
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 19 June 2024
Sec. Infectious Diseases: Pathogenesis and Therapy
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1406983
This article is part of the Research Topic Progressing the Understanding and Management of Bloodstream Infections View all 5 articles
 Shiori Kitaya1,2*†
Shiori Kitaya1,2*† Hajime Kanamori1†
Hajime Kanamori1† Ryo Ishii2
Ryo Ishii2 Makoto Katsumi3
Makoto Katsumi3 Ryoukichi Ikeda4
Ryoukichi Ikeda4 Kenjiro Higashi2
Kenjiro Higashi2 Risako Kakuta2
Risako Kakuta2 Akira Ohkoshi2
Akira Ohkoshi2 Yukio Katori2
Yukio Katori2Introduction: Compared to other cancers, research on bloodstream infection in head and neck cancer is scarce, lacking comparative studies on persistent versus transient bacteremia outcomes.
Methods: This retrospective survey examined patients with head and neck cancer undergoing blood culture at our center from June 2009 to May 2023. Blood culture-positive cases suspected of infection were divided into persistent bacteremia and transient bacteremia groups. We investigated their clinical, epidemiological, and microbiological features, including risk factors for persistent bacteremia and mortality. The primary outcome was 90-day mortality.
Results: In this 97-patient cohort, 14 (14%) cases were assigned to the persistent bacteremia group. Catheter-related bloodstream infections were the leading cause of infection in both groups, consistently contributing to a high proportion of overall bloodstream infections. The mortality rate was generally higher in the persistent bacteremia group than in the transient bacteremia group (odds ratio [OR], 2.6; 95% confidence interval [CI], 0.6–11.1), particularly in the non-clearance subgroup (OR, 9; 95% CI, 0.5–155.2). Pyogenic spondylitis was a key risk factor for persistent bacteremia, while hypoalbuminemia increased mortality.
Conclusion: In patients with bacteremia and head and neck cancer, persistent bacteremia was associated with higher mortality than was transient bacteremia. Adittionally, bacteremia clearance in persistent bacteremia is thus crucial for prognostic improvement.
Head and neck cancer (HNC) is the fifth most common malignant tumor in the world and the eighth leading cause of carcinoma-related mortality (1, 2). Chemoradiotherapy (CRT), a common treatment for HNC, can lead to skin and pharyngeal mucosal damage, neutropenia, and immunosuppression (3). Specifically, it can lead to an increased risk of infectious complications, including bloodstream infections (BSIs), both during and after radiotherapy (RT) or CRT. Notably, patients undergoing RT or CRT experience 90-and 180-day overall mortality rates of 3–4% and 7–10%, respectively (4–7), with BSI implicated in 10% of non-carcinoma-related early deaths (8).
Persistent bacteremia (PB) is associated with adverse clinical outcomes, such as prolonged hospitalization and higher mortality rates (9). It is attributed to various organisms, including Staphylococcus aureus (10), gram-negative rods (GNR) (11), and Candida spp. (12). Furthermore, our previous investigation revealed that for gram-positive cocci (GPC), GNR, and Candida spp., the non-clearance group of PB had a higher mortality rate than did the clearance group (13). Although studies on BSI in patients with HNC have been conducted (8, 14–16), none have compared the outcomes between PB and transient bacteremia (TB) in BSI among patients with HNC, nor have they compared outcomes based on clearance of bacteremia. Accordingly, the objectives of this retrospective observational study were to (1) compare clinical outcomes, including mortality rates, between patients with HNC having PB versus TB, (2) compare clinical outcomes based on clearance of bacteremia, and (3) analyze the risk factors leading to PB and mortality in patients with HNC.
This retrospective, single-center, observational study was conducted at a tertiary care center at Tohoku University Hospital, Sendai, Miyagi, Japan. We investigated electronic clinical charts and hospital records to gather research variables from patients with HNC who underwent blood culture (BC) at the Department of Otolaryngology, Head and Neck Surgery, Tohoku University Hospital, between June 2009 and May 2023. All patients with HNC diagnosed with BSI were eligible for inclusion in this study. Exclusion criteria included possible contaminants, such as coagulase-negative staphylococci, Propionibacterium spp., and Corynebacterium spp.
The clinical characteristics were retrospectively examined using electronic clinical charts, hospital records, and microbiological data. Detailed information regarding the collected anamnestic and clinical data is provided in the Supplementary method S1. Microbial data (associated infection sites, organisms, and susceptibility) were extracted from the Infectious Diseases Department database. The focus of infection was identified by infectious disease specialists based on detailed physical examinations and confirmation of information in medical records. The primary outcome of this study was the 90-day mortality rate. Secondary outcomes included the risk factors for PB and mortality.
This study was conducted in accordance with the Declaration of Helsinki and approved by the Human Ethical and Clinical Trial Committee of Tohoku University Hospital (2018–1-736). The requirement for patient consent was waived due to the retrospective nature of the study.
The definitions of BC collection, BSI, PB, follow-up BC, PB duration, PB clearance, contamination, neutropenia, intravascular devices, source control, and adequacy of antimicrobial therapy were adopted from our previous report (13). Bacteremia was classified as nosocomially acquired, healthcare-related, or community-acquired by applying previously described criteria (17). The quick Sequential Organ Failure Assessment (qSOFA) score was calculated based on previously established definitions (18). Comorbidities were identified using the Charlson Comorbidity Scoring System (19). The 90-day mortality rate was defined as death within 90 days of the first BC collection.
The histological type and tumor grading were assessed in accordance with the International Classification of Diseases for Oncology, third edition (20), third edition first revision (21), and third edition second revision (22). Tumor staging was performed based on the Union for International Cancer Control TNM classification, including the sixth (2002) (23), seventh (2010) (24), and eighth (2017) editions (25). Immunohistochemical analysis of p16 expression in patients with oropharyngeal cancer served as an indicator of human papillomavirus status. Oral and laryngopharyngeal mucositis and dermatitis were classified according to the Common Terminology Criteria for Adverse Events Version 4.0 (26).
Details pertaining to the methodology for identifying microorganisms, measuring antimicrobial susceptibility, and performing statistical analyses are provided in the Supplementary methods S2, S4.
Figures 1A,B illustrate the temporal changes in hospitalization morbidity rates per 1,000 patient days and mortality rates for the PB and TB groups. Hospitalization morbidity rates per 1,000 patient days and mortality rates exhibited similar trends over time, with both groups peaking in 2011. In more recent years, an increased trend was observed in hospitalization morbidity and mortality rates for TB cases. Catheter-related bloodstream infection (CRBSI) accounted for a significant proportion of the TB and PB groups, serving as a focal point of infection.
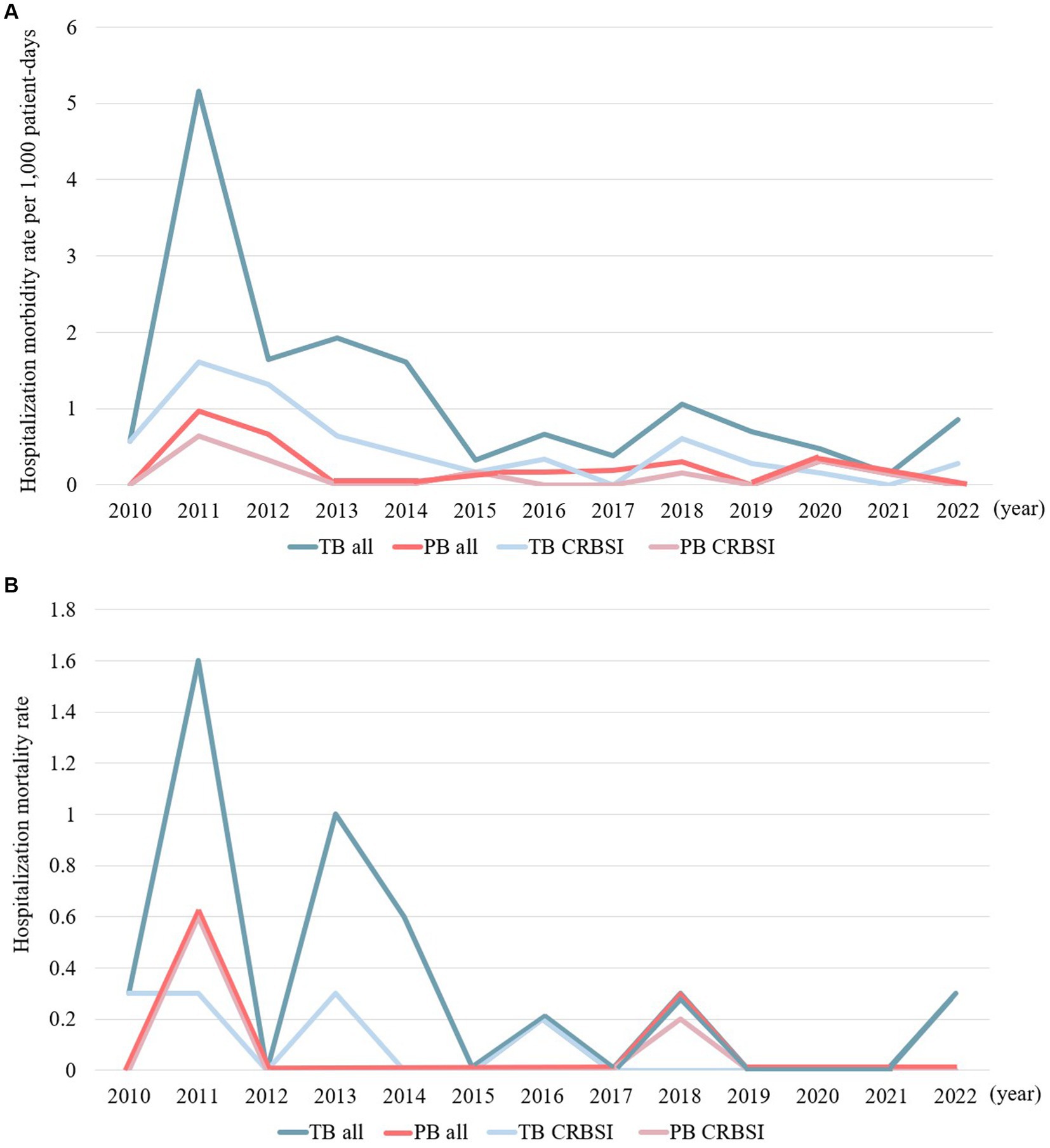
Figure 1. (A) Temporal trends in the hospitalization morbidity rates per 1,000 patient days in cases of persistent and transient bacteremia. (B) Temporal trends in hospitalization mortality rates in cases of persistent and transient bacteremia.
The clinical characteristics of PB and TB in patients with HNC are shown in Table 1. During the study period, there were 97 cases of positive BCs in patients with HNC; of these, 14 (14%) exhibited PB. Significantly fewer cases of inappropriate antimicrobial use were observed in the PB group (0 cases; 0%) than in the TB group (23 cases; 28%; OR, 0; CI not applicable). In contrast, the number of cases with insufficient source control measures was significantly higher in the PB group than in the TB group (odds ratio [OR], 6.2; 95% confidence interval [CI] 1.4–27.1, p = 0.023).

Table 1. Differences in clinical characteristics between the persistent and transient bacteremia groups in patients with head and neck cancer.
In terms of infection sites, CRBSI was the most common infection in the PB and TB groups (8 cases [57%] and 36 cases [43%], respectively). In the PB group, pyogenic spondylitis (4 cases; 29%) and thrombophlebitis (2 cases; 14%) were the next most prevalent, whereas respiratory (10 cases; 12%) and urinary tract infections (8 cases; 10%) were more frequent in the TB group. The proportion of patients with pyogenic spondylitis was significantly higher in the PB group than in the TB group (OR, 16.2; 95% CI 2.6–100, p = 0.004). Additionally, the incidence of thrombophlebitis tended to be higher in the PB group than in the TB group, although it was not statistically significant (OR, 4.4; 95% CI 0.7–29.4).
Regarding the pharmacological treatment regimen, the proportion of biotherapy, including cetuximab + fluorouracil + cisplatin, cetuximab-RT, and nivolumab, showed a tendency to be higher in the PB group than in the TB group, although the difference was not statistically significant (OR, 4.4 [95% CI 0.7–29.4], OR, 6.3 [95% CI 0.4–107.2], and OR, 6.3 [95% CI 0.4–107.2], respectively). When comparing the 90-day mortality rates between the PB and TB groups, the PB group tended to exhibit a higher mortality rate (OR, 2.6; 95% CI 0.6–11.1), although no statistically significant difference was observed. Additionally, the group in which PB clearance was not achieved tended to have a higher mortality rate than the group in which PB clearance was achieved (OR, 9; 95% CI 0.5–155.2).
Regarding the causative microorganisms of PB and TB in patients with HNC, an overview of the bacterial species based on Gram staining is shown in Figure 2. Detailed bacterial genera and species names are presented in Supplementary Tables S2, S3, highlighting the variation in bacterial species between PB and TB cases, as well as between instances of mortality and survival. GPC tended to occur in a higher proportion of patients in the PB group than in the TB group (67% vs. 55%; OR, 1.6 [95% CI 0.5–5]). Conversely, there was a significantly lower proportion of GNR in the PB group than in the TB group (7% vs. 34%; OR, 0.1 [95% CI 0–1.1], p = 0.037). In the PB group, the most frequently identified causative pathogen was methicillin-resistant S. aureus (MRSA; 33%), followed by methicillin-susceptible S. aureus (MSSA) and Candida parapsilosis (13%). In contrast, the most frequently identified causative pathogen in the TB group was Staphylococcus epidermidis (20%), followed by MRSA (13%), MSSA (9%), and Pseudomonas aeruginosa (7%).
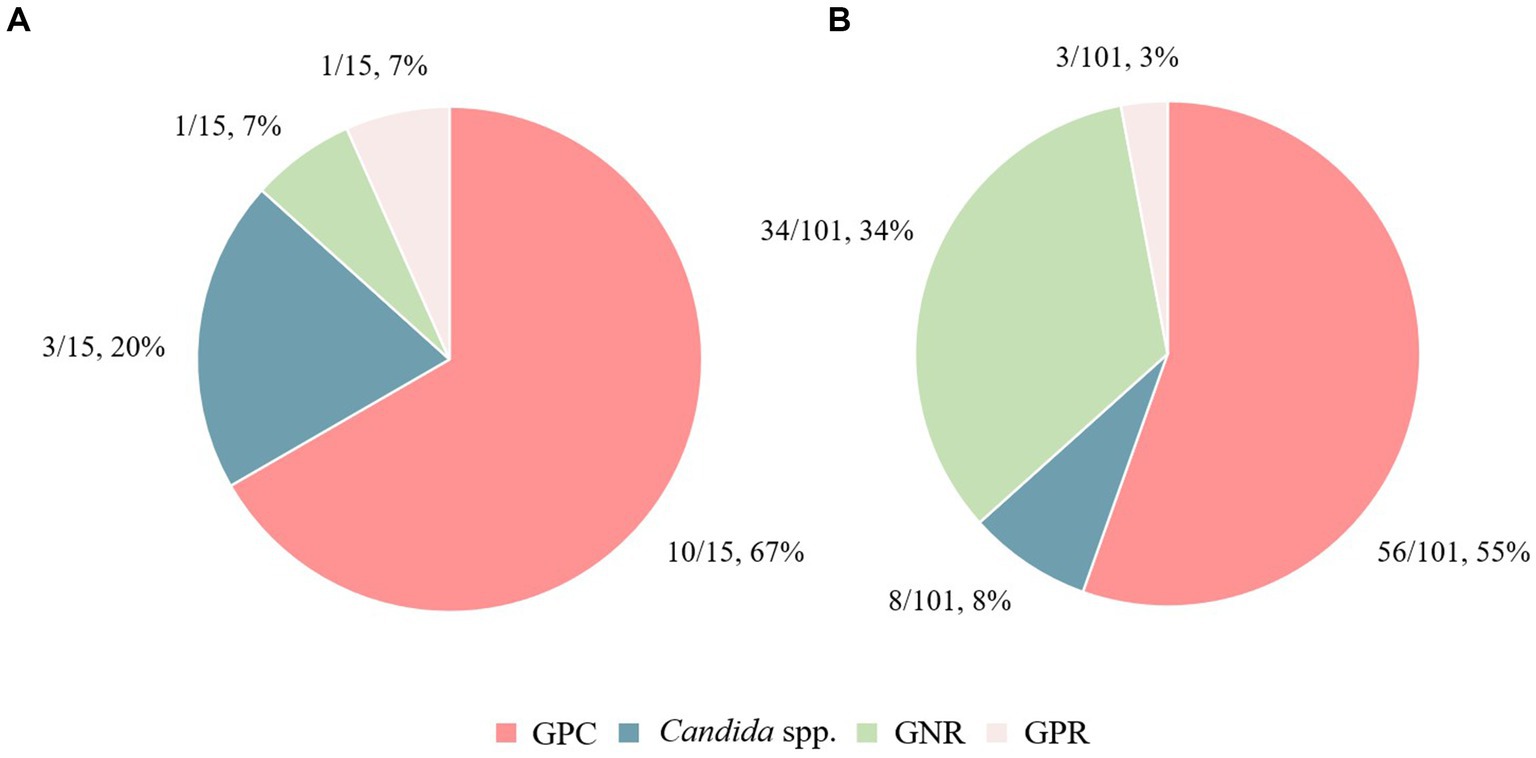
Figure 2. Differences in causative bacterial species between the persistent bacteremia and transient bacteremia groups. The causative bacterial species in (A) the persistent bacteremia group and (B) the transient bacteremia group are shown. GPC, Gram-positive cocci; GPR, Gram-positive rods; GNR, Gram-negative rods.
Table 2 presents the multivariate logistic regression analysis results. The factor independently associated with PB in patients with HNC was pyogenic spondylitis (adjusted odds ratio [aOR], 22.1; 95% CI 2–239.2], p = 0.011). Factors independently associated with mortality in patients with HNC were serum albumin levels (aOR, 0.1; 95% CI 0–0.8, p = 0.023) and clinical stages I–II (aOR, 12.9; 95% CI 1.8–93.4, p = 0.011).
The hospitalization morbidity rate per 1,000 patient days and hospitalization mortality rate both peaked in 2011. Furthermore, the hospitalization morbidity and mortality rates in the TB group has exhibited an increasing trend since 2022. Since 2011, the medical team managing HNC has actively implemented infection control measures, specifically by increasing opportunities for BC collection and ensuring the collection of two sets of BCs (Supplementary Figure S1). Additionally, until mid-2011, the primary chemotherapeutic regime was docetaxel + cisplatin + fluorouracil, resulting in a high incidence of febrile neutropenia due to hematologic toxicity. These factors may have contributed significantly to the notable increase in hospitalization morbidity rate per 1,000 patient days and hospitalization mortality rate in 2011. More recently, there has been a renewed increase in the hospitalization morbidity and mortality rates in the TB group in our study. It is imperative to monitor infection control measures and treatment management within hospital wards to prevent further increases in the mortality rate of patients with BSIs and HNC.
Recent technological advancements in the healthcare industry have improved surgical techniques, including those used for treating HNC. In the field of chemotherapy, molecular targeted therapy and immunotherapy have emerged alongside conventional treatment regimens (27, 28). Despite the presumed improvement in hygiene management during patient care, the number of CRBSI cases in our department remains high. In the Department of Otolaryngology and Head and Neck Surgery, laryngoscopy is frequently performed during hospitalization to monitor the treatment progress and airway management. Additionally, procedures such as suction with sputum dispersion and postoperative wound care are often performed within the examination units of the ward. In patients with HNC, central venous catheters or ports are frequently inserted for chemotherapy and nutritional supplementation. In these patients, there is a potential risk of contracting CRBSI, especially during techniques involving aerosol dispersion, where droplets may adhere to the catheter surroundings or be transmitted through contact with healthcare personnel. Although our department generally follows the CRBSI guidelines (29), infection prevention strategies focused solely on managing the catheter insertion site have limitations. Thus, it is desirable to reduce the environmental bacterial load alongside catheter management. Our hospital’s infection control team regularly inspects various medical and surgical units, providing infection management guidance. Particularly for patients with antimicrobial-resistant bacteria, the infection control team recommend isolated treatment units, frequent cleaning and disinfection of equipment and chairs, and environmental disinfection using ultraviolet irradiation devices (30). In addition to managing the catheter-insertion site, implementing these environmental disinfection measures to lower the environmental bacterial load is crucial to decrease the incidence of infections, including CRBSI, in patients with HNC. Furthermore, fostering collaboration between the infection control team and medical staff to increase infection management awareness, informed by current issues, proves to be an effective approach.
RT induces endothelial damage and vascular narrowing, increasing the risk of delayed wound healing and infection (31). Moreover, chemotherapy can cause microcirculatory disturbances and mucositis (32). In our study, CRT was the most common treatment modality in patients with pyogenic spondylitis, accounting for half of the cases (3/6 cases; 50%). Contrastingly, a previous study reported cases of patients with oropharyngeal cancer who developed inflammation of the vertebral bodies and intervertebral discs after oral surgery (33). Therefore, postoperative patients should be monitored for pyogenic spondylitis, similar to those receiving CRT.
Primarily, pyogenic spondylitis is treated with long-term antimicrobial therapy (34). Surgical intervention, however, is recommended in instances of spinal instability, vertebral destruction, abscess formation in the lumbar muscles, and nerve damage (35, 36). In this study, 33% (2/6 cases) underwent debridement for pyogenic spondylitis, while 67% (4/6 cases) did not. Notably, 75% (3/4 cases) of those without source control progressed to PB. In patients with HNC, their compromised overall condition or nutritional status due to cancer may discourage invasive interventions. Hence, this may be a contributing factor to the high proportion of cases for which source control was not implemented to treat pyogenic spondylitis. Multivariate analysis highlighted pyogenic spondylitis as a PB risk factor. Thus, the timely implementation of source control, when necessary, may contribute to the prevention of progression to PB. Furthermore, close monitoring for PB progression is essential, particularly in cases lacking source control.
Various bacterial species such as S. aureus (10), Enterococcus spp. (37), Gram-negative bacteria (11), and Candida spp. (12) are reportedly associated with PB and worsened prognosis, particularly leading to increased mortality rates. Cancer status is considered a risk factor for PB (37), and PB is regarded as a risk factor for increased mortality in patients with cancer (38, 39). In this study, even among patients with HNC, the PB group showed a stronger tendency toward higher mortality compared to that of the TB group. Therefore, in patients with HNC who develop PB, careful consideration should be given to appropriate treatment, active management of the infectious focus, and comprehensive systemic management.
In this study, a tendency toward higher mortality rates was observed in cases for which PB clearance was not confirmed (50%, 2/4 cases) compared to that with cases with confirmed clearance (10%, 1/10 cases; OR, 9 [95% CI, 0.5–155.2]). Our previous study demonstrated a similar trend in cases associated with GPC, GNR, and Candida spp., with the groups without PB clearance having a higher mortality rate than that of those with clearance (13). The results of this study suggest that even in cases with PB in patients with HNC, confirming its clearance may improve patient prognosis.
In a retrospective study at our university hospital, 13% of BC-positive patients exhibited PB, 53% of which were caused by GPC (13). Among patients with HNC, the PB incidence rate was approximately 14%, aligning closely with the general hospital population’s rate. Notably, 67% of these PB cases were due to GPC, a marginally higher proportion compared to the general hospital population. Regarding the characteristics of causative pathogens in BSI owing to differences in cancer types, GNRs are predominantly isolated in BSI among patients with cancers other than HNCs (38). Contrastingly, GPC are predominantly isolated in BSI among patients with HNC (16). In our study on bacteremia in patients with HNC, GPC also emerged as the most frequent pathogen (13). Integrating these findings with our current study results reveals distinct characteristics in the causative pathogens of BSI, which vary based on differences in cancer types. Despite the variation, GPC remain an important causative agent, even in patients with HNC.
Malnutrition or enhanced vascular permeability can lead to low serum albumin levels, which are linked to heightened infection and BSI risks (16). Patients with esophageal cancer with low pretreatment serum albumin levels reportedly have a higher incidence of complications, such as BSIs, respiratory failure, arrhythmias, and heart failure (40). Furthermore, patients with low pretreatment serum albumin levels had higher postoperative mortality rates, and pretreatment serum albumin levels have been identified as predictors of survival (40). In patients with HNC, low albumin levels are also a BSI risk factor, escalating both early and overall mortality rates (16). Our multivariate analysis identified low albumin levels as a mortality risk factor. Thus, in patients with HNC, serum albumin levels are crucial prognostic factors and valuable for prognostic assessment alongside other clinical metrics.
Patients in clinical stages I and II of HNC exhibited a higher mortality risk compared to those in clinical stages III and IV. However, since the number of cases in clinical stages I and II in this study was small (n = 18 cases), the results may have been influenced by sample size bias (Supplementary Table S4).
In conclusion, to the best of our knowledge, this is the first study to investigate clinical features of PB in patients with HNC. Although this study was a single-center retrospective investigation with limitations in generalizability, it was conducted over a long period of 15 years, making the results significant. The key findings are summarized as follows: (1) Peaks in hospitalization morbidity rates per 1,000 patient days and mortality rates in the PB and TB groups were observed in 2011. An increasing trend has recently been observed in hospitalization morbidity and mortality rates for the TB group. (2) CRBSI was the main infectious focus in both groups, with a consistently high contribution to BSI. (3) Patients with HNC with pyogenic spondylitis had an elevated PB risk, particularly without source control. (4) MRSA-related BSI in patients with HNC led more often to PB and higher mortality than did MSSA-related BSI. (5) Pyogenic spondylitis increased PB risk, while hypoalbuminemia increased mortality in patients with HNC. (6) PB had a higher mortality rate than did TB, thereby underscoring the need for PB clearance to improve prognosis.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving humans were approved by the Human Ethical and Clinical Trial Committee of Tohoku University Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the requirement for patient consent was waived due to the retrospective nature of the study.
SK: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. HK: Writing – review & editing, Investigation. RIs: Writing – review & editing, Data curation. MK: Writing – review & editing, Data curation. RIk: Writing – review & editing, Funding acquisition. KH: Writing – review & editing. RK: Writing – review & editing. AO: Writing – review & editing. YK: Funding acquisition, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Takeda Science Foundation and Department of Community Medicine of Hearing Loss, Tohoku University Graduate School of Medicine.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1406983/full#supplementary-material
HNC, head and neck cancer; CRT, chemoradiotherapy; BSIs, bloodstream infections; RT, radiotherapy; PB, persistent bacteremia; GNR, gram-negative rods; GPC, gram-positive cocci; TB, transient bacteremia; BC, blood culture; qSOFA, quick sequential organ failure assessment; CRBSI, catheter-related bloodstream infection; OR, odds ratio; CI, confidence interval; MRSA, methicillin-resistant S. aureus; MSSA, methicillin-susceptible S. aureus; aOR, adjusted odds ratio
1. Maños, M, Giralt, J, Rueda, A, Cabrera, J, Martinez-Trufero, J, Marruecos, J, et al. Multidisciplinary management of head and neck cancer: first expert consensus using Delphi methodology from the Spanish Society for Head and Neck Cancer (part 1). Oral Oncol. (2017) 70:58–64. doi: 10.1016/j.oraloncology.2017.04.004
2. Rueda, A, Giralt, J, Mañós, M, Lozano, A, Sistiaga, A, García-Miragall, E, et al. Multidisciplinary management of head and neck cancer: first expert consensus using Delphi methodology from the Spanish Society for Head and Neck Cancer (part 2). Oral Oncol. (2017) 70:65–72. doi: 10.1016/j.oraloncology.2017.04.005
3. Verastegui, EL, Morales, RB, Barrera-Franco, JL, Poitevin, AC, and Hadden, J. Long-term immune dysfunction after radiotherapy to the head and neck area. Int Immunopharmacol. (2003) 3:1093–104. doi: 10.1016/S1567-5769(03)00013-4
4. Gaubatz, ME, Bukatko, AR, Simpson, MC, Polednik, KM, Adjei Boakye, E, Varvares, MA, et al. Racial and socioeconomic disparities associated with 90-day mortality among patients with head and neck cancer in the United States. Oral Oncol. (2019) 89:95–101. doi: 10.1016/j.oraloncology.2018.12.023
5. Hamilton, SN, Tran, E, Berthelet, E, Wu, J, and Olson, R. Early (90-day) mortality after radical radiotherapy for head and neck squamous cell carcinoma: a population-based analysis. Head Neck. (2018) 40:2432–40. doi: 10.1002/hed.25352
6. Talani, C, Mäkitie, A, Beran, M, Holmberg, E, Laurell, G, and Farnebo, L. Early mortality after diagnosis of cancer of the head and neck — a population-based nationwide study. PLoS One. (2019) 14:e0223154. doi: 10.1371/journal.pone.0223154
7. Jensen, KH, Vogelius, I, Kristensen, CA, Andersen, E, Overgaard, J, Eriksen, JG, et al. Early mortality after radical radiotherapy in head and neck cancer – a nationwide analysis from the Danish head and neck Cancer group (DAHANCA) database. Clin Oncol (R Coll Radiol). (2021) 33:57–63. doi: 10.1016/j.clon.2020.07.004
8. Jensen, KH, Vogelius, I, Moser, CE, Andersen, E, Eriksen, JG, Johansen, J, et al. Bloodstream infections in head and neck cancer patients after curative-intent radiotherapy: a population-based study from the Danish head and neck Cancer group database. Br J Cancer. (2021) 125:458–64. doi: 10.1038/s41416-021-01430-w
9. Minejima, E, Mai, N, Bui, N, Mert, M, Mack, WJ, She, RC, et al. Defining the breakpoint duration of Staphylococcus aureus bacteremia predictive of poor outcomes. Clin Infect Dis. (2020) 70:566–73. doi: 10.1093/cid/ciz257
10. Kuehl, R, Morata, L, Boeing, C, Subirana, I, Seifert, H, Rieg, S, et al. Defining persistent Staphylococcus aureus bacteraemia: secondary analysis of a prospective cohort study. Lancet Infect Dis. (2020) 20:1409–17. doi: 10.1016/S1473-3099(20)30447-3
11. Maskarinec, SA, Park, LP, Ruffin, F, Turner, NA, Patel, N, Eichenberger, EM, et al. Positive follow-up blood cultures identify high mortality risk among patients with gram-negative bacteraemia. Clin Microbiol Infect. (2020) 26:904–10. doi: 10.1016/j.cmi.2020.01.025
12. Fraser, VJ, Jones, M, Dunkel, J, Storfer, S, Medoff, G, and Dunagan, WC. Candidemia in a tertiary care hospital: epidemiology, risk factors, and predictors of mortality. Clin Infect Dis. (1992) 15:414–21. doi: 10.1093/clind/15.3.414
13. Kitaya, S, Kanamori, H, Baba, H, Oshima, K, Takei, K, Seike, I, et al. Clinical and epidemiological characteristics of persistent bacteremia: a decadal observational study. Pathogens. (2023) 12:212. doi: 10.3390/pathogens12020212
14. Tjoa, T, Rathi, VK, Goyal, N, Yarlagadda, BB, Barshak, MB, Rich, DL, et al. Pneumonia, urinary tract infection, bacteremia, and Clostridioides difficile infection following major head and neck free and pedicled flap surgeries. Oral Oncol. (2021) 122:105541. doi: 10.1016/j.oraloncology.2021.105541
15. Uraguchi, K, Makino, T, Kariya, S, Noda, Y, Marunaka, H, Doi, A, et al. Candidemia in patients with head and neck cancer: mortality and a novel risk factor. Support Care Cancer. (2022) 30:5921–30. doi: 10.1007/s00520-022-07010-9
16. Marín, M, Gudiol, C, Castet, F, Oliva, M, Peiró, I, Royo-Cebrecos, C, et al. Bloodstream infection in patients with head and neck cancer: a major challenge in the cetuximab era. Clin Transl Oncol. (2019) 21:187–96. doi: 10.1007/s12094-018-1905-5
17. Friedman, ND, Kaye, KS, Stout, JE, McGarry, SA, Trivette, SL, Briggs, JP, et al. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. (2002) 137:791–7. doi: 10.7326/0003-4819-137-10-200211190-00007
18. Lind, ML, Phipps, AI, Mooney, S, Liu, C, Fohner, A, Patel, K, et al. Predictive value of 3 clinical criteria for sepsis (quick sequential organ failure assessment, systemic inflammatory response syndrome, and national early warning score) with respect to short-term mortality in allogeneic hematopoietic cell transplant recipients with suspected infections. Clin Infect Dis. (2021) 72:1220–9. doi: 10.1093/cid/ciaa214
19. Charlson, M, Szatrowski, TP, Peterson, J, and Gold, J. Validation of a combined comorbidity index. J Clin Epidemiol. (1994) 47:1245–51. doi: 10.1016/0895-4356(94)90129-5
20. Fritz, A, Percy, C, Jack, A, Fritz, A, Percy, C, Jack, A, et al. International classification of diseases for oncology (ICD-O). 3rd ed. Geneva, Switzerland: World Health Organization (2000).
21. Fritz, A, Percy, C, Jack, A, Shanmugaratnam, K, Sobin, L, Parkin, D, et al. International classification of diseases for oncology (ICD-O), 1st rev. 3rd ed. Geneva, Switzerland: World Health Organization (2013).
22. Fritz, A, Percy, C, Jack, A, Shanmugaratnam, K, Sobin, L, Parkin, D, et al. International classification of diseases for oncology (ICD-O), 2nd rev. 3rd ed. Geneva, Switzerland: World Health Organization (2019).
23. Greene, FL, Page, DL, and Fleming, ID. TNM classification of malignant tumors. 6th ed. New York: Springer (2002).
24. Sobin, LH, Gospodarowicz, MK, and Wittekind, C. TNM classification of malignant tumors. 7th ed. Oxford, UK: Wiley-Blackwell (2010).
25. Brierley, JD, Gospodarowicz, MK, and Wittekind, C. TNM classification of malignant tumors. 8th ed. Oxford, UK: Wiley-Blackwell (2017).
26. Basch, E, Reeve, BB, Mitchell, SA, Clauser, SB, Minasian, LM, Dueck, AC, et al. Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst. (2014) 106:dju244. doi: 10.1093/jnci/dju244
27. Vermorken, JB, Mesia, R, Rivera, F, Remenar, E, Kawecki, A, Rottey, S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. (2008) 359:1116–27. doi: 10.1056/NEJMoa0802656
28. Ferris, RL, Blumenschein, G, Fayette, J, Guigay, J, Colevas, AD, Licitra, L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. (2016) 375:1856–67. doi: 10.1056/NEJMoa1602252
29. O’Grady, NP, Alexander, M, Burns, LA, Dellinger, EP, Garland, J, Heard, SO, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. (2011) 39:S1–S34. doi: 10.1016/j.ajic.2011.01.003
30. Kanamori, H, Rutala, WA, Gergen, MF, Sickbert-Bennett, EE, Knelson, LP, Anderson, DJ, et al. Microbial assessment of health care-associated pathogens on various environmental sites in patient rooms after terminal room disinfection. Open Forum Infect Dis. (2021) 8:ofab008. doi: 10.1093/ofid/ofab008
31. Debnam, JM, Garden, AS, and Ginsberg, LE. Benign ulceration as a manifestation of soft tissue radiation necrosis: imaging findings. AJNR Am J Neuroradiol. (2008) 29:558–62. doi: 10.3174/ajnr.A0886
32. Naidu, MU, Ramana, GV, Rani, PU, Mohan, IK, Suman, A, and Roy, P. Chemotherapy-induced and/or radiation therapy-induced oral mucositis—complicating the treatment of cancer. Neoplasia. (2004) 6:423–31. doi: 10.1593/neo.04169
33. Honda, K, Asato, R, Tsuji, J, Kanda, T, Watanabe, Y, Mori, Y, et al. Pyogenic spondylodiscitis after transoral surgery for oropharyngeal cancer. Auris Nasus Larynx. (2013) 40:320–2. doi: 10.1016/j.anl.2012.05.002
34. Zimmerli, W . Clinical practice. Vertebral osteomyelitis. N Engl J Med. (2010) 362:1022–9. doi: 10.1056/NEJMcp0910753
35. Kuklo, TR, Potter, BK, Bell, RS, Moquin, RR, and Rosner, MK. Single-stage treatment of pyogenic spinal infection with titanium mesh cages. J Spinal Disord Tech. (2006) 19:376–82. doi: 10.1097/01.bsd.0000203945.03922.f6
36. Lee, CY, Wu, MH, Cheng, CC, Huang, TJ, Huang, TY, Lee, CY, et al. Comparison of gram-negative and gram-positive hematogenous pyogenic spondylodiscitis: clinical characteristics and outcomes of treatment. BMC Infect Dis. (2016) 16:735. doi: 10.1186/s12879-016-2071-4
37. Bussini, L, Rosselli Del Turco, E, Pasquini, Z, Scolz, K, Amedeo, A, Beci, G, et al. Risk factors for persistent enterococcal bacteraemia: a multicentre retrospective study. J Glob Antimicrob Resist. (2022) 29:386–9. doi: 10.1016/j.jgar.2022.05.003
38. Royo-Cebrecos, C, Gudiol, C, Ardanuy, C, Pomares, H, Calvo, M, and Carratalà, J. A fresh look at polymicrobial bloodstream infection in cancer patients. PLoS One. (2017) 12:e0185768. doi: 10.1371/journal.pone.0185768
39. Royo-Cebrecos, C, Laporte-Amargós, J, Peña, M, Ruiz-Camps, I, Puerta-Alcalde, P, Abdala, E, et al. Pseudomonas aeruginosa bloodstream infections in patients with cancer: differences between patients with hematological malignancies and solid tumors. Pathogens. (2022) 11:1132. doi: 10.3390/pathogens11101132
Keywords: catheter-related bloodstream infection, clearance of bacteremia, head and neck cancer, mortality rate, hypoalbuminemia, persistent bacteremia, pyogenic spondylitis
Citation: Kitaya S, Kanamori H, Ishii R, Katsumi M, Ikeda R, Higashi K, Kakuta R, Ohkoshi A and Katori Y (2024) Clinical characteristics and outcomes of persistent bacteremia in patients with head and neck cancer in a tertiary care hospital. Front. Med. 11:1406983. doi: 10.3389/fmed.2024.1406983
Received: 25 March 2024; Accepted: 07 June 2024;
Published: 19 June 2024.
Edited by:
Leonard Peruski, Wadsworth Center, United StatesReviewed by:
Alfred Otoe Ankrah, Korle Bu Teaching Hospital, GhanaCopyright © 2024 Kitaya, Kanamori, Ishii, Katsumi, Ikeda, Higashi, Kakuta, Ohkoshi and Katori. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shiori Kitaya, c2hpb3JpLmtpdGF5YS5iN0B0b2hva3UuYWMuanA=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.