- 1School of Medicine, Nankai University, Tianjin, China
- 2Department of General Surgery, The First Medical Center, Chinese PLA General Hospital, Beijing, China
- 3Department of Gastrointestinal Surgery, Peking University International Hospital, Beijing, China
Introduction: The combination of neoadjuvant immunotherapy and chemotherapy (NICT) has become a common treatment regimen for locally advanced gastric cancer (LAGC). However, the safety and efficacy of radical gastrectomy following NICT (NICT-G) remain controversial. This study aimed to analyze the risk factors influencing postoperative complications (POCs) after NICT-G. Additionally, it aimed to construct a nomogram to provide a clinical reference for predicting POCs.
Methods: This study included 177 patients who received NICT-G at the Chinese PLA General Hospital First Medical Center from January 2020 to January 2024. Univariable and multivariable logistic regression models were used to evaluate the risk factors influencing POCs, and a nomogram model was constructed. To evaluate the discrimination and accuracy of the nomogram model, the area under the receiver operating characteristic curve (AUC) and the calibration curve were measured.
Results: In 177 patients who received NICT-G, the pathological complete response and major pathological response rates were 15.8% and 45.2%, respectively, whereas the rates of the overall and severe treatment-related adverse events were 71.8% and 15.8%, respectively. In addition, 43 (24.3%) patients developed overall POCs (Clavien–Dindo classification ≥ II). Univariable and multivariable logistic analyses showed that age ≥70 years, greater estimated blood loss, platelet/lymphocyte ratio (PLR) ≤196, neutrophil/lymphocyte ratio (NLR) >1.33, non-R0 resection, and body mass index (BMI) < 18.5 kg/m2 were independent risk factors for overall POCs (p < 0.05). The nomogram model developed using the abovementioned variables showed that the AUC (95% confidence interval [CI]) was 0.808 (95% CI): 0.731–0.885 in predicting the POC risk. The calibration curves showed that the prediction curve of the nomogram was a good fit for the actual POCs (Hosmer–Lemeshow test: χ2 = 5.76, P = 0.451).
Conclusion: The independent risk factors for overall POCs in the NICT-G were age ≥ 70 years, greater estimated blood loss, PLR ≤ 196, NLR > 1.33, non-R0 resection, and BMI < 18.5 kg/m2. The nomogram model developed based on the abovementioned indicators showed better accuracy in predicting the POC risk.
1 Introduction
According to the statistics from GLOBOCAN 2020 (1), gastric cancer (GC) is the fifth most common cancer worldwide, and it poses a high risk of tumor-related deaths. China has a high incidence of GC, with locally advanced gastric cancer (LAGC) representing a significant portion GC cases in China (2). In addition to radical gastrectomy, performing perioperative treatments such as chemotherapy, immunotherapy, and targeted therapy is equally essential. These approaches achieve better therapeutic effects and improve the long-term prognosis for patients with LAGC.
One of the most crucial perioperative treatments for LAGC is neoadjuvant therapy. The concept of ypTNM stages, aimed at facilitating the broad application and precise evaluation of tumor regression after neoadjuvant therapy in LAGC, was first proposed in the GC staging criteria of the American Joint Committee on Cancer (AJCC), 8th edition. Recently, the combination of neoadjuvant immunotherapy and chemotherapy (NICT) has become the preferred treatment regimen for LAGC due to its superior tumor regression, acceptable treatment tolerance, and perioperative safety compared to using neoadjuvant chemotherapy alone (3, 4). The KEYNOTE-585 study demonstrated that patients who received NICT [pembrolizumab plus fluorouracil + oxaliplatin + docetaxel (FLOT)] did not reach the threshold for statistical significance (p = 0.0178) in event-free survival (5); however, a higher proportion of pathological complete response (pCR) indicated a potential survival benefit for patients with LAGC (6).
Surgeons rely on perioperative safety as a key factor when performing radical gastrectomy following NICT (NICT-G). Previous studies have reported that NICT-G and gastrectomy following neoadjuvant chemotherapy have comparable postoperative complications (POCs) and recovery (7, 8). However, based on our clinical experience, some potential risks caused by NICT-G may increase surgical difficulty and even lead to POCs, including a decreased immune system and physical status during NICT, increased tissue fragility, and disruptions of anatomical gaps. Therefore, this explorative retrospective study aimed to present the therapeutic effects of NICT, analyze the risk factors affecting POCs following NICT-G, and construct a nomogram to provide a clinical reference for predicting POCs following NICT-G.
2 Materials and methods
2.1 Patients
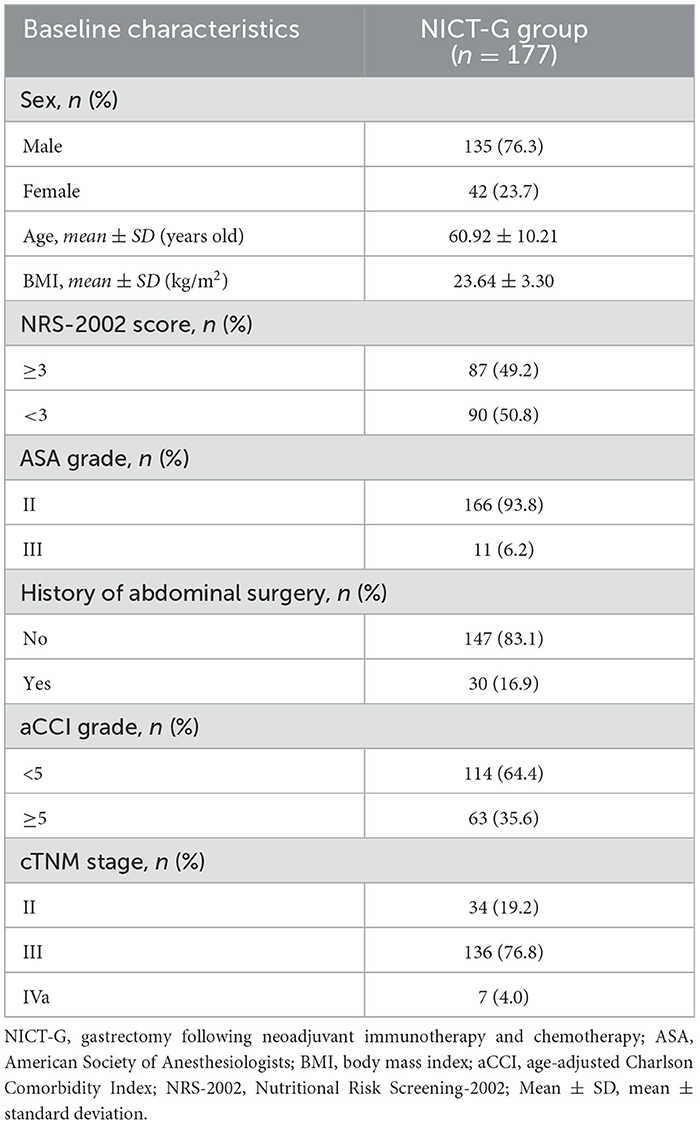
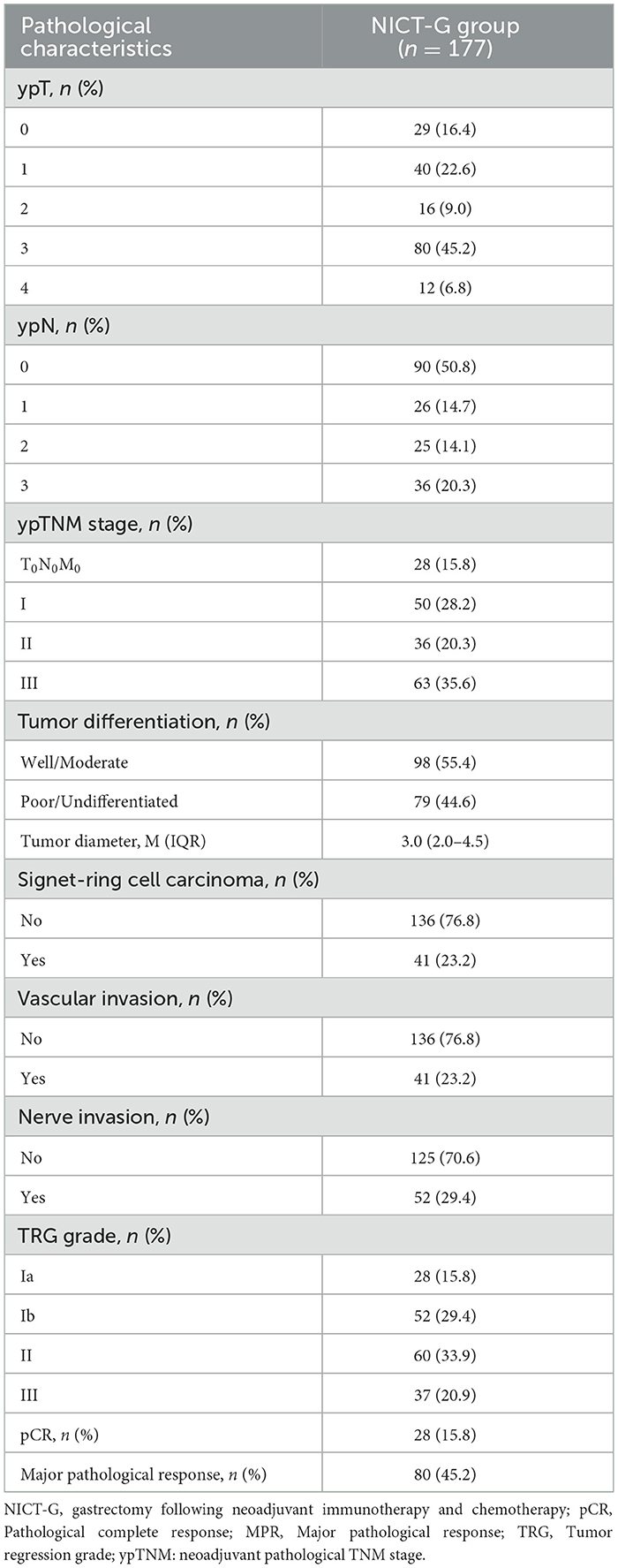
A total of 177 patients who received NICT-G in the Chinese PLA General Hospital First Medical Center from January 2020 to January 2024 were included in this study. The inclusion criteria were as follows: (1) patients in the cT2N + M0 or T3 − 4bNanyM0 (clinical TNM stages II–Iva) clinical stage, according to the AJCC 8th edition GC staging criteria; (2) those with pathologically confirmed gastric adenocarcinoma; and (3) those who received NICT simultaneously. The exclusion criteria were as follows: (1) patients who received targeted therapy, radiotherapy, or other antitumor treatments rather than NICT before surgery; (2) those with residual GC or a history of gastric surgery before NICT; (3) those with other gastric tumors, such as mesenchymal tumor and lymphoma; and (4) those lacking integrated clinical and pathological data. We collected data on sex, age, body mass index (BMI), age-adjusted Charlson Comorbidity Index (aCCI) score, the American Society of Anesthesiologists (ASA) grade, the Nutritional Risk Screening-2002 (NRS-2002) score, history of abdominal surgery, cTNM stages, neoadjuvant pathological tumor (ypT) stage, neoadjuvant pathological node (ypN) stages, ypTNM stages, tumor differentiation, tumor diameter, signet ring cell carcinoma, and vascular and nerve invasion as baseline characteristics (Tables 1, 2). The ethics committee of the Chinese PLA General Hospital First Medical Center approved this study (approval No. S2023-190-01), and all patients provided informed consent before perioperative treatment.
2.2 Neoadjuvant therapy and therapeutic effect evaluation
All patients in this study accepted simultaneous chemotherapy and immunotherapy preoperatively. The following were the explicit neoadjuvant regimens: (1) SOX (S-1 combined with oxaliplatin), (2) XELOX (capecitabine combined with oxaliplatin) and SAP (S-1 combined with nab-paclitaxel), (3) FOLFOX (oxaliplatin combined with fluorouracil), and (4) FLOT (fluorouracil + oxaliplatin + docetaxel). Neoadjuvant immunotherapy regimens were totally programmed as death-1 inhibitors, which included nivolumab, pembrolizumab, toripalimab, camrelizumab, and sintilimab. During NICT, we used the Common Terminology Criteria for Adverse Events criteria (version 5.0) to define the category and severity of treatment-related adverse events (TRAEs) (9).
The therapeutic effects of NICT were evaluated using abdominal enhanced computed tomography (CT) and postoperative pathological results. Following the Response Evaluation Criteria in Solid Tumors criteria (version 1.1), abdominal enhanced CT scans were performed every 6 weeks preoperatively to evaluate the radiologic response. The Response Evaluation Criteria in Solid Tumors criteria (version 1.1) were divided into complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) (10). The pathological TNM stages after neoadjuvant therapy were defined as ypTNM stages according to the 8th edition of the AJCC Cancer Staging Manual, which was especially used for tumor staging after neoadjuvant therapy. The ypTNM stages divided patients with non-metastatic LAGC after receiving neoadjuvant therapy into three stages: stage I, stage II, and stage III. The ypT and ypN stages were determined by the remaining tumor cells located in the deepest layer of the gastric wall and metastasis in the regional lymph nodes. The neoadjuvant pathological T0 (ypT0) and neoadjuvant pathological N0 (ypN0) stages indicated that no residual tumor existed in the gastric wall and regional lymph nodes, respectively, after neoadjuvant therapy. For evaluating the tumor regression grade (TRG) from pathological results, Becker's standard was used as follows: (1) TRG1a, no residual tumor cells; (2) TRG1b, < 10% residual tumor cells; (3) TRG2, 10%−50% residual tumor cells; and (4) TRG3, >50% residual tumor cells (11). pCR and major pathological response (MPR) were defined as TRG1a (ypT0N0M0) and TRG1a/1b, respectively.
2.3 Perioperative indicators
Previous studies have suggested that preoperative laboratory indices hold value in POC prediction (12, 13). Therefore, we collected data on the hemoglobin level, leukocyte, neutrophil, lymphocyte, and platelet counts, and the serum albumin level in the peripheral blood. Furthermore, we calculated combined laboratory indicators, including the neutrophil/lymphocyte ratio (NLR, neutrophil count/lymphocyte count), the platelet/lymphocyte ratio (PLR, platelet count/lymphocyte count), Onodera's prognostic nutritional index (PNI) score (10 × albumin [g/dL] + 0.005 × lymphocyte count/mm3) (14), and the systemic immune-inflammation (SII) index score (platelet count × neutrophil count/lymphocyte count) (15).
2.4 Surgery and postoperative recovery
Radical gastrectomy plus D2 lymphadenectomy was performed 4–6 weeks following the completion of NICT. The surgical details were consistent with the Japanese GC Treatment Guidelines (6th edition) (16). Operation time and estimated blood loss were recorded during the NICT-G, and data on the retrieved lymph nodes, first flatus days, postoperative hospitalized days, and 30-day POCs were collected from the postoperative medical records and follow-up visits. The Clavien–Dindo classification was used to define POC severity (17). Owing to the limitations of a retrospective study, we defined POCs ≥Clavien–Dindo Grade II as overall POCs and POCs ≥Clavien–Dindo Grade IIIa as severe POCs. Postoperative treatment was initiated 4 weeks after the operation.
2.5 Variables for risk factors
We conducted a binary logistic regression analysis to explore the risk factors for the overall POCs after the NICT-G. The cut-off values of the BMI, PNI, NLR, PLR, and SII index were selected using the Youden Index from the receiver operating characteristic (ROC) curve and clinical preference. The following clinical and operative variables were investigated as possible risk factors for POCs after the NICT-G: sex (male or female), age (< 70 years or ≥70 years), BMI (< 18.5 kg/m2, 18.5 to < 24.0 kg/m2, 24.0 to < 28.0 kg/m2, or ≥28.0 kg/m2), tumor resection (proximal, distal, or total), ASA grade (II or III), severe TRAEs (yes or no), aCCI score (< 5 or ≥5), NRS-2002 score (< 3 or ≥3), surgical approach (open, laparoscopic, or robotic), R0 resection (yes or no), tumor diameter (< 2 cm or ≥2 cm), operation time (< 250 min or ≥250 min), PNI score (< 45 or ≥45), SII index (≤ 260 or >260), NLR (≤ 1.33 or >1.33), and PLR (≤ 196 or >196).
2.6 Statistical analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences version 26.0 (IBM SPSS Statistics, Chicago, IL, USA) and R version 4.2.2 (R Project, the Institute of Statistics and Mathematics, Vienna, Austria). Continuous data with normal distribution were expressed as means ± standard deviations, whereas categorical variables were presented as numbers (percentages). Continuous data with skewed distribution were expressed as medians (interquartile ranges). A binary logistic regression analysis was conducted for variable analysis, and factors with a p-value of < 0.15 were included in the multivariable logistic analysis. Independent risk factors were used to construct a nomogram prediction model using R packages. We used the 1,000 bootstrap replications method for drawing the calibration curve; subsequently, the Hosmer–Lemeshow goodness-of-fit test was conducted to evaluate the goodness of fit. The predictive performance of the risk model was shown using the ROC curve and quantified using the area under the ROC curve [area under the receiver operating characteristic curve (AUC)]. A p-value of < 0.05 was considered statistically significant.
3 Results
3.1 Clinicopathological characteristics
Overall, 177 patients with LAGC who underwent NICT-G were enrolled in the final analysis. The clinicopathological characteristics of the enrolled patients are presented in Tables 1, 2. Most of the enrolled patients were male individuals (76.3%), with an average age of 60.92 ± 10.21 years and a BMI of 23.64 ± 3.30 kg/m2. Of the enrolled patients, 34 (19.2%) were classified as having cTNM stage II, 136 (76.8%) as having cTNM stage III, and 7 (4.0%) as having cTNM stage Iva.
Regarding pathological characteristics, the patients were classified into ypTNM stages as follows: 28 (15.8%) were at stage T0N0M0, 50 (28.2%) were at stage I, 36 (20.3%) were at stage II, and 63 (35.6%) were at stage III. The median tumor diameters were 3.0 (2.0–4.5) cm. Furthermore, 41 (23.2%) of the enrolled patients had vascular invasion and 52 (29.4%) had nerve invasion. According to Becker's standard, which evaluated the TRG, 28 (15.8%) patients acquired TRG1a with pCR, while 80 (45.2%) patients acquired TRG1a/1b with MPR.
3.2 Treatment regimens and TRAEs
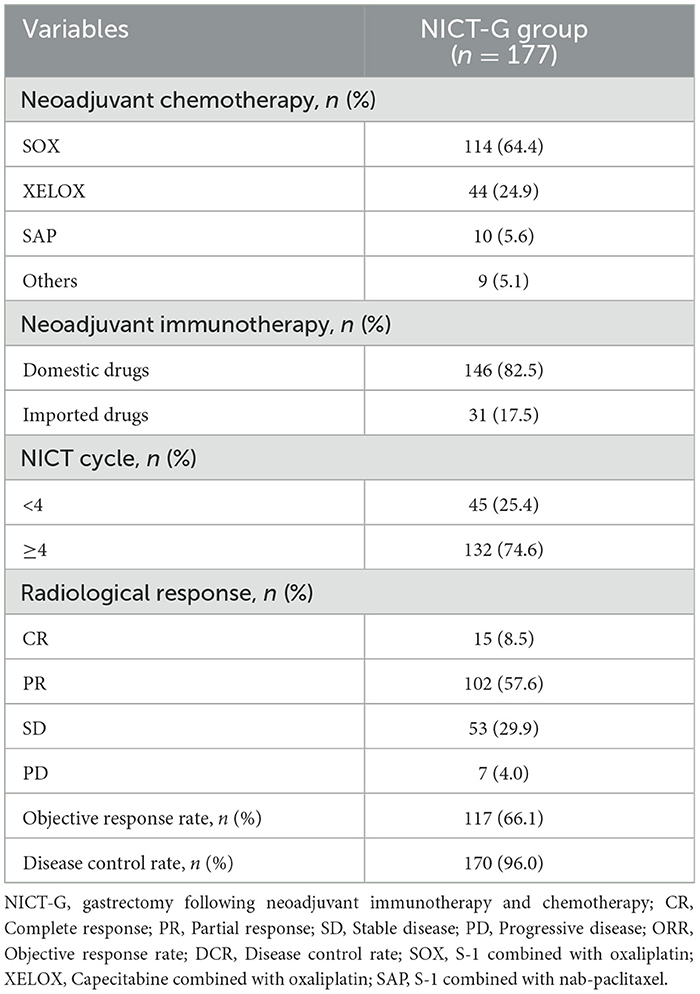
The percentage of patients who received ≥4 cycles of NICT was 74.6% (132/177 patients). Most patients (82.5%) accepted domestic neoadjuvant immunotherapy regimens (e.g., sintilimab, tislelizumab, and camrelizumab). The proportions of complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD), which reflected the radiological response, were 8.5%, 57.6%, 29.9%, and 4.0%, respectively. The objective response and disease control rates were 66.1% and 96.0%, respectively (Table 3).
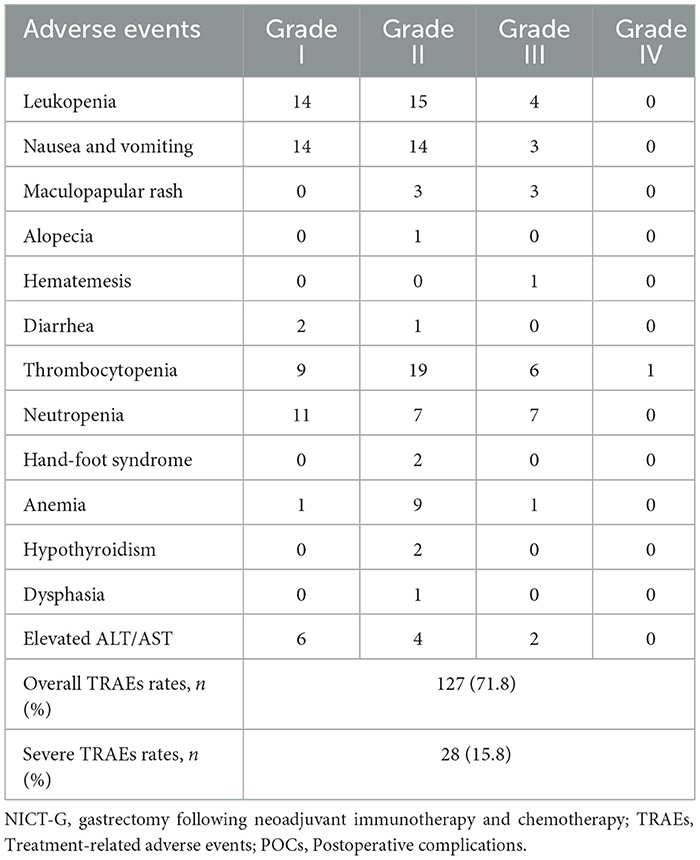
The TRAEs experienced by the patients during NICT are presented in Table 4. Leukopenia, nausea and vomiting, thrombocytopenia, and neutropenia were the top four most common TRAEs during the NICT. The rates of the overall and severe TRAEs were 71.8% and 15.8%, respectively. No patients died during the NICT.
3.3 Surgical safety, postoperative recovery, and complications
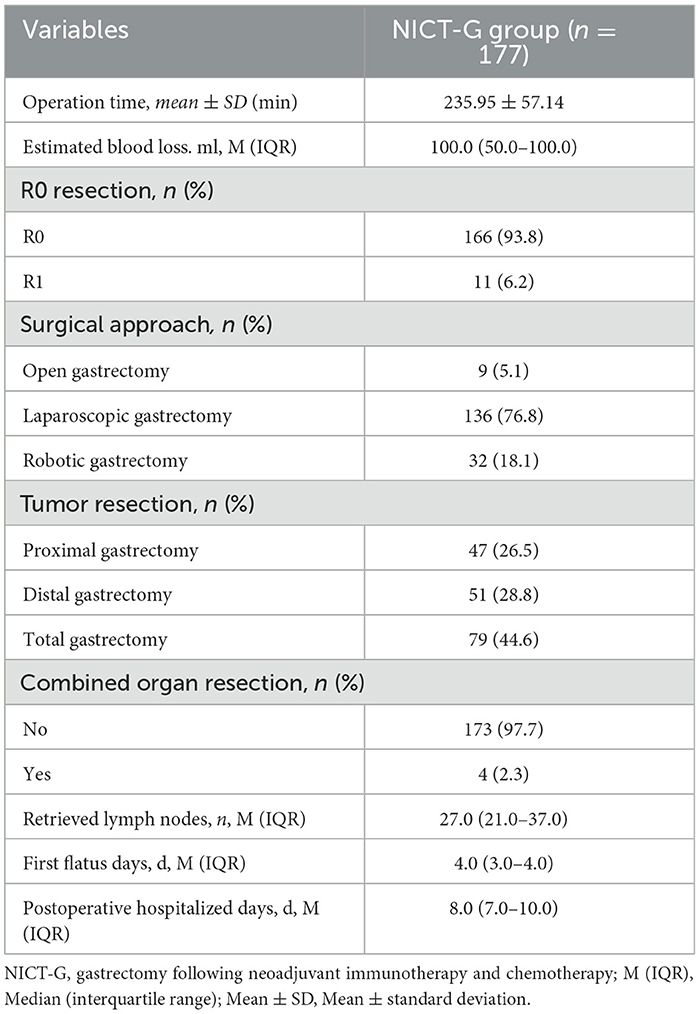
The perioperative treatment and recovery of the patients who received NICT-G are shown in Table 5. Patients in the NICT-G group required an average of 235.95 ± 57.14 min of operation time, and the estimated blood loss was 100.0 (50.0–100.0) mL. Most patients (94.9%) accepted minimally invasive gastrectomy following NICT, and the R0 resection rate of all the enrolled patients was 93.8%. The first flatus and the postoperative hospitalized days were 4.0 (3.0–4.0) and 8.0 (7.0–10.0) days, respectively.
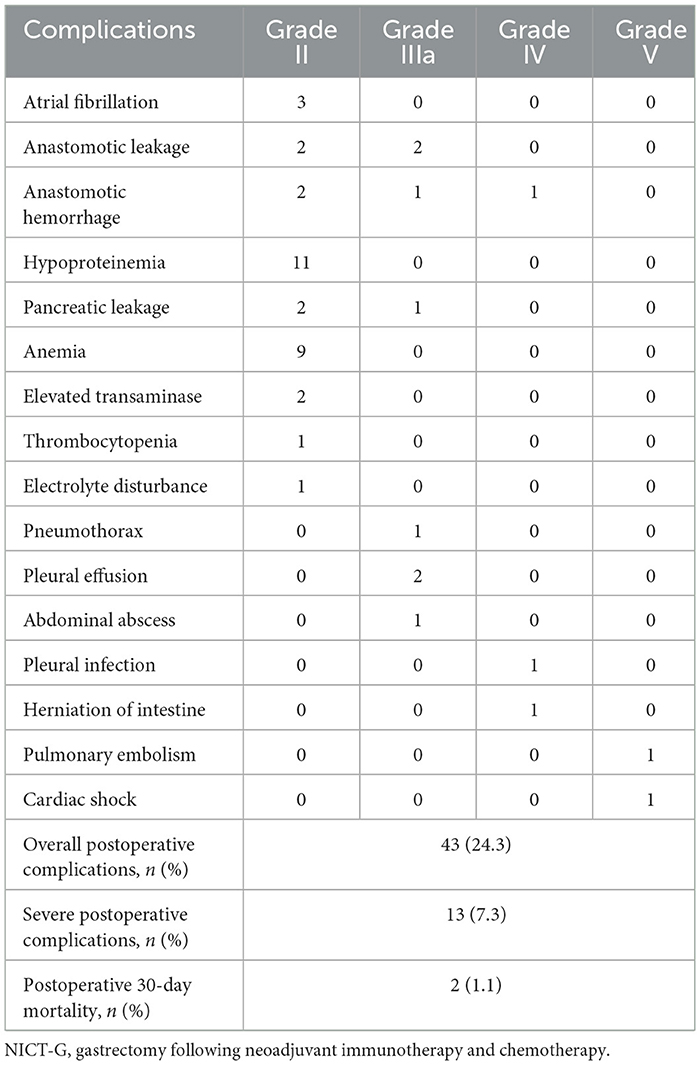
Moreover, 43 (24.3%) patients developed POCs (Clavien–Dindo classification ≥ II) (Table 6). Hypoproteinemia (11/43), anemia (9/43), anastomotic leakage (4/43), and anastomotic hemorrhage (4/43) were the four most common complications. The prevalence of severe complication (Clavien–Dindo classification ≥ IIIa) and postoperative mortality were 7.3% and 1.1%, respectively.
3.4 Risk factors for POCs in the NICT-G group
The univariable - and multivariable logistic regression results used to explore the risk factors for POCs following NICT-G are shown in Table 7 and Figure 1. We placed indicators obtained by the univariable logistic regression with a p-value of < 0.15 into the multivariable analysis and observed that age ≥70 years (odds ratio [OR] [95% confidence interval (CI)]: 4.148 [1.257–13.692], P = 0.020), greater estimated blood loss (OR [95% CI]: 1.006 [1.002–1.010], P = 0.004), PLR ≤ 196 (OR [95% CI]: 7.295 [1.265–42.060], P = 0.026), NLR > 1.33 (OR [95% CI]: 5.683 [1.656–19.501], P = 0.006), non-R0 resection (OR [95%CI]: 5.528 [1.088–28.086], P = 0.039), and BMI < 18.5 kg/m2 (OR [95% CI]: 30.471 [2.635–352.323], P = 0.006) were significant independent risk factors for overall POCs.
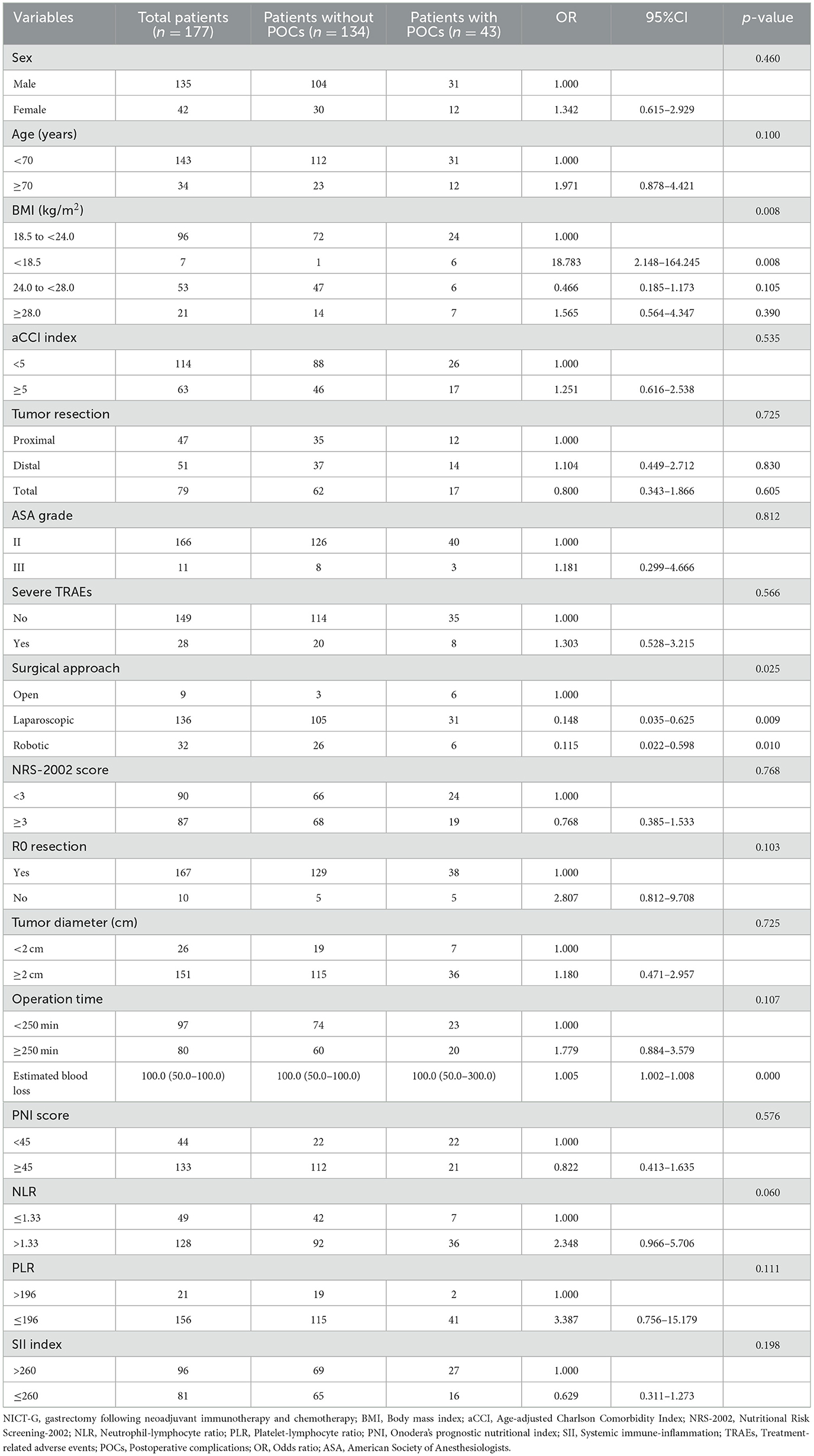
Table 7. The characteristics of and univariable analysis of LAGC patients with and without POCs after NICT-G.
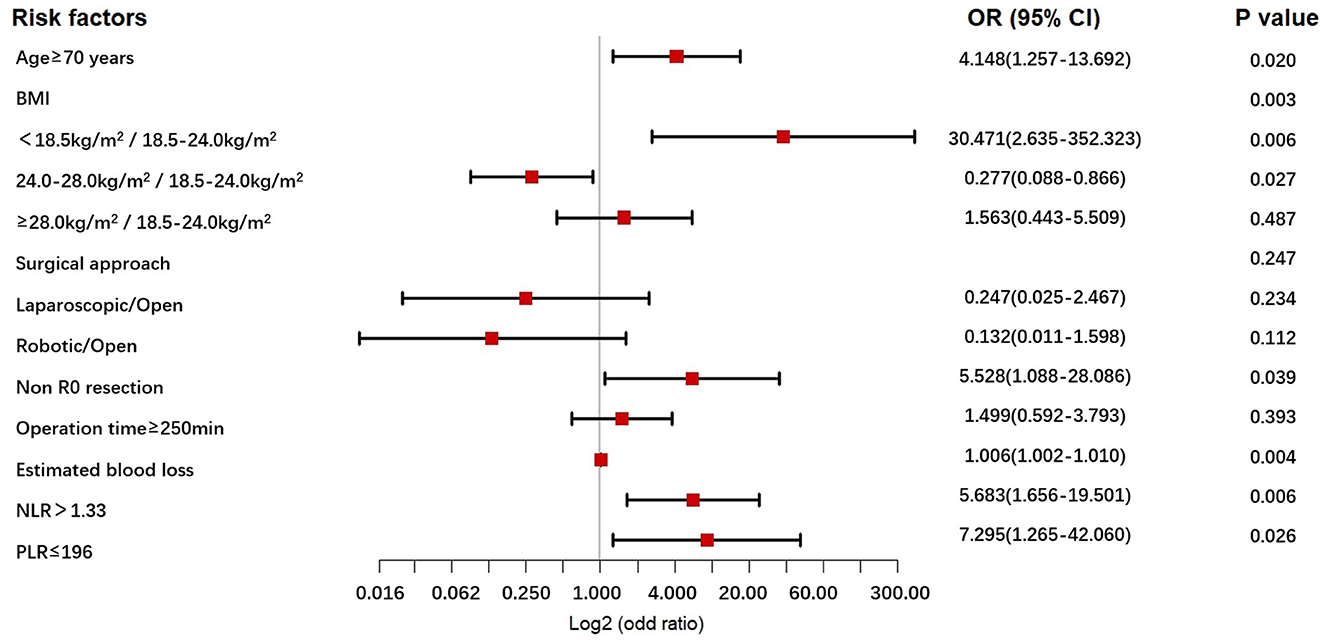
Figure 1. The forest plot for multivariable analysis for POCs in the NICT-G patients. POCs, postoperative complications; NICT-G, gastrectomy following neoadjuvant immunotherapy and chemotherapy.
3.5 Construction and validation of the nomogram for predicting POCs
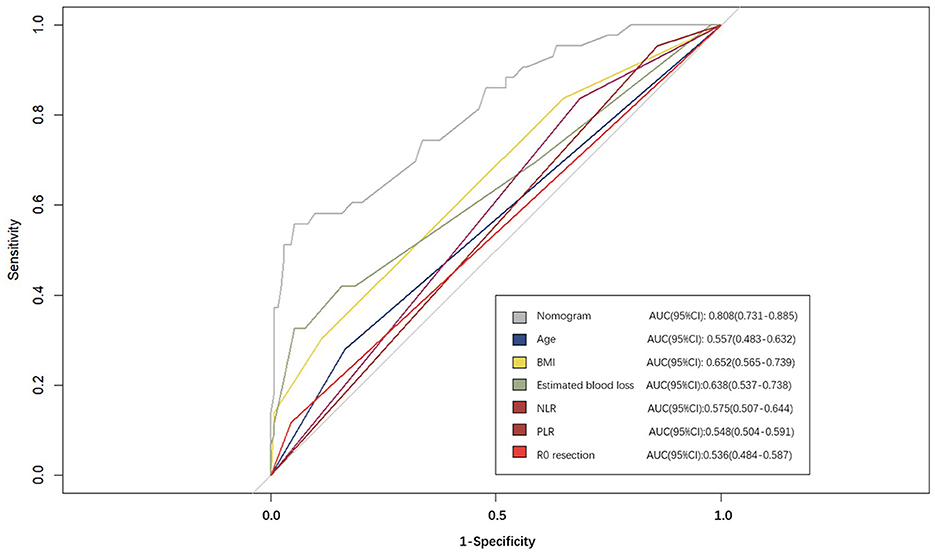
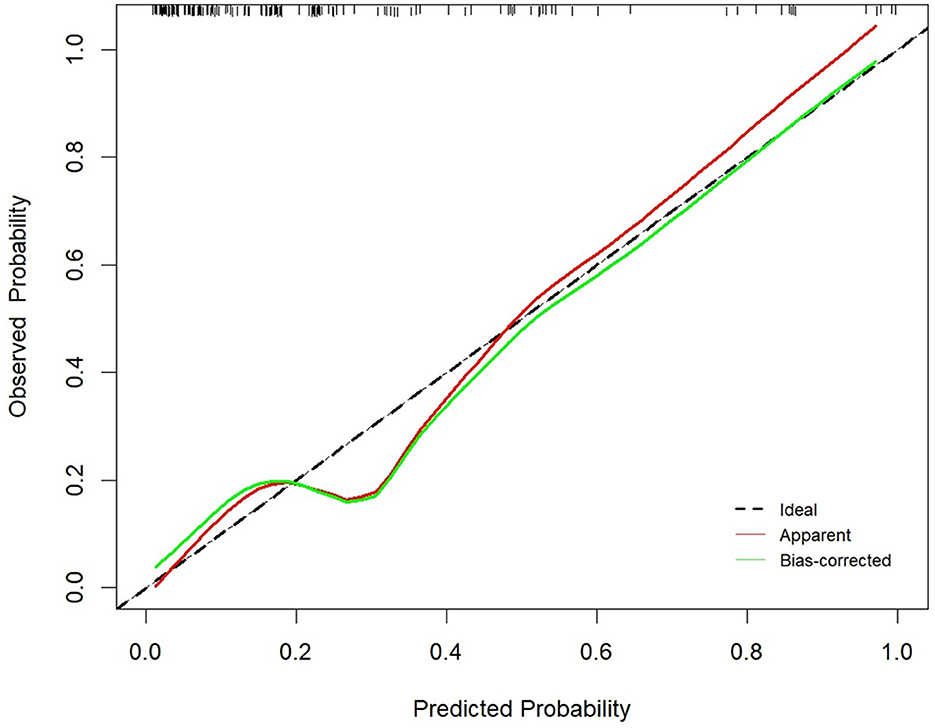
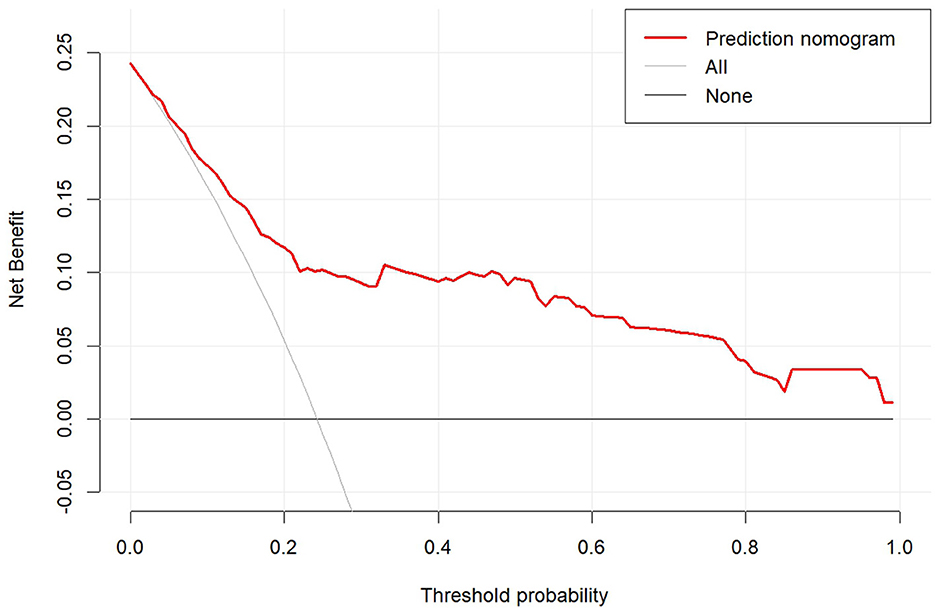
A nomogram was constructed based on the six independent predictive factors derived from the multivariable analysis (Figure 2). The corresponding point for each factor was assigned based on the condition of the patients and the OR, which could be calculated by drawing a vertical line upward (e.g., a patient with NLR > 1.33 would receive 26 points). The sum of the points for each item equaled the total score. Then, the corresponding point on the total point axis was located, and a vertical line was drawn downward to predict the risk of POCs after NICT-G. The ROC curve showed that the AUC of the combination of six variables was 0.808 (95% CI): 0.731–0.885, which was superior to any single risk factor (Figure 3). The calibration plot based on the 1,000 bootstrap replications method showed good adaptability between the prediction and observation (Hosmer–Lemeshow test: χ2 = 5.76, P = 0.451) (Figure 4). The decision curve analysis demonstrated that our predictive model showed good benefits for different threshold probabilities between 10% and 100%, indicating favorable clinical utility (Figure 5).
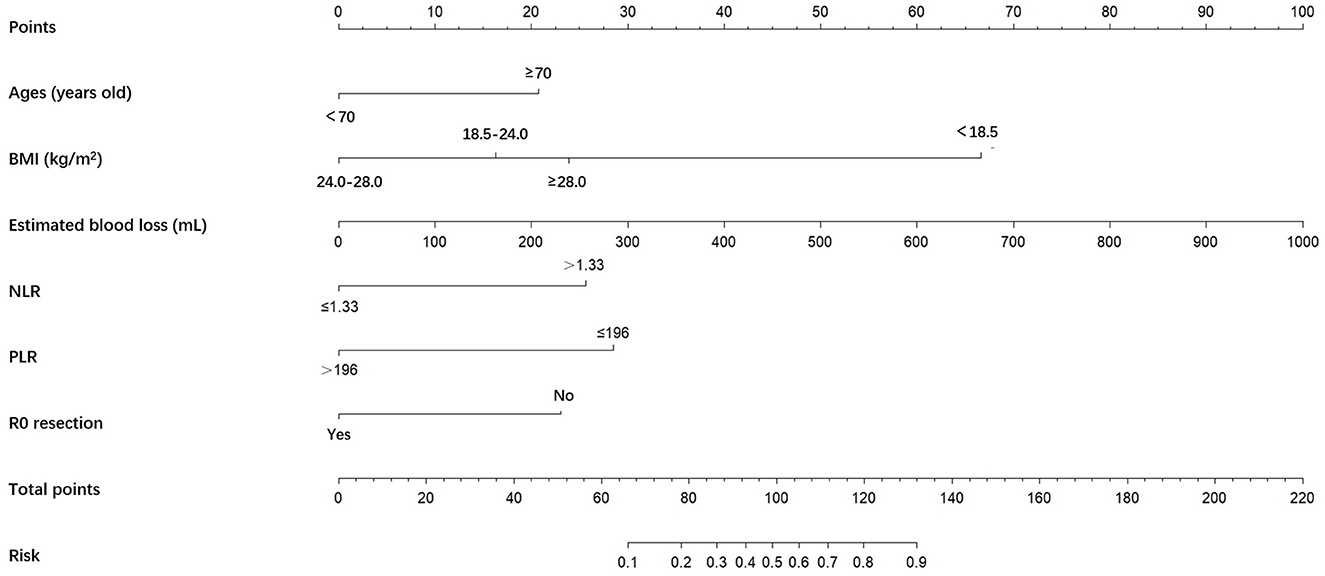
Figure 2. A nomogram predicting the risk of POCs for patients with NICT-G. The point for each factor was according to the condition of patients and odds ratio. The sum of the scores for each item is equal to the total points, then find the corresponding point on the total points axis and make a vertical line down to predict the risk of the POCs after NICT-G. POCs, postoperative complications; NICT-G, gastrectomy following neoadjuvant immunotherapy and chemotherapy.
4 Discussion
In the last 2 years, NICT for patients with LAGC has been gradually gaining recognition owing to its superior antitumor effects compared with neoadjuvant chemotherapy alone. However, it remains unclear whether NICT affects surgical safety and elevates the potential risk of POCs. In the study, we observed that NICT showed satisfactory tumor regression and acceptable TRAEs in 177 patients with LAGC. Meanwhile, six independent risk factors for predicting POCs were detected, and a risk prediction model for predicting POCs in patients receiving NICT-G was successfully developed, which could facilitate preoperative assessment to elevate perioperative safety.
Medication safety and antitumor efficacy are crucial for promoting the application of NICT. A recent meta-analysis of 1,074 patients with resectable gastric/gastroesophageal junction tumors demonstrated that patients receiving NICT had a 24% pCR rate and a 49% MPR rate, with a 28% incidence of severe TRAEs (18). A study by Yuan et al. involving 54 patients with LAGC treated with NICT (toripalimab combined with SOX/XELOX) showed that a higher proportion of patients achieved TRG0/1 and exhibited a manageable safety profile compared to those treated with chemotherapy alone (3). Better tumor regression may translate into survival benefit. Lin et al. reported that the proportions of ypT0, ypN0, and pCR rates in the NICT (camrelizumab + nab-paclitaxel + S-1) group were significantly higher. The patients in the NICT group had a longer average time to recurrence (18.9 vs. 13.1 months, P = 0.004) than those in the chemotherapy group (19). In our NICT-G cohort, 15.8% of the patients achieved pCR and 45.2% achieved MPR, indicating higher rates than those reported in previous studies involving patients with LAGC treated with neoadjuvant chemotherapy (3, 20). The acceptable incidence of the overall and severe TRAEs (71.8% and 15.8%, respectively) further supports the safety and efficacy of NICT among patients with LAGC.
Surgeons focus more on surgical safety and postoperative recovery following neoadjuvant therapy. Our findings showed that patients in the NICT-G group had an average operation time of 235.95 ± 57.14 min and an estimated blood loss of 100.0 (50.0–100.0) mL, which were consistent with our previous studies on upfront gastrectomy (21). The values from these studies were also acceptable in terms of the first flatus and postoperative hospitalized days in the NICT-G group. We attribute these positive results to the high proportion of minimally invasive gastrectomy (94.9%) and the use of enhanced recovery after surgery (ERAS) in our cohort. The use of high-definition magnified imaging and precise surgical procedures during laparoscopy or the use of surgical robots may help counteract the negative effects of tissue brittleness, increased exudation, and blurred anatomical gaps caused by NICT, while the application of ERAS can promote the postoperative recovery of gastrointestinal function (22).
POCs are key indicators for evaluating short-term outcomes in patients with NICT-G. In this study, 43 (24.3%) patients developed POCs with a Clavien–Dindo classification of ≥II. Moreover, hypoproteinemia, anemia, anastomotic leakage, and anastomotic hemorrhage were the four most common POCs in the NICT-G group, suggesting that surgeons did not pay attention to the perioperative nutritional status, anastomotic safety, and exact hemostasis. We detected six risk factors through the multivariable analysis and developed a prediction model to assess the potential risk of POCs following NICT-G. Our nomogram showed good calibration (Hosmer–Lemeshow test: χ2 = 5.76, P = 0.451), excellent predictive ability (0.808 [95% CI: 0.731–0.885]), and good positive net benefit.
Previous studies have suggested that some laboratory indices, including NLR, PLR, PNI, and SII, play a certain role in the prediction of POCs (23, 24). A study conducted by Wang found that a higher NLR was an independent risk factor for infectious POCs following gastrectomy (25). Furthermore, Inaoka et al. reported that the low PLR group exhibited an increased incidence of POCs for clinical T2 − 4 GC (26). In our study, although the TRAEs of myelosuppression caused by NICT may have affected the changes in preoperative laboratory indicators, we noted that a lower preoperative PLR and a higher preoperative NLR were independent risk factors for POCs following NICT-G, indicating a good predictive value. This result was likely due to the ability of NLR and PLR to reflect systemic inflammatory responses, immune disorders, and potential microvascular thrombosis, which are strongly associated with POCs, particularly infectious POCs (27).
Older patients are prone to developing POCs owing to the high proportion of comorbidity and decreased physical status. A study by the Dutch Upper Gastrointestinal Cancer Audit group proved that age ≥ 70 years is an independent risk factor for predicting POCs following radical gastrectomy (28). Moreover, Yu et al. showed a close correlation between POCs and older age in patients who underwent gastrectomy following neoadjuvant chemotherapy (29). Although neoadjuvant therapy does not increase surgical complications following gastrectomy (30), the dual influence of NICT combined with surgery may increase the risk of POCs, especially for older patients. The results of our study showed that age ≥ 70 years was strongly associated with POCs following NICT-G, indicating that surgeons must emphasize the perioperative management of older patients. The requirements for the perioperative management of older patients are as follows: (1) a dynamic evaluation of the nutritional status with proper intervention, (2) effective treatment for TRAEs during NICT, (3) symptomatic treatment for comorbidities, and (4) a comprehensive preoperative assessment of the physical status by a multidisciplinary team (31).
According to the Chinese BMI classification, underweight is defined as < 18.5 kg/m2, normal weight as 18.5 to < 24 kg/m2, overweight as 24 to < 28 kg/m2, and obesity as ≥28 kg/m2 (32). A lower BMI score often reflects poor nutritional status, especially for patients who undergo NICT before surgery. Hirahara et al. found that a lower BMI score was associated with impaired nutritional status among patients and that the patients subsequently developed POCs (33). Kim's study indicated that a lower BMI score was an independent risk factor for pulmonary POCs after neoadjuvant therapy followed by surgery for non-small-cell lung cancer (34). Cohen et al. observed that neoadjuvant therapy-related nutritional deterioration increased the risk of POCs after cystectomy (35). Moreover, other previous studies have also demonstrated that patients with a lower BMI score have a higher risk of developing Petersen's hernia, experiencing more severe POCs, and having poorer prognosis after gastrectomy (36, 37). In our study, we found that patients with a lower BMI score of < 18.5 kg/m2 were prone to developing POCs such as hypoproteinemia and anemia, which indicated poor nutritional status during perioperative periods. Thus, based on mature minimally invasive technology and stable surgical safety, attention should be paid to perioperative nutritional assessment and symptomatic supportive treatment to reduce the risk of POCs in patients who undergo NICT-G.
This study had some limitations. First, selection bias may have existed in this single-institutional retrospective study. Second, POCs with a Clavien–Dindo classification of grade I were not included in the analysis owing to the limitations of a retrospective cohort (38). To systematically evaluate POCs following NICT-G, prospective studies should be conducted. Third, the effect of NICT on POCs was not included in this study. Future studies should focus on comparing the short-term outcomes between patients with LAGC receiving NICT and patients with LAGC not receiving NICT before gastrectomy. Finally, this study lacked external validation due to the limited sample size. Future studies should perform external validation, which could demonstrate the exact predictive value of the nomogram (39).
5 Conclusion
This study demonstrated the antitumor effects, medication safety, and perioperative recovery of patients with LAGC who underwent NICT-G in a large single-institutional cohort. Furthermore, we analyzed six independent risk factors, including age ≥ 70 years, greater estimated blood loss, PLR ≤ 196, NLR > 1.33, non-R0 resection, and BMI < 18.5 kg/m2, which could predict POCs following NICT-G. Meanwhile, we developed a new nomogram prediction model with good discrimination and calibration. This model could assist surgeons in individualizing perioperative management by predicting the potential risk of POC development after NICT-G.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors on reasonable request, without undue reservation.
Ethics statement
This study was approved by the Ethics Committee of the Chinese PLA General Hospital (approval No. S2023-190-01). The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from Electronic medical record system. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
HC: Conceptualization, Data curation, Visualization, Writing – original draft, Writing – review & editing. SZ: Conceptualization, Data curation, Writing – original draft. LS: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. ZY: Data curation, Writing – original draft, Writing – review & editing. QX: Data curation, Writing – review & editing. JG: Data curation, Writing – review & editing. LC: Funding acquisition, Supervision, Validation, Writing – review & editing. JC: Conceptualization, Funding acquisition, Supervision, Validation, Writing – review & editing. BW: Conceptualization, Data curation, Funding acquisition, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82073192, 82273231, and 62133010), the Beijing Science and Technology Program (Z221100007422125), and the Chinese People's Liberation Army General Hospital Medical Engineering Laboratory Project (2022SYSZZKY16).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
NICT-G, Gastrectomy following neoadjuvant immunotherapy and chemotherapy; GC, Gastric cancer; LAGC, Locally advanced gastric cancer; ASA, American Society of Anesthesiologists; BMI, Body mass index; aCCI, Age-adjusted Charlson Comorbidity Index; NRS-2002, Nutritional Risk Screening-2002; pCR, Pathological complete response; MPR, Major pathological response; CR, Complete response; PR, Partial response; SD, Stable disease; PD, Progressive disease; ORR, Objective response rate; DCR, Disease control rate; TRG, Tumor regression grade; NLR, Neutrophil-lymphocyte ratio; PLR, Platelet-lymphocyte ratio; PNI, Onodera's prognostic nutritional index; SII, Systemic immune-inflammation; OR, Odds ratio; TRAEs, Treatment-related adverse events; POCs, Postoperative complications; AUC, The area under the receiver operating characteristic curve; Median (interquartile range).
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Qi J, Li M, Wang L, Hu Y, Liu W, Long Z, et al. National and subnational trends in cancer burden in China, 2005-20: an analysis of national mortality surveillance data. Lancet Public Health. (2023) 8:e943–55. doi: 10.1016/S2468-2667(23)00211-6
3. Yuan SQ, Nie RC, Jin Y, Liang CC, Li YF, Jian R, et al. Perioperative toripalimab and chemotherapy in locally advanced gastric or gastro-esophageal junction cancer: a randomized phase 2 trial. Nat Med. (2024) 30:552–9. doi: 10.1038/s41591-023-02721-w
4. Huang X, Fang J, Huang L, Chen H, Chen H, Chai T, et al. SOX combined with sintilimab versus SOX alone in the perioperative management of locally advanced gastric cancer: a propensity score-matched analysis. Gastric Cancer. (2023) 26:1040–50. doi: 10.1007/s10120-023-01431-z
5. Shitara K, Rha SY, Wyrwicz LS, Oshima T, Karaseva N, Osipov M, et al. Neoadjuvant and adjuvant pembrolizumab plus chemotherapy in locally advanced gastric or gastro-oesophageal cancer (KEYNOTE-585): an interim analysis of the multicentre, double-blind, randomised phase 3 study. Lancet Oncol. (2024) 25:212–24. doi: 10.1016/S1470-2045(23)00541-7
6. Sinnamon AJ, Savoldy M, Mehta R, Dineen SP, Peña LR, Lauwers GY, et al. Tumor regression grade and overall survival following gastrectomy with preoperative therapy for gastric cancer. Ann Surg Oncol. (2023) 30:3580–9. doi: 10.1245/s10434-023-13151-w
7. Su J, Guo W, Chen Z, Wang L, Liu H, Zhao L, et al. Safety and short-term outcomes of laparoscopic surgery for advanced gastric cancer after neoadjuvant immunotherapy: A retrospective cohort study. Front Immunol. (2022) 13:1078196. doi: 10.3389/fimmu.2022.1078196
8. Cui H, Liang W, Cui J, Song L, Yuan Z, Chen L, et al. Safety and feasibility of minimally invasive gastrectomy after neoadjuvant immunotherapy for locally advanced gastric cancer: a propensity score-matched analysis in China. Gastroenterol Rep. (2024) 12:goae005. doi: 10.1093/gastro/goae005
9. US Department of Health and Human Services. Common Terminology Criteria for Adverse Events. Version 5.0. Published November 27, 2017. (2020). Available online at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf
10. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 11). Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
11. Becker K, Mueller JD, Schulmacher C, Ott K, Fink U, Busch R, et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer. (2003) 98:1521–30. doi: 10.1002/cncr.11660
12. Miyamoto R, Inagawa S, Sano N, Tadano S, Adachi S, Yamamoto M. The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients. Eur J Surg Oncol. (2018) 44:607–12. doi: 10.1016/j.ejso.2018.02.003
13. Puértolas N, Osorio J, Jericó C, Miranda C, Santamaría M, Artigau E, et al. Effect of perioperative blood transfusions and infectious complications on inflammatory activation and long-term survival following gastric cancer resection. Cancers (Basel). (2022) 15:144. doi: 10.3390/cancers15010144
14. Sasahara M, Kanda M, Ito S, Mochizuki Y, Teramoto H, Ishigure K, et al. The preoperative prognostic nutritional index predicts short-term and long-term outcomes of patients with stage II/III gastric cancer: analysis of a multi-institution dataset. Dig Surg. (2020) 37:135–44. doi: 10.1159/000497454
15. Hirahara N, Tajima Y, Matsubara T, Fujii Y, Kaji S, Kawabata Y, et al. Systemic immune-inflammation index predicts overall survival in patients with gastric cancer: a propensity score-matched analysis. J Gastrointest Surg. (2021) 25:1124–33. doi: 10.1007/s11605-020-04710-7
16. Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer. (2023) 26:1–25. doi: 10.1007/s10120-022-01331-8
17. Katayama H, Kurokawa Y, Nakamura K, Ito H, Kanemitsu Y, Masuda N, et al. Extended Clavien-Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg Today. (2016) 46:668–85. doi: 10.1007/s00595-015-1236-x
18. Wang J, Tong T, Zhang G, Jin C, Guo H, Liu X, et al. Evaluation of neoadjuvant immunotherapy in resectable gastric/gastroesophageal junction tumors: a meta-analysis and systematic review. Front Immunol. (2024) 15:1339757. doi: 10.3389/fimmu.2024.1339757
19. Lin JL, Lin M, Lin GT, Zhong Q, Lu J, Zheng CH, et al. Oncological outcomes of sequential laparoscopic gastrectomy after treatment with camrelizumab combined with nab-paclitaxel plus S-1 for gastric cancer with serosal invasion. Front Immunol. (2024) 15:1322152. doi: 10.3389/fimmu.2024.1322152
20. Zhang X, Liang H, Li Z, Xue Y, Wang Y, Zhou Z, et al. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol. (2021) 22:1081–92. doi: 10.1016/S1470-2045(21)00297-7
21. Gao Y, Xi H, Qiao Z, Li J, Zhang K, Xie T, et al. Comparison of robotic- and laparoscopic-assisted gastrectomy in advanced gastric cancer: updated short- and long-term results. Surg Endosc. (2019) 33:528–34. doi: 10.1007/s00464-018-6327-5
22. Tian Y, Cao S, Liu X, Li L, He Q, Jiang L, et al. Randomized controlled trial comparing the short-term outcomes of enhanced recovery after surgery and conventional care in laparoscopic distal gastrectomy (GISSG1901). Ann Surg. (2022) 275:e15–21. doi: 10.1097/SLA.0000000000004908
23. Mungan I, Dicle ÇB, Bektaş S, Sari S, Yamanyar S, Çavuş M, et al. Does the preoperative platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio predict morbidity after gastrectomy for gastric cancer? Mil Med Res. (2020) 7:9. doi: 10.1186/s40779-020-00234-y
24. Schietroma M, Romano L, Schiavi D, Pessia B, Mattei A, Fiasca F, et al. Systemic inflammation response index (SIRI) as predictor of anastomotic leakage after total gastrectomy for gastric cancer. Surg Oncol. (2022) 43:101791. doi: 10.1016/j.suronc.2022.101791
25. Wang C, Huang HZ, He Y, Yu YJ, Zhou QM, Wang RJ, et al. A new nomogram based on early postoperative NLR for predicting infectious complications after gastrectomy. Cancer Manag Res. (2020) 12:881–9. doi: 10.2147/CMAR.S238530
26. Inaoka K, Kanda M, Uda H, Tanaka Y, Tanaka C, Kobayashi D, et al. Clinical utility of the platelet-lymphocyte ratio as a predictor of postoperative complications after radical gastrectomy for clinical T2-4 gastric cancer. World J Gastroenterol. (2017) 23:2519–26. doi: 10.3748/wjg.v23.i14.2519
27. Pang W, Lou N, Jin C, Hu C, Arvine C, Zhu G, et al. Combination of preoperative platelet/lymphocyte and neutrophil/lymphocyte rates and tumor-related factors to predict lymph node metastasis in patients with gastric cancer. Eur J Gastroenterol Hepatol. (2016) 28:493–502. doi: 10.1097/MEG.0000000000000563
28. Nelen SD, Bosscha K, Lemmens VEPP, Hartgrink HH, Verhoeven RHA, de Wilt JHW, et al. Morbidity and mortality according to age following gastrectomy for gastric cancer. Br J Surg. (2018) 105:1163–70. doi: 10.1002/bjs.10836
29. Yu H, Xu L, Yin S, Jiang J, Hong C, He Y, et al. Risk factors and prognostic impact of postoperative complications in patients with advanced gastric cancer receiving neoadjuvant chemotherapy. Curr Oncol. (2022) 29:6496–507. doi: 10.3390/curroncol29090511
30. Yan Y, Yang A, Lu L, Zhao Z, Li C, Li W, et al. Impact of Neoadjuvant therapy on minimally invasive surgical outcomes in advanced gastric cancer: an international propensity score-matched study. Ann Surg Oncol. (2021) 28:1428–36. doi: 10.1245/s10434-020-09070-9
31. Zietlow KE, Wong S, Heflin MT, McDonald SR, Sickeler R, Devinney M, et al. Geriatric preoperative optimization: a review. Am J Med. (2022) 135:39–48. doi: 10.1016/j.amjmed.2021.07.028
32. Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. (2021) 9:373–92. doi: 10.1016/S2213-8587(21)00045-0
33. Hirahara N, Tajima Y, Fujii Y, Kaji S, Kawabata Y, Hyakudomi R, et al. Prediction of postoperative complications and survival after laparoscopic gastrectomy using preoperative Geriatric Nutritional Risk Index in elderly gastric cancer patients. Surg Endosc. (2021) 35:1202–9. doi: 10.1007/s00464-020-07487-7
34. Kim HE Yu WS, Lee CY, Lee JG, Kim DJ, Park SY. Risk factors for pulmonary complications after neoadjuvant chemoradiotherapy followed by surgery for non-small cell lung cancer. Thorac Cancer. (2022) 13:361–8. doi: 10.1111/1759-7714.14263
35. Cohen S, Gal J, Freifeld Y, Khoury S, Dekel Y, Hofman A, et al. Nutritional status impairment due to neoadjuvant chemotherapy predicts post-radical cystectomy complications. Nutrients. (2021) 13:4471. doi: 10.3390/nu13124471
36. Liu S, Hu Q, Song P, Tao L, Ai S, Miao J, et al. Risk factor and surgical outcome of petersen's hernia after gastrectomy in gastric cancer. Front Oncol. (2021) 11:765509. doi: 10.3389/fonc.2021.765509
37. Chen HN, Chen XZ, Zhang WH, Yang K, Chen XL, Zhang B, et al. The impact of body mass index on the surgical outcomes of patients with gastric cancer: a 10-year, single-institution cohort study. Medicine. (2015) 94:e1769. doi: 10.1097/MD.0000000000001769
38. Umeki Y, Shibasaki S, Nakauchi M, Serizawa A, Nakamura K, Akimoto S, et al. Safe implementation of robotic distal gastrectomy performed by non-endoscopic surgical skill qualification system-qualified surgeons. Surg Today. (2023) 53:192–7. doi: 10.1007/s00595-022-02553-0
Keywords: gastric cancer, neoadjuvant therapy, immunotherapy, nomogram, postoperative complication
Citation: Cui H, Zhang S, Sun L, Yuan Z, Xu Q, Gao J, Chen L, Cui J and Wei B (2024) Risk factor analysis and nomogram construction of postoperative complications for patients with locally advanced gastric cancer who received neoadjuvant immunotherapy and chemotherapy. Front. Med. 11:1405704. doi: 10.3389/fmed.2024.1405704
Received: 23 March 2024; Accepted: 03 July 2024;
Published: 26 July 2024.
Edited by:
Jianming James Tang, University of Alabama at Birmingham, United StatesReviewed by:
Shi Yan, Army Medical University, ChinaShihori Tanabe, National Institute of Health Sciences (NIHS), Japan
Copyright © 2024 Cui, Zhang, Sun, Yuan, Xu, Gao, Chen, Cui and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Wei, d2VpYm9AMzAxaG9zcGl0YWwuY29tLmNu; Jianxin Cui, Y3VpamlhbnhpbkAzMDFob3NwaXRhbC5jb20uY24=
†These authors have contributed equally to this work and share first authorship
 Hao Cui
Hao Cui Sijin Zhang1†
Sijin Zhang1† Qixuan Xu
Qixuan Xu Jingwang Gao
Jingwang Gao Bo Wei
Bo Wei