- 1Department of Neurology, Chongqing Emergency Medical Center, Chongqing University Central Hospital, Chongqing University, Chongqing, China
- 2Clinical Medical College, Ya'an Vocational and Technical College, Ya'an, China
- 3Department of Ultrasonography, Chongqing Emergency Medical Center, Chongqing University Central Hospital, Chongqing University, Chongqing, China
Meningoencephalitis, an infectious disease affecting the nervous system, is primarily caused by a variety of pathogens. Non-tuberculous mycobacteria (NTM) have emerged as the leading causative agent of infections worldwide, but central nervous system infections resulting from NTM are infrequent in individuals with functioning immune systems. This case report highlights the diagnosis and treatment of a 26-year-old female patient who developed headaches 2 months post double eyelid surgery and was subsequently diagnosed with NTM meningoencephalitis through metagenomic next-generation sequencing (mNGS) analysis of cerebrospinal fluid. The patient underwent a comprehensive diagnostic and therapeutic protocol, resulting in a positive clinical outcome.
Introduction
Meningoencephalitis is a neurologic infectious disease caused by a variety of pathogens, including bacteria, viruses, fungi, spirochetes, and rickettsia (1). Non-tuberculous Mycobacteria (NTM), also known as environmental or atypical Mycobacteria, are Mycobacterium species other than Mycobacterium tuberculosis complex or Mycobacterium leprae. While the pathogenicity of NTM is generally lower than that of tuberculous mycobacteria, they have increasingly emerged as a significant cause of infection worldwide due to changes in the infectious disease landscape (2). Infections can manifest in various anatomical locations, such as the lungs, skin, and soft tissues, with the detection frequency of NTM exhibiting an upward trend in recent years (3, 4). Nevertheless, instances of central nervous system infection are infrequent, particularly among immunocompetent individuals (4). Hence, a comprehensive assessment encompassing species diversity, pathogenicity, geographical variations, modes of transmission, and predisposing factors is imperative for the accurate diagnosis of NTM meningoencephalitis. Additionally, the potential role of invasive procedures and implantation of foreign materials in cosmetic surgery, a trend that has gained popularity in recent times (5), should not be underestimated.
Case presentation
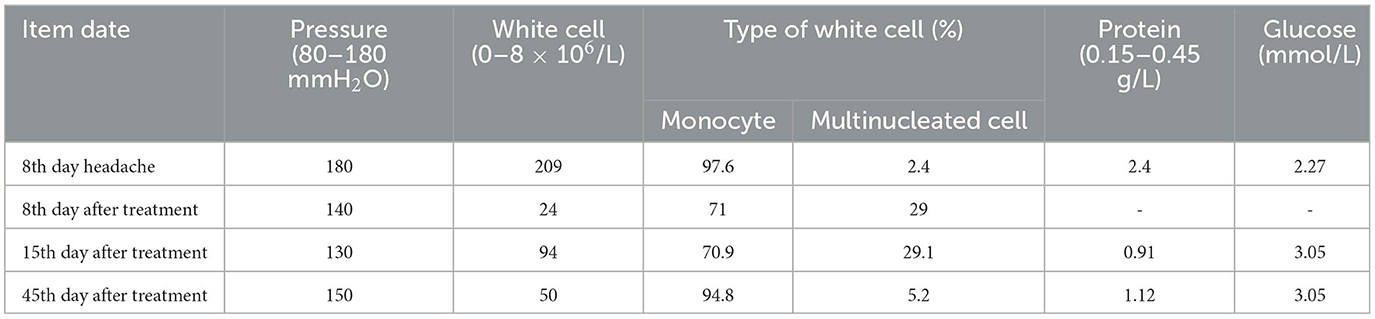
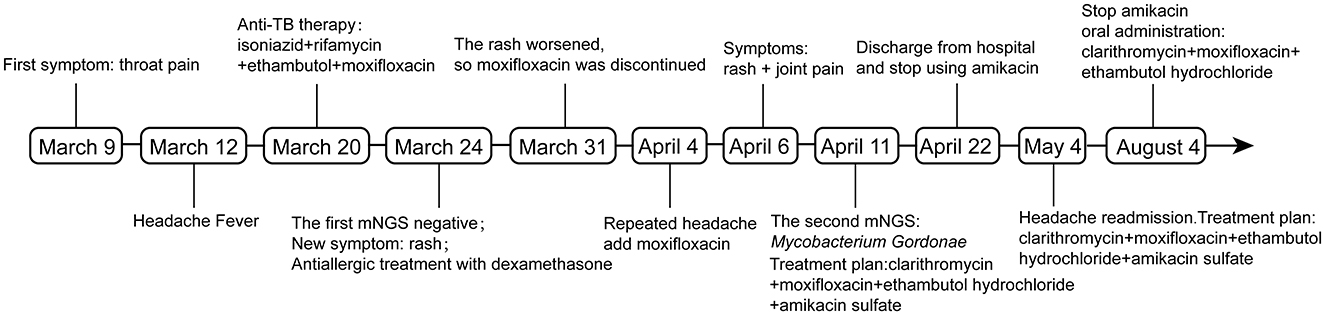
A 26-year-old Chinese female patient who underwent double eyelid surgery 2 months prior presented with night sweats, loss of appetite, and a subsequent 15 kg weight loss. On 9 March 2021, she reported symptoms of a sore throat, followed by temporal pain and fever (a peak temperature of 38.2°C) 3 days later. Following 4 days of antimicrobial therapy (cefalosporin and levofloxacin) at a community hospital, her sore throat had resolved. However, she had to be referred to our facility for persistent temporal headaches. Upon physical examination, her weight was 53 kg, vital signs were stable, and her cranial nerves, motor system, and sensory system were found to be normal. However, the Babinskis sign and meningeal irritation signs (Neck Stiffness, Kernig's Sign, and Brudzinski's Sign) were positive. The lumbar puncture demonstrated normal intracranial pressure but revealed elevated white blood cell and protein levels in the cerebrospinal fluid (Table 1). The metagenomic next-generation sequencing (mNGS) of the cerebrospinal fluid metagenome (by KingMed Diagnostics Group Co., Ltd.), acid-fast bacillus staining and ink staining were all normal. Chest CT showed a few striated bands within the middle lobe of the right lung and segmental atelectasis in the basal segment of the lower lobe of the left lung (Figures 1A, B). Enhanced CT imaging of the skull was normal. Immunodeficiency syndromes and autoimmune diseases were excluded based on the results of blood specimen testing. Considering her medical history, presenting symptoms of headache and fever, positive Babinskis sign and meningeal irritation signs, as well as the results of cerebrospinal fluid analysis, the possibility of meningoencephalitis (potentially tuberculosis or NTM) should be considered. From March 20th, 2021, four drugs were used (isoniazid, 0.6 g/day through intravenous drip; rifamycin, 0.6 g/day per os; ethambutol 0.75 g/day per os; and moxifloxacin 0.4 g through intravenous drip). The next day, the headache symptoms of the patients were relieved. On the 8th day after treatment, the pressure of cerebral effusion was normal, and the number of white blood cells and monocytes in cerebrospinal fluid decreased. During this period, she developed a rash, and the treatment with dexamethasone did not relieve it. Considering moxifloxacin allergy, the rash improved after suspension, and the remaining three drugs continued to be used according to the treatment guidelines for tuberculous meningoencephalitis. On the 15th day, the patient developed a severe headache again. The initial quadruple anti-tuberculosis treatment is definitely effective, but the regular triple tuberculosis patients have repeated conditions. To control the condition, we restart intravenous injection of moxifloxacin. Subsequently, the patient developed a systemic rash with joint pain. At this time, the second mNGS of cerebrospinal fluid was conducted to identify the pathogen. Upon reanalysis of the original mNGS data, 7 nucleic acid fragments of Mycobacterium gordonae were dentified in raw data (Supplementary Table 1). Despite the low number of M. gordonae sequences, the possibility of laboratory contamination or detection error was excluded as this bacterial sequence was not found in the cerebrospinal fluid mNGS of other patients from the same batch in the laboratory. In conclusion, she was diagnosed with M. gordonae meningoencephalitis. The adjusted treatment plan was clarithromycin 0.75 g/day per os, moxifloxacin 0.8 g/day per os, ethambutol hydrochloride 0.75 g/day per os and amikacin sulfate 0.8 g/day through intravenous drip. And her condition was relieved again. After 1 month of treatment, the patient was discharged from the hospital. Because the patient refused to continue to use amikacin for intravenous injection, she continued to take clarithromycin, moxifloxacin and ethambutol orally after discharge according to China 2020 Guidelines for Diagnosis and Treatment of Non-tuberculous Mycobacterium (6). On the 10th day after discharge, she was readmitted to the hospital with recurrent headaches with nausea. She was no infections were found in her urinary, gynecological, or gastrointestinal examinations. Head MRI showed no abnormality in the brain parenchyma, and chest CT showed no obvious changes. And cerebrospinal fluid pressure is normal, and white blood cells and monocytes in cerebrospinal fluid are increased. We still consider the diagnosis first: NTM meningoencephalitis. Four drugs were used (including clarithromycin 0.75 g/day per os; moxifloxacin 0.8 g/day per os; ethambutol hydrochloride 0.75 g/day per os and amikacin sulfate 0.8 g/day through intravenous drip). After 3 days, the symptoms were relieved. After 3 months of intravenous amikacin infusion, the condition was stabilized and discontinued. After 18 months, she stopped all drug treatment, and her condition was stable without recurrence. The complete treatment process is illustrated in Figure 2.
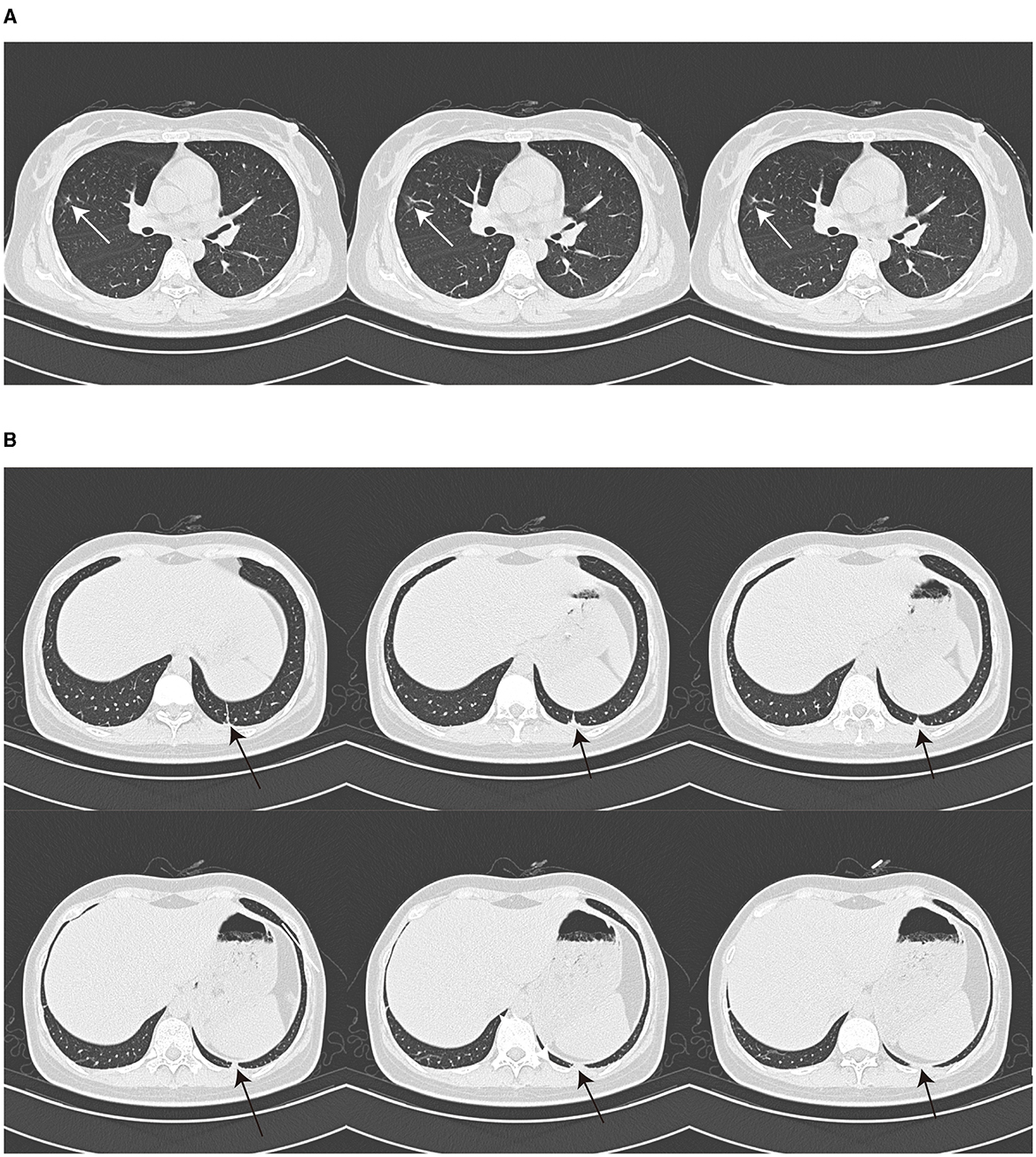
Figure 1. Chest CT image. Chest CT showed a few striated bands in the middle lobe of the right lung [(A), arrow] and segmental atelectasis in the basal segment of the lower lobe of the left lung [(B), arrow].
Discussion and conclusion
Initially, it was believed that NTM primarily caused infections in immunocompromised individuals, including those with HIV infection, affecting organs such as the lungs, skin, and soft tissues, with rare transmission through organs (7). Individuals with conditions such as HIV infection, solid organ transplants, corticosteroid use, long-term hemodialysis for kidney failure, diabetes, and malignant tumors are considered at risk for M. gordonae infection, and the manifestations of infection are also varied (8, 9). There have been reports of clinically significant infections in immunocompetent individuals, regardless of age, gender, or immune status (9–13). M. gordonae infection can be triggered by various factors, the most significant of which are invasive procedures and new surgical interventions (14). Our case study further supports this finding, highlighting the significance of the patient's prior double eyelid surgery.
M. gordonae is frequently found in tap water (15, 16) and medical system water (14, 17). There exists a potential risk of NTM infection following invasive eyelid surgery in a hospital setting. In this case the patient presented with night sweats, loss of appetite, weight loss, followed by sore throat and temporal pain after cosmetic eyelid surgery and was finally diagnosed with intracranial infection caused by M. gordonae. The association between this type of infection and double eyelid surgery cannot be disregarded. It is well-established that the facial vein and ocular vein play a crucial role in draining venous blood from the facial region, eyeball, and ocular appendages. The ocular vein connects with the facial vein anteriorly and communicates with intracranial veins posteriorly. Furthermore, the ocular vein lacks valves to regulate blood flow direction, potentially allowing facial infections to spread intracranially through the ocular vein (18). The invasive nature of double eyelid surgery may increase the risk of infectious agents entering the surgical site (19). Our case reports suggest a need for reevaluation of the potential severity of surgical complications associated with this common medical cosmetic procedure.
Prior studies have indicated that NTM infections in immunocompetent individuals, initially presenting as meningoencephalitis, exhibit a rapid progression and pose challenges in diagnosis, often resulting in mortality within a 2-week timeframe and necessitating post-mortem confirmation. Conversely, survivors typically display localized lesions, such as intracranial encapsulated abscesses, with diagnosis typically confirmed through post-surgical pathological examination (20). Currently, routine microbial culture remains the diagnostic tool for NTM infections. However, some NTM cultures necessitate the utilization of specialized media, the adherence to incubation temperatures, or the extension of the incubation period. And the presence of a lower pathogenic microorganism load in cerebrospinal fluid, posing challenges to clinical diagnosis due to the limited sensitivity of traditional diagnostic methods such as cultures and smears. In contrast, metagenomic next-generation sequencing (mNGS) offers improved sensitivity and specificity in detecting pathogenic bacteria compared to culture methods (21, 22). mNGS is capable of identifying pathogenic microorganisms in various clinical specimens, including cerebrospinal fluid (23–25). Hence, mNGS offers direct detection of seven nucleic acid fragments of M. gordonae, presenting significant implications for early diagnosis. The concurrent utilization of mNGS, cultures, and smears, along with meticulous comparison of results obtained from the same set of samples, may represent an optimal approach for attaining expedited and precise diagnostic outcomes.
Four efficacious anti-M. gordonae medications were selected for the treatment regimen: clarithromycin, moxifloxacin, ethambutol hydrochloride, and amikacin. Clarithromycin (26, 27), a macrolide antibiotic, exerts its bacteriostatic effects by inhibiting protein synthesis through the obstruction of the 50S ribosomal subunit. Over the past two decades, clarithromycin has been the cornerstone of treatment for NTM infections due to its broad-spectrum efficacy against various NTM species, including M. gordonae. Furthermore, clarithromycin is capable of crossing the blood-brain barrier (BBB) in cases of meningoencephalitis. The recommended dosage for adults weighing <50 kg is 500–750 mg per day, whereas adults weighing 50 kg or more should receive 750–1,000 mg per day, with a maximum daily dose of 1,000 mg. Moxifloxacin (26–28) functions by inhibiting and preventing the replication and transcription of bacterial DNA, thereby exerting a sterilizing effect on the bacteria. It exhibits a more pronounced efficacy against slow-growing NTM, such as M. gordonae, while also demonstrating antibacterial activity against fast-growing NTM. Additionally, moxifloxacin has been shown to possess favorable permeability across the BBB. The established dosage regimen for adult's ranges from 400 to 800 mg per day, administered either orally or intravenously once daily. In this case, the patient exhibited a skin rash and joint pain following intravenous administration, which are recognized as common allergy and adverse effects. Nonetheless, these symptoms resolved upon transitioning to oral administration, suggesting that the route of administration may impact the manifestation of drug side effects. The concomitant use of moxifloxacin with other anti-NTM agents has demonstrated an enhancement in the drug's efficacy. It must be noted that moxifloxacin is associated with cardiotoxicity, specifically the prolongation of the Q-T interval. Ethambutol (25, 29) is the most commonly utilized and essential pharmaceutical agent in the treatment of NTM disease. It functions by inhibiting the synthesis of the cell wall of Mycobacterium and demonstrates antibacterial activity against M. gordonae. The recommended dosage for adults is 15–25 mg/kg/day, with a maximum daily limit of 1,250 mg. Amikacin (26, 30) exhibits a more pronounced bactericidal effect against M. gordonae, with an adult dosage of 15–20 mg/kg/day and a maximum daily limit of 1.0 g. The administration of this treatment is conducted through either intramuscular injection or intravenous infusion. However, the cessation of amikacin during the treatment course led to an extended duration of therapy, potentially affecting the ultimate therapeutic outcome. Subsequent to the second intervention, the patient expressed a commitment to adhere to the prescribed treatment regimen, which includes combination pharmacotherapy and prolonged intravenous infusions. The treatment protocol for this case indicates the necessity of considering several critical factors: the selection of appropriate therapeutic agents must be guided by the specific type of infectious agent, the pattern of drug resistance, and the site of infection. Notably, clarithromycin, ethambutol, and amikacin possess the capability to traverse the BBB and exert antimicrobial effects within the central nervous system, particularly when inflammation has compromised the integrity of the BBB. Furthermore, rigorous safety monitoring and management and treatment management are essential for all patients undergoing treatment for NTM disease. Currently, there is an absence of standardized treatment protocols for NTM infections affecting the central nervous system. This case study could provide valuable insights for the diagnosis and management of such patients in the future.
This case represents the initial documentation of M. gordonae meningoencephalitis arising from eyelid surgery, and notably, the first instance in a non-immunocompromised individual where the condition presented as meningoencephalitis and was successfully treated. Meningoencephalitis caused by NTM infection is characterized by its swift and aggressive progression. Utilization of mNGS can aid in the prompt identification of NTM infection, yet it is imperative to meticulously compare laboratory parameters and test outcomes from a consistent set of samples to arrive at a comprehensive assessment. Furthermore, timely diagnosis and appropriate, comprehensive, consistent, and sustained pharmacological treatment yield favorable clinical results. Additionally, the first documented cases of NTM meningoencephalitis linked to medical cosmetic surgery highlight the emergence of novel disease risks, particularly in light of the growing prevalence of medical cosmetic surgery.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Chongqing Emergency Medical Center Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
DY: Writing – review & editing, Data curation. XD: Data curation, Writing – review & editing. JC: Investigation, Writing – review & editing. YZ: Investigation, Methodology, Writing – original draft. XW: Writing – review & editing. JZ: Data curation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Chongqing Science and Health Joint Medical Research Project (2022MSXM112).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1416272/full#supplementary-material
References
2. Donohue MJ. Epidemiological risk factors and the geographical distribution of eight Mycobacterium species. BMC Infect Dis. (2021) 21:258. doi: 10.1186/s12879-021-05925-y
3. Yu X, Liu P, Liu G, Zhao L, Hu Y, Wei G, et al. The prevalence of non-tuberculous mycobacterial infections in mainland China: systematic review and meta-analysis. J Infect. (2016) 73:558–67. doi: 10.1016/j.jinf.2016.08.020
4. Huang JJ, Li YX, Zhao Y, Yang WH, Xiao M, Kudinha T, et al. Prevalence of nontuberculous mycobacteria in a tertiary hospital in Beijing, China, January 2013 to December 2018. BMC Microbiol. (2020) 20:158. doi: 10.1186/s12866-020-01840-5
5. American Society for Dermatologic Surgery. Consumer Survey on Cosmetic Dermatologic Procedures, Data Were Collected From 3,503 Consumers Through a Blind Online Survey in 2023 (2023). Available at: https://www.asds.net/consumer-survey (accessed September 25, 2023).
6. Branch CMAT. Guidelines for the diagnosis and treatment of nontuberculous mycobacterial diseases (2020 edition). Chin J Tubercul Respirat Dis. (2020) 43:918–46. doi: 10.3760/cma.j.cn112147-20200508-00570
7. Lim A, Chotirmall SH, Fok E, Verma A, De PP, Goh SK, et al. Profiling non-tuberculous mycobacteria in an Asian setting: characteristics and clinical outcomes of hospitalized patients in Singapore. BMC Pulm Med. (2018) 18:85. doi: 10.1186/s12890-018-0637-1
8. Pamparino S, Valente I, Tagliafico A, Dentone C, Bassetti M, Mennella S, et al. A very rare case of mycobacterium gordonae infection of the breast. Breast J. (2020) 26:2229–32. doi: 10.1111/tbj.14086
9. Chang HY, Tsai WC, Lee TF, Sheng WH. Mycobacterium gordonae infection in immunocompromised and immunocompetent hosts: a series of seven cases and literature review. J Formos Med Assoc. (2021) 120:524–32. doi: 10.1016/j.jfma.2020.06.029
10. Asija A, Prasad A, Eskridge E. Disseminated Mycobacterium gordonae infection in an immunocompetent host. Am J Ther. (2011) 18:e75–7. doi: 10.1097/MJT.0b013e3181e32e55
11. Chen Y, Jiang J, Jiang H, Chen J, Wang X, Liu W, et al. Mycobacterium gordonae in patient with facial ulcers, nosebleeds, and positive T-SPOTTB test, China. Emerg Infect Dis. (2017) 23:1204–6. doi: 10.3201/eid2307.162033
12. Freyne B, Curtis N. Mycobacterium gordonae skin infection in an immunocompetent child. Pediatr Infect Dis J. (2017) 36:523–5. doi: 10.1097/INF.0000000000001549
13. Bilolikar VK, Ilyas AM. Mycobacterial infections of the hand. Hand. (2022) 17:772–9. doi: 10.1177/1558944720940064
14. Satta Y, Yamashita M, Matsuo Y, Kiyokawa H, Sato Y, Takemura H, et al. Non-tuberculous mycobacterial pseudo-outbreak of an intestinal culture specimen caused by a water tap in an endoscopy unit. Intern Med. (2020) 59:2811–5. doi: 10.2169/internalmedicine.5188-20
15. Oriani AS, Sierra F, Baldini MD. Effect of chlorine on Mycobacterium gordonae and Mycobacterium chubuense in planktonic and Biofilm State. Int J Mycobacteriol. (2018) 7:122–7. doi: 10.4103/ijmy.ijmy_30_18
16. Tortone CA, Oriani DS, Staskevich AS, Oriani AS, Gino LM, Marfil MJ, et al. Species diversity of non-tuberculous mycobacteria isolated from aquatic environments of General Pico city, Province of La Pampa (Argentina). Rev Argent Microbiol. (2019) 51:259–67. doi: 10.1016/j.ram.2018.08.005
17. Arfaatabar M, Karami P, Khaledi A. An update on prevalence of slow-growing mycobacteria and rapid-growing mycobacteria retrieved from hospital water sources in Iran—a systematic review. Germs. (2021) 11:97–104. doi: 10.18683/germs.2021.1245
18. Siwetz M, Widni-Pajank H, Hammer N, Pilsl U, Bruneder S, Wree A, et al. The course and variation of the facial vein in the face-known and unknown facts: an anatomical study. Medicina. (2023) 59:1479. doi: 10.3390/medicina59081479
19. Liu J, Song B. Review of complications in double eyelid surgery. Indian J Ophthalmol. (2022) 70:1460–5. doi: 10.4103/ijo.IJO_1518_21
20. Talati NJ, Rouphael N, Kuppalli K, Franco-Paredes C. Spectrum of CNS disease caused by rapidly growing mycobacteria. Lancet Infect Dis. (2008) 8:390–8. doi: 10.1016/S1473-3099(08)70127-0
21. Huang Z, Zhang C, Fang X, Li W, Zhang C, Zhang W, et al. Identification of musculoskeletal infection with non-tuberculous mycobacterium using metagenomic sequencing. J Infect. (2019) 78:158–69. doi: 10.1016/j.jinf.2018.10.002
22. Wang S, Chen Y, Wang D, Wu Y, Zhao D, Zhang J, et al. The feasibility of metagenomic next-generation sequencing to identify pathogens causing tuberculous meningitis in cerebrospinal fluid. Front Microbiol. (2019) 10:1993. doi: 10.3389/fmicb.2019.01993
23. Parize P, Muth E, Richaud C, Gratigny M, Pilmis B, Lamamy A, et al. Untargeted next-generation sequencing-based first-line diagnosis of infection in immunocompromised adults: a multicentre, blinded, prospective study. Clin Microbiol Infect. (2017) 23:574.e1–e6. doi: 10.1016/j.cmi.2017.02.006
24. Ai JW, Li Y, Cheng Q, Cui P, Wu HL, Xu B, et al. Diagnosis of local hepatic tuberculosis through next-generation sequencing: smarter, faster and better. Clin Res Hepatol Gastroenterol. (2018) 42:178–81. doi: 10.1016/j.clinre.2018.04.007
25. Miao Q, Ma Y, Wang Q, Pan J, Zhang Y, Jin W, et al. Microbiological diagnostic performance of metagenomic next-generation sequencing when applied to clinical practice. Clin Infect Dis. (2018) 67:S231–40. doi: 10.1093/cid/ciy693
26. Li G, Pang H, Guo Q, Huang M, Tan Y, Li C, et al. Antimicrobial susceptibility and MIC distribution of 41 drugs against clinical isolates from China and reference strains of nontuberculous mycobacteria. Int J Antimicrob Agents. (2017) 49:364–74. doi: 10.1016/j.ijantimicag.2016.10.024
27. Shen Y, Wang X, Jin J, Wu J, Zhang X, Chen J, et al. In vitro susceptibility of Mycobacterium abscessus and Mycobacterium fortuitum isolates to 30 antibiotics. Biomed Res Int. (2018) 2018:4902941. doi: 10.1155/2018/4902941
28. Maurer FP, Pohle P, Kernbach M, Sievert D, Hillemann D, Rupp J, et al. Differential drug susceptibility patterns of Mycobacterium chimaera and other members of the Mycobacterium avium-intracellulare complex. Clin Microbiol Infect. (2019) 25:379.e1–e7. doi: 10.1016/j.cmi.2018.06.010
29. Brown-Elliott BA, Woods GL. Antimycobacterial susceptibility testing of nontuberculous mycobacteria. J Clin Microbiol. (2019) 57:e00834–19. doi: 10.1128/JCM.00834-19
30. Griffith DE, Eagle G, Thomson R, Aksamit TR, Hasegawa N, Morimoto K, et al. Amikacin liposome inhalation suspension for treatment-refractory lung disease caused by Mycobacterium avium complex (CONVERT). A prospective, open-label, randomized study. Am J Respir Crit Care Med. (2018) 198:1559–69. doi: 10.1164/rccm.201807-1318OC
Keywords: meningoencephalitis, Mycobacterium gordonae, mNGS, double eyelid surgery, case report
Citation: Yuan D, Ding X, Chen J, Zhao Y, Wang X and Zhu J (2024) Case report: A rare case of meningoencephalitis caused by Mycobacterium gordonae. Front. Med. 11:1416272. doi: 10.3389/fmed.2024.1416272
Received: 21 April 2024; Accepted: 23 September 2024;
Published: 23 October 2024.
Edited by:
Vishwanath Venketaraman, Western University of Health Sciences, United StatesReviewed by:
Anand Kumar Maurya, All India Institute of Medical Sciences, Bhopal, IndiaAlfonso Martin Cabello Vilchez, Peruvian University Cayetano Heredia, Peru
Copyright © 2024 Yuan, Ding, Chen, Zhao, Wang and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Zhu, OTk1OTQ4MTcyQHFxLmNvbQ==
†These authors have contributed equally to this work
 Dezhi Yuan1†
Dezhi Yuan1† Jie Zhu
Jie Zhu