- 1Department of Gynecology, Ningbo No. 2 Hospital, Ningbo, Zhejiang, China
- 2Department of Pathology, Ningbo Clinical Pathology Diagnosis Center, Ningbo, Zhejiang, China
Malacoplakia is a rare chronic granulomatous disease that mostly affects the gastrointestinal tract and urinary tract of immunocompromised patients; malacoplakia rarely effects the female reproductive tract. Here, we report a 56-year-old patient who underwent thymectomy for thymoma and myasthenia gravis prior to developing cervical and vaginal malacoplakia. The patient presented with recurrent vaginal bleeding. We discovered that there were alterations in the cervical cauliflower pattern during colposcopy, which is suggestive of cervical cancer. Pathological examination of the lesion tissue showed that a large number of macrophages aggregated, and M-G bodies with concentric circles and refractive properties were observed between cells. Immunostaining for CD68 and CD163 was positive, and special staining for D-PAS and PAS was positive. The discovery of Escherichia coli in bacterial culture can aid in the diagnosis of malacoplakia. Following surgery, we performed vaginal lavage with antibiotics in addition to resection of local cervical and vaginal lesions. This study provides a fresh perspective on the management of genital malacoplakia.
1 Introduction
A relatively rare granulomatous condition called malacoplakia primarily impacts the urinary system, but it can also affect the gastrointestinal tract, testicles, prostate, and other organs (1). The causes of malacoplakia and its pathophysiology are unclear. Nonetheless, most studies indicate a strong correlation between infection and the onset of malacoplakia (2). These infections were primarily caused by Acidophilus, Klebsiella species, and Escherichia coli. Malacoplakia has also been linked to immunodeficiency, which is thought to be caused by a malfunction in the process of killing intracellular bacteria (3). There is currently little research on malacoplakia in the female vaginal canal. Diagnosing and treating malacoplakia are more difficult for professionals due to its unusual clinical presentation, and clinical misdiagnosis is highly common. The two main treatments for cervical malacoplakia are hysterectomy and antibiotic therapy (2, 4–12).
However, the disadvantages of hysterectomy are obvious, including short-term infections, peripheral organ damage, increased morbidity, and long-term complications such as pelvic organ prolapse and urinary incontinence (13). At present, the treatment and prognosis of malacoplakia patients with uterine preservation are unclear. The cases and treatments reported below provide new ideas for the clinical treatment of genital tract malacoplakia (14).
2 Case reports
At the beginning of 2021, a 56-year-old Chinese woman who complained of recurrent vaginal bleeding for one week visited our hospital. The patient had previously undergone thymectomy due to myasthenia gravis combined with type B2 thymoma, and she had no history of diabetes, AIDS, tuberculosis, etc. Gynecological examination of the patient at the time of treatment revealed that the 5 * 5 cm cauliflower-like mass on the anterior lip of the cervix involved the vault and easily bled when touched. A gynecologic ultrasound revealed that the cervix was enlarged with solid tumor formation. The outpatient department was highly suspicious of a cervical malignant tumor, so further colposcopy (Figure 1A) and tissue biopsy were performed. Colposcopy revealed that the cervical surface showed cauliflower-like changes, involving the upper 1/3 of the posterior vaginal wall, the upper 1/3 of the left vaginal wall, the front segment of the right vaginal wall, and the front 1/2 of the vaginal wall. No obvious white epithelium was observed, and the iodine test was negative. The microscope showed that a large number of macrophages (tissue cells) aggregated, and concentric and refractive small bodies, called MG bodies, were seen between tissue cells. They can be seen inside or outside the cytoplasm of macrophages (tissue cells) and are characteristic for diagnosing soft spot disease. Immunohistochemical staining for CD68 and CD163 was used to identify tissue cells, while D-PAS and PAS were used to visualize MG bodies. The discovery of Escherichia coli in bacterial culture can aid in the diagnosis of soft spot disease. Antibiotics be combined with surgical hysterectomy based on the results of drug sensitivity tests. Due to religious beliefs, the patient refused uterine removal, so she was given 0.2 g intravenous amikacin once a day according to the drug sensitivity test. After one month of treatment, the vaginal bleeding of the patient stopped, the colposcopy mass was smaller than before, and the focus was limited to the cervical surface in the second month (Figure 1B). At the end of 2022, after the patient was infected with COVID-19 and had mild COVID-19 pneumonia, vaginal bleeding occurred again. Pelvic magnetic resonance imaging (MRI) revealed a large cervical space occupying the protrusion into the vagina; this space was thought to be a result of malacoplakia, although cervical malignancies were not excluded (Figure 2). After communicating with the patient, the patient asked to keep their uterus, and after signing the informed consent form, cervical lesion resection + vaginal wall lesion resection was performed. Microscopy revealed a diffuse inflammatory lesion with a large number of tissue cell clusters (Figure 3A, 100X). In the background of tissue cells, there were varying numbers of plasma cells and lymphocytes, and there may have been bleeding and a small amount of neutrophils (Figure 3B, 100X). Characteristic soft spot bodies (Michaelis Gutmann bodies, MG bodies) were also observed inside and outside the tissue cells. Soft spot bodies were round or oval in shape, with clear boundaries, refractive, alkaline homogeneous shapes, or ring-like structures resembling “owl's eyes” (Figure 3C, 400X). MG bodies are formed by incomplete degradation of bacterial calcification. Immunohistochemistry revealed CD68- and CD163-positive tissue cells (Figures 3D, E, 200X), while PAS staining revealed purplish red soft macular bodies (Figure 3F, 400X). After surgery, the method of antibiotic administration was changed, and tobramycin/dexamethasone eye ointment + 0.2 g amikacin were mixed with local vaginal lavage. More than one year after surgery, the disease is well controlled, and there has been no recurrence.
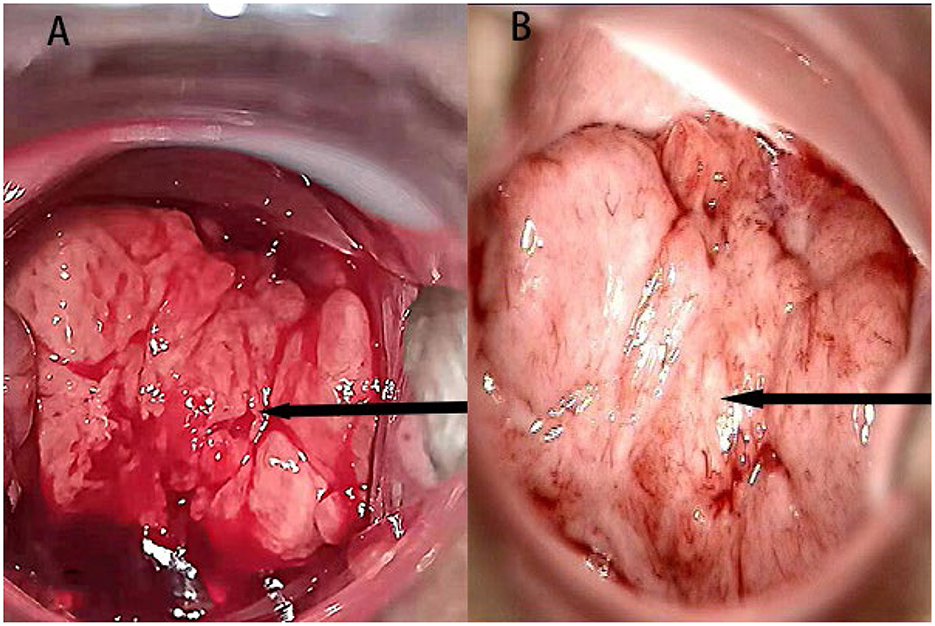
Figure 1. (A) A colposcopic image at the time of initial diagnosis; the arrow points to the lesion. (B) Review of the colposcopic image in the second month of treatment; the arrow points to the lesion.
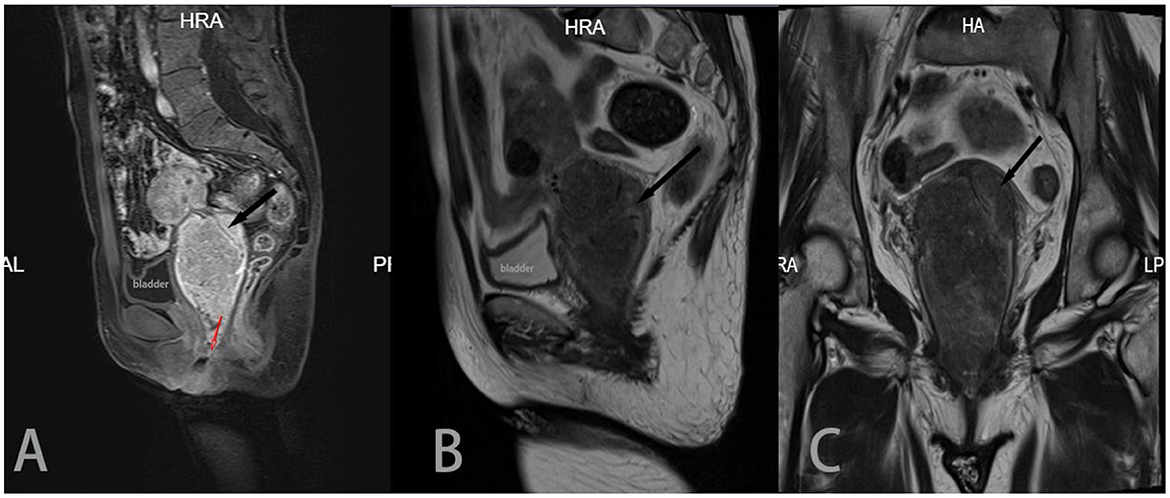
Figure 2. Sagittal T2 (A), sagittal T1 enhancement (B) and coronal T2 (C) images all show a large cervical mass protruding into the vagina, with a size of approximately 50 * 58 * 91 mm. The mass invaded the cervical interstitium (black arrow), and the surrounding low-signal basal ring was still apparent. In (B), and the lesion appeared in the vagina, occupying 2/3 of the vaginal cavity (red arrow). The uterine body was compressed and moved upward.

Figure 3. Pathological images of cervical lesions. Microscopy revealed a diffuse inflammatory lesion with a large number of tissue cell clusters [(A), 100X]. In the background of tissue cells, there were varying numbers of plasma cells and lymphocytes, and there may have been bleeding and a small amount of neutrophils [(B), 100X]. Characteristic soft spot bodies (Michaelis Gutmann bodies, MG bodies) were also observed inside and outside the tissue cells. Soft spot bodies were round or oval in shape, with clear boundaries, refractive, alkaline homogeneous shapes, or ring-like structures resembling “owl's eyes” [(C), 400X]. MG bodies are formed by incomplete degradation of bacterial calcification. Immunohistochemistry revealed CD68- and CD163-positive tissue cells [(D, E), 200X), while PAS staining revealed purplish red soft macular bodies [(F), 400X].
3 Discussion
Malacoplakia has been recognized since before 1900 as a very rare disease that is characterized by defects in the mononuclear phagocyte system (15). Malacoplakia related to the female reproductive system is rare. Malacoplakia is usually associated with an immunosuppressive state, indicating that immunity plays an important role in its pathogenesis. Different manifestations of malacoplakia include malignant tumors, SOT, human immunodeficiency virus (HIV), and autoimmune diseases (16–18).
3.1 Demographic patterns of malacoplakia of the cervix: age and racial disparities
A previous study revealed that malacoplakia had a high prevalence in the southwestern United States, with patients ranging in age from as young as 6 weeks to as old as 85 years of age (19), usually with the highest prevalence in patients >50 years of age (20). The most recent case of cervical malacoplakia is reported in this paper. To date, a total of 14 cases of cervical malacoplakia have been reported, including that in the present paper, among which the youngest woman was only 27 years old, the oldest patient was 83 years old, and there were 11 cases in women over 50 years old, accounting for 78.57% of all cases; these ages are close to the ages of onset of other malacoplakia diseases (2, 4–12).
3.2 Pathogenesis of malacoplakia of the cervix
The etiology and pathogenesis of cervical malacoplakia are still unclear, and the main underlying mechanisms are various microbial infections and immune dysfunction (21). In this case, Escherichia coli was found in the vaginal secretions, so we believe that microbial infection may be one of the important factors involved in the pathogenesis of cervical malacoplakia. Moreover, the occurrence of malacoplakia is related to functional defects in macrophages, which block the degradation of phagocytic bacteria by lysosomes, resulting in excessive undigested bacterial debris in the cytoplasm (22–24). Our patient had previously undergone a thymectomy for myasthenia gravis with type B2 thymoma. Thymoma-associated myasthenia gravis is a paraneoplastic disease, and myasthenia gravis is the most widely reported autoimmune disease associated with thymoma (25). There is evidence that cholinergic receptor agonists, such as chlormethine, combined with antibiotics may improve the function of macrophages by correcting lysosomal defects. Therefore, we speculate that cholinergic receptor antibodies in malacoplakia patients may affect the function of macrophages, thus driving the phagocytosis of pathogenic bacteria in patients (26). Therefore, we hypothesized that the patient's previous history of myasthenia gravis combined with thymoma may have induced the development of malacoplakia.
3.3 Challenges in the clinical diagnosis of malacoplakia of the cervix: overlapping symptoms
Most patients with malacoplakia present with abnormal vaginal bleeding and ulcerative changes in the cervix, which can be seen with the naked eye (27). The similarity of clinical manifestations often leads us to overlook cervical malacoplakia and misdiagnose it as a malignant tumor of the cervix (28). Our patient saw a doctor due to abnormal vaginal bleeding. During gynecological examination and colposcopy, a cauliflower-like cervical tumor was found. Ultrasound revealed that the cervix was enlarged with a solid tumor-like appearance. Because cervical cancer was suspected, we conducted a colposcopy examination and found that the lesion was soft, yellow, slightly raised, and fused into a 5 * 5 cm cauliflower-like plaque. A histological biopsy was taken. However, to our surprise, the pathological report suggested that this was a case of malacoplakia. Here, we emphasize the clinical significance of malacoplakia in the differential diagnosis of gynecological diseases. Malacoplakia may mimic malignant tumors, which can be a challenge for obstetricians and gynecologists.
3.4 Identification of malacoplakia in the genital tract
To date, there have been fewer than 40 reported cases of female genital malacoplakia, 14 of which were cervical malacoplakia (including this case); most of these patients presented with vaginal and endometrial soft spots, and ovarian and fallopian tube invasion was rarer (29). Malacoplakia involving the cervix, endometrium and vagina has similar clinical manifestations, mainly abnormal uterine bleeding, postmenopausal vaginal bleeding and increased secretions (30). The ultrasound characteristics of endometrial malacoplakia include anechoic fluid expansion in the endometrial cavity in the acute stage, irregular and heterogeneous thickening, and endometrial hypopogenicity in the chronic stage; gynecological examinations generally have no specific findings (31). The imaging characteristics of cervical malacoplakia include a hypoechoic space in the cervix. Cervical lesions can be detected via gynecological examination and confirmed via cervical lesion biopsy. Vaginal malacoplakia can be detected through gynecological examination, vaginal lesions, and biopsy, and abnormalities can be detected in the uterus and cervix (7). Ovarian and tubal malacoplakia often manifest as abdominal pain and abdominal discomfort before surgery, and imaging can reveal space in the accessory area. Operations can show that lesions directly spread and invade the surrounding tissues, similar to tumors, but postoperative pathology will suggest malacoplakia (32).
3.5 Pathological features of malacoplakia of the cervix
Malacoplakia is a chronic granulomatous disease that is characterized by a large number of tissue cells that are visible under the microscope, with a background of small lymphocytes, plasma cells, and neutrophils (26). Among them, circular or oval shaped cells are observed, with clear boundaries, refraction, alkaline homogeneity, or a ring-like structure resembling “owl's eyes”. Immunohistochemically, there are more CD68 and CD163 positive tissue cells, and unstained circular or oval vacuolar structures can be seen inside tissue cells (29). The MG bodies are specifically stained with D-PAS. PAS can mark MG bodies, which are purple–red in color. The diagnosis is consistent with soft spot disease. Due to the rarity of this disease, its clinical symptoms and general features are nonspecific, and there is a lack of sufficient understanding. Thus, misdiagnosing this disease as a malignant tumor, especially using frozen specimens obtained during surgery, is easy, and misdiagnosis and missed diagnoses can occur. Therefore, differential diagnosis is necessary. (1) The differential diagnosis for endometrial poorly differentiated carcinoma is as follows: when malacoplakia occurs in the uterine cavity, it often manifests as vaginal bleeding, menstrual changes, ultrasound detection of a space occupying the uterine cavity, and microscopic masses of tissue cells that are easily mistaken for epithelial cells. Especially during intraoperative frozen sectioning, due to the lack of fixed tissue and atypical cell morphology, malacoplakia can be misdiagnosed as poorly differentiated endometrial carcinoma. In endometrial cancer, CK (AE1/AE3) and vimentin are positive, while CD68 is negative, and the Ki67 proliferation index is significantly greater than that in malacoplakia (33, 34). (2) The differential diagnosis for malignant melanoma is as follows: when malacoplakia is accompanied by bleeding, the lesion appears dark brown. Under a microscope, tissue cells are prone to morphology similar to that of malignant melanoma cells. Malignant melanoma cells exhibit obvious atypia, with large purple–red nucleoli visible and melanin visible in the cytoplasm. The immunohistochemical markers HMB-45, Melan-A, and S-100 are positive (35). (3) Xanthogranulomatous and histiocytic endometritis are commonly observed in postmenopausal women and are characterized by vaginal bleeding or fluid flow, often accompanied by cervical stenosis or pyometra with generally brownish yellow brittle tissue. Microscopically, patients with xanthogranulomatous and histiocytic endometritis show a large number of tissue cells with eosinophilic or foam-like cytoplasm. The cytoplasm is also rich in lipids or hemosiderin. There are also plasma cells, lymphocytes and neutrophils in the background (36, 37). Unlike malacoplakia, these patients lack characteristic MG bodies. This disease can be distinguished based on medical history.
3.6 Individualized management of malacoplakia of the cervix
Currently, there are no definitive guidelines for the treatment of malacoplakia. The main therapeutic approaches are antimicrobial therapy, a reduction in the use of immunosuppressive drugs and surgical treatment (23, 24). Quinolone antimicrobials (methotrexate, ciprofloxacin) have good cell membrane penetration and are therapeutically effective. However, quinolones block neuromuscular transmission, and there is a possibility of myasthenia gravis (38, 39). Early antimicrobial treatment before malacoplakia causes severe and extensive pathological damage can prevent this pathological damage (40). In this case, the patient was sensitive to aminoglycoside antibiotics, so we administered amikacin and tobramycin ointment for anti-infection treatment. In addition, another treatment strategy is immunotherapy. Cholinergic receptor agonists and vitamin C can alleviate immune dysfunction. Ascorbic acid can enhance lysozyme damage caused by immune deficiency. Therefore, the combination of antibiotics, vitamin C, and cholinergic drugs may have a certain effect (41–44).
Surgery may be recommended when conventional drug therapy fails (2, 4–12). Thirteen cases of cervical malacoplakia have been reported; three patients were treated with antibiotics, five were treated with hysterectomy, and the remaining five were either not treated or died before treatment. Hysterectomy was performed in all surgically treated patients, which may be related to the difficulty of distinguishing cervical malacoplakia from cervical malignancy (29) (Table 1). Due to differences in culture and religious beliefs, most Chinese women want to preserve the uterus. For our patient, we intravenously administered antibiotics they were sensitive to immediately after diagnosis. In the first month, the cervical space occupation tended to decrease. However, after infection with COVID-19, the cervical space occupied became larger. This effect may be related to COVID-19 attacking the immune system (45, 46). Due to the special anatomical properties of the cervix, we first performed a colposcopic biopsy before the operation, and the pathology confirmed cervical malacoplakia. Therefore, we developed a personalized operation involving the resection of cervical lesions and vaginal wall lesions. We also administered a special vaginal lavage after surgery. The optimal duration of antibiotic therapy for patients with malacoplakia is unclear, and typically ranges from 12 weeks to 6 months (14). We chose to administer the drug vaginally for 3 months continuously, and the patient did not relapse within 12 months after surgery.
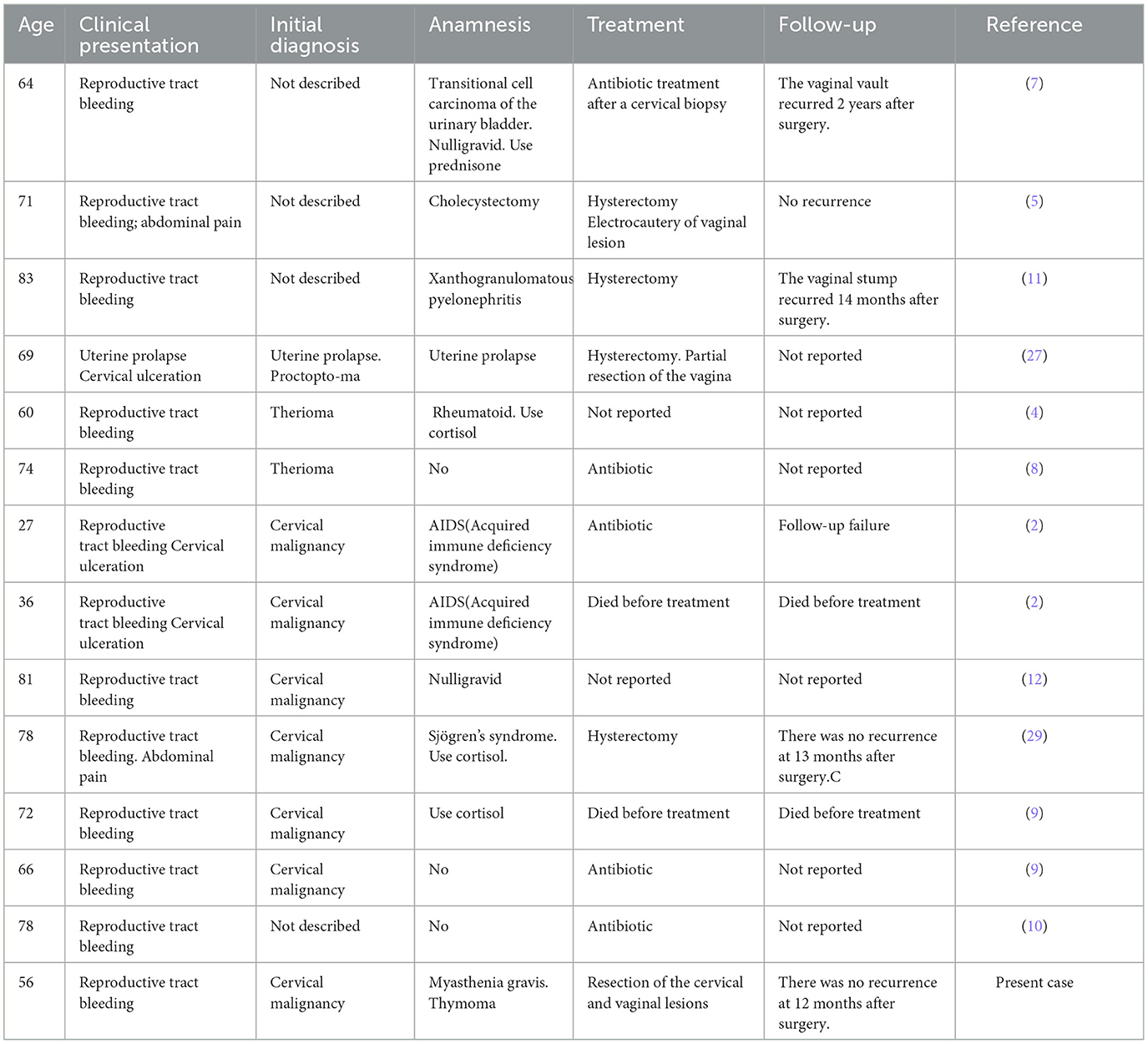
Table 1. Summary of the previous cases reported of malacoplakia of the uterine cervix in literature along with our case.
4 Conclusion
Malacoplakia is a rare systemic disease that is usually seen in immunocompromised patients, and the common treatment regimen is intravenous or oral antibiotics combined with total hysterectomy. In our case, we used the first treatment protocol involving antibiotic vaginal lavage after combined resection of cervical and vaginal lesions, which was a new approach for the treatment of cervical malacoplakia. Early and accurate diagnosis and individualized treatment are the basis for improving patient prognosis, and we hope that in future studies, we can explore the etiology, pathogenesis, imaging characteristics, and treatment modalities of cervical malacoplakia in greater depth to improve the quality of life of patients and the cure rate of this disease.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Ningbo No.2 Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JL: Writing – original draft, Writing – review & editing. JM: Software, Writing – original draft, Writing – review & editing. JW: Data curation, Writing – review & editing. ZZ: Funding acquisition, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from Zhu Xiu Shan Talent Project of Ningbo No. 2 Hospital (2023HMJQ11), Hwamei Research Foundation of Ningbo No. 2 Hospital (2018HMZD26), and Hwamei Research Foundation of Ningbo No. 2 Hospital (2019HMKY11).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Xia Z, Du D, Zhang Z, Hu Z, Liu Z. Malakoplakia of urinary bladde: 3 cases reports and review. Int Urol Nephrol. (2024) 56:1779–84. doi: 10.1007/s11255-023-03920-7
2. Ramdial PK, Sing Y, Chotey NA, Bagratee JS. Concomitant malacoplakia and granuloma inguinale of the cervix in acquired immune deficiency syndrome. Int J Gynecol Pathol. (2008) 27:282–7. doi: 10.1097/PGP.0b013e31815788fc
3. Kohl SK, Hans CP. Cutaneous malakoplakia. Arch Pathol Lab Med. (2008) 132:113–7. doi: 10.5858/2008-132-113-CM
4. Stewart CJ, Thomas MA. Malacoplakia of the uterine cervix and endometrium. Cytopathology. (1991) 2:271–5. doi: 10.1111/j.1365-2303.1991.tb00498.x
5. Willén R, Stendahl U, Willén H, Tropé. Malacoplakia of the cervix and corpus uteri: a light microscopic, electron microscopic, and X-ray microprobe analysis of a case. Int J Gynecol Pathol. (1983) 2:201–8. doi: 10.1097/00004347-198302000-00011
6. Agnarsdóttir M, Hahn L, Sellgren U, Willén R. Malacoplakia of the cervix uteri and vulva. Acta Obstet Gynecol Scand. (2004) 83:214–6. doi: 10.1080/j.0001-6349.2004.077c.x
7. Chalvardjian A, Picard L, Shaw R, Davey R, Cairns JD. Malacoplakia of the female genital tract. Am J Obstet Gynecol. (1980) 138:391–4. doi: 10.1016/0002-9378(80)90134-9
8. Wiltenburg W, Wouters M. Malacoplakie van de tractus genitalis bij een vrouw met postmenopauzaal bloedverlies. Nederlands tijdschrift voor geneeskunde: Ned Tijdschr Geneeskd (2003).
10. Falcón-Escobedo R, Mora-Tiscareño A, Pueblitz-Peredo SJAC. Malacoplakia of the uterine cervix. Histologic, cytologic and ultrastructural study of a case. Acta Cytol. (1986) 30:281–4.
11. Chen K, Hendricks EJO. Gynecology. Malakoplakia of the female genital tract. Acta Cytol. (1985) 65:84S−7S.
12. Hall V. Malakoplakia of the cervix uteri. J Obstet Gynaecol. (1996) 16:62. doi: 10.3109/01443619609028393
13. Ramdhan RC, Loukas M, Tubbs RS. Anatomical complications of hysterectomy: a review. Clin Anat. (2017) 30:946–52. doi: 10.1002/ca.22962
14. Kinsella PM, Smibert OC, Whitlam JB, Steven M, Masia R, Gandhi RG, et al. Successful use of azithromycin for Escherichia coli-associated renal allograft Malakoplakia: a report of two cases. Eur J Clin Microbiol Infect Dis. (2021) 40:2627–31. doi: 10.1007/s10096-021-04270-x
15. Yousef GM, Naghibi B, Hamodat MM. Malakoplakia outside the urinary tract. Arch Pathol Lab Med. (2007) 131:297–300. doi: 10.5858/2007-131-297-MOTUT
16. Toubes-Klingler E, Prabhu VC, Bernal K, Poage D, Swindells S. Malacoplakia of the cranium and cerebrum in a human immunodeficiency virus-infected man. Case report. J Neurosurg. (2006) 104:432–5. doi: 10.3171/jns.2006.104.3.432
17. Shawaf AZ, Boushi LA, Douri TH. Perianal cutaneous malakoplakia in an immunocompetent patient. Dermatol Online J. (2010) 16:10. doi: 10.5070/D332P0W3T8
18. Alsaeed M, Mursi M, Eltayeb N, Kuriry H, Albaghli S, Alrusayni Y. Bifocal malakoplakia in a patient living with HIV: case report. AIDS Res Ther. (2024) 21:3. doi: 10.1186/s12981-024-00592-w
19. Sinclair-Smith C, Kahn LB, Cywes S. Malacoplakia in childhood. Case report with ultrastructural observations and review of the literature. Arch Pathol. (1975) 99:198–203.
21. Medlicott S, Magi-Galluzzi C, Jimenez RE, Trpkov K. Malakoplakia associated with prostatic adenocarcinoma: report of 4 cases and literature review. Ann Diagn Pathol. (2016) 22:33–7. doi: 10.1016/j.anndiagpath.2016.03.004
23. Stanton MJ, Maxted W. Malacoplakia: a study of the literature and current concepts of pathogenesis, diagnosis and treatment. J Urol. (1981) 125:139–46. doi: 10.1016/S0022-5347(17)54940-X
24. Abdou NI, NaPombejara C, Sagawa A, et al. Malakoplakia: evidence for monocyte lysosomal abnormality correctable by cholinergic agonist in vitro and in vivo. N Engl J Med. (1977) 297:1413–9. doi: 10.1056/NEJM197712292972601
25. Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol. (2015) 14:1023–36. doi: 10.1016/S1474-4422(15)00145-3
26. Lee E, Park H, Park S, Cho HR, Park KS, Park J, et al. Outgrowing skin involvement in malakoplakia after kidney transplantation: A case report. Transplant Proc. (2022) 54:1627–31. doi: 10.1016/j.transproceed.2022.03.055
27. Agnarsdóttir M, Hahn L, Sellgren U, Willén R. Malacoplakia of the cervix uteri and vulva. Acta Obstet Gynecol Scand. (2004) 83:214–6. doi: 10.1111/j.0001-6349.2004.077c.x
28. Arafah M, Rashid S, Tulbah A, Akhtar M. Carcinomas of the uterine cervix: comprehensive review with an update on pathogenesis, nomenclature of precursor and invasive lesions, and differential diagnostic considerations. Adv Anat Pathol. (2021) 28:150–70. doi: 10.1097/PAP.0000000000000300
29. Saco A, Rakislova N, Marimon L, Torne A, Diaz-Feijoo B, et al. Malacoplakia of the uterine cervix: a case report. Pathogens. (2021) 10:343. doi: 10.3390/pathogens10030343
30. d'Amati A, Bellitti E, Resta L. Unexpected endometrial malacoplakia related to abortion and placental rests retention: a case report. Diagn Pathol. (2020) 15:88. doi: 10.1186/s13000-020-01014-x
31. Antonella V, Francesca C, Rosalba DN, et al. Tracking endometrial malacoplakia through the evolution of 2D and 3D ultrasound and histopathological features. Cureus. (2024) 16:e52268. doi: 10.7759/cureus.52268
33. Stewart CJ, Crook ML. PAX2 and cyclin D1 expression in the distinction between cervical microglandular hyperplasia and endometrial microglandular-like carcinoma: a comparison with p16, vimentin, and Ki67. Int J Gynecol Pathol. (2014) 34:90–100. doi: 10.1097/PGP.0000000000000107
34. Turashvili G, Hanley K. Practical updates and diagnostic challenges in endometrial carcinoma. Arch Pathol Lab Med. (2023) 148:78–98. doi: 10.5858/arpa.2022-0280-RA
35. Kollabathula A, Gupta P, Das CK, Awasthi D, Srinivasan R. Malignant uterine perivascular epithelioid cell tumor: histopathologic and immunohistochemical characterization of a rare tumor in a post-menopausal woman. Int J Clin Exp Pathol. (2021) 14:993–9.
36. Malik CA, Dudani S, Mani BN. Xanthogranulomatous endometritis presenting as pyometra and mimicking carcinoma on imaging. J Midlife Health. (2016) 7:88–90. doi: 10.4103/0976-7800.185326
37. Makkar M, Gill M, Singh D. Xanthogranulomatous endometritis: an unusual pathological entity mimicking endometrial carcinoma. Ann Med Health Sci Res. (2013) 3:S48–9. doi: 10.4103/2141-9248.121222
38. Gunduz A, Turedi S, Kalkan A, Nuhoglu I. Levofloxacin induced myasthenia crisis. Emerg Med J. (2006) 23:662. doi: 10.1136/emj.2006.038091
39. Sieb JP. Fluoroquinolone antibiotics block neuromuscular transmission. Neurology. (1998) 50:804–7. doi: 10.1212/WNL.50.3.804
40. Gao P, Hu Z, Du D. Malakoplakia of the bladder near the ureteral orifice: a case report. J Int Med Res. (2021) 49:3000605211050799. doi: 10.1177/03000605211050799
41. Zurier RB, Weissmann G, Hoffstein S, Kammerman S, Tai HH. Mechanisms of lysosomal enzyme release from human leukocytes. II Effects of cAMP and cGMP, autonomic agonists, and agents which affect microtubule function. J Clini Investigat. (1974) 53:297–309. doi: 10.1172/JCI107550
42. Dasgupta P, Womack C, Turner AG, Blackford HN. Malacoplakia: von Hansemann's disease. BJU Int. (1999) 84:464–9. doi: 10.1046/j.1464-410x.1999.00198.x
43. Purnell SD, Davis B, Burch-Smith R, Coleman P. Renal malakoplakia mimicking a malignant renal carcinoma: a patient case with literature review. BMJ Case Rep. (2015) 2015:bcr2014208652. doi: 10.1136/bcr-2014-208652
44. Dias PHGF, Slongo LE, Romero FR, Paques GR, Gomes RPX, Carlos DAR, et al. Retroperitoneal sarcoma-like malakoplakia. Rev Assoc Med Bras. (2011) 57:615–6. doi: 10.1590/S0104-42302011000600005
45. Sun Y, Luo B, Liu Y, Wu Y, Chen Y. Immune damage mechanisms of COVID-19 and novel strategies in prevention and control of epidemic. Front Immunol. (2023) 14:1130398. doi: 10.3389/fimmu.2023.1130398
Keywords: malacoplakia, malignant tumor of the cervix, pathology, vaginal bleeding, case report
Citation: Li J, Mi J, Wang J and Zhuo Z (2024) Case report: A rare case of malacoplakia resembling a malignant tumor of the cervix: a case report and review of the literature. Front. Med. 11:1409239. doi: 10.3389/fmed.2024.1409239
Received: 29 March 2024; Accepted: 13 May 2024;
Published: 04 June 2024.
Edited by:
Rafał Watrowski, Helios Hospital Müllheim, GermanyReviewed by:
Antonio d'Amati, University of Bari Aldo Moro, ItalyAngel Danchev Yordanov, Medical University Pleven, Bulgaria
Copyright © 2024 Li, Mi, Wang and Zhuo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhihong Zhuo, emh1b3poaWhvbmcxQDE2My5jb20=
†These authors have contributed equally to this work
 Jiaorong Li
Jiaorong Li Jiaying Mi1†
Jiaying Mi1† Zhihong Zhuo
Zhihong Zhuo