
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 15 July 2024
Sec. Hepatobiliary Diseases
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1401900
This article is part of the Research Topic Treatment and Prognostic Assessment of Liver Cirrhosis and Its Complications, Volume II View all 21 articles
 Jun Tie1*†
Jun Tie1*† Xulong Yuan1†
Xulong Yuan1† Ying Zhu1
Ying Zhu1 Kai Li1
Kai Li1 Xiaoyuan Gou1
Xiaoyuan Gou1 Na Han1
Na Han1 Jing Niu1
Jing Niu1 Jiao Xu1
Jiao Xu1 Wenlan Wang2
Wenlan Wang2 Yongquan Shi1*
Yongquan Shi1*Objectives: Nonselective beta blockers (NSBBs) or endoscopic therapies are currently recommended by guidelines for preventing the first variceal bleed in patients with high-risk varices. However, there is a lack of detailed treatment strategies for patients who are intolerant to both NSBBs and endoscopic approaches. Our study aimed to assess the efficacy and safety of variceal embolization as a primary prophylaxis method in cirrhosis patients who are not suitable candidates for NSBBs or endoscopic treatments.
Methods: The study included 43 cirrhotic patients with high-risk varices who were candidates for primary prophylaxis against variceal bleeding. These patients underwent variceal embolization at the Xijing Hospital between January 2020 and June 2022. The primary endpoint was the occurrence of bleeding from varices, and the secondary endpoints were the recurrence of varices and the emergence of complications.
Results: The procedure of variceal embolization had a success rate of 93.0% (40 out of 43 patients). Over a 2-year follow-up period, the rate of variceal bleeding was 11.6% (5 out of 43 patients), the recurrence rate of varices was 14.0% (6 out of 43 patients), and the rate of severe complications was limited to 2.3% (1 out of 43 patients).
Conclusion: Variceal embolization is a viable primary prophylactic intervention for cirrhotic patients who are at risk of variceal bleeding when standard treatments, such as NSBBs or endoscopic therapies, are difficult to perform.
Variceal bleeding is a serious complication of portal hypertension secondary to cirrhosis. At the point of diagnosis, approximately 50% of patients with cirrhosis are found to have esophageal and/or gastric varices (EGVs). Patients without EGV at diagnosis develop varices at an annual rate of approximately 8%, and patients with small EGV pose a 22% annual risk of progression to medium or large varices. Additionally, 10–15% of patients with EGV experience their initial variceal bleeding each year, and the mortality rate for the first episode of variceal bleeding is high, with approximately 15% of patients dying from this initial bleeding (1, 2). Therefore, primary prophylaxis to prevent the first episode of variceal bleeding is essential in the management of cirrhosis.
Current guidelines recommend non-selective beta-blockers (NSBBs) as the first-line treatment for preventing the first variceal hemorrhage in patients with high-risk EGV. If NSBBs are contraindicated or not tolerated, endoscopic band ligation (EBL) is the preferred alternative for esophageal varices (EVs). For high-risk GVs, endoscopic cyanoacrylate injection (ECI) is recommended when NSBBs are not suitable (3–6). However, the guidelines currently lack detailed treatment strategies for patients who are unable to tolerate both NSBBs and endoscopic procedures.
Variceal embolization encompasses a range of interventional procedures aimed at occluding varicose veins. This approach is minimally invasive, technically straightforward, and has been confirmed to have efficacy and safety in both acute variceal bleeding treatment and secondary prophylaxis (7–9). Notably, in the treatment of GV, embolization with cyanoacrylate is superior to ECI (10). Given the efficacy and safety profile of variceal embolization, we hypothesize that it could also be a viable option for primary prophylaxis against variceal bleeding. This study aims to assess the effectiveness and safety of variceal embolization as a primary prophylactic intervention in cirrhosis patients who are not candidates for NSBBs and endoscopic treatments.
This was a single-arm retrospective observational study. It consecutively enrolled patients with cirrhosis and high-risk EGV who were admitted to Xijing Hospital from January 2020 to June 2022. Follow-up for the last patient extended beyond 6 months. Written informed consent was obtained from all patients for the use of their data in this research. The study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Air Force Medical University (approval number: KY20232008-C-1).
The inclusion criteria were as follows: (1) patients who are aged between 18 and 75 years; (2) patients with cirrhosis diagnosed by biopsy or a combination of medical history, etiology, physical examination, clinical presentation, biochemical testing, and imaging; (3) patients with high-risk EGV confirmed by endoscopic examination; (4) patients difficult to be treated with NSBBs and EBL/ECI as evaluated by two endoscopists; and (5) patients receiving variceal embolization with cyanoacrylate and/or coil for the prevention of EGV bleed. The exclusion criteria were as follows: patients with (1) a history of variceal bleeding; (2) hepatocellular carcinoma (HCC) beyond the Milan criteria; (3) malignant tumors excluding HCC; (4) main portal vein thrombus of over 50% or cavernous transformation of the portal vein; (5) recurrent or refractory ascites; (6) end-stage renal disease with renal replacement treatment; (7) cardiorespiratory failure; (8) human immunodeficiency virus infection or acquired immune deficiency syndrome; or (9) lack of baseline data and/or follow-up data.
The following data were collected for all patients: (1) baseline data including age, sex, etiology of liver disease, liver function, variceal type, and enhanced CT/MRI; (2) treatment information including access to the operation, embolization details, and operation-related complications; and (3) follow-up data including main symptoms, variceal bleeding, blood biochemistry, the changes of varices, and overall survival.
Endoscopic evaluations of varices were performed at 1, 3, and 6 months after the initial procedure and subsequently at 6-month intervals to monitor variceal changes. The study was concluded for each patient either upon the occurrence of an endpoint event, which was either variceal bleeding or recurrence, or after a minimum follow-up duration of 6 months post-embolization.
Our study utilized two approaches to variceal embolization: percutaneous transhepatic varices embolization (PTVE) and transjugular varices embolization (TJVE). PTVE involves accessing the varices via a percutaneous transhepatic route, known for its ease of operation. TJVE, alternatively, uses a transjugular intrahepatic route, which is preferred in patients with severe thrombocytopenia or significant ascites due to its reduced risk of abdominal hemorrhage. The criteria for selecting the approach were based on platelet counts and the presence of ascites: PTVE for patients with platelet counts above 50 × 103/μL without ascites and TJVE for those with counts below 50× 103/μL or with significant ascites.
The procedures were performed by Dr. Tie Jun, who possesses extensive experience with over a decade in conducting transjugular intrahepatic portosystemic shunt (TIPS) operations and has a professional record of conducting over 1,000 procedures. The TJVE procedure was conducted as follows: Initially, the femoral artery was accessed using the Seldinger technique, and then the superior mesenteric artery was selectively intubated for indirect portal vein angiography. Following this, the internal jugular vein was punctured, and a 0.035-inch guide wire (Terumo, Tokyo, Japan) was introduced through it into the inferior vena cava. Over this guide wire, the RUPS 100 puncture set (Cook, Chicago, United States) was advanced into the inferior vena cava. Subsequently, a portal vein branch was accessed from the right hepatic vein or the inferior vena cava, with placement confirmed by pushing a small amount of contrast agent. The guide wire was then maneuvered to the distal segment of the splenic or superior mesenteric vein for direct portal vein angiography, which facilitated the assessment of the varices in terms of location, morphology, size, and blood flow velocity. The 5F angiographic catheter was used to selectively intubate the varices. The varices were embolized with steel coils of appropriate diameter until the blood flow velocity of the varices slowed significantly. Then, a coaxial microcatheter (Boston Scientific Corporation, Massachusetts, United States) was inserted into the varices. The mixture of cyanoacrylate and iodide oil was slowly injected to make them flow slowly along the varicose veins and distribute in the reticular branches of the distal varicose veins until they were completely embolized. The ratio of iodide oil to cyanoacrylate was 3:1. For varicose veins with diameters greater than 10 mm, the ratio of iodide oil to cyanoacrylate was reduced. After the embolization was completed, the end of the angiography catheter was placed at the splenic hilum, and direct portal vein angiography was performed again to confirm whether the varicose veins were completely embolized (Figure 1A).
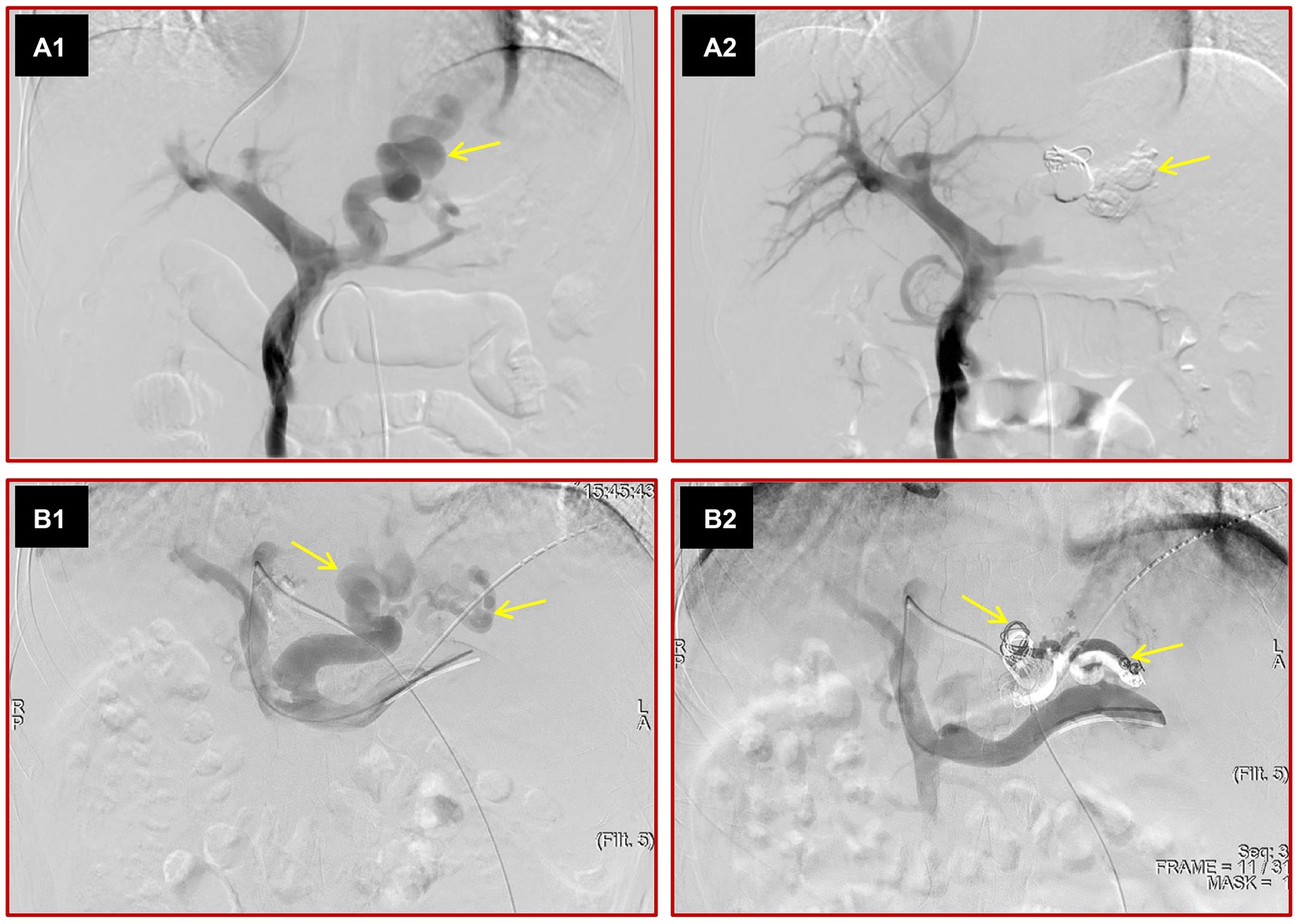
Figure 1. Images depict the two kinds of variceal embolization methods. The portal vein was punctured from the hepatic vein through internal jugular vein access, direct portal vein angiography was performed, and large varices (yellow arrow) were seen (A1). After variceal embolization with cyanoacrylate and steel coils, the varices disappeared on direct portal angiography (A2). The left portal vein was punctured by a percutaneous transhepatic approach, direct portal vein angiography was performed, and various large varices (yellow arrows) were observed (B1). After variceal embolization with cyanoacrylate and steel coils, the varices were not seen (B2).
The main PTVE procedure was as follows. The intrahepatic portal vein branch was punctured under ultrasound guidance. The guide wire was adjusted to the splenic vein or distal superior mesenteric vein, and the 5F angiographic catheter was advanced over the guide wire. Then, direct portal vein angiography was performed. The variceal embolization process was the same as that for TJVE. After variceal embolization, the puncture path was sealed with steel coils and cyanoacrylate (Figure 1B).
High-risk EGV: grade I varices and red signs, grade 2–3 varices, and GVs larger than 10 mm in diameter (4, 5). EVs type 1 refers to grade I varices with positive red signs. EVs type 2 refers to grade 2–3 varices with or without positive red signs.
The types of high-risk EGVs are EV and GV. The GV was classified into gastroesophageal varices 1 (GOV1), gastroesophageal varices 2 (GOV2), isolated GV 1 (IGV1), and isolated GV 2 (IGV2), according to the Sarin classification (11).
Variceal bleeding: the presence of medium or large varices and red signs or active bleeding; the presence of blood in the stomach without any cause other than large varices (12, 13).
Variceal eradication: complete disappearance of varices or the presence of varices smaller than 5 mm in diameter and no red signs (14).
Variceal recurrence: the presence of varices larger than 5 mm in diameter after initial eradication (14).
Successful variceal embolization: direct portal angiography without visible varices after the operation and endoscopic variceal eradication at 1 month after variceal embolization (Figure 2).
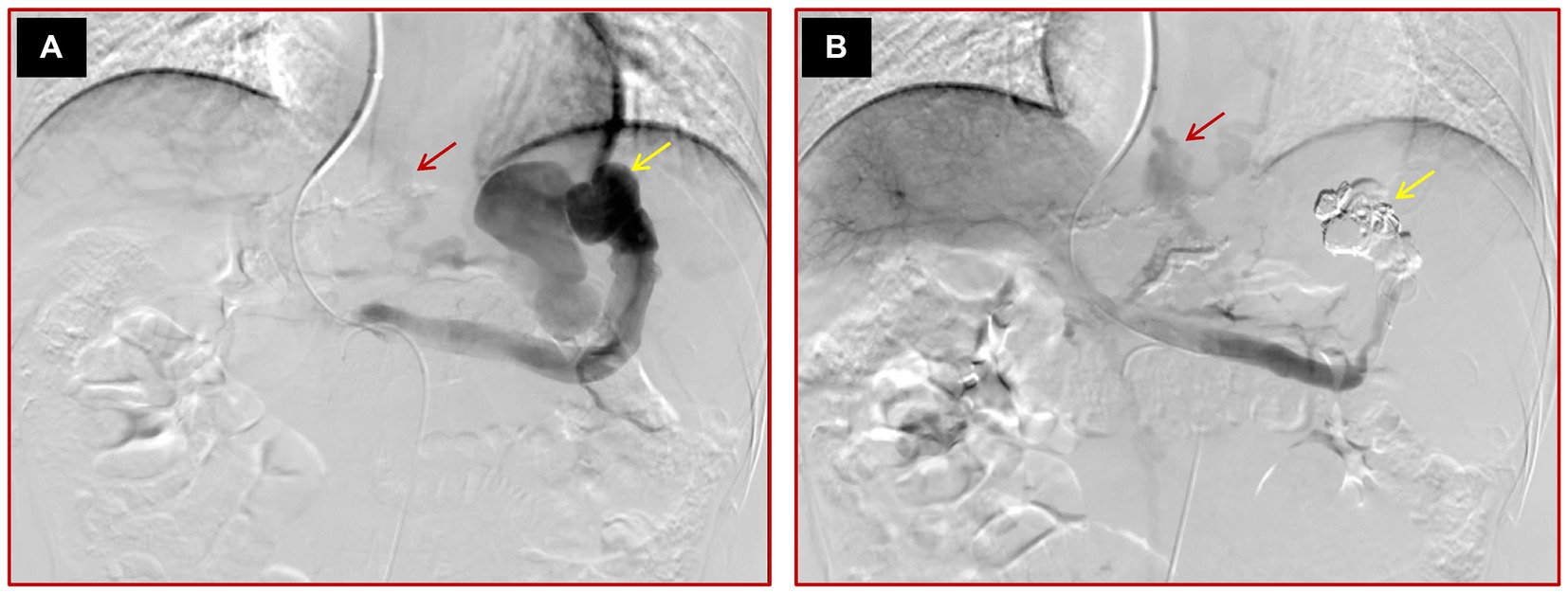
Figure 2. Images of a 45-year-old male cirrhotic patient with high-risk EV and IGV1 who failed variceal embolization. (A) The initial direct portal vein angiography reveals multiple varices and a prominent spontaneous splenorenal shunt, highlighted by yellow arrows. (B) Post-embolization angiography demonstrates that while the conspicuous spontaneous splenorenal shunt is no longer detectable, signifying partial procedural success, some varices persist, as indicated by a red arrow, suggesting incomplete occlusion.
Ascites occurrence: the new onset of any detectable ascites post-procedure in patients with no prior history of ascites before undergoing variceal embolization.
Worsening of ascites/hydrothorax: an increase in the ascites/hydrothorax volume from minimal to moderate or large, as determined by imaging or physical examination post-procedure.
All continuous variables are expressed as the median (range). Categorical variables are expressed as counts and percentages.
Between January 2020 and June 2022, a total of 73 consecutive patients underwent variceal embolization for high-risk EGV. However, 14 patients were excluded based on the exclusion criteria; 2 were over the age of 75 years, and 12 suffered from non-cirrhotic portal hypertension. An additional 16 patients were excluded for various reasons: 3 had a prior history of variceal bleeding, 10 patients had HCC who did not meet the Milan criteria, 1 was diagnosed with colon cancer, 1 was undergoing renal replacement therapy for end-stage renal disease, and 1 had a cavernous transformation of the portal vein. Consequently, 43 patients were eligible and enrolled in the study (Figure 3). The specific reasons for patients being unsuitable for NSBBs and endoscopic treatments are detailed in Table 1.
Of these participants, the median age was 54 years, with a range from 18 to 74 years. Male patients constituted 44.2% (n = 19), and 48.8% (n = 21) had hepatitis B virus-related cirrhosis. The median Child–Pugh score was 6, ranging from 5 to 12, and the median Model for End-Stage Liver Disease (MELD) score was 10, ranging from 4 to 22. Regarding varices types, 23.3% (n = 10) of the patients exhibited EVs, while 76.7% (n = 33) displayed GVs. Among the GV subgroup, 25.6% (n = 11) had GOV1, 23.3% (n = 10) had GOV2, 9.3% (n = 4) had IGV1, and 18.6% (n = 8) had a combination of more than one variceal type. Treatment modalities were nearly evenly split, with 51.2% (n = 22) undergoing transjugular variceal embolization (TJVE) and 48.8% (n = 21) receiving percutaneous transhepatic variceal embolization (PTVE). The follow-up period varied among participants, with the shortest being 77 days and the longest extending to 924 days. The median follow-up duration was 573 days. A comprehensive baseline characteristics of the patient cohort are detailed in Table 2.
Out of 43 patients, 93.0% (40 patients) achieved successful embolization, as confirmed by the absence of visible varices on post-operative portal angiography. During the follow-up, five patients (11.6%) experienced variceal bleeding; these individuals were subsequently treated with TIPS therapy. The median interval from embolization to bleeding was 40 days, ranging from 3 to 454 days. Notably, bleeding occurred within 6 weeks for the three patients whose embolization was not successful, whereas it occurred at 250 days and 454 days for the two patients with initial successful procedures. This suggests that failed embolization may lead to an early increase in portal vein pressure and subsequent bleeding from residual varices. In contrast, no short-term bleeding was observed in patients with successful embolization. Over 2 years, the variceal bleeding rate remained at 11.6%. Additionally, 14.0% of patients experienced a recurrence of high-risk EGV, with a median time to recurrence of 195 days. Among the 13 patients with pre-existing hepatic ascites or hydrothorax, 30.8% experienced a worsening of these conditions, typically within 43 days. Conversely, 20.0% of patients without initial ascites and hydrothorax developed ascites, generally after a median time of 371 days. The study recorded a 7.0% mortality rate (three patients) during the follow-up period, but no deaths were directly attributed to variceal bleeding as per the data in Table 3.
Abdominal pain was the most frequent complication, occurring in 51.2% of cases (22/43). Generally, the pain resolved within a week of symptomatic treatment. Nausea and vomiting were reported in 30.2% (13/43) of patients. Fever was present in 7.0% (3/43), while portal vein thrombosis was noted in one case (2.3%). There was one instance of abdominal hemorrhage (2.3%), which required hepatic artery embolization for management. Subcutaneous hematoma at the puncture site, either in the internal jugular vein or femoral artery, was observed in 4.7% (2/43) of the patients. These hematomas typically resolve with local compression between 2 and 4 weeks post-operation. Ectopic embolization of cyanoacrylate occurred in 4.7% (2/43) of patients due to large spontaneous portosystemic shunts. However, the embolization particles were small, and the scope of embolization was limited, resulting in no clinical symptoms, and consequently, no intervention was required for these cases, as shown in Table 4. There were no fatal complications reported. Overall, the rate of severe complications, such as abdominal hemorrhage, was low, at only 2.3% (1/43).
Current guidelines recommend NSBBs or EBL/ECI for the prevention of initial variceal bleeding in patients with cirrhosis. However, these options are not viable for patients who are intolerant to both treatments. In this retrospective study, we assessed the effectiveness and safety of variceal embolization as primary prophylaxis in 43 patients with cirrhosis who were difficult to treat using NSBBs and EBL/ECI. The findings indicated a high success rate of 93.0% for variceal embolization. Over 2 years, the rate of variceal bleeding was 11.6%, and the recurrence of EGV was 14.0%. Importantly, there were no fatal complications associated with the procedure. This study is the first to demonstrate that variceal embolization can be a safe and effective primary prophylactic measure against variceal bleeding in patients who are not candidates for NSBBs and EBL/ECI.
This study suggests that variceal embolization is an effective option for the primary prevention of variceal bleeding. For patients on NSBBs, a reduction in the risk of bleeding is only observed in those who exhibit a hepatic venous pressure gradient (HVPG) response. The HVPG response rate to NSBBs in patients with cirrhosis varies between 30 and 60% (15–17), implying that at least approximately 40% of patients may not benefit from NSBB therapy. Comparatively, the long-term variceal bleeding rate within 2 years for patients undergoing EBL spans from 8.5 to 23%, with an associated mortality rate of approximately 4.6% from variceal bleeding (18–20). Additionally, the actuarial probability of bleeding from GV over a median follow-up of 26 months was reported to be 13% in patients receiving ECI for primary prophylaxis (21). In contrast, the 2-year variceal bleeding rate in this study was 11.6% among patients receiving variceal embolization, with no occurrences of variceal bleeding-related mortality.
This study also demonstrated that variceal embolization is safe. Up to 20% of patients with cirrhosis are intolerant to NSBBs (22), with approximately 3.7% experiencing serious adverse reactions (23). Additionally, the use of NSBBs in patients with Child–Pugh class C liver function remains controversial. There is potential for lethal hemorrhage from recent post-procedure ulcers after EBL (2). ECI in patients with high-risk GV has 3% serious complications and 7% overall mortality (21). In this study, severe complications occurred in only 2.3% (1 out of 43) of the cases following variceal embolization, with no fatalities reported. These findings suggest that variceal embolization is comparable to the methods recommended by the current guidelines in terms of safety.
This study used an enhanced variceal embolization technique, contributing to its efficacy and safety. Variceal embolization encompasses all interventional procedures aimed at occluding varicose veins, primarily through retrograde or anterograde approaches. Retrograde procedures, such as balloon-occluded retrograde transvenous obliteration (BRTO), are limited to patients with spontaneous splenorenal shunts. In contrast, anterograde embolization, which includes balloon-occluded antegrade transvenous obliteration (BATO) and PTVE, applies to a broader range of varices. In this study, the anterograde embolization technique was selected for its versatility. We opted for a non-balloon-assisted embolization method, avoiding the complexities of maneuvering balloon catheters into tortuous varices, which can lead to procedural failure and increased complications associated with balloon catheter indwelling, as seen in classical BRTO. A variety of catheters, such as the Cobra, MIK, and microcatheters, were utilized for the successful catheterization of varices, achieving almost a 100% success rate. Instead of using sclerosing agents, which have been associated with serious complications such as hemolysis and renal impairment (24), this study used a combination of steel coils and cyanoacrylate tissue adhesives. This approach not only shortened the operation time but also minimized complications (25, 26). The steel coils reduced blood flow, while the cyanoacrylate ensured complete and reticular embolization, effectively preventing ectopic embolization without the need for a balloon catheter post-operation. Cyanoacrylate was injected into an extensive variceal network, targeting the lower esophageal, periesophageal, paraesophageal, gastric cardiac, and perforating veins. This comprehensive and occlusive embolization strategy significantly reduced the likelihood of a short-term recurrence of EGV.
This study is subject to certain limitations. First, the study is retrospective in nature even though the data were collected prospectively. The findings require validation through prospective, large-sample studies. Second, the determination of when endoscopic treatments were difficult was subjective. Since both EBL and ECI require specific expertise, and because the proficiency of endoscopists can vary widely, the decision to prevent variceal bleeding is often influenced by the level of available local expertise at a given center. To minimize these limitations, particularly those associated with technical proficiency and interpretive variability, decisions were made collaboratively by two experienced endoscopists. This approach aimed to ensure a more standardized assessment and reduce the potential bias in determining the suitability of endoscopic treatment options.
In conclusion, the results of this study indicate that variceal embolization is a viable and safe alternative to the established standard treatments for the primary prophylaxis of variceal bleeding. This technique offers a definitive treatment pathway for patients who are intolerant to NSBBs or endoscopic therapies.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the Medical Ethics Committee of the First Affiliated Hospital of Air Force Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
JT: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. XY: Methodology, Data curation, Resources, Validation, Visualization, Writing – review & editing. YZ: Investigation, Methodology, Resources, Validation, Writing – review & editing. KL: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – review & editing. XG: Resources, Writing – review & editing. NH: Data curation, Resources, Software, Validation, Visualization, Writing – review & editing. JN: Data curation, Investigation, Methodology, Resources, Validation, Visualization, Writing – review & editing. JX: Resources, Validation, Visualization, Writing – review & editing. WW: Software, Validation, Writing – review & editing. YS: Software, Supervision, Validation, Visualization, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Key R&D Program of Shaanxi Province (2020ZDLSF01-05).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. European Association for the Study of the Liver. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. (2018) 69:406–60. doi: 10.1016/j.jhep.2018.03.024
2. Garcia-Tsao, G, and Bosch, J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. (2010) 362:823–32. doi: 10.1056/NEJMra0901512
3. de Franchis, R, Bosch, J, Garcia-Tsao, G, Reiberger, T, Ripoll, C, Abraldes, JG, et al. Baveno vii - renewing consensus in portal hypertension. J Hepatol. (2022) 76:959–74. doi: 10.1016/j.jhep.2021.12.022
4. Tripathi, D, Stanley, AJ, Hayes, PC, Patch, D, Millson, C, Mehrzad, H, et al. UK guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut. (2015) 64:1680–704. doi: 10.1136/gutjnl-2015-309262
5. Garcia-Pagan, JC, Barrufet, M, Cardenas, A, and Escorsell, A. Management of gastric varices. Clin Gastroenterol Hepatol. (2014) 12:919–928.e1. quiz e51-2. doi: 10.1016/j.cgh.2013.07.015
6. Gralnek, IM, Camus Duboc, M, Garcia-Pagan, JC, Fuccio, L, Karstensen, JG, Hucl, T, et al. Endoscopic diagnosis and management of esophagogastric variceal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. (2022) 54:1094–120. doi: 10.1055/a-1939-4887
7. Tian, X, Wang, Q, Zhang, C, Liu, F, Cui, Y, Liu, F, et al. Modified percutaneous transhepatic variceal embolization with 2-Octylcyanoacrylate for bleeding gastric varices: long-term follow-up outcomes. AJR Am J Roentgenol. (2011) 197:502–9. doi: 10.2214/AJR.10.6005
8. Widrich, WC, Robbins, AH, and Nabseth, DC. Transhepatic embolization of varices. Cardiovasc Intervent Radiol. (1980) 3:298–303. doi: 10.1007/BF02552748
9. Zhang, CQ, Liu, FL, Liang, B, Xu, HW, Xu, L, Feng, K, et al. A modified percutaneous transhepatic varices embolization with 2-Octyl cyanoacrylate in the treatment of bleeding esophageal varices. J Clin Gastroenterol. (2009) 43:463–9. doi: 10.1097/MCG.0b013e31817ff90f
10. Wang, J, Tian, XG, Li, Y, Zhang, CQ, Liu, FL, Cui, Y, et al. Comparison of modified percutaneous Transhepatic variceal embolization and endoscopic cyanoacrylate injection for gastric variceal rebleeding. World J Gastroenterol. (2013) 19:706–14. doi: 10.3748/wjg.v19.i5.706
11. Sarin, SK, Lahoti, D, Saxena, SP, Murthy, NS, and Makwana, UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology. (1992) 16:1343–9. doi: 10.1002/hep.1840160607
12. Mallet, M, Rudler, M, and Thabut, D. Variceal bleeding in cirrhotic patients. Gastroenterol Rep. (2017) 5:185–92. doi: 10.1093/gastro/gox024
13. de Franchis, RBaveno V Faculty. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. (2010) 53:762–8. doi: 10.1016/j.jhep.2010.06.004
14. de Franchis, R. Updating consensus in portal hypertension: report of the Baveno III consensus workshop on definitions, methodology and therapeutic strategies in portal hypertension. J Hepatol. (2000) 33:846–52. doi: 10.1016/s0168-8278(00)80320-7
15. Schwarzer, R, Kivaranovic, D, Paternostro, R, Mandorfer, M, Reiberger, T, Trauner, M, et al. Carvedilol for reducing portal pressure in primary prophylaxis of variceal bleeding: a dose-response study. Aliment Pharmacol Ther. (2018) 47:1162–9. doi: 10.1111/apt.14576
16. Villanueva, C, Albillos, A, Genesca, J, Abraldes, JG, Calleja, JL, Aracil, C, et al. Development of Hyperdynamic circulation and response to Beta-blockers in compensated cirrhosis with portal hypertension. Hepatology. (2016) 63:197–206. doi: 10.1002/hep.28264
17. De Binary, K, Das, D, Sen, S, Biswas, PK, Mandal, SK, Majumdar, D, et al. Acute and 7-day portal pressure response to carvedilol and propranolol in Cirrhotics. J Gastroenterol Hepatol. (2002) 17:183–9. doi: 10.1046/j.1440-1746.2002.02674.x
18. Tripathi, D, Ferguson, JW, Kochar, N, Leithead, JA, Therapondos, G, McAvoy, NC, et al. Randomized controlled trial of carvedilol versus variceal band ligation for the prevention of the first variceal bleed. Hepatology. (2009) 50:825–33. doi: 10.1002/hep.23045
19. Shah, HA, Azam, Z, Rauf, J, Abid, S, Hamid, S, Jafri, W, et al. Carvedilol vs. esophageal variceal band ligation in the primary prophylaxis of variceal hemorrhage: a multicentre randomized controlled trial. J Hepatol. (2014) 60:757–64. doi: 10.1016/j.jhep.2013.11.019
20. Baiges, A, Hernandez-Gea, V, and Bosch, J. Pharmacologic prevention of variceal bleeding and rebleeding. Hepatol Int. (2018) 12:68–80. doi: 10.1007/s12072-017-9833-y
21. Mishra, SR, Sharma, BC, Kumar, A, and Sarin, SK. Primary prophylaxis of gastric variceal bleeding comparing cyanoacrylate injection and Beta-blockers: a randomized controlled trial. J Hepatol. (2011) 54:1161–7. doi: 10.1016/j.jhep.2010.09.031
22. Reiberger, T, and Mandorfer, M. Beta adrenergic blockade and decompensated cirrhosis. J Hepatol. (2017) 66:849–59. doi: 10.1016/j.jhep.2016.11.001
23. Vijayaraghavan, R, Jindal, A, Arora, V, Choudhary, A, Kumar, G, and Sarin, SK. Hemodynamic effects of adding simvastatin to carvedilol for primary prophylaxis of variceal bleeding: a randomized controlled trial. Am J Gastroenterol. (2020) 115:729–37. doi: 10.14309/ajg.0000000000000551
24. Saad, WE, and Darcy, MD. Transjugular intrahepatic portosystemic shunt (Tips) versus balloon-occluded retrograde transvenous obliteration (Brto) for the management of gastric varices. Semin Intervent Radiol. (2011) 28:339–49. doi: 10.1055/s-0031-1284461
25. Nakai, M, Ikoma, A, Higashino, N, Inagaki, T, and Sonomura, T. Balloon-occluded retrograde Transvenous obliteration of a gastric varix with the use of an N-butyl cyanoacrylate-Lipiodol-ethanol mixture. J Vasc Interv Radiol. (2018) 29:1325–7. doi: 10.1016/j.jvir.2018.02.010
26. Kim, DJ, Darcy, MD, Mani, NB, Park, AW, Akinwande, O, Ramaswamy, RS, et al. Modified balloon-occluded retrograde Transvenous obliteration (Brto) techniques for the treatment of gastric varices: vascular plug-assisted retrograde transvenous obliteration (Parto)/coil-assisted retrograde Transvenous obliteration (carto)/balloon-occluded Antegrade Transvenous obliteration (Bato). Cardiovasc Intervent Radiol. (2018) 41:835–47. doi: 10.1007/s00270-018-1896-1
Keywords: variceal embolization, primary prophylaxis, cirrhosis, hypertension, portal, variceal bleeding
Citation: Tie J, Yuan X, Zhu Y, Li K, Gou X, Han N, Niu J, Xu J, Wang W and Shi Y (2024) Efficacy and safety of variceal embolization for primary prophylaxis in cirrhosis patients with challenges in standard treatments: preliminary results. Front. Med. 11:1401900. doi: 10.3389/fmed.2024.1401900
Received: 16 March 2024; Accepted: 28 June 2024;
Published: 15 July 2024.
Edited by:
Xingshun Qi, General Hospital of Northern Theater Command, ChinaReviewed by:
Yuzheng Zhuge, Nanjing Drum Tower Hospital, ChinaCopyright © 2024 Tie, Yuan, Zhu, Li, Gou, Han, Niu, Xu, Wang and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Tie, dGllanVuNzc3NkAxNjMuY29t; Yongquan Shi, c2hpeXF1YW5AZm1tdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.