- Department of Endocrinology, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, China
Introduction: Previous studies have demonstrated a correlation between the ratio of alanine aminotransferase to high-density lipoprotein cholesterol (ALT/HDL-C) in the serum and the risk of diabetes. However, no existing study has investigated the association between insulin resistance (IR) and ALT/HDL-C. Therefore, this study aims to explore the association between ALT/HDL-C and IR in American adults.
Methods: A total of 7,599 adults selected from the National Health and Nutrition Examination Survey (NHANES) in 2013 to 2020 were studied. IR was assessed based on the homeostatic model assessment of insulin resistance (HOMA-IR). And the association between IR and ALT/HDL-C was assessed through multiple logistic regression, generalized smooth curve fitting and subgroup analyses.
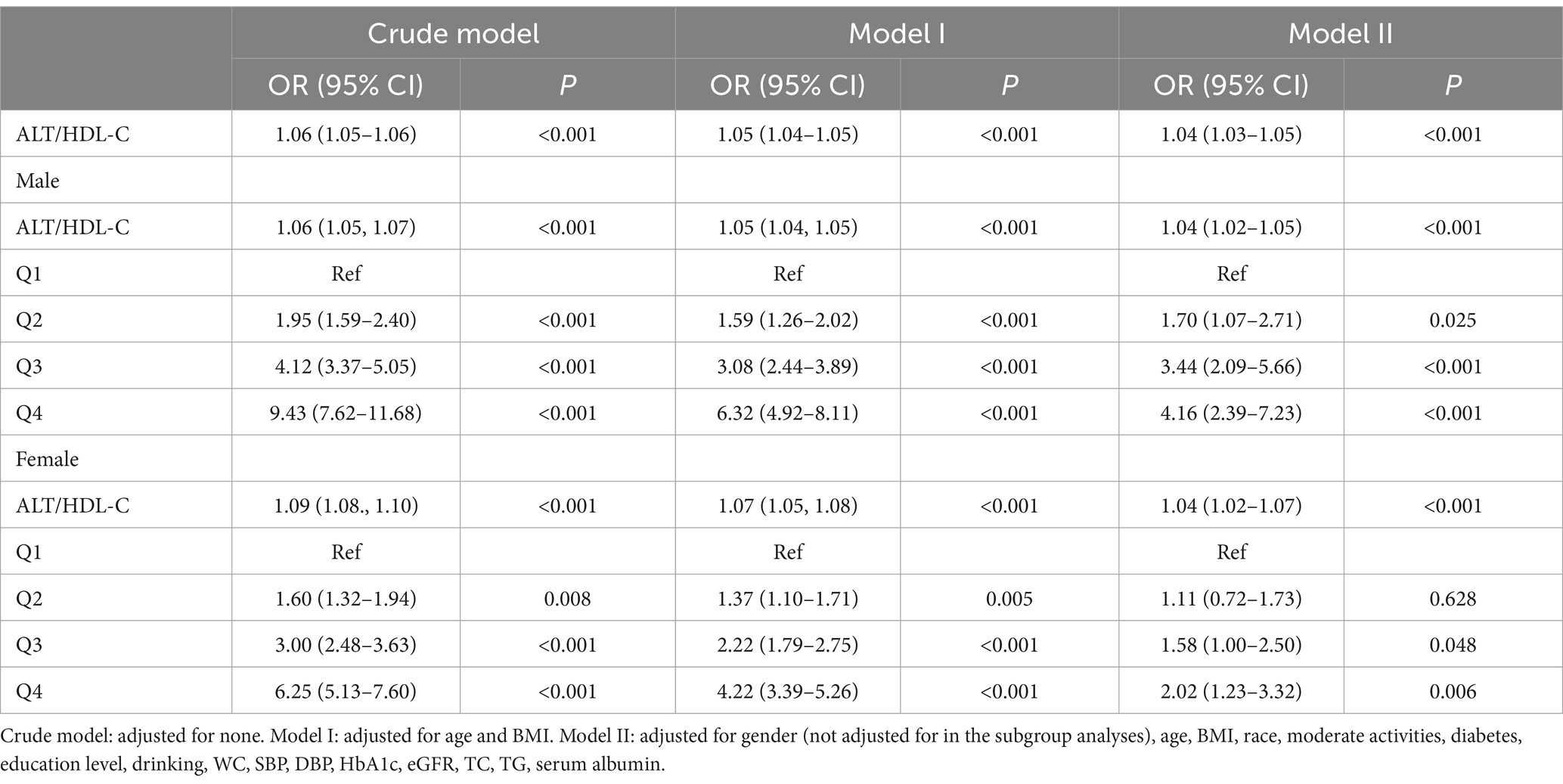
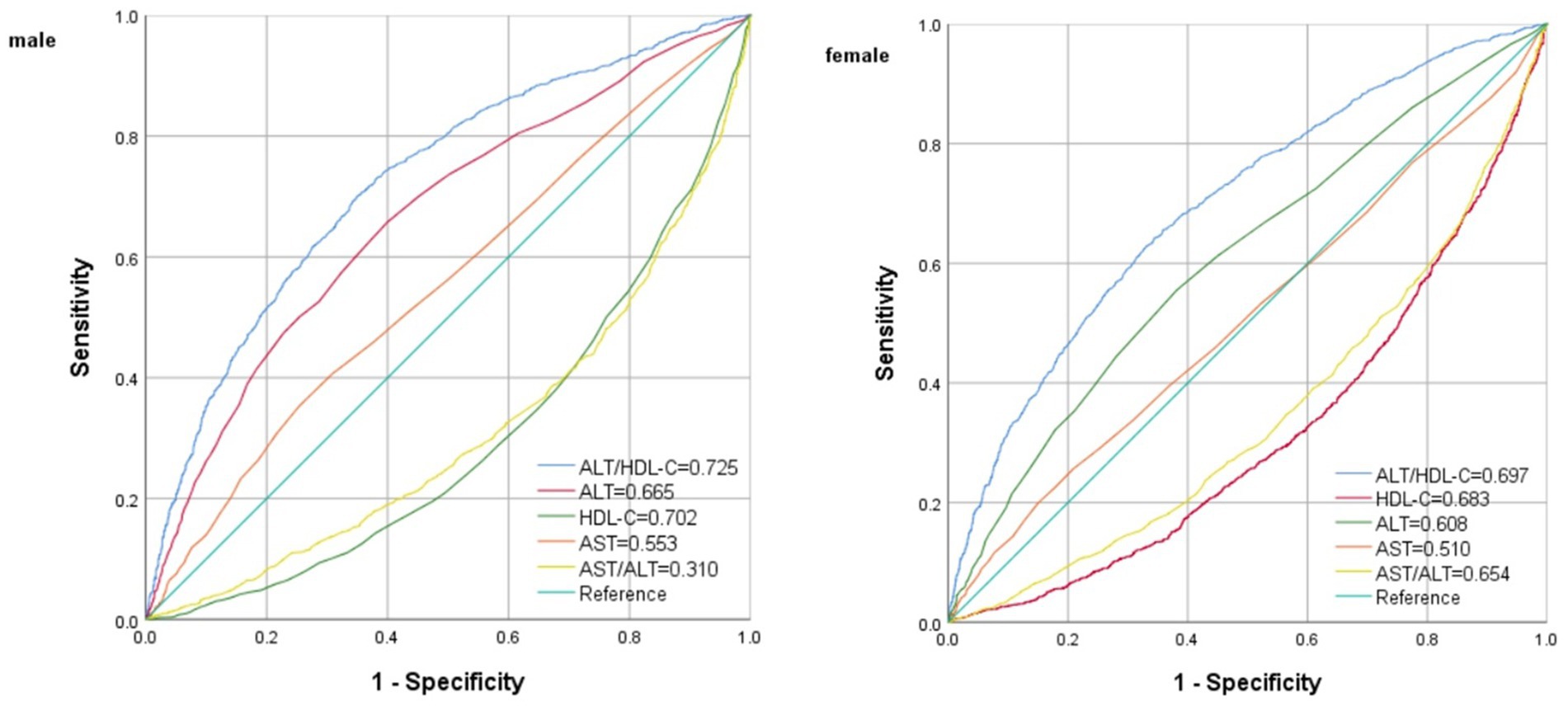
Results: Multiple logistic regression analysis indicated a significant correlation between IR and ALT/HDL-C, with odds ratios (OR) of 1.04 (95% CI = 1.02–1.05) in males and 1.04 (95% CI = 1.02–1.07) in females. A non-linear association and saturation effect between ALT/HDL-C and IR risk were identified, with an inverted L shaped curve and an inflection point at 33.62. The area under the ROC curve (AUC) of ALT/HDL-C was significantly larger (AUC = 0.725 for males and 0.696 for females, all p < 0.01) compared with the use of ALT, HDL-C, AST and AST/ALT. Subgroup analysis showed a significantly higher independent association in obese individuals and individuals aged ≥50 years (All P interaction <0.05).
Conclusion: Elevated ALT/HDL-C demonstrates a significant correlation with IR, which can be used as a potential indicator of IR in American adults.
Introduction
IR is widely recognized as a significant contributing factor in various pathological conditions, including diabetes, atherosclerosis, hypertension and metabolic syndrome (MetS). Therefore, the accurate measurement on IR is of utmost importance. The hyperinsulinemic-euglycemic clamp is considered as the gold standard for IR. However, its routine clinical application is hindered by issues related to replicability, cost, accessibility and reproducibility (1–5). As an alternative, HOMA-IR is considered as an index widely used in adults (6). Although HOMA-IR is commonly adopted in adults, its reliance on fasting plasma insulin measurements poses challenges within clinical settings. Consequently, there is a demand for a diagnostic test with accuracy, cost-effectiveness and simplicity in predicting IR.
ALT is commonly considered as an epidemiological marker for NAFLD, which is associated with an increased risk of developing diabetes (7). Furthermore, there is evidence suggesting that elevated ALT levels are linked to hepatic IR, potentially contributing to the onset of diabetes (8). Additionally, decreased HDL-C levels have been implicated in the pathogenesis of IR and MetS (9–13). Recently, Cao et al. conducted a study investigating the combination of HDL-C and ALT, and the findings suggest that the ALT/HDL-C ratio serves as a valuable novel predictor for the risk in the development of diabetes in Japanese (14). He et al. conducted a study on a total of 116,251 Chinese people and found a positive correlation between the risk in the development of diabetes and the ALT/HDL-C (15). However, the existence of an association between IR risk and ALT/HDL-C remains unclear. In order to explore this hypothesis, the current study sought to analyze the correlation between ALT/HDL-C and IR through a substantial sample of American adults derived from NHANES.
Methods
Research subjects
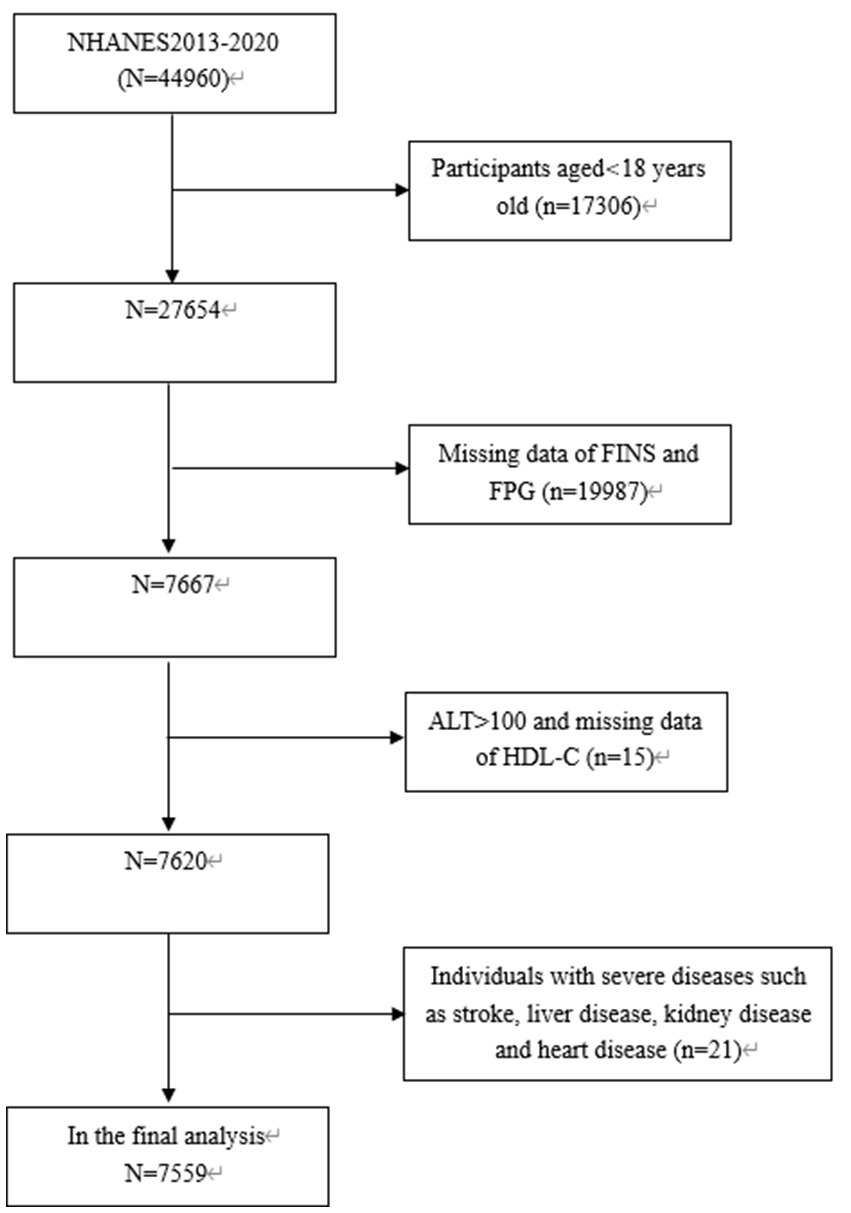
The data analyzed in this study were obtained from NHANES (2013–2020), with a stratified, multi-stage probability and complex sample of the uninstituted population in America. The cross-sectional surveys were conducted by NCHS. Further information regarding NHANES methods can be accessed at www.cdc.gov/nchs/NHANEs/.
The study focused exclusively on subjects aged 18 years old and above (n = 27,654), among which, 20,055 subjects were eliminated: (1) Those with missing data on fasting insulin (FINS), ALT, HDL-C or FPG; (2) Those with ALT levels exceeding 100 IU/L, such as elevated levels are predominantly indicative of liver damage resulting from different forms of acute and chronic hepatitis; (3) Those with severe diseases such as stroke, heart disease, kidney disease and inflammatory disease. Consequently, the final analysis involved 7,599 subjects aged 18–80 years old (Figure 1).
The implementation of NHANES was granted approval by NCHS Ethics Review Board, and all subjects provided written informed consent (16).
Anthropometric measurements
The following data were collected at admission, such as history of diabetes, alcohol intake, race, physical activity, education and physical measurements, including weight, waist circumference, height and blood pressure. Subjects who were obesity were defined as BMI ≥ 30 kg/m2, those with normal weight or overweight were defined as BMI <30 kg/m2.
TC, HbA1c, LDL-C, FINS, UA, FPG, triglycerides (TG), ALT, creatinine, AST, albumin and HDL-C were collected in blood samples. Less than 3% of values missed in total. Multiple imputation was performed for missing values. eGFR was estimated with the Modification of Diet in Renal Diseases (2). The detailed measuring method and acquisition process of each variable are available at www.cdc.gov/nchs/nhanes.
Assessment of IR
IR was assessed through the HOMA-IR formula, and HOMA-IR was calculated as multiplied FPG (mmol/L) by FINS (IU/L) divided by 22.5 (2). IR was defined as HOMA-IR values equal to or greater than 2.69 (17, 18).
Statistical analysis
It was worth noting that there were gender disparities in ALT, HDL, and ALT/HDL-C, and separate analyses were necessary for males and females. The assessment on normality for continuous variables involved expressing them as either median and interquartile range or mean ± standard deviation. In order to evaluate the differences between the two groups, t-test or Mann–Whitney U test was adopted for continuous variables, and chi-square tests were adopted for categorical variables. Furthermore, the association between ALT/HDL-C and metabolic risk factors was explored through Spearmen’s correlation. The subjects were divided into groups based on their ALT/HDL-C levels (≤12.94, 12.94–18.68, 18.68–16.08, ≥28.08 in the male group, ≤8.37, 8.37–11.43, 11.43–16.33, ≥16.33 in the female group). Variables demonstrating clinical significance and statistical significance in the univariate analysis (p < 0.05) were incorporated into the multivariate analyses. The association between ALT/HDL-C quartiles and the presence of IR was assessed with binary logistic regression models. In Model 1, no covariate was adjusted; In Model 2, adjustment was made for BMI and age. Based on Model 2, the race, moderate activities, diabetes, SBP, education level, WC, drinking, TC, HbA1c, DBP, serum albumin, eGFR, TG were added as covariates to Model 3. Subgroup analyses stratified by BMI (<30 kg/m2 and ≥ 30 kg/m2), gender (male and female), diabetes (yes and no), age (<50 and ≥ 50 years), moderate activities (yes and no), education level (high school or above and less than high school) and drinking (yes and no) were conducted (19–22). To examine the potential effect modification within subgroups, interaction terms were employed between subgroup indicators, followed by likelihood ratio tests. In order to identify potential nonlinear relationship between IR probabilities and ALT/HDL-C, generalized smooth curve fitting were adopted. ROC curve analyses were performed to evaluate the diagnostic efficacy of ALT/HDL-C in detecting IR. The statistical analysis was conducted with EmpowerStats software and R, with significance at p < 0.05.
Results
Characteristics of participants
As shown in Table 1, the prevalence of IR reached 46.0% in both genders. HOMA-IR, age, proportion individuals with diabetes, WC, BMI, SBP, DBP, HbA1c, FPG, FINS, TG, uric acid, ALT and ALT/HDL-C levels were all higher in IR subjects than those in non-IR subjects with both genders (p < 0.001). Furthermore, the proportion of moderate activities and HDL-C levels were lower in IR subjects than those in non-IR subjects with both genders.
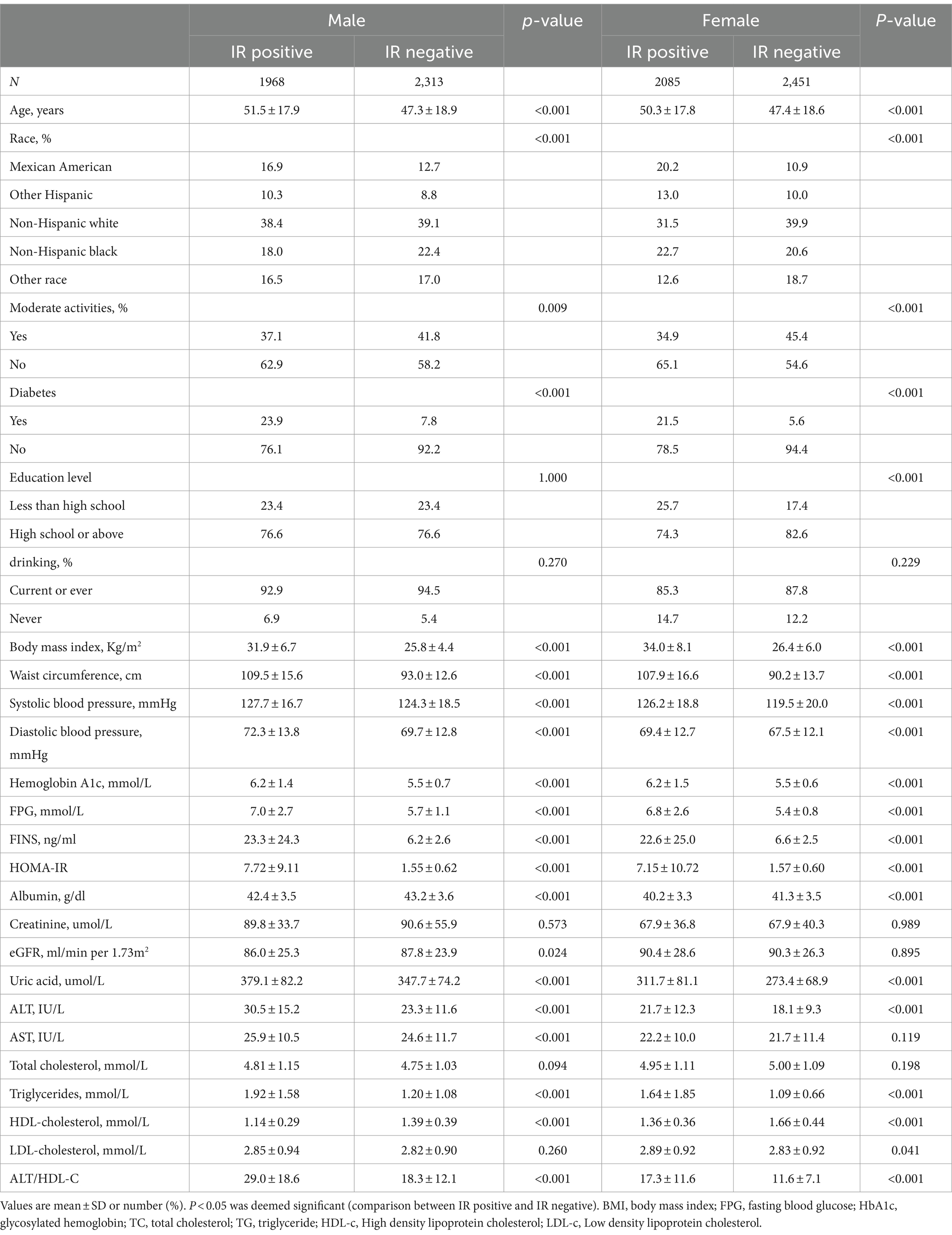
Table 1. Baseline characteristics of the study population stratified by insulin resistance and gender.
Correlation between clinical parameters and Alt/HDL-C
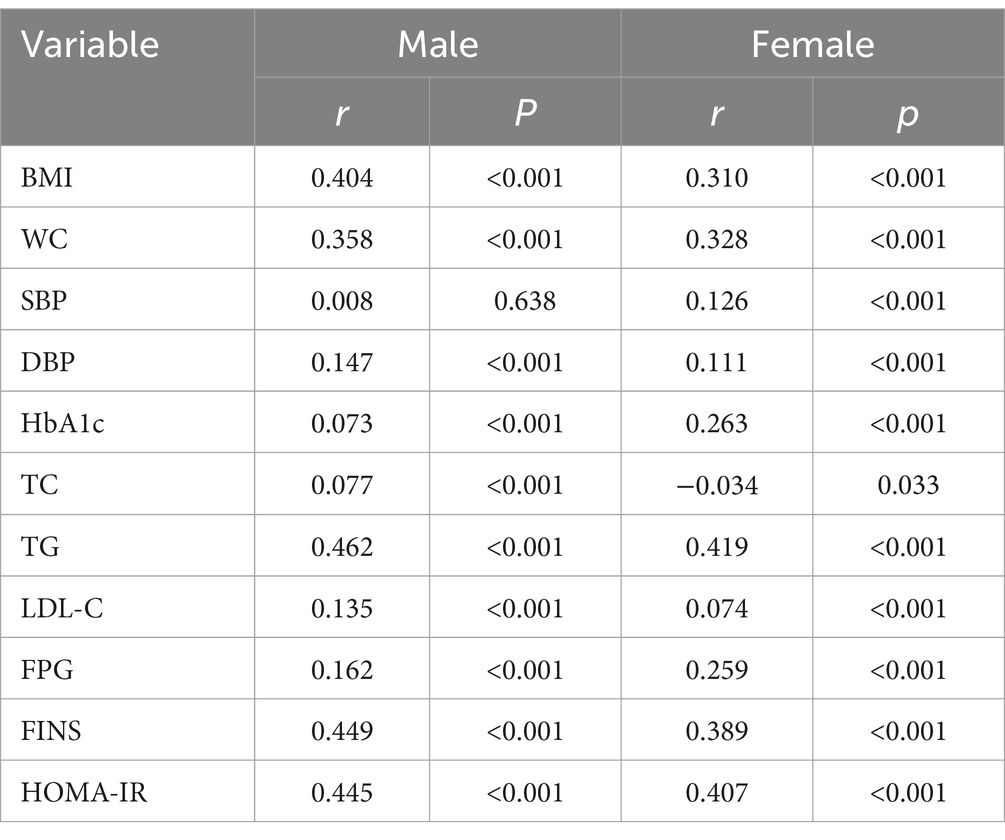
The correlation between metabolic parameters and ALT/HDL-C was analyzed with Spearman’s correlation and the results are shown in Table 2. The analysis revealed positive correlation between ALT/HDL-C and BMI, HbA1c, WC, TG, FPG, DBP, FINS, LDL-C, HOMA-IR in all subjects.
Correlation between IR and Alt/HDL-C
Table 3 shows binary logistic analyses for the correlation between ALT/HDL-C quartiles with IR in subjects. In the unadjusted model, ALT/HDL-C was positively correlated with IR (OR = 1.06 in males and 1.09 in females). The association still existed in Model 2 (OR = 1.05 in males and 1.07 in females) and Model 3 (OR = 1.04 in both genders). In order to further investigate the association between IR and ALT/HDL-C, smooth curve fittings and generalized additive model were adopted (Table 4 and Figure 2). Among all subjects, the correlation between ALT/HDL-C and IR risk exhibited an inverted L-shaped curve, with inflection points at 33.62.
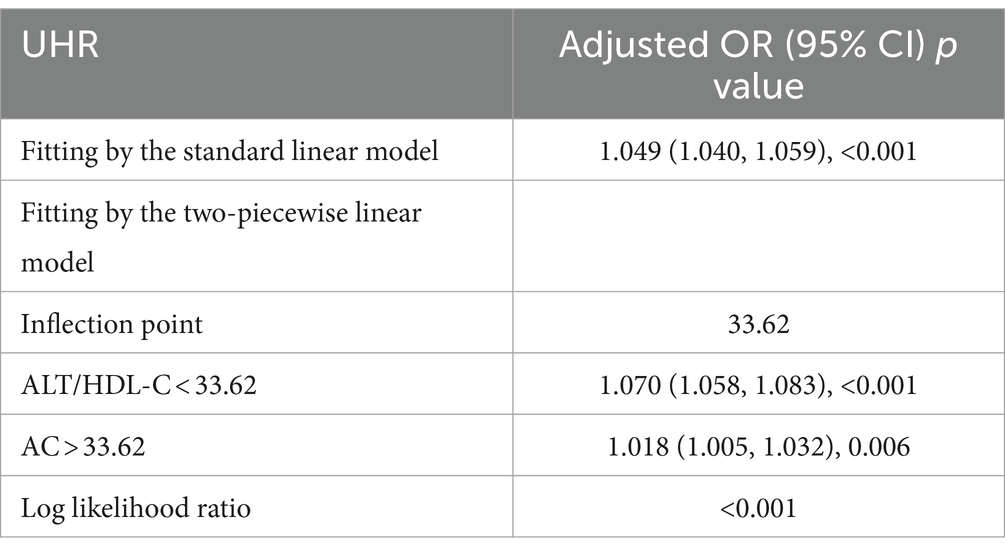
Table 4. Threshold effect analysis of ALT/HDL-C on IR using the two-piecewise linear regression model.
Subgroup analysis on the correlation between IR and Alt/HDL-C
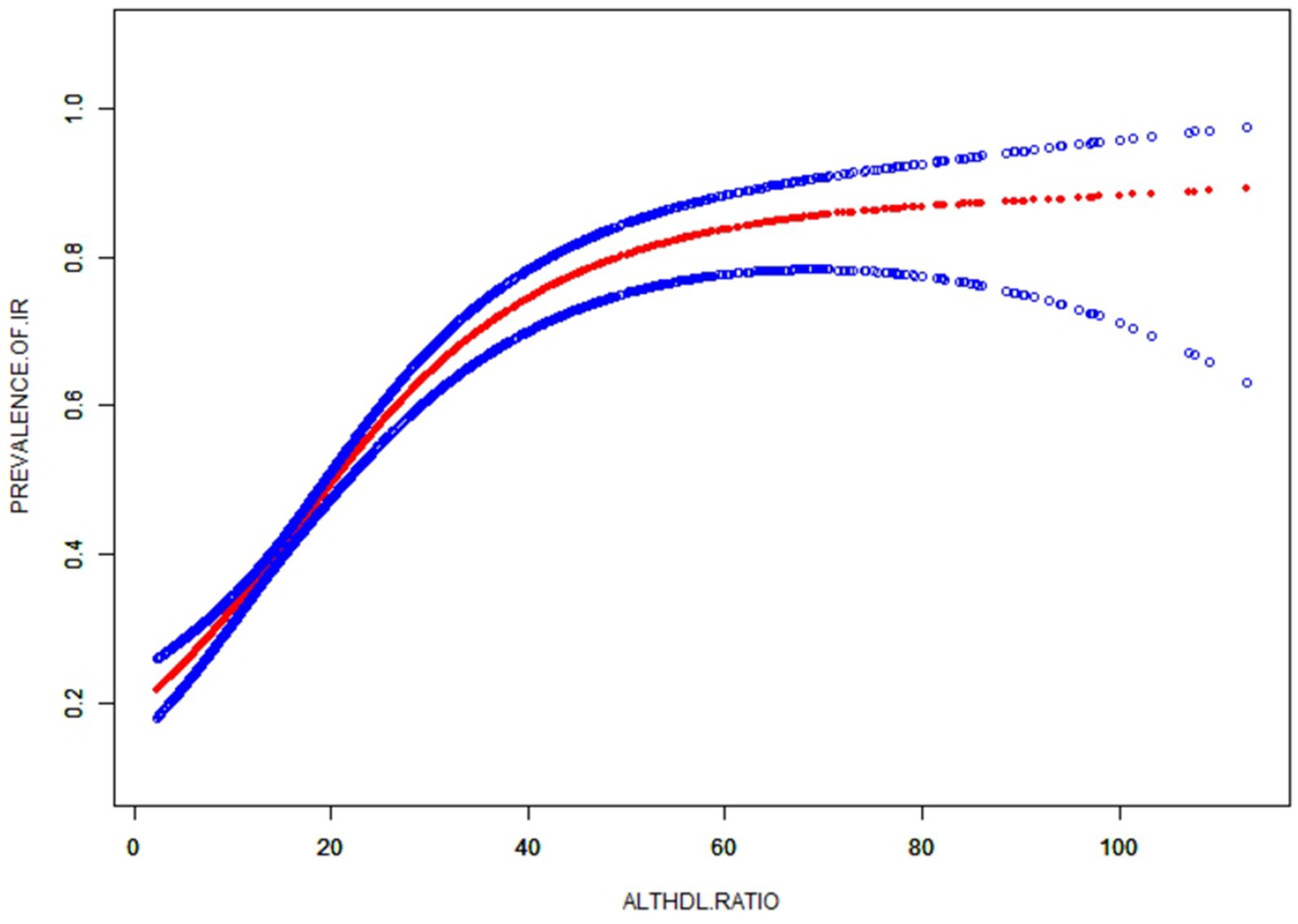
Subgroup analysis was conducted based on age, diabetes, sex, BMI, moderate activities and education level to investigate the association between ALT/HDL-C and the risk of IR in various commonly categorized populations. The findings indicate that there are significant statistical differences (p < 0.05) in the association between the ALT/HDL-C ratio and the risk of IR across various age and BMI groups. According to Table 5, the likelihood of developing IR exhibits notable disparities across distinct age groups, with individuals aged 50 and above displaying a heightened risk in comparison to those at the age < 50 (p = 0.003). In the subgroup analysis based on BMI, it was found that obese subjects have a higher risk of IR associated with the ALT/HDL-C ratio compared to non-obese subjects (OR: non-obese 1.03 VS obese 1.06; p = 0.044). Furthermore, upon employing smooth curve fittings to characterize the non-linear association, it was observed that the positive correlation between IR and ALT/HDL-C levels persisted in the majority of groups (Figure 3).
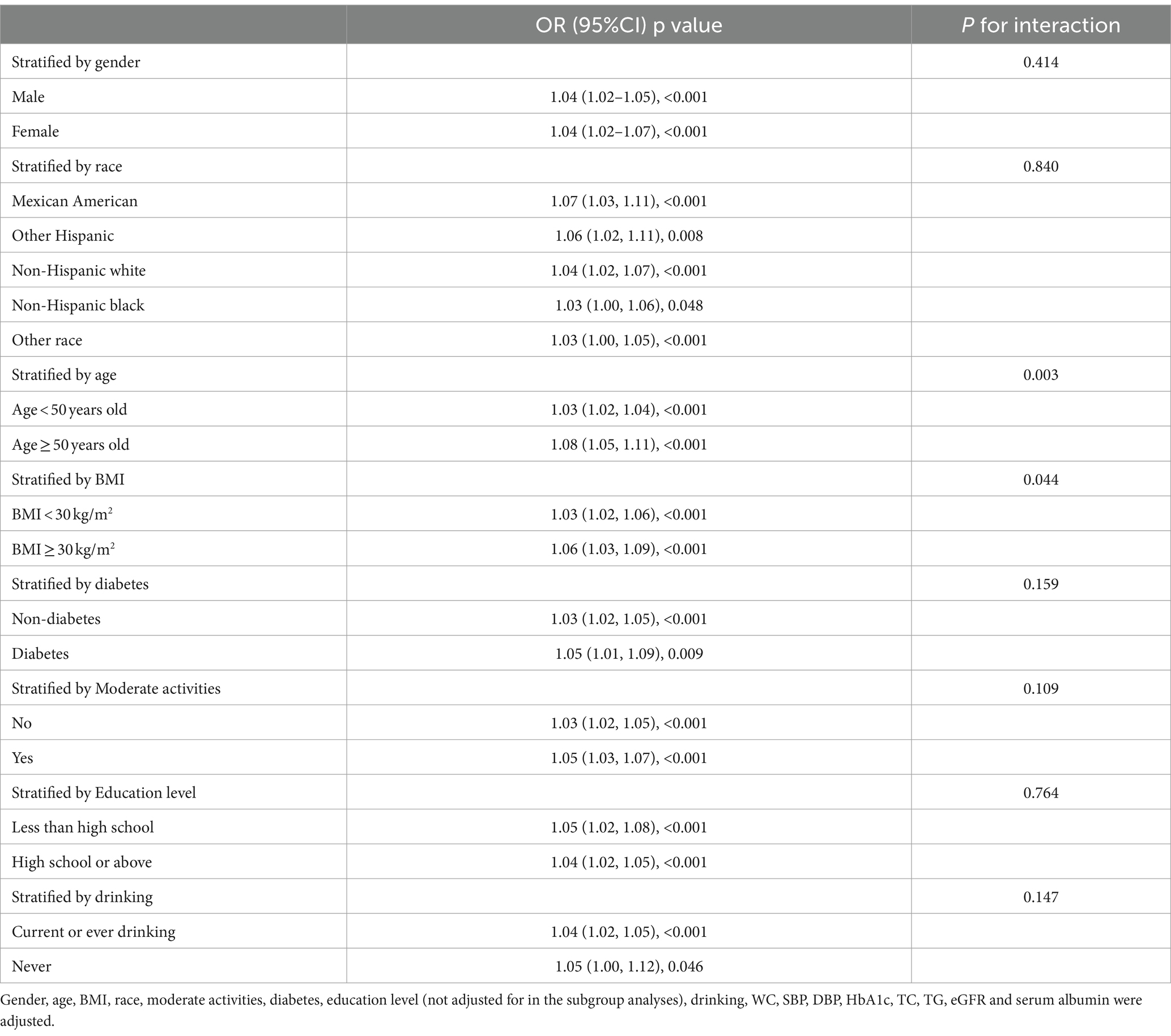
Table 5. Association between ALT/HDL and insulin resistance stratified by gender, age, race and BMI.
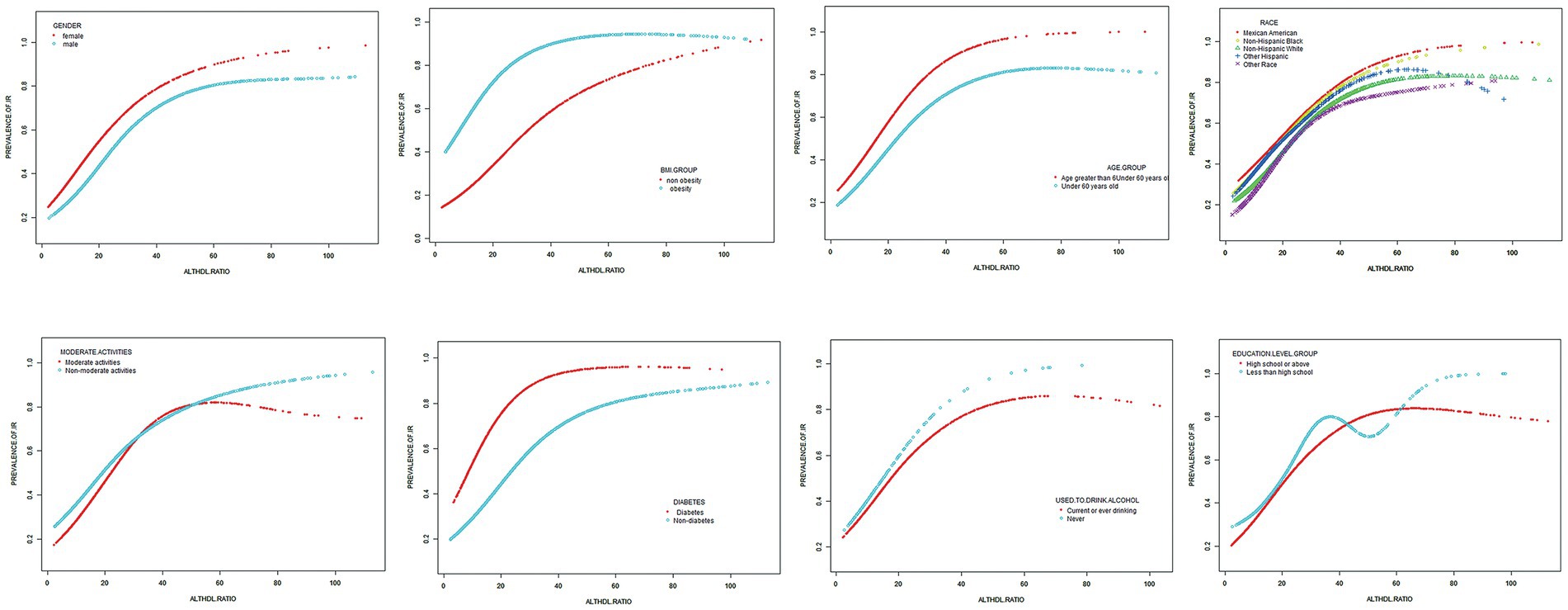
Figure 3. Subgroups analysis for the association between ALT/HDL-C and prevalence of IR by gender, age, race, BMI, diabetes, moderate activities, education level and drinking.
Predictive value of Alt/HDL-C for IR
The ROC curve in Figure 4 presents the diagnostic performance of ALT/HDL-C, HDL-C, ALT, AST and AST/ALT in identifying IR. Table 6 demonstrates that the AUC for ALT/HDL-C in the ROC analysis was 0.725 (95% CI: 0.709–0.742) for males and 0.696 (95% CI, 0.680–0.713) for females, exceeding ALT, HDL-C, AST and AST/ALT (p < 0.001), which suggests that ALT/HDL-C may serve as a superior indicator of IR compared with ALT or HDL-C alone, although its diagnostic accuracy remains somewhat limited.
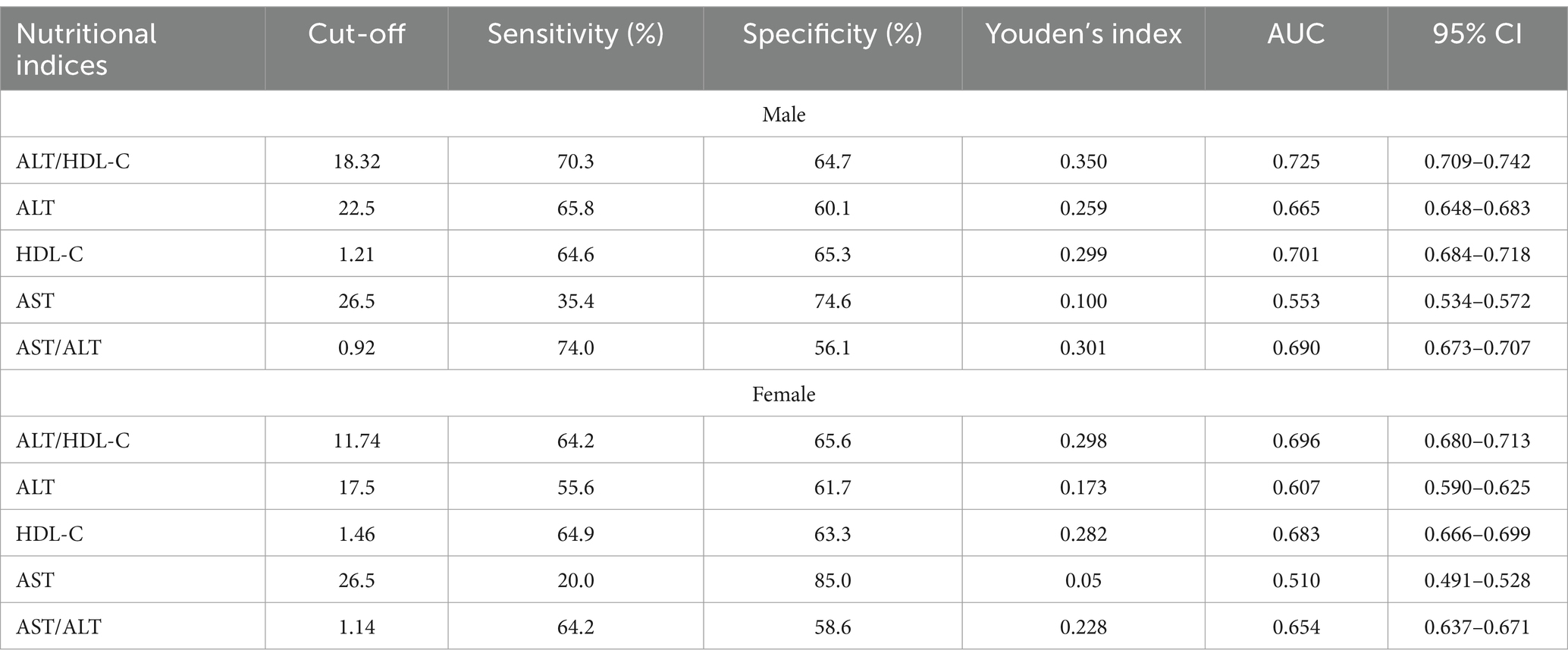
Table 6. The results of ROC analysis of ALT/HDL, ALT, HDL-C, AST, AST/ALT and TG/HDL for the diagnosis of IR.
Discussion
This study aims to investigate the connection between IR and ALT/HDL-C. Subsequently, stratified analyses were conducted to ascertain a notably stronger association between the two variables in subjects aged 50 and above, and those with obesity. Moreover, the ROC analysis demonstrated a substantial enhancement in the capacity to identify IR when utilizing the ALT/HDL-C ratio as compared to HDL-C, ALT, AST/ALT and AST. These findings indicate that ALT/HDL-C has the potential to be a valuable and straightforward biomarker for assessing the risk of IR.
The liver enzyme ALT is found to be strongly associated with hepatic fat accumulation and has also been linked to obesity and various components of MetS. Elevated ALT levels have been found to be associated with a higher prevalence of diabetes, MetS and cardiovascular diseases (23, 24). Additionally, ALT is considered as a predictive factor for non-alcoholic steatosis (25) and has been associated with both cardiovascular risk and IR in adolescents (26, 27). A reduction in HDL-C concentration is observed as a manifestation of MetS. Moreover, an elevation in HDL-C is widely recognized as a protective factor against IR (28). Furthermore, recent studies have proposed that the combination of HDL-C and ALT may serve as a more sensitive and novel biomarker for assessing inflammatory and metabolic disorders (14, 15).
As suggested in the study of Cao et al., the ALT/HDL-C composite index demonstrated enhanced predictive capabilities for diabetes compared to individual parameters (14). This amalgamation holds promise for potential applications in metabolic diseases. Based on the strong associations observed between the commonly employed atherosclerosis indicator HDL-C and the liver enzyme marker ALT with NAFLD (29–32). It is postulated that the amalgamation of ALT and HDL-C ratios is intricately associated with IR, potentially augmenting the capacity to detect IR.
The ALT/HDL-C ratio, the combination of the parameters of ALT and HDL-C, is predominantly investigated in clinical research (14). Conversely, establishing the threshold for ALT/HDL-C typically necessitates the accumulation of substantial clinical research evidence, thereby mitigating the selective disregard of chronic disease risks resulting from excessively high thresholds. In this study, the efficacy of various liver enzyme indicators was additionally compared in detecting IR. The findings demonstrate that ALT/HDL-C outperforms AST and AST/ALT significantly in identifying IR. The findings collectively indicate that the combination of HDL-C and ALT can potentially serve as a valuable tool for monitoring and diagnosing chronic diseases. The results obtained from the ROC analysis of this study suggest that an ALT/HDL-C (18.32 in males and 11.74 in females) can be considered as a screening threshold for identifying IR.
Furthermore, a more comprehensive subgroup analysis conducted in this study revealed intriguing findings, particularly highlighting a stronger association between IR and ALT/HDL-C in obese individuals and those aged 50 years and above. The main analysis of this study was presented as follows: Obese subjects generally exhibited elevated levels of ALT (33, 34) and decreased levels of HDL-C compared to non-obese subjects (35–38). A numerical analysis reveals that an increase in ALT or a decrease in HDL-C leads to an elevation in the ALT/HDL-C ratio. According to the findings, an elevated ALT/HDL-C ratio was indicative of an increased risk of IR in obese subjects. Additionally, advanced age and obesity were associated with poorer metabolic outcomes (39–41). Consequently, the heightened risk of IR observed in these populations in relation to ALT/HDL-C may be influenced by other metabolic pathways that contribute to these unfavorable outcomes.
Furthermore, an intriguing discovery of a previously unreported non-linear correlation between IR and ALT/HDL-C was made. It is likely that there is a saturating effect of IR risk when ALT/HDL-C reaches 33.62. The findings have the potential to provide new insights into the treatment and prevention of IR.
There are possible mechanistic explanations for the correlation between ALT/HDL-C and IR. It has been established that heightened levels of serum transaminases are linked to physical inactivity and obesity (42). Subjects with these characteristics exhibit lipid accumulation in hepatocytes and other tissues. ALT is recognized as the most reliable indicator of hepatic lipid accumulation (43). The elevated ALT can be interpreted as an indicator of subclinical systemic inflammation, with a correlation observed between ALT and proinflammatory molecules such as cytokines, CRP, and TNF-α (44). These molecules play a direct role in the pathogenesis of IR by modulating their signaling pathways (45). Additionally, ALT is primarily responsible for the conversion of alanine to pyruvate during hepatic glucose regulation, a process crucial for gluconeogenesis and closely linked to IR and the development of diabetes (46–48). HDL-C possesses various beneficial effects including reverse cholesterol transport, which can mitigate atherosclerosis, as well as anti-thrombotic, vasodilatory, anti-inflammatory and antiapoptotic properties (49). This study suggests that ALT/HDL-C, a combination of the lipid metabolism and inflammatory response, may serve as a potential indicator for IR.
The strengths of investigation encompass the extensive sample size and the nationwide representativeness of the United States. In addition, various confounding factors, including diabetes, age, drinking status, gender, physical activity and BMI, have been taken into account. Nevertheless, there are certain constraints in this study. First of all, the cross-sectional studies do not allow us to establish a cause-and-effect association between ALT/HDL-C and IR. Secondly, HOMA-IR was adopted as a means to assess IR. Although it is not taken as a gold standard, it is widely used because of its practicality. Thirdly, the AUC for ALT/HDL-C in the ROC analysis was close to 0.7, indicating limited diagnostic ability. Fourthly, it is crucial to verify the connection between ALT/HDL-C and IR in diverse populations and ethnicities, as this study solely focuses on American.
Conclusion
In conclusion, findings from this investigation conducted on American adults reveal a positive correlation between IR and ALT/HDL-C. This association is particularly significant among individuals with obesity and those aged 50 or older. It may be an effective indicator to identify IR in American and prevent disease progression.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: NHANES, www.cdc.gov/nchs/NHANEs/.
Ethics statement
The studies involving humans were approved by National Center for Health Statistics Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XZ: Writing – original draft, Conceptualization. JX: Conceptualization, Data curation, Investigation, Writing – original draft. HD: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Wenzhou Municipal Science and Technology Bureau (Y20220612 to Dr. Huifang Dai) and the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (2019330727 to Dr. Xinhe Zhou).
Acknowledgments
We would like to thank the NHANES database for providing the data source for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tam, CS, Xie, W, Johnson, WD, Cefalu, WT, Redman, LM, and Ravussin, E. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes Care. (2012) 35:1605–10. doi: 10.2337/dc11-2339
2. Wallace, TM, Levy, JC, and Matthews, DR. Use and abuse of HOMA modeling. Diabetes Care. (2004) 27:1487–95. doi: 10.2337/diacare.27.6.1487
3. Espinel-Bermudez, MC, Robles-Cervantes, JA, Del Sagrario Villarreal-Hernandez, L, Villasenor-Romero, JP, Hernandez-Gonzalez, SO, Gonzalez-Ortiz, M, et al. Insulin resistance in adult primary care patients with a surrogate index, Guadalajara, Mexico, 2012. J Investig Med. (2015) 63:247–50. doi: 10.1097/JIM.0000000000000130
4. Borai, A, Livingstone, C, and Ferns, GA. The biochemical assessment of insulin resistance. Ann Clin Biochem. (2007) 44:324–42. doi: 10.1258/000456307780945778
5. Rudvik, A, and Mansson, M. Evaluation of surrogate measures of insulin sensitivity - correlation with gold standard is not enough. BMC Med Res Methodol. (2018) 18:64. doi: 10.1186/s12874-018-0521-y
6. Burke, JP, Hale, DE, Hazuda, HP, and Stern, MP. A quantitative scale of acanthosis nigricans. Diabetes Care. (1999) 22:1655–9. doi: 10.2337/diacare.22.10.1655
7. Sung, KC, Jeong, WS, Wild, SH, and Byrne, CD. Combined influence of insulin resistance, overweight/obesity, and fatty liver as risk factors for type 2 diabetes. Diabetes Care. (2012) 35:717–22. doi: 10.2337/dc11-1853
8. Wu, CZ, Hsieh, CH, Lu, CH, Pei, D, Chen, JS, and Chen, YL. First-phase insulin secretion is positively correlated with alanine aminotransferase in young adults. Adv Clin Exp Med. (2021) 30:35–40. doi: 10.17219/acem/128229
9. Leavens, KF, and Birnbaum, MJ. Insulin signaling to hepatic lipid metabolism in health and disease. Crit Rev Biochem Mol Biol. (2011) 46:200–15. doi: 10.3109/10409238.2011.562481
10. von Eckardstein, A, and Sibler, RA. Possible contributions of lipoproteins and cholesterol to the pathogenesis of diabetes mellitus type 2. Curr Opin Lipidol. (2011) 22:26–32. doi: 10.1097/MOL.0b013e3283412279
11. Han, T, Cheng, Y, Tian, S, Wang, L, Liang, X, Duan, W, et al. Changes in triglycerides and high-density lipoprotein cholesterol may precede peripheral insulin resistance, with 2-h insulin partially mediating this unidirectional relationship: a prospective cohort study. Cardiovasc Diabetol. (2016) 15:154. doi: 10.1186/s12933-016-0469-3
12. Rachek, LI. Free fatty acids and skeletal muscle insulin resistance. Prog Mol Biol Transl Sci. (2014) 121:267–92. doi: 10.1016/B978-0-12-800101-1.00008-9
13. Karhapaa, P, Malkki, M, and Laakso, M. Isolated low HDL cholesterol. An insulin-resistant state. Diabetes. (1994) 43:411–7.
14. Cao, C, Hu, H, Han, Y, Yuan, S, Zheng, X, Zhang, X, et al. The nonlinear correlation between alanine aminotransferase to high-density lipoprotein cholesterol ratio and the risk of diabetes: a historical Japanese cohort study. BMC Endocr Disord. (2023) 23:124. doi: 10.1186/s12902-023-01382-7
15. He, S, Yu, C, Kuang, M, Qiu, J, Yang, R, Zhang, S, et al. Alanine aminotransferase to high-density lipoprotein cholesterol ratio is positively correlated with the occurrence of diabetes in the Chinese population: a population-based cohort study. Front Endocrinol (Lausanne). (2023) 14:1266692. doi: 10.3389/fendo.2023.1266692
16. Zipf, G, Chiappa, M, Porter, KS, Ostchega, Y, Lewis, BG, and Dostal, J. National health and nutrition examination survey: plan and operations, 1999-2010. Vital Health Stat. (2013) 1:1–37.
17. Zhou, X, and Xu, J. Association between serum uric acid-to-high-density lipoprotein cholesterol ratio and insulin resistance in patients with type 2 diabetes mellitus. J Diabetes Investig. (2023) 15:113–20. doi: 10.1111/jdi.14086
18. Zhou, M, Zhu, L, Cui, X, Feng, L, Zhao, X, He, S, et al. The triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio as a predictor of insulin resistance but not of beta cell function in a Chinese population with different glucose tolerance status. Lipids Health Dis. (2016) 15:104. doi: 10.1186/s12944-016-0270-z
19. Zeng, P, Cai, X, Yu, X, and Gong, L. Markers of insulin resistance associated with non-alcoholic fatty liver disease in non-diabetic population. Sci Rep. (2023) 13:20470. doi: 10.1038/s41598-023-47269-4
20. Di Murro, E, Di Giuseppe, G, Soldovieri, L, Moffa, S, Improta, I, Capece, U, et al. Physical activity and type 2 diabetes: in search of a personalized approach to improving beta-cell function. Nutrients. (2023) 15:202. doi: 10.3390/nu15194202
21. Ortega, FB, Ruiz, JR, Hurtig-Wennlof, A, Meirhaeghe, A, Gonzalez-Gross, M, Moreno, LA, et al. Physical activity attenuates the effect of low birth weight on insulin resistance in adolescents: findings from two observational studies. Diabetes. (2011) 60:2295–9. doi: 10.2337/db10-1670
22. Arjmand, B, Ebrahimi Fana, S, Ghasemi, E, Kazemi, A, Ghodssi-Ghassemabadi, R, Dehghanbanadaki, H, et al. Metabolic signatures of insulin resistance in non-diabetic individuals. BMC Endocr Disord. (2022) 22:212. doi: 10.1186/s12902-022-01130-3
23. Hartman, C, Rennert, HS, Rennert, G, Elenberg, Y, and Zuckerman, E. Prevalence of elevated liver enzymes and comorbidities in children and adolescents with overweight and obesity. Acta Paediatr. (2021) 110:985–92. doi: 10.1111/apa.15469
24. Porter, SA, Pedley, A, Massaro, JM, Vasan, RS, Hoffmann, U, and Fox, CS. Aminotransferase levels are associated with cardiometabolic risk above and beyond visceral fat and insulin resistance: the Framingham heart study. Arterioscler Thromb Vasc Biol. (2013) 33:139–46. doi: 10.1161/ATVBAHA.112.300075
25. Vos, MB, Abrams, SH, Barlow, SE, Caprio, S, Daniels, SR, Kohli, R, et al. NASPGHAN clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children: recommendations from the expert committee on NAFLD (ECON) and the north American Society of Pediatric Gastroenterology, hepatology and nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr. (2017) 64:319–34. doi: 10.1097/MPG.0000000000001482
26. Klein, M, Iazzettii, L, Speiser, P, Carey, D, Shelov, S, Accacha, S, et al. Alanine transferase: an independent indicator of adiposity related comorbidity risk in youth. J Diabetes. (2015) 7:649–56. doi: 10.1111/1753-0407.12221
27. Patel, DA, Srinivasan, SR, Chen, W, and Berenson, GS. Serum alanine aminotransferase and its association with metabolic syndrome in children: the Bogalusa heart study. Metab Syndr Relat Disord. (2011) 9:211–6. doi: 10.1089/met.2010.0086
28. Anan, F, Yonemochi, H, Masaki, T, Takahashi, N, Fukunaga, N, Teshima, Y, et al. High-density lipoprotein cholesterol and insulin resistance are independent and additive markers of left ventricular hypertrophy in essential hypertension. Hypertens Res. (2007) 30:125–31. doi: 10.1291/hypres.30.125
29. Chen, ZW, Chen, LY, Dai, HL, Chen, JH, and Fang, LZ. Relationship between alanine aminotransferase levels and metabolic syndrome in nonalcoholic fatty liver disease. J Zhejiang Univ Sci B. (2008) 9:616–22. doi: 10.1631/jzus.B0720016
30. Schindhelm, RK, Diamant, M, Dekker, JM, Tushuizen, ME, Teerlink, T, and Heine, RJ. Alanine aminotransferase as a marker of non-alcoholic fatty liver disease in relation to type 2 diabetes mellitus and cardiovascular disease. Diabetes Metab Res Rev. (2006) 22:437–43. doi: 10.1002/dmrr.666
31. Omagari, K, Takamura, R, Matsutake, S, Ichimura, M, Kato, S, Morikawa, S, et al. Serum alanine aminotransferase concentration as a predictive factor for the development or regression of fatty liver. J Clin Biochem Nutr. (2011) 49:200–6. doi: 10.3164/jcbn.11-27
32. Chang, Y, Ryu, S, Sung, E, and Jang, Y. Higher concentrations of alanine aminotransferase within the reference interval predict nonalcoholic fatty liver disease. Clin Chem. (2007) 53:686–92. doi: 10.1373/clinchem.2006.081257
33. Samadi, N, Cembrowski, GS, and Chan, J. Effect of waist circumference on reference intervals of liver-related enzyme tests in apparently healthy adult Mexican Americans, black and white Americans. Clin Biochem. (2007) 40:206–12. doi: 10.1016/j.clinbiochem.2006.11.007
34. Bekkelund, SI, and Jorde, R. Alanine aminotransferase and body composition in obese men and women. Dis Markers. (2019) 2019:1695874. doi: 10.1155/2019/1695874
35. Davis, CE, Williams, DH, Oganov, RG, Tao, SC, Rywik, SL, Stein, Y, et al. Sex difference in high density lipoprotein cholesterol in six countries. Am J Epidemiol. (1996) 143:1100–6. doi: 10.1093/oxfordjournals.aje.a008686
36. Kauma, H, Savolainen, MJ, Heikkila, R, Rantala, AO, Lilja, M, Reunanen, A, et al. Sex difference in the regulation of plasma high density lipoprotein cholesterol by genetic and environmental factors. Hum Genet. (1996) 97:156–62. doi: 10.1007/BF02265258
37. Mooradian, AD, Albert, SG, and Haas, MJ. Low serum high-density lipoprotein cholesterol in obese subjects with normal serum triglycerides: the role of insulin resistance and inflammatory cytokines. Diabetes Obes Metab. (2007) 9:441–3. doi: 10.1111/j.1463-1326.2006.00636.x
38. Anagnostis, P, Stevenson, JC, Crook, D, Johnston, DG, and Godsland, IF. Effects of menopause, gender and age on lipids and high-density lipoprotein cholesterol subfractions. Maturitas. (2015) 81:62–8. doi: 10.1016/j.maturitas.2015.02.262
39. Nannini, DR, Joyce, BT, Zheng, Y, Gao, T, Liu, L, Yoon, G, et al. Epigenetic age acceleration and metabolic syndrome in the coronary artery risk development in young adults study. Clin Epigenetics. (2019) 11:160. doi: 10.1186/s13148-019-0767-1
40. Pucci, G, Alcidi, R, Tap, L, Battista, F, Mattace-Raso, F, and Schillaci, G. Sex-and gender-related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: a review of the literature. Pharmacol Res. (2017) 120:34–42. doi: 10.1016/j.phrs.2017.03.008
41. Ramirez-Manent, JI, Jover, AM, Martinez, CS, Tomas-Gil, P, Marti-Lliteras, P, and Lopez-Gonzalez, AA. Waist circumference is an essential factor in predicting insulin resistance and early detection of metabolic syndrome in adults. Nutrients. (2023) 15:257. doi: 10.3390/nu15020257
42. Villegas, R, Xiang, YB, Elasy, T, Cai, Q, Xu, W, Li, H, et al. Liver enzymes, type 2 diabetes, and metabolic syndrome in middle-aged, urban Chinese men. Metab Syndr Relat Disord. (2011) 9:305–11. doi: 10.1089/met.2011.0016
43. Mohamed, J, Nazratun Nafizah, AH, Zariyantey, AH, and Budin, SB. Mechanisms of diabetes-induced liver damage: the role of oxidative stress and inflammation. Sultan Qaboos Univ Med J. (2016) 16:e132–41. doi: 10.18295/squmj.2016.16.02.002
44. Wang, CS, Chang, TT, Yao, WJ, Wang, ST, and Chou, P. Impact of increasing alanine aminotransferase levels within normal range on incident diabetes. J Formos Med Assoc. (2012) 111:201–8. doi: 10.1016/j.jfma.2011.04.004
45. Chen, L, Chen, R, Wang, H, and Liang, F. Mechanisms linking inflammation to insulin resistance. Int J Endocrinol. (2015) 2015:508409. doi: 10.1155/2015/508409
46. Hanley, AJ, Wagenknecht, LE, Festa, A, D'Agostino, RB Jr, and Haffner, SM. Alanine aminotransferase and directly measured insulin sensitivity in a multiethnic cohort: the insulin resistance atherosclerosis study. Diabetes Care. (2007) 30:1819–27. doi: 10.2337/dc07-0086
47. Qian, K, Zhong, S, Xie, K, Yu, D, Yang, R, and Gong, DW. Hepatic ALT isoenzymes are elevated in gluconeogenic conditions including diabetes and suppressed by insulin at the protein level. Diabetes Metab Res Rev. (2015) 31:562–71. doi: 10.1002/dmrr.2655
48. Ballestri, S, Zona, S, Targher, G, Romagnoli, D, Baldelli, E, Nascimbeni, F, et al. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol. (2016) 31:936–44. doi: 10.1111/jgh.13264
Keywords: obesity, race, alanine aminotransferase, insulin resistance, diabetes
Citation: Zhou X, Xu J and Dai H (2024) The ratio of alanine aminotransferase to high-density lipoprotein cholesterol is positively correlated with the insulin resistance in American adults: a population-based cohort study. Front. Med. 11:1418364. doi: 10.3389/fmed.2024.1418364
Edited by:
Serafino Fazio, Federico II University Hospital, ItalyReviewed by:
Alessandra Cuomo, University of Naples Federico II, ItalyEnrique Torres Rasgado, Meritorious Autonomous University of Puebla, Mexico
Copyright © 2024 Zhou, Xu and Dai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huifang Dai, ZGFpaGZAMTI2LmNvbQ==
 Xinhe Zhou
Xinhe Zhou Jing Xu
Jing Xu