- 1Department of Pharmacy, Chengdu Second People’s Hospital, Chengdu, Sichuan, China
- 2Department of Respiratory and Critical Care Medicine, Chengdu Second People’s Hospital, Chengdu, Sichuan, China
- 3Department of Pharmacy, Central People’s Hospital of Zhanjiang, Zhanjiang, Guangdong, China
Background: Psittacosis is a zoonotic disease with a low incidence rate and a lack of specificity in clinical manifestations, making it prone to be missed, misdiagnosed, and even cause delayed treatment for patients. Metagenomic next-generation sequencing (mNGS) was successfully performed for the diagnosis of a young patient with psittacosis progressing to acute respiratory distress syndrome (ARDS), and precisely targeted antibiotic treatment was promptly administered. Additionally, a comprehensive review was conducted on 68 cases of psittacosis complicated with ARDS, with the goal of improving the clinical awareness of this disease.
Case presentation: This study reports a 37-year-old young female who was infected with Chlamydia psittaci (C. psittaci) after contact with parrots and eventually developed ARDS. The patient initially developed fever and sore throat, followed by cough and expectoration. Despite receiving empirical anti-infection treatment, the condition continued to progress rapidly, and severe dyspnea developed within a short period of time. She was subsequently transferred to the intensive care unit (ICU) and underwent tracheal intubation and mechanical ventilation due to acute respiratory failure. After the DNA sequence of C. psittaci in bronchoalveolar lavage fluid (BALF) was detected through mNGS, the patient received targeted antibiotic treatment with doxycycline and moxifloxacin, and her clinical symptoms gradually improved.
Conclusion: Epidemiological investigations and the application of mNGS are crucial for the early identification and diagnosis of psittacosis. For suspected psittacosis patients, the application of mNGS technology could promote early identification of pathogens and targeted antimicrobial therapy, which might improve patient prognosis. In addition, young psittacosis patients without underlying disease should also be vigilant about the possibility of developing severe cases.
1 Introduction
Psittacosis, also known as ornithosis, is caused by infection with Chlamydia psittaci (C. psittaci). Moreover, C. psittaci is a gram-negative and specialized intracellular parasitic pathogen, which is also one of the species in the Chlamydia family that is pathogenic to humans or animals (1). It can directly or indirectly infect the host, leading to the onset of psittacosis. Specifically, psittacosis spreads mainly between birds and can be transmitted to humans through birds or other animals in specific situations (2). In addition, it can also be transmitted from human to human (3, 4). The interpersonal transmission of C. psittaci is regarded as a newly emerging public health risk, especially for healthcare workers and their close contacts (5). Psittacosis mainly manifests as pneumonia, but only approximately 1% of community-acquired pneumonia is caused by psittacosis (6). Although the incidence rate is relatively low, C. psittaci pneumonia accounts for a high proportion (8%) of severe community-acquired pneumonia cases (7). The asymptomatic spread of C. psittaci and inadequate laboratory detection techniques may lead to a serious underestimation of the current global number of psittacosis cases. In recent years, with the migration of migratory birds and the increase in the number of pet birds, reports of animals and humans infected with C. psittaci have increased annually, indicating that C. psittaci poses a potential threat to animal husbandry and human health. However, owing to nonspecific clinical symptoms, the lack of laboratory testing and diagnostic tools, and insufficient clinical understanding of the disease, psittacosis is often ignored in the diagnosis process, leading to delayed treatment in clinical practice, which undoubtedly increases the health burden of psittacosis worldwide.
Unfortunately, there is currently no effective vaccine to prevent psittacosis. If not treated promptly after infection, a critical illness may develop in a short period of time, seriously affecting the patient’s prognosis. Rapid and accurate identification of clinical pathogens and timely targeted antibiotic treatment are key strategies for the successful treatment of psittacosis. With the application of metagenomic next-generation sequencing (mNGS) technology in the clinic, many atypical pathogens can be validly detected. An increasing number of cases of C. psittaci pneumonia have been clearly diagnosed, providing timely and effective targeted treatment for clinical practice.
Previous studies have shown limited case reports of psittacosis complicated with acute respiratory distress syndrome (ARDS). However, the hospitalization mortality rate of severe ARDS patients is relatively high, ranging from 26 to 60%, posing a serious threat to public life and health safety (8, 9). Herein, this is a report of a young female patient infected with C. psittaci who was diagnosed by mNGS and progressed to ARDS. In addition, we summarized the epidemiology, clinical manifestations, laboratory data, complications and treatment strategies of 68 patients with severe C. psittaci pneumonia complicated with ARDS to improve the clinical understanding of the diagnosis and treatment of this disease.
2 Case presentation
A 37-year-old female patient was admitted to the Department of Respiratory and Critical Care Medicine at Chengdu Second People’s Hospital on February 27, 2023, due to cough and expectoration. The patient presented with sore throat and fever (39.3°C) 3 days before admission. The patient subsequently took ceftizoxime (0.2 g po bid) and diclofenac sodium sustained-release tablets (0.1 g po qd), but the above symptoms did not improve. The patient has good past health without personal or family history. Epidemiological investigations revealed that the patient purchased two parrots from a pet store on January 25, 2023. A parrot died on February 19, 2023, and the patient cleared its body and feces.
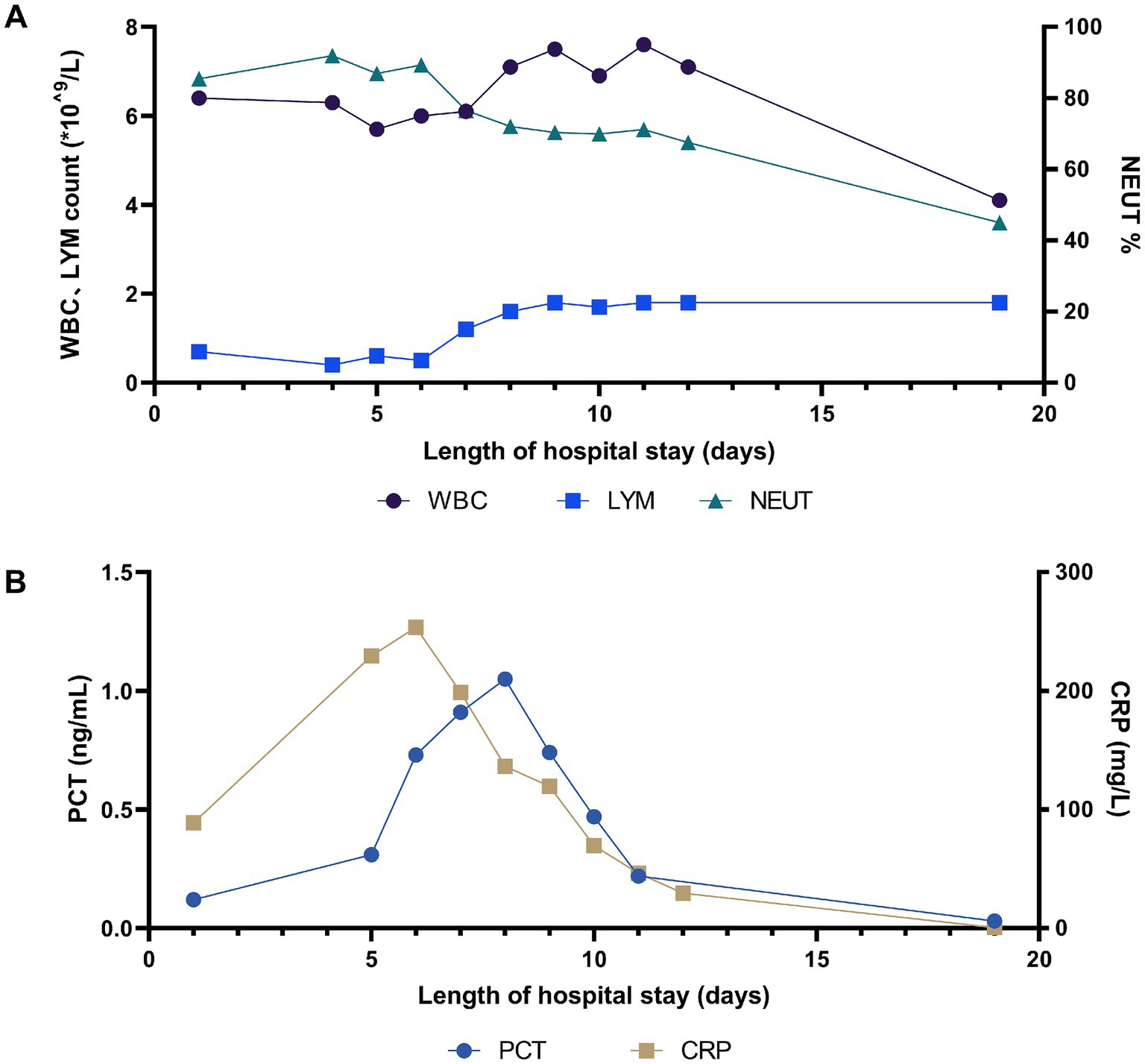
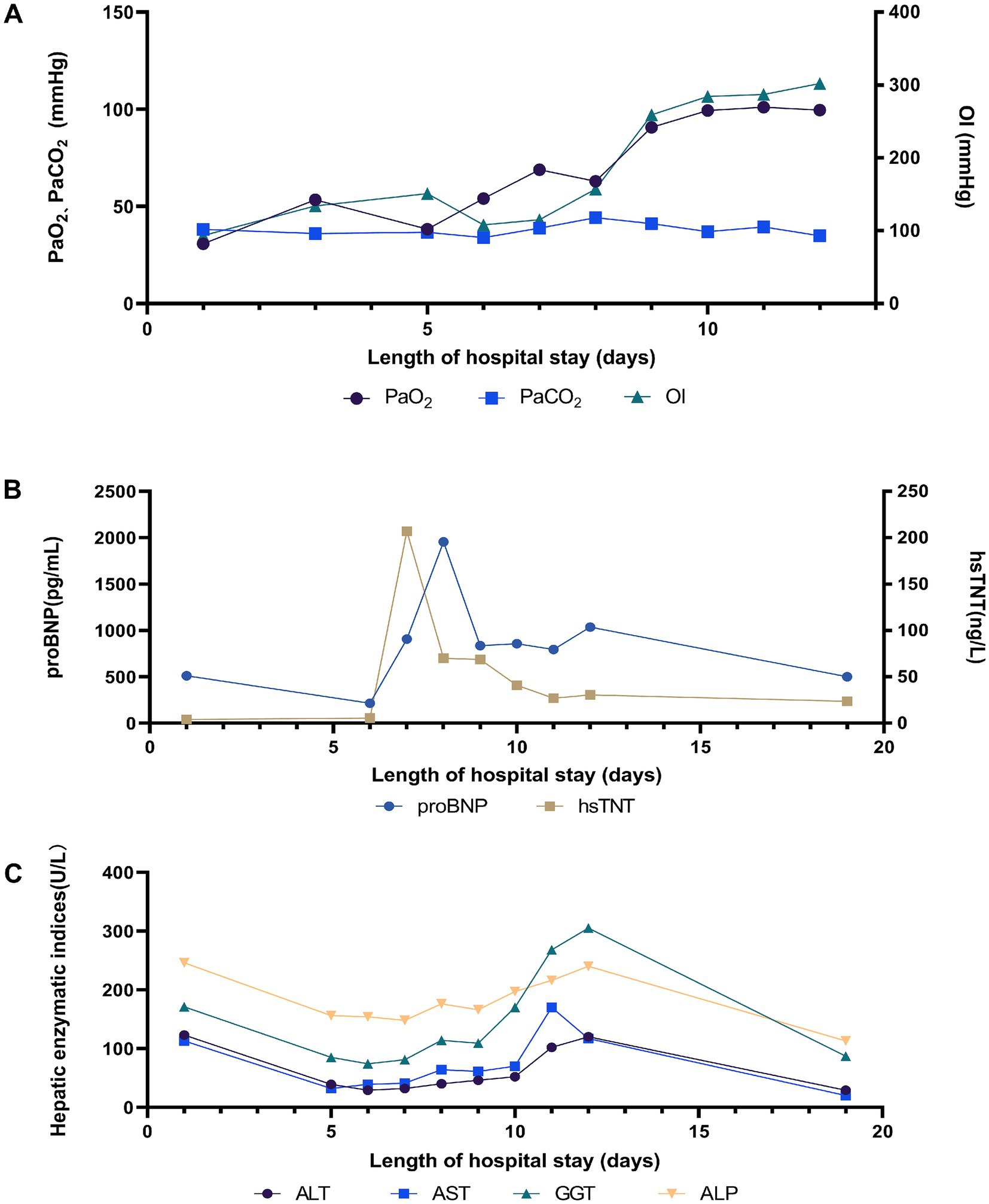
Physical examination on admission revealed that body temperature, heart rate (HR), respiratory rate, and blood pressure were 39.3°C, 99 beats/min, 22 beats/min, and 97/63 mmHg, respectively. Initial lung computed tomography (CT) revealed multiple patchy and nodular high-density shadows in both lungs, mainly involving the upper and lower lobes of the right lung. It was partially consolidated with right pleural effusion and mediastinal lymph node enlargement, as shown in Figures 1A–C. In addition, the patient was fully conscious and experienced mild shortness of breath. Chest auscultation revealed wet rales in both lungs. Laboratory tests on admission revealed a white blood cell (WBC) count of 6.4 × 109·L−1, neutrophils (NEUT) count of 5.5 × 109·L−1, C-reactive protein (CRP) of 88.8 mg/L, procalcitonin (PCT) of 0.12 ng/mL, alanine aminotransferase (ALT) of 123 U/L, aspartate aminotransferase (AST) of 113 U/L, alkaline phosphatase (ALP) of 246 U/L, and gamma-glutamyl transpeptidase (GGT) of 171 U/L. Arterial blood gas analysis (ABGA) revealed that the pH, oxygen partial pressure (PaO2), carbon dioxide partial pressure (PaCO2), oxygenation index (OI), and HCO3− and K+ concentrations were 7.47, 30.7 mmHg, 38.2 mmHg, 93.2 mmHg, 27.6 mmol/L and 3.1 mmol/L, respectively. The patient was diagnosed with community-acquired pneumonia and type I respiratory failure, who received oxygen therapy through a facemask at a rate of 4 L/min and a fraction of inspired oxygen (FiO2) 37%. In addition, the patient also received treatment with polyene phosphatidylcholine capsules (456 mg po tid), diclofenac sodium sustained-release tablets (0.1 g po qd), administered cefazoxime (2.25 g ivgtt q8h), and nebulized inhalation of acetylcysteine (0.3 g inh tid).
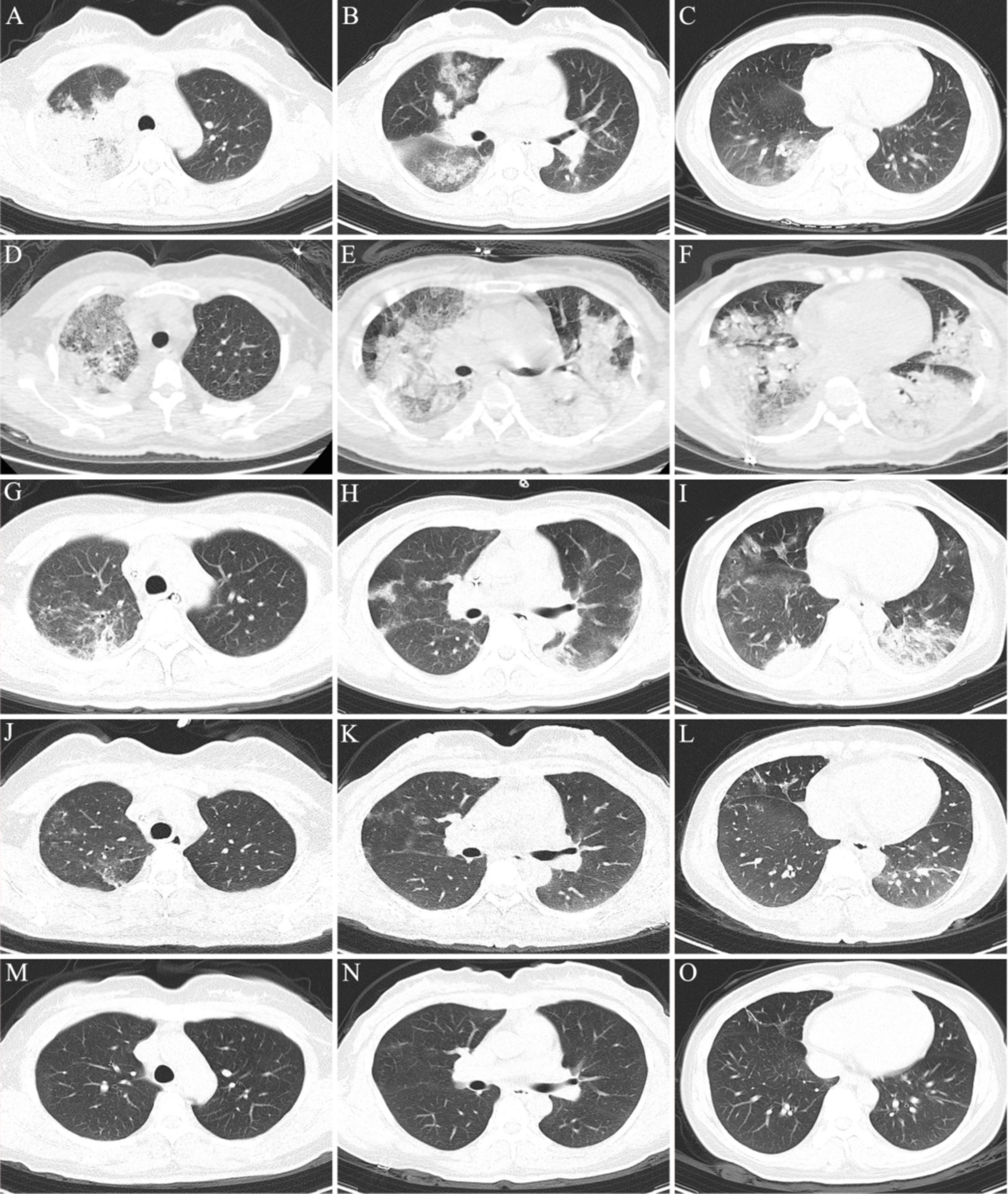
Figure 1. Chest CT scan of the patient during hospitalization and follow-up. (A–C) On the 1th day after admission; (D–F) on the 6th day after admission; (G–I) on the 12th day after admission; (J–L) on the day of discharge; (M–O) on the 17th day of follow-up after discharge.
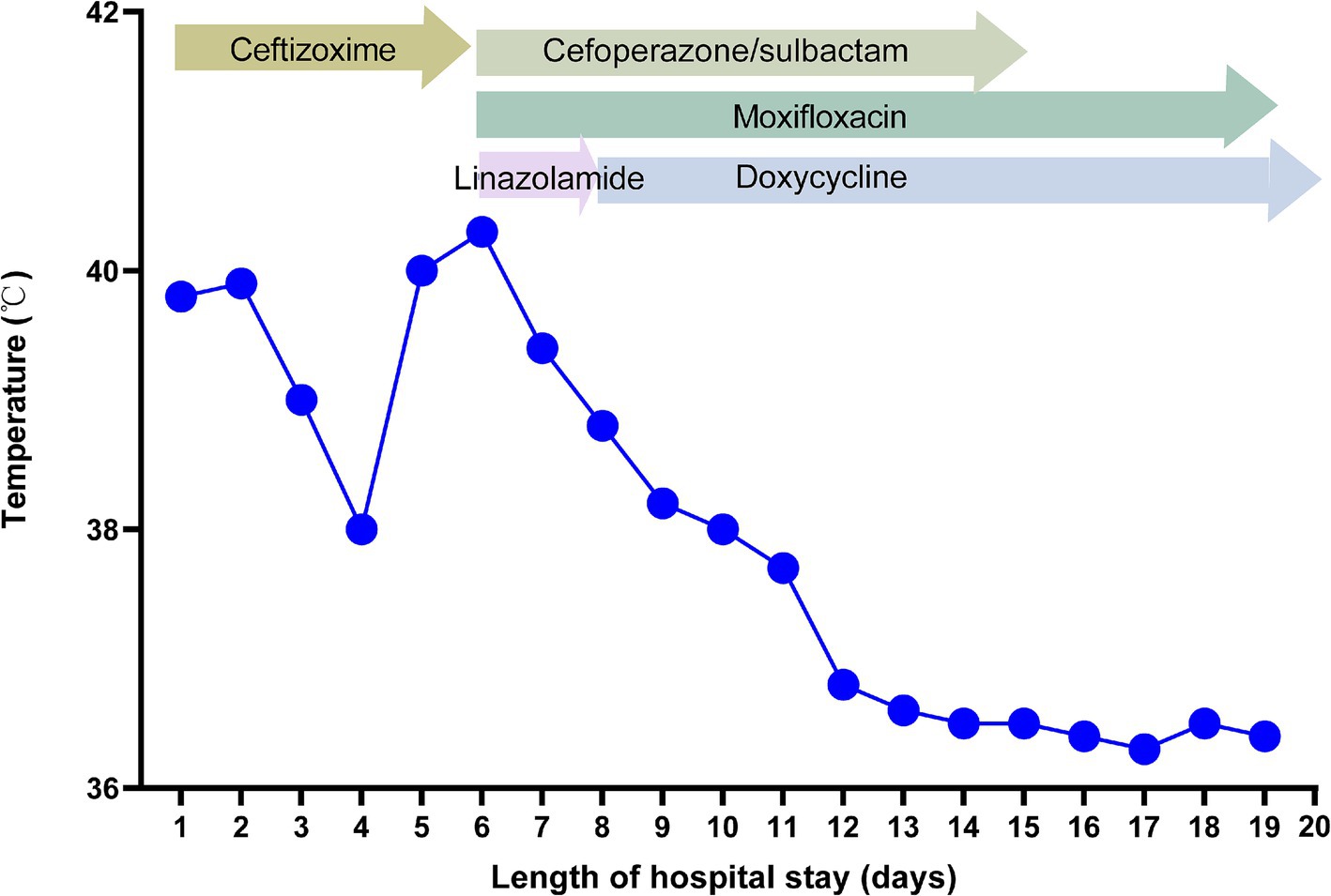
On the 5th day after admission, the patient was in a poor mental state and had a fever with a maximum body temperature above 40°C, lip cyanosis, and a respiratory rate of more than 30 beats/min. Breathing sounds thicker, and wet rales in both lungs were audible. The oxygen saturation (SpO2) was 60 ~ 70%, and the patient immediately received noninvasive mechanical ventilation (ST mode, IPAP 15cmH2O, EPAP 4cmH2O, FiO2 40%). The sputum and blood culture results for typical bacteria and fungi, as well as the relevant tests for Mycobacterium tuberculosis were negative. Serological tests for novel coronavirus (2019-nCoV) nucleic acid, influenza virus nucleic acid, (1,3)-β-D-glucan (BDG), galactomannan (GM), the capsular polysaccharide antigen of Cryptococcus, and IgM antibodies against respiratory pathogens were negative. In addition, the serum antinuclear antibodies (ANAs), antineutrophil autoantibodies, and immunoglobulin concentrations were within the normal ranges.
On the 6th day after admission, the patient developed a high fever with a maximum body temperature of 40.3°C, accompanied by drowsiness and confusion. Moreover, there was significant shortness of breath, with a respiratory rate of approximately 40 beats/min. SpO2 continued to decrease and could not be maintained at normal levels after slight activity. Arterial blood gas analysis (ABGA) revealed that the pH, PaO2, PaCO2, SpO2, PaO2/FiO2, SpO2/FiO2, and PaO2/FiO2 were 7.53, 54.0 mmHg, 33.9 mmHg, 88.6%, 90.0 mmHg, 147.7, and < 100 mmHg, respectively. In accordance with the ‘Berlin Definition’, the patient was diagnosed with severe ARDS (10). Chest CT revealed multiple plaques and high-density shadows in both lungs with bronchograms, partial consolidation, and partial interstitial involvement. The infection was significantly aggravated, with a slight increase in pleural effusion, as shown in Figures 1D–F. The patient subsequently developed refractory respiratory failure and was transferred to the intensive care unit (ICU), where she received tracheal intubation and invasive mechanical ventilation (V-A/C mode, VTE 360 mL, PEEP 8 cmH2O, FiO2 100%). To reduce the risk of ventilator-induced lung injury (VILI) and increase the effective alveolar ventilation area, some lung-protective ventilation strategies, such as low tidal volume (6 mL/kg), low plateau pressure (20 cmH2O), high end-expiratory pressure (8 cmH2O), and prone position ventilation have been implemented. The antibiotics were subsequently adjusted to cefoperazone/sulbactam (3 g ivgtt q8h) combined with moxifloxacin (0.4 g ivgtt qd) for treatment. Owing to the rapid progression of the disease, vancomycin was added, but due to the redness of the patient’s skin after intravenous infusion, it was immediately adjusted to linezolid (0.6 g ivgtt q12h). Moreover, samples of the bronchoalveolar lavage fluid (BALF) were collected and tested via mNGS for etiological diagnosis.
On the 8th day after admission, the BALF results were reported, and 70 sequence reads corresponding to C. psittaci were identified, with a relative abundance of 0.49%, as shown in Figure 2. Considering the recent contact history with parrots, the patient was finally diagnosed with C. psittaci pneumonia. The patient was subsequently adjusted to receive a combination therapy of targeted antibiotics: moxifloxacin (0.4 g ivgtt qd) and doxycycline (0.1 g po q12h). After effective treatment, the patient’s clinical symptoms gradually improved. Owing to improvements in respiratory function, invasive mechanical ventilation was withdrawn after 5 days of clinical application. On the 11th day after admission, echocardiography revealed a decrease in ventricular septal contractile activity, mild pericardial effusion, and a decrease in the left ventricular ejection fraction (LVEF: 48%). On the 12th day after admission, chest CT showed significant absorption of pulmonary inflammation and reduced pleural effusion compared with before, as shown in Figures 1G–I. Considering the absence of a history of underlying cardiac disease and no significant abnormalities were observed on the electrocardiogram after admission, it was speculated that the patient’s cardiac changes were related to infection with C. psittaci. On the 13th day after admission, the patient’s condition improved with temperature returned to normal (Figure 3) and she was transferred to a general ward for subsequent treatment. Owing to timely and accurate targeted antibiotic treatment, the patient recovered (Figures 4, 5) and was discharged 7 days later. On the day of discharge, chest CT revealed further absorption of pulmonary inflammation, and no pleural effusion was observed, as shown in Figures 1J–L. The patient continued to receive doxycycline treatment outside the hospital for 12 days. On the 17th day of follow-up after discharge, a chest CT revealed a small amount of patchy and linear high-density shadows, as shown in Figures 1M–O, and the rest completely recovered.
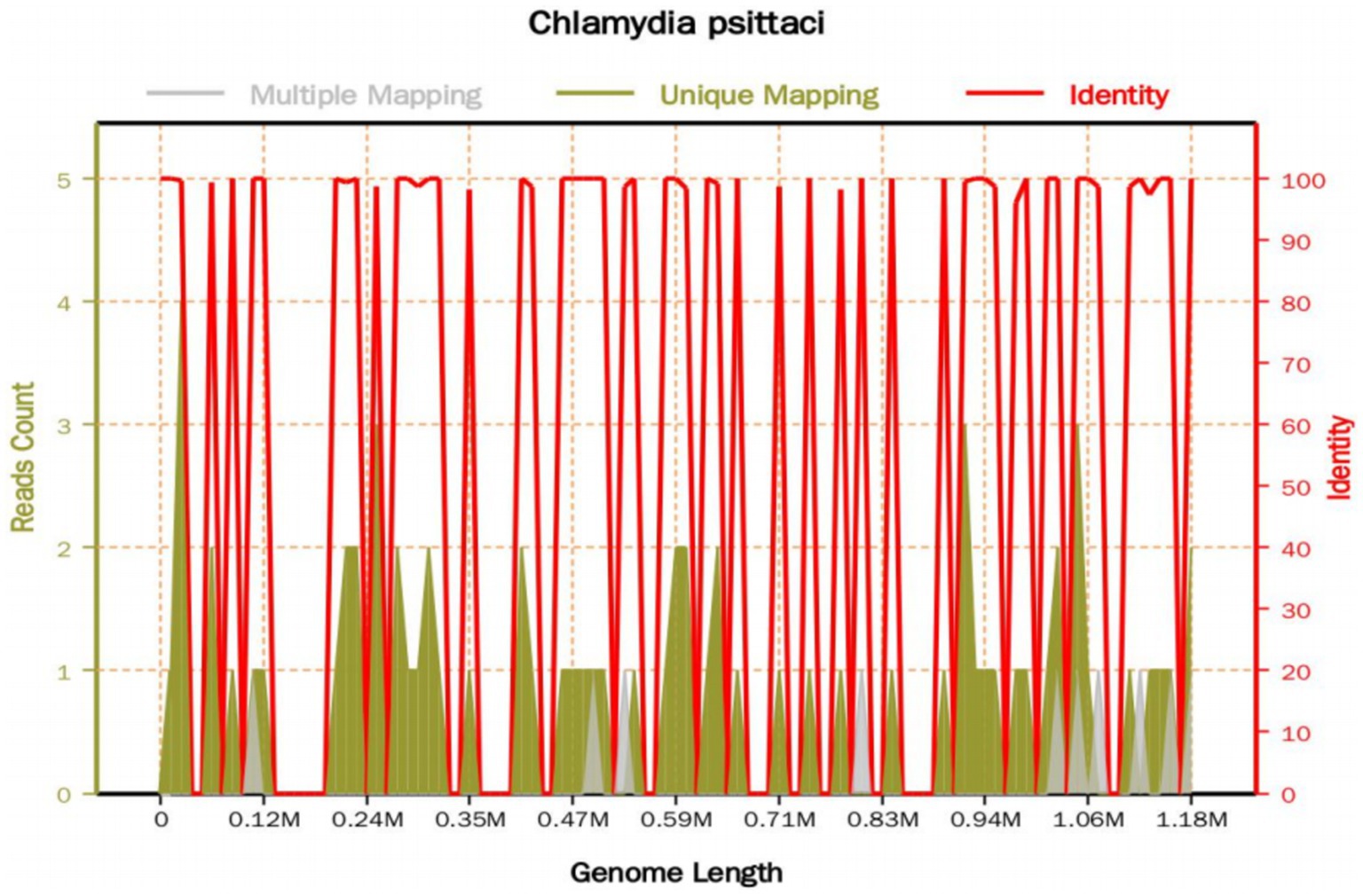
Figure 2. The mNGS sequencing results of the BALF sample. The total base number of the detected genome is 1,179,220 (bp), of which the total length covered by the C. psittaci sequence is 5,764 (bp), with a coverage of 0.488798% and an average depth of 1.04X. A total of 70 sequence reads corresponding to C. psittaci were identified.
3 Literature review
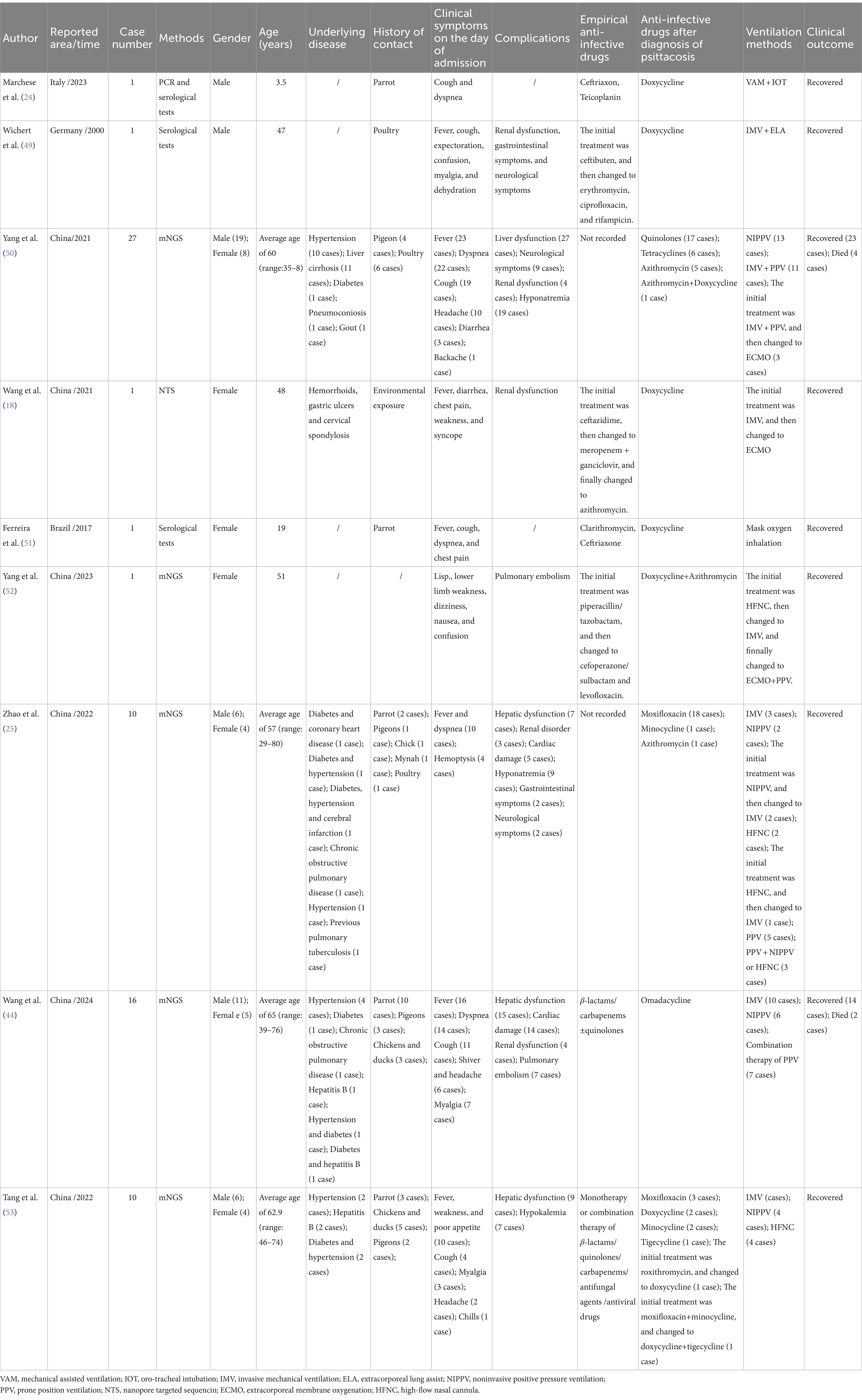
We searched the PubMed and Embase databases for articles on ARDS caused by C. psittaci published before January 31, 2024. The search strategies were “psittacosis” and “acute respiratory distress syndrome,” and a total of 79 articles were retrieved. Excluding duplicate studies or those with incomplete original data, a total of 9 articles were included for further study, with a total of 68 patients, including 45 males (66.2%) and 23 females (33.8%), as shown in Figure 6A. Detailed information is shown in Table 1. Among them, 64 cases were detected for C. psittaci through mNGS, 1 case was detected through nanopore targeted sequencing (NTS), 2 cases were confirmed through serological testing, and 1 case was confirmed through both serological testing and polymerase chain reaction (PCR) (Figure 6B). In particular, the majority of cases (n = 45, 66.2%) had a history of contact with birds and poultry, or exposure to the environment. Forty-six patients (67.6%) were reported to have underlying diseases, including hypertension, diabetes, cirrhosis and hepatitis B. In addition, 62 patients (91.2%) had fever upon admission. With the progression of the disease, most patients also experienced organ damage such as the liver, kidneys, heart, digestive system, and nervous system, in addition to developing into ARDS. The liver was the most commonly affected, including 58 cases (85.3%), and liver function involvement was reported to be unrelated to the underlying diseases of hepatitis B or cirrhosis. After the corresponding treatment, their liver function recovered. All patients received different ventilation methods to improve hypoxia. Notably, it was found that renal dysfunction might be a high-risk factor for patients with psittacosis complicated with ARDS, as the proportion of renal dysfunction in the deceased group was significantly higher than that in the survived group (83.3% vs 12.9%, p = 0.001) through Fisher’s exact test (Supplementary Table S1). Sixteen patients (23.5%) received positive pressure ventilation (PPV), and six patients (8.8%) received extracorporeal membrane oxygenation (ECMO) or extracorporeal lung assist (ELA) replacement therapy because of the difficulties in maintaining oxygenation during mechanical ventilation treatment. After being diagnosed with C. psittaci pneumonia, all patients received a single or combination therapy of tetracyclines (doxycycline, minocycline, tigecycline or omadacycline), quinolones (levofloxacin or moxifloxacin), or macrolides (azithromycin or roxithromycin), as shown in Figure 6C. Finally, 62 cases (91.2%) recovered and 6 cases (8.8%) died.

Figure 6. Gender (A), pathogen detection methods (B), and anti-infective drugs after diagnosis of psittacosis (C) of the 68 patients in our literature review.
4 Discussion
Psittacosis has erupted and spread in multiple regions worldwide (11, 12), with an increasing trend annually (5). Due to its potential to cause collective infection and illness during the epidemic, its infectivity and pathogenicity cannot be ignored, as it poses a significant threat to public health. Failure to diagnose diseases or identify pathogens in a timely manner could lead to delayed usage of effective antibiotics, resulting in high mortality rates in severe cases. Although there has been an increase in cases of psittacosis in recent years, psittacosis is still considered a rare disease, and there are still few reports on cases of ARDS caused by psittacosis. In addition, due to the diverse clinical manifestations and insufficient clinical understanding of the disease, it is often misdiagnosed or missed. Therefore, it is necessary to report and review cases of ARDS caused by psittacosis to promote the clinical understanding and management of the disease.
Chlamydia psittaci is a recognized zoonotic pathogen that is infectious to birds and poultry such as parrots, pigeons, chickens, and ducks, as well as mammals like horses, sheep, and cows. It can be transmitted to humans through the urine, feces, and other excreta of infected animals. In recent years, cases of human-to-human transmission have also been reported. The incubation period of psittacosis is usually 5 to 14 days, which may last up to 1 month (13). Patients often have a history of contact with birds and poultry (14, 15). Cleaning wild bird feeders or direct contact with feces contaminated with C. psittaci are considered important risk factors for infection (16). In addition, indirect or environmental contact with C. psittaci is also associated with infection with psittacosis (2, 17). In this report, the patient had contact with dead parrots before the onset of psittacosis, so dead parrots were highly likely to be the source of the pathogen. Our literature review reported a total of 68 cases, of which 44 had contact with birds or poultry. In addition, 1 patient had no clear history of contact, but lived in a community with large forests and birds (18). Therefore, in addition to inquiring about the patient’s direct contact history, it is also necessary to conduct a detailed investigation of the patient’s travel history, residential history, and environmental exposure, which may provide favorable clues for clinical diagnosis. However, patients without a clear history of contact are also common and cannot be ruled out.
Psittacosis is common in middle-aged and elderly people, with more males than females (19), and a lower risk of infection in children (20, 21). Psittacosis during pregnancy is rare, but it is highly likely to cause illness and death in pregnant women and fetuses (22, 23). According to reports, a 3.5-year-old child with C. psittaci-induced ARDS improved after treatment (24), whereas a pregnant woman discovered fetal death 2 days after undergoing tracheal intubation (25). Additionally, some reports have suggested that age (>65 year) and males are risk factors for severe C. psittaci pneumonia (19). This study indicates that among the cases with C. psittaci-induced ARDS, male patients accounted for a relatively large proportion, mainly middle-aged and elderly patients, which is consistent with previous reports. However, in this study, we reported a healthy young woman who eventually progressed to ARDS. Therefore, young women without underlying disease should also be vigilant about the possibility of developing into severe illness.
Chlamydia psittaci mainly infects the mucosal surface of human and animal hosts, causing various diseases (26). Initial replication begins in respiratory mucosal epithelial cells and macrophages, resulting in sepsis. Then, replication is carried out in epithelial cells and parenchymal tissues of the whole body (27), and various organs and systems may be affected. The clinical manifestations are diverse, and the severity of the disease varies from asymptomatic to severe (28). The respiratory system is most commonly affected, and almost all patients experience fever (26). Symptoms usually appear suddenly, and some patients may experience dyspnea. Severe cases may progress to ARDS (18), multiple organ dysfunction syndrome (MODS) (29, 30), and even death. Most patients also experience fatigue, muscle pain, central nervous system symptoms, and gastrointestinal symptoms (19). Notably, ARDS is usually associated with high mortality rates. An epidemiological study of ICU patients from 50 countries revealed that the incidence of ARDS was 10.4% of ICU admissions, and the hospital mortality rate was 46.1% for those with severe ARDS (8). Patients with psittacosis commonly progress to ARDS. A retrospective multicenter study involving 75 patients with psittacosis revealed that over half of the patients (41/74, 55.4%) developed ARDS, with 6 deaths and a mortality rate of 14.6% (31). However, owing to the limited sample scale, the overall incidence rate and mortality of ARDS caused by psittacosis still needs to be further determined through large-scale researches. Compared with general ARDS treatment strategies, timely use of antibiotics that can effectively inhibit C. psittaci is crucial for targeted therapy of psittacosis-related ARDS, in addition to respiratory support, nutritional support, fluid management, and symptomatic treatments such as glucocorticoids, sedatives, and muscle relaxants.
In the case reported in this study, the patient presented with fever as the primary manifestation, followed by cough, expectoration, and chills during the course of the disease. Her respiratory condition deteriorated rapidly, with dyspnea and ARDS, so she received mechanical ventilation. The pathogen was subsequently identified through mNGS, and timely treatment was provided. In addition to respiratory involvement, the liver and heart were also affected, which might be related to C. psittaci invading the liver and heart. In the literature review of this study, all patients with C. psittaci pneumonia who developed ARDS received oxygen therapy, severe cases received mechanical ventilation, and when NIPPV and PPV were insufficient to increase blood oxygen saturation, ECMO treatment was received. Almost all patients were diagnosed with ARDS combined with septic shock, and severe cases progressed to MODS. Among the 68 patients, 6 (8.8%) died due to multiple organ failure caused by secondary infection. Respiratory support, especially mechanical ventilation, plays an important role in the treatment of ARDS patients. Thus, the adoption of lung- protective ventilation strategies is recommended for all ARDS patients (32). Owing to the high metabolic status of ARDS patients, appropriate nutritional support should be provided timely (33). To alleviate the systemic inflammatory response, glucocorticoids can be used for severe ARDS patients, which can reduce the mortality rate (34). Additionally, sedatives should only be used in the early stages of patients with moderate-to-severe ARDS, and when there is still agitation and human–machine confrontation, muscle relaxants can be used (35). ARDS is a high-risk factor for deep vein thrombosis (DVT) and pulmonary embolism. Therefore, preventive anticoagulant therapy may be considered for ARDS patients without high-risk bleeding factors.
Early identification of the pathogen is crucial for targeted antibiotic treatment, which can reduce the mortality rate of ARDS caused by C. psittaci. However, for many hospitalized patients, timely and accurate diagnosis still poses challenges (36). Failure or delay in the diagnosis of infection may lead to prolonged hospital stays, readmission, and increased mortality rates (37, 38). In addition, undiagnosed patients always require empirical broad-spectrum antimicrobial therapy, increasing the risk of adverse drug reactions and antibiotic resistance (39). As a new approach to identify pathogens, mNGS has obvious advantages in detecting rare and complex pathogens in difficult cases, which is considered as a promising detection tool in the clinical diagnosis of unknown infections, such as C. psittaci (40). NTS is a fourth-generation sequencing technology that can provide faster and more comprehensive information for clinical diagnosis compared to mNGS. However, owing to its limitations, such as high cost and low popularity, there are few reports on its application in detecting C. psittaci. Although mNGS and NTS cannot replace traditional methods, they can serve as important supplements to microbiological pathogen testing tools and provide evidence for diagnosis in clinical. Among the 68 patients in our literature review, 64 were diagnosed with mNGS and 1 was diagnosed with NTS. There is no colonized C. psittaci in the normal human body, and C. psittaci belongs to intracellular bacteria. Due to the relatively small number of pathogens released into body fluids such as blood, sputum, and BALF, the detection sensitivity and positivity rate are relatively low. Therefore, once the DNA sequence of C. psittaci is detected, its potential as a pathogen must be considered. In our literature review, a patient with 8 DNA sequences of C. psittaci detected by mNGS still developed into a severe case (25). And in our case report, 70 DNA sequences of C. psittaci were detected in the BALF sample of the patient through mNGS. Therefore, even if a small amount of the C. psittaci DNA sequence was detected, it could be diagnosed as psittacosis, and targeted anti-infection treatment should be initiated in a timely manner.
Chlamydia psittaci is naturally resistant to β-lactams due to the lack of a cell wall, while tetracyclines and macrolides are effective drugs for treatment. Tetracyclines are regarded as the main recommended antibiotics for treating psittacosis. Among them, doxycycline is the preferred agent due to its strong intracellular activity and extensive clinical medication record (41). Over 90% of patients experience fever reduction within 48 h of taking doxycycline (42). In addition, minocycline has good antimicrobial activity and has been successfully applied in clinical practice (42). Tigecycline and omadacycline are the new generation of novel tetracycline antibiotics. Among them, tigecycline has a wide antibacterial spectrum and can be used as an alternative treatment for severe C. psittaci pneumonia, especially when patients are infected with other bacteria (43). Omadacycline has a higher concentration in the lungs than in the plasma and is eliminated mainly through feces. In patients with impaired liver and renal function and elderly individuals, the dosage does not need to be adjusted when omadacycline is used (44). However, owing to the immature clinical application of tigecycline and omadacycline, further large-scale clinical studies are still needed to observe whether their efficacy is superior to that of other tetracyclines. Macrolides, such as roxithromycin or azithromycin, can be used as alternative options for patients with a history of tetracycline allergy, as well as children, pregnant women, and other patients who are prohibited from using tetracyclines. In vitro studies have shown that fluoroquinolones are effective against C. psittaci (45), mainly by interfering with topoisomerase, thereby inhibiting the DNA synthesis of C. psittaci. However, the therapeutic effects of fluoroquinolones are not comparable to those of tetracyclines and macrolides (46), which may lead to treatment failure. At present, large-scale case–control studies comparing the efficacy differences between monotherapy and combination therapy are lacking. For patients with severe C. psittaci pneumonia or those with poor initial drug treatment, combination therapy may be preferable. In addition, antibiotic resistance is a clinical issue that needs attention. According to previous reports, genes related to the antibiotic resistance of C. psittaci include 16S rRNA, 23S rRNA, and rpoB, which are associated with different mechanisms leading to antibiotic resistance (47, 48). The abuse of antibiotics may further exacerbate the problem of microbial resistance, posing a threat to global public health safety. Therefore, precise targeted antimicrobial therapy is crucial.
Although the incidence rate of psittacosis is relatively low, it may lead to death in severe cases because of its strong pathogenicity, which poses a serious threat to human health. The nonspecific clinical manifestations and the lack of conventional detection methods pose challenges in the diagnosis of psittacosis. The contact history of birds and poultry before the onset of the disease has a suggestive effect on the diagnosis, and early identification needs to be combined with the actual medical history. Preliminary diagnosis can be quickly confirmed through mNGS, and once confirmed, targeted antimicrobial therapy should be administered as soon as possible. In addition, antibiotic resistance in Chlamydia remains a threat, and the development of new drugs against Chlamydia is urgently needed. At present, the prevention methods for psittacosis pneumonia are not yet mature, and it is necessary to explore new prevention methods or develop vaccines to prevent the spread and outbreak of this disease. Moreover, society and medical institutions need to attach great importance to the potential threat of C. psittaci, and it is recommended to include it in routine screening for respiratory pathogens. With the development of modern molecular biology technology and in-depth research on new drugs, more timely and accurate diagnostic methods for C. psittaci infection, as well as safer and more effective treatments, will provide strong support for clinical workers.
5 Conclusion
The clinical manifestations of C. psittaci pneumonia are diverse. For patients with severe C. psittaci pneumonia combined with ARDS, if not treated in a timely manner, it may be life-threatening. This study demonstrated the importance value of epidemiological investigations and the application of mNGS for early identification and diagnosis of psittacosis. As a highly promising detection tool, mNGS technology can help quickly and accurately identify pathogens, which contributes to promote the progress of targeted antimicrobial therapy and improve the prognosis of patients with severe infections. In addition, young psittacosis patients without underlying diseases should also be vigilant about the possibility of developing into severe cases.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the ethical committee of Chengdu second people‘s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YZh: Conceptualization, Resources, Writing – original draft. YZo: Data curation, Methodology, Validation, Writing – original draft. LZ: Investigation, Validation, Writing – original draft. HW: Investigation, Validation, Writing – original draft. Y-WZ: Data curation, Validation, Writing – original draft. X-RG: Data curation, Validation, Writing – original draft. NL: Data curation, Funding acquisition, Supervision, Validation, Visualization, Writing – review & editing. YL: Conceptualization, Formal analysis, Project administration, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1418241/full#supplementary-material
References
1. Bachmann, NL, Polkinghorne, A, and Timms, P. Chlamydia genomics: providing novel insights into chlamydial biology. Trends Microbiol. (2014) 22:464–72. doi: 10.1016/j.tim.2014.04.013
2. Chan, J, Doyle, B, Branley, J, Sheppeard, V, Gabor, M, Viney, K, et al. An outbreak of psittacosis at a veterinary school demonstrating a novel source of infection. One Health. (2017) 3:29–33. doi: 10.1016/j.onehlt.2017.02.003
3. Wallensten, A, Fredlund, H, and Runehagen, A. Multiple human-to-human transmission from a severe case of psittacosis, Sweden, January-February 2013. Euro Surveill. (2014) 19:20937. doi: 10.2807/1560-7917.es2014.19.42.20937
4. Zhang, Z, Zhou, H, Cao, H, Ji, J, Zhang, R, Li, W, et al. Human-to-human transmission of Chlamydia psittaci in China, 2020: an epidemiological and aetiological investigation. Lancet Microbe. (2022) 3:e512–20. doi: 10.1016/s2666-5247(22)00064-7
5. Liu, S, Cui, Z, Carr, MJ, Meng, L, Shi, W, and Zhang, Z. Chlamydia psittaci should be a notifiable infectious disease everywhere. Lancet Microbe. (2023) 4:e62–3. doi: 10.1016/s2666-5247(22)00306-8
6. Hogerwerf, L, de Gier, B, Baan, B, and van der Hoek, W. Chlamydia psittaci (psittacosis) as a cause of community-acquired pneumonia: a systematic review and meta-analysis. Epidemiol Infect. (2017) 145:3096–105. doi: 10.1017/s0950268817002060
7. Wu, X, Li, Y, Zhang, M, Li, M, Zhang, R, Lu, X, et al. Etiology of severe community-acquired pneumonia in adults based on metagenomic next-generation sequencing: a prospective multicenter study. Infect Dis Ther. (2020) 9:1003–15. doi: 10.1007/s40121-020-00353-y
8. Bellani, G, Laffey, JG, Pham, T, Fan, E, Brochard, L, Esteban, A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. (2016) 315:788–800. doi: 10.1001/jama.2016.0291
9. Paolone, S. Extracorporeal membrane oxygenation (ECMO) for lung injury in severe acute respiratory distress syndrome (ARDS): review of the literature. Clin Nurs Res. (2017) 26:747–62. doi: 10.1177/1054773816677808
10. Grasselli, G, Calfee, CS, Camporota, L, Poole, D, Amato, MBP, Antonelli, M, et al. ESICM guidelines on acute respiratory distress syndrome: definition, phenotyping and respiratory support strategies. Intensive Care Med. (2023) 49:727–59. doi: 10.1007/s00134-023-07050-7
11. Shaw, KA, Szablewski, CM, Kellner, S, Kornegay, L, Bair, P, Brennan, S, et al. Psittacosis outbreak among Workers at Chicken Slaughter Plants, Virginia and Georgia, USA, 2018. Emerg Infect Dis. (2019) 25:2143–5. doi: 10.3201/eid2511.190703
12. Yao, W, Chen, X, Wu, Z, Wang, L, Shi, G, Yang, Z, et al. A cluster of psittacosis cases in Lishui, Zhejiang Province, China, in 2021. Front Cell Infect Microbiol. (2022) 12:1044984. doi: 10.3389/fcimb.2022.1044984
13. Beeckman, DS, and Vanrompay, DC. Zoonotic Chlamydophila psittaci infections from a clinical perspective. Clin Microbiol Infect. (2009) 15:11–7. doi: 10.1111/j.1469-0691.2008.02669.x
14. Burnard, D, and Polkinghorne, A. Chlamydial infections in wildlife-conservation threats and/or reservoirs of 'spill-over' infections? Vet Microbiol. (2016) 196:78–84. doi: 10.1016/j.vetmic.2016.10.018
15. Dai, N, Li, Q, Geng, J, Guo, W, and Yan, W. Severe pneumonia caused by Chlamydia psittaci: report of two cases and literature review. J Infect Dev Ctries. (2022) 16:1101–12. doi: 10.3855/jidc.16166
16. Rehn, M, Ringberg, H, Runehagen, A, Herrmann, B, Olsen, B, Petersson, AC, et al. Unusual increase of psittacosis in southern Sweden linked to wild bird exposure, January to April 2013. Euro Surveill. (2013) 18:20478. doi: 10.2807/ese.18.19.20478-en
17. Branley, J, Bachmann, NL, Jelocnik, M, Myers, GS, and Polkinghorne, A. Australian human and parrot Chlamydia psittaci strains cluster within the highly virulent 6BC clade of this important zoonotic pathogen. Sci Rep. (2016) 6:30019. doi: 10.1038/srep30019
18. Wang, L, Shi, Z, Chen, W, Du, X, and Zhan, L. Extracorporeal membrane oxygenation in severe acute respiratory distress syndrome caused by Chlamydia psittaci: a case report and review of the literature. Front Med. (2021) 8:731047. doi: 10.3389/fmed.2021.731047
19. Ni, Y, Zhong, H, Gu, Y, Liu, L, Zhang, Q, Wang, L, et al. Clinical features, treatment, and outcome of psittacosis pneumonia: a multicenter study. Infect Dis. (2023) 10:ofac518. doi: 10.1093/ofid/ofac518
20. Palmer, SR. Psittacosis in man – recent developments in the UK: a review. J R Soc Med. (1982) 75:262–7. doi: 10.1177/014107688207500412
21. Rybarczyk, J, Versteele, C, Lernout, T, and Vanrompay, D. Human psittacosis: a review with emphasis on surveillance in Belgium. Acta Clin Belg. (2020) 75:42–8. doi: 10.1080/17843286.2019.1590889
22. Katsura, D, Tsuji, S, Kimura, F, Tanaka, T, Eguchi, Y, and Murakami, T. Gestational psittacosis: a case report and literature review. J Obstet Gynaecol Res. (2020) 46:673–7. doi: 10.1111/jog.14217
23. Tantengco, OAG. Gestational psittacosis: an emerging infection. Lancet Microbe. (2022) 3:e728. doi: 10.1016/s2666-5247(22)00191-4
24. Marchese, S, Marchese, G, Paviglianiti, G, Lapi, M, Ottoveggio, G, Pipitone, G, et al. A pediatric case of Chlamydia psittaci caused severe acute respiratory distress syndrome (ARDS) in Italy. Ital J Pediatr. (2023) 49:107. doi: 10.1186/s13052-023-01497-6
25. Zhao, ZL, Tang, X, He, CW, Liu, YL, Li, XY, Wang, R, et al. Clinical characteristics and outcomes of acute respiratory distress syndrome caused by severe Chlamydia psittaci pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. (2022) 45:1015–21. doi: 10.3760/cma.j.cn112147-20220221-00139
26. Cheong, HC, Lee, CYQ, Cheok, YY, Tan, GMY, Looi, CY, and Wong, WF. Chlamydiaceae: diseases in primary hosts and zoonosis. Microorganisms. (2019) 7:146. doi: 10.3390/microorganisms7050146
27. Vanrompay, D, Ducatelle, R, Haesebrouck, F, and Hendrickx, W. Primary pathogenicity of an European isolate of Chlamydia psittaci from Turkey poults. Vet Microbiol. (1993) 38:103–13. doi: 10.1016/0378-1135(93)90078-l
28. Smith, KA, Bradley, KK, Stobierski, MG, and Tengelsen, LA. Compendium of measures to control Chlamydophila psittaci (formerly Chlamydia psittaci) infection among humans (psittacosis) and pet birds, 2005. J Am Vet Med Assoc. (2005) 226:532–9. doi: 10.2460/javma.2005.226.532
29. Meijer, R, Van Biezen, P, Prins, G, and Boiten, HJ. Multi-organ failure with necrotic skin lesions due to infection with Chlamydia psittaci. Int J Infect Dis. (2021) 106:262–4. doi: 10.1016/j.ijid.2021.03.091
30. Zhang, H, Zhan, D, Chen, D, Huang, W, Yu, M, Li, Q, et al. Next-generation sequencing diagnosis of severe pneumonia from fulminant psittacosis with multiple organ failure: a case report and literature review. Ann Transl Med. (2020) 8:401. doi: 10.21037/atm.2020.03.17
31. Liu, K, Wu, L, Chen, G, Zeng, D, Zhong, Q, Luo, L, et al. Clinical characteristics of Chlamydia psittaci infection diagnosed by metagenomic next-generation sequencing: a retrospective multi-center study in Fujian, China. Infect Drug Resist. (2024) 17:697–708. doi: 10.2147/idr.S443953
32. Fan, E, Del Sorbo, L, Goligher, EC, Hodgson, CL, Munshi, L, Walkey, AJ, et al. An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. (2017) 195:1253–63. doi: 10.1164/rccm.201703-0548ST
33. Guo, Y, Cheng, J, and Li, Y. Influence of enteral nutrition initiation timing on curative effect and prognosis of acute respiratory distress syndrome patients with mechanical ventilation. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. (2018) 30:573–7. doi: 10.3760/cma.j.issn.2095-4352.2018.06.014
34. Chaudhuri, D, Nei, AM, Rochwerg, B, Balk, RA, Asehnoune, K, Cadena, RS, et al. Executive summary: guidelines on use of corticosteroids in critically ill patients with Sepsis, acute respiratory distress syndrome, and community-acquired pneumonia focused update 2024. Crit Care Med. (2024) 52:833–6. doi: 10.1097/ccm.0000000000006171
35. Moss, M, Huang, DT, Brower, RG, Ferguson, ND, Ginde, AA, Gong, MN, et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. (2019) 380:1997–2008. doi: 10.1056/NEJMoa1901686
36. Messacar, K, Parker, SK, Todd, JK, and Dominguez, SR. Implementation of rapid molecular infectious disease diagnostics: the role of diagnostic and antimicrobial stewardship. J Clin Microbiol. (2017) 55:715–23. doi: 10.1128/jcm.02264-16
37. Glimaker, M, Johansson, B, Grindborg, Ö, Bottai, M, Lindquist, L, and Sjolin, J. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis. (2015) 60:1162–9. doi: 10.1093/cid/civ011
38. Weiss, SL, Fitzgerald, JC, Balamuth, F, Alpern, ER, Lavelle, J, Chilutti, M, et al. Delayed antimicrobial therapy increases mortality and organ dysfunction duration in pediatric sepsis. Crit Care Med. (2014) 42:2409–17. doi: 10.1097/ccm.0000000000000509
39. Llor, C, and Bjerrum, L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf. (2014) 5:229–41. doi: 10.1177/2042098614554919
40. Gu, W, Deng, X, Lee, M, Sucu, YD, Arevalo, S, Stryke, D, et al. Rapid pathogen detection by metagenomic next-generation sequencing of infected body fluids. Nat Med. (2021) 27:115–24. doi: 10.1038/s41591-020-1105-z
41. Stewardson, AJ, and Grayson, ML. Psittacosis. Infect Dis Clin N Am. (2010) 24:7–25. doi: 10.1016/j.idc.2009.10.003
42. Yung, AP, and Grayson, ML. Psittacosis--a review of 135 cases. Med J Aust. (1988) 148:228–33. doi: 10.5694/j.1326-5377.1988.tb99430.x
43. Liu, J, and Gao, Y. Tigecycline in the treatment of severe pneumonia caused by Chlamydia psittaci: a case report and literature review. Front Med. (2022) 9:1040441. doi: 10.3389/fmed.2022.1040441
44. Wang, DX, Xiao, LX, Deng, XY, and Deng, W. Omadacycline for the treatment of severe pneumonia caused by Chlamydia psittaci complicated with acute respiratory distress syndrome during the COVID-19 pandemic. Front Med. (2023) 10:1207534. doi: 10.3389/fmed.2023.1207534
45. Donati, M, Rodrìguez Fermepin, M, Olmo, A, D'apote, L, and Cevenini, R. Comparative in-vitro activity of moxifloxacin, minocycline and azithromycin against Chlamydia spp. J Antimicrob Chemother. (1999) 43:825–7. doi: 10.1093/jac/43.6.825
46. Wu, HH, Feng, LF, and Fang, SY. Application of metagenomic next-generation sequencing in the diagnosis of severe pneumonia caused by Chlamydia psittaci. BMC Pulm Med. (2021) 21:300. doi: 10.1186/s12890-021-01673-6
47. Benamri, I, Azzouzi, M, Sanak, K, Moussa, A, and Radouani, F. An overview of genes and mutations associated with Chlamydiae species' resistance to antibiotics. Ann Clin Microbiol Antimicrob. (2021) 20:59. doi: 10.1186/s12941-021-00465-4
48. Lu, H, Yuan, J, Wu, Z, Wang, L, Wu, S, Chen, Q, et al. Distribution of drug-resistant genes in alveolar lavage fluid from patients with psittacosis and traceability analysis of causative organisms. Front Microbiol. (2023) 14:1182604. doi: 10.3389/fmicb.2023.1182604
49. Wichert, A, Lukasewitz, P, Häuser, MH, Bittersohl, J, and Lennartz, H. ARDS in fulminant ornithosis and treatment with extracorporeal lung assist. Int J Artif Organs. (2000) 23:371–4. doi: 10.1177/039139880002300605
50. Yang, F, Li, J, Qi, B, Zou, L, Shi, Z, Lei, Y, et al. Clinical symptoms and outcomes of severe pneumonia caused by Chlamydia psittaci in Southwest China. Front Cell Infect Microbiol. (2021) 11:727594. doi: 10.3389/fcimb.2021.727594
51. Ferreira, VL, Silva, MV, Bassetti, BR, Pellini, ACG, and Raso, TF. Intersectoral action for health: preventing psittacosis spread after one reported case. Epidemiol Infect. (2017) 145:2263–8. doi: 10.1017/s0950268817001042
52. Yang, SL, Gao, Y, Han, ZY, Du, X, Liu, W, Jin, SG, et al. Successful treatment of near-fatal pulmonary embolism and cardiac arrest in an adult patient with fulminant psittacosis-induced severe acute respiratory distress syndrome after veno-venous extracorporeal membrane oxygenation rescue: a case report and follow-up. Heliyon. (2023) 9:e20562. doi: 10.1016/j.heliyon.2023.e20562
Keywords: Chlamydia psittaci , acute respiratory distress syndrome, epidemiological investigation, metagenomic next-generation sequencing, case report
Citation: Zhou Y, Zou Y, Zhou L, Wei H, Zou Y-W, Guo X-R, Ye Y-Q, Li N and Lu Y (2024) Acute respiratory distress syndrome caused by Chlamydia psittaci: a case report and literature review. Front. Med. 11:1418241. doi: 10.3389/fmed.2024.1418241
Edited by:
Michael Marceau, Université Lille Nord de France, FranceReviewed by:
B. M. Munasinghe, Queen Elizabeth the Queen Mother Hospital, United KingdomJun Chu, China Agricultural University, China
Copyright © 2024 Zhou, Zou, Zhou, Wei, Zou, Guo, Ye, Li and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Lu, bHV5dW4wNzE0QDE2My5jb20=
 Yan Zhou1
Yan Zhou1 Ya Zou
Ya Zou Lu Zhou
Lu Zhou Hua Wei
Hua Wei Yun Lu
Yun Lu