doi: 10.1371/journal.pone.0084391
256. Truyens, M, Lobatón, T, Ferrante, M, Bossuyt, P, Vermeire, S, Pouillon, L, et al. Effect of 5-hydroxytryptophan on fatigue in quiescent inflammatory bowel disease: a randomized controlled trial. Gastroenterology. (2022) 163:1294–1305.e3. doi: 10.1053/j.gastro.2022.07.052
257. Rathi, A, Jadhav, SB, and Shah, N. A randomized controlled trial of the efficacy of systemic enzymes and probiotics in the resolution of post-COVID fatigue. Medicines. (2021) 8:47. doi: 10.3390/medicines8090047
258. Venturini, L, Bacchi, S, Capelli, E, Lorusso, L, Ricevuti, G, and Cusa, C. Modification of immunological parameters, oxidative stress markers, mood symptoms, and well-being status in CFS patients after probiotic intake: observations from a pilot study. Oxidative Med Cell Longev. (2019) 2019:1–10. doi: 10.1155/2019/1684198
259. Yoo, J-W, Shin, Y-J, Ma, X, Son, Y-H, Jang, H-M, Lee, CK, et al. The alleviation of gut microbiota-induced depression and colitis in mice by anti-inflammatory probiotics NK151, NK173, and NK175. Nutrients. (2022) 14:2080. doi: 10.3390/nu14102080
260. Hospital MG, BV WBI . Effect of a probiotic mixture on the gut microbiome and fatigue in patients with quiescent inflammatory Bowel disease. (2017). Available at: https://ClinicalTrials.gov/show/NCT03266484
261. Faraj, LJ, Takanti, LV, and Tavakoli, HR. The gut-brain axis: literature overview and psychiatric applications. Fed Pract. (2021) 38:356. doi: 10.12788/fp.0159
262. Tarn, J, Legg, S, Mitchell, S, Simon, B, and Ng, W-F. The effects of noninvasive Vagus nerve stimulation on fatigue and immune responses in patients with primary Sjögren’s syndrome. Neuromodul Technol Neural Interface. (2019) 22:580–5. doi: 10.1111/ner.12879
263. Aranow, C, Atish-Fregoso, Y, Lesser, M, Mackay, M, Anderson, E, Chavan, S, et al. Transcutaneous auricular vagus nerve stimulation reduces pain and fatigue in patients with systemic lupus erythematosus: a randomised, double-blind, sham-controlled pilot trial. Ann Rheum Dis. (2021) 80:203–8. doi: 10.1136/annrheumdis-2020-217872
Keywords: fatigue, Crohn’s disease, ulcerative colitis, tiredness, gut-brain axis
Citation: Truyens M, Lernout H, De Vos M, Laukens D and Lobaton T (2024) Unraveling the fatigue puzzle: insights into the pathogenesis and management of IBD-related fatigue including the role of the gut-brain axis. Front. Med. 11:1424926. doi: 10.3389/fmed.2024.1424926
Edited by:
Wenqing Cao, New York University, United StatesReviewed by:
Daniele Noviello, University of Milan, ItalyRaffaele Pellegrino, University of Campania Luigi Vanvitelli, Italy
Copyright © 2024 Truyens, Lernout, De Vos, Laukens and Lobaton. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marie Truyens, TWFyaWUuVHJ1eWVuc0B1Z2VudC5iZQ==
†These authors have contributed equally to this work and share first authorship
 Marie Truyens
Marie Truyens Hannah Lernout
Hannah Lernout Martine De Vos1
Martine De Vos1