- 1The 2nd Clinical Medical College of Jinan University, Shenzhen, China
- 2Shenzhen Eye Institute, Shenzhen Eye Hospital, Jinan University, Shenzhen, China
- 3Department of Ophthalmology, Jinzhou Medical University, Jinzhou, China
Background: Despite reports suggesting a link between obesity and keratoconus, the causal relationship is not fully understood.
Methods: We used genome-wide association study (GWAS) data from public databases for a two-sample Mendelian randomization analysis to investigate the causal link between body mass index (BMI) and keratoconus. The primary method was inverse variance weighted (IVW), complemented by different analytical techniques and sensitivity analyses to ensure result robustness. A meta-analysis was also performed to bolster the findings’ reliability.
Results: Our study identified a significant causal relationship between BMI and keratoconus. Out of 20 Mendelian randomization (MR) analyses conducted, 9 showed heterogeneity or pleiotropy. Among the 11 analyses that met all three MR assumptions, 4 demonstrated a significant causal difference between BMI and keratoconus, while the remaining 7 showed a positive trend but were not statistically significant. Meta-analysis confirmed a significant causal relationship between BMI and keratoconus.
Conclusion: There is a significant causal relationship between BMI and keratoconus, suggesting that obesity may be a risk factor for keratoconus.
1 Introduction
Keratoconus (KC) is a relatively common congenital developmental anomaly characterized by thinning of the central or paracentral cornea, increased curvature, bilateral asymmetry, and progressive expansion. It initially presents as myopia and irregular astigmatism, leading to vision loss in later stages due to corneal stromal scarring, protrusion, and thinning (1, 2). Globally, its prevalence is estimated to be between 1:375 and 1:2,000 individuals, with a significantly higher rate among younger populations (3). In the early stages, the progression of keratoconus can be slowed with collagen cross-linking, while later stages may require corneal transplantation to restore vision (3). Therefore, identifying other modifiable risk factors for keratoconus is crucial for its prevention and management.
However, the pathogenesis of keratoconus is still unclear, but it is generally considered a multifactorial disease influenced by environmental, genetic, metabolic, viral infection, lifestyle, and immune factors (4, 5).
Body mass index (BMI, kg/m2) is commonly used to measure obesity, with BMI ≥25 considered overweight and BMI ≥30 classified as obese (6). Previous studies have shown that an increase in BMI is associated with a variety of diseases. At a systemic level, higher BMI is linked to a greater risk of type 2 diabetes, cardiovascular diseases, and sleep apnea (7, 8). Locally, when focusing on the eyes, higher BMI is also significantly associated with age-related cataracts, age-related macular degeneration, and diabetic retinopathy (9, 10).
To date, the impact of high BMI on keratoconus remains unclear. Past reports have indicated that in Australian patients (11), a higher BMI was not associated with an increased risk of keratoconus; a recent study in Iran (12) found no significant difference in BMI between keratoconus patients and control subjects. However, recent findings suggest that a high BMI may be a risk factor for keratoconus. Research by Eliasi et al. (13) indicated that in the adolescent population, those who are overweight (OR = 1.42, p < 0.05) and obese (OR = 1.50, p < 0.05) have a higher likelihood of developing keratoconus; Ren et al. (14) suggested that individuals with a high BMI may be more prone to eye rubbing, thereby increasing the risk of KC. Clarifying the causal relationship between high BMI and keratoconus could enhance strategies for keratoconus detection and prevention. However, despite reports of a significant correlation between obesity and keratoconus, the causal link remains unclear due to the influence of confounding factors and the limited number of related studies.
Mendelian randomization (MR) is an emerging method (15) for testing causal relationships between risk factors and various phenotypes. The fundamental principle involves inferring the influence of biological factors on disease by utilizing the effects of genotypes, which are randomly distributed in nature, on phenotypes. Using genetic variants as instrumental variables can effectively reduce biases and confounding factors, thereby increasing the accuracy of causal inference.
To ascertain whether there is a causal relationship between obesity and keratoconus, this study intends to use the two-sample Mendelian randomization (MR) analysis approach to explore the causal link between BMI and keratoconus.
2 Workflow and study design
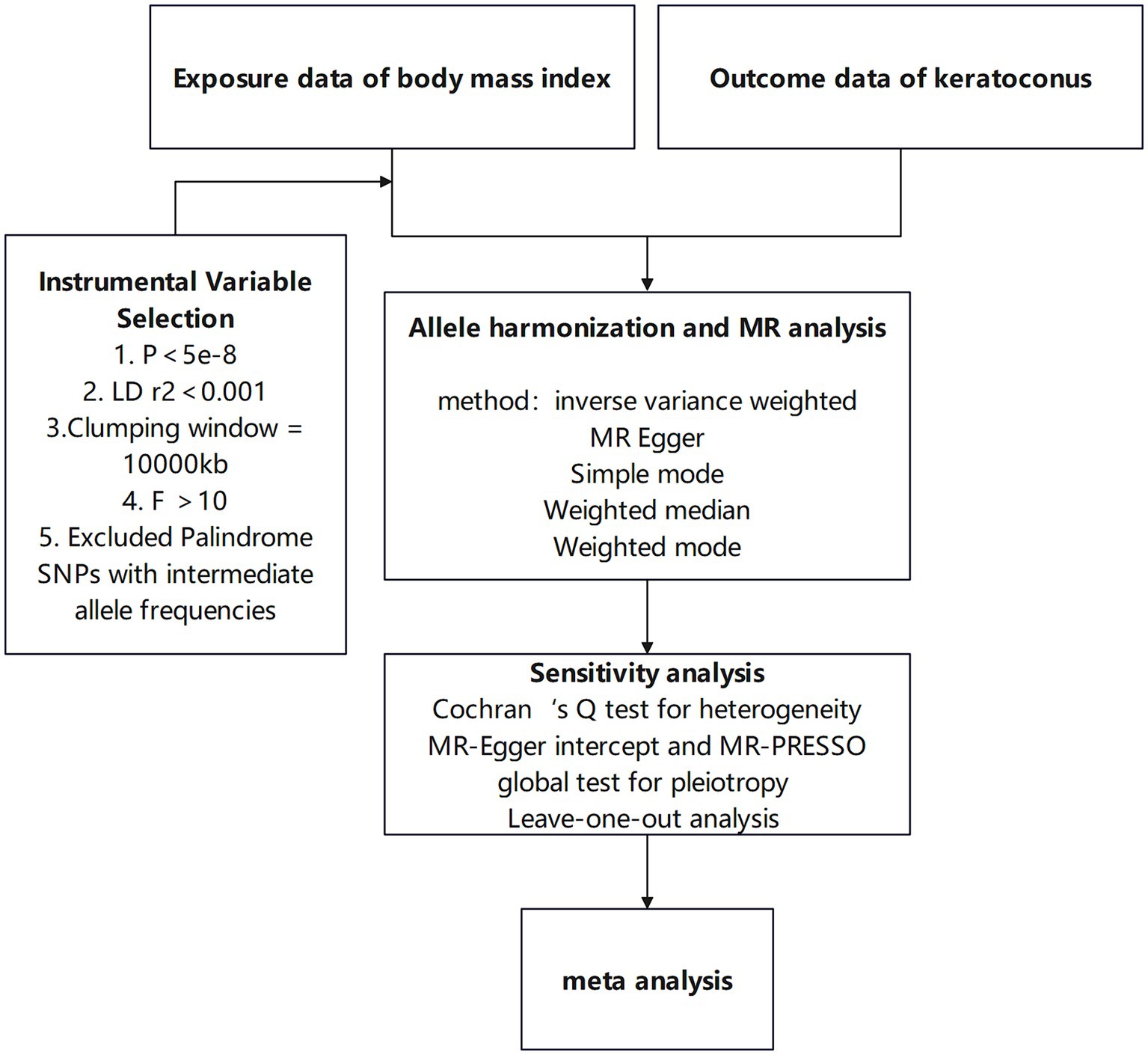
This study utilizes a two-sample Mendelian randomization approach to investigate the causal relationship between BMI and keratoconus, enhancing result robustness through heterogeneity tests and horizontal pleiotropy tests. Meta-analysis is subsequently used to further ensure the robustness of the findings. The specific workflow is shown in Figure 1. This research employs various BMI-associated single-nucleotide polymorphisms (SNPs) as instrumental variables to assess the causal relationship between BMI and keratoconus. MR analysis must satisfy the following three assumptions: first, the instrumental variables related to the exposure must be closely related to the exposure itself; second, the selected instrumental variables must not be related to any confounding factors between the exposure and outcome; and third, the instrumental variables can only influence the outcome through the exposure, meaning in this study, the chosen instrumental variables can only affect keratoconus through BMI. The study design is illustrated in Figure 2.
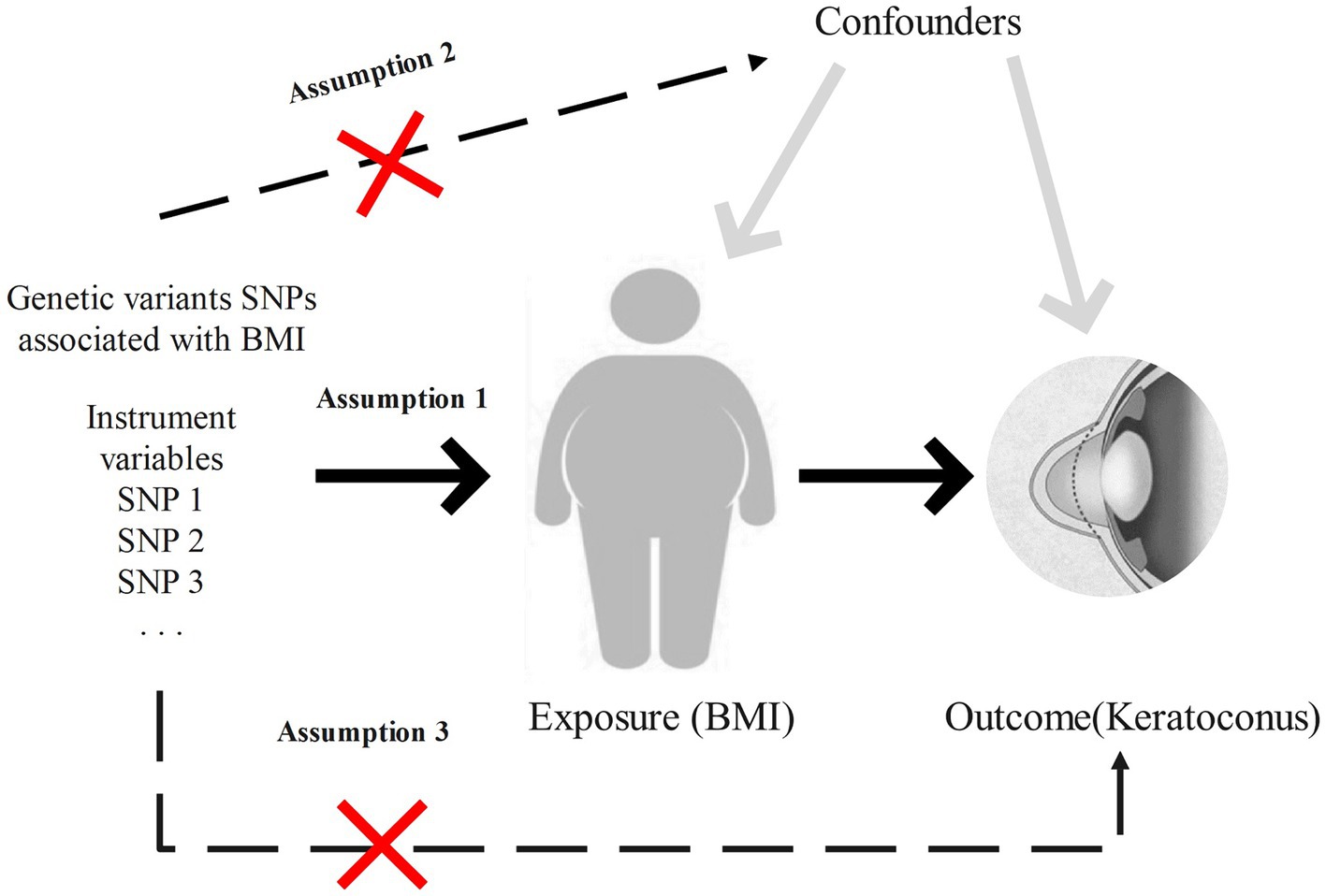
Figure 2. Study design and key assumptions of Mendelian randomization. Assumption 1: the instrumental variables are closely related to the exposure. Assumption 2: the instrumental variables are not associated with any confounding factors. Assumption 3: the instrumental variables, aside from exposure, are not directly related to keratoconus.
3 Materials and methods
3.1 Data sources
GWAS data related to keratoconus were sourced from the GWAS Catalog1 and Finngen R9.2 The dataset from the GWAS Catalog was obtained from a large-scale GWAS, for which we used the first-phase meta-analysis, including 2,116 cases and 24,626 European ancestry controls. The GWAS data from Finngen R9 included 311 cases and 209,287 European ancestry controls.
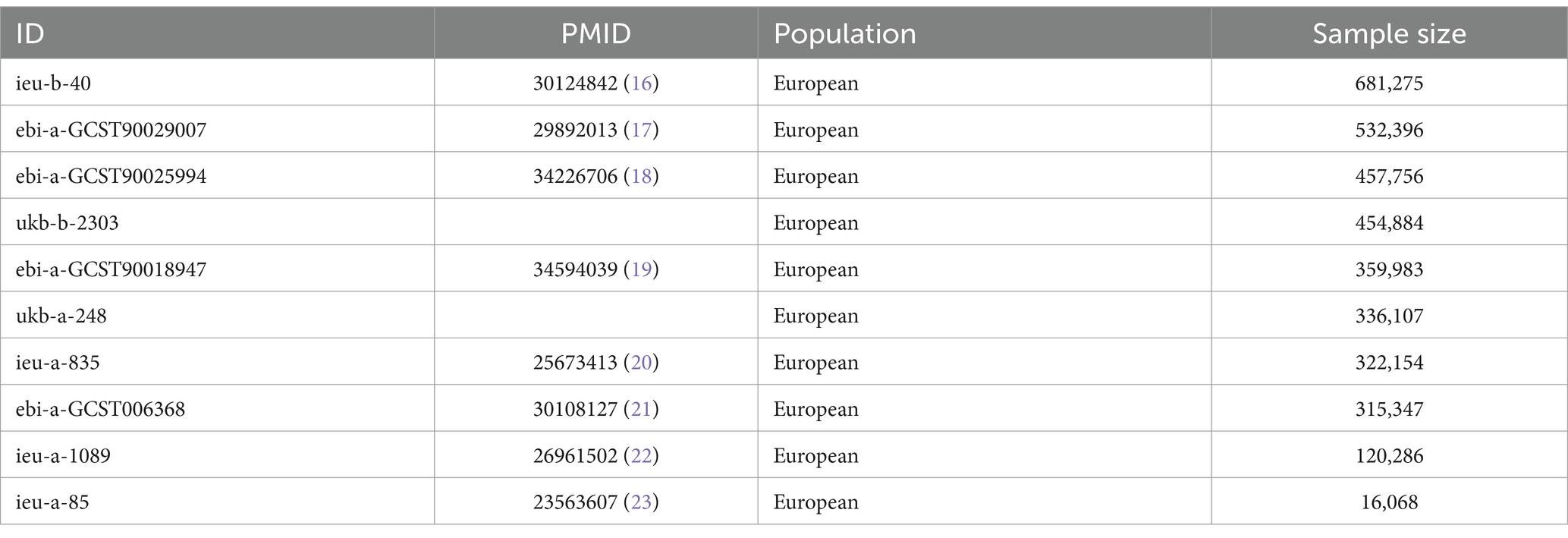
To acquire as much BMI-related GWAS data as possible, this study searched Open GWAS3 using “body mass index” as the search term, obtaining all BMI-related GWAS data for European populations. After eliminating duplicate studies, 10 BMI-related GWAS datasets were used for further research, with GWAS IDs being ebi-a-GCST006368, ebi-a-GCST90018947, ebi-a-GCST90025994, ebi-a-GCST90029007, ieu-a-85, ieu-a-835, ieu-a-1089, ieu-b-40, ukb-a-248, and ukb-b-2303. Detailed information about all the BMI-related GWAS datasets is presented in Table 1.
3.2 Instrumental variable selection
To select instrumental variables highly related to BMI, we filtered SNPs based on a standard of p < 5 × 10−8, relaxing the criterion to p < 5 × 10−6 if fewer than 5 SNPs were selected. To ensure the independence between SNPs. We removed linkage disequilibrium based on an R2 < 0.001 and distance of Kb < 10,000. Furthermore, weak instrumental variables were eliminated if they had an F value (F = (βexposure/SEexposure)2, where βexposure and SEexposure are the effect value and standard error of the exposure, respectively) <10. Finally, all palindromic SNPs with intermediate allele frequencies were excluded.
3.3 Mendelian randomization analysis
This study employed a two-sample Mendelian randomization approach to explore the causal relationship between BMI and keratoconus, with weighted median as the primary analysis method, supplemented by MR Egger, simple mode, weighted median, and weighted mode as auxiliary analysis methods. Cochran’s Q test was used to assess the heterogeneity of the instrumental variables, with p > 0.05 indicating no heterogeneity; MR PRESSO and MR Egger’s intercept tests were used to determine the presence of horizontal pleiotropy, with p > 0.05 suggesting no pleiotropy, among them, MRPRESSO can detect the residual validity and outliers of MR analysis, which can correct horizontal pleiotropy by removing outliers and further evaluate the robustness of causal relationships. Additionally, leave-one-out sensitivity analysis was conducted to determine if the results were driven by a single strong SNP.
3.4 Meta-analysis
This study incorporated results that met the three fundamental assumptions of MR analysis into meta-analysis to ensure the robustness of the findings. Before conducting meta-analysis, heterogeneity testing was performed on all results. If significant heterogeneity was observed among the results (I2 > 50%), a random-effects model was used; otherwise, a fixed-effects model was employed.
3.5 Data analysis
All analyses were conducted using R 4.3.1 and relevant R packages including “TwoSampleMR (0.5.7),” “MRPRESSO (1.0),” and “metafor.”
4 Result
4.1 Obtaining instrumental variables
After stringent selection and removal of linkage disequilibrium, this study acquired 10 sets of SNPs significantly associated with BMI, with the number of SNPs per set ranging from 7 to 475. Detailed data on the instrumental variables used for MR analysis in each dataset can be found in Supplementary material S1.
4.2 Mendelian randomization analysis
This study conducted a total of 20 MR analyses (10 for BMI X 2 for KC), and after excluding 9 results with heterogeneity and horizontal pleiotropy, 11 were considered to have neither. Detailed results of Cochran’s Q test, MR PRESSO, and MR Egger’s intercept test can be found in Supplementary material S2. Interestingly, among all results, 4 indicated a significant causal relationship between BMI and KC, with outcome data for these 4 results all coming from the FinnGen R9 database.
Specifically, when the exposure data came from a GWAS by Hoffmann et al. (21) involving 315,347 individuals of European ancestry, the OR (IVW) was 1.69, 95% CI was 1.03 to 2.76, p = 0.037. Although not statistically significant, the OR values for MR Egger, simple mode, weighted mode, and weighted median were all >1, consistent with the IVW method. When the exposure data was from Loh et al. (17) involving 532,396 individuals of European ancestry, the OR (IVW) was 1.53, 95% CI was 1.08 to 2.16, p = 0.016, with OR values for the other four auxiliary methods also >1. In the MR analysis of data from Yengo et al. (16) involving 681,275 individuals, the OR (IVW) was 1.68, 95% CI was 1.17 to 2.39, p = 0.005, even though the other four methods did not show statistically significant results. In the MR analysis of data from Ben Elsworth et al. involving 454,884 individuals of European ancestry, the OR (IVW) was 1.83, 95% CI was 1.24 to 2.59, p = 0.001, with the other four methods not showing statistically significant results, but again, all OR values were >1, consistent with the IVW method trend. Scatter plots for all results are shown in Figure 3. Leave-one-out sensitivity analysis results are presented in Figure 4.
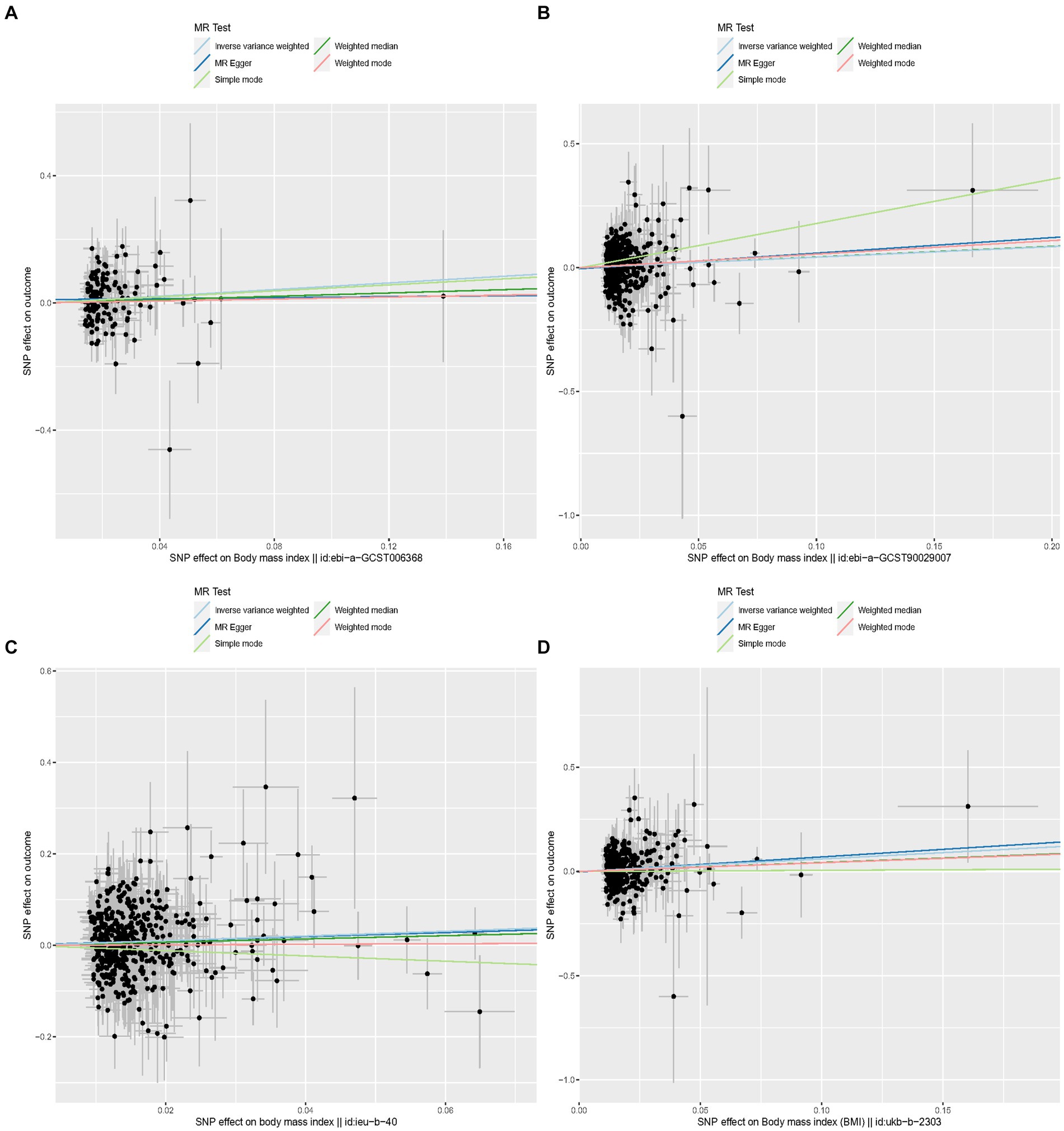
Figure 3. Scatter plots for MR analyses of the causal effect of proinsulin levels on keratoconus. Each line shows the slope corresponding to the estimated MR effect each method. (A) The ID number of the GWAS data related to BMI is ebi-a-GCST006368. (B) The ID number of the GWAS data related to BMI is ebi-a-GCST90029007. (C) The ID number of the GWAS data related to BMI is ieu-b-40. (D) The ID number of the GWAS data related to BMI is ukb-b-2303.
Figure 4. Leave-one-out sensitivity analysis shows that the results are not caused by a strong single SNP. (A) The ID number of the GWAS data related to BMI is ebi-a-GCST006368. (B) The ID number of the GWAS data related to BMI is ebi-a-GCST90029007. (C) The ID number of the GWAS data related to BMI is ieu-b-40. (D) The ID number of the GWAS data related to BMI is ukb-b-2303.
4.3 Meta analysis
This study included these 11 results in a meta-analysis. Heterogeneity testing revealed no heterogeneity among all results, thus a fixed-effects model was adopted for the meta-analysis. The results showed that BMI is a risk factor for keratoconus. IVW: OR = 1.38, 95% CI (1.238–1.54), p < 0.0001 (Figure 5A); MR Egger: OR = 1.40, 95% CI (1.06–1.84), p = 0.0172 (Figure 5B); simple mode: OR = 1.20, 95% CI (0.88–1.63), p = 0.2415 (Figure 5C); weighted mode: OR = 1.23, 95% CI (0.98–1.54), p = 0.076 (Figure 5D); weighted median: OR = 1.26, 95% CI (1.07–1.48), p = 0.0054 (Figure 5E). Detailed results of heterogeneity testing for the meta-analysis are shown in Figure 5F. Furthermore, the funnel plots can be found in Supplementary Figures S1–S4.
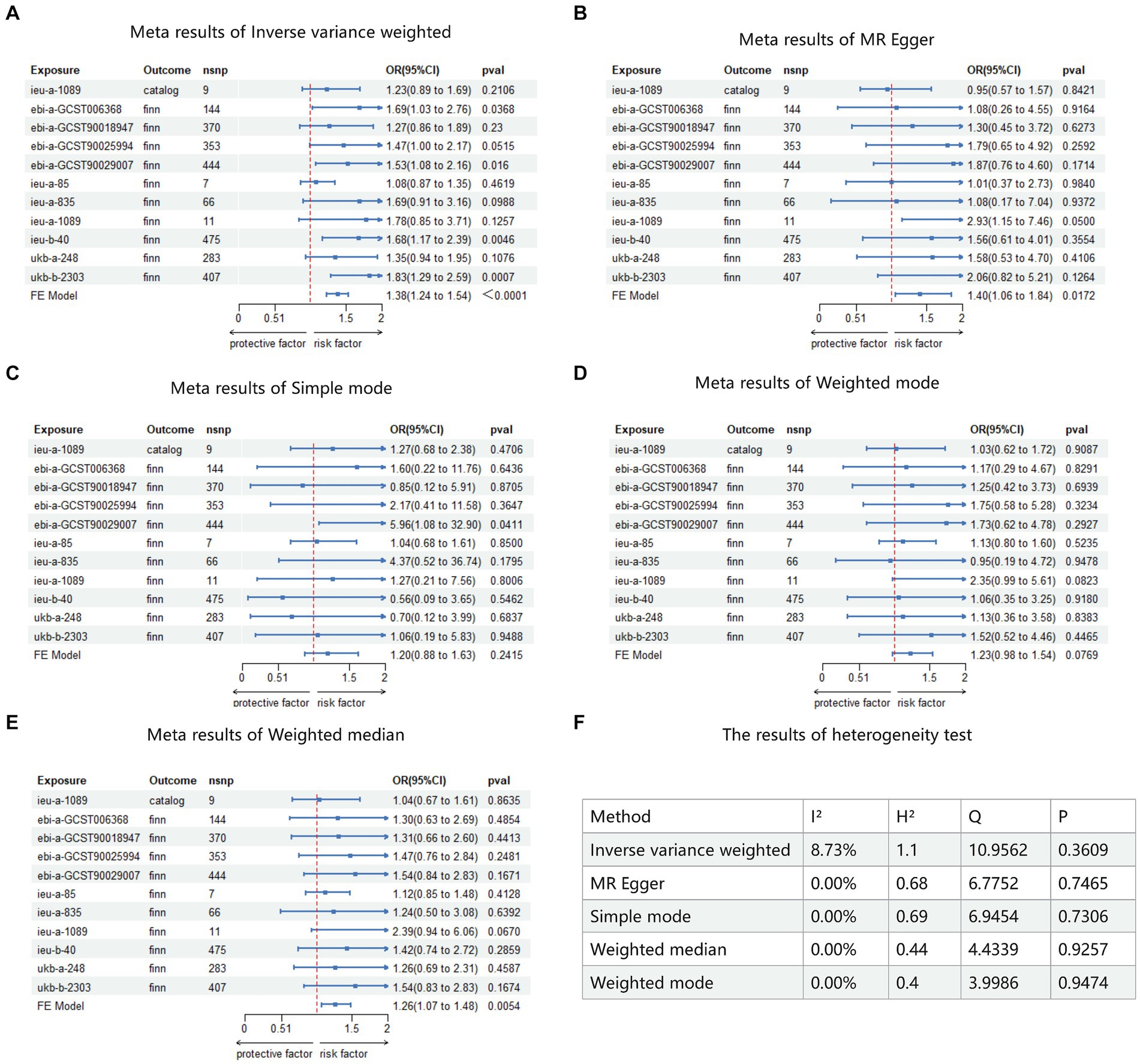
Figure 5. Meta analysis result of MR. Catalog: GWAS data for KC sourced from GWAS Catalog. Finn: GWAS data for KC sourced from FinnGen R9. FE model: fixed-effects model used for meta-analysis. (A) Meta analysis result of inverse variance weighted. (B) Meta analysis result of MR Egger. (C) Meta analysis result of simple mode. (D) Meta analysis result of weighted mode. (E) Meta analysis result of weighted median. (F) The result of heterogeneity test.
5 Discussion
Given the low natural incidence rate and lengthy disease course of keratoconus (KC), it is challenging to conduct prospective studies on the causal relationship between keratoconus and BMI in the real world. Retrospective studies are susceptible to confounding factors and reverse causality, which can render the results unreliable. Therefore, this study employs genetic variants related to BMI as instrumental variables, utilizing “natural random allocation” to assess whether these genes are associated with the risk of keratoconus. This approach helps to eliminate the influence of confounding factors and reverse causality that may exist in observational studies, providing more reliable evidence of causality.
In this study, the results of 11 Mendelian randomization (MR) analyses appeared inconsistent, possibly due to differences in sequencing results and study populations. However, the meta-analysis ultimately confirmed BMI as a risk factor for KC, with an ORIVW of 1.38, 95% CI (1.24–1.54), p < 0.001. This means for each unit increase in BMI, the risk of developing keratoconus increases by 38%. This suggests a strong causal relationship between higher BMI values and keratoconus, implying that individuals with obesity are at a higher risk of developing KC.
Although the pathophysiological mechanisms by which obesity causes keratoconus are not yet clear, past research has proposed several potential mechanisms.
Inflammatory responses may be a significant cause of keratoconus induced by obesity. Obesity is a systemic chronic metabolic inflammation (24), with upregulation of inflammatory markers such as tumor necrosis factor (TNF-α), interleukins (IL)-6, IL-1β, and CCL2 in the adipose tissue of obese individuals (24, 25). Additionally, organs such as the liver, pancreas, brain, and muscles experience increased exposure to inflammation in the state of obesity, leading to the development of various diseases (26). This chronic inflammation persists over time, and when such “inflammation” occurs in the eye, it can lead to changes in ocular inflammation, resulting in chronic damage to the ocular surface (25). Traditionally, keratoconus (KC) has been viewed as a non-inflammatory disease (27). However, with more in-depth research in recent years, a close association between the onset of KC and inflammation has been discovered (28). Previous studies have confirmed an increased expression of various inflammatory markers such as interleukins, metalloproteinases, and tumor necrosis factors in the corneal tissues and tear samples of patients with keratoconus (29–31). Interleukin 1β (IL-1β) and tumor necrosis factor α (TNF-α) have been shown to reduce the synthesis of collagen, induce apoptosis in corneal cells, and increase the activity of matrix metalloproteinases (MMPs) (31). Interestingly, the expression of MMPs in obese individuals is significantly higher compared to that in normal individuals. Mazor et al. (32) observed an increased expression and activity of MMP-2 in the brain tissues of obese patients, which in turn can reflexively promote obesity. MMPs, including MMP-1, MMP-3, MMP-7, and MMP-13, are enzymes secreted in response to cytokines and growth factors, responsible for the degradation of extracellular matrix proteins (31). In patients with keratoconus (KC), an increase in the proteolytic activity and expression levels of various matrix metalloproteinases (MMPs) and cytokines has been observed compared to healthy controls (29). Compared to healthy individuals and those with other corneal diseases, MMP levels are elevated, and levels of protease inhibitors are reduced in keratoconus specimens (33, 34), which may be a significant factor in the development of KC due to obesity. Furthermore, Dou et al. (35) have discovered through single-cell sequencing that inflammatory markers such as interleukins, metalloproteinases, and chemokines (e.g., CXCL1) are elevated in keratoconus tissue, further confirming the association between the onset of keratoconus and chronic inflammatory responses. Thus, chronic inflammation might be the underlying connection between obesity and the development of keratoconus.
Increased frequency of eye rubbing may be a key factor in the development of keratoconus due to obesity. Previous research indicates that eye rubbing can alter the mechanical stress on the cornea, leading to increased friction between the cornea and the conjunctiva, which causes remodeling of the corneal stromal microstructure and reduces its mechanical stability (36). Research by Dou et al. (35) found that the expression of biomechanical homeostasis regulators, including YAP1 and TEAD1, is elevated in the corneal group of patients with keratoconus, suggesting a biomechanical homeostasis imbalance in the corneal tissues of these patients. Moreover, an increased frequency of eye rubbing can promote the expression of various protease genes in the corneal matrix, including MMP1, MMP3, CTSD, CTSK, etc. (35, 37). This indicates that eye rubbing can not only change the mechanical stress on the cornea but also trigger biochemical reactions, causing an imbalance in corneal matrix protein dissolution and inducing the occurrence of keratoconus.
Recent reports have indicated that high BMI values can lead to damage to corneal epithelial nerves and an increase in dendritic cells in the cornea (38), suggesting that obesity can not only directly cause changes in corneal nerve structures and inflammatory states but also lead to eye discomfort, increase the frequency of eye rubbing in patients, and raise the risk of developing keratoconus. Additionally, it has been reported that obese patients are more prone to eyelid laxity and dermatochalasis compared to healthy individuals, which increases mechanical stress on the cornea from the eyelids, thereby increasing the risk of keratoconus development (13).
Despite recent studies focusing on the relationship between obesity and keratoconus, their findings have not fully ruled out other confounding factors and selection biases. Mendelian randomization (MR) analysis, however, akin to randomized controlled trials, is less susceptible to biases arising from confounding, selection biases, and reverse causality, thereby minimizing the limitations of previous research. Nevertheless, this study still has certain limitations. Firstly, although genetic predictions suggest that high BMI may be one of the risk factors for keratoconus, this study did not provide a detailed explanation of the pathological and physiological mechanisms involved. Secondly, as the GWAS sequencing data for keratoconus predominantly stem from individuals of European ancestry, the MR analysis in this study is limited to European populations, hence the results may not be fully generalizable to other ethnicities.
This study identified a significant causal relationship between high BMI, indicative of obesity, and keratoconus in the European population using Mendelian randomization (MR) methodology, and demonstrated the robustness of this association through various sensitivity analysis methods. Further meta-analysis validated the study findings, further strengthening the causal relationship between obesity and keratoconus. The findings of this study suggest that obesity may be a risk factor for keratoconus in the European population. In clinical management activities targeting obese patients, greater attention should be paid to the potential impact of obesity on ocular health. Furthermore, future research efforts are anticipated to involve more comprehensive whole-genome sequencing data and large-scale multicenter studies to validate the biological mechanisms underlying the association between obesity and keratoconus, elucidating the pathogenesis of keratoconus in greater detail for improved prevention and treatment of both conditions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for the studies involving humans because all data obtained from publicly available databases. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements because all data obtained from publicly available databases.
Author contributions
JW: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. FL: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. JM: Formal analysis, Writing – original draft. DG: Software, Writing – original draft. FZ: Data curation, Writing – original draft. JS: Data curation, Visualization, Writing – review & editing. SD: Data curation, Writing – original draft. WY: Supervision, Writing – review & editing. PG: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Shenzhen Science and Technology Program (JCYJ20230807114605011).
Acknowledgments
Thanks to Ming Li (MD, Shenzhen Eye Hospital, Jinan University, Shenzhen Eye Institute, Shenzhen, 518040, China) for his guidance on this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1402108/full#supplementary-material
Footnotes
References
1. Meyer, JJ, Gokul, A, Vellara, HR, and McGhee, CNJ. Progression of keratoconus in children and adolescents. Br J Ophthalmol. (2023) 107:176–80. doi: 10.1136/bjophthalmol-2020-316481
2. Jones-Jordan, LA, Walline, JJ, Sinnott, LT, Kymes, SM, and Zadnik, K. Asymmetry in keratoconus and vision-related quality of life. Cornea. (2013) 32:267–72. doi: 10.1097/ICO.0b013e31825697c4
3. Deshmukh, R, Ong, ZZ, Rampat, R, Alió del Barrio, JL, Barua, A, Ang, M, et al. Management of keratoconus: an updated review. Front Med. (2023) 10:1212314. doi: 10.3389/fmed.2023.1212314
4. Song, M, Fang, QY, Seth, I, Baird, PN, Daniell, MD, and Sahebjada, S. Non-genetic risk factors for keratoconus. Clin Exp Optom. (2023) 106:362–72. doi: 10.1080/08164622.2022.2062222
5. Chen, S, Li, XY, Jin, JJ, Shen, RJ, Mao, JY, Cheng, FF, et al. Genetic screening revealed latent keratoconus in asymptomatic individuals. Front Cell Dev Biol. (2021) 9:650344. doi: 10.3389/fcell.2021.650344
6. Garrow, JS . Three limitations of body mass index. Am J Clin Nutr. (1988) 47:553. doi: 10.1093/ajcn/47.3.553
7. Piche, ME, Tchernof, A, and Despres, JP. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res. (2020) 126:1477–500. doi: 10.1161/CIRCRESAHA.120.316101
8. Badran, M, and Joseph, V. Sleep apnea and diet-induced obesity-the female advantage on the spotlight. Sleep. (2023) 46:zsad174. doi: 10.1093/sleep/zsad174
9. Niazi, S, Moshirfar, M, Dastjerdi, MH, Niazi, F, Doroodgar, F, and Ambrósio, R Jr. Association between obesity and age-related cataract: an updated systematic review and dose-response meta-analysis of prospective cohort studies. Front Nutr. (2023) 10:1215212. doi: 10.3389/fnut.2023.1215212
10. Zhang, QY, Tie, LJ, Wu, SS, Lv, PL, Huang, HW, Wang, WQ, et al. Overweight, obesity, and risk of age-related macular degeneration. Invest Ophthalmol Vis Sci. (2016) 57:1276–83. doi: 10.1167/iovs.15-18637
11. Pihlblad, MS, and Schaefer, DP. Eyelid laxity, obesity, and obstructive sleep apnea in keratoconus. Cornea. (2013) 32:1232–6. doi: 10.1097/ICO.0b013e318281e755
12. Mohaghegh, S, Kangari, H, Masoumi, SJ, Bamdad, S, Rahmani, S, Abdi, S, et al. Prevalence and risk factors of keratoconus (including oxidative stress biomarkers) in a cohort study of shiraz university of medical science employees in Iran. BMC Ophthalmol. (2023) 23:188. doi: 10.1186/s12886-023-02934-0
13. Eliasi, E, Bez, M, Megreli, J, Avramovich, E, Fischer, N, Barak, A, et al. The association between keratoconus and body mass index: a population-based cross-sectional study among half a million adolescents. Am J Ophthalmol. (2021) 224:200–6. doi: 10.1016/j.ajo.2020.11.021
14. Ren, S, Tu, R, Xu, L, Gu, Y, Fan, Q, Wang, Q, et al. A high body mass index strengthens the association between the time of eye rubbing and keratoconus in a Chinese population: a case control study. BMC Public Health. (2023) 23:2032. doi: 10.1186/s12889-023-16937-5
15. Wang, H, Zhang, Z, Zou, L, Zhang, J, Jia, Z, Zhao, L, et al. Peripheral artery disease mediating the effect of metabolic syndrome related diseases on lower limb ulcers: Mendelian randomization analysis. Front Endocrinol. (2024) 15:1345605. doi: 10.3389/fendo.2024.1345605
16. Yengo, L, Sidorenko, J, Kemper, KE, Zheng, Z, Wood, AR, Weedon, MN, et al. Meta-analysis of genome-wide association studies for height and body mass index in approximately 700000 individuals of European ancestry. Hum Mol Genet. (2018) 27:3641–9. doi: 10.1093/hmg/ddy271
17. Loh, PR, Kichaev, G, Gazal, S, Schoech, AP, and Price, AL. Mixed-model association for biobank-scale datasets. Nat Genet. (2018) 50:906–8. doi: 10.1038/s41588-018-0144-6
18. Barton, AR, Sherman, MA, Mukamel, RE, and Loh, PR. Whole-exome imputation within UK Biobank powers rare coding variant association and fine-mapping analyses. Nat Genet. (2021) 53:1260–9. doi: 10.1038/s41588-021-00892-1
19. Sakaue, S, Kanai, M, Tanigawa, Y, Karjalainen, J, Kurki, M, Koshiba, S, et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat Genet. (2021) 53:1415–24. doi: 10.1038/s41588-021-00931-x
20. Locke, AE, and Kahali, B. Genetic studies of body mass index yield new insights for obesity biology. Nature. (2015) 518:197–206. doi: 10.1038/nature14177
21. Hoffmann, TJ, Choquet, H, Yin, J, Banda, Y, Kvale, MN, Glymour, M, et al. A large multiethnic genome-wide association study of adult body mass index identifies novel loci. Genetics. (2018) 210:499–515. doi: 10.1534/genetics.118.301479
22. Wood, AR, Tyrrell, J, Beaumont, R, Jones, SE, Tuke, MA, Ruth, KS, et al. Variants in the FTO and CDKAL1 loci have recessive effects on risk of obesity and type 2 diabetes, respectively. Diabetologia. (2016) 59:1214–21. doi: 10.1007/s00125-016-3908-5
23. Berndt, SI, Gustafsson, S, Mägi, R, Ganna, A, Wheeler, E, Feitosa, MF, et al. Genome-wide meta-analysis identifies 11 new loci for anthropometric traits and provides insights into genetic architecture. Nat Genet. (2013) 45:501–12. doi: 10.1038/ng.2606
24. Cox, AJ, West, NP, and Cripps, AW. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. (2015) 3:207–15. doi: 10.1016/S2213-8587(14)70134-2
25. Gregor, MF, and Hotamisligil, GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. (2011) 29:415–45. doi: 10.1146/annurev-immunol-031210-101322
26. Rohm, TV, Meier, DT, Olefsky, JM, and Donath, MY. Inflammation in obesity, diabetes, and related disorders. Immunity. (2022) 55:31–55. doi: 10.1016/j.immuni.2021.12.013
27. Krachmer, JH, Feder, RS, and Belin, MW. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. (1984) 28:293–322. doi: 10.1016/0039-6257(84)90094-8
28. di Martino, E, Ali, M, and Inglehearn, CF. Matrix metalloproteinases in keratoconus—too much of a good thing? Exp Eye Res. (2019) 182:137–43. doi: 10.1016/j.exer.2019.03.016
29. Balasubramanian, SA, Mohan, S, Pye, DC, and Willcox, MD. Proteases, proteolysis and inflammatory molecules in the tears of people with keratoconus. Acta Ophthalmol. (2012) 90:e303–9. doi: 10.1111/j.1755-3768.2011.02369.x
30. Lema, I, Sobrino, T, Duran, JA, Brea, D, and Diez-Feijoo, E. Subclinical keratoconus and inflammatory molecules from tears. Br J Ophthalmol. (2009) 93:820–4. doi: 10.1136/bjo.2008.144253
31. McMonnies, CW . Inflammation and keratoconus. Optom Vis Sci. (2015) 92:e35–41. doi: 10.1097/OPX.0000000000000455
32. Mazor, R, Friedmann-Morvinski, D, Alsaigh, T, Kleifeld, O, Kistler, EB, Rousso-Noori, L, et al. Cleavage of the leptin receptor by matrix metalloproteinase-2 promotes leptin resistance and obesity in mice. Sci Transl Med. (2018) 10:eaah6324. doi: 10.1126/scitranslmed.aah6324
33. Sawaguchi, S, Yue, BY, Sugar, J, and Gilboy, JE. Lysosomal enzyme abnormalities in keratoconus. Arch Ophthalmol. (1989) 107:1507–10. doi: 10.1001/archopht.1989.01070020581044
34. Shetty, R, Ghosh, A, Lim, RR, Subramani, M, Mihir, K, Reshma, AR, et al. Elevated expression of matrix metalloproteinase-9 and inflammatory cytokines in keratoconus patients is inhibited by cyclosporine a. Invest Ophthalmol Vis Sci. (2015) 56:738–50. doi: 10.1167/iovs.14-14831
35. Dou, S, Wang, Q, Zhang, B, Wei, C, Wang, H, Liu, T, et al. Single-cell atlas of keratoconus corneas revealed aberrant transcriptional signatures and implicated mechanical stretch as a trigger for keratoconus pathogenesis. Cell Discov. (2022) 8:66. doi: 10.1038/s41421-022-00397-z
36. McMonnies, CW, Korb, DR, and Blackie, CA. The role of heat in rubbing and massage-related corneal deformation. Cont Lens Anterior Eye. (2012) 35:148–54. doi: 10.1016/j.clae.2012.01.001
37. Balasubramanian, SA, Pye, DC, and Willcox, MD. Effects of eye rubbing on the levels of protease, protease activity and cytokines in tears: relevance in keratoconus. Clin Exp Optom. (2013) 96:214–8. doi: 10.1111/cxo.12038
Keywords: keratoconus, BMI, obesity, Mendelian randomization, inflammatory factors
Citation: Wang J, Liu F, Mo J, Gong D, Zheng F, Su J, Ding S, Yang W and Guo P (2024) Exploring the causal relationship between body mass index and keratoconus: a Mendelian randomization study. Front. Med. 11:1402108. doi: 10.3389/fmed.2024.1402108
Edited by:
Majid Moshirfar, University of Utah, United StatesCopyright © 2024 Wang, Liu, Mo, Gong, Zheng, Su, Ding, Yang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weihua Yang, YmVuYmVuMDYwNkAxMzkuY29t; Ping Guo, MjYwNzIxMjg1OEBxcS5jb20=
†These authors have contributed equally to this work
 Jiaoman Wang
Jiaoman Wang Fangyuan Liu1
†
Fangyuan Liu1
† Di Gong
Di Gong Weihua Yang
Weihua Yang