
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med., 03 June 2024
Sec. Regulatory Science
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1400585
This article is part of the Research TopicThe Integrity of Randomized Clinical TrialsView all 4 articles
Clinical trials (CTs) are essential for medical advancements but face significant challenges, particularly in professional training and role clarity. Principal investigators, clinical research coordinators (CRCs), nurses, clinical trial pharmacists, and monitors are key players. Each faces unique challenges, such as maintaining protocol compliance, managing investigational products, and ensuring data integrity. Clinical trials’ complexity and evolving nature demand specialized and ongoing training for these professionals. Addressing these challenges requires clear role delineation, continuous professional development, and supportive workplace environments to improve retention and trial outcomes. Enhanced training programs and a collaborative approach are essential for the successful conduct of clinical trials and the advancement of medical research.
CTs are typically conducted within a healthcare setting where the researchers are usually clinicians, being the healthcare system responsible for two missions: improving patients’ health and advancing therapeutics, often in collaboration with the industry. This dual purpose of clinical assistance and research requires adequate support ecosystems characterized by a highly demanding organizational framework (1, 2) that has as a benchmark the Guideline for Good Clinical Practice (GCP) of the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use for Conducting Clinical Trials Guidelines (ICH) (3).
GCP is the international standard for ethical and scientific quality in clinical trial design, conduct, and reporting to protect trial participants’ rights, safety, and well-being (4). On the other hand, different regulations on CTs, such as Title 21, Chapter 1 of the Code of Federal Regulation (5) in the United States, and Regulation (EU) No 536/2014 of the European Parliament and of the Council of 16 April 2014 on clinical trials on medicinal products for human use (6) in Europe frame the definition of the responsibilities of the different participants in CTs. These regulations emphasize the necessity for clinical trials to be conducted by professionals who are not only ethically and scientifically trained but also well-versed in the nuances of clinical trial execution.
In this sense, the Declaration of Helsinki highlights the need for adequately trained professionals. It explicitly indicates that “Medical research involving human subjects must be conducted only by individuals with the appropriate ethics and scientific education, training, and qualifications. Research on patients or healthy volunteers requires the supervision of a competent and appropriately qualified physician or other health care professional.”(7).
As regulation, complexity, and requirements in CTs are increasing, support structures and professional expertise are more demanding (8–10), especially in the deployment of initial phases of clinical research (11). Moreover, new challenges such as centering opportunities around people and equity, guaranteeing accountability, and improving efficiency must be addressed (12). In this scenario, specific professional profiles are recommended to provide adequate support for CTs, such as principal investigators, sub-investigators, clinical study nurses, clinical research coordinators, study coordinators, pharmacists, and clinical monitors (clinical research associates) (13–16).
However, the literature recognizes that there are no standard definitions of the different profiles involved in CTs, highlights an overlap of functions, and points out the lack of specific training for these professionals involved in the development of CTs and a standard organizational model for defining high-quality support teams (2, 13, 17–19). Despite the relevance of the professional profiles and roles in CTs, there still needs to be literature reviews that address regulations, activities, profiles, qualifications required, training opportunities, and challenges in this field.
As we noted that the aggregate information available is quite limited, we consulted with Chat GPT-4, now recognized as a source of information for healthcare professionals (20). We asked Chat GPT-4: “Which are the main CT professional support profiles involved in implementing CTs in the clinical arena?” (21). The support profiles identified by Chat GPT-4 are reflected in Table 1. During a conversation with Chat GPT-4, it came to our attention that not all of the professionals identified are necessarily directly involved in the clinical setting. So then, we asked Chat GPT-4 to provide us with the outstanding profiles of the referred in Table 1. The second question launched to Chat GPT-4 was: Which of the profiles listed are the more relevant in the clinical arena? The top five profiles selected by Chat GPT-4 were: principal investigator, CRC, clinical research associate, biostatistician, and regulatory affairs specialist. This result showed discrepancies with the information we had previously gathered from the literature review, underscoring the importance of reviewing multiple sources to understand this topic comprehensively.
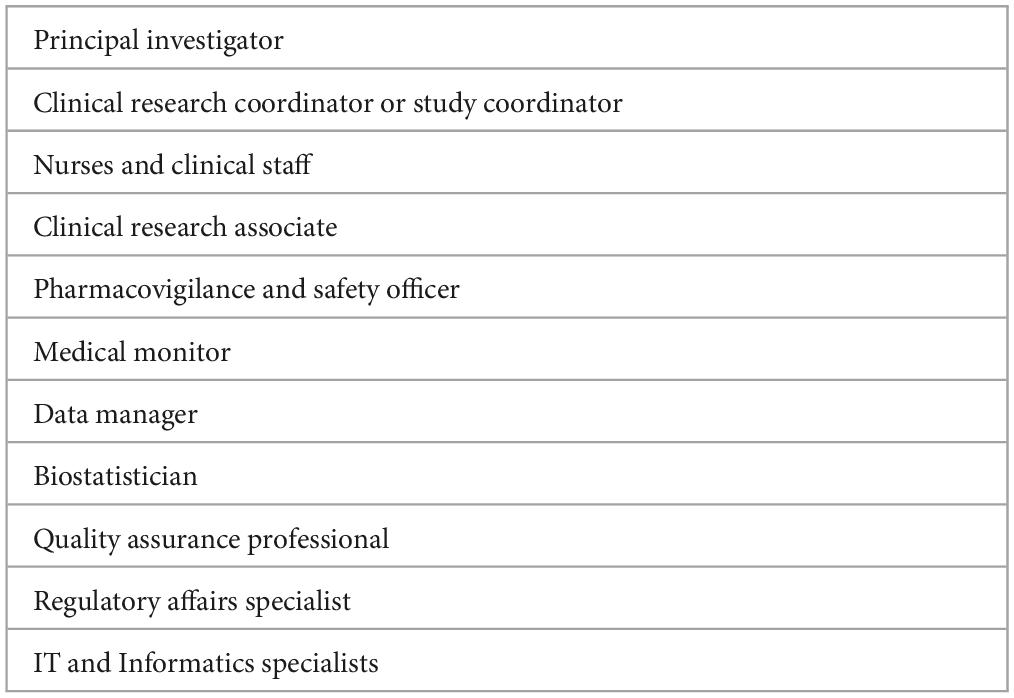
Table 1. Professional clinical trial support profiles in the clinical arena identified by Chat GPT-4.
This paper aims to thoroughly explore the intricate roles and responsibilities that define the backbone of clinical trial operations. It discusses the distinct challenges these professionals face, from ensuring rigorous protocol compliance and managing sensitive investigational products to safeguarding participant well-being and data accuracy. Additionally, it highlights the critical need for ongoing professional development and robust institutional support to sustain workforce competence and morale amidst the pressures of clinical trial demands.
For the initial phase of the review, PubMed was utilized to identify relevant published literature between 2000 and 2023. The search strategy involved specific descriptors related to each of the professional profiles being analyzed (“investigator”, “Clinical Research Nurse”, Clinical Trial Nurse”, Clinical Research Coordinator”, “Clinical Trial Pharmacist”, “Clinical Trial Monitor”, “Clinical Research Associates”) with the following descriptors: “qualifications,” “profiles,” “roles,” “challenges,” and “training.” This approach ensured a focused retrieval of relevant literature.
Additionally, the search included an examination of the existing literature reviews on the different CT professional profiles and a thorough analysis of the bibliographies of the identified documents to capture further pertinent sources. This iterative process of reviewing bibliographies helped to identify additional relevant materials that might not have been captured in the initial database searches.
Moreover, the review was extended to examine major international regulations pertinent to the professional profiles under discussion, specifically the guidelines from the International Council for Harmonization (ICH) and key regulations from European and US authorities. This aspect of the methodology ensured that the review was informed by current regulatory frameworks, which could impact professional roles and responsibilities.
Original documents related to the topics being analyzed were also scrutinized to ensure the inclusion of primary sources and firsthand information, which provided a solid foundation for the analysis and conclusions of the review. This comprehensive methodological approach facilitated a thorough exploration of the professional profiles, highlighting various challenges and training needs within the context of current regulatory and professional standards.
GCP (3), the Code of Federal Regulations in the United States (22), Regulation (EU) No 536/2014 of the European Parliament (6), and other international standards, guidelines, and regulations (23–28), establish that the principal investigator’s responsibilities in CTs are broad. They include adequate expertise, providing resources needed, deploying the trial tasks in compliance with the protocol accordingly regulation and reporting, and guaranteeing high standards of medical care (Table 2).
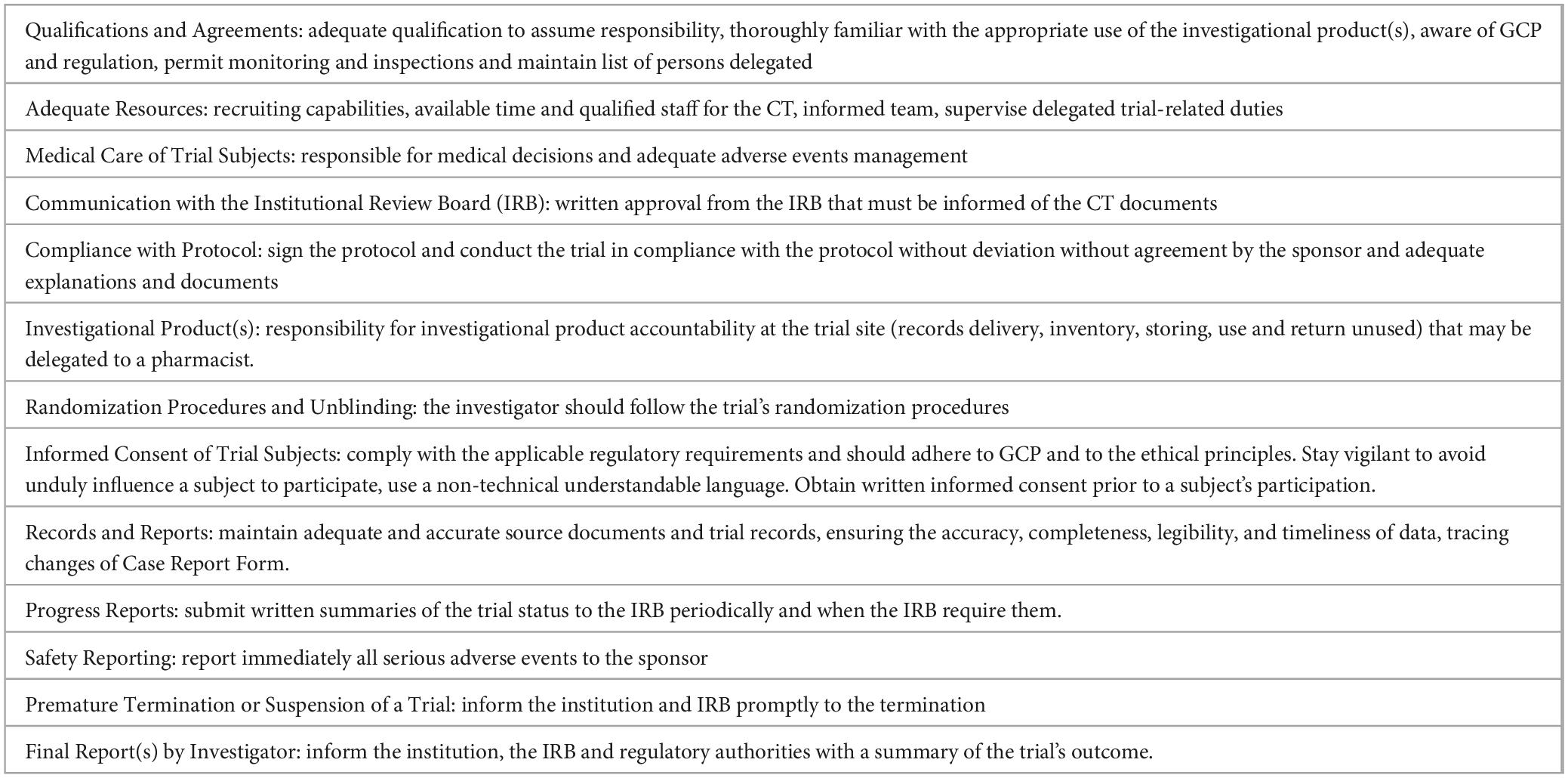
Table 2. Principal investigator responsibilities in clinical trials [adapted from (3)].
In the US, the investigator must sign Form FDA 1572, an agreement to provide certain information to the sponsor and to assure that he/she will comply with FDA regulations related to the conduct of a clinical investigation of an investigational drug or biologic (22, 25, 29) (Table 3). The FDA 1572 has two purposes: (1) to provide the sponsor with information about the investigator’s qualifications and the clinical site that will enable the sponsor to establish and document that the investigator is qualified and the site is an appropriate location where to conduct the clinical investigation, and (2) to inform the investigator of his obligations and obtain the investigator’s commitment to follow pertinent FDA regulations (29).
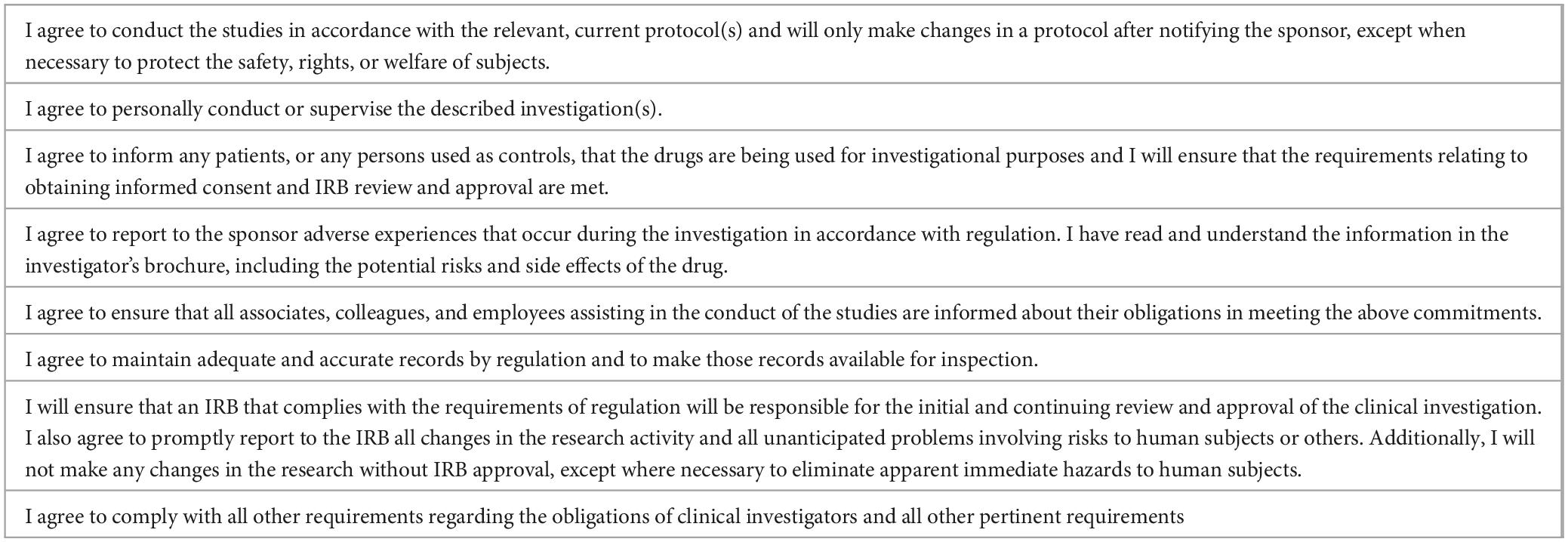
Table 3. Commitments assumed by the investigator in the FDA 1572 form (29).
Accordingly to European (6) and US (5) regulations, the sponsor is responsible for selecting an investigator for each CT, who may further appoint one or more sub-investigators at the trial site to perform crucial trial-related procedures and make critical decisions. These sub-investigators could be associates, residents, or research fellows. However, it is of utmost importance that these tasks are performed in a manner that prioritizes the safety of the subjects and the reliability and robustness of the data generated at the CT site (3, 6, 30).
The CT team is under the investigator’s responsibility. In this sense, the Food and Drug Administration (FDA) (28) and the GCP (3) emphasize this role in the designation and supervision, ensuring that any individual delegated is qualified by education, training, and experience. The CT team is responsible for managing the daily activities of the trial; however, the investigator is ultimately responsible for ensuring that the trial is conducted in compliance with European (6) and US (5) regulations and that accurate records are maintained. The investigator is responsible for protocol violations or discrepancies (25). The FDA specifically focuses on four areas of investigator’s supervision: adequate delegation of study-related tasks, adequate qualification and training of the CT staff to conduct and understand the study, adequate supervision and involvement in the ongoing study, and adequate supervision or oversight of any third parties involved (28).
Informed consent is one of the critical responsibilities of the investigator in CTs (3, 30). Conducting research is an important endeavor, but it is crucial to prioritize the well-being of the participants and to ensure the purpose and risks of the study are disclosed and discussed with them. International regulation highlights that the investigator must inform through informed consent about the purpose of the research, the expected duration of the subject’s participation, the procedures to be followed, and any foreseeable risks or discomforts to the subject (5, 6, 31). Furthermore, it is the investigator’s responsibility to make it clear that patients’ decision to participate will not affect their future medical care. It is indispensable to avoid any hint of coercion when presenting the study, as patients often trust their doctors and may feel compelled to participate if asked (32).
The literature suggests that informed consent forms are often unclear and poorly understood by participants, emphasizing the need for improving communication and comprehension (33). Even more, some subjects may not even be aware that they are participating in research, highlighting the ethical imperative for investigators to ensure transparency and understanding (34). The investigator may delegate informed consent to team members adequately qualified by education, training, and experience. If present, information related to the investigator’s financial relationships or interests must be provided to the participants through informed consent (35). The roles of investigators in the informed consent process are particularly important in some areas, such as pediatric oncology, where doctors must balance their clinical and research roles, highlighting the need for transparent communication and ethical behavior (36). Also, the context of emergency CTs has been challenging for investigators (37).
Reporting is also a relevant responsibility of the investigator in CTs (3, 25). It includes reporting of adverse events by the investigator to the sponsor and the IRB (38), which is required by the different regulatory agencies (5, 6). These reports of severe events must occur without undue delay as they can affect the safety of the volunteers involved in the CTs and even the trial’s pertinence (39). Other reports that are the investigator’s responsibility are progress reports (usually annually) and the final report to inform the Institution and the IRB (3, 5, 38). Financial disclosures to the sponsor are also relevant and should be updated during the investigation and after the completion of the study according to different regulations (25).
The FDA (5), the European regulation (6), and GCP (3) require investigators qualified by training, education, and experience, although these normative and recommendations do not detail the competencies requirements. Moreover, the qualification standards for investigators vary widely among countries (40, 41). Generally, European countries demand GPC training every 2–3 years (41), and in the US, the National Center for Advancing Translational Science (NCATS) recommends undergoing training every 3 years (14). The IRB is responsible for reviewing the principal investigator’s qualifications as part of the trial application to the IRB and can ask questions and request documents such as curriculum vitae (3, 35).
The investigator is usually a physician, although other profiles have been recognized as physician associates/assistants (42), pharmacists (43, 44), and nurses (45). However, the proportion of CTs with physician associates/assistants as investigators is low (14 in 145.398 in the US 2007–2020) (42), and of pharmacists too (523 in 2009 in the US) (46). Even more, some local regulations may prevent non-physician profiles as pharmacists from being principal investigators (47).
Successful clinical research heavily relies on education and training, as highlighted by various studies (14, 48). However, the relevance of clinical research is typically only a tiny part of basic medical training (40). Clinical specialties provide a significant opportunity for the training of new clinical researchers. Several medical specialty training requirements proposed by the European Union of Medical Specialists include mentions of clinical research with specific reference to CTs (49). Recent recommendations to improve CT investigator training promote using more effective methods of adult learning and focus on real needs, changing the behavior of study personnel and considering the potential challenges associated with a particular protocol, as well as the most common deviations that have occurred in protocols similar in design or therapeutic area (50, 51).
The sponsor usually certifies investigator qualifications before participation in CTs through GCP training (3, 52). Most biopharma companies have developed their own GCP courses, requiring each trialist to attend as a condition for participating in their trials (40). These programs have significant disparities and require substantial time that often does not prepare clinicians for the hands-on investigator responsibilities (53). Moreover, the current content of some GCP training materials is considered redundant, unengaging, and uninteresting (52). Various programs aim to qualify clinical research investigators through mentoring involving multiple activities and clinical departments (14, 54) (Table 4). Despite these recognized drawbacks, investigator certification is associated with improved data quality in clinical research (55). Recommendations to evaluate the impact of GPC training have also been proposed (14).
It is worth noting that participating in a trial allows clinicians to be at the forefront of a particular area of medicine. It often enables researchers and their staff to meet with researchers from the same country or worldwide to exchange ideas and plan future collaborations (56).
Investigators must be aware of their enormous responsibility as their workload significantly increases with more legal, regulatory, financial, and administrative issues (27). CTs demand strict adherence to the protocol, precise and comprehensive documentation of clinical care, and accountability for oversight of all activities related to managing the trial subjects and monitoring the investigational product. Increased trial complexity is derived, at least partly, from new designs that have resulted in significant challenges for investigators, including multiple sample collections, multiple committee approvals, more stringent patient monitoring, complex drug administration, and multiple protocol modifications (57). This has contributed to increased disappointment, less interest in participating in multicenter trials, and a decline in clinicians willing to become clinical investigators (27, 28, 58).
Different barriers for investigators to participate in CTs have been identified as institutional support, infrastructure issues, staff support, administrative burden, time requirements, workload balance, data and safety reporting requirements, and dissatisfaction with finance-related issues (58–61). Accordingly, to these identified barriers, the Clinical Trials Transformation Initiative (CTTI) Strengthening the Investigator Community Project (62) has developed some recommendations focused on reinforcement of four critical categories of site-based research activity: developing site-based research infrastructure and staff, optimizing trial execution and conduct, improving site budget development and contract negotiations, and discovering opportunities for conducting additional trials (58, 63).
The high turnover of CT investigators, believed to lead to inefficiency, instability, and increased costs in conducting CTs, is of concern. A study of the U.S. FDA’s Bioresearch Monitoring Information System showed a decline by approximately one-third of investigators submitting a Form from 1999 to 2015. The proportion of investigators involved in only one trial increased, signaling potential adverse trends in the clinical investigator workforce (64), and yearly, about 40% of unique investigators decide not to participate in another FDA-regulated trial (61).
One of the main operational challenges recognized for CT investigators is recruitment (58, 65). Some studies demonstrate that investigators are optimistic and overconfident in predicting outcomes concerning the statistical significance of their primary outcome and completing recruitment of their registered target enrollment by trial closure (66). Fulfilling patient eligibility criteria and ensuring no exclusion criteria apply before enrolling a patient in a study are two of the most common sources of significant protocol violations (24). The main barriers investigators encounter in recruiting are institutional or clinic time, reimbursement constraints, perceived risk, and treatment preferences (67, 68). Proposals to increase the engagement of investigators other than financial incentives to improve recruitment have been proposed as authorship and letters of accomplishment (66).
Recruitment of under-represented populations in CTs is also a concern, with investigators identifying time constraints and implementation issues as barriers (69). Recommendations for investigators to address this issue have been developed to improve patient communication and provide resources such as transportation or specific apps (69). Published experiences indicate that it may be successfully tackled by offering adequate tools for investigators and monitoring their impact (70). Some studies analyze gender distributions in participants in CTs, detecting a high disbalance with female under-representation (71–73), although this disbalance has improved in the last decade (74, 75). Studies indicate that this distribution is related to gender investigator bias as CT led by women enrolled more female participants (71).
When a physician considers enrolling a patient in a trial, a new relationship develops between the patient and the physician. This relationship could conflict with the traditional doctor-patient relationship. When considering the various treatment arms of a trial (including any placebo arms), the investigator could doubt whether one arm or the other is more effective (76). The “dual-role consent” physician-investigators is particularly relevant to informed consent, especially when they have preexisting treatment relationships, and present recommendations indicate the need to consider the ethical acceptability of dual-role consent that varies with the features of each study. This dual research participation, when approximates usual care, becomes increasingly acceptable—even preferable—for physicians to seek consent for research from their patients (77–79). In pediatric clinical research, the understanding of parents and adolescents is crucial, and the dual role of the physician/investigator presents challenges in communicating the goals of phase 1 trials to families (80).
CTs are changing rapidly due to advancements in technology and new designs. Implementing these novel CT designs, such as basket, platform, and umbrella trials, which have become valuable tools in modern drug development and regulatory processes, adds complexity and new challenges (57, 81). Innovative approaches such as blockchain protocols have been proposed to enhance transparency and traceability of consent, reflecting the evolving landscape of informed consent in clinical research (82). Artificial intelligence tools can combine patient data (demographic, laboratory, imaging, and other -omics) to match patients with those complex inclusion criteria, ensuring recruitment fitness. They may help interpret data and transform it into usable insights to improve patient safety (83). Digital technologies can also improve informed consent and data collection, including patient-reported outcomes, endpoint generation, and compliance monitoring (84, 85).
Digitalizing CTs is undoubtedly an opportunity to improve CTs’ participant access, engagement, trial-related metrics, and intervention efficiency. The use of digital technologies in CTs must consider user attitudes, practices, expectations, and preferences, as potential participants may view using their data (86). Investigators are generally willing to implement new technologies in CT. However, the use of various platforms, interoperability issues, limited technological support, and the potential increased burden caused by them are challenges identified by investigators for its implementation in CTs (87). Still, it also implies the need for new guidance for deploying CTs and new skills in the professionals involved (84, 88).
Although the need for clinical research nurses (CRNs) has emerged as the vanguard of public awareness during the COVID-19 pandemic, this nursing specialty has supported clinical research for decades (89). An estimated 10,000 nurses are involved in clinical research work in the United States and around 12.000 in the United Kingdom, across a broad spectrum of roles and settings (90). Independent of the position, the demand for CT nurses is increasing (91, 92).
Different names are used to design nurses dedicated to clinical research, some related to specific roles of nurses in CTs, such as CT nurses, study nurses, research nurses, research nurse coordinators, and clinical research nurses (93–96). Concerning the job titles, a variety has been identified among nurses that give support to CTs as a biostatistician, clinical nurse specialist, clinical research associate, coverage analyst, medical data review manager, protocol interpreter, and rural nurse specialist, which reflects a broad spectrum of roles and positions of the nurses (89).
The National Institutes of Health Clinical Center considers CRN a nursing practice focusing on clinical research. It includes care provided to research participants and activities supporting protocol implementation, data collection, and research participant protection. In addition to providing and coordinating clinical care, CRNs have a central role in assuring ongoing maintenance of informed consent, integrity of protocol procedures, accuracy of research data collection, and education of participants (96, 97).
Some reviews classify CRN roles in four areas: participants and managers of CTs, caregivers and protectors of subjects, coordinators of research teams, and educators (96). In an international study, a variety of deployed roles of CRNs have been identified (Table 5), being the ones more uniformly present in the nine countries involved, acting as a central contact for participants and the research team, documenting participant data, monitoring patient safety, educating participants, participate in the informed consent, assessing, and advocating participants in CTs, reporting adverse events, and documenting patient care and process specimens (93). Another recent European study identified that the most frequently assigned duty for CRNs was administering investigational medicinal products, processing blood samples, and shipping clinical samples (96). Concerning time spent by CRNs in CTs, patient care is the main task of CRNs, which includes monitoring patients for adverse events, teaching participants about the study, reporting potential patient adverse events, recording patient research data, or explaining study procedures to patients (98).
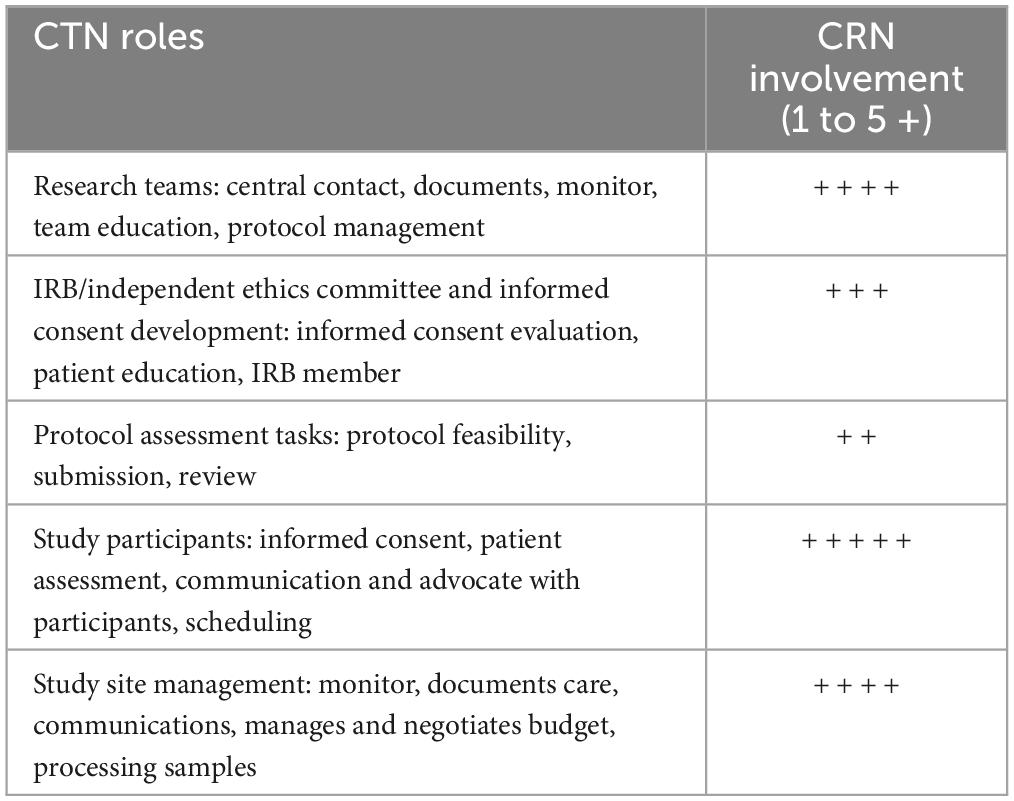
Table 5. Main roles areas for CRNs and their level of deployment the world [Modified of (93)].
The role of CRNs is particularly relevant in some CT activities that require clinical expertise, dialogue skills, and proximity to the patient, such as in the deployment of informed consent. CRNs can assist in designing, reviewing, and approving informed consent (96, 99). CRN guidance and organizational skills in communication and proximity to the patient are also relevant in educating participants about consent documentation and helping them understand the information on the consent form. This supporting role for informed consent is especially relevant in cases of limited health literacy that conditions a lack of comprehension of CT consent documents, increased anxiety during the informed consent process, and even the success in CT enrollment (100).
The literature supports nurses’ relevance in CTs, as they promote an increase in recruitment (101), enhance subject retention (102), and increase general study efficiency (103). Nurses are also viewed as specifically relevant in deploying retention strategies based on individualizing and identifying threats to CT participants’ retention (102). Some experiences in the UK National Health Services highlight the role of CRNs in building local research, changing research culture, informing the communities, networking, and patient public involvement (104).
Independently of the various roles and responsibilities of the CRNs, the literature highlights that they must continually balance the requirements for protocol integrity and data quality with the clinical needs, comfort, and safety of research participants (105, 106).
In this plethora of roles, two are the central conceptual positions of nurses in clinical research: CRN and research nurse coordinators (95). CRNs, or clinical research care nurses, are clinical research staff nurses with a central focus on the care of research participants. They support study implementation within the context of the care delivery setting. Research nurse coordinators are primarily responsible for study coordination and data management, with particular focus in managing subject recruitment and enrollment, consistency of study implementation, data management and integrity, and compliance with regulatory requirements and reporting (107).
National Institutes of Health have differentiated CRNs’ skills into five categories: care coordination and continuity of clinical practice, human subjects’ protection, study management, and contribution to science (108). These five categories are deployed through 53 different activities, which comprise the full range of practice of clinical nurses providing research-based patient care and study coordinators managing studies (108). Others have proposed an eight-dimensional model of CRNs: care, work-study, expert, lead, prepare, data, advance science, and ethics (98). More recently, an expert team has designed a CRN training model based on the theoretical basis of position competence through literature study, qualitative interviews, and the Delphi method (109).
CRN was recognized as a specialty in the United States by the American Nurses Association in 2016, a relevant step that creates a conceptual basis for developing specialty practice tools such as job descriptions, practice standards, competency assessment, educational content, and certification (110). In this sense, the creation of the International Association of Clinical Research Nurses (IACRN) as a professional nursing organization dedicated to defining, validating, and advancing the specialty practice of clinical research nursing focused on “maintaining the equilibrium between the care of the research participant and fidelity to the research protocol” has been a breakthrough. The IACRN supports the professional development of CRNs with specific resources and meetings (110) and has created a curriculum for nursing school adoption and integration of research nursing within accredited programs (111). Oncology CRN has been considered a subspecialty (112), and some specific resources have been developed, such as the Manual for Clinical Trials Nursing (113), a description of their competencies (114, 115), and a CTs Nurse Questionnaire used to determine the various activities of the CRNs within oncology (116).
Australia, where CRN is also a specialty, has developed CRN standards (Table 6). In Europe, a recent survey has identified the presence of specific educational courses for research nurses in the majority of countries. However, the length of the research nurse education and the providers of research nurse courses exhibited significant variation, ranging from workers’ unions to individual institutions or hospitals. Completing the GCP course and renewing the GCP accreditation are regularly mandatory for CRNs in most countries (96).

Table 6. CRN Australian standards for practice (233).
Specific CT training for nurses may positively impact their confidence, ability to discuss CTs, and perceived behavioral norms surrounding such talks. A randomized CT demonstrated that an online video-based educational program increased oncology nurses’ intention to discuss CT with patients and increased self-reported discussions about CT compared to a text-based educational intervention (117).
Recent data indicate that the number of CRNs in the United States is decreasing (118), which is a matter of concern (119), considering that few nursing schools describe research nursing as a potential career (111). While it’s important for nurse professionals to conduct research (120), CRNs may face difficulties balancing regulatory compliance requirements, research integrity, recruitment targets, and eligibility criteria with their responsibility to prioritize the needs of research subjects and advocate for them. More research is needed to address these challenges (121, 122). Transitioning from nurse to CRN is, per se, a challenge and takes time. It implies thinking like a researcher rather than a clinician, adapting to functioning independently, gain and using skills in data management (123).
The perception of CRNs about their participation in CTs has been analyzed, concluding that most of them remark that they contribute to the clinical research enterprise through knowledge of the research process and nursing expertise in the care of clinical research participants, with an outstanding role in participant care and safety (89). Some studies have analyzed the main barriers for nurses participating in CTs. Lack of knowledge and confidence, concerns about the skills needed to communicate effectively in various patient situations, and the need for explicit norms regarding their role have been identified as relevant barriers (124). Issues like threats to voluntariness, measures to safeguard voluntariness as a time to consider participation, the inclusion of vulnerable groups, and the questionable exclusion of certain groups due to language or cognitive barriers concern CRNs (125).
The decentralization of CTs boosted by the COVID-19 pandemic implies a new model of working for nurses in CTs as home care is incorporated as standard practice. It implies that digital technologies are required, and updating nurses’ competencies is essential in this new CT deployment model, implying an added effort (111).
Clinical research coordinators (CRCs) are also known as “study coordinators,” “data managers,” “clinical trial administrators”, and “clinical research associates” (126–128). They are not described or defined in the GCP (129). The Association of Clinical Research Professionals (ACRP) (130) states that a CRC, or Study Site Coordinator, works at a clinical research site under the immediate direction of a principal investigator whose research activities are conducted under Good Clinical Practice regulations.
The tasks developed by the CRCs may be classified into eight domains (Table 7) and are most frequently related to the domain of monitoring activities, to a lesser extent to administrative activities, being researcher-related activities tertiary (126). Among other delegated tasks, CRCs perform site preparation, patient screening and recruitment, patient enrollment, conduct and ensure the quality of case report forms, maintain source documents, and ensure site quality (11, 131–133). CRCs are complementary in providing informed consent to investigators (134, 135). So, although the investigator is responsible for the overall conduct of the CT, the CRCs are responsible for many of the daily activities (136). CRCs may also help in the protocol assessment during the CT conceptualization and evaluation regarding operational logistics or procedures issues (135).
Although CRCs usually work in direct contact with healthcare, patients, and patient information, in Western countries, their hiring frequently depends on the investigator and is not linked directly to healthcare systems (137). In Japan, CRCs work under the discretion of their hospital and, in general, support clinical trials in various areas (138).
From the organizational point of view, CRCs may have different levels of responsibilities. Some clinical trial units have established first and second levels of CRCs that are differentiated by the type of studies they manage (minimal risk vs. non-minimal risk CTs) and the kind of tasks (abstracting and screening vs. regulatory and start-up approval). Others have established four skill levels with different experience and salaries (129, 139).
Some critical elements for a good CRC have been considered related to the “five 5 Cs”: coordination, connection, commitment, communication, and collaboration, highlighting the relevance of their responsibilities and networking abilities in CTs (136). It has been valued that CRCs mobilize altruism to deploy their activities, motivate participants to adhere to study protocols, and manage the tension between research and care (140).
Investigators recognize the critical role of the CRCs in the informed consent process, as they have more detailed knowledge of research-related logistics, such as the duration and frequency of visits, possible costs, and compensation, and can offer information about the potential participation requirements. CRCs usually have more time for supporting information, offer a more friendly language, and demonstrate a high degree of that may promote voluntariness (138). CT participants may be more comfortable saying no to coordinators than physician-investigators, which mitigates physician-investigators negative dual role (141). In this sense, CRC’s cultural and racial diversity may facilitate recruitment and CT participant engagement and diminish bias in recruiting marginal populations (142).
CRCs also facilitate adequate billing of CTs. Billing requires information and communication from many sources that must be coordinated, and complex decisions must be documented to avoid common pitfalls covered by the CRC’s role and expertise. Given the potential errors in this process, a CRC can provide the right information punctually to each stakeholder to facilitate an organized and transparent process (143).
CRCs’ responsibilities impact the quality of CTs in a relevant way, and some published audits highlight their relevance (144) and are considered an essential element for an optimal CT unit infrastructure. In an Italian survey, more than 80% of participating sites associated improving the quality of clinical research with implementing a coordinator as a team member (145). CRCs also positively impact scientific output (146).
Different CRC workload assessment tools have also been developed that consider CT complexity to define the optimal number of studies to be assigned to each CRC based on its complexity. These tools consider issues like type of funding (profit or non-profit CTs), frequency of visits, and number of on-site patient access and centralized procedures (147).
The Joint Task Force for Clinical Trial Competency of the Association of Clinical Research Professionals (ACRP) has defined core competencies for Clinical Research Coordinators (148). These competencies are described in a guideline and a certification pathway comprising eight domains. The domains are categorized as entry-level, intermediate, and senior CRCs. The guideline provides self-assessment and competence gap analysis tools for CRCs. The certification pathway includes personalized professional development plans. The guideline and certification pathway is a roadmap for research sites to support CRCs’ hiring, assessment, and development (Table 8). These core competencies are differentiated by the clinical study method (observational vs. interventional) and are associated with potential utilization and outcomes, including training initiatives, job descriptions, and policy development (149).

Table 8. Group of Domains of competencies of clinical research coordinators developed by the Association of Clinical Research Professionals. Modified from (148).
The literature has reflected some discussion about the optimal CRC background. Some consider nurses to be the best profile for CRCs with the advantage of having a possible dual role (clinical and CTs support role) that allows them to act as delegates for the investigating physician (including concomitant medications and adverse events (150) while others believe that the general background is considered an advantage (126). Various general skills needed for CRCs are service orientation, negotiation, management of personnel resources, and instructing abilities (133).
Specific CT training of CRCs is based on GCP as the international standard. Although different formal programs are available, surveys to CRCs indicate that these are considered helpful in the initial introduction as a new employee and for refreshing baseline knowledge, although experience, day-to-day practice, observing peers and colleagues, and having mentors are the essential elements to learning (151). Formal training in GCP can improve protocol adherence and clinical trial quality, and one retrospective study data indicates that the number of protocol deviations was significantly lower if the CRCs were certified in GCP (152). Also, educating and training senior CRCs of new teams is considered necessary (2).
Different tools for competency self-assessment have been developed as the various versions of the Competency Index for Clinical Research Professionals, with excellent psychometric properties and a good ability to distinguish between experienced and non-experienced CRCs (153).
Despite CRCs’ recognized role in CT deployment, some discussions have been opened about their invisible role, and they have sometimes been referred to as “phantom investigators” (145). This is due, at least in part, to the fact that specific certifications infrequently recognize the CRC professional profile (129), and no formal CRC position exists in institutions’ staff, as required by national healthcare workforce regulations (127). For example, in the United States, the Bureau of Labor Statistics does not recognize CRCs (129). However, the demand growth in the market research management of CRCs is objectively relevant (154).
Also, some studies reflect CRC’s inadequate professional identity, suboptimal remuneration, and lack of peer support and recognition (155). CRCs’ job satisfaction and retention have been issues of discussion as turnover is relatively high and impacts productivity and emotional costs. An adequate salary, greater respect, collaboration, and engagement from the principal investigator are significantly associated with higher retention (155, 156). Over-delegation of responsibilities and under-supervision to CRCs is another issue of discussion identified in warning letters of the regulatory agencies (156). In this frame, some have warned about the risk of high turnover, lack of identity of job description, and lack of institutional support (157).
Some proposals for clarifying the competencies for specific clinical research roles have been established by the Special Programme for Research and Training in Tropical Diseases (TDR) of the World Health Organization (158) and by the Multi-Regional CTs Center at Harvard University (13). As the CRC job is not well delineated and competencies are increasingly broad, difficulties have been described in its recruitment, due at least in part to its description in job offers. A guideline for human resources units recruiting CRCs has been elaborated to overcome this barrier (139).
Several challenges for the CRCs in CTs are related to recruitment, specifically in racial and ethnic minorities. Fronting to this population implies overcoming language and cultural barriers and emotional challenges associated with requesting participation from seriously ill patients. Adaptive and technical strategies to improve recruitment of underrepresented CT participants from the CRCs’ point of view have also been identified (159).
CRCs’ burnout is a concern (160). Causes for CRCs’ burnout are diverse and associated with job dissatisfaction, perceived daily work overload, low endurance, and nurturance personality traits. As the burnout risk of CRCs has been identified with high rates accordingly, some studies about some team-based interventions have been developed demonstrating that they are affordable with good results (139, 161).
CT pharmacists have differentiating roles and expertise that impact the organization and activities of CTs (162). Some specifications about the pharmacist’s responsibilities in CTs are described in the GCP (3), which defines the need for dispensing records kept at the pharmacy and notes that an investigator should delegate responsibility for the storage and accountability of investigational products to an appropriate pharmacist. In the United States, the Code of Federal Regulations defines issues related to investigational product management (163).
Pharmacists CT support is usually provided by an investigational drug research service (clinical research pharmacy) that can be a small-scale operation with a part-time pharmacist or a large-scale operation with a team of dedicated clinical research pharmacists, technicians, and coordinators. Usually, it is a component of a more prominent pharmacy organization, such as a hospital pharmacy (162). Specific investigational drug service pharmacists are present in many centres (164), and the literature highlights that investigational drug service management is especially relevant in supporting early-phase CTs (165). Guidelines (162), best practices standards (165, 166), and frameworks (164) for investigational drug services provided by pharmacists have been published, making medication management a central issue of their tasks.
The Society of Hospital Pharmacists of Australia (166) defines standard roles (Table 9), services (Table 10), and the following objectives for investigational drug research services:

Table 9. Role of the clinical trials pharmacist accordingly the Society of Hospital Pharmacists of Australia (166).
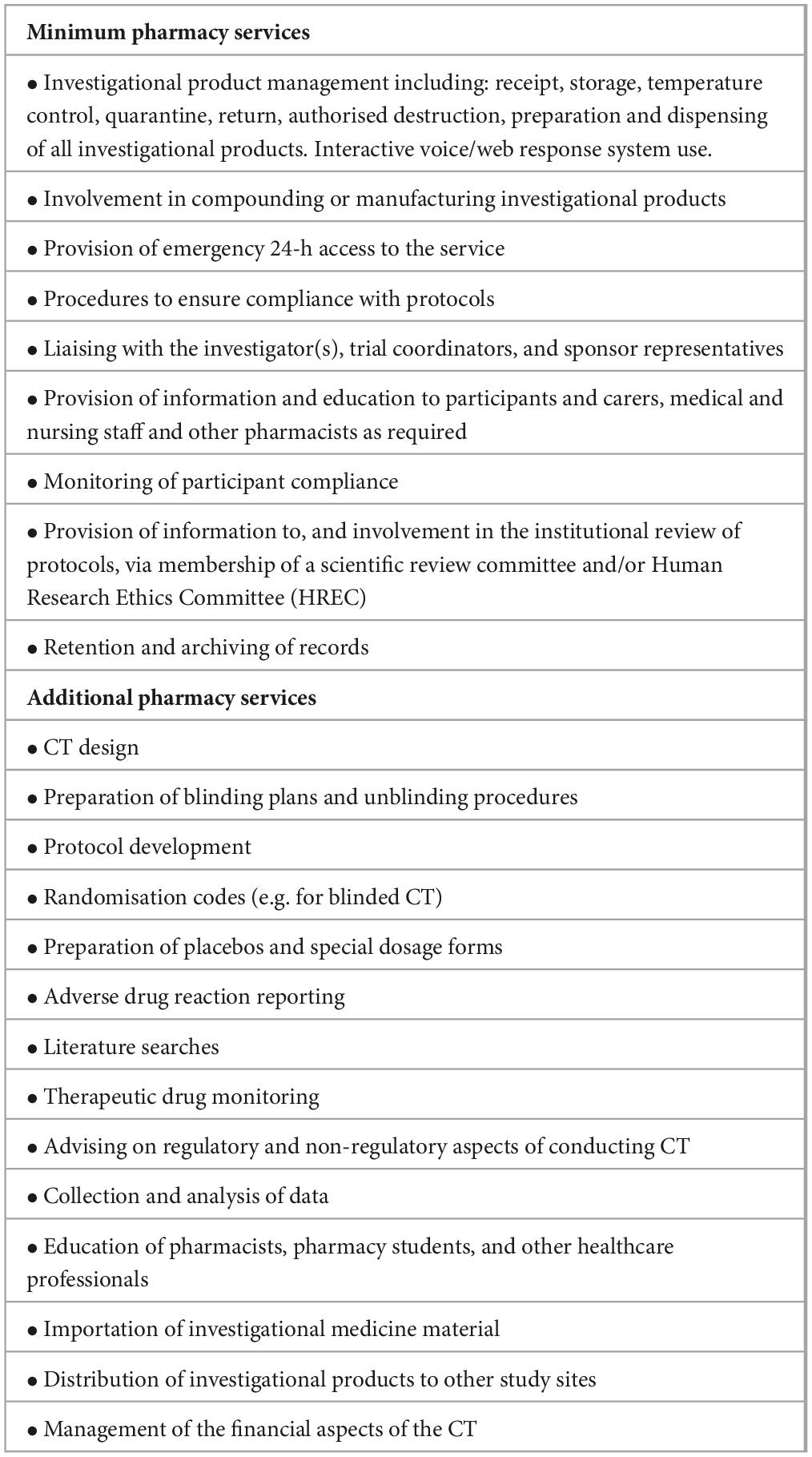
Table 10. Minimum and additional services required for a clinical trials pharmacy service [adapted from (166)].
• Provide safe and ethical use of investigational products by ensuring they are appropriate for use and are procured, handled, stored, and used safely and correctly.
• Apply the principles of best pharmacy practice to evaluate new investigational products or medicines.
• Ensure that the pharmacy aspects of investigational product use comply with relevant legislation, standards, guidelines, and local or institutional policies.
• Consider the safety and welfare of participants and the protection of their legal and ethical rights, including confidentiality and privacy.
Differentiating requirements for investigational drug research services according to the complexity of the supported CTs has been proposed. For example, for first-in-human CTs, adaptive trials, and multidrug regimens that have a high risk for adverse events or drug–drug interactions, high standards have been recommended. These imply the needing for an on-call investigational drug service pharmacist to conduct concomitant medication reviews, assess potential drug–drug interactions, monitor renal or hepatic function as a basis for dose adjustment, deliver initial patient education on the investigational drug, and advise patients on any necessary dose modifications (164).
Some of the CT pharmacist’s roles are specific to investigational drug services such as frozen storage, drug accountability, expiration date tracking, manufacturing, compounding, labelling, blinding of investigational drugs, subject and patient training, dispensing and dosing, monitoring drug events, importation and distribution of investigational medicine material, and maintaining written and up-to-date Standard Operating Procedures for the handling of investigational products (164–169). However, other pharmacists’ roles may overlap with other CT support profiles, such as CT design, protocol development, volunteers’ recruitment, adverse drug reaction reporting, data collection and analysis, education, financial management, or IRB participation (170, 171).
In a national survey developed in the United States, 26 pharmacy services supported cancer CTs over 61 centres. Services were classified as pretrial implementation support, trial implementation support, patient medication profile review, medication therapy engagement, medication therapy management, and miscellaneous. The most common pharmacy-performed services (over 60% of respondents) were a clinical review of investigational drug prescribed orders before dispensing, the recommendation for dose adjustments, a review of trial protocol for scientific merit, the development of training material for research staff, and pharmacist IRB membership. The least frequently performed services (under 10% of respondents) were presenting or publishing trial findings, serving as study co-investigator, consenting patients for trial participation, and serving as principal investigator (172).
The potential impact of pharmacists in CTs is relevant in the medication reconciliation process and especially in identifying protocol-prohibited concomitant medications (173). Despite the recognized role of pharmacists in drug interaction screening during eligibility assessment, they are only sometimes involved in this phase. However, pharmacists were more frequently involved in drug interaction screening for enrolled patients with medication changes (174, 175).
During a patient’s CT participation, pharmacists can create a personalized ‘concomitant medication review guide’ in table presentation. This guide can assist other clinicians in preventing and assessing drug-drug interactions by listing critical medication-use information (176). This has been considered of particular relevance in CTs as blind study participants to treatment groups impact product appearance and packaging, lacking differentiating features such as colour or font, being necessary, including a warning about investigational use (168).
The comprehensive medication review, and specifically the resolution of drug–drug interactions, for participants in oncology CTs has been considered crucial as nearly 70% of the patients may have drug interactions (176, 177). The relevance of this role is reinforced by the fact that frequently the CTs protocols do not asses adequately the interactions (178) and that patients enrolling in CTs often require medication changes to meet eligibility requirements (177).
In IRB participation, pharmacists add complementary relevant expertise (162, 179). For example, in the protocol design, a pharmacist may define the parts related to drug information and medication management, including investigational drug management. This would cover the drug information section of the research protocol, as well as the assessment of the informed consent form for appropriate language on benefits and hazards related to adverse drug reactions, drug information supplements, patient logs, or other adherence procedures. In the review of the protocol for the IRB, pharmacists have specific training to evaluate the quality and practicability of the drug supply management, administration, and dosing strategy, the pertinence of additional regulatory requirements (e.g., Risk Evaluation and Mitigation Strategy programs), and medication safety strategies (165, 179, 180).
Pharmacists may also participate in the screening, assessing, and recruiting volunteers in CTs (181–185). Several experiences involving pharmacists in managing patients in clinical research in primary care have been communicated, with studies focused on pharmacological treatment of acute low back pain (181), preventive intervention of cardiovascular events (185), atrial fibrillation screening (183), and assessing outcomes of enhanced chronic disease care through patient education (186).
The benefits of pharmacist participation in CTs have been described. Different surveys indicate that pharmacists consider that their participation in CTs implies improved relationships and communication with other healthcare professionals, enhanced training and knowledge, exploration of personal interests, and development of the pharmacy profession (172, 187, 188).
New models of work using remote communication workflows have implied readaptation of the investigational drug services. Site qualification visits, site initiation visits, sponsor monitoring visits during a clinical trial, mailing of oral investigational products to patients, and virtual sponsor audits have been widely implemented following the coronavirus disease 2019 (COVID-19) pandemic outbreak and are now standards. Its use must be adapted to the characteristics of each CT and patient (163).
Specific certifications for clinical trial pharmacists are less abundant than other CT support profiles (189). The American Society of Health-System Pharmacists (ASHSP) has defined five required competency areas, 14 goals, 51 objectives, and 305 criteria for investigational drugs services and research pharmacy residence programs (Table 11). This program defines the 69 key topics designed to provide comprehensive training for the residents across a spectrum of research concepts, regulatory requirements, performance measures, information technology, and leadership principles (190, 191).
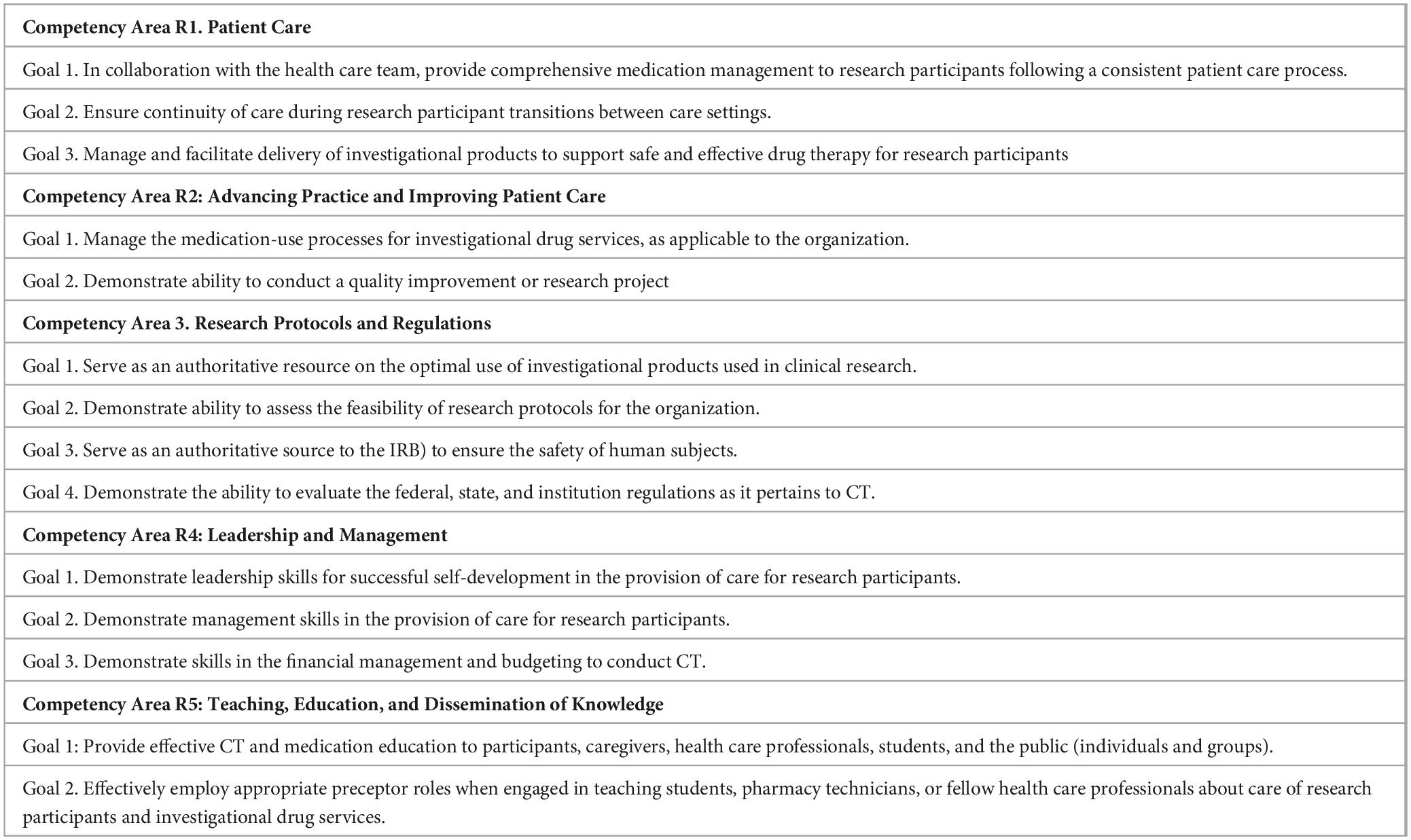
Table 11. Required competency areas and goals defined by the for investigational drugs and research pharmacy residencies (190).
Accordingly, several programs focused on Investigational Drugs & Research pharmacy residency training have been developed, some accredited by reference associations such as the ASHSP. The training program requires at least 3,000 h of fellowship devoted to research-related activities over 2 years. Detailed information about the research pharmacy residency in reference hospitals is accessible (192, 193).
Implementing investigational drug services and high-quality drug management in CTs implies a comprehensive approach. It needs to provide adequate resources and implement detailed processes that involve computer software, barcoding, expiration date tracking, order templates, clinical decision support, and pharmacy-independent double checks at the point of dispensing, in addition to many of the legal and accreditation requirements already mentioned (165).
One of the main concerns for CT pharmacists is medication safety. Safe practices in medication management in CTs have not been standardized, and several areas of the operative medication-use process may pose specific safety risks during clinical research. For example, the repackaging process implies contamination risks. Storing investigational drugs may require particular facilities as they must be separated by protocol and strength to reduce the risk of incorrect product selection during dispensing (165, 168).
Perceptions toward practice-based research of pharmacists have also been analyzed in the literature, showing a consistently high degree of interest in research in general, with percentages of 70–87%. However, studies show high variability in the proportion of pharmacists who had experience with practice-based research (30–77%) and were confident they could conduct practice-based research (34–73%) (172, 188). Different surveys indicate that the main barriers identified are low research training and management priority, lack of funding, time, resources, and culture, as well as opportunities and awareness of opportunities (172, 187, 188).
Although not specific to CTs, deploying computerized provider order entry systems is complex. It implies a straight collaboration framework with clinicians to contribute to the safer use of medications (194). Integrating electronic health records in CTs can increase generalizability, reduce costs and time, expand the research fields, and associate with various stakeholder benefits (195). However, it poses significant challenges, including infrastructure costs, interoperability, standardization, data quality, ethics, privacy, and data security considerations. The implementation involves different stakeholders in the healthcare arena, CT pharmacists being one essential (196).
3D printing helps facilitate rapid CT prototyping, automate compounding processes in pharmacies, and ensure precise dosages and drug combinations while enhancing precision, efficiency, and patient safety (197, 198). However, there are still obstacles, such as regulatory considerations, material selection, and quality control. Despite these challenges, 3D printing is a promising technology that can revolutionize pharmaceutical innovation due to ongoing advancements.
The CT monitor (or clinical research associate) is responsible for overseeing the progress of a CT and ensuring that it is conducted, recorded, and reported according to the protocol, standard operation procedures, GCP (3), and the Medicines for Human Use Regulations (199). GCP (3) explicitly states that the sponsor should appoint monitors according to the extent and nature of monitoring based on considerations such as the objective, purpose, design, complexity, blinding, size, and endpoints of CTs.
Information about monitoring principles and different guidance are available on the websites of the leading international regulatory agencies such as the European Medicines Agency, FDA, Medicines and Healthcare Products Regulatory Agency, or other research or research supporting institutions such as CTs Transformation Initiative, Health Research Authority, International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, National Institute for Health Research, TransCelerate Biopharma Inc or UK Trial Manager Network (199).
Five main purposes have been defined for CT monitors: that the rights and well-being of the human subjects are protected, the reported trial data are accurate, complete, and verifiable from source documents, the conduct of the trial is following the currently approved protocol/amendment(s), GCP and the applicable regulatory requirements, improve the way the trial is running and prevention of pitfalls (3, 199, 200). According to GCP (3), these five aims are deployed in a list of responsibilities (Table 12).
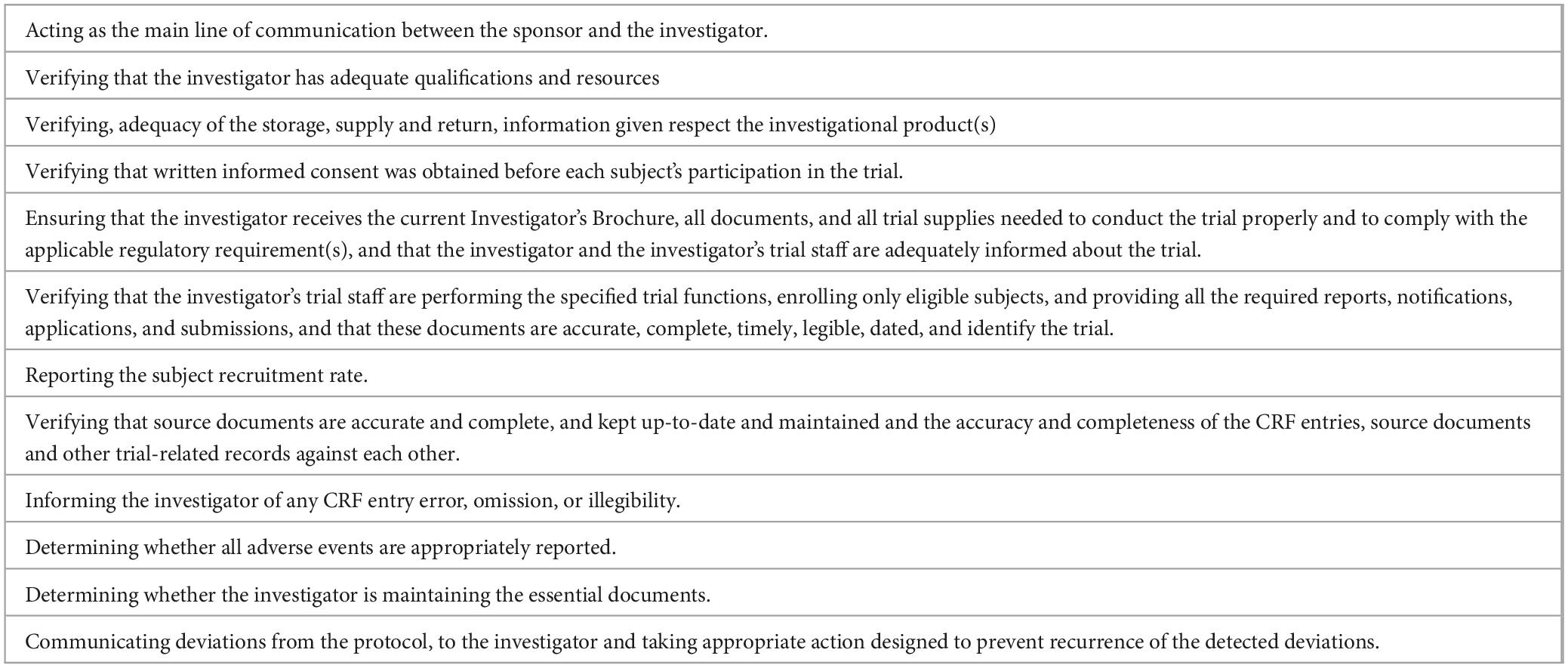
Table 12. Monitor responsibilities [modified from (3)].
In daily life, monitoring activity requires deploying all the skills related to the different roles they are involved in, such as trainers, planners, trouble-shooters, negotiators, detectives, or psychologists (201). Some of the specific tasks of the monitor during the initial CT visit include determining whether the administrative file is complete and in order, checking that the protocol is signed, and attaching the investigator’s curriculum vitae. After this initial step, the monitor ensures the application and respect of good clinical practices, particularly strict adherence to the protocol, reviews the trial data after having consulted the clinical files, checks their authenticity and coherence, determines that the randomization order has been respected, assures the providing of the necessary products to the investigator for conducting the trial such as drug packages and materials for biological assays, and ensures that communication is maintained throughout the trial (200, 202–204).
Each described CT monitor responsibility is deployed according to standard operating procedures that itemize every task that must be deployed. For example, pharmacy monitoring includes documentation and delegation log review, protocol and SOP compliance, investigational medicinal product storage check, labelling expiry dates randomization, accountability, and breaking blind procedure, and each of these elements includes detailed activities (200, 205).
Planning is a relevant issue related to monitoring activities. A monitoring plan is a relevant part of the activity to ensure optimal monitoring of the CT, according to the specific risks to human subject protection and data integrity of each CT. The plan should describe the monitoring strategy, the responsibilities of all parties involved, the different monitoring methods, and the rationale for their use. The plan should also emphasize monitoring critical data and processes (3).
Although the monitors are external to the organizations, they must have direct access to the original medical records of the trial subjects to verify the trial procedures and/or data (199). This must be done without violating the subject’s confidentiality, to the extent permitted by applicable laws and regulations, and the subject must authorize such access by signing a written informed consent form (3).
The cost of monitoring is relevant. It has been estimated that the average cost per site visit of a monitor in an oncology CT is around 1.500$ (206), and about 25% of the cost of a CT is occasioned by on-site monitoring activities (207). Monitoring activity before, during, and after the CTs is usually developed on-site. However, central monitoring is also an option for specific circumstances considering each CT’s objective, purpose, design, complexity, blinding, size, and endpoints (3).
The Association of Clinical Research Professionals (ACRP) has defined a Core Competency Framework for Clinical Study Monitoring with four different levels (entry-level, intermediate, senior, and clinical lead), which include domain, competency, and expectation (Table 13). More deeply considered issues are related to managing confidential information, the informed consent process, and the need to communicate with the team’s participants in the CTs (130).
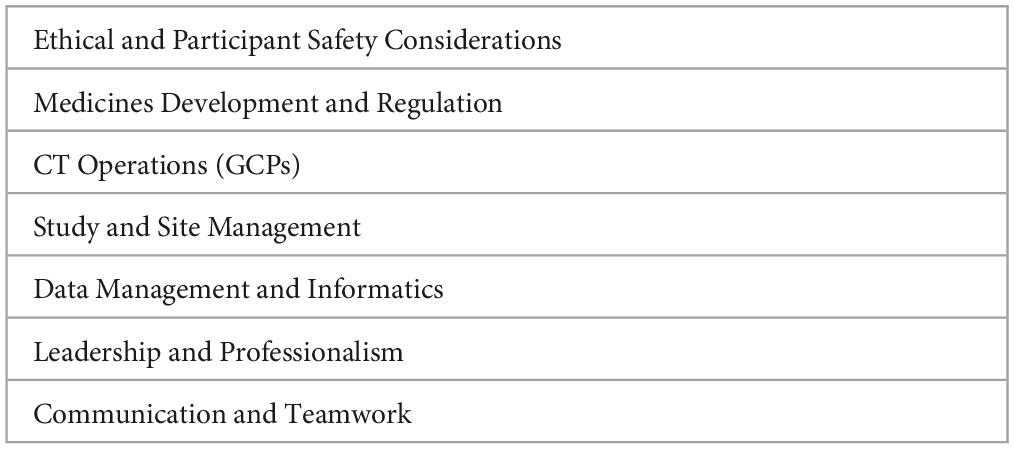
Table 13. Core competency framework for clinical study monitoring (130).
Indeed, becoming a clinical trial monitor does not necessarily require formal education, as various pathways are available for anyone with a high school diploma or higher (208). Information concerning the academic background of the monitors is limited. In a Children’s Oncology Group report from the National Cancer Institute (209) that analyzed the cause of turnover in a survey of over 456 monitors, the level of education was a bachelor’s degree at 47% and a graduate degree at 28% (210).
Despite the absence of a certification requirement, obtaining certification in the United States can offer more job prospects and even higher salaries (210). Various training programs for clinical trial monitor training are available (211, 212). The Society of Clinical Research Associates (SOCRA) (213, 214), the Association of Clinical Research Professionals (ACRP), Certified Clinical Research Professionals Society (CCRPS) (215), among others, provide certifications. Also, different University programs are available (208).
Updated statistics from the United States indicate that the average salary of a clinical trial monitor is $73,700/year (216). A senior clinical trial monitor (minimum 2 years of clinical monitoring experience, remote work) is around $ 120.000–135.000 per year, and some employment offers include a benefits package and an annual performance bonus. A lead clinical trial monitor reaches salaries (accordingly published offers in the United States) of around $150.000–170.000/year (217).
There is a growing concern about the effectiveness and efficiency of monitoring practices and a need for empirical evidence to determine which practices best achieve the goals of trial monitoring. Risk-based monitoring has been proposed as an efficient way to cover most of the activities developed by on-site monitoring or even detect flaws better and sooner than on-site monitoring (177, 178, 218, 219). Risk-based monitoring combines centralized follow-up for most of the reviews and on-site for further support and has been studied with results that suggest that it improves data quality regarding data points of major importance to trial outcomes, efficacy, and significant safety requiring less than 50% of extensive on-site monitoring resources (204, 219–222). Based on this data, international recommendations promote risk-based monitoring (3, 223, 224), although most small single-site academic studies apply traditional approaches (91, 221, 225).
CT monitor turnovers are high, and data from different countries indicate that the percentage of annual turnover in 2015–2019 was around 25% due at least in part to a low salary, unmanageable workload, lack of career advancement, and limited research commitment from the medical team (226). The main factors considered to monitor for staying in their jobs are a supportive principal investigator and satisfaction working with colleagues (209).
The CT monitor position and adaptation capability are recognized as valuable. On the other hand, CT monitor are motivated and excited about how they can contribute to the health and well-being of others and their career by rapidly learning critical skills and fast adaptation capabilities. During the COVID-19 pandemic, a rapid transition to decentralizing to monitoring activity has occurred (227). In this scenario, companies are creating retention programs focused on building more connections among professionals and reducing “travel fatigue”, creating a professional pathway and annual incentives (226).
While CT monitors currently play a crucial role in healthcare, their importance is projected to diminish in centralized models where patients can provide much of the necessary information directly and through electronic health records in an automated way. Wearables have immense potential for automated patient monitoring and will be an integral part of the future of healthcare models alongside CT monitoring (228).
CTs are becoming increasingly complex and require trained teams of expert professionals to support various activities with maximum rigor, adhering to ethical and technical international requirements. Healthcare professionals, including principal investigators, CRCs, nurses, pharmacists, and monitors, have pivotal roles in the successful execution of CT. Clearly defining roles, providing adaptive and continuous professional training, and fostering a culture of collaboration and communication are pivotal in ensuring success in this field. Addressing recruitment, retention, and professional growth challenges is also essential.
Although professional profiles and roles of the experts involved in CTs are not well delineated, some roles and functions are recognized internationally as essential for its deployment, especially in highly complex CTs. In this sense, a relevant advance has taken place in recent years. As CTs gain weight as a relevant part of the activity of worldwide leader healthcare systems, better delineation of roles, training, and a higher degree of professionalization are desirable.
Across all roles, there is a clear need for more standardized, formalized, and accessible training programs to ensure that professionals are equipped to meet the demands of their roles in clinical trials. Also, a collaborative approach and enhanced training programs tailored to each professional role’s specific needs and challenges within the CT framework are convenient. All professionals involved in clinical trials must navigate a complex landscape of ethical and regulatory requirements and new technology advances, underscoring the need for continuous education and adherence to ethical standards.
Specific strategies are needed to front the challenges related to the professionals involved in CT in the healthcare arena. Principal investigators should focus on enhancing protocol compliance through rigorous training in GCP and regulatory updates, ensuring they are well-versed in the latest standards and delegated in adequately trained teams. For clinical research coordinators, implementing project management tools and comprehensive training in patient interaction and data management systems is advised to effectively manage the trial’s increased complexity. Monitors could benefit from advanced training in risk-based monitoring techniques and digital monitoring tools to enhance the efficiency and effectiveness of site visits and remote monitoring tasks. Nurses, who often face direct patient care challenges, should receive ongoing support in patient management systems and be given access to continuous education in trial-specific procedures and treatments. These steps will enhance individual performance and improve clinical trials’ overall functioning, ensuring data integrity, participant safety, and regulatory compliance.
Digitalization can streamline many aspects of CT processes, from patient recruitment and data collection to protocol compliance and real-time monitoring. This improves efficiency, enhances data integrity, and reduces the administrative burden on staff. For instance, electronic health records and mobile health applications could be more extensively utilized to facilitate seamless communication and accurate data tracking.
Future studies could explore the specific impacts of digital technologies on trial efficiency and participant safety, the effectiveness of new training modalities, and the long-term benefits of these innovations on clinical trial outcomes. Research into the development of a standardized framework for integrating digital tools across various stages of clinical trials could also provide valuable insights and guidance for the field.
GP: Writing–review and editing, Writing–original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Conceptualization. BS-S: Writing–review and editing, Writing–original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Conceptualization.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Instituto de Investigación Marqués de Valdecilla (IDIVAL) (INT/A23).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Farber GK, Weiss L. Core facilities: Maximizing the return on investment. Sci Transl Med. (2011) 3:95cm21. doi: 10.1126/scitranslmed.3002421
2. Croghan IT, Viker SD, Limper AH, Evans TK, Cornell AR, Ebbert JO, et al. Developing a clinical trial unit to advance research in an academic institution. Contemp Clin Trials. (2015) 45:270–6. doi: 10.1016/j.cct.2015.10.001
4. Fleischmann R. Primer: Establishing a clinical trial unit – regulations and infrastructure. Nat Clin Pract Rheumatol. (2007) 3:234–9. doi: 10.1038/ncprheum0461
5. Food and Drug Administration. Code of federal regulations 21 CFR chapter I subchapter D- drugs for human use. Silver Spring, MD: Food and Drug Administration (2023).
6. European Commission. Regulation (EU) No 536/2014 of the European parliament and of the council of 16 april 2014 on clinical trials on medicinal products for human use, and repealing directive 2001/20/EC. Brussels: European Commission (2014).
7. World Medical Association. Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects. Ferney-Voltaire: World Medical Association (2022).
8. Lee C, Werner TL, Deal AM, Krise-Confair CJ, Bentz TA, Cummings TM, et al. Clinical trial metrics: The complexity of conducting clinical trials in North American cancer centers. JCO Oncol Pract. (2021) 17:e77–93. doi: 10.1200/OP.20.00501
9. Getz KA, Campo RA. Trial watch: Trends in clinical trial design complexity. Nat Rev Drug Discov. (2017) 16:307. doi: 10.1038/nrd.2017.65
10. Jones HM, Curtis F, Law G, Bridle C, Boyle D, Ahmed T. Evaluating follow-up and complexity in cancer clinical trials (EFACCT): An eDelphi study of research professionals’ perspectives. BMJ Open. (2020) 10:e034269. doi: 10.1136/bmjopen-2019-034269
11. Fujiwara N, Ochiai R, Shirai Y, Saito Y, Nagamura F, Iwase S, et al. Qualitative analysis of clinical research coordinators’ role in phase I cancer clinical trials. Contemp Clin Trials Commun. (2017) 8:156–61. doi: 10.1016/j.conctc.2017.09.009
12. Wolinetz CD, Tabak LA. Transforming clinical research to meet health challenges. JAMA. (2023) 329:1740–1. doi: 10.1001/jama.2023.3964
13. Sonstein SA, Jones CT. Joint task force for clinical trial competency and clinical research professional workforce development. Front Pharmacol. (2018) 9:1148. doi: 10.3389/fphar.2018.01148
14. Shanley TP, Calvin-Naylor NA, Divecha R, Wartak MM, Blackwell K, Davis JM, et al. Enhancing clinical research professionals’ training and qualifications (ECRPTQ): Recommendations for good clinical practice (GCP) training for investigators and study coordinators. J Clin Transl Sci. (2017) 1:8–15. doi: 10.1017/cts.2016.1
15. Calvin-Naylor NA, Jones CT, Wartak MM, Blackwell K, Davis JM, Divecha R, et al. Education and training of clinical and translational study investigators and research coordinators: A competency-based approach. J Clin Transl Sci. (2017) 1:16–25. doi: 10.1017/cts.2016.2
16. Baer AR, Zon R, Devine S, Lyss AP. The clinical research team. J Oncol Pract. (2011) 7:188–92. doi: 10.1200/JOP.2011.000276
17. Mora V, Colantuono S, Fanali C, Leonetti A, Wlderk G, Pirro MA, et al. Clinical research coordinators: Key components of an efficient clinical trial unit. Contemp Clin Trials Commun. (2023) 32:101057. doi: 10.1016/j.conctc.2023.101057
18. Tang C, Hess K, Sanders D, Davis S, Kurzrock R, Lee JJ, et al. Efficient clinical research infrastructure and trial performance: Assessment of a dedicated clinical trials unit within an academic cancer center. Eur J Cancer. (2016) 69:779. doi: 10.1016/s0959-8049(16)32779-4
19. Hind D, Reeves BC, Bathers S, Bray C, Corkhill A, Hayward C, et al. Comparative costs and activity from a sample of UK clinical trials units. Trials. (2017) 18:203. doi: 10.1186/s13063-017-1934-3
20. Lee P, Goldberg C, Kohane I. The AI revolution in medicine: GPT-4 and beyond. Boston, MA: Pearson Education, Inc (2023).
22. Food and Drug Administration. Code of federal regulations: 21 CFR Part 312 subpart D – responsibilities of sponsors and investigators. Silver Spring, MD: Food and Drug Administration (2023).
23. Polk HC, Bowden TA, Rikkers LF, Balch CM, Organ CH, Murie JA, et al. Scientific data from clinical trials: Investigators’ responsibilities and rights. Arch Surg. (2002) 137:1. doi: 10.1097/00005373-200206000-00001
24. Baer AR, Devine S, Beardmore CD, Catalano R. Clinical investigator responsibilities. J Oncol Pract. (2011) 7:124–8. doi: 10.1200/JOP.2010.000216
25. Feehan AK, Garcia-Diaz J. Investigator responsibilities in clinical research. Ochsner J. (2020) 20:44–9. doi: 10.31486/toj.19.0085
26. Sweedler JV. So you want to be a principal investigator? Anal Chem. (2014) 86:7159. doi: 10.1021/ac502543w
27. Saleh M, Naik G. So you want to be a principal investigator. J Oncol Pract. (2018) 14:e384–92. doi: 10.1200/JOP.18.00011
28. Food and Drug Administration. Investigator Responsibilities - protecting the rights, safety, and welfare of study subjects. Silver Spring, MD: Food and Drug Administration (2009).
29. Food and Drug Administration. Frequently asked questions – statement of investigator (form FDA 1572) information sheet guidance for irbs, clinical investigators, and sponsors. Silver Spring, MD: Food and Drug Administration (2010).
30. Food and Drug Administration. Informed consent guidance for IRBs, clinical investigators, and sponsors. Silver Spring, MD: Food and Drug Administration (2023).
31. Nijhawan LP, Janodia MD, Muddukrishna BS, Bhat KM, Bairy KL, Udupa N, et al. Informed consent: Issues and challenges. J Adv Pharm Technol Res. (2013) 4:134–40. doi: 10.4103/2231-4040.116779
32. Kelch RP. Maintaining the public trust in clinical research. N Engl J Med. (2002) 346:285–7. doi: 10.1056/NEJM200201243460413
33. Colombo C, Mayrhofer MT, Kubiak C, Battaglia S, Matei M, Lavitrano M, et al. The CORBEL matrix on informed consent in clinical studies: A multidisciplinary approach of research infrastructures building enduring life-science services. BMC Med Ethics. (2021) 22:95. doi: 10.1186/s12910-021-00639-x
34. Joffe S, Cook EF, Cleary PD, Clark JW, Weeks JC. Quality of informed consent: A new measure of understanding among research subjects. J Natl Cancer Inst. (2001) 93:139–47. doi: 10.1093/jnci/93.2.139
35. Food and Drug Administration. IRB responsibilities for reviewing the qualifications of investigators, adequacy of research sites, and the determination of whether an IND/IDE is needed. Silver Spring, MD: Food and Drug Administration (2013).
36. Dekking SA, van der Graaf R, Kars MC, Beishuizen A, de Vries MC, van Delden JJ. Balancing research interests and patient interests: A qualitative study into the intertwinement of care and research in paediatric oncology. Pediatr Blood Cancer. (2015) 62:816–22. doi: 10.1002/pbc.25444
37. Carazo S, Folkesson E, Anglaret X, Beavogui AH, Berbain E, Camara AM, et al. Challenges in preparing and implementing a clinical trial at field level in an Ebola emergency: A case study in Guinea, West Africa. PLoS Negl Trop Dis. (2017) 11:e0005545. doi: 10.1371/journal.pntd.0005545
38. Rivera SM. Clinical research from proposal to implementation: What every clinical investigator should know about the institutional review board. J Investig Med. (2008) 56:975–84. doi: 10.2310/JIM.0b013e31818e1da9
39. Simon GE, Shortreed SM, Rossom RC, Penfold RB, Sperl-Hillen JAM, O’Connor P. Principles and procedures for data and safety monitoring in pragmatic clinical trials. Trials. (2019) 20:690. doi: 10.1186/s13063-019-3869-3
40. Boeynaems JM, Canivet C, Chan A, Clarke MJ, Cornu C, Daemen E, et al. A European approach to clinical investigator training. Front Pharmacol. (2013) 4:112. doi: 10.3389/fphar.2013.00112
41. Magnin A, Iversen VC, Calvo G, Čečetková B, Dale O, Demlova R, et al. European survey on national training activities in clinical research. Trials. (2019) 20:616. doi: 10.1186/s13063-019-3702-z
42. Nalepinski CEG. PAs as principal investigators of FDA-regulated clinical trials. JAAPA. (2023) 36:40–2. doi: 10.1097/01.JAA.0000931416.49268.b2
43. Bayat M, Davis L, Knoell D, Korth-Bradley J, Munger M, Shaefer M, et al. The clinical pharmacist as principal investigator: A commentary from the american college of clinical pharmacy. Pharmacotherapy. (2000) 20:35165. doi: 10.1592/phco.20.6.599.35165
44. Ali S, Karakitsos D. Clinical pharmacists as principal investigators in clinical trials”. Encyclop Pharm Pract Clin Pharm. (2019) 18:444. doi: 10.1016/B978-0-128-12735-3.00144-8
45. Rosenzweig MQ, Bender CM, Brufsky AM. The nurse as principal investigator in a pharmaceutically sponsored drug trial: Considerations and challenges. Oncol Nurs Forum. (2005) 32:293–9. doi: 10.1188/05.ONF.293-299
46. Burton ME, Munger MA, Bednarczyk EM, Davis LE, Davis GA, Elliott ME, et al. Update: The clinical pharmacist as principal investigator. Pharmacotherapy. (2010) 30:1314. doi: 10.1592/phco.30.12.1314
47. Okubanjo T, Gonzalez LS. Pharmacists as investigators in FDA-regulated drug trials. Am J Health Syst Pharm. (2008) 65:1010–1. doi: 10.2146/ajhp070649
48. Glasziou P, Altman DG, Bossuyt P, Boutron I, Clarke M, Julious S, et al. Reducing waste from incomplete or unusable reports of biomedical research. Lancet. (2014) 383:267–76. doi: 10.1016/S0140-6736(13)62228-X
50. University of Maryland. Clinical investigator training course. College Park, MD: University of Maryland (2023).
51. Washington University in St. Louis. Graduate certificate in clinical investigation | clinical research training center. St. Louis, MO: Washington University in St. Louis (2023).
52. Swezey T, McGuire FH, Hurley P, Panhuis J, Goldstein K, Chuck T, et al. More than a box to check: Research sponsor and clinical investigator perspectives on making GCP training relevant. Contemp Clin Trials Commun. (2020) 19100606. doi: 10.1016/j.conctc.2020.100606
53. Saleh M, Naik G, Jester P, Joiner C, Westfall E, Kimberlin DW, et al. Clinical investigator training program (CITP) – a practical and pragmatic approach to conveying clinical investigator competencies and training to busy clinicians. Contemp Clin Trials Commun. (2020) 19:100589. doi: 10.1016/j.conctc.2020.100589
54. Moskowitz J, Thompson JN. Enhancing the clinical research pipeline: Training approaches for a new century. Acad Med. (2001) 76:307–15. doi: 10.1097/00001888-200104000-00004
55. Akroyd D, Kathryn H. Does PI certification make a difference?” Applied clinical trials. (2018). Available online at: https://www.appliedclinicaltrialsonline.com/view/does-pi-certification-make-difference (accessed December 12, 2023).
56. Lader EW, Cannon CP, Ohman EM, Newby LK, Sulmasy DP, Barst RJ, et al. The clinician as investigator: Participating in clinical trials in the practice setting: Appendix 1: fundamentals of study design. Circulation. (2004) 109:e302–4. doi: 10.1161/01.CIR.0000128807.57602.61
57. Cecchini M, Rubin EH, Blumenthal GM, Ayalew K, Burris HA, Russell-Einhorn M, et al. Challenges with novel clinical trial designs: Master protocols. Clin Cancer Res. (2019) 25:2049–57. doi: 10.1158/1078-0432.CCR-18-3544
58. Dombeck CB, Hinkley T, Fordyce CB, Blanchard K, Roe MT, Corneli A. Continued investigator engagement: Reasons principal investigators conduct multiple FDA-regulated drug trials. Contemp Clin Trials Commun. (2019) 17:100502. doi: 10.1016/j.conctc.2019.100502
59. Corneli A, Pierre C, Hinkley T, Lin L, Fordyce CB, Hamre G, et al. One and done: Reasons principal investigators conduct only one FDA-regulated drug trial. Contemp Clin Trials Commun. (2017) 6:31–8. doi: 10.1016/j.conctc.2017.02.009
60. Paget SP, Caldwell PH, Murphy J, Lilischkis KJ, Morrow AM. Moving beyond ‘not enough time’: Factors influencing paediatric clinician’ participation in research. Intern Med J. (2017) 47:299–306. doi: 10.1111/imj.13351
61. Getz K, Brown C, Stergiopoulos S, Beltre C. Baseline assessment of a global clinical investigator landscape poised for structural change. Ther Innov Regul Sci. (2017) 51:575–81. doi: 10.1177/2168479017701504
63. Fordyce CB, Malone K, Forrest A, Hinkley T, Corneli A, Topping J, et al. Improving and sustaining the site investigator community: Recommendations from the clinical trials transformation initiative. Contemp Clin Trials Commun. (2019) 16:100462. doi: 10.1016/j.conctc.2019.100462
64. Fordyce CB, Roe MT, Pierre C, Hinkley T, Hamre G, Tenaerts P, et al. Trends in clinical trial investigator workforce and turnover: An analysis of the U.S. FDA 1572 BMIS database. Contemp Clin Trials Commun. (2019) 15:100380. doi: 10.1016/j.conctc.2019.100380
65. Unger JM, Vaidya R, Hershman DL, Minasian LM, Fleury ME. Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. J Natl Cancer Inst. (2019) 111:245–55. doi: 10.1093/jnci/djy221
66. Mentz RJ, Peterson ED. Site principal investigators in multicenter clinical trials: Appropriately recognizing key contributors. Circulation. (2017) 135:1185–7. doi: 10.1161/CIRCULATIONAHA.116.026650
67. Somkin CP, Altschuler A, Ackerson L, Geiger AM, Greene SM, Mouchawar J, et al. Organizational barriers to physician participation in cancer clinical trials. Am J Manag Care. (2005) 11:413–21.
68. Jacobs SR, Weiner BJ, Reeve BB, Weinberger M, Minasian LM, Good MJ. Organizational and physician factors associated with patient enrollment in cancer clinical trials. Clin Trials. (2014) 11:565–75. doi: 10.1177/1740774514536000
69. Clark LT, Watkins L, Piña IL, Elmer M, Akinboboye O, Gorham M, et al. Increasing diversity in clinical trials: Overcoming critical barriers. Curr Probl Cardiol. (2019) 44:148–72. doi: 10.1016/j.cpcardiol.2018.11.002
70. Parra A, Karnad AB, Thompson IM. Hispanic accrual on randomized cancer clinical trials: A call to arms. J Clin Oncol. (2014) 32:1871–3. doi: 10.1200/JCO.2013.51.7946
71. Yong C, Suvarna A, Harrington R, Gummidipundi S, Krumholz HM, Mehran R, et al. Temporal trends in gender of principal investigators and patients in cardiovascular clinical trials. J Am Coll Cardiol. (2023) 81:428–30. doi: 10.1016/j.jacc.2022.10.038
72. Luong VTT, Ho C, Aedo-Lopez V, Segelov E. Gender profile of principal investigators in a large academic clinical trials group. Front Surg. (2022) 9:962120. doi: 10.3389/fsurg.2022.962120
73. Nguyen Q, Luc JGY, Lawton JS, Chikwe J, Cornwell LD, Simpson K, et al. Sex representation among principal investigators in cardiac surgery clinical trials in the united states: The glass ceiling and room for improvement. Ann Surg. (2022) 276:e1101–6. doi: 10.1097/SLA.0000000000004961
74. Jou J, Brodsky A, Charo L, Binder P, Saenz C, Eskander RN, et al. Trends and geographic variation in women’s representation as principal investigators (PI) in phase 3 gynecologic oncology clinical trials. Gynecol Oncol. (2021) 162:389–93. doi: 10.1016/j.ygyno.2021.05.037
75. Ludmir EB, Moningi S, Mainwaring W, Miller AB, Lin T, Jethanandani A, et al. Women’s Representation among Lead Investigators of clinical trials in oncology. Int J Radiat Oncol Biol Phys. (2019) 105:E465–6. doi: 10.1016/j.ijrobp.2019.06.1430
76. Freedman B. Equipoise and the ethics of clinical research. In: Friedmann W, Sprecher DA editors. Human experimentation and research. Milton Park: Routledge (2017). doi: 10.4324/9781315244426-17
77. American Medical Association. “AMA code of medical ethics’ opinions on clinical research | journal of ethics”. Chicago, IL: American Medical Association (2023).
78. Morain SR, Joffe S, Largent EA. When is it ethical for physician-investigators to seek consent from their own patients? Am J Bioeth. (2019) 19:11–8. doi: 10.1080/15265161.2019.1572811
79. CIOMS. Revised CIOMS international ethical guidelines for health-related research involving humans. JAMA. (2017) 317:135–6. doi: 10.1001/jama.2016.18977
80. Baker JN, Leek AC, Salas HS, Drotar D, Noll R, Rheingold SR, et al. Suggestions from adolescents, young adults, and parents for improving informed consent in phase 1 pediatric oncology trials. Cancer. (2013) 119:4154–61. doi: 10.1002/cncr.28335
81. Eichler HG, Sweeney F. The evolution of clinical trials: Can we address the challenges of the future? Clin Trials. (2018) 15:27–32. doi: 10.1177/1740774518755058
82. Benchoufi M, Porcher R, Ravaud P. Blockchain protocols in clinical trials: Transparency and traceability of consent. F1000Res. (2017) 6:66. doi: 10.12688/f1000research.10531.5
83. Askin S, Burkhalter D, Calado G, El Dakrouni S. Artificial intelligence applied to clinical trials: Opportunities and challenges. Health Technol (Berl). (2023) 13:203–13. doi: 10.1007/s12553-023-00738-2
84. Inan OT, Tenaerts P, Prindiville SA, Reynolds HR, Dizon DS, Cooper-Arnold K, et al. Digitizing clinical trials. NPJ Digit Med. (2020) 3:101. doi: 10.1038/s41746-020-0302-y
85. Tan RKJ, Wu D, Day S, Zhao Y, Larson HJ, Sylvia S, et al. Digital approaches to enhancing community engagement in clinical trials. NPJ Digit Med. (2022) 5:37. doi: 10.1038/s41746-022-00581-1
86. Rosa C, Marsch LA, Winstanley EL, Brunner M, Campbell ANC. Using digital technologies in clinical trials: Current and future applications. Contemp Clin Trials. (2021) 100:106219. doi: 10.1016/j.cct.2020.106219
87. Polhemus AM, Kadhim H, Barnes S, Zebrowski SE, Simmonds A, Masand SN, et al. Accelerating adoption of patient-facing technologies in clinical trials: A pharmaceutical industry perspective on opportunities and challenges. Ther Innov Regul Sci. (2019) 53:8–24. doi: 10.1177/2168479018801566
88. De Brouwer W, Patel CJ, Manrai AK, Rodriguez-Chavez IR, Shah NR. Empowering clinical research in a decentralized world. NPJ Digit Med. (2021) 4:102. doi: 10.1038/s41746-021-00473-w
89. Jones CT, Griffith CA, Fisher CA, Grinke KA, Keller R, Lee H, et al. Nurses in clinical trials: Perceptions of impact on the research enterprise. J Res Nurs. (2022) 27:50–65. doi: 10.1177/17449871211073757
90. ANA, IACRN. Clinical research nursing: Scope and standards of practice. Silver Spring, MD: American Nurses Association International Association of Clinical Research Nurses (2015).
91. Olsen R, Bihlet AR, Kalakou F, Andersen JR. The impact of clinical trial monitoring approaches on data integrity and cost–a review of current literature. Eur J Clin Pharmacol. (2016) 72:399–412. doi: 10.1007/s00228-015-2004-y
92. Park HJ, Yu S. The role of clinical trial nurses: Transitioning from clinicians to clinical research coordinators. Int J Nurs Pract. (2022) 28:e12943. doi: 10.1111/ijn.12943
93. Brinkman-Denney S. An international comparison of the clinical trials nurse role. Nurs Manag (Harrow). (2013) 20:32–40. doi: 10.7748/nm2013.12.20.8.32.e1060
94. Ness EA, Royce C. Clinical Trials and the Role of the Oncology Clinical Trials Nurse. Nurs Clin North Am. (2017) 52:133–48. doi: 10.1016/j.cnur.2016.10.005
95. Braun-Inglis C, Boehmer LM, Zitella LJ, Hoffner B, Shvetsov YB, Berenberg JL, et al. Role of oncology advanced practitioners to enhance clinical research. J Adv Pract Oncol. (2022) 13:107–19. doi: 10.6004/jadpro.2022.13.2.2
96. Salmanton-García J, Stewart FA, Wipfler P, Hofstraat SHI, Bruijning-Verhagen P, Cornely OA. Education pathways and key tasks for research nurses in Europe, results from a VACCELERATE online survey. Nurse Educ Pract. (2024) 77:103953. doi: 10.1016/j.nepr.2024.103953
97. NIH Clinical Center. Nursing at the NIH clinical center. Bethesda, MD: NIH Clinical Center (2024).
98. Purdom MA, Petersen S, Haas BK. Results of an oncology clinical trial nurse role delineation study. Oncol Nurs Forum. (2017) 44:589–95. doi: 10.1188/17.ONF.589-595
99. Poston RD, Buescher CR. The essential role of the clinical research nurse (CRN). Urol Nurs. (2010) 30:55–63. doi: 10.7257/1053-816x.2010.30.1.55
100. Burks AC, Keim-Malpass J. Health literacy and informed consent for clinical trials: A systematic review and implications for nurses. Nurs Res Rev. (2019) 9:s207497. doi: 10.2147/nrr.s207497
101. Isaacman DJ, Reynolds EA. Effect of a research nurse on patient enrollment in a clinical study. Pediatr Emerg Care. (1996) 12:340–2. doi: 10.1097/00006565-199610000-00004
102. Good M, Schuler L. Subject retention in a controlled clinical trial. J Adv Nurs. (1997) 26:351–5. doi: 10.1046/j.1365-2648.1997.1997026351.x
103. McKinney J, Vermeulen W. Research nurses play a vital role in clinical trials. Oncol Nurs Forum. (2000) 27:28.
105. Alt-White AC, Pranulis MF. Addressing nurses’ ethical concerns about research in critical care settings. Nurs Adm Q. (2006) 30:67–75. doi: 10.1097/00006216-200601000-00010
106. Grady C, Edgerly M. Science, technology, and innovation: Nursing responsibilities in clinical research. Nurs Clin North Am. (2009) 44:471–81. doi: 10.1016/j.cnur.2009.07.011
107. Wallen G, Fisher C. Clinical research nursing: A new domain of practice: principles and practice of clinical research. Amsterdam: Elsevier (2018). doi: 10.1016/B978-0-12-849905-4.00039-3
108. CRN Domain of Practice Committee. Building the foundation for clinical research nursing. domain of practice for the specialty of clinical research nursing. Bethesda, MD: CRN 2010 Domain of Practice Committee (2009).
109. Sun J, Shan WC, Liu JM, Zhang QQ, Ye Y, Huang ST, et al. Construction of clinical research nurse training program based on position competence. World J Clin Cases. (2023) 11:7363–71. doi: 10.12998/wjcc.v11.i30.7363
110. International Association of Clinical Research Nurses. About IACRN. Pittsburgh, PA: International Association of Clinical Research Nurses (2023).
111. Capili B, Baker L, Thangthaeng N, Legor K, Larkin ME, Jones CT. Development and evaluation of a clinical research nursing module for undergraduate nursing schools: Expanding clinical research nurses’ outreach. J Res Nurs. (2022) 27:68–77. doi: 10.1177/17449871211070972
112. Ness E. The oncology clinical research nurse study co-ordinator: Past, present, and future. Asia Pac J Oncol Nurs. (2020) 7:237–42. doi: 10.4103/apjon.apjon_10_20
113. The Oncology Nursing Society. Manual for clinical trials nursing”. Controlled clinical trials. Pittsburgh, PA: The Oncology Nursing Society (2015).
114. Lubejko B, Good M, Weiss P, Schmieder L, Leos D, Daugherty P. Oncology clinical trials nursing: Developing competencies for the novice. Clin J Oncol Nurs. (2011) 15:637–43. doi: 10.1188/11.CJON.637-643
115. Ness E, Ermete R, Leos D, Schmieder L, Behrens L, Brassil KJ, et al. Oncology clinical trials nurse competencies. Pittsburgh, PA: Oncology Nursing Society (2016).
116. Ehrenberger HE, Lillington L. Development of a measure to delineate the clinical trials nursing role. Oncol Nurs Forum. (2004) 31:E64–8. doi: 10.1188/04.ONF.E64-E68
117. Margevicius S, Daly B, Schluchter M, Flocke S, Manne S, Surdam J, et al. Randomized trial of a web-based nurse education intervention to increase discussion of clinical trials. Contemp Clin Trials Commun. (2021) 22:100789. doi: 10.1016/j.conctc.2021.100789
118. Smiley RA, Allgeyer RL, Shobo Y, Lyons KC, Letourneau R, Zhong E, et al. The 2022 national nursing workforce survey. J Nurs Regul. (2023) 14:9. doi: 10.1016/S2155-8256(23)00047-9
119. Johnson E. Trouble for trials – The worrying state of the research nurse workforce. Contemp Clin Trials. (2022) 120:106878. doi: 10.1016/j.cct.2022.106878
120. Challinor JM, Alqudimat MR, Teixeira TOA, Oldenmenger WH. Oncology nursing workforce: Challenges, solutions, and future strategies. Lancet Oncol. (2020) 21:e564–74. doi: 10.1016/S1470-2045(20)30605-7
121. Larkin ME, Beardslee B, Cagliero E, Griffith CA, Milaszewski K, Mugford MT, et al. Ethical challenges experienced by clinical research nurses: A qualitative study. Nurs Ethics. (2019) 26:172–84. doi: 10.1177/0969733017693441
122. Höglund AT, Helgesson G, Eriksson S. Ethical dilemmas and ethical competence in the daily work of research nurses. Health Care Anal. (2010) 18:239–51. doi: 10.1007/s10728-009-0126-z
123. Shields AM, Larue EM. Transitioning from clinician to clinical research coordinator. Am J Nurs. (2010) 110:26–7. doi: 10.1097/01.NAJ.0000366159.25823.55
124. Campagnaro EL, Margevicius S, Daly BJ, Eads JR, Kinzy TG, Liu TM, et al. Health care worker attitudes about clinical trials at a comprehensive cancer center. J Clin Oncol. (2013) 31:e20633. doi: 10.1200/jco.2013.31.15_suppl.e20633
125. Godskesen T, Björk J, Juth N. Challenges regarding informed consent in recruitment to clinical research: A qualitative study of clinical research nurses’ experiences. Trials. (2023) 24:801. doi: 10.1186/s13063-023-07844-6
126. Rico-Villademoros F, Hernando T, Sanz JL, López-Alonso A, Salamanca O, Camps C, et al. The role of the clinical research coordinator–data manager–in oncology clinical trials. BMC Med Res Methodol. (2004) 4:6. doi: 10.1186/1471-2288-4-6
127. Caminiti C, Maglietta G, Frau I, Peruzzotti G, Felisi M, van Dijk A. Presence and activities of clinical research coordinators at Italian Health Care Institutions: A national cross-sectional survey. J Clin Transl Sci. (2021) 6:e1. doi: 10.1017/cts.2021.872
128. Vanhoff D, Hesser T, Kelly KP, Freyer D, Stork S, Sung L. Facilitating accrual to cancer control and supportive care trials: The clinical research associate perspective. BMC Med Res Methodol. (2013) 13:154. doi: 10.1186/1471-2288-13-154
129. Association of Clinical Research Professionals. Are clinical research coordinators recognized as professionals? Clin Res. (2021) 35:2056.
130. Association of Clinical Research Professionals. ACRP Core competency framework for clinical study monitoring. Alexandria, VA: Association of Clinical Research Professionals (2021).
131. Association of Clinical Research Professionals. (2023). Available online at: https://acrpnet.org/ (accessed December 29, 2023).
132. Campasano M, Itani KMF. Regulatory considerations: The clinical research coordinator. En Clinical trials design in operative and non operative invasive procedures. Cham: Springer (2017). doi: 10.1007/978-3-319-53877-8_33
133. Cingi C, Muluk NB. The duties of a clinical research coordinator: Quick guide to good clinical practice. Switzerland: Springer (2017). doi: 10.1007/978-3-319-44344-7_10
134. Loh WY, Butow PN, Brown RF, Boyle F. Ethical communication in clinical trials. Issues faced by data managers in obtaining informed consent. Cancer. (2002) 95:2414–21. doi: 10.1002/cncr.10994
135. Pelke S, Easa D. The role of the clinical research coordinator in multicenter clinical trials. J Obstet Gynecol Neonatal Nurs. (1997) 26:279–85. doi: 10.1111/j.1552-6909.1997.tb02143.x
136. Willis C, Bratcher K, Kenworthy-Heinige T, McBurney C, Asghar A, Beck D, et al. The anatomy of a great clinical research coordinator. Clin Res. (2018) 32:4865.
137. Cagnazzo C, Testoni S, Guarrera AS, Stabile S, Taverniti C, Federici I, et al. [Clinical research coordinators: A crucial resource]. Recenti Prog Med. (2019) 110:65–7. doi: 10.1701/3112.31000
138. Yanagawa H, Akaishi A, Miyamoto T, Takai S, Nakanishi R, Irahara M. Role of clinical research coordinators in promoting clinical trials of drugs for surgical patients. Int Arch Med. (2008) 1:26. doi: 10.1186/1755-7682-1-26
139. Fisher E, Thomas RS, Higgins MK, Williams CJ, Choi I, McCauley LA. Finding the right candidate: Developing hiring guidelines for screening applicants for clinical research coordinator positions. J Clin Transl Sci. (2021) 6:e20. doi: 10.1017/cts.2021.853
140. Fisher JA, Kalbaugh CA. Altruism in clinical research: Coordinators’ orientation to their professional roles. Nurs Outlook. (2012) 60:143–8. doi: 10.1016/j.outlook.2011.10.002
141. Morain SR, Barlevy D, Joffe S, Largent EA. Physician-investigator, research coordinator, and patient perspectives on dual-role consent in oncology: A qualitative study. JAMA Netw Open. (2023) 6:e2325477. doi: 10.1001/jamanetworkopen.2023.25477
142. Shihabuddin BS, Fritter J, Ellison AM, Cruz AT. Diversity among research coordinators in a pediatric emergency medicine research collaborative network. J Clin Transl Sci. (2022) 7:e46. doi: 10.1017/cts.2022.448
143. Jackson M. Good financial practice and clinical research coordinator responsibilities. Semin Oncol Nurs. (2020) 36:150999. doi: 10.1016/j.soncn.2020.150999
144. MedCity. Top 10 FDA warning letter findings for 2011. (2023). Available online at: https://medcitynews.com/2012/02/top-10-fda-warning-letter-findings-for-clinical-investigators-in-2011/ (accessed November 24, 2023).
145. Cinefra M, Cagnazzo C, McMahon L, Arizio F, Campora S, Camisa R, et al. The critical role of the clinical research coordinator for clinical trials: A survey in oncology. Med Access Point Care. (2017) 1:15. doi: 10.5301/MAAPOC.0000015
146. Otto C, Siewe J, Zarghooni K, Kaulhausen T, Sauerland S, Eysel P, et al. [Clinical research in orthopaedics–creation of a clinical trial unit in orthopaedics/trauma surgery]. Z Orthop Unfall. (2010) 148:145–8. doi: 10.1055/s-0029-1240660
147. Fabbri F, Gentili G, Serra P, Vertogen B, Andreis D, Dall’Agata M, et al. How many cancer clinical trials can a clinical research coordinator manage? The clinical research coordinator workload assessment tool. JCO Oncol Pract. (2021) 17:e68–76. doi: 10.1200/JOP.19.00386
148. Association of Clinical Research Professionals. Core competency guidelines for clinical research coordinators. Alexandria, VA: Association of Clinical Research Professionals (2023).
149. Sonstein S, Seltzer J, Li R, Silva H, Jones CT, Daemen E. Moving from compliance to competency: A harmonized core competency framework for the clinical research professional. Clin Res. (2014) 28:3.
150. Raybuck JA. The clinical nurse specialist as research coordinator in clinical drug trials. Clin Nurse Spec. (1997) 11:15–9. doi: 10.1097/00002800-199701000-00010
151. Mozersky JT, Antes AL, Baldwin K, Jenkerson M, DuBois JM. How do clinical research coordinators learn Good Clinical Practice? A mixed-methods study of factors that predict uptake of knowledge. Clin Trials. (2020) 17:166–75. doi: 10.1177/1740774519893301
152. Haeusler JMC. Certification in good clinical practice and clinical trial quality: A retrospective analysis of protocol adherence in four multicenter trials in the USA. Clin Res Regul Affairs. (2009) 26:1596. doi: 10.1080/10601330902911893
153. Hornung CA, Kerr J, Gluck W, Jones CT. The competency of clinical research coordinators: The importance of education and experience. Ther Innov Regul Sci. (2021) 55:1231–8. doi: 10.1007/s43441-021-00320-w
154. Association of Clinical Research Professionals. Special report: An assessment of the adequacy of the clinical research workforce. Alexandria, VA: Association of Clinical Research Professionals (2023).
155. Buchanan DA, Goldstein J, Pfalzer AC, Lin YC, Kang H, Claassen DO. Empowering the clinical research coordinator in academic medical centers. Mayo Clin Proc Innov Qual Outcomes. (2020) 5:265–73. doi: 10.1016/j.mayocpiqo.2020.09.014
156. Speicher LA, Fromell G, Avery S, Brassil D, Carlson L, Stevens E, et al. The critical need for academic health centers to assess the training, support, and career development requirements of clinical research coordinators: Recommendations from the Clinical and Translational Science Award Research Coordinator Taskforce. Clin Transl Sci. (2012) 5:470–5. doi: 10.1111/j.1752-8062.2012.00423.x
157. Stabile S, Cenna R, Sinna V, Veronica F, Mannozzi F, Federici I, et al. Clinical trial units and clinical research coordinators: A system facing crisis? AboutOpen. (2023) 10:2508. doi: 10.33393/ao.2023.2508
158. Julé A, Furtado T, Boggs L, van Loggerenberg F, Ewing V, Vahedi M, et al. Developing a globally applicable evidence-informed competency framework to support capacity strengthening in clinical research. BMJ Glob Health. (2017) 2:e000229. doi: 10.1136/bmjgh-2016-000229
159. Haley SJ, Southwick LE, Parikh NS, Rivera J, Farrar-Edwards D, Boden-Albala B. Barriers and strategies for recruitment of racial and ethnic minorities: Perspectives from neurological clinical research coordinators. J Racial Ethn Health Disparities. (2017) 4:1225–36. doi: 10.1007/s40615-016-0332-y
160. Gwede CK, Johnsson DJ, Roberts C, Cantor AB. Burnout in clinical research coordinators in the United States. Oncol Nurs Forum. (2005) 32:1123–30. doi: 10.1188/05.onf.1123-1130
161. Mascaro JS, Palmer PK, Ash MJ, Peacock C, Sharma A, Escoffery C, et al. Feasibility, acceptability, and preliminary effectiveness of a compassion-centered team intervention to improve clinical research coordinator resilience and well-being. JCO Oncol Pract. (2021) 17:e936–46. doi: 10.1200/OP.21.00120
162. Kay SC, Luke DG, Tamer HR. ASHP guidelines for the management of investigational drug products. Am J Health Syst Pharm. (2018) 75:561–73. doi: 10.2146/ajhp170812
163. Finnes HD, Child B, DeFrates S, Kinsman K, Thorne A, Lentz S, et al. Adapting investigational drug services during a pandemic: Recommendations for future preparedness from the hematology/oncology pharmacy association investigational drug services special interest group. Am J Health Syst Pharm. (2023) 80:e67–73. doi: 10.1093/ajhp/zxac267
164. Saunders J, Murli S, Rudek MA, Khandoobhai A, DeLisa A, Goodrich A, et al. Evaluation of clinical pharmacy services for phase 1 clinical trials. J Hematol Oncol Pharm. (2022) 12:131–7.
165. Hematology/Oncology Pharmacy Association. Investigational drug service. Best practice standards. Milwaukee: Hematology/Oncology Pharmacy Association (2014).
166. Slobodian P, Challen J, Ching M, Hong E, Nikolajevic-Sarunac J, Shum B, et al. Standard of practice in clinical trials for pharmacy services. J Pharm Pract Res. (2020) 50:429–44. doi: 10.1002/jppr.1676
167. Demotes-Mainard J, Chêne G, Libersa C, Pignon JP. Clinical research infrastructures and networks in France: Report on the French ECRIN workshop. Therapie. (2005) 60:183–99. doi: 10.2515/therapie:2005023
168. Brown JN, Britnell SR, Stivers AP, Cruz JL. Medication safety in clinical trials: role of the pharmacist in optimizing practice, collaboration, and education to reduce errors. Yale J Biol Med. (2017) 90:125–33.
169. Adcock S, Atiee G, Sramek J. Pharmacy services in a Phase I clinical research unit. Research Triangle Park, NC: World Clinica Data (2023).
170. Brown A, Dixon D, Eatock J, Meenan BJ, Young T. A survey of success factors in new product development in the medical devices industry. Proceedings of the IEMC-Europe 2008 – 2008 IEEE international engineering management conference, Europe: Managing engineering, technology and innovation for growth. Berlin: (2008). doi: 10.1109/IEMCE.2008.4617987
171. Raehl CL, Miller DE, Foster TS. Pharmacist involvement in institutional review of clinical trials. Am J Hosp Pharm. (1981) 38:334–9.
172. Khandoobhai A, Poi M, Kelley K, Mirtallo J, Lopez B, Griffith N. National survey of comprehensive pharmacy services provided in cancer clinical trials. Am J Health Syst Pharm. (2017) 74:S35–41. doi: 10.2146/ajhp160628
173. Redic KA, Skyles A, Zaccardelli J. Potential role of a pharmacist to enhance medication-related aspects of clinical trials conducted in a dedicated clinical research unit. Contemp Clin Trials Commun. (2017) 6:55–7. doi: 10.1016/j.conctc.2017.03.003
174. Goodin S. Pharmacists’ critical role in cancer clinical trials. Am J Health Syst Pharm. (2018) 75:592–3. doi: 10.2146/ajhp180038
175. Hertz DL, Siden R, Modlin J, Gabel LL, Wong SF. Drug interaction screening in SWOG clinical trials. Am J Health Syst Pharm. (2018) 75:607–12. doi: 10.2146/ajhp170449
176. McGahey KE, Weiss GJ. Reviewing concomitant medications for participants in oncology clinical trials. Am J Health Syst Pharm. (2017) 74:580–6. doi: 10.2146/ajhp151052
177. Wisinski KB, Cantu CA, Eickhoff J, Osterby K, Tevaarwerk AJ, Heideman J, et al. Potential cytochrome P-450 drug-drug interactions in adults with metastatic solid tumors and effect on eligibility for Phase I clinical trials. Am J Health Syst Pharm. (2015) 72:958–65. doi: 10.2146/ajhp140591
178. Marcath LA, Coe TD, Hoylman EK, Redman BG, Hertz DL. Prevalence of drug-drug interactions in oncology patients enrolled on national clinical trials network oncology clinical trials. BMC Cancer. (2018) 18:1155. doi: 10.1186/s12885-018-5076-0
179. Phillips MS, Abdelghany O, Johnston S, Rarus R, Austin-Szwak J, Kirkwood C. Navigating the institutional review board (IRB) process for pharmacy-related research. Hosp Pharm. (2017) 52:105–16. doi: 10.1310/hpj5202-105
180. Donehew GR, Schaumberg JP. The pharmacist’s role as a member of the institutional review board. Hosp Pharm. (1979) 14:508–9.
181. Abdel Shaheed C, Maher CG, Williams KA, McLachlan AJ. Participation of pharmacists in clinical trial recruitment for low back pain. Int J Clin Pharm. (2014) 36:986–94. doi: 10.1007/s11096-014-9985-y
182. Fletcher JM, Saunders-Smith T, Manns BJ, Tsuyuki R, Hemmelgarn BR, Tonelli M, et al. Pharmacist and patient perspectives on recruitment strategies for randomized controlled trials: A qualitative analysis. BMC Med Res Methodol. (2020) 20:270. doi: 10.1186/s12874-020-01140-6
183. Lowres N, Neubeck L, Salkeld G, Krass I, McLachlan AJ, Redfern J, et al. Feasibility and cost-effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies. The SEARCH-AF study. Thromb Haemost. (2014) 111:1167–76. doi: 10.1160/TH14-03-0231
184. Peytremann-Bridevaux I, Bordet J, Santschi V, Collet TH, Eggli M, Burnand B. Community-based pharmacies: An opportunity to recruit patients? Int J Public Health. (2013) 58:319–22. doi: 10.1007/s00038-012-0383-8
185. Tsuyuki RT, Al Hamarneh YN, Jones CA, Hemmelgarn BR. The effectiveness of pharmacist interventions on cardiovascular risk: The multicenter randomized controlled RxEACH trial. J Am Coll Cardiol. (2016) 67:2846–54. doi: 10.1016/j.jacc.2016.03.528
186. Campbell DJ, Tonelli M, Hemmelgarn B, Mitchell C, Tsuyuki R, Ivers N, et al. Assessing outcomes of enhanced chronic disease care through patient education and a value-based formulary study (ACCESS)-study protocol for a 2×2 factorial randomized trial. Implement Sci. (2016) 11:131. doi: 10.1186/s13012-016-0491-6
187. Watson M, Bewick BM, Tharmanathan P. The current role and perceived benefits and barriers of secondary care pharmacists facilitating patient participation in Clinical Trials of Investigational Medicinal Products (CTIMPs) conducted within the NHS: A cross-sectional survey. J Eval Clin Pract. (2020) 26:142–8. doi: 10.1111/jep.13104
188. Reali S, Lee T, Bishop J, Mirkov S, Johnson J, McCourt E, et al. Attitudes, barriers and facilitators of hospital pharmacists conducting practice-based research: A systematic review. J Pharm Pract Res. (2021) 51:192–202. doi: 10.1002/jppr.1741
189. American Society of Hospital Pharmacy. Investigational drug services certificate. Bethesda, MD: American Society of Health-System Pharmacists (2024).
190. American Society of Health-System Pharmacists. Required competency areas, goals, and objectives for postgraduate year two (PGY2) investigational drugs and research pharmacy residencies. Bethesda, MD: American Society of Health-System Pharmacists (2023).
191. Wascher M, Mighty J, Brown V, Ashby D, Rudek MA, Nesbit T, et al. Establishing an investigational drugs and research residency at an academic medical center. Am J Health Syst Pharm. (2019) 76:1862–7. doi: 10.1093/ajhp/zxz175
192. Johns Hopkins Medicine. Investigational drug service | department of pharmacy. Baltimore, MD: Johns Hopkins Medicine (2023).
193. University of Michigan. “(PGY1 and PGY2 Combined) investigational drugs and research pharmacy residency. Ann Arbor, MI: University of Michigan (2024).
194. Vélez-Díaz-Pallarés M, Pérez-Menéndez-Conde C, Bermejo-Vicedo T. Systematic review of computerized prescriber order entry and clinical decision support. Am J Health Syst Pharm. (2018) 75:1909–21. doi: 10.2146/ajhp170870
195. Lombardo G, Couvert C, Kose M, Begum A, Spiertz C, Worrell C, et al. Electronic health records (EHRs) in clinical research and platform trials: Application of the innovative EHR-based methods developed by EU-PEARL. J Biomed Inform. (2023) 148:104553. doi: 10.1016/j.jbi.2023.104553
196. McCord KA, Hemkens LG. Using electronic health records for clinical trials: Where do we stand and where can we go? CMAJ. (2019) 191:E128–33. doi: 10.1503/cmaj.180841
197. Seoane-Viaño I, Trenfield SJ, Basit AW, Goyanes A. Translating 3D printed pharmaceuticals: From hype to real-world clinical applications. Adv Drug Deliv Rev. (2021) 174:553–75. doi: 10.1016/j.addr.2021.05.003
198. Huanbutta K, Burapapadh K, Sriamornsak P, Sangnim T. Practical application of 3D printing for pharmaceuticals in hospitals and pharmacies. Pharmaceutics. (2023) 15:1877. doi: 10.3390/pharmaceutics15071877
199. Love SB, Yorke-Edwards V, Ward E, Haydock R, Keen K, Biggs K, et al. What is the purpose of clinical trial monitoring? Trials. (2022) 23:836. doi: 10.1186/s13063-022-06763-2
200. Molloy SF, Henley P. Monitoring clinical trials: A practical guide. Trop Med Int Health. (2016) 21:1602–11. doi: 10.1111/tmi.12781
201. Shah KA. day in the life of a monitor! Perspect Clin Res. (2012) 3:32–4. doi: 10.4103/2229-3485.92305
202. Manasco P, Bhatt DL. Evaluating the evaluators – Developing evidence of quality oversight effectiveness for clinical trial monitoring: Source data verification, source data review, statistical monitoring, key risk indicators, and direct measure of high risk errors. Contemp Clin Trials. (2022) 117:106764. doi: 10.1016/j.cct.2022.106764
203. Yeshaswini A, Allada TS, Mujeebuddin CS. Monitoring in clinical trial. Int J Pharm Sci Rev Res. (2020) 63:32–9.
204. Klatte K, Pauli-Magnus C, Love SB, Sydes MR, Benkert P, Bruni N, et al. Monitoring strategies for clinical intervention studies. Cochrane Database Syst Rev. (2021) 12:MR000051. doi: 10.1002/14651858.MR000051.pub2
205. UKCRC Registered CTU Network. Monitoring handbook – UKCRC CTU network guidance. Leeds: UKCRC Registered CTU Network (2021).
206. Favalli G, Vermorken JB, Vantongelen K, Renard J, Van Oosterom AT, Pecorelli S. Quality control in multicentric clinical trials. An experience of the EORTC Gynecological Cancer Cooperative Group. Eur J Cancer. (2000) 36:1125–33. doi: 10.1016/s0959-8049(00)00090-3
207. Eisenstein EL, Lemons PW, Tardiff BE, Schulman KA, Jolly MK, Califf RM. Reducing the costs of phase III cardiovascular clinical trials. Am Heart J. (2005) 149:482–8. doi: 10.1016/j.ahj.2004.04.049
208. Coursera staff. How to become a clinical research associate. Mountain View, CA: Coursera staff (2023).
209. Owens Pickle EE, Borgerson D, Espirito-Santo A, Wigginton S, Devine S, Stork S. the clinical research associate retention study: A report from the children’s oncology group. J Pediatr Oncol Nurs. (2017) 34:414–21. doi: 10.1177/1043454217723861
210. Brewer B. How to become a clinical research associate. (2023). Available online at: https://www.medicaltechnologyschools.com/health-sciences/how-to-become-a-clinical-research-associate (accessed December 12, 2023).
211. CCRPS. Clinical research associate certification -CRA certification. Jacksonville, FL: CCRPS (2024).
212. Association of Clinical Research Professionals. Course catalog – training for clinical research professionals. Alexandria, VA: Association of Clinical Research Professionals (2024).
213. The Society of Clinical Research Associates. SOCRA the society of clinical research associates. Chalfont, PA: The Society of Clinical Research Associates (2024).
214. Association of Clinical Research Professionals. Certification. Alexandria, VA: Association of Clinical Research Professionals (2023).
215. CCRPS. Clinical research training - clinical research certification. Jacksonville, FL: CCRPS (2023).
216. Payscale. Clinical research associate (CRA) salary in 2024. (2024). Available online at: https://www.payscale.com/research/US/Job=Clinical_Research_Associate_(CRA)/Salary (accessed February 18, 2024).
217. LinkedIn. Employ offers «clinical research associate» in the United States. (2024). Available online at: https://www.linkedin.com/jobs/search/?currentJobId=3818043745&geoId=103644278&keywords=Clinical%20Research%20Associate&location=United%20States (accessed February 18, 2024).
218. Bakobaki JM, Rauchenberger M, Joffe N, McCormack S, Stenning S, Meredith S. The potential for central monitoring techniques to replace on-site monitoring: Findings from an international multi-centre clinical trial. Clin Trials. (2012) 9:257–64. doi: 10.1177/1740774511427325
219. Brosteanu O, Schwarz G, Houben P, Paulus U, Strenge-Hesse A, Zettelmeyer U, et al. Risk-adapted monitoring is not inferior to extensive on-site monitoring: Results of the ADAMON cluster-randomised study. Clin Trials. (2017) 14:584–96. doi: 10.1177/1740774517724165
220. Beever D, Swaby L. An evaluation of risk-based monitoring in pragmatic trials in UK clinical trials units. Trials. (2019) 20:556. doi: 10.1186/s13063-019-3619-6
221. Hurley C, Sinnott C, Clarke M, Kearney P, Racine E, Eustace J, et al. Perceived barriers and facilitators to Risk Based Monitoring in academic-led clinical trials: A mixed methods study. Trials. (2017) 18:423. doi: 10.1186/s13063-017-2148-4
222. Andersen JR, von Sehested C, Byrjalsen I, Popik S, Follin AB, Bihlet AR. Impact of monitoring approaches on data quality in clinical trials. Br J Clin Pharmacol. (2023) 89:1756–66. doi: 10.1111/bcp.15615
223. European Medicines Agency. Reflection paper on risk based quality management in clinical trials. Amsterdam: European Medicines Agency (2013).
224. Food and Drug Administration. Guidance for industry oversight of clinical investigations — a risk-based approach to monitoring. Silver Spring, MD: Food and Drug Administration (2013).
225. Houston L, Martin A, Yu P, Probst Y. Time-consuming and expensive data quality monitoring procedures persist in clinical trials: A national survey. Contemp Clin Trials. (2021) 103:106290. doi: 10.1016/j.cct.2021.106290
226. BDO Alliance USA. 2020/2021 CRO INSIGHTS REPORT: While compensation levels and practices remain status quo, turnover continues to be high. Chicago, IL: BDO Alliance USA (2023).
228. Hardman TC, Aitchison R, Scaife R, Edwards J, Slater G. The future of clinical trials and drug development: 2050. Drugs Context. (2023) 12:2023. doi: 10.7573/dic.2023-2-2
229. Food and Drug Administration. Clinical investigator training course (CITC). Silver Spring, MD: Food and Drug Administration (2023).
230. Mayo Clinic College of Medicine and Science. Clinician investigator training program – residencies and fellowships – mayo clinic college of medicine & science. Rochester, MN: Mayo Clinic College of Medicine and Science (2024).
231. Weil Cornell Medicine. Advanced certificate program in clinical & translational investigation. New York, NY: Weil Cornell Medicine (2023).
232. PharmaTrain. PharmaTrain federation. (2023). Available online at: https://www.pharmatrain.eu/index (accessed December 23, 2023).
Keywords: clinical trial, investigator, clinical research coordinator, clinical trial monitoring, nurse, pharmacist, ICH (international conference of harmonization) guidelines
Citation: Peralta G and Sánchez-Santiago B (2024) Navigating the challenges of clinical trial professionals in the healthcare sector. Front. Med. 11:1400585. doi: 10.3389/fmed.2024.1400585
Received: 22 March 2024; Accepted: 13 May 2024;
Published: 03 June 2024.
Edited by:
Khalid Saeed Khan, University of Granada, SpainReviewed by:
Murtaza Hussain, Metrics Research Pvt. Ltd, PakistanCopyright © 2024 Peralta and Sánchez-Santiago. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Galo Peralta, ZGlyZWNjaW9uZ2VzdGlvbkBpZGl2YWwub3Jn
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.