- 1Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University, Daegu, Republic of Korea
- 2Department of Orthopaedic Surgery, College of Medicine, Yeungnam University, Daegu, Republic of Korea
- 3Division of Endocrinology and Metabolism, Department of Internal Medicine, College of Medicine, Yeungnam University, Daegu, Republic of Korea
The continuous monitoring of the health status of patients is essential for the effective monitoring of disease progression and the management of symptoms. Recently, health monitoring using non-contact sensors has gained interest. Therefore, this study aimed to investigate the use of non-contact sensors for health monitoring in hospital settings and evaluate their potential clinical applications. A comprehensive literature search was conducted using PubMed to identify relevant studies published up to February 26, 2024. The search terms included “hospital,” “monitoring,” “sensor,” and “non-contact.” Studies that used non-contact sensors to monitor health status in hospital settings were included in this review. Of the 38 search results, five studies met the inclusion criteria. The non-contact sensors described in the studies were radar, infrared, and microwave sensors. These non-contact sensors were used to obtain vital signs, such as respiratory rate, heart rate, and body temperature, and were then compared with the results from conventional measurement methods (polysomnography, nursing records, and electrocardiography). In all the included studies, non-contact sensors demonstrated a performance similar to that of conventional health-related parameter measurement methods. Non-contact sensors are expected to be a promising solution for health monitoring in hospital settings.
1 Introduction
Health monitoring is crucial in hospital settings for early detection and prevention of diseases as well as for assessing the effectiveness of treatments (1, 2). Regular monitoring of health status enables the early detection of disease onset or health-related issues and helps identify the risk of comorbidities and complications (3). Moreover, monitoring disease progression can help decelerate or prevent deterioration (4, 5). Health monitoring of patients being treated in hospitals can also help track patient recovery and increase treatment efficiency (6, 7). Furthermore, patients admitted to hospitals often experience post-hospital syndrome characterized by temporary frailty and an increased risk of readmission due to inactivity or sleep deprivation following admission (8). Monitoring of the heart rate (HR) and sleep can help reduce the incidence of post-hospital syndrome (8). Considering various clinical situations, hospital health monitoring is perceived as both important and essential.
Traditionally, contact-based devices (e.g., electrocardiogram recorders, continuous blood glucose monitoring devices, and respiratory belts) have been utilized in clinical settings for hospital-based health monitoring. Electrocardiograms measure vital signs via patches attached to the skin that receive electrical signals; however, they carry the risk of skin disorders owing to patch usage (9, 10). Invasive devices can also induce fear in patients (11), whereas wearable devices can cause inconvenience owing to difficulties in wearing the devices (12). Additionally, general vital sign monitors can induce psychological distress in patients owing to their bulky size (13). Moreover, the cables connecting the patient to the device can cause discomfort when the patients move (14, 15). Non-contact sensors have been developed to overcome the limitations of conventional devices in health monitoring. Through non-contact sensors, patients can monitor their health status in real-time without wearing specific equipment, thereby improving convenience and comfort in daily life (16, 17). Additionally, non-contact sensors pose a minimal risk of skin disorders because they do not directly touch the patient’s body and can reduce psychological burdens owing to their small size and inconspicuous nature (18, 19). Non-contact sensors can be particularly useful in hospital environments where the risk of infection is high. Hospitals are recognized as places where infection control is critical. Thus, non-contact sensing technology can play a significant role in reducing the spread of infections within hospitals by minimizing direct contact between patients and healthcare providers (20). In addition, non-contact sensing technology is known to be effective for quickly monitoring the health status of many patients. Non-contact thermometers or heart rate monitors can reduce the waiting time necessary for medical examinations and increase efficiency by reducing the workload of healthcare providers, as they do not require direct intervention. Several previous studies have demonstrated the performance of non-contact sensors in health monitoring. In 2019, Michler et al. (21) highlighted the usefulness of radar-based non-contact sensors in measuring the HR and respiratory rate (RR). In 2022, He et al. (22) reported that non-contact sensors based on depth cameras and radar could accurately detect the RR and respiratory patterns. Similarly, in 2022, Talukdar et al. (23) reported that a camera-based non-contact sensor using remote photoplethysmography (PPG) technology could be a novel solution for monitoring HR, RR, oxygen saturation, and blood pressure. Non-contact sensors in health monitoring appear to overcome the limitations of traditional methods and have the potential to be useful in hospital settings.
In this study, we compared the performance of traditional measuring devices with that of non-contact sensors for health monitoring in hospital settings. We conducted an in-depth examination of the current limitations of non-contact sensing technology and assessed the potential clinical applicability of non-contact sensors. In addition, by proposing future research directions, we aim to provide new perspectives in the fields of medicine and sensing technologies.
2 Methods
2.1 Search strategy
We searched the PubMed database for relevant studies published up to February 26, 2024. The following search terms were used: “hospital,” “monitoring,” “sensor,” and “non-contact.” The inclusion criteria for the studies were as follows: (1) studies of non-contact sensors used to monitor health status in hospitals and (2) studies that compared the performance of non-contact sensors and traditional methods for health monitoring. The exclusion criteria were as follows: (1) studies involving only healthy individuals; (2) studies involving wearable devices; (3) reviews, conference presentations, letters to the editor, or other unidentified types of articles; (4) studies published in languages other than English owing to the authors’ limited language abilities.
2.2 Data extraction
All search results were exported to Endnote 20 software. Two independent reviewers (Y. J. C. and M. C. C.) confirmed the retrieved studies and selected eligible ones. We checked the titles and abstracts to select studies that met the selection criteria and read the full texts to determine which articles were to be included in this study. Any disagreements between the reviewers were resolved through discussion.
3 Results
3.1 Selection of studies
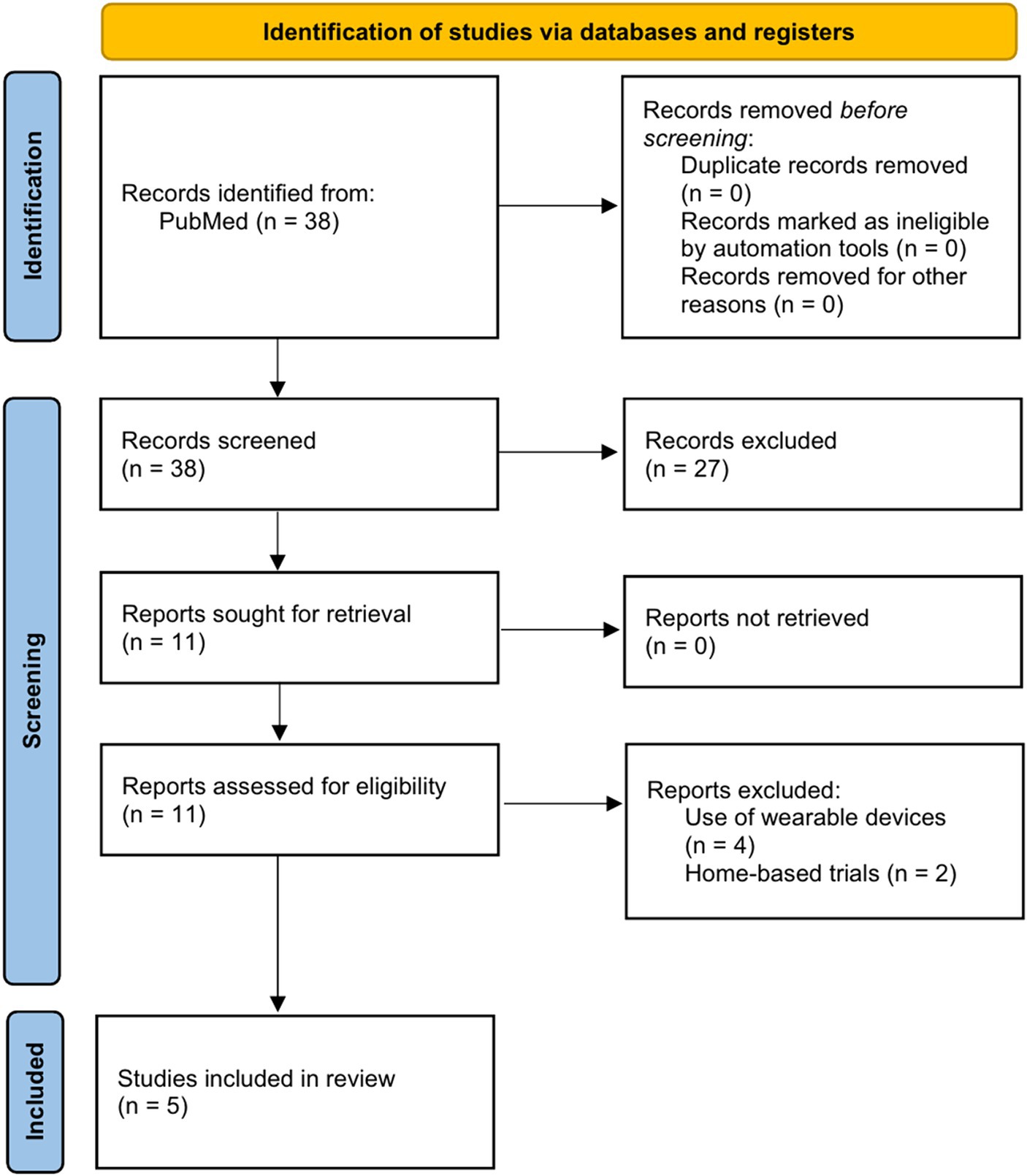
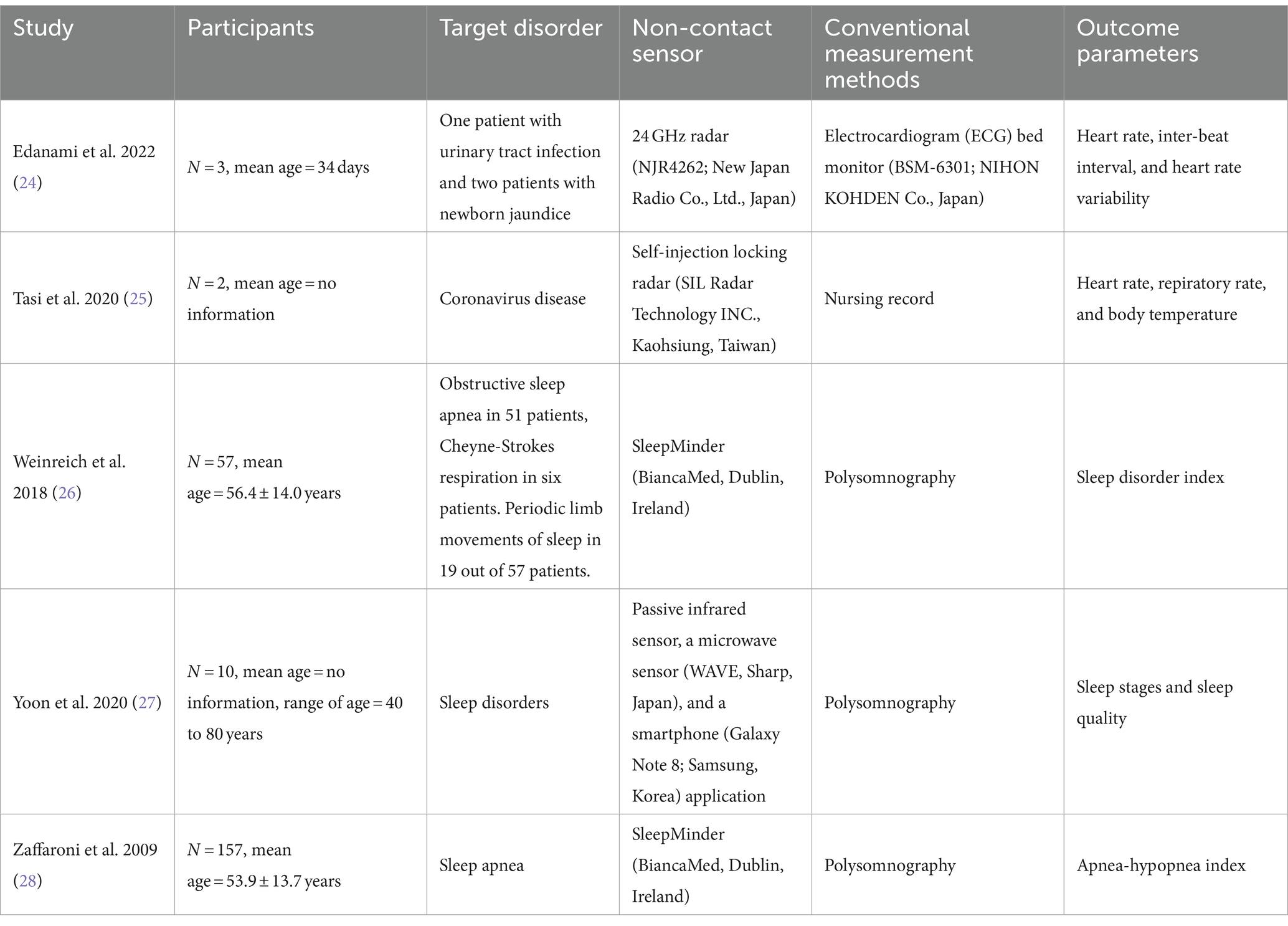
Of the 38 studies, 27 were excluded after checking the titles and abstracts of the studies that met the selection criteria. On checking the full texts of the remaining 11, four were excluded because they included wearable devices and two were excluded because they were conducted in a home environment. Finally, five studies (24–28) were included in this narrative review (Figure 1). The characteristics of the included studies are summarized in Table 1.
3.2 Summary of selected studies
In 2009, Zaffaroni et al. (28) compared the effectiveness of non-contact sensors (SleepMinder, BiancaMed, Dublin, Ireland) and polysomnography (PSG) in estimating the apnea-hypopnea index (AHI) of 157 participants with suspected sleep apnea. SleepMinder is a radar sensor-based non-contact sleep status monitoring device, developed to measure the breathing status and body movement during sleep. SleepMinder extracts respiratory signals from a patient’s chest movements and is typically placed facing the patient’s upper body because it has directionality that allows it to measure movement only in front of the sensor (29). In addition, it has a limited range and can only respond to objects within 2.5 meters of the sensor (29). The operating principle of SleepMinder is similar to that of the Doppler effect, a phenomenon in which the wave frequency changes depending on the relative motion of the wave source (26). SleepMinder detects and analyzes the phase shifts occurring in moving objects, such as breathing. Radiofrequency energy at 5.8 GHz was transmitted as two short pulses, each 5 ns long (30). The two pulses serve as the main transmit pulse, which is reflected from the object and received by the sensor, and the mixer pulse, which generates a signal proportional to the phase change of the main transmit pulse inside the receiver. SleepMinder operates at a low power with an average radiated power of 0.25 mW, meeting the safety and regulatory guidelines for radiofrequency devices (29). PSG is considered the gold standard for diagnosing sleep disorders (31). In PSG, apnea is generally defined as the complete cessation of breathing or a decrease in airflow of 90% or more lasting for more than 10 s (32). Hypopnea is typically defined as a 50% or greater decrease in airflow or a 3% or greater decrease in oxygen saturation lasting >10 s (32). AHI is a scale that determines the severity of sleep apnea and is classified into normal (AHI < 5), mild (5 ≤ AHI < 15), moderate (15 ≤ AHI < 30), and severe (AHI ≥ 30) (28). In Zaffaroni et al.’s study (28), SleepMinder was installed along with PSG to record biomotion signals simultaneously. The correlation coefficient between AHI estimates from SleepMinder and PSG was 0.91, indicating a strong positive correlation.
In 2018, Weinreich et al. (26) evaluated the performance of SleepMinder in detecting obstructive sleep apnea (OSA) and periodic limb movements during sleep (PLMS). Fifty-seven patients with sleep disorders, including 19 with PLMS, participated in this study. The levels of OSA and PLMS are typically determined using the AHI and periodic limb movement index (PLMI), respectively. Weinreich et al. (26) introduced a new sleep disorder index (SDI), the sum of the AHI and PLMI, and compared the agreement between SleepMinder-derived SDI and PSG-derived SDI. The correlation coefficient of the association between the SDIs generated by SleepMinder and PSG was 0.79, indicating a strong positive correlation. According to Zaffaroni et al. (28) and Weinreich et al. (26), SleepMinder is a viable alternative to conventional methods of detecting OSA and PLMS.
In 2020, Tasi et al. (25) applied a non-contact sensor to monitor the vital signs and body movements of patients with coronavirus disease (COVID-19) in an isolation ward. The non-contact sensor was a non-contact self-injection locking (SIL) radar (SIL Radar Technology Inc., Kaohsiung, Taiwan). The SIL radar was developed to measure vital signs, and it includes the following components: (1) receive antenna, (2) transmit antenna, (3) differential voltage-controlled oscillator with an injection port, (4) frequency demodulator composed of a mixer and a delay line, (5) low-pass filter, (6) bandpass filter, and (7) digital signal processor with built-in analog-to-digital converter and digital-to-analog converter (33). The continuous wave signal emitted from the oscillator embedded in the SIL radar is reflected by the target and injected back into the same oscillator, thereby forming an SIL state (34). The generated signals were modulated by a voltage-controlled oscillator, processed, and analyzed using a digital signal processor (33). The SIL radar can operate at a distance of 4 m from objects and is capable of stable vital sign measurement, even at an operating frequency of 3.6 GHz and an output power of 0 dBm (35). In a study by Tasi et al. (25), an SIL radar was fixed to the ward ceiling to detect a patient’s body temperature and HR. The monitoring results of body temperature and HR from the SIL radar for two COVID-19 patients were compared with the nurses’ records. The p-value obtained using Fisher’s exact test was 0.139 for body temperature and 0.292 for HR, indicating that the data collected by the SIL radar and nursing records were not significantly different. Additionally, the SIL radar can detect human face and body movements, allowing for real-time confirmation of patients’ coughing and breathing movements. Coughing and dyspnea are considered significant symptoms of COVID-19. Therefore, the SIL radar allows for continuous monitoring of COVID-19 patients’ conditions, enabling clinicians to save time compared to the conventional method of direct observation. Moreover, continuous health monitoring of patients in nursing stations (clean zones) can reduce the risk of infection.
In 2020, Yoon et al. (27) demonstrated the feasibility of using a non-contact sensor for sleep monitoring compared with PSG in a sample of 10 participants with sleep disorders. The non-contact sleep monitoring device consisted of a passive infrared sensor, a microwave sensor (WAVE, Sharp, Japan), and a smartphone (Galaxy Note 8, Samsung, Korea). The activity of the object was detected by an infrared sensor and recorded every 2 s, whereas the RR and HR were measured every 200 ms by a microwave sensor and then averaged over minutes. Changes in the RR- and HR-related frequencies over time were visualized in graphs and checked using the smartphone. The collected activity, RR, and HR data were used to predict sleep stages and evaluate sleep quality. Sleep was classified into four stages: awake, rapid eye movement (REM), light, and deep sleep. Additionally, the total sleep time, sleep efficiency, and wake after sleep onset (WASO) were used to evaluate sleep quality. Sleep efficiency was defined as the percentage of total time spent sleeping in bed, and WASO was defined as the time spent awake after sleep onset. The accuracy of the non-contact sensors in estimating sleep stages was 98.65%, and a significant positive correlation was found between the non-contact sensors and PSG for evaluating sleep quality (total sleep time, r = 0.97; sleep efficiency, r = 0.996; WASO, r = 0.99).
In 2022, Edanami et al. (24) evaluated the heart-signal detection performance of a non-contact medical radar-based vital sign monitoring system. Their sample included three infants in the neonatal intensive care unit (NICU); an electrocardiogram (ECG) bed monitor (BSM-6301, NIHON KOHDEN Co., Japan) simultaneously measured heart signals with a non-contact sensor. The non-contact sensor included a 24-GHz radar (NJR4262, New Japan Radio Co., Ltd., Japan) and signal acquisition and analysis software. The distance between the radar sensor and the target object was 5 cm, and the radar detected the movement of the object by continuously emitting radio waves of a certain frequency and receiving the reflected waves. Heartbeat peaks were estimated from the heart signal to obtain the interbeat interval (IBI) and heart rate variability (HRV) values. IBI was defined as the time interval between two neighboring heartbeat peaks, and HRV was estimated as a time series of IBI. The agreement between the non-contact radar sensor and ECG for the HR was 99%, and the correlation coefficients of the associations between the non-contact sensor and ECG for the IBI, low-frequency (LF), and high-frequency (HF) HRV were 0.82, 0.98, and 0.95, respectively. These results indicate the excellent performance of radar-based non-contact sensors in analyzing infant heart signals.
4 Discussion
This study explored the application of non-contact sensors for health monitoring in hospital settings. Based on the included studies, non-contact sensors are believed to have ample potential for monitoring cardiac and respiratory activities and body movements during sleep (26–28), vital signs of patients in COVID-19 isolation wards (25), and cardiac activity of infants in NICU settings (24). Additionally, monitoring health status using non-contact sensors can alleviate the excessive workload of the nursing staff, and real-time monitoring can help the hospital personnel detect the risk to patients in the ward at any time (25).
Of the five included studies (24–28), four (24–26, 28) used radar-based non-contact sensors, and one (27) used an infrared sensor and a microwave sensor to detect body movement and parameters related to cardiac and respiratory activities. Radar sensors detect the position and velocity of an object by analyzing the signals that radio waves reach and then reflect (36). Infrared sensors determine the presence of an object by detecting the infrared radiation generated by the heat emitted by the object (37). The energy level of the infrared light changes depending on the temperature of the object, allowing it to detect changes in the temperature or position of the object (37, 38). Microwave sensors detect movement by emitting microwaves and receiving signals reflected from objects (39). The operating principles of radar sensors and microwave sensors are rather similar, and when radar sensors and microwave sensors are combined, they are classified as microwave radar sensors (39). Radar and infrared sensors are commonly used as non-contact sensors for monitoring health status, and this technology is becoming more sophisticated with the introduction of algorithms for accurate signal processing and analysis (24). However, the health status that can be monitored using non-contact sensors is limited to vital signs such as HR, RR, blood pressure, body temperature, and oxygen saturation. Blood glucose is often cited as a major concern in health-conscious people (40, 41). The most widely used device for measuring blood glucose is continuous glucose monitoring (CGM), which is an invasive method for checking blood glucose levels by inserting a needle into the skin (11). Although CGM is known to help manage blood glucose profiles, it has been reported to be underutilized by people with diabetes, citing fear of invasive equipment and the hassle of wearing the devices (42, 43). Recently, various sensors such as colorimetric and fluorescent sensors have been developed as wearable sensors for health monitoring (44). Colorimetric and fluorescence sensors can be used to collect and analyze various biochemical information, such as glucose or chloride ion concentration (45–47). The development of a device that can analyze the composition of sweat using non-contact sensors is expected to provide significant benefits for blood glucose management in health-conscious individuals or patients with diabetes. Furthermore, the development of technologies capable of accurately recognizing sleep positions has recently gained interest (48). Movements of the body during sleep are closely correlated with sleep quality and health outcomes (e.g., sleep apnea or sudden infant death syndrome), making them an important factor in health monitoring (49, 50). In 2022, Islam et al. (51) developed a technology using a microwave Doppler radar that could accurately measure cardiopulmonary movement patterns in three sleep positions: supine, side, and prone. Applying this technology in hospital settings, such as the NICU or wards for patients with sleep disorders, could enable the immediate detection of potentially risky situations during sleep, allowing for prompt early intervention.
With the growing interest in non-contact sensors for health monitoring, related technologies are constantly being developed. Many previous studies have reported that non-contact sensors provide more accurate measurements than standard laboratory methods for measuring vital signs (52–54). However, non-contact sensors are not widely used in clinical practice. Despite the abundance of evidence supporting their superior performance, non-contact sensors are yet to become commercially available for several reasons.
First, if the results of a standard measurement tool, which serves as a benchmark for evaluating the accuracy of non-contact sensors, are deemed unreliable, there is a potential risk of misinterpreting the performance of the non-contact sensor. One example is the controversial reliability of PSG, which is the standard method for determining sleep disorders. In 2008, Levendowski et al. (55) reported that high night-to-night variability in OSA could compromise the reliability of PSG results. In 2022, Lee et al. (56) conducted a meta-analysis to evaluate the inter-rater reliability of manual scoring of sleep stages using PSG results. Pooling results from 11 articles, they reported that inter-rater reliability was high for wake (stage W) and REM sleep (stage R), “moderate” for moderate sleep (stage N2) and deep sleep (stage N3), and “fair” for light sleep (stage N1). Overall, the results of the PSG were considered reliable, but the results were poor at certain stages, suggesting that validity needs improvement. These issues should be considered when interpreting the results and evaluating the performance of non-contact sensors.
Second, the measurement performance of non-contact sensors using PPG can vary depending on skin type, sex, or surrounding environment. In 2020, Nowara et al. (57) reported that dark skin types significantly affected the PPG sensor measurement results, with women tending to have a slightly lower measurement accuracy. In 2023, Zhao et al. (58) reported that the non-isothermal nature of the human body can cause discrepancies in readings when non-contact infrared sensors measure the body temperature at a location different from that of a reference device. In addition, blood flow or skin thickness at a particular measurement site can have an impact and environmental factors such as ambient temperature or humidity can act as confounding variables (58, 59). Studies reporting the performance of non-contact sensors have focused on small populations and not on multicenter and multicultural populations. Furthermore, these studies were not designed to consider all human characteristics. Future studies should evaluate the performance of non-contact sensors in diverse healthcare settings and individuals with different characteristics to produce generalizable results.
Third, separating cardiac and respiratory signals perfectly remains a significant challenge. As the amplitude of the heartbeat signal is smaller than that of the respiratory signal, it can be easily distorted by the harmonics of the respiratory signal, necessitating advanced techniques to clearly distinguish between cardiac and respiratory signals (60). In 2023, Uddin et al. (61) developed a system using a microwave Doppler radar that can classify normal breathing, apnea, and hypopnea patterns through HRV-based feature extraction. HRV refers to the variation in time intervals between consecutive heartbeats, and the HF and LF components of HRV exhibit significant changes under different breathing patterns (61). Therefore, future research should consider using HRV as a key biomarker to advance the technologies for separating cardiac and respiratory signals. Another approach is to use ultra-wide band (UWB) sensors. Several previous studies demonstrated that UWB is highly resistant to multipath effects, making it excellent for separating cardiopulmonary signals (62–64).
Fourth, the technology for processing the signals caused by random body movements (RBM) has limitations. During sleep, the signals generated by unexpected RBM are much larger than those obtained from regular chest movements, which can obscure the respiratory signals (65). Previous studies set the maximum amplitude and normal breathing rate standards for respiratory signals based on data collected over a period of time from participants in a static position after falling asleep. Sudden increases in amplitude beyond these standards were classified as unexpected RBM, and signals from these movements were excluded from the analysis (65, 66). However, this signal-processing method is difficult to apply in clinical settings. In the technology development stage, biosignals are generally measured and verified within a defined range of movements of healthy volunteers. However, in clinical settings, patients exhibit various clinical characteristics, including sudden muscle spasms, seizures during sleep, apnea, and hypopnea, making it difficult to predict their sleep behavior patterns. Therefore, future research should consider a wider range of movement characteristics during the development process to enable non-contact sensing technology to learn from diverse output signals. In addition, there is a need for further advancements in techniques to precisely separate the noise caused by movement from respiratory signals.
Finally, concerns regarding privacy violations must be addressed. Sensor devices collect and analyze user biometric information; hence, the risk of violation of personal information cannot be ignored (67). To address this issue, clear and easy-to-understand privacy policies must be published to help users recognize that non-contact sensors pose a low risk of privacy infringement and leakage (68). Additionally, installing non-contact sensors for real-time monitoring increases the risk of users feeling like they are being watched, and they may feel uncomfortable being recorded all day (69). Before applying the device to the user, the personal information to be collected should be disclosed, the monitoring procedures should be explained in detail, and consent must be obtained.
Owing to these technical limitations, current non-contact sensors may be perceived as less reliable. However, in hospital environments, where accurate health monitoring results are expected, a compromise should be sought. We discuss the following trade-off scenario: a large-scale temperature detection system can quickly screen many people, but the accuracy of individual measurements may be lower. In situations where it is crucial to quickly determine the infection status of a large group, even at the expense of individual accuracy, non-contact sensing technology can be adopted. For instance, at the hospital entrance, basic screening and non-contact temperature checks can be used to identify patients with potential COVID-19 symptoms. In this case, minimizing the infection risk holds greater value than achieving a precise diagnosis, thus justifying the use of non-contact sensors. When using non-contact methods to monitor large groups and selectively re-examine those who exceed certain thresholds, several advantages emerge: (1) it significantly reduces the time required compared to measuring each individual with contact-based equipment, (2) it alleviates the excessive workload of healthcare providers, (3) it saves costs related to labor and equipment use, and (4) it alleviates the psychological burden of infection risk for both patients and healthcare providers. By prioritizing infection prevention over absolute reliability, non-contact solutions can be used as the primary health monitoring method to maximize their benefits.
In conclusion, non-contact sensors are expected to be suitable alternatives to contact devices for health monitoring in hospital settings. However, challenges regarding performance enhancement and privacy protection remain unaddressed. In the future, non-contact sensors are expected to overcome these limitations and be widely used for full-cycle health monitoring.
Author contributions
YJC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. GWL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. JSM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MCC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Research Foundation of Korea grant funded by the Korean government (MSIT) (no. RS-2023-00219725).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Downey, CL, Chapman, S, Randell, R, Brown, JM, and Jayne, DG. The impact of continuous versus intermittent vital signs monitoring in hospitals: a systematic review and narrative synthesis. Int J Nurs Stud. (2018) 84:19–27. doi: 10.1016/j.ijnurstu.2018.04.013
2. Jensen-Doss, A, Haimes, EMB, Smith, AM, Lyon, AR, Lewis, CC, Stanick, CF, et al. Monitoring treatment Progress and providing feedback is viewed favorably but rarely used in practice. Admin Pol Ment Health. (2018) 45:48–61. doi: 10.1007/s10488-016-0763-0
3. Becking-Verhaar, FL, Verweij, RPH, de Vries, M, Vermeulen, H, van Goor, H, and Huisman-de Waal, GJ. Continuous vital signs monitoring with a wireless device on a general ward: a survey to explore Nurses' experiences in a post-implementation period. Int J Environ Res Public Health. (2023) 20:5794. doi: 10.3390/ijerph20105794
4. Downey, C, Randell, R, Brown, J, and Jayne, DG. Continuous versus intermittent vital signs monitoring using a wearable, wireless patch in patients admitted to surgical wards: pilot cluster randomized controlled trial. J Med Internet Res. (2018) 20:e10802. doi: 10.2196/10802
5. Subbe, CP, Duller, B, and Bellomo, R. Effect of an automated notification system for deteriorating ward patients on clinical outcomes. Crit Care. (2017) 21:52. doi: 10.1186/s13054-017-1635-z
6. Fang, C, He, B, Wang, Y, Cao, J, and Gao, S. EMG-centered multisensory based technologies for pattern recognition in rehabilitation: state of the art and challenges. Biosensors. (2020) 10:85. doi: 10.3390/bios10080085
7. Stawiarska, E, and Stawiarski, M. Assessment of patient treatment and rehabilitation processes using electromyography signals and selected industry 4.0 solutions. Int J Environ Res Public Health. (2023) 20:3754. doi: 10.3390/ijerph20043754
8. Patel, V, Orchanian-Cheff, A, and Wu, R. Evaluating the validity and utility of wearable Technology for Continuously Monitoring Patients in a hospital setting: systematic review. JMIR Mhealth Uhealth. (2021) 9:e17411. doi: 10.2196/17411
9. Fruytier, LA, Janssen, DM, Campero Jurado, I, van de Sande, DA, Lorato, I, Stuart, S, et al. The utility of a novel electrocardiogram patch using dry electrodes technology for arrhythmia detection during exercise and prolonged monitoring: proof-of-concept study. JMIR Form Res. (2023) 7:e49346. doi: 10.2196/49346
10. Nigusse, AB, Mengistie, DA, Malengier, B, Tseghai, GB, and Langenhove, LV. Wearable smart textiles for long-term electrocardiography monitoring-a review. Sensors. (2021) 21:4174. doi: 10.3390/s21124174
11. Hilliard, ME, Levy, W, Anderson, BJ, Whitehouse, AL, Commissariat, PV, Harrington, KR, et al. Benefits and barriers of continuous glucose monitoring in young children with type 1 diabetes. Diabetes Technol Ther. (2019) 21:493–8. doi: 10.1089/dia.2019.0142
12. Ferguson, C, Hickman, LD, Turkmani, S, Breen, P, Gargiulo, G, and Inglis, SC. "wearables only work on patients that wear them": barriers and facilitators to the adoption of wearable cardiac monitoring technologies. Cardiovasc Digit Health J. (2021) 2:137–47. doi: 10.1016/j.cvdhj.2021.02.001
13. Knutsson, SE, and Bergbom, IL. Custodians' viewpoints and experiences from their child's visit to an ill or injured nearest being cared for at an adult intensive care unit. J Clin Nurs. (2007) 16:362–71. doi: 10.1111/j.1365-2702.2005.01517.x
14. Dzisko, M, Lewandowska, A, and Wudarska, B. Can the standard configuration of a cardiac monitor Lead to medical errors under a stress induction? Sensors. (2022) 22:3536. doi: 10.3390/s22093536
15. Kapoor, M, and Greenough, G. Home sleep tests for obstructive sleep apnea (OSA). J Am Board Fam Med. (2015) 28:504–9. doi: 10.3122/jabfm.2015.04.140266
16. Corman, BHP, Rajupet, S, Ye, F, and Schoenfeld, ER. The role of unobtrusive home-based continuous sensing in the Management of Postacute Sequelae of SARS CoV-2. J Med Internet Res. (2022) 24:e32713. doi: 10.2196/32713
17. Lim, YG, Hong, KH, Kim, KK, Shin, JH, Lee, SM, Chung, GS, et al. Monitoring physiological signals using nonintrusive sensors installed in daily life equipment. Biomed Eng Lett. (2011) 1:11–20. doi: 10.1007/s13534-011-0012-0
18. Manullang, MCT, Lin, YH, Lai, SJ, and Chou, NK. Implementation of thermal camera for non-contact physiological measurement: a systematic review. Sensors. (2021) 21:7777. doi: 10.3390/s21237777
19. Vitazkova, D, Foltan, E, Kosnacova, H, Micjan, M, Donoval, M, Kuzma, A, et al. Advances in respiratory monitoring: a comprehensive review of wearable and remote technologies. Biosensors. (2024) 14:90. doi: 10.3390/bios14020090
20. Islam, SM, Fioranelli, F, and Lubecke, VM. Can radar remote life sensing technology help combat COVID-19? Front Comms Net. (2021) 2:648181. doi: 10.3389/frcmn.2021.648181
21. Michler, F, Shi, K, Schellenberger, S, Steigleder, T, Malessa, A, Hameyer, L, et al. A clinically evaluated interferometric continuous-wave radar system for the contactless measurement of human vital parameters. Sensors. (2019) 19:2492. doi: 10.3390/s19112492
22. He, S, Han, Z, Iglesias, C, Mehta, V, and Bolic, M. A real-time respiration monitoring and classification system using a depth camera and radars. Front Physiol. (2022) 13:799621. doi: 10.3389/fphys.2022.799621
23. Talukdar, D, De Deus, LF, and Sehgal, N. Evaluation of a camera-based monitoring solution against regulated medical devices to measure heart rate, respiratory rate, oxygen saturation, and blood pressure. Cureus. (2022) 14:e31649. doi: 10.7759/cureus.31649
24. Edanami, K, Kurosawa, M, Yen, HT, Kanazawa, T, Abe, Y, Kirimoto, T, et al. Remote sensing of vital signs by medical radar time-series signal using cardiac peak extraction and adaptive peak detection algorithm: performance validation on healthy adults and application to neonatal monitoring at an NICU. Comput Methods Prog Biomed. (2022) 226:107163. doi: 10.1016/j.cmpb.2022.107163
25. Tsai, CY, Chang, NC, Fang, HC, Chen, YC, and Lee, SS. A novel non-contact self-injection-locked radar for vital sign sensing and body movement monitoring in COVID-19 isolation Ward. J Med Syst. (2020) 44:177. doi: 10.1007/s10916-020-01637-z
26. Weinreich, G, Terjung, S, Wang, Y, Werther, S, Zaffaroni, A, and Teschler, H. Validation of a non-contact screening device for the combination of sleep-disordered breathing and periodic limb movements in sleep. Sleep Breath. (2018) 22:131–8. doi: 10.1007/s11325-017-1546-x
27. Yoon, YS, Hahm, J, Kim, KK, Park, SK, and Oh, SW. Non-contact home-adapted device estimates sleep stages in middle-aged men: a preliminary study. Technol Health Care. (2020) 28:439–46. doi: 10.3233/THC-192036
28. Zaffaroni, A, de Chazal, P, Heneghan, C, Boyle, P, Mppm, PR, and McNicholas, WT. SleepMinder: an innovative contact-free device for the estimation of the apnoea-hypopnoea index. Annu Int Conf IEEE Eng Med Biol Soc. (2009) 2009:7091–4. doi: 10.1109/IEMBS.2009.5332909
29. De Chazal, P, Fox, N, O'Hare, E, Heneghan, C, Zaffaroni, A, Boyle, P, et al. Sleep/wake measurement using a non-contact biomotion sensor. J Sleep Res. (2011) 20:356–66. doi: 10.1111/j.1365-2869.2010.00876.x
30. Ballal, T, Heneghan, C, Zaffaroni, A, Boyle, P, de Chazal, P, Shouldice, R, et al. A pilot study of the nocturnal respiration rates in COPD patients in the home environment using a non-contact biomotion sensor. Physiol Meas. (2014) 35:2513–27. doi: 10.1088/0967-3334/35/12/2513
31. Rundo, JV, and Downey, R 3rd. Polysomnography. Handb Clin Neurol. (2019) 160:381–92. doi: 10.1016/B978-0-444-64032-1.00025-4
32. Shamim-Uzzaman, Q, Singh, S, and Chowdhuri, S. Hypopnea definitions, determinants and dilemmas: a focused review. Sleep Sci Pract. (2018) 2:7. doi: 10.1186/s41606-018-0023-1
33. Wang, FK, Tang, MC, Su, SC, and Horng, TS. Wrist pulse rate monitor using self-injection-locked radar technology. Biosensors. (2016) 6:54. doi: 10.3390/bios6040054
34. Wang, FK, Wu, CTM, Horng, TS, Tseng, CH, Yu, SH, Chang, CC, et al. Review of self-injection-locked radar systems for non-contact detection of vital signs. IEEE J Electromagn RF Microw Med Biol. (2020) 4:294–307. doi: 10.1109/JERM.2020.2994821
35. Wang, FK, Li, CJ, Hsiao, CH, Horng, TS, Lin, J, Peng, KC, et al. A novel vital-sign sensor based on a self-injection-locked oscillator. IEEE Trans Microw Theory Techn. (2010) 58:4112–20. doi: 10.1109/TMTT.2010.2087349
36. Ogunrinde, I, and Bernadin, S. Deep camera-radar fusion with an attention framework for autonomous vehicle vision in foggy weather conditions. Sensors. (2023) 23:6255. doi: 10.3390/s23146255
37. Politi, S, Aloisi, A Jr, Bartoli, V, Guglietta, A, and Magnifica, F. Infrared thermography images acquisition for a technical perspective in screening and diagnostic processes: protocol standardized acquisition. Cureus. (2021) 13:e19931. doi: 10.7759/cureus.19931
38. Zheng, S, Zhou, C, Jiang, X, Huang, J, and Xu, D. Progress on infrared imaging technology in animal production: a review. Sensors. (2022) 22:705. doi: 10.3390/s22030705
39. Thi Phuoc Van, N, Tang, L, Demir, V, Hasan, SF, Duc Minh, N, and Mukhopadhyay, S. Review-microwave radar sensing systems for search and rescue purposes. Sensors. (2019) 19:2879. doi: 10.3390/s19132879
40. Kesavadev, J, Misra, A, Saboo, B, Aravind, SR, Hussain, A, Czupryniak, L, et al. Blood glucose levels should be considered as a new vital sign indicative of prognosis during hospitalization. Diabetes Metab Syndr. (2021) 15:221–7. doi: 10.1016/j.dsx.2020.12.032
41. Kuwahara, K, Yamamoto, S, Honda, T, Nakagawa, T, Ishikawa, H, Hayashi, T, et al. Improving and maintaining healthy lifestyles are associated with a lower risk of diabetes: a large cohort study. J Diabetes Investig. (2022) 13:714–24. doi: 10.1111/jdi.13713
42. Divan, V, Greenfield, M, Morley, CP, and Weinstock, RS. Perceived burdens and benefits associated with continuous glucose monitor use in type 1 diabetes across the lifespan. J Diabetes Sci Technol. (2022) 16:88–96. doi: 10.1177/1932296820978769
43. Tanenbaum, ML, Hanes, SJ, Miller, KM, Naranjo, D, Bensen, R, and Hood, KK. Diabetes device use in adults with type 1 diabetes: barriers to uptake and potential intervention targets. Diabetes Care. (2017) 40:181–7. doi: 10.2337/dc16-1536
44. Kaur, B, Kumar, S, and Kaushik, BK. Novel wearable optical sensors for vital health monitoring systems-a review. Biosensors. (2023) 13:181. doi: 10.3390/bios13020181
45. Ardalan, S, Hosseinifard, M, Vosough, M, and Golmohammadi, H. Towards smart personalized perspiration analysis: an IoT-integrated cellulose-based microfluidic wearable patch for smartphone fluorimetric multi-sensing of sweat biomarkers. Biosens Bioelectron. (2020) 168:112450. doi: 10.1016/j.bios.2020.112450
46. Choi, J, Bandodkar, AJ, Reeder, JT, Ray, TR, Turnquist, A, Kim, SB, et al. Soft, skin-integrated multifunctional microfluidic systems for accurate colorimetric analysis of sweat biomarkers and temperature. ACS Sens. (2019) 4:379–88. doi: 10.1021/acssensors.8b01218
47. Kim, SB, Zhang, Y, Won, SM, Bandodkar, AJ, Sekine, Y, Xue, Y, et al. Super-absorbent polymer valves and colorimetric chemistries for time-sequenced discrete sampling and chloride analysis of sweat via skin-mounted soft microfluidics. Small. (2018) 14:e1703334. doi: 10.1002/smll.201703334
48. Islam, SMM. Radar-based remote physiological sensing: progress, challenges, and opportunities. Front Physiol. (2022) 13:955208. doi: 10.3389/fphys.2022.955208
49. Oksenberg, A, and Silverberg, DS. Avoiding the supine posture during sleep for patients with mild obstructive sleep apnea. Am J Respir Crit Care Med. (2009) 180:101. doi: 10.1164/ajrccm.180.1.101
50. Goldberg, N, Rodriguez-Prado, Y, Tillery, R, and Chua, C. Sudden infant death syndrome: a review. Pediatr Ann. (2018) 47:e118–23. doi: 10.3928/19382359-20180221-03
51. Islam, SMM, and Lubecke, VM. Sleep posture recognition with a dual-frequency microwave doppler radar and machine learning classifiers. IEEE Sens Lett. (2022) 6:1–4. doi: 10.1109/LSENS.2022.3148378
52. Kagiyama, N, Hiki, M, Matsue, Y, Dohi, T, Matsuzawa, W, Daida, H, et al. Validation of telemedicine-based self-assessment of vital signs for patients with COVID-19: a pilot study. J Telemed Telecare. (2023) 29:600–6. doi: 10.1177/1357633X211011825
53. Crinion, SJ, Tiron, R, Lyon, G, Zaffaroni, A, Kilroy, H, Doheny, E, et al. Ambulatory detection of sleep apnea using a non-contact biomotion sensor. J Sleep Res. (2020) 29:e12889. doi: 10.1111/jsr.12889
54. Harrington, N, Bui, QM, Wei, Z, Hernandez-Pacheco, B, DeYoung, PN, Wassell, A, et al. Passive longitudinal weight and cardiopulmonary monitoring in the home bed. Sci Rep. (2021) 11:24376. doi: 10.1038/s41598-021-03105-1
55. Levendowski, DJ, Zack, N, Rao, S, Wong, K, Gendreau, M, Kranzler, J, et al. Assessment of the test-retest reliability of laboratory polysomnography. Sleep Breath. (2009) 13:163–7. doi: 10.1007/s11325-008-0214-6
56. Lee, YJ, Lee, JY, Cho, JH, and Choi, JH. Interrater reliability of sleep stage scoring: a meta-analysis. J Clin Sleep Med. (2022) 18:193–202. doi: 10.5664/jcsm.9538
57. Nowara, EM, McDuff, D, and Veeraraghavan, A. A meta-analysis of the impact of skin tone and gender on non-contact photoplethysmography measurements. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition Workshops. Vancouver, BC: IEEE. (2020) 284–285.
58. Zhao, Y, and Bergmann, JHM. Non-contact infrared thermometers and thermal scanners for human body temperature monitoring: a systematic review. Sensors. (2023) 23:7439. doi: 10.3390/s23177439
59. Foster, J, Lloyd, AB, and Havenith, G. Non-contact infrared assessment of human body temperature: the journal temperature toolbox. Temperature. (2021) 8:306–19. doi: 10.1080/23328940.2021.1899546
60. Kebe, M, Gadhafi, R, Mohammad, B, Sanduleanu, M, Saleh, H, and Al-Qutayri, M. Human vital signs detection methods and potential using radars: a review. Sensors. (2020) 20:1454. doi: 10.3390/s20051454
61. Uddin, SD, Hossain, MS, and Islam, SM. Heart rate variability-based obstructive sleep apnea events classification using microwave Doppler radar. IEEE J Electromagn RF Microw Med Biol. (2023) 7:416–24. doi: 10.1109/JERM.2023.3317304
62. Islam, SM, Boric-Lubecke, O, Lubecke, VM, Moadi, AK, and Fathy, AE. Contactless radar-based sensors: recent advances in vital-signs monitoring of multiple subjects. IEEE Microw Mag. (2022) 23:47–60. doi: 10.1109/MMM.2022.3140849
63. Li, C, Lubecke, VM, Boric-Lubecke, O, and Lin, J. A review on recent advances in Doppler radar sensors for noncontact healthcare monitoring. IEEE Trans Microw Theory Techn. (2013) 61:2046–60. doi: 10.1109/TMTT.2013.2256924
64. Xie, Z, Zhou, B, Cheng, X, Schoenfeld, E, and Ye, F. Passive and context-aware in-home vital signs monitoring using co-located uwb-depth sensor fusion. ACM Trans Comput Healthc. (2022) 3:1–31. doi: 10.1145/3549941
65. Baboli, M, Singh, A, Soll, B, Boric-Lubecke, O, and Lubecke, VM. Wireless sleep apnea detection using continuous wave quadrature Doppler radar. IEEE Sensors J. (2019) 20:538–45. doi: 10.1109/JSEN.2019.2941198
66. Islam, SM, Rahman, A, Yavari, E, Baboli, M, Boric-Lubecke, O, and Lubecke, VM. Identity authentication of OSA patients using microwave Doppler radar and machine learning classifiers. In 2020 IEEE radio and wireless symposium (RWS). IEEE: San Antonio, TX, USA. (2020) 251–254.
67. Pato, JN, and Millett, LINational Research Council (US) Whither Biometrics Committee. Cultural, social, and legal considerations In: LI Millett, editor. Biometric recognition: challenges and opportunities. Washington, DC: National Academies Press (2010)
68. Dery, L, and Jelnov, A. Privacy-accuracy consideration in devices that collect sensor-based information. Sensors. (2021) 21:4684. doi: 10.3390/s21144684
Keywords: health monitoring, non-contact, sensor, hospital, review
Citation: Choo YJ, Lee GW, Moon JS and Chang MC (2024) Application of non-contact sensors for health monitoring in hospitals: a narrative review. Front. Med. 11:1421901. doi: 10.3389/fmed.2024.1421901
Edited by:
Shaoxiong Sun, The University of Sheffield, United KingdomReviewed by:
Shekh Md Mahmudul Islam, University of Dhaka, BangladeshYu Zhao, Bohai University, China
Yichao Yuan, Rutgers University, United States
Copyright © 2024 Choo, Lee, Moon and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Sung Moon, bWpzNzkxMkB5dS5hYy5rcg==; Min Cheol Chang, d2hlZWw2MzNAZ21haWwuY29t
†These authors have contributed equally to this work and share first authorship
 Yoo Jin Choo
Yoo Jin Choo Gun Woo Lee
Gun Woo Lee Jun Sung Moon
Jun Sung Moon Min Cheol Chang
Min Cheol Chang