
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med. , 20 November 2024
Sec. Pulmonary Medicine
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1410724
This article is part of the Research Topic The Role of Mucosal Surfaces on Asthma and Respiratory Disease View all 6 articles
Tracheobronchopathia osteoplastica (TO) is a rare benign lesion of the airways, characterized by multiple nodular proliferations of bone and/or cartilaginous tissue in the submucosa of the trachea and bronchi. In this paper, we present a case of a 63-year-old male patient who was admitted to the hospital due to cough and blood in his sputum. The patient was diagnosed with TO through examination and pathological biopsy. The exact pathogenesis of this disease remains unclear, but it may be related to multiple factors such as chronic infection and inflammation. The clinical manifestations are diverse, with no symptoms or only mild cough in the early stage, and persistent cough, dyspnea, and other symptoms may appear as the disease progresses. Diagnosis mainly relies on pathological biopsy, while treatment is mainly symptomatic, including inhalation of glucocorticoids, airway interventional therapy, and stent placement. Overall, TO progresses slowly and has a good prognosis, but early diagnosis and elimination of predisposing factors are crucial for treatment.
Tracheobronchopathia osteoplastica (TO) is an extremely rare benign lesion of the airways encountered in clinical practice (1). Its main pathological feature is the presence of multiple nodular proliferations of bone and/or cartilaginous tissue in the submucosa of the trachea and bronchi, which protrude into the lumen, forming specific lesion manifestations (1). Although most patients do not exhibit obvious symptoms or may only have a mild cough (2), bronchoscopic examination reveals multiple nodules on the anterior and lateral walls of the trachea and bronchi. These nodules, with diameters ranging from 1 to 5 millimeters, may be scattered or fused into patches, exhibiting morphological changes resembling pebbles or stalactites (3). In severe cases, these lesions may also involve the membranous portion and glottis region, providing important diagnostic clues. Given the rarity of the disease and the non-specificity of its symptoms, the diagnostic process is often challenging. However, early accurate diagnosis and treatment significantly impact the prognosis of patients. Therefore, deepening the understanding of TO is crucial. This article aims to enhance the medical community’s cognitive level of this disease by presenting a detailed case report of TO.
A 63-year-old male presented to our hospital with a two-year history of cough and intermittent blood-tinged sputum for 20 days. Two years prior, the patient developed paroxysmal irritating cough and expectoration without apparent provocation, unaccompanied by post-exertional wheezing or shortness of breath. Despite treatment at a local clinic, the cough and expectoration persisted for more than 3 months annually. Twenty days before admission, the patient noticed blood in his sputum after physical activity, without chest tightness or pain. He was subsequently hospitalized at Shiquan County Hospital, where relevant investigations did not support a diagnosis of tuberculosis. The patient was discharged after 1 week when the hemoptysis ceased, but the cough and expectoration continued intermittently. The patient presented to our outpatient clinic yesterday with recurrent blood-tinged sputum and was admitted for further evaluation of suspected pneumonia, chronic bronchitis, and the etiology of hemoptysis. Since the onset of symptoms, the patient denied fever, night sweats, fatigue, or weight changes. His past medical history was significant for a diagnosis of Pseudomonas aeruginosa pneumonia and hamartoma at Tangdu Hospital in 2017, treated with bronchoscopic surgery. He also had a two-year history of working in the coal mining industry. The patient had a history of smoking for 10 years, with a daily consumption of 2–3 cigarettes, and had quit smoking for 5 years. There were no similar medical records of tracheobronchial ossification among other family members. The patient was primarily engaged in farming, had lived locally for a long time, had no history of itinerant life, exposure to toxic gases or special chemicals, or adverse social history. Physical examination revealed hyperresonant lungs on percussion, coarse breath sounds on auscultation, and no abnormalities in the cardiovascular, abdominal, or neurological systems. The initial diagnoses were acute exacerbation of chronic bronchitis and hemoptysis of unknown etiology. Initial treatment included anti-infection therapy (piperacillin-tazobactam 4.5 g q8h), hemostasis (thrombin lyophilized powder 2,000 IU via nebulization bid), cough suppression and expectoration facilitation (Suhuang cough relief capsules 1.35 g tid, carbocisteine oral solution 25 mL tid).
Post-admission investigations included an electrocardiogram, pulmonary function tests, echocardiography, routine biochemical tests, lung tumor markers, and TBNK lymphocyte subset analysis, all of which were unremarkable. GeneXpert testing on bronchoalveolar lavage fluid was negative for tuberculosis. Cytological examination of the bronchoalveolar lavage fluid from the right upper lobe did not reveal any evidence of malignancy. A chest CT scan showed multiple calcified and non-calcified nodules protruding into the lumen of the trachea and main bronchi, with irregular luminal contours, sparing the posterior tracheal wall (membranous portion). Bronchoscopy revealed nodular lesions of unknown etiology in the trachea and bilateral bronchi (Figure 1). Biopsy of these nodular tissues revealed ossification within the bronchial mucosa and focal surface epithelial squamous metaplasia (Figure 2). Re-examination of chest CT scan: compared with the previous results, the lesions remain largely unchanged with no significant variation (Figure 3). The patient was subsequently treated with antibiotics (cefoxitin sodium 1 g q8h, levofloxacin hydrochloride injection 0.5 g qd), cough suppression and expectoration facilitation (Suhuang cough relief capsules 1.35 g tid, inhaled acetylcysteine solution 0.3 g tid).
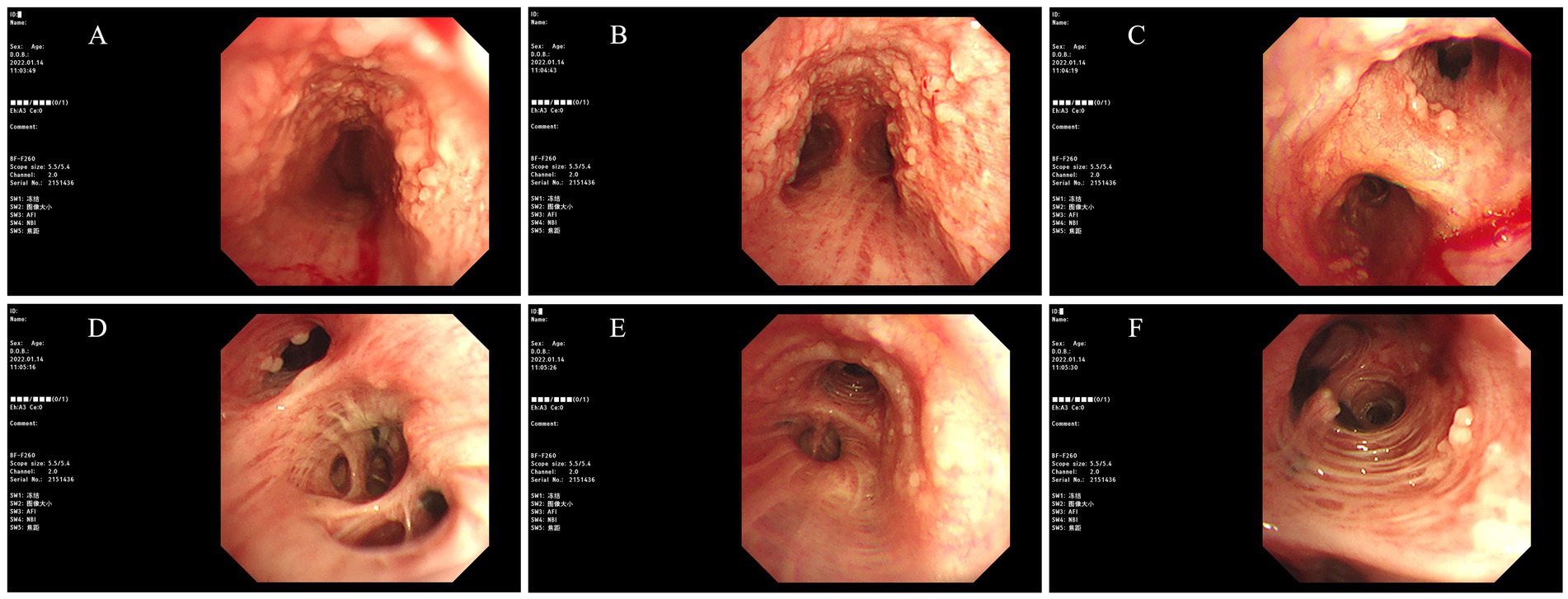
Figure 1. Images obtained under bronchoscopy (Bronchoscopy revealed nodular lesions of unknown etiology in the trachea and bilateral bronchi. ((A) Trachea, (B) Carina, (C) Right Main Bronchus, (D) Right Intermediate Bronchus, (E) Left Main Bronchus, (F) Left Upper Lobe Bronchus)).
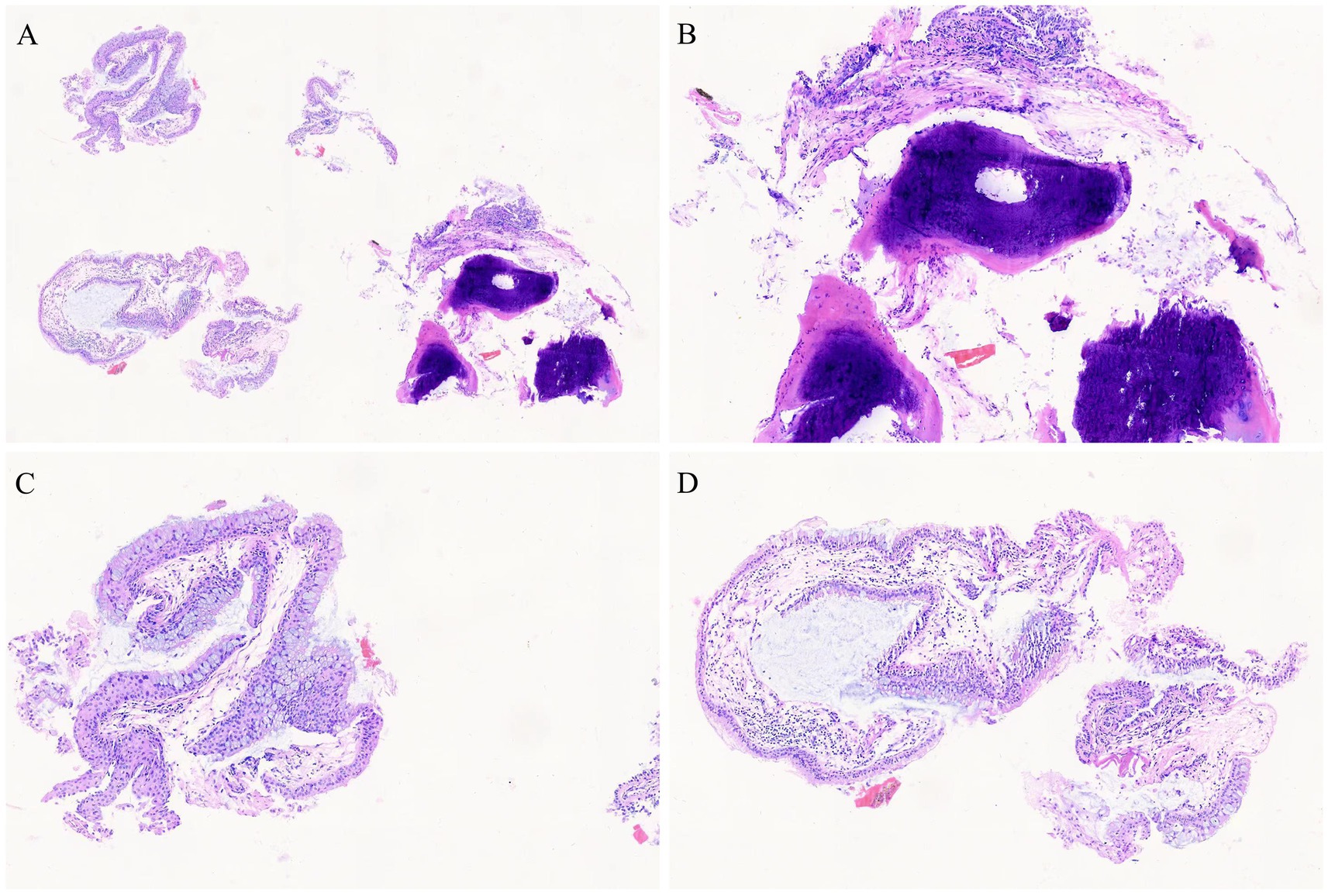
Figure 2. Pathological images (biopsy of these nodular tissues revealed ossification within the bronchial mucosa and focal surface epithelial squamous metaplasia, HE staining, (A) 4 × 10, (B–D) 10 × 10).
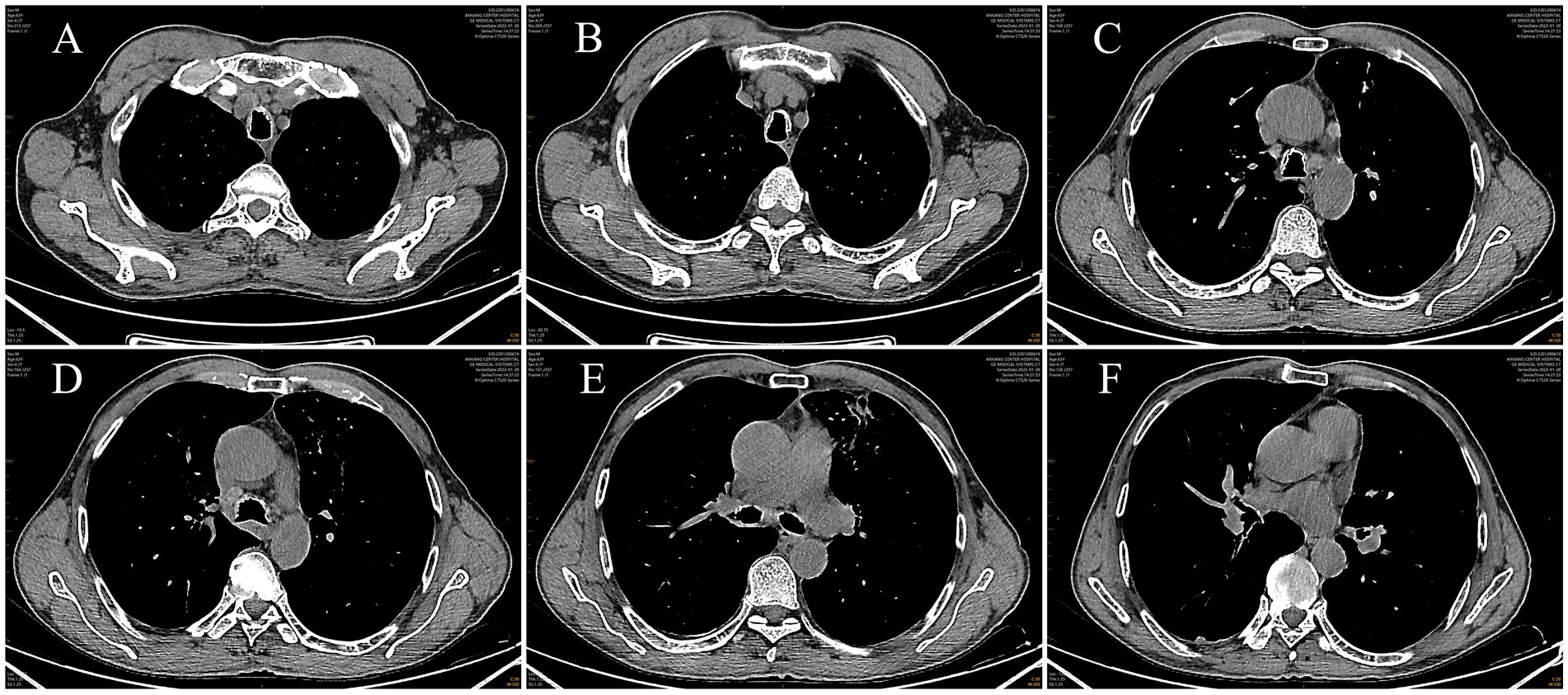
Figure 3. Images of mediastinal window from chest CT scan(a chest CT scan showed multiple calcified and non-calcified nodules protruding into the lumen of the trachea and main bronchi, with irregular luminal contours, sparing the posterior tracheal wall (membranous portion). ((A, B) Level Above the Aortic Arch, (C, D) Level at the Lower End of the Trachea, (E) Level of the Tracheal Bifurcation, (F) Level of the Right Middle Lobe Bronchus)).
The patient was discharged with a diagnosis of tracheobronchopathia osteoplastica and acute exacerbation of chronic bronchitis [differential diagnosis: (1) pulmonary mycosis, (2) HIV-associated pneumocystis pneumonia, (3) silicotic pulmonary fibrosis, (4) lung cancer]. Following the aforementioned treatment, the patient’s hemoptysis resolved, and his cough and expectoration improved. The patient requested discharge and was advised to enhance self-care, avoid exposure to cold, undergo periodic bronchoscopy, and follow up in the outpatient clinic. Two years later, telephone follow-up revealed that the patient had not experienced recurrent hemoptysis and only had occasional cough and expectoration.
TO, a rare disease whose precise pathogenic mechanism remains enigmatic, is widely believed by scholars to be potentially influenced by multiple factors, including chronic infection, inflammation, congenital genetics, degenerative processes, chemical irritants, and mechanical injuries (4–10). Notably, persistent stimuli, such as chronic inflammation, continuously act upon the cartilaginous, bony structures, and fibrous connective tissue within the tracheal rings. This stimulation facilitates the gradual formation of chondromas and osteophytes, which subsequently develop into multiple nodules within the airway. Over time, these fibrous connective tissues undergo a process of chondrification and ossification, ultimately evolving into TO.
Furthermore, Virchow’s research has further elucidated the pivotal role of bone morphogenetic protein 2 (BMP-2) in the progression of this disease. In synergy with transforming growth factor beta 1 (TGF-β1), BMP-2 can induce chondrification of the submucosal connective tissue (11), thereby promoting the formation of chondromas from cartilage and osteophytes from bony structures. These lesions manifest as multiple hard nodules on the surface of the airway (12, 13). Under microscopic examination, these nodules exhibit a smooth, bony appearance (14). Due to the presence of these nodules, some of the luminal spaces become obstructed, impeding the drainage of secretions. Consequently, the structure and function of the bronchial mucosa become abnormal, ultimately giving rise to clinical symptoms such as cough, expectoration, and recurrent infections (15).
TO manifests clinically in a variety of ways, with many patients exhibiting no significant symptoms or only mild discomfort in the early stages of the disease. However, as the condition gradually progresses, some patients may experience persistent cough, while others may develop symptoms of dyspnea. In cases of infection, patients may also cough up purulent secretions. During the early asymptomatic phase, TO is highly susceptible to misdiagnosis or missed diagnosis. Nevertheless, thanks to continuous advancements in bronchoscopy and CT technology, clinicians’ understanding of TO has gradually deepened. The severity of this disease is closely related to multiple factors, including the presence of concomitant infection, the degree of airway stenosis, and the specific location of nodules within the airway. Therefore, once patients exhibit symptoms closely related to airway function, such as cough, wheezing, dry cough, hemoptysis, chest pain, and hoarseness, prompt diagnosis should be made through electronic bronchoscopy and CT three-dimensional reconstruction. It is important to note that if some patients do not receive active and effective treatment, the disease may progress to life-threatening conditions such as respiratory failure in its later stages.
The diagnosis of TO primarily relies on pathological biopsy, which serves as the gold standard for confirming this disease. In the case of this patient, the final diagnosis was also established through pathological biopsy. However, due to the hardness of the lesion site in TO, performing a biopsy requires a certain level of experience to ensure the acquisition of high-quality specimens. In terms of treatment, there are currently no specific therapeutic approaches targeting TO, and management primarily involves symptomatic relief based on the patient’s specific symptoms. Studies have suggested that inhaled corticosteroids may help slow the progression of the disease (16). With the continuous development of airway interventional techniques, interventional therapies including argon gas, plasma radiofrequency, microwave coagulation, electrocautery, and laser can be considered for patients with airway stenosis (17, 18). Additionally, stent placement is an effective method for addressing severe airway stenosis.
Overall, TO is a slowly progressive disease with a favorable prognosis. However, clinicians need to fully understand the characteristics of this disease, make an early diagnosis, and actively seek possible predisposing factors. Timely elimination of these predisposing factors is crucial for the treatment of patients.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the Ethics Committee of Ankang Central Hospital, approval number 2024 No. 022. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
JL: Writing – original draft. YD: Writing – original draft. QH: Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors would like to thank the editors and reviewers for their valuable comments and suggestions to improve the quality of the paper.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1410724/full#supplementary-material
1. Sun, J, Xie, L, Su, X, and Zhang, X. Tracheobronchopathia osteochondroplastica: case report and literature review. Respir Med Case Rep. (2015) 15:14–7. doi: 10.1016/j.rmcr.2015.03.003
2. Agarwal, A, Klair, J, Joiner, A, and Meena, NK. Nodular trachea: tracheobronchopathia osteochondroplastica. BMJ Case Rep. (2015) 2015:bcr2015209860. doi: 10.1136/bcr-2015-209860
3. Wang, W, Hu, H, Liu, M, and Wang, J. Tracheobronchopathia osteochondroplastica: five cases report and literature review. Ear Nose Throat J. (2020) 99:NP111–8. doi: 10.1177/0145561319897982
4. Guo, R, Zhou, M, Wei, X, and Niu, L. Clinical characteristics of six cases of tracheobronchopathia osteochondroplastica. Can Respir J. (2020) 2020:8685126. doi: 10.1155/2020/8685126
5. Härmä, RA, and Suurkari, S. Tracheopathia chondro-osteoplastica. A clinical study of thirty cases. Acta Otolaryngol. (1977) 84:118–23. doi: 10.3109/00016487709123949
6. Leske, V, Lazor, R, Coetmeur, D, Crestani, B, Chatté, G, and Cordier, JF. Tracheobronchopathia osteochondroplastica: a study of 41 patients. Medicine. (2001) 80:378–90. doi: 10.1097/00005792-200111000-00004
7. Nienhuis, DM, Prakash, UB, and Edell, ES. Tracheobronchopathia osteochondroplastica. Ann Otol Rhinol Laryngol. (1990) 99:689–94. doi: 10.1177/000348949009900903
8. Song, JY, Kim, BG, Zo, S, Park, S, Han, JH, and Kim, H. Tracheobronchopathia osteochondroplastica (TPO) associated with tracheobronchial amyloidosis (TBA). Respir Med Case Rep. (2020) 31:101158. doi: 10.1016/j.rmcr.2020.101158
9. Sousa, M, Silva, J, and Rodrigues, B. Coincident tuberculosis and tracheobronchopathia osteochondroplastica in a patient. Arch Bronconeumol. (2017) 53:343. doi: 10.1016/j.arbres.2016.11.019
10. Luo, T, Zhou, H, and Meng, J. Clinical characteristics of tracheobronchopathia osteochondroplastica. Respir Care. (2019) 64:196–200. doi: 10.4187/respcare.05867
11. Tajima, K, Yamakawa, M, Katagiri, T, and Sasaki, H. Immunohistochemical detection of bone morphogenetic protein-2 and transforming growth factor beta-1 in tracheopathia osteochondroplastica. Virchows Arch. (1997) 431:359–63. doi: 10.1007/s004280050111
12. Jindal, S, Nath, A, Neyaz, Z, and Jaiswal, S. Tracheobronchopathia osteochondroplastica—a rare or an overlooked entity? J Radiol Case Rep. (2013) 7:16–25. doi: 10.3941/jrcr.v7i3.1305
13. Pounder, DJ, and Pieterse, AS. Tracheopathia osteoplastica: a study of the minimal lesion. J Pathol. (1982) 138:235–9. doi: 10.1002/path.1711380305
14. Nielsen, SW, Stevens, JR, Dion, GR, and Howard, NS. Dyspnea, dysphonia, and cough: varied presentations of tracheobronchopathia osteochondroplastica. Ann Otol Rhinol Laryngol. (2015) 124:829–33. doi: 10.1177/0003489415586845
15. Dharia, I, and Bielamowicz, S. Surgical management in tracheobronchopathia osteochondroplastica: a case study. Laryngoscope. (2021) 131:E911–3. doi: 10.1002/lary.29095
16. Zhang, XB, Zeng, HQ, Cai, XY, and Zhang, YJ. Tracheobronchopathia osteochondroplastica: a case report and literature review. J Thorac Dis. (2013) 5:E182–4. doi: 10.3978/j.issn.2072-1439.2013.08.64
17. Prakash, UB . Tracheobronchopathia osteochondroplastica. Semin Respir Crit Care Med. (2002) 23:167–76. doi: 10.1055/s-2002-25305
Keywords: tracheobronchopathia osteoplastica, trachea, bronchus, case report, diagnosis
Citation: Lai J, Deng Y and He Q (2024) Tracheobronchopathia osteoplastica: a clinical case report of a 63-year-old man. Front. Med. 11:1410724. doi: 10.3389/fmed.2024.1410724
Received: 02 April 2024; Accepted: 11 November 2024;
Published: 20 November 2024.
Edited by:
Shradha Wali, McGill University Health Centre, CanadaReviewed by:
Basim Dubaybo, Wayne State University, United StatesCopyright © 2024 Lai, Deng and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingmin He, MTMwOTUwMzczNTNAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.