
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 11 July 2024
Sec. Geriatric Medicine
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1404152
This article is part of the Research Topic Molecular and Cellular Changes in Skeletal Muscle, Cartilage, Bone and Tendon During Aging View all articles
Background: The systemic inflammatory response index (SIRI) is a novel composite biomarker of inflammation. However, there is limited information on its use in the context of osteoporotic fractures. Hence, this study aimed to investigate the association between baseline SIRI values and bone turnover markers (BTMs) in Chinese patients diagnosed with osteoporotic fractures (OPFs), to offer a more precise method for assessing bone health and inflammation in clinical settings.
Methods: A retrospective cross-sectional study was conducted on 3,558 hospitalized patients with OPFs who required surgery or hospitalization at the First People’s Hospital of Kunshan City from January 2017 to July 2022. Baseline measurements of SIRI, β-CTX (beta-C-terminal telopeptide of type I collagen), and P1NP (procollagen type I N-terminal propeptide) were obtained. The analyses were adjusted for variables, including age, sex, body mass index (BMI), and other initial laboratory and clinical findings. Furthermore, multivariable logistic regression, smooth curve fitting, and threshold analysis were also performed.
Results: The results revealed a negative correlation between baseline SIRI values and both β-CTX and P1NP levels. After adjusting for covariates in the regression analysis, each unit increase in SIRI was found to be inked to a reduction of 0.04 (β = −0.04; 95% confidence interval [CI], −0.05 to −0.03; with p-value <0.001) in β-CTX levels and a decrease of 3.77 (β = 3.77; 95% CI, 5.07 to 2.47; with p-value <0.001) in P1NP levels. Furthermore, a curvilinear relationship and threshold effect were also identified. Turning points were identified at SIRI values of 1.41 and 1.63 on the adjusted smooth curve.
Conclusion: The results showed a negative correlation between the baseline SIRI value and β-CTX level, as well as the level of P1NP. This suggests a possible link between the systemic inflammatory response and reduced bone metabolism. If these findings are verified, SIRI has the potential to function as a predictive indicator for BTMs. Nevertheless, additional research is necessary to verify these findings.
Osteoporosis (OP) is a common disorder of bone metabolism defined by reduced bone mass and degradation of bone tissue (1), often leading to fragile and easily fractured bones (2), especially in women over the age of 55 and men over the age of 65. OP is diagnosed according to internationally recognized definitions when the bone density is >2.5 standard deviations below the mean of young healthy individuals (3). Most patients have secondary causes of OP, ranging from endocrine disorders to chronic inflammation and genetic diseases, which can contribute to the development of the disease (4). The prevalence rate of OP in the elderly population in China is approximately 39.4% (5). OP imposes a significant burden on both patients’ health and the economy (6, 7). It increases the risk of fractures, reducing the patients’ quality of life and increasing the likelihood of hospitalization, disability, and potentially even mortality (8). Global estimates indicate that an OP associated fracture occurs every three seconds, resulting in about 8.9 million instances each year (9).
Inflammation is known to promote bone resorption and is acknowledged as a risk factor for OP (10). Other factors, apart from inflammation, are also associated with the development of OP including age, sex, genetic factors, malnutrition, lack of exercise, smoking, marital status, and excessive alcohol consumption (11, 12). Early screening, diagnosis, and therapy are critical for the prevention and management of OP since they can decrease the likelihood of fractures and enhance patients’ quality of life (13).
Bone turnover markers (BTMs) are biochemical indicators that reflect the dynamic interplay between bone formation and resorption and can be measured in the serum, plasma, or urine (14, 15). In the present study, procollagen type 1 N-terminal propeptide (P1NP), and beta-C-terminal telopeptide of type I collagen (β-CTX) were studied as representative BTMs. These are among the most important BTMs. P1NP is a crucial constituent of the bone matrix and is released during the synthesis of type I collagen synthesis and its subsequent integration into the bone matrix, and thus qualifies as a reliable indicator of bone formation (16). P1NP levels are correlated with osteoblast activity (17).
β-CTX is a peptide fragment of collagen that is released into the bloodstream during the process of bone resorption and thus serves as an indicator of bone metabolism (18). The link between β-CTX and OP is well-established (19). Both β-CTX and P1NP are used routinely to monitor OP or predict the prognosis of individuals with disorders of bone metabolism.
The systemic inflammatory response index (SIRI) is a newly developed biomarker for inflammation that takes into account the absolute numbers of neutrophils, monocytes, and lymphocytes. The SIRI indicates the magnitude of the body’s inflammatory reaction (20). Previous studies have reported its extensive application in various cardiovascular diseases including stroke (21), ischemic heart disease (22), acute coronary syndrome (20), and aortic dissection (23). In addition, the SIRI has been employed in studies involving cervical cancer (24), COVID-19 (25), and ankylosing spondylitis (26). However, the correlation between the SIRI and OP, particularly with BTMs, is relatively unexplored with minimal data. To fill this gap, the present study aimd to determine the relation between the baseline SIRI and BTM levels (β-CTX and P1NP) in individuals with osteoporotic fractures (OPFs) to fill this research gap.
The study received approval from the Ethics Committee of the Affiliated Kunshan Hospital of Jiangsu University, Suzhou, China (approval No. 2021–06-015-K01), and adhered to the principles outlined in the Declaration of Helsinki. The patients’ identities were concealed to ensure an unbiased investigation. All patients provided written informed consent.
In the present study, a retrospective cross-sectional analysis was performed using patient data collected from January 2017 to July 2022. The medical information of the patients was obtained from Kunshan Hospital, affiliated with Jiangsu University, Suzhou, China. The study involved a cohort of 3,558 patients with OPF who underwent surgical procedures or required hospitalization. All participants received blood tests while they were hospitalized. The diagnosis of OP was established based on the following criteria: (1) The existence of bone instability and fractures without any accompanying metabolic bone disorders, coupled with a standard bone mineral density (BMD) (T-score); (2) verification of osteoporosis (OP) using a T-score of −2.5 or below, even in the absence of a prevailing bone fracture (27). The exclusion criteria were as follows: (1) Missing or incomplete records; (2) Multiple or pathological hip fractures; (3) Diagnosed with other diseases that interfere with bone metabolism (such as thyroid diseases, parathyroid-related diseases, diabetes, gonadal diseases); (4) Presence of an autoimmune disease such as systemic lupus erythematosus; (5) Long-term use of drugs that affect bone metabolism; (6) SIRI>15 (28).
Levels of SIRI, β-CTX, and P1NP were assessed in a cohort of 732 individuals. The complete blood counts of the patients were determined and the SIRI was computed as the exposure variable. Neutrophils, monocytes, and lymphocytes were measured using flow cytometry with nuclear staining on the Sysmex XN-10 (B4) hematology analyzer. The outcome variables investigated were the levels of β-CTX and P1NP. The β-CTX and P1NP levels in patients were measured using automated electrochemiluminescence immunoassays (ECLIA) from Roche Diagnostics in (Mannheim, Germany). All measurements were collected using the same instrument and the same experienced operator following standardized protocols.
The covariate variables including age, sex, body mass index (BMI), high-density lipoprotein (HDL), total cholesterol (TC), hypertension, diabetes, calcium, platelet count, smoking status, alcohol consumption, and the Charlson comorbidity index (CCI) (29) were measured and recorded. Calcium levels were measured using a Beckman AU5800 automated biochemistry analyzer, employing the Arsenazo III method. Platelet counts were determined by flow cytometry with impedance on a Sysmex XN-10 (B4) hematology analyzer, while HDL was measured on the Beckman AU5800 automated biochemistry analyzer employing the direct approach. All clinical indicators were assessed within three days of admission.
The data related to demographics, laboratory tests, and clinical outcomes are presented as either the median with the interquartile range (the 25th and 75th percentiles) or the mean ± standard deviation (SD). The data are presented in the form of frequencies (expressed as percentages) for each category. Categorical data were analyzed using either Pearson’s chi-square test or Fisher’s exact test for univariate analysis. Independent-sample tests were used to compare normally distributed continuous data, while the Mann–Whitney U test was used for non-normally distributed continuous data. The association between the attributes of OPFs and the BTMs, β-CTX, and P1NP, was also investigated using univariate analysis.
The Generalized Estimating Equation (GEE) and Generalized Additive Model (GAM) are two common statistical modeling approaches. GEE models the average response and correlations by specifying a working correlation structure, suitable for handling correlated data such as longitudinal or clustered data. In contrast, the GAM employs flexible nonparametric smoothing functions to explore complex nonlinear relationships between the response and predictors, without assuming parametric forms. Both require specifying the response distribution, formulating the mean model, and using iterative algorithms to estimate parameters. Researchers can then evaluate model fit and perform statistical inference.
The GEE was employed for appropriate adjustment of covariates and investigation of the independent relationship between SIRI levels and β-CTX and P1NP in OPFs. The models that were developed included unadjusted and slightly adjusted models, referred to as Model 1 and Model 2, respectively, as well as the fully adjusted model, termed Model 3. Firstly, a variance inflation factor (VIF) analysis was conducted to detect any collinearity among the covariates. Subsequently, decisions were taken to modify these elements based on the following criteria: (1) A modification in the matched odds ratio (OR) by ≥10% was observed upon the addition or removal of covariates in the basic or full model, respectively; (2) Variables that satisfied criterion 1 or had a p-value less than 0.1 in the univariate model (30). Model 3 employed both criteria 1 and 2 to adjust for covariates. This resulted in the development of three models, namely, Model 1, which was left unadjusted, and Model 2 (minimally adjusted model), which included covariate adjustments for age, sex, BMI, smoking status, alcohol consumption, hypertension, and diabetes CCI levels, and Model 3, which additionally included covariates such as calcium, HDL, total cholesterol, and the platelet count.
The GAM was used to detect possible non-linear associations. After the identification of these correlations, a two-piecewise linear regression model was used to determine the threshold effects in the resulting smoothing curves. A recursive approach was used to independently determine the inflection point, employing a maximum-likelihood model when the curves exhibited a clear ratio (31). The robustness of the studies and changes among patient subgroups were assessed by conducting subgroup analyses, stratifying patients based on specific covariates. Subgroup interactions and modifications were analyzed using the likelihood ratio test (LRT).
The R packages from The R Foundation1 and Empower Stats from X&Y Solutions, Inc., MA, USA2 were utilized for all analyses. A significance criterion of p < 0.05, using a two-tailed test, was employed.
According to the eligibility criteria depicted in Figure 1, a total of 732 patients were treated between January 1, 2017, to July 27, 2022, were ultimately included in the analysis. Table 1 summarizes the baseline characteristics of the hospitalized patients (n = 732), of whom 33.61% were male and 66.39% female, with a mean age of 69.04 ± 11.02 years.
The subjects in this study exhibited a mean Systemic Immune-Inflammation Index (SIRI) value of 2.40 ± 2.18, a mean β-CTX value of 0.53 ± 0.28 ng/mL, and a mean P1Np value of 57.72 ± 30.72 μg/L. Observations were conducted to identify variations in the co-variate variables among the patient cohort.
A univariate analysis was performed to investigate the relation between β-CTX and P1NP with covariate variables (Table 2). No significant relationships were found between the investigated variables and β-CTX or P1NP in the patients with OPF.
Three models were used in the subsequent phase to analyze the correlation between SIRI and both BTMs (β-CTX and P1NP) in participants with OP (Table 3). In the unadjusted Model 1, there was a significant correlation between SIRI and β-CTX (β = −0.03, 95% CI: −0.04 to −0.02, p < 0.001) (β is the regression coefficient in the linear regression model, representing the magnitude and direction of the association between SIRI and BTMs). Similarly, a significant correlation was found between SIRI and P1NP (β = −2.87, 95% CI: −3.70 to −1.70, p < 0.001). After adjusting for variables including age, sex, BMI, smoking status, alcohol intake, CCI, hypertension, and diabetes in Model 2, the observed relationships were consistent. The SIRI remained significantly associated with both β-CTX (β = −0.03, 95% CI: −0.04 to −0.02, p < 0.001) and P1NP (β = −2.83, 95% CI: −4.02 to −1.64, p < 0.001). Expanding on Model 2, Model 3 included further adjustments for HDL, TC, calcium, and platelet count, and consistently showed a negative correlation. The SIRI remained significantly associated with β-CTX (β = −0.04, 95% CI: −0.05 to −0.03, p < 0.001) and P1NP (β = −3.77, 95% CI: −5.07 to −2.47, p < 0.001).
Additional subgroup analysis was conducted to assess the robustness of Model 3 by categorizing patients with OPF according to various characteristics such as age, sex, BMI, smoking status, alcohol consumption, CCI, hypertension, diabetes, HDL, TC, calcium, and platelet count. Adjustment was established for those covariates that were not utilized for stratification. The studies showed consistent patterns in the results, with no detected interactions due to stratification (all p < 0.05, Table 4).
The link between SIRI β-CTX and P1NP was then evaluated using graphical techniques to determine if it was linear or nonlinear (Figure 2). The GAM estimation revealed that, after accounting for covariate variables, there were distinct nonlinear relationships between SIRI and BTMs in the OPF population in the study. These associations were modeled using segmented linear regression, with the identified breakpoints (K-values) being 1.41 and 1.63, respectively (Table 5). To the left of the thresholds, there was a stronger negative correlation between SIRI and β-CTX, as well as with P1NP.
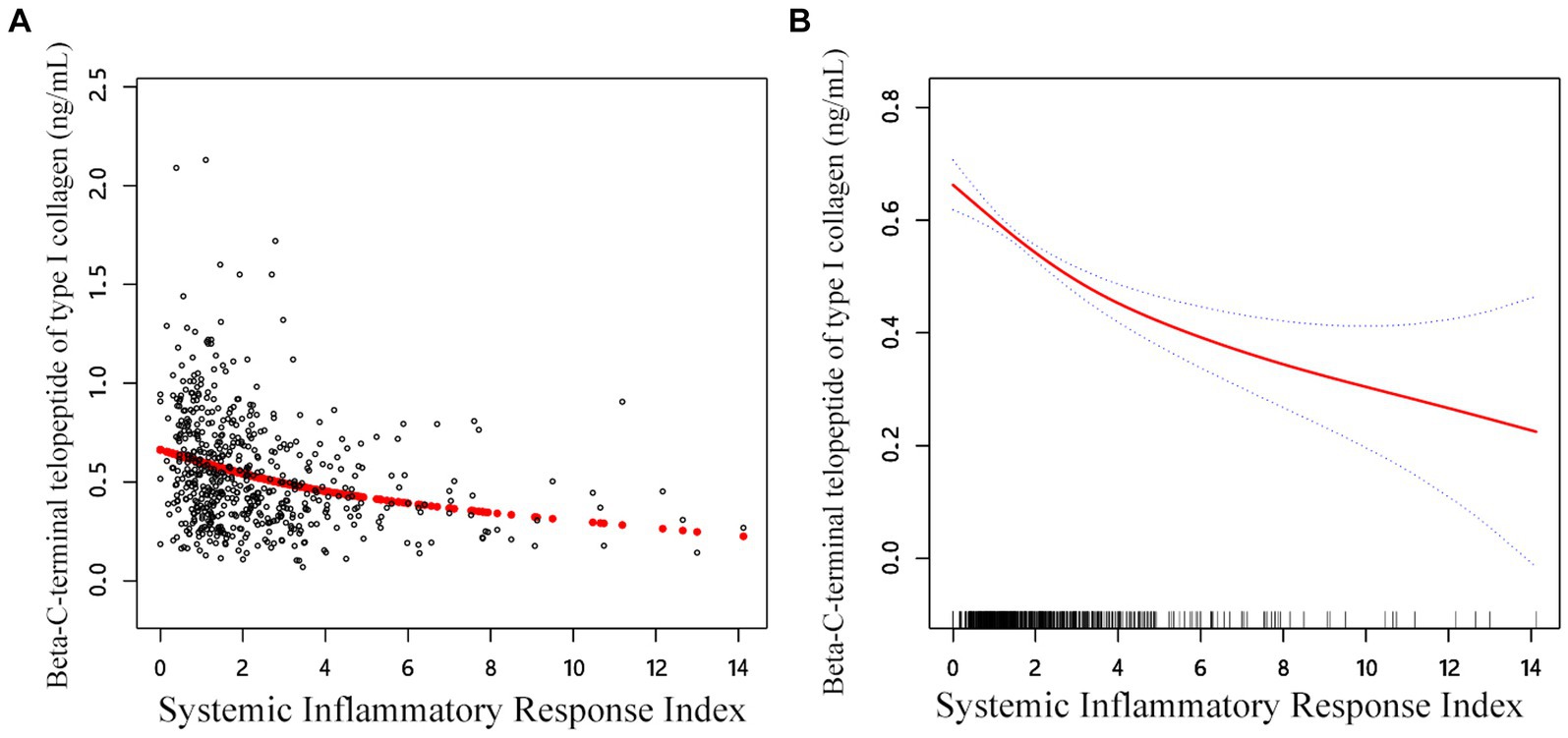
Figure 2. Adjusted smoothed curve analysis revealing the interplay between SIRI and β-CTX: (A) Each black point represents a single participant sample. (B) Solid red line represents the smooth curve fit between variables. Blue bands represent the 95% of confidence interval from the fit. Age, sex, BMI, smoke, drink, CCI, hypertension, diabetes, HDL, TC, calcium, and platelet count were adjusted.
In the threshold studies for SIRI and β-CTX, the effect size on the left side of the threshold was −0.13 (95% CI: −0.20 to −0.06, p = 0.002). The effect size on the right side of the threshold was −0.03 (95% CI: −0.04 to −0.02, p < 0.001). Regarding the SIRI and P1NP thresholds, the effect size on the left side of the threshold was −13.64 (95% CI: −20.17 to −7.11, p < 0.001). The effect size on the right side of the threshold was −2.35 (95% CI: −3.94 to −0.77, p = 0.003) (Figure 3).
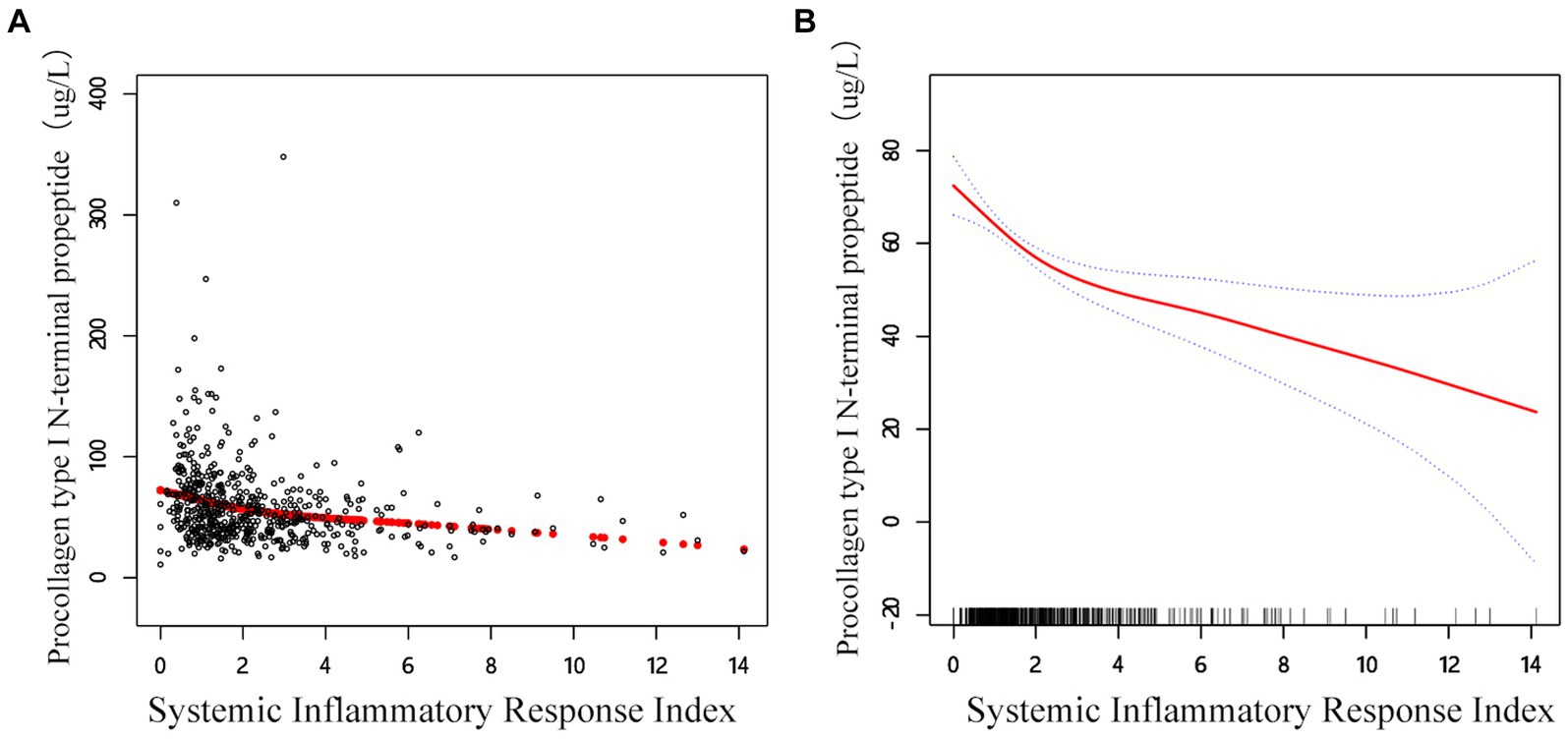
Figure 3. Adjusted smoothed curve analysis revealing the interplay between SIRI and P1NP: (A) Each black point represents a single participant sample. (B) Solid red line represents the smooth curve fit between variables. Blue bands represent the 95% of confidence interval from the fit. Age, sex, BMI, smoke, drink, CCI, hypertension, diabetes, HDL, TC, calcium, and platelet count are adjusted.
This cross-sectional study aimed to investigate the association between the SIRI and BTMs, including β-CTX and P1NP, in 3,558 patients hospitalized for osteoporotic fractures that required surgical intervention. The findings suggest that increased inflammation is associated with decreased bone metabolism.
The association between inflammation and OP has recently received attention, as the pivotal role of inflammation in the pathogenesis of OP is well-recognized. Clinical observations have indicated that systemic OP often co-exists with systemic periodic inflammation, while localized OP tends to be associated with regional inflammatory processes (32). Normal bone metabolism in the human body is dependent on achieving an equilibrium between bone resorption and formation (33). It is reported that various pro-inflammatory cytokines, including, IL-1, IL-6, TNF-α (34), and CRP, may cause persistent systemic and subclinical inflammation (35, 36). In response to these inflammatory stimuli, blood cells, especially neutrophils, are activated and recruited to the site (37), while other immune cells assume residence in the bone marrow (38). The SIRI value indicates alterations in blood parameters, indicative of a disturbance in the steady state of bone metabolism and a subsequent decline in the bone metabolism level (39). Inflammatory mechanisms underlying OP have also been proposed in recent years (40, 41). It is evident that inflammation alters bone homeostasis, resulting in reduced bone mass, weaker bone strength, and decreased bone density, as well as ultimately a decrease in bone turnover, all of which contribute to adverse outcomes in OPFs (42, 43). Increased circulating levels of pro-inflammatory cytokines and immune cells directly or indirectly affect bone turnover through various pathways (44, 45). To date, several studies have developed delivery systems using extracellular vesicles (EVs) secreted by mesenchymal stem cells (MSCs), targeting both the suppression of bone resorption and the promotion of bone formation and angiogenesis (46). This suggests the potential of anti-inflammatory/immune-based therapies for the treatment of OP.
The SIRI is a promising inflammatory marker that provides a comprehensive reflection of the body’s immune and inflammatory status (47). However, to the best of our knowledge, there is still insufficient information and evidence on the association between the SIRI and bone metabolism. Therefore, the objectives of the present study were to investigate the correlation between the SIRI and BTMs in patients with OPF to assess the predictive value of the SIRI in OPFs. We demonstrated a significant negative correlation between SIRI and BTMs (β-CTX and P1NP), indicating that increased systemic inflammation is associated with reduced bone metabolism, which typically suggests a poorer prognosis. These findings also suggest that SIRI values may serve as a tool for evaluating the risk of OPFs. Consistent with prior research (42, 48, 49), the present study demonstrated that a strong inflammatory response is detrimental to the prognosis of individuals with OP.
Notable advantages of this study encompass a nationally representative population and statistical models that adjusted for various important confounding factors. It is believed that these research results may apply to the general population. However, the study is subject to several limitations. First, the study used a cross-sectional design, which is only able to establish associations rather than demonstrate the temporal relationship between the SIRI and changes in BTMs. Furthermore, bone fractures can elicit acute-phase responses, leading to alterations in blood parameters. The study was unable to exclude the potential influence of fractures on inflammatory biomarkers, and thus represents a limitation of the research. The pathogenesis of OP is influenced by both hereditary and non-genetic factors. However, this data analysis largely focused on controlling for specific demographic and lifestyle variables. Further prospective research is required for a comprehensive understanding of the relevant linkages. In addition, the sample size was relatively small, consisting of only 732 subjects that could be analyzed. This calls for more extensive research in the future, involving detailed studies on real patient populations with different diseases.
In conclusion, the finding of the study established a significant negative relationship between the SIRI and BTMs (β-CTX and P1NP) in individuals with OPFs. The results imply that increased inflammation is linked to reduced bone metabolism, often indicative of an unfavorable prognosis. These findings underscore the harmful impact of inflammation in OP and suggest that the assessment of SIRI values could serve as a valuable tool for evaluating the risk and prognosis of OPFs. Further research is required to unravel the underlying relationship between inflammation and OP and to validate the predictive utility of the SIRI in more extensive and diverse patient cohorts.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the IRB of Affiliated Kunshan Hospital of Jiangsu University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
PZ: Data curation, Formal analysis, Investigation, Resources, Software, Writing – original draft, Writing – review & editing. KL: Data curation, Methodology, Resources, Software, Supervision, Validation, Writing – review & editing. CL: Resources, Software, Supervision, Validation, Writing – review & editing. M-zX: Data curation, Resources, Software, Supervision, Validation, Writing – original draft. Y-wY: Investigation, Methodology, Resources, Supervision, Validation, Writing – original draft. H-qS: Resources, Software, Supervision, Validation, Writing – review & editing. YY: Supervision, Validation, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by China Postdoctoral Science Foundation (CN) (2022M711439), Elderly Health Research Project of Jiangsu Province (CN) (LKZ2022020), Special Funding for Jiangsu Province Science and Technology Plan (Key Research and Development Program for Social Development) (CN) (BE2023738), Suzhou Collaborative Innovation Research Project of Medical and Industrial Integration (CN) (SLJ2022023).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1404152/full#supplementary-material
1. Rachner, TD, Khosla, S, and Hofbauer, LC. Osteoporosis: now and the future. Lancet. (2011) 377:1276–87. doi: 10.1016/S0140-6736(10)62349-5
2. Chow, SKH, Ho, CY, Wong, HW, Chim, YN, Wong, RM-Y, and Cheung, WH. Efficacy of low-magnitude high-frequency vibration (LMHFV) on musculoskeletal health of participants on wheelchair: a study protocol for a single-blinded randomised controlled study. BMJ Open. (2020) 10:e038578. doi: 10.1136/bmjopen-2020-038578
3. Battaglia, Y, Bellasi, A, Bortoluzzi, A, Tondolo, F, Esposito, P, Provenzano, M, et al. Bone mineral density changes in long-term kidney transplant recipients: a real-life cohort study of native vitamin D supplementation. Nutrients. (2022) 14:323. doi: 10.3390/nu14020323
4. Ebeling, PR, Nguyen, HH, Aleksova, J, Vincent, AJ, Wong, P, and Milat, F. Secondary osteoporosis. Endocr Rev. (2022) 43:240–313. doi: 10.1210/endrev/bnab028
5. Salari, N, Darvishi, N, Bartina, Y, Larti, M, Kiaei, A, Hemmati, M, et al. Global prevalence of osteoporosis among the world older adults: a comprehensive systematic review and meta-analysis. J Orthop Surg. (2021) 16:669. doi: 10.1186/s13018-021-02821-8
6. Dimai, HP, Redlich, K, Peretz, M, Borgström, F, Siebert, U, and Mahlich, J. Economic burden of osteoporotic fractures in Austria. Health Econ Rev. (2012) 2:12. doi: 10.1186/2191-1991-2-12
7. Bleibler, F, Rapp, K, Jaensch, A, Becker, C, and König, H-H. Expected lifetime numbers and costs of fractures in postmenopausal women with and without osteoporosis in Germany: a discrete event simulation model. BMC Health Serv Res. (2014) 14:284. doi: 10.1186/1472-6963-14-284
8. López, E, Ibarz, E, Herrera, A, Mateo, J, Lobo-Escolar, A, Puértolas, S, et al. A mechanical model for predicting the probability of osteoporotic hip fractures based in DXA measurements and finite element simulation. Biomed Eng Online. (2012) 11:84. doi: 10.1186/1475-925X-11-84
9. Johnston, CB, and Dagar, M. Osteoporosis in older adults. Med Clin North Am. (2020) 104:873–84. doi: 10.1016/j.mcna.2020.06.004
10. Nor Muhamad, ML, Ekeuku, SO, Wong, S-K, and Chin, K-Y. A scoping review of the skeletal effects of Naringenin. Nutrients. (2022) 14:4851. doi: 10.3390/nu14224851
11. Hendrickx, G, Boudin, E, and Van Hul, W. A look behind the scenes: the risk and pathogenesis of primary osteoporosis. Nat Rev Rheumatol. (2015) 11:462–74. doi: 10.1038/nrrheum.2015.48
12. Zhou, Y, Zhu, X, Zhang, M, Li, Y, Liu, W, Huang, H, et al. Association between dietary inflammatory index and bone density in lactating women at 6 months postpartum: a longitudinal study. BMC Public Health. (2019) 19:1076. doi: 10.1186/s12889-019-7409-6
13. Lems, WF. Fracture risk estimation may facilitate the treatment gap in osteoporosis. Ann Rheum Dis. (2015) 74:1943–5. doi: 10.1136/annrheumdis-2015-208245
14. Löfdahl, E, Ahmed, S, Ahmed, A, and Rådegran, G. Plasma biomarkers for clinical assessment of bone mineral density in heart transplanted patients—a single-center study at Skåne University Hospital in Lund. Transpl Int. (2022) 35:10161. doi: 10.3389/ti.2022.10161
15. Lane, NE, Saag, K, O’Neill, TJ, Manion, M, Shah, R, Klause, U, et al. Real-world bone turnover marker use: impact on treatment decisions and fracture. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. (2021) 32:831–40. doi: 10.1007/s00198-020-05734-0
16. Han, S, Gong, F, Xue, Y, Wang, C, and Qi, X. Development of a Chemiluminescence assay for Total N-terminal Propeptide of type I collagen and its evaluation in lung transplantation. J Anal Methods Chem. (2022) 2022:2711414–9. doi: 10.1155/2022/2711414
17. Wu, C, Kato, TS, Pronschinske, K, Qiu, S, Naka, Y, Takayama, H, et al. Dynamics of bone turnover markers in patients with heart failure and following haemodynamic improvement through ventricular assist device implantation. Eur J Heart Fail. (2012) 14:1356–65. doi: 10.1093/eurjhf/hfs138
18. Craven, BC, Giangregorio, LM, Alavinia, SM, Blencowe, LA, Desai, N, Hitzig, SL, et al. Evaluating the efficacy of functional electrical stimulation therapy assisted walking after chronic motor incomplete spinal cord injury: effects on bone biomarkers and bone strength. J Spinal Cord Med. (2017) 40:748–58. doi: 10.1080/10790268.2017.1368961
19. Smilic, TN, Novakovic, TR, Markovic-Jovanovic, SR, Smilic, LLJ, Mitic, JS, and Radunovic, ML. The relevance of Osteoclastic and osteoblastic activity markers follow-up in patients on Antiresorptive osteoporosis treatment. J Clin Densitom. (2018) 21:322–8. doi: 10.1016/j.jocd.2017.06.030
20. Dziedzic, EA, Gąsior, JS, Tuzimek, A, Paleczny, J, Junka, A, Dąbrowski, M, et al. Investigation of the associations of novel inflammatory biomarkers-systemic inflammatory index (SII) and systemic inflammatory response index (SIRI)-with the severity of coronary artery disease and acute coronary syndrome occurrence. Int J Mol Sci. (2022) 23:9553. doi: 10.3390/ijms23179553
21. Zhang, Y, Xing, Z, Zhou, K, and Jiang, S. The predictive role of systemic inflammation response index (SIRI) in the prognosis of stroke patients. Clin Interv Aging. (2021) 16:1997–2007. doi: 10.2147/CIA.S339221
22. Dziedzic, EA, Gąsior, JS, Tuzimek, A, Dąbrowski, M, and Jankowski, P. The association between serum vitamin D concentration and new inflammatory biomarkers-systemic inflammatory index (SII) and systemic inflammatory response (SIRI)-in patients with ischemic heart disease. Nutrients. (2022) 14:4212. doi: 10.3390/nu14194212
23. Zhao, Y, Hong, X, Xie, X, Guo, D, Chen, B, Fu, W, et al. Preoperative systemic inflammatory response index predicts long-term outcomes in type B aortic dissection after endovascular repair. Front Immunol. (2022) 13:992463. doi: 10.3389/fimmu.2022.992463
24. Han, K, Shi, D, Yang, L, Wang, Z, Li, Y, Gao, F, et al. Prognostic value of systemic inflammatory response index in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Ann Med. (2022) 54:1667–77. doi: 10.1080/07853890.2022.2083671
25. Fois, AG, Paliogiannis, P, Scano, V, Cau, S, Babudieri, S, Perra, R, et al. The systemic inflammation index on admission predicts in-hospital mortality in COVID-19 patients. Mol Basel Switz. (2020) 25:5725. doi: 10.3390/molecules25235725
26. Wu, J, Yan, L, and Chai, K. Systemic immune-inflammation index is associated with disease activity in patients with ankylosing spondylitis. J Clin Lab Anal. (2021) 35:e23964. doi: 10.1002/jcla.23964
27. Aibar-Almazán, A, Voltes-Martínez, A, Castellote-Caballero, Y, Afanador-Restrepo, DF, del Carmen Carcelén-Fraile, M, and López-Ruiz, E. Current status of the diagnosis and Management of Osteoporosis. Int J Mol Sci. (2022) 23:9465. doi: 10.3390/ijms23169465
28. Zhang, J, Jiang, J, Qin, Y, Zhang, Y, Wu, Y, and Xu, H. Systemic immune-inflammation index is associated with decreased bone mass density and osteoporosis in postmenopausal women but not in premenopausal women. Endocr Connect. (2023) 12:e220461. doi: 10.1530/EC-22-0461
29. Charlson, ME, Carrozzino, D, Guidi, J, and Patierno, C. Charlson comorbidity index: a critical review of Clinimetric properties. Psychother Psychosom. (2022) 91:8–35. doi: 10.1159/000521288
30. Kernan, WN, Viscoli, CM, Brass, LM, Broderick, JP, Brott, T, Feldmann, E, et al. Phenylpropanolamine and the risk of hemorrhagic stroke. N Engl J Med. (2000) 343:1826–32. doi: 10.1056/NEJM200012213432501
31. Liu, S, Wang, X, Lu, Y, Li, T, Gong, Z, Sheng, T, et al. The effects of intraoperative cryoprecipitate transfusion on acute renal failure following orthotropic liver transplantation. Hepatol Int. (2013) 7:901–9. doi: 10.1007/s12072-013-9457-9
32. Yun, AJ, and Lee, PY. Maldaptation of the link between inflammation and bone turnover may be a key determinant of osteoporosis. Med Hypotheses. (2004) 63:532–7. doi: 10.1016/S0306-9877(03)00326-8
33. Dirckx, N, Van Hul, M, and Maes, C. Osteoblast recruitment to sites of bone formation in skeletal development, homeostasis, and regeneration. Birth Defects Res C. (2013) 99:170–91. doi: 10.1002/bdrc.21047
34. Beck, GR, Ha, S-W, Camalier, CE, Yamaguchi, M, Li, Y, Lee, J-K, et al. Bioactive silica based nanoparticles stimulate bone forming osteoblasts, suppress bone esorbing osteoclasts, and enhance bone mineral density in vivo. Nanomed. (2012) 8:793–803. doi: 10.1016/j.nano.2011.11.003
35. Furman, D, Campisi, J, Verdin, E, Carrera-Bastos, P, Targ, S, Franceschi, C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. (2019) 25:1822–32. doi: 10.1038/s41591-019-0675-0
36. Santoro, A, Guidarelli, G, Ostan, R, Giampieri, E, Fabbri, C, Bertarelli, C, et al. Gender-specific association of body composition with inflammatory and adipose-related markers in healthy elderly Europeans from the NU-AGE study. Eur Radiol. (2019) 29:4968–79. doi: 10.1007/s00330-018-5973-2
37. Herrero-Cervera, A, Soehnlein, O, and Kenne, E. Neutrophils in chronic inflammatory diseases. Cell Mol Immunol. (2022) 19:177–91. doi: 10.1038/s41423-021-00832-3
38. Caputa, G, Castoldi, A, and Pearce, EJ. Metabolic adaptations of tissue-resident immune cells. Nat Immunol. (2019) 20:793–801. doi: 10.1038/s41590-019-0407-0
39. Nakano, S, Inoue, K, Xu, C, Deng, Z, Syrovatkina, V, Vitone, G, et al. G-protein Gα13 functions as a cytoskeletal and mitochondrial regulator to restrain osteoclast function. Sci Rep. (2019) 9:4236. doi: 10.1038/s41598-019-40974-z
40. Fischer, V, and Haffner-Luntzer, M. Interaction between bone and immune cells: implications for postmenopausal osteoporosis. Semin Cell Dev Biol. (2022) 123:14–21. doi: 10.1016/j.semcdb.2021.05.014
41. Ginaldi, L, Di Benedetto, MC, and De Martinis, M. Osteoporosis, inflammation and ageing. Immun Ageing A. (2005) 2:14. doi: 10.1186/1742-4933-2-14
42. Liu, Y-C, Yang, T-I, Huang, S-W, Kuo, Y-J, and Chen, Y-P. Associations of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio with osteoporosis: a Meta-analysis. Diagnostics. (2022) 12:2968. doi: 10.3390/diagnostics12122968
43. Wehmeyer, C, Pap, T, Buckley, CD, and Naylor, AJ. The role of stromal cells in inflammatory bone loss. Clin Exp Immunol. (2017) 189:1–11. doi: 10.1111/cei.12979
44. Ginaldi, L, and De Martinis, M. Osteoimmunology and beyond. Curr Med Chem. (2016) 23:3754–74. doi: 10.2174/0929867323666160907162546
45. Redlich, K, and Smolen, JS. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat Rev Drug Discov. (2012) 11:234–50. doi: 10.1038/nrd3669
46. Cui, Y, Guo, Y, Kong, L, Shi, J, Liu, P, Li, R, et al. A bone-targeted engineered exosome platform delivering si RNA to treat osteoporosis. Bioact Mater. (2021) 10:207–21. doi: 10.1016/j.bioactmat.2021.09.015
47. Chu, M, Luo, Y, Wang, D, Liu, Y, Wang, D, Wang, Y, et al. Systemic inflammation response index predicts 3-month outcome in patients with mild acute ischemic stroke receiving intravenous thrombolysis. Front Neurol. (2023) 14:1095668. doi: 10.3389/fneur.2023.1095668
48. Qu, L, Zuo, X, Yu, J, Duan, R, and Zhao, B. Association of inflammatory markers with all-cause mortality and cardiovascular mortality in postmenopausal women with osteoporosis or osteopenia. BMC Womens Health. (2023) 23:487. doi: 10.1186/s12905-023-02631-6
Keywords: systemic inflammatory response index, bone turnover markers, osteoporotic fractures, inflammation, osteoporosis
Citation: Zhou P, Lu K, Li C, Xu M-z, Ye Y-w, Shan H-q and Yin Y (2024) Association between systemic inflammatory response index and bone turnover markers in Chinese patients with osteoporotic fractures: a retrospective cross-sectional study. Front. Med. 11:1404152. doi: 10.3389/fmed.2024.1404152
Received: 20 March 2024; Accepted: 02 July 2024;
Published: 11 July 2024.
Edited by:
Meihong Xu, Peking University, ChinaCopyright © 2024 Zhou, Lu, Li, Xu, Ye, Shan and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Yin, eXktMTk3MjNAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
‡ORCID: Peng Zhou, orcid.org/0009-0006-7726-9114
Ke Lu, orcid.org/0000-0002-0029-7874
Chong Li, orcid.org/0000-0002-1526-221X
Min-zhe Xu, orcid.org/0000-0002-7094-2189
Yao-wei Ye, orcid.org/0000-0001-7405-2733
Hui-qiang Shan, orcid.org/0000-0003-2038-1755
Yi Yin, orcid.org/0000-0002-8385-4153
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.