- 1The Dermatology Department of Shanxi Provincial People’s Hospital, Five Hospital of Shanxi Medical University, Taiyuan, China
- 2Department of Dermatology, Universitätsklinikum Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany
- 3Department of Minimally Invasive Spine Surgery, The Affiliated Shanxi Provincial People’s Hospital of Shanxi Medical University, Taiyuan, China
Acquired reactive perforating collagenosis (ARPC) is a rare dermatological disorder condition defined by the perforation of altered collagen fibers through the epidermis. The presence of underlying conditions such as diabetes or renal disease is helpful in the ARPC diagnosis. Although skin rashes related to ARPC have been reported, the exact causative factors and mechanisms remain unclear. Here, we present a unique case of ARPC triggered by trauma in a 67-year-old male without concurrent systemic alterations. The diagnosis of ARPC with eosinophilia was made following comprehensive diagnostic testing, including clinical presentation, histological results, and blood tests, ruling out other possible diseases. Intriguingly, the histopathological examination revealed collagen penetration into the epidermis at different tissue sections. In addition, we reviewed existing literature on ARPC, which documented the causation. To help confirm the diagnosis, clinicians have to pay attention to traumatic triggers for ARPC and its rare manifestation with eosinophilia.
Introduction
Reactive perforating collagenosis (RPC), first described by Mehregan et al. (1), is typically considered a rare dermatological condition disorder defined by the transepidermal elimination of altered collagen fibers through the epidermis. It can be classified into two variants: inherited RPC (IRPC) and acquired RPC (ARPC) (2). ARPC is more frequently observed in adult population and is commonly associated with underlying systemic conditions, notably diabetes mellitus, chronic kidney disease, and hypertension (3). It can also be associated by cirrhosis, pulmonary fibrosis, hypothyroidism or hyperthyroidism, and malignant tumors, with reports of ARPC occurring during pregnancy (4). Additionally, drugs such as efalizumab, infliximab, and rituximab, as well as skin irritation from mosquito bites, hemodialysis, and laser hair removal, can induce the onset of this disease, with hemodialysis being the most significant risk factor (5). However, cases of ARPC caused by trauma without any underlying disease and increased eosinophilia in patients have been rarely reported.
We present a case of ARPC with eosinophilia. The uniqueness of this case lies in the ARPC being attributed to a scratch from a branch, the absence of commonly associated systemic conditions, such as diabetes mellitus and chronic kidney disease, and the presence of peripheral eosinophilia. We believe that providing an updated review of ARPC in the medical literature would help clarify certain uncertain points regarding its etiology, differential diagnosis, and pathogenesis.
Case description
A 67-year-old male patient presented to the dermatology outpatient clinic with a chief complaint of intensely pruritic papules lesions on his bilateral lower limbs, which had persisted for 2 months. The patient reported subsequent spread of the lesions to his trunk and abdomen (Figure 1). He recalled that the onset was subsequent to a trauma from a branch while exercising without using medicine or having comorbidities before the onset of this episode, resulting in several erythematous, itchy cutaneous lesions at the same location. Following that, scattered papules and nodules appeared, causing severe itching and sleep disturbances. There was no reported history of diabetes mellitus, hypertension, or chronic renal disease in this family (although genetic analysis was not obtained). He sought treatment at an external clinic multiple times and received topical medication (specifics unknown), with no significant improvement observed. On physical examination, crateriform lesions with an erythematous base and crusty substance inside (Figures 1C,D), along with similar but smaller lesions on his trunk and abdomen (Figures 1A,B). Routine serum laboratory tests revealed in all four tests the absolute eosinophil count > 0.63 × 109/L (normal reference range 0.05 × 109/L to 0.5 × 109/L) and a percentage of blood eosinophils > 7.7% (normal reference range 0.5 to 5%). Other routine serum laboratory investigations, including liver function tests, renal function tests, serum glucose level, urinalysis and stool exam, were all within normal limits.
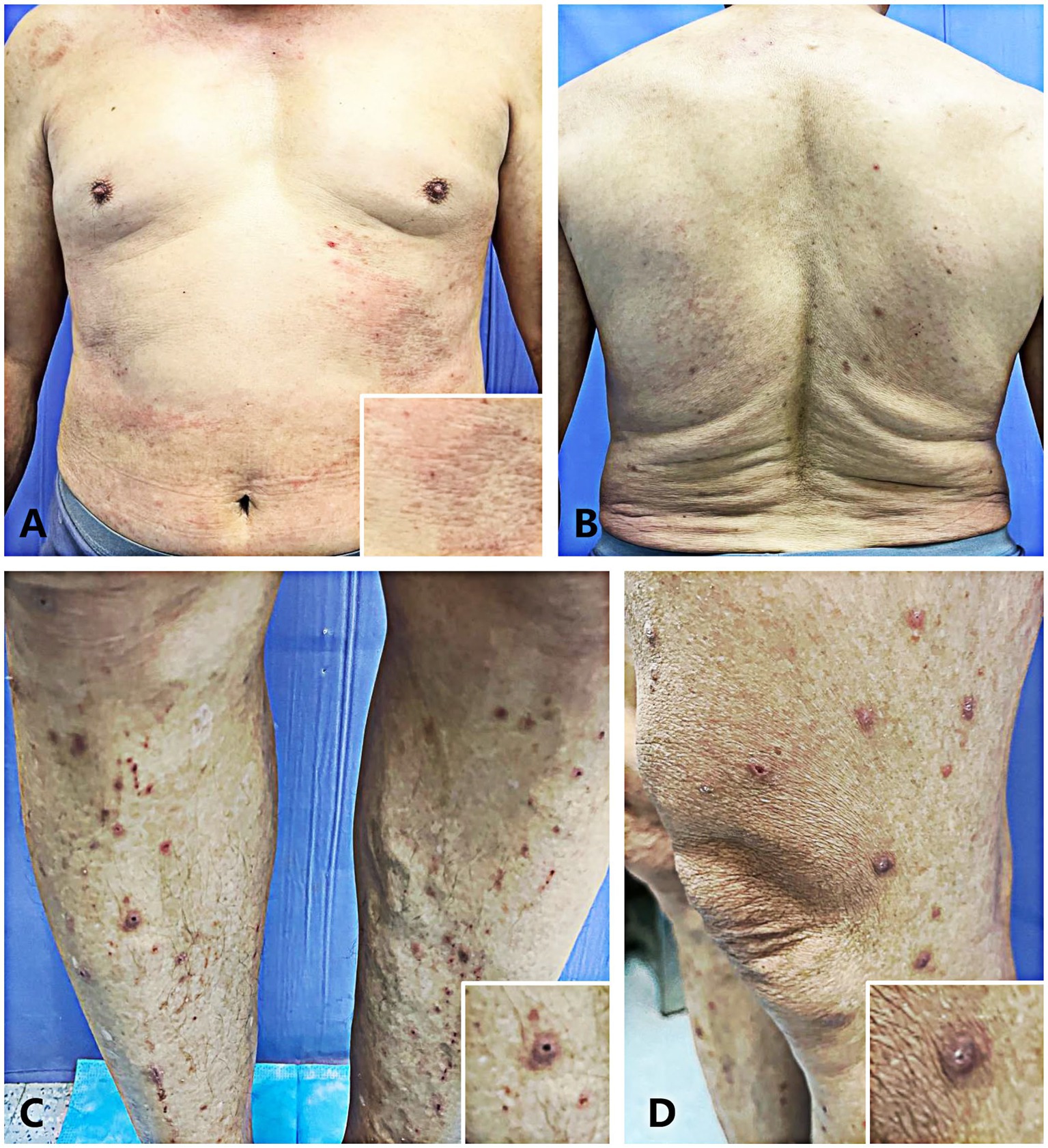
Figure 1. (A,B) The patient’s lesions presented as flat reddish macules and papules on the abdomen (A) and trunk (B). (C,D) The patient presented with multiple well-circumscribed keratotic papules bilaterally distributed on the lower extremities.
A skin biopsy was performed on a representative lesion located on the lower extremity. Histopathological examination revealed degenerated collagen bundles that had been eliminated to form a cup-shaped epidermal depression (Figure 2A). High-power view highlighted the degenerating collagen bundles, vertically oriented, and the basophilic debris within the crater. Numerous neutrophils and lymphocytic infiltration were seen directly under the depression (Figure 2B). The findings were consistent with reactive perforating collagenosis, as evidenced by the presence of collagen elimination before (Supplementary Figures 1A,B) and after complete penetration through the epidermis (Figures 2A,B; Supplementary Figures 1C,D). Masson’s trichrome staining showed that collagen bundles perforate the base of the epidermal erosion (Figure 2C). High-power view highlighted the degenerating collagen bundles, vertically oriented, and the basophilic debris within the crater (Figure 2D).
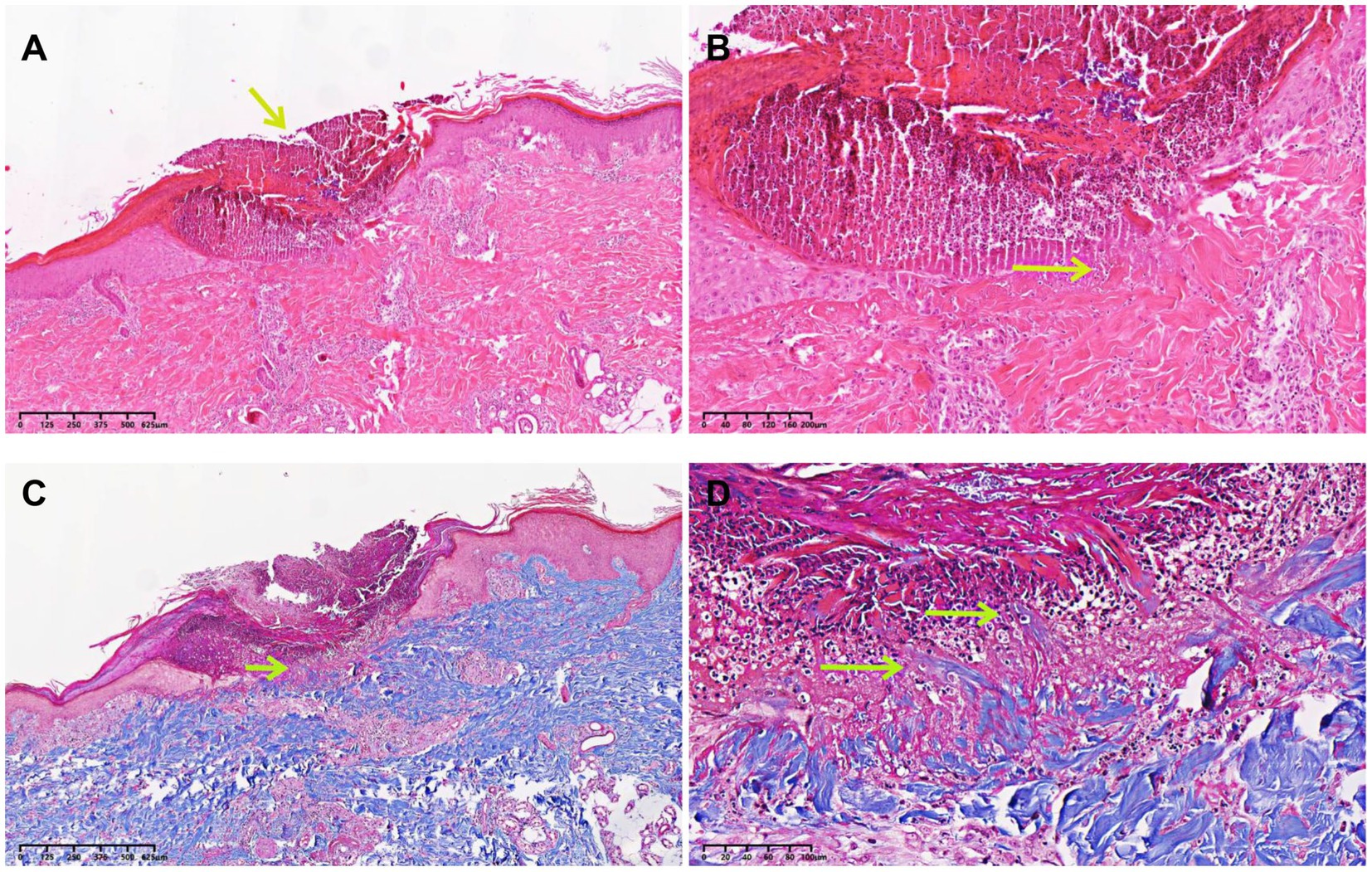
Figure 2. Histopathological examination of the biopsy specimen. (A) A skin biopsy sample from the papule located on the lower extremity demonstrated degenerated collagen bundles that had been eliminated to form a cup-shaped epidermal depression (HE, ×10, arrow). (B) High-power view highlighted the degenerating collagen bundles, vertically oriented, and the basophilic debris within the crater. Numerous neutrophils and lymphocytic infiltration were seen directly under the depression (HE, ×100, arrow). (C) Collagen bundles perforated the base of the epidermal erosion (Masson’s trichrome staining, ×10, arrow). (D) High-power view highlighted the degenerating collagen bundles, vertically oriented, and the basophilic debris within the crater (Masson’s trichrome staining, ×100, arrows).
A diagnosis of ARPC with eosinophilia was established. Treatment with topical halomethasone monohydrate at a dosage of 3 g per day for 2 weeks, in combination with oral antihistamine therapy consisting of levocetirizine at 5 mg per day and tripterygium glycoside at 60 mg per day for a duration of 5 weeks. This therapeutic regimen effectively alleviated the patient’s pruritus and prevented further scratching of the affected areas. After 2 months of treatment, the skin lesions showed significant resolution, with only temporary post-inflammatory hyperpigmentation remaining at the original lesion sites. The patient was informed of the importance of regular follow-up and continued to be monitored accordingly.
Discussion
ARPC caused by trauma without any underlying disease and increased eosinophilia in patients have been rarely reported. Mehregan et al. (1) suggested that trauma in genetically susceptible individuals leads to collagen necrobiosis in the dermal papillae. Herzinger et al. (6) showed that immune activation may be involved in the development of ARPC, with collagen types IV and VII from the basal membrane identified in the plug of ARPC skin lesions. Other studies suggested that the transepidermal elimination of collagen may be a response to chronic scratching or rubbing in pruritic conditions (7). The investigation of ARPC is of paramount importance for several reasons, including its rare documentation in the published literature, the crucial role of histopathological examination in establishing the diagnosis, and its frequent association with underlying systemic disorders (8). Consistent with the literature (9), our patient presented with a disseminated cutaneous eruption involving the abdomen, trunk, and lower extremities. Histopathological examination of the skin biopsy revealed a well-circumscribed area of necrosis containing a keratotic plug. Within this necrotic region, parakeratotic cells and lymphocytic infiltration were observed. Notably, sparse collagen fibers in the dermis were found to perforate the epidermis, forming a cup-shaped structure surrounded by acanthosis and hyperkeratosis of the epidermis. Furthermore, a lymphocytic infiltrate was present in the superficial dermis and perivascular regions. Intriguingly, the histopathological examination captured the penetration of collagen into the epidermis at different tissue sections.
The differential diagnosis for this case primarily includes Kyrle’s disease (KD), perforating folliculitis (PF), and elastosis perforans serpiginosa (EPS) (7, 10), while ARPC, KD, PF, and EPS share the common features of transepidermal elimination of altered dermal components, the differentiation is made based on the types of epidermal damage and the characteristics of the eliminated material. Distinguishing ARPC from other perforating dermatoses can be challenging, and the clinical presentation and histopathological examination are crucial for accurate diagnosis. In KD, the lesions typically present as papules filled with conical keratotic plugs, often located near hair follicles. The pathological characteristics of KD include epidermal invagination, visible eosinophilic material fragments, absence of elastic fiber tissue, and a perforating base with granulomatous inflammatory reaction (11, 12). ARPC lesions are typically larger and deeper, while KD or PF manifests as smaller and more numerous rashes. The biggest difference between KD and ARPC is the presence of a granulomatous inflammatory reaction in KD, which is absent in ARPC (13). PF presents with erythematous, follicular papules measuring a few millimeters across, with a white, central keratotic plug. The lesions are disseminated and primarily found on hair-bearing extremity surfaces (14). The pathological manifestations of PF include significantly dilated hair follicles, filled with incompletely keratinized keratotic plugs, fragments of eosinophilic material, and coiled hair. In the present case, the skin lesions did not exhibit perifollicular growth, and histopathological examination revealed the absence of discernible follicular structures. ARPC and EPS are distinct skin conditions with different characteristics. ARPC is characterized by large, deep lesions, often associated with systemic diseases such as diabetes or chronic renal failure. In contrast, EPS presents with small papular lesions that form a serpiginous pattern and is frequently associated with connective tissue disorders like pseudoxanthoma elasticum. Additionally, ARPC typically affects adults, while EPS is more common in children and young adults (15). In addition, Alves et al. reported a case of perforating granuloma annulare (perforating GA) that should be considered in the differential diagnosis of ARPC (16). The pathological manifestations of perforating GA show progressively degenerated dermal collagen fibers, surrounded by a large number of epithelioid cells and infiltrated by other chronic inflammatory cells, whereas ARPC show no evidence of the epithelioid cells. Their case did not support a perforating collagenosis, which generally presents as multiple keratotic papules on the extensor surfaces of the limbs (17).
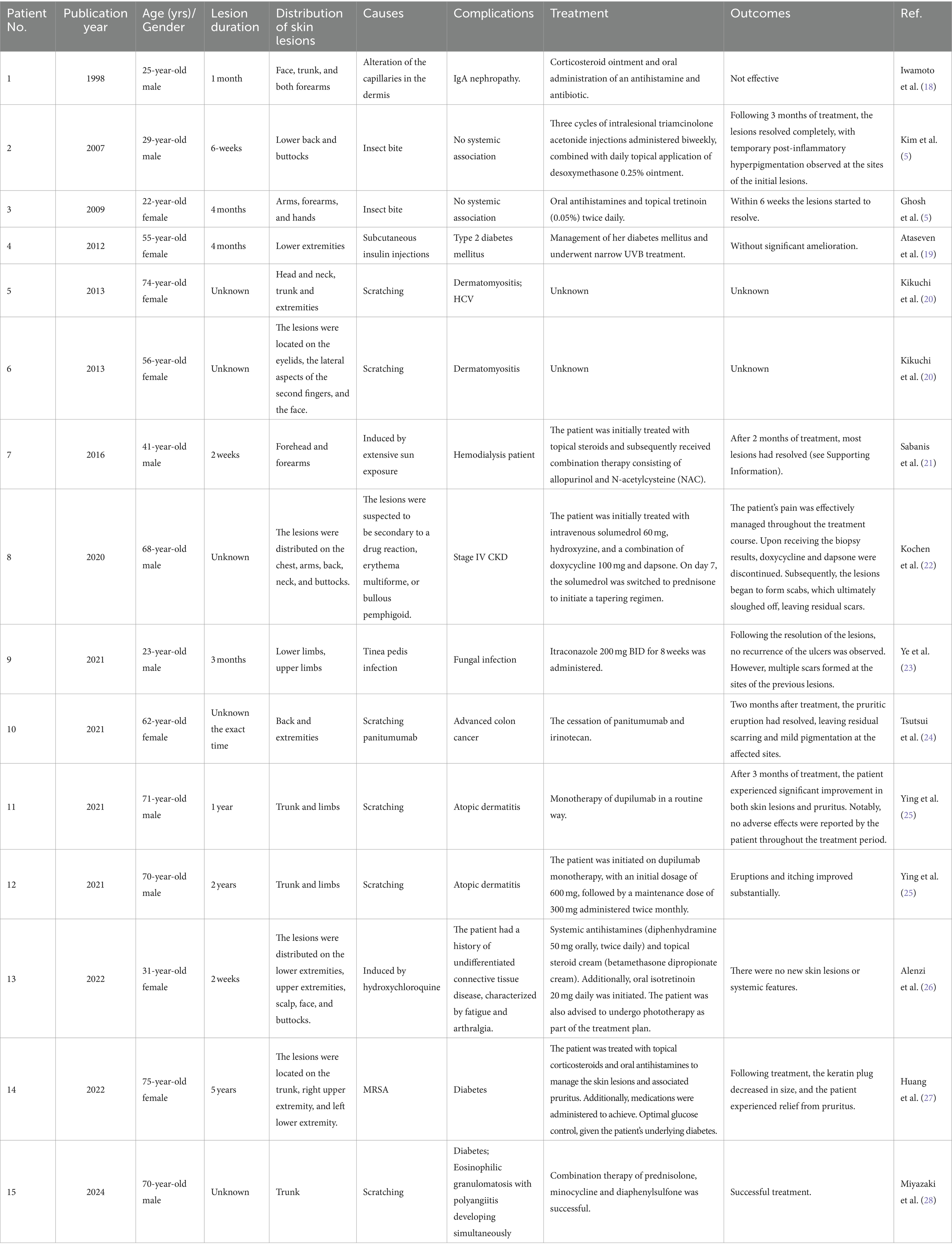
We reviewed cases of ARPC reported in the literature and summarized their causes, clinical characteristics, complications, and prognoses (Table 1). The typical clinical presentation of RPC involves multiple round plaques and nodules with central hyperkeratotic plugs, most commonly distributed on the extremities and back, with occasional involvement of the face and neck. Pruritus is a frequent symptom associated with RPC. Interestingly, in contrast to previous reports, the Koebner phenomenon was not mentioned in the majority of cases reviewed in this study (29). Among the reported patients with RPC, the most frequently associated comorbidities included diabetes mellitus, chronic kidney failure (with or without dialysis), lymphoma, IgA nephropathy, and acquired immunodeficiency syndrome (AIDS). However, it is noteworthy that the exact etiological factors were not specified in the majority of the reported cases (18, 30–32). Kim and Ghosh reported cases of ARPC developing following insect bites (5, 33). Miyazaki et al. described a case of ARPC and eosinophilic granulomatosis with polyangiitis (EGPA) occurring concurrently in a patient with diabetes (28). Their findings suggest that the increased secretion of matrix metalloproteinases (MMPs) in patients with EGPA may contribute to collagen degeneration and deposition in the context of diabetes. Furthermore, they hypothesized that this process could be associated with the transepidermal elimination of altered collagen, potentially facilitated by the weakening of the basement membrane. In contrast to the case reported by Miyazaki et al., our patient presented with ARPC induced by trauma from a branch, accompanied by eosinophilia. Notably, the patient had no family history of similar dermatological conditions or other systemic disorders, such as diabetes mellitus and renal failure, which have been previously associated with RPC. Therefore, this is the first reported case of ARPC induced by trauma combined with eosinophilia without other systemic disorders. Consequently, establishing a diagnosis of ARPC based solely on the presence of other systemic diseases poses a challenge in this case.
This case presents several interesting aspects. Firstly, the patient did not have a significant family history of similar lesions, which initially was not given much consideration. Secondly, the patient reported a history of trauma from scratching by a tree branch preceding the onset of the rash. We hypothesize that ARPC could have been triggered by increased eosinophils due to exposure to microorganisms or insect eggs on the tree branch, although concrete evidence to support this theory is lacking. Notably, previous literature has documented cases where insect bites led to the development of ARPC (5, 33). The pathogenesis of ARPC remains incompletely understood. Mehregan et al. (1) initially proposed that superficial trauma may contribute to the development of ARPC. Ghosh et al. (5) reported a case of ARPC following an insect bite and considered the transepidermal elimination of collagen as a reaction pattern resulting from chronic scratching or rubbing in a subset of pruritic patients. Additionally, Miyazaki et al. (28) showed a case of ARPC and eosinophilic granulomatosis with polyangiitis (EGPA) developing concurrently in a diabetic patient, suggesting that increased secretion of MMP-2 and MMP-9 in EGPA might be associated with collagen degeneration in the skin. Furthermore, although the pathogenesis of ARPC is unknown, overexpression of transforming growth factor-3 (TGF-β3) has been seen in many ARPC patients. TGF-β, matrix metalloproteinase-1, and tissue inhibitor of metalloproteinase-1 immunoreactivity were significantly increased in the lesions of ARPC (34), indicating the crucial function of these factors in regulating epidermal homeostasis, postponing the re-epithelialization and remodeling, and changing extracellular matrix protein metabolism (7). In the present case, mild superficial trauma or inflammatory cell infiltration may have contributed to the development of ARPC, resulting in the intense affinity for hematoxylin in the connective tissue of the papillary dermis or the wall of the superficial capillaries. Subsequently, parakeratosis and epidermal atrophy with transepidermal elimination of collagen occurred. Moreover, increased eosinophils may have been caused by exposure to microorganisms or insect eggs on the tree branch, potentially stimulating the secretion of MMPs (such as MMP-2 and MMP-9). The precise mechanisms underpinning the pathogenesis of ARPC require further investigation. Thirdly, common comorbid disorders typically associated with ARPC, such as hypothyroidism, hyperparathyroidism, dermatomyositis, or liver dysfunction, were absent in this case. However, the patient did not have a long-standing history of diabetes or renal failure. Fourth, it is worth mentioning that the absolute eosinophil count in all four tests was >0.63 × 109/L (normal reference range 0.05 × 109/L to 0.5 × 109/L) and the percentage of blood eosinophils was >7.7% (normal reference range 0.5% to 5%). Reports suggest that eosinophils, along with neutrophils, can release MMPs upon stimulation by tumor necrosis factor-α (TNF-α) (35). This heightened MMP secretion could potentially contribute to collagen degeneration in the skin in this specific case.
Conclusion
We report a unique case of ARPC triggered by trauma and accompanied by eosinophilia, in the absence of concurrent systemic disorders. To the best of our knowledge, this represents the first documented case of ARPC associated with eosinophilia and lacking a familial history or systemic conditions such as diabetes mellitus and renal failure. Further investigations are necessary to validate the potential link between ARPC and trauma-induced eosinophilia. Additionally, elucidating the underlying mechanism and causal relationship between eosinophilia and ARPC warrants comprehensive exploration.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
JL: Validation, Writing – original draft. PZ: Data curation, Investigation, Supervision, Writing – review & editing. XZ: Methodology, Software, Writing – review & editing. JG: Investigation, Resources, Writing – review & editing. HH: Software, Writing – review & editing. JQ: Investigation, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1415545/full#supplementary-material
References
1. Mehregan, AH, Schwartz, OD, and Livingood, CS. Reactive perforating collagenosis. Arch Dermatol. (1967) 96:277–82. doi: 10.1001/archderm.1967.01610030055009
2. Pai, VV, Naveen, KN, Athanikar, SB, Shastri, DU, and Rai, V. Familial reactive perforating collagenosis: a report of two cases. Indian J Dermatol. (2014) 59:287–9. doi: 10.4103/0019-5154.131405
3. Sutedja, EK, Widjaya, MRH, Dharmadji, HP, Achdiat, PA, and Tsaqilah, L. Coexistent lichen amyloidosis and acquired reactive perforating Collagenosis in type 2 diabetes mellitus and post-thyroidectomy hypothyroidism due to papillary thyroid carcinoma: a rare case. Int Med Case Rep J. (2022) 15:745–52. doi: 10.2147/IMCRJ.S391199
4. Lavery, MJ, Winters, S, Lorenzelli, D, Low, SE, Sharma, N, and Ngan, K. Acquired reactive perforating collagenosis in association with pregnancy. Eur J Obstet Gynecol Reprod Biol. (2020) 253:339–41. doi: 10.1016/j.ejogrb.2020.07.055
5. Ghosh, SK, Bandyopadhyay, D, and Chatterjee, G. Acquired reactive perforating collagenosis following insect bite. Indian J Dermatol Venereol Leprol. (2009) 75:306–7. doi: 10.4103/0378-6323.51269
6. Herzinger, T, Schirren, CG, Sander, CA, Jansen, T, and Kind, P. Reactive perforating collagenosis--transepidermal elimination of type IV collagen. Clin Exp Dermatol. (1996) 21:279–82. doi: 10.1111/j.1365-2230.1996.tb00094.x
7. Fei, C, Wang, Y, Gong, Y, Xu, H, Yu, Q, and Shi, Y. Acquired reactive perforating collagenosis: a report of a typical case. Medicine. (2016) 95:e4305. doi: 10.1097/MD.0000000000004305
8. Karpouzis, A, Giatromanolaki, A, Sivridis, E, and Kouskoukis, C. Acquired reactive perforating collagenosis: current status. J Dermatol. (2010) 37:585–92. doi: 10.1111/j.1346-8138.2010.00918.x
9. Prados-Carmona, A, and De la Torre Gomar, FJ. Acquired reactive perforating Collagenosis. N Engl J Med. (2023) 389:e37. doi: 10.1056/NEJMicm2301877
10. Kulkarni, SK, and Rangan, K. A rare complication of acute myocardial infarction: intra-myocardial dissecting hematoma. Echocardiography. (2019) 36:182–3. doi: 10.1111/echo.14195
11. Ataseven, A, Ozturk, P, Kucukosmanoglu, I, and Kurtipek, GS. Kyrle's disease. BMJ Case Rep. (2014) 2014:bcr2013009905. doi: 10.1136/bcr-2013-009905
12. Nair, PA, Jivani, NB, and Diwan, NG. Kyrle's disease in a patient of diabetes mellitus and chronic renal failure on dialysis. J Family Med Prim Care. (2015) 4:284–6. doi: 10.4103/2249-4863.154678
13. Sorhage, B, Glowania, HJ, and Schafer, R. Kyrle disease--a case report. Z Hautkr. (1990) 65:847–50.
14. Diez-Madueno, K, Buendia Castano, D, Alonso-Garcia, S, and de la Cueva, DP. Perforating folliculitis. Med Clin. (2022) 159:456. doi: 10.1016/j.medcli.2022.07.007
15. de Rezende, LN, Nunez, MG, Clavery, TG, Constancio, EG, Rochael, MC, Pires, GJ, et al. Elastosis perforans serpiginosa. Indian Dermatol Online J. (2014) 5:236–7. doi: 10.4103/2229-5178.131156
16. Alves, J, Barreiros, H, and Bartolo, E. Perforating granuloma Annulare—an unusual subtype of a common disease. Healthcare. (2014) 2:338–45. doi: 10.3390/healthcare2030338
17. Hoque, SR, Ameen, M, and Holden, CA. Acquired reactive perforating collagenosis: four patients with a giant variant treated with allopurinol. Br J Dermatol. (2006) 154:759–62. doi: 10.1111/j.1365-2133.2005.07111.x
18. Iwamoto, I, Baba, S, and Suzuki, H. Acquired reactive perforating collagenosis with IgA nephropathy. J Dermatol. (1998) 25:597–600. doi: 10.1111/j.1346-8138.1998.tb02464.x
19. Ataseven, A, and Kayacetin, S. Acquired reactive perforating collagenosis. Eur J Med. (2012) 44:51–3. doi: 10.5152/eajm.2012.11
20. Kikuchi, N, Ohtsuka, M, and Yamamoto, T. Acquired reactive perforating collagenosis: a rare association with dermatomyositis. Acta Derm Venereol. (2013) 93:735–6. doi: 10.2340/00015555-1562
21. Sabanis, N, Paschou, E, Gavriilaki, E, Kalaitzoglou, A, Papanikolaou, D, Vasileiou, S, et al. Acquired reactive perforating collagenosis and pseudoporphyric bullous dermatosis in a hemodialysis patient. Hemodial Int. (2016) 20:E14–8. doi: 10.1111/hdi.12402
22. Kochen, D, Sohal, RJ, and Nat, A. Reactive perforating Collagenosis; an uncontrolled pruritus that left you scratching your head. Cureus. (2020) 12:e9175. doi: 10.7759/cureus.9175
23. Ye, B, Cao, Y, and Liu, Y. Successful treatment of acquired reactive perforating collagenosis with itraconazole. Eur J Med Res. (2021) 26:74. doi: 10.1186/s40001-021-00542-6
24. Tsutsui, K, Namikawa, K, Mori, T, Kato, K, Jinnai, S, Nakama, K, et al. Case of acquired reactive perforating collagenosis induced by panitumumab for colon cancer. The. J Dermatol. (2020) 48:e114–5. doi: 10.1111/1346-8138.15718
25. Ying, Y, Shuang, C, and Zhen-Ying, Z. Dupilumab may be an alternative option in the treatment of acquired reactive perforating collagenosis combined with AD. Immun Inflamm Dis. (2022) 10:e574. doi: 10.1002/iid3.574
26. Alenzi, F. Reactive perforating collagenosis and systemic lupus erythematosus: a rare case report. Medicine. (2022) 101:e32138. doi: 10.1097/MD.0000000000032138
27. Huang, F, Ren, W, Wang, M, Li, X, and Pan, M. Acquired reactive perforating collagenosis combined with MRSA: a case report. Med Int. (2023) 3:69. doi: 10.3892/mi.2023.69
28. Miyazaki, A, Taki, T, Mori, S, Fujishiro, R, Arai, Y, Yamada, M, et al. Acquired reactive perforating collagenosis and eosinophilic granulomatosis with polyangiitis developing simultaneously in a diabetic patient. J Cutan Immunol Allergy. (2024) 7:594. doi: 10.3389/jcia.2024.12594
29. Faver, IR, Daoud, MS, and Su, WP. Acquired reactive perforating collagenosis. Report of six cases and review of the literature. J Am Acad Dermatol. (1994) 30:575–80. doi: 10.1016/S0190-9622(94)70065-6
30. Fernandes, KA, Lima, LA, Guedes, JC, Lima, RB, D'Acri, AM, and Martins, CJ. Acquired perforating dermatosis in a patient with chronic renal failure. An Bras Dermatol. (2016) 91:10–3. doi: 10.1590/abd1806-4841.20164619
31. Rao, G, Kollipara, H, Satya, R, and Attili, S. Acquired reactive perforating collagenosis: case series. Indian. Dermatol Online J. (2023) 14:72. doi: 10.4103/idoj.idoj_373_22
32. Saenz Aguirre, A, Martínez-González, MI, García-Río, I, Caton Santaren, B, and González-Pérez, R. Acquired reactive perforating collagenosis in a patient with chronic renal failure and nephrogenic systemic fibrosis. Australas J Dermatol. (2021) 62:e141–2. doi: 10.1111/ajd.13438
33. Kim, EJ, Kim, MY, Kim, HO, and Park, YM. Acquired reactive perforating collagenosis triggered by insect bite. J Dermatol. (2007) 34:677–9. doi: 10.1111/j.1346-8138.2007.00351.x
34. Gambichler, T, Birkner, L, Stucker, M, Othlinghaus, N, Altmeyer, P, and Kreuter, A. Up-regulation of transforming growth factor-beta3 and extracellular matrix proteins in acquired reactive perforating collagenosis. J Am Acad Dermatol. (2009) 60:463–9. doi: 10.1016/j.jaad.2008.06.006
Keywords: ARPC, eosinophilia, skin rash, traumatic trigger, case report
Citation: Liu J, Zhao P, Zhang X, Gao J, Han H and Qin J (2024) Acquired reactive perforating collagenosis triggered by trauma with eosinophilia: a case report and literature review. Front. Med. 11:1415545. doi: 10.3389/fmed.2024.1415545
Edited by:
Andreas Recke, University of Lübeck, GermanyReviewed by:
Özay Gököz, Hacettepe University, TürkiyeNeusa Sakai Valente, University of São Paulo, Brazil
Copyright © 2024 Liu, Zhao, Zhang, Gao, Han and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junxia Qin, cWluanVueGlhMTk3OUAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Jie Liu
Jie Liu Peng Zhao1†
Peng Zhao1†