
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Med., 04 June 2024
Sec. Hepatobiliary Diseases
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1399665
This article is part of the Research TopicChronic Hepatitis B Management: Current Status and Future DirectionsView all 14 articles
 Wenjuan Zhao1
Wenjuan Zhao1 Yi Liu1
Yi Liu1 Mengdi Zhang2
Mengdi Zhang2 Zixin Cui1
Zixin Cui1 Zhan Qu1
Zhan Qu1 Yiyang Li1
Yiyang Li1 Meijuan Wan1
Meijuan Wan1 Wen Wang1
Wen Wang1 Yunru Chen1
Yunru Chen1 Lei Shi1
Lei Shi1 Jianzhou Li1
Jianzhou Li1 Feng Ye1*
Feng Ye1*Background: This study was aimed at investigating the dynamics of lipids and the effect of TAF on the lipid profile of patients including fatty liver disease in CHB patients.
Methods: The data of TC, LDL-c, HDL-c, TG, and TC/HDL ratio were collected at baseline, 24 weeks, 48 weeks, 72 weeks, and 96 weeks. CHB patients with fatty liver at baseline were further analyzed in a subgroup.
Results: A total of 137 CHB patients treated with TAF were enrolled in this study. During 96 weeks of TAF treatment, there was no significant change in TC, LDL-c, HDL-c, and TG level (P > 0.05). The TC/HDL-c ratio was increased with no significant change (+0.24, P > 0.05). In CHB patients with fatty liver (n = 48), TC, LDL-c, and TC/HDL-c ratio increased gradually during TAF treatment, TG levels increased to 146.63 mg/dL at 48 weeks (P = 0.057) and then decreased, but there was still no significant change compared with the baseline level by 96 weeks (P > 0.05).
Conclusion: TAF treatment had a low effect on the lipid profile of CHB patients over the course of 96 weeks, and it was safe even in patients with fatty liver.
Clinical trial registration: [https://www.chictr.org.cn/showproj.html?proj=65123], identifier [ChiCTR2000041005].
Chronic hepatitis B (CHB) is a systemic infectious disease caused by persistent infection with the hepatitis B virus (HBV) (1). It is estimated that approximately 296 million people worldwide are chronically infected with HBV, which could cause as many as 820,000 deaths annually due to cirrhosis, hepatocellular carcinoma, and other complications resulting from the progression of CHB (2).
Tenofovir alafenamide (TAF) is a new effective and well-tolerated nucleotide analogue after Tenofovir disoproxil fumarate (TDF) and entecavir (ETV) (3). TAF targets the RNA-dependent DNA polymerase of HBV and exhibits more potent antiviral activity (4). Therefore, TAF, as a drug that inhibits HBV replication, has become the first-line antiviral drugs recommended by major clinical guidelines for the treatment of HBV infection (5).
Antiviral therapy for CHB was considered a long-term treatment (6). This has increased the concern of physicians and patients about drug-related adverse effects. The effect of TAF on lipids has been the focus of recent attention, but the conclusions are controversial. In clinical studies of TAF-containing regimens treated HBV or HIV-infected patients, it was found that there was weight gain and a significant increase in lipid levels during the treatment (7–9). However, there are also studies found that the majority of patients treated with TAF with elevated LDL cholesterol have either a history of dyslipidemia or elevated baseline lipids, or both (10). And Jeong’s study also showed that patients treated with TAF did not elevated lipids compared to HBV-uninfected controls (11).
Meanwhile, CHB combined with nonalcoholic fatty liver disease (NAFLD) is also becoming more common in clinical practice; it is estimated that 25%–30% of CHB patients have combined NAFLD (12). Studies have shown that obesity, high BMI, hyperlipidemia, and type 2 diabetes mellitus increased the risk of comorbidity with NAFLD in CHB patients (13, 14). Therefore, it is necessary to evaluate the lipid safety and the risk of cardiovascular disease during treatment with TAF, especially in patients with CHB combined with NAFLD. Previous studies suggest that long-term antiviral therapy in such patients may increase body fat mass and visceral fat area through virological suppression (15). However, there are a few studies suggesting that TAF-induced dyslipidemia has little effect on the incidence of NAFLD (16).
Therefore, to better understand the changes of lipid levels during TAF treatment, especially in patients with fatty liver, this prospective cohort study aims to investigate the changes in lipid levels after long-term antiviral therapy with TAF in CHB patients and the effects of patients with CHB combined with fatty liver.
This was a prospective cohort study conducted in the Department of Infection of the First Affiliated Hospital of Xi’an Jiaotong University. Inclusion criteria were as follows: (1) patients with age > 18 years old; (2) meeting the diagnostic criteria for CHB in the Chinese 2019 Guidelines for the Prevention and Control of Chronic Hepatitis B(HBsAg and/or HBV DNA positive for more than 6 months); (3) receiving TAF therapy (25 mg/dose) for at least 6 months and not receiving other antiretroviral therapy.
Patients who had one of the following criteria were excluded: (1) patients co-infected with HIV or hepatitis C virus; (2) patients with poorly controlled malignancies including hepatocellular carcinoma; (3) patients with a history of allergy to nucleotide analogues (NAs); (4) patients with undergoing lipid-lowering therapy; (5) women who were pregnant or breastfeeding during the treatment; (6) patients with other causes of steatosis such as alcohol consumption, obesity, metabolic diseases, etc. Written informed consents were obtained from all patients in the study.
The study was approved by the Ethics Committee of our hospital (Ethics No. XJTU1AF2020LSL-024) and was registered under the Chinese Clinical Trial Registry (ChiCTR2000041005).
At baseline, 24W, 48W, 72W and 96W of TAF treatment, the included patients were examined HBV-DNA load measured with the Roche COBAS® TaqMan® HBV test (Lower limit of detection, 20 IU/mL) and HBsAg quantification by an Elecsys 2010 immunoanalyzer (Roche). The serum low-density lipoprotein-cholesterol (LDL-c), triglyceride (TG), TC, HDL-c levels as well as the TC/HDL ratio, ALT, AST were measured by fully automated biochemistry and the controlled attenuation parameter (CAP) was measured by transient elastography in the Laboratory Department of the hospital. Trends in lipids using TAF 96W were analyzed according to a continuous follow-up cohort.
To investigate the lipid safety of TAF in a special population, stratified analysis was conducted on the effect of TAF on lipid safety according to the presence or absence of comorbid hypercholesterolemia (TC > 5.2 mmol/L or LDL ≥ 2.5 mmol/L) and fatty liver (CAP > 240 dB/m) at baseline.
Factors influencing TAF-related lipid changes in treatment-naive CHB patients were analyzed by linear regression.
Descriptive statistics for demographic and clinical variables were expressed as mean ± standard deviation, median (interquartile spacing), count (n), and proportion (%). Data comparing changes in lipid levels at different time points before and after TAF treatment were compared using the Student’s t-test for normally distributed continuous variables and the Kruskal-Wallis test for non-normally distributed continuous variables. The chi-square test between multiple groups was used for dichotomous data.
Spearman’s correlation analysis was used to evaluate the relationship between TAF-related lipid changes and age, HBsAg levels, AST, ALT, and CAP values. The main influencing factors of TAF-related lipid changes were also investigated by multiple linear regression analysis. Data analysis was performed using SPSS 26.0 statistical software, and the significance level was set at p < 0.05.
All procedures performed in this study were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethics standards.
A total of 154 CHB patients treated with TAF were were screened. Among them, 17 were excluded (11 treated with TAF combined with TDF, ETV, or IFN,2 started using lipid-lowering drugs, 2 stopped due to pregnancy, 2 were not available for cirrhosis decompensation or HCC, Figure 1). Baseline characteristics of 137 CHB patients were presented in Table 1. Mean Age was 40.71 ± 10.41 years, 62.04% were male. Subgroup analysis was performed according to whether the baseline lipid were hypercholesterolemia or not. There was no difference between the hypercholesterolemia group (n = 81) and the non-hypercholesterolemia group (n = 56) in terms of the baseline characteristics (P > 0.05), except for the differences in lipid profile and TC/HDL ratio.
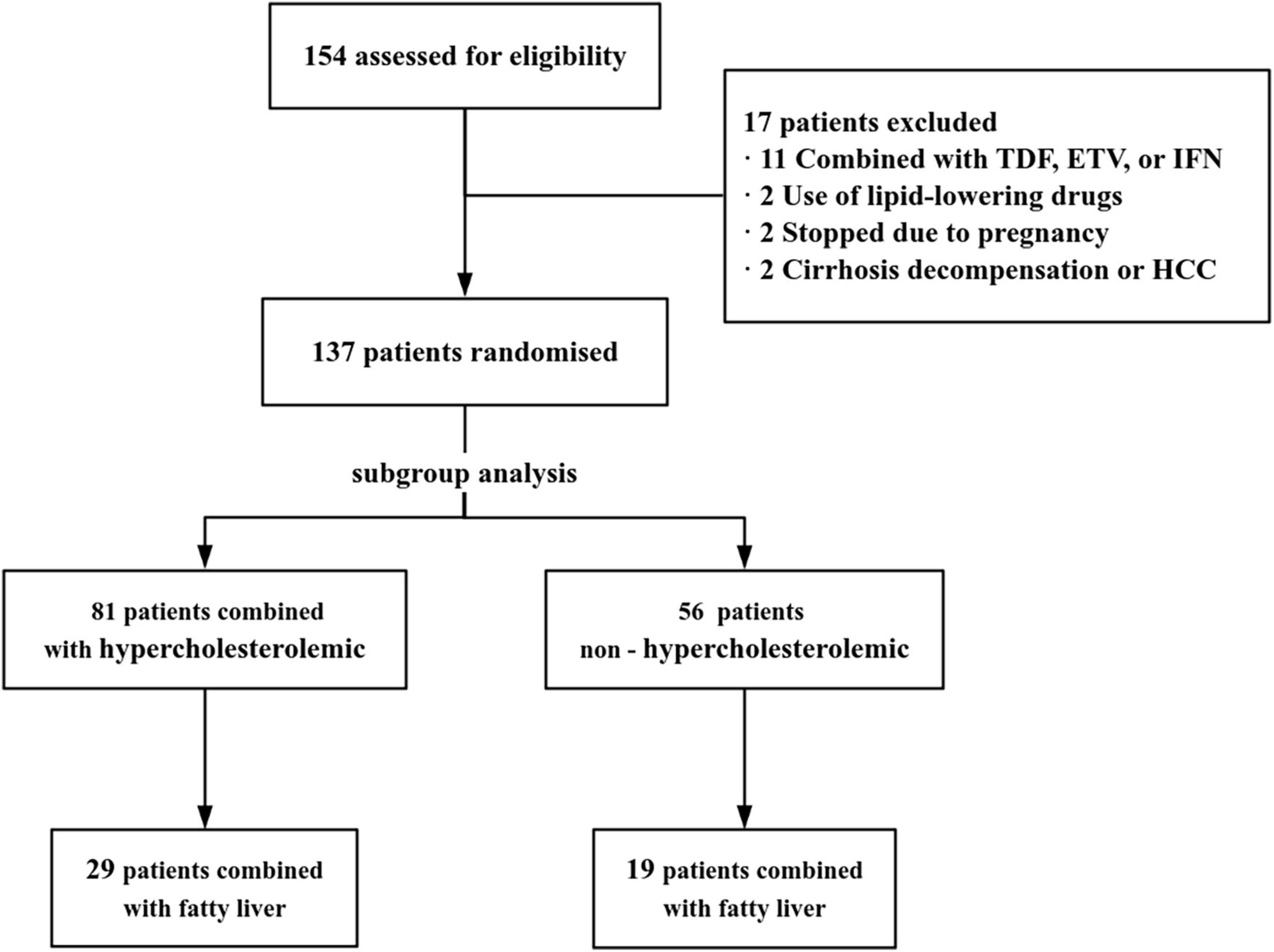
Figure 1. Study flowchart. TDF, tenofovir disoproxil fumarate; ETV, entecavir; IFN, interferon; HCC, hepatocellular carcinoma.
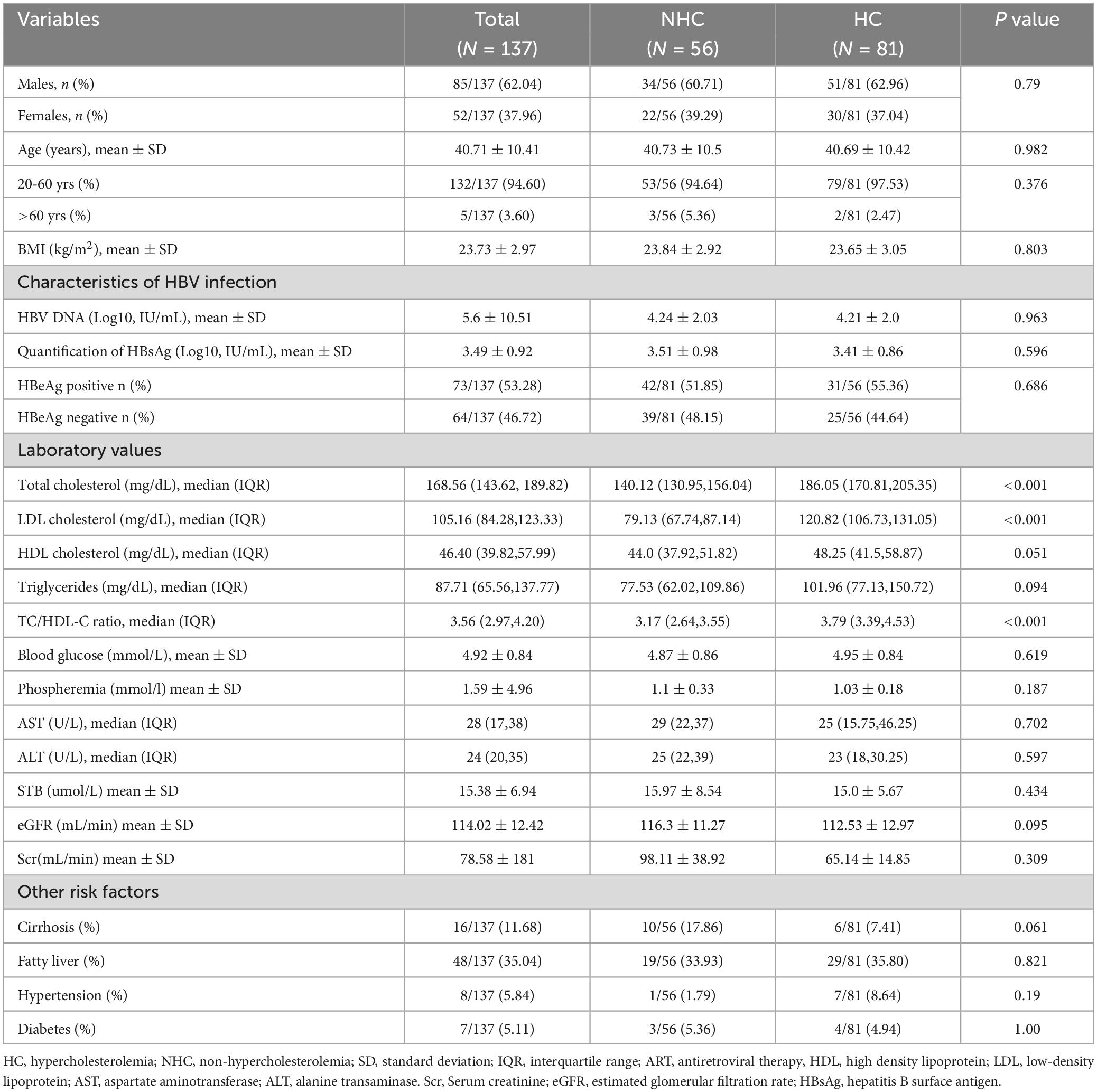
Table 1. Baseline characteristics of the study population and comparison between patients with and without baseline hypercholesterolemia (HC).
The proportion of patients with HBV DNA < 20 IU/mL was significantly higher after 24 W, 48 W, 72 W, and 96 W of TAF treatment, compared with baseline (65.57%, 78.21%, 87.18%, 83.33% vs. 18.11%, P < 0.001) (Figure 2). The proportion of patients with ALT < 40 U/L was significantly higher after 24 W, 48 W, 72W and 96 W of treatment, compared with baseline (85.27%, 89.02%, 92.86%, 93.10% vs. 76.52%, P < 0.05) (Figure 2). In addition, the proportion of HBeAg-negative patients increased to 51.85% and serum HBsAg levels gradually decreased to 3.21 ± 0.97 log IU/mL after 96W of TAF treatment.
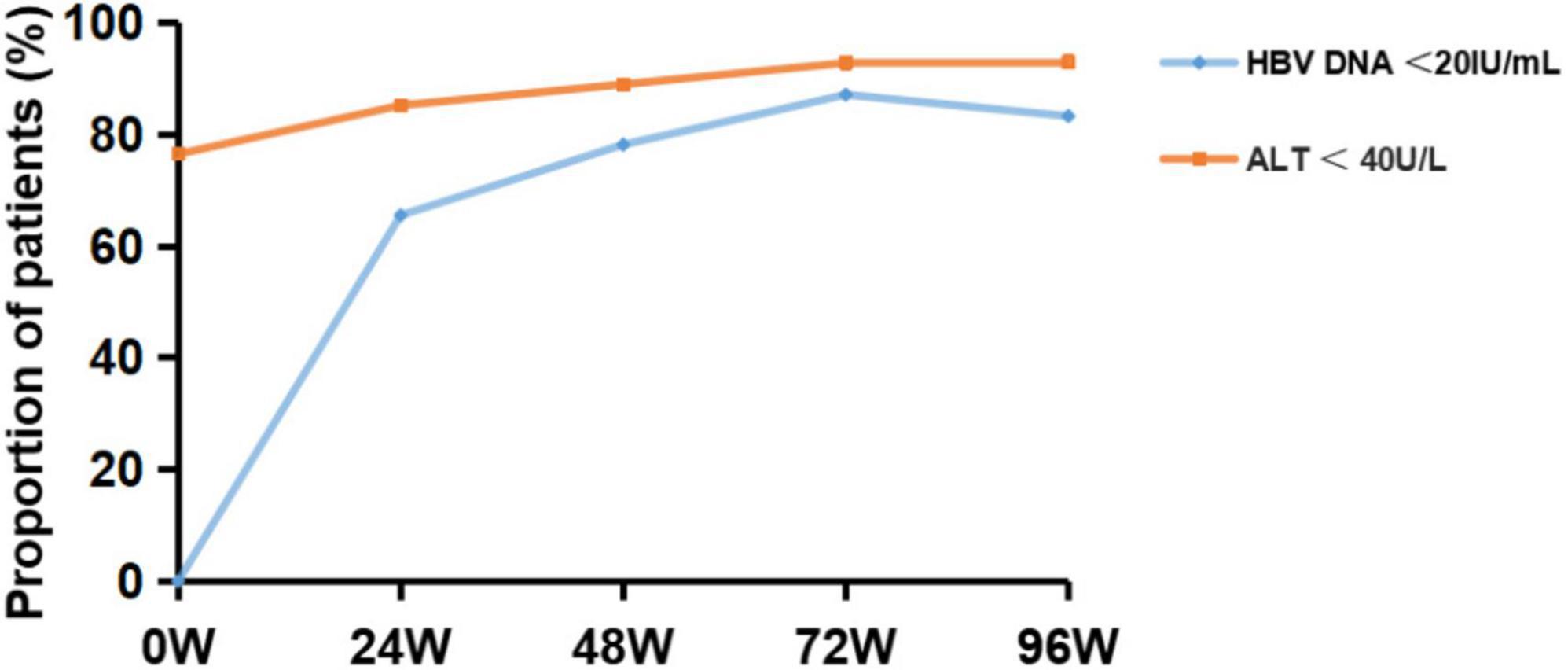
Figure 2. Virological response outcome before and after 24, 48, 72 and 96 weeks of TAF treatment in HBV patients. HBV-DNA, hepatitis B virus-deoxyribonucleic acid; ALT, alanine transaminase.
Cross-sectional results showed that TC and LDL-c levels gradually increased during treatment with TAF, increased to 174.94 mg/dL (+6.38mg/dL, P > 0.05) and 107.47 mg/dL (+2.32 mg/dL, P > 0.05) at 72W respectively, gradually decreased after 72 W, and slightly decreased relative to the baseline at 96 W, but there was no statistical difference (P > 0.05). HDL-c levels did not change significantly during 96 W of treatment with TAF (P > 0.05). TG levels gradually increased before 48 W, increased to 116.07 mg/dL (+28.35mg/dL, P = 0.009)at 48 W, and then gradually decreased after 48 W. There was no significant change relative to the baseline at 96 W (+0.90mg/dL, P > 0.05). TC/HDL levels slightly increased during the use of TAF, and increased to 3.80 by 96 W (+0.24, P > 0.05) (Figure 3A).
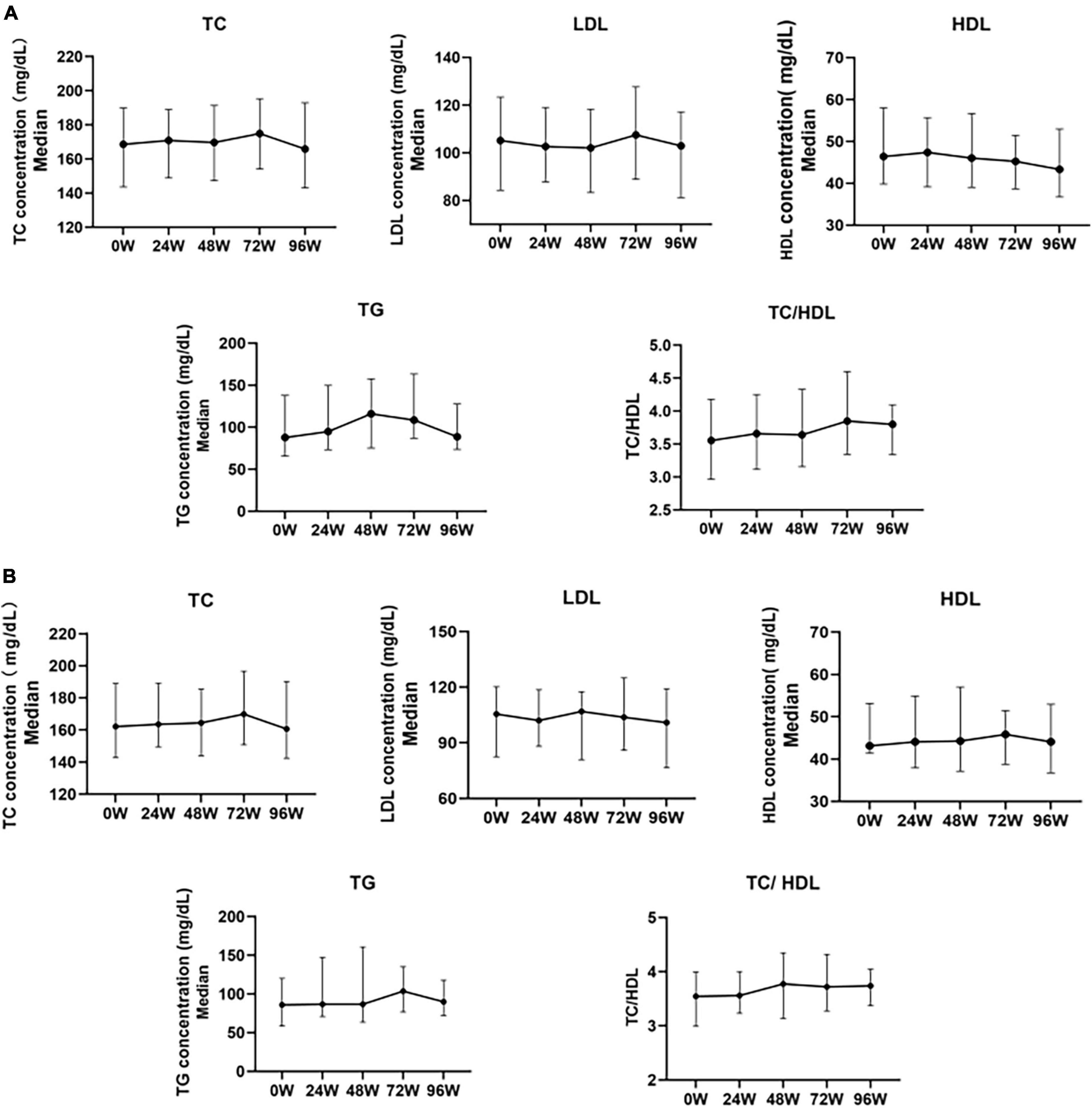
Figure 3. Changes in serum lipoproteins before and after 24, 48, 72, and 96 weeks of univariate analysis of TAF treatment in HBV patients. (A) Changes in Lipid Levels in Overall Patients After Treatment with TAF (0W: n = 137, 24W: n = 137, 48W: n = 89, 72W: n = 62, 96W: n = 60). (B) Changes in blood lipid profile in a small sample of HBV patients with a complete follow-up of 96 weeks after treatment with TAF (n = 36). TC, Total Cholesterol; LDL, Low Density Lipoprotein; HDL, High Density Lipoprotein; TG, Triglyceride. The data were shown as the median (IQR).
In the 96W cohort study (N = 36), the changes in TC, LDL-c, HDL-c, TG, and TC/HDL ratio after TAF treatment were generally consistent with the overall lipid changes in the above cross-sections (Figure 3B).
TC and LDL levels in non-hypercholesterolemic CHB patients increased significantly at 24 W (P < 0.05). Serum TC and LDL levels increased to 150.39 mg/dL (+10.05 mg/dL, P = 0.017) and 84.28 mg/dL (+5.03 mg/dL, P = 0.007) respectively, and then gradually declined after 24 W. There was no significant change at 96W compared with baseline levels (+4.25 mg/dL and +2.71 mg/dL, P > 0.05). TG levels increased to 116.95mg/dL at 48W, which was a significant increase compared with the baseline (+39.43 mg/dL, P = 0.027), gradually decreased after 48W. There was no significant change at 96W compared with baseline levels (+5.76 mg/dL, P > 0.05) as showed in Figure 4.
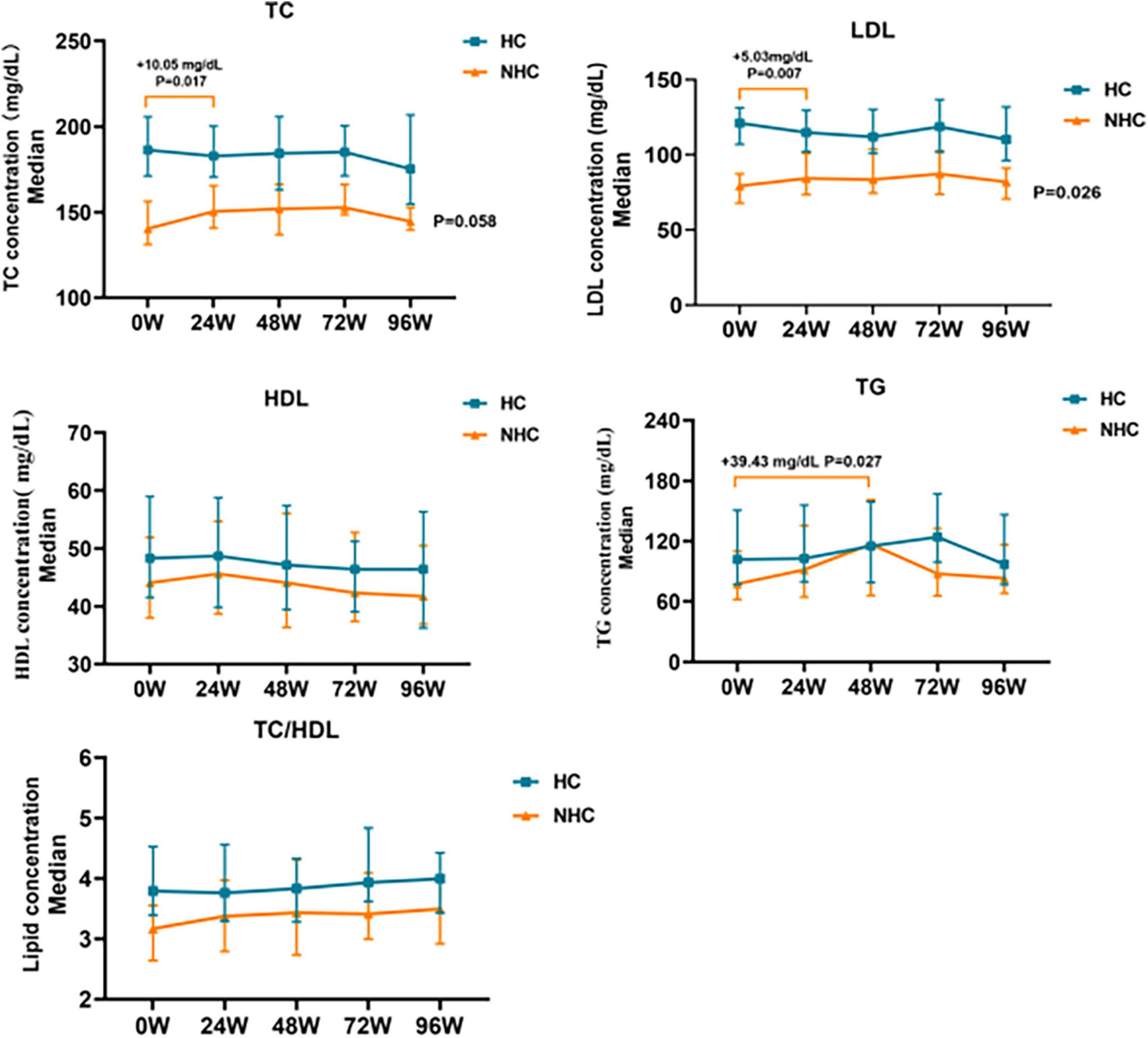
Figure 4. Changes in relative baseline lipid levels after TAF treatment in HBV patients with NHC (n = 56) and HC (n = 81). NHC, non-hypercholesterolemia; HC, hypercholesterolemia; TC, Total Cholesterol; LDL, Low Density Lipoprotein; HDL, High Density Lipoprotein; TG, Triglyceride. The data were shown as the median (IQR).
During treatment with TAF in patients combined with hypercholesterolemia, TC and LDL levels gradually decreased before 72 W and gradually increased after 72 W, but none of them were statistically different (P > 0.05). TG levels did not change significantly during treatment with TAF (P > 0.05), as shown in Figure 4.
Regardless of whether hypercholesterolemia was combined or not, HDL levels gradually decreased in CHB patients treated with TAF, TC/HDL ratio slightly increased in CHB patients with TAF, increased to 4.0 (+0.20, P > 0.05) and 3.49 (+0.33, P > 0.05) by 96 W, but none of them were statistically different (P > 0.05), as shown in Figure 4.
Lipid levels and lipid ratios were not significantly correlated with age and HBsAg levels, while they were positively correlated with CAP values for fatty liver (P < 0.05) in patients after 24 weeks of TAF treatment. In addition, the levels of TC, LDL, and HDL were significantly and positively correlated with AST and ALT after TAF treatment (P < 0.05), as shown in Table 2. Therefore, a multiple regression analysis of the influencing factors was further performed.
Linear regression analysis with CAP value as the dependent variable showed that CAP value was the influencing factor of lipid levels or ratios after TAF treatment(P < 0.05), as shown in Table 3. Linear regression analysis with AST and ALT as the dependent variables showed that AST and ALT were the influencing factors of cholesterol (TC, LDL, and HDL) levels (Table 4).
Because the CAP value of fatty liver is an effect factor in lipid levels, our subgroup analysis found that TC and LDL-c levels gradually increased at 96 W in CHB patients with fatty liver treated with TAF, which increased to 196.7 mg/dL (+28.76 mg/dL, P = 0.06) and 127.2 mg/dL (+22.58 mg/dL, P > 0.05), respectively. TG levels gradually increased to 146.63 mg/dL (+37.67 mg/dL, P = 0.057) at 48 W, and then gradually decreased, and there was no significant change at 96 W compared to baseline. There was no significant change in HDL-c levels during TAF treatment (P > 0.05).
None of the lipid profiles of CHB patients with non-fatty liver disease also changed significantly (P > 0.05) during treatment with TAF (Figure 5).

Figure 5. Changes in the lipid levels during TAF treatment in HBV patients with fatty liver (n = 48) and non-fatty liver (n = 89). TC, Total Cholesterol; LDL, Low Density Lipoprotein; HDL, High Density Lipoprotein; TG, Triglyceride. The data were shown as median (IQR).
Regardless of whether fatty liver was combined or not, the TC/HDL ratio slightly increased in CHB patients treated with TAF, increased to 4.16 (+0.30, P > 0.05) and 3.58 (+0.11, P > 0.05) at 96 W (Figure 5).
With the wide application of TAF, the lipid safety of TAF is a great clinical concern (17). In this study, through cross-sectional and cohort studies, the lipid profiles were demonstrated in CHB patients treated with TAF, in patients including hypercholesterolemia and fatty liver disease.
TAF is the first-line antiviral drug treatment for CHB patients, with potent antiviral effects (18). The results of our study also showed high complete viral suppression rate and ALT normalization rate during TAF treatment, which were consistent with the previous clinical studies (19, 20)
Previous studies have found that a greater increase in TC levels was observed in patients with CHB receiving TAF compared to those receiving ETV (16). In this study, it was firstly found that TAF had low effect on lipids in a cross-sectional study, and furthermore, a long-term cohort study was conducted to clarify that the use of TAF in CHB patients for 96W was safe.
The results of our study showed that a gradual decrease in LDL-c levels during TAF treatment. TC, HDL-c and TG levels increased at 24W, then gradually decreased after 24W and were lower than the baseline at 96W. None of the lipid changes showed significant differences. Similar studies have also shown a slight increase in lipids in HIV patients within one year of antiretroviral therapy, and lipid changes were static and not significantly different after 48 W, and TAF may not cause deterioration in the lipid profile of the subjects (11, 21). In our cohort study, the results were generally consistent with the lipid changes in the preceding cross-sections. In stratified analysis, CHB patients combined with non-hypercholesterolemic showed no significant changes in lipid profiles, especially for TC and LDL-c, after 96W of TAF treatment. Thus, lipid dynamics in CHB patients were safe during 96W of TAF treatment.
In our study, insignificant changes were found in lipid levels in CHB patients with TAF for 96W. Also, studies such as Chan et al. suggested that the effect of TAF on lipid levels was not clinically significant (22). However, a retrospective multicentering study showed significantly elevated levels of hypercholesterolemia, HDL-C, LDL-C, non-HDL-C, and oxidized LDL in CHB patients who switched from TDF to TAF, with an increased incidence of dyslipidemia (from 33% to 39%) (23). Therefore, the conclusions of this study need to be confirmed by longer-term studies.
Patients with CHB combined with hypercholesterolemia showed a trend towards lower TC and LDL-C levels with TAF for 96W, but there was no significant difference, and no significant change in TG levels. Similar findings also found that in HIV patients diagnosed with hypercholesterolemia at baseline, HDL-c was elevated after TAF treatment, whereas TC and LDL-levels did not change significantly during TAF treatment (24, 25).
The TC/HDL ratio is recognized as an important cumulative indicator of cardiovascular risk (26, 27). The results of this study showed a slight increase in TC/HDL ratio after TAF treatment. More importantly, the TC/HDL ratio of patients with hypercholesterolemia did not change significantly after TAF treatment. Other studies have shown that compared with baseline, no significant increase in overall ASCVD risk or any ASCVD risk category was observed during the 96-week treatment period, which was similar in the TAF and TDF groups (28). Hong’s study also showed that the association between long-term TDF or TAF therapy and changes in lipid profiles may influence or increase the impact on cardiovascular risk to some extent, if at all, but is not one of the main determinants (29). Therefore, TAF in patients with dyslipidemia may not increase the risk of cardiovascular disease. However, longer-term follow-up of lipid profiles in patients using TAF is necessary to further assess the impact on CVD risk.
Through multiple regression analysis, our study confirmed that baseline ALT and AST levels and CAP values were related to lipid safety during TAF treatment. Previous studies have shown that aminotransferase levels were significantly associated with hepatic inflammation caused by HBV viral load (30, 31). In addition, there is a negative correlation between hepatic steatosis and HBV activity (32), and it has been shown that accumulation of saturated fatty acids initiates an immune response that inhibits HBV replication; accelerates HBsAg and HBV-DNA clearance by increasing apoptosis of HBV-infected cells (33). It is hypothesized that TAF treatment reduces HBV viral load, promotes hepatic recovery, reduces aminotransferase levels, and further restores lipid metabolism.
CAP measurements can reflect the degree of hepatic steatosis (34). Based on this, our subgroup analysis based on whether CHB patients had comorbid fatty liver or not, which revealed that CHB patients with fatty liver did not have significant changes in their lipid profile after TAF treatment, and the results of our study did not show a significant effect on TAF-related lipid change in patients with fatty liver, which requires further observation.
The studies of HIV-infected patients have found an association between TAF use and hepatic steatosis (35). Relatively few studies have been conducted on whether TAF use exacerbates steatosis in patients with CHB, however, some research indicated TAF may increase patient weight (36). Therefore, CHB combined with fatty liver may also be a risk factor for TAF-related safety, and further randomized controlled and large-sample trials are needed to validate the changes in lipid profile after TAF treatment, to assess the risk of cardiovascular disease for the population with fatty liver disease, and to provide a rational basis for the use of antiviral therapy in the fatty liver population.
In this study, we observed the dynamic changes in lipids after antiviral therapy in patients with CHB who were initially treated with TAF. However, there’s limitation in our study, the sample size of our study was small, and lack of other antiviral drug treatments as control group. In the future, a larger sample size, well controlled study should be designed and conducted to improve the rigor and objectivity of the study.
TAF treatment had a low effect on the lipid profile of CHB patients over the course of 96 weeks, and it was safe in patients combined with fatty liver and hypercholesterolemia. Further effects of TAF use on lipids and the potential role on cardiovascular disease risk require more prolonged observation as well as mechanistic studies.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the First Affiliated Hospital of Xi’an Jiaotong University Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
WZ: Data curation, Formal analysis, Writing–original draft, Writing–review and editing. YL: Methodology, Writing–review and editing. MZ: Formal analysis, Writing–review and editing. ZC: Formal analysis, Writing–review and editing. ZQ: Data curation, Writing–review and editing. YL: Formal analysis, Writing–review and editing. MW: Data curation, Writing–review and editing. WW: Data curation, Formal analysis, Writing–review and editing. YC: Data curation, Writing–review and editing. LS: Data curation, Investigation, Writing–review and editing. JL: Data curation, Formal analysis, Writing–review and editing. FY: Formal analysis, Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of the article. This work was supported by the Key Scientific Research Project of Traditional Chinese Medicine Inheritance Innovation and Development of “Qin Medicine; Shaanxi Provincial Department of Science and Technology, Shaanxi Provincial Natural Science Basic Research Program Project (2020JM-399); Project of Chinese Medicine Administration of Shaanxi Province (2021-ZZ-JC01,2021-03-ZZ-003,2022-SLRH-YQ-011) and Clinical Research Project of the First Affiliated Hospital of Xi’an Jiaotong University (XJTU1AF-CRF-2020-018).
We would like to thank all the sponsor and Dr. Deyan Lu for providing CAP value datas from fibroscan in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Yuen M, Chen D, Dusheiko G, Janssen H, Lau D, Locarnini S. Hepatitis B virus infection. Nat Rev Dis Primers. (2018) 4:18036. doi: 10.1038/nrdp.2018.35
2. Jeng W-J, Papatheodoridis GV, Lok ASF. Hepatitis B. Lancet. (2023) 401:1039–52. doi: 10.1016/S0140-6736(22)01468-4
3. Terrault NA, Lok ASF, Mcmahon BJ, Chang K-M, Hwang JP, Jonas MM. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. (2018) 67:1560–99. doi: 10.1002/hep.29800
4. Babusis D, Phan TK, Lee WA, Watkins WJ, Ray AS. Mechanism for effective lymphoid cell and tissue loading following oral administration of nucleotide prodrug GS-7340. Mol Pharmaceutics. (2013) 10:459–66. doi: 10.1021/mp3002045
5. European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. (2017) 67:370–98.
6. Agarwal K, Fung SK, Nguyen TT, Cheng W, Sicard E, Ryder SD. Twenty-eight day safety, antiviral activity, and pharmacokinetics of tenofovir alafenamide for treatment of chronic hepatitis B infection. J Hepatol. (2015) 62:533–40. doi: 10.1016/j.jhep.2014.10.035
7. Byun KS, Choi J, Kim J-H, Lee YS, Lee HC, Kim YJ. Tenofovir alafenamide for drug-resistant hepatitis B: A randomized trial for switching from Tenofovir Disoproxil Fumarate. Clin Gastroenterol Hepatol. (2022) 20(2):427.e–37.e. doi: 10.1016/j.cgh.2021.04.045
8. Kauppinen KJ, Aho I, Sutinen J. Switching from tenofovir alafenamide to tenofovir disoproxil fumarate improves lipid profile and protects from weight gain. AIDS. (2022) 36:1337–44. doi: 10.1097/QAD.0000000000003245
9. Mallon PWG, Brunet L, Fusco JS, Prajapati G, Beyer A, Fusco GP, et al. Lipid changes after switch from TDF to TAF in the OPERA cohort: LDL cholesterol and triglycerides. Open Forum Infectious Dis. (2022) 9:ofab621. doi: 10.1093/ofid/ofab621
10. Buti M, Gane E, Seto WK, Chan HLY, Chuang W-L, Stepanova T. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. (2016) 1:196–206. doi: 10.1016/S2468-1253(16)30107-8
11. Jeong J, Shin JW, Jung SW, Park EJ, Park NH. Tenofovir alafenamide treatment may not worsen the lipid profile of chronic hepatitis B patients: A propensity score-matched analysis. Clin Mol Hepatol. (2022) 28:254–64. doi: 10.3350/cmh.2021.0314
12. Hui RWH, Seto WK, Cheung KS, Mak LY, Liu KSH, Fung J, et al. Inverse relationship between hepatic steatosis and hepatitis B viremia: results of a large case-control study. J Viral Hepatitis. (2018) 25:97–104. doi: 10.1111/jvh.12766
13. Pais R, Rusu E, Zilisteanu D, Circiumaru A, Micu L, Voiculescu M, et al. Prevalence of steatosis and insulin resistance in patients with chronic hepatitis B compared with chronic hepatitis C and non-alcoholic fatty liver disease. Eur J Internal Med. (2015) 26:30–6. doi: 10.1016/j.ejim.2014.12.001
14. Zhu L, Jiang J, Zhai X, Baecker A, Peng H, Qian J. Hepatitis B virus infection and risk of non-alcoholic fatty liver disease: a population-based cohort study. Liver Int. (2019) 39:70–80. doi: 10.1111/liv.13933
15. Yao J, Zhou L, Hua X, Kong M, Chen Y, Duan Z. Effects of nucleos(t)ide analogs on body composition in HBV-infected men: an age- and BMI-matched, cross-sectional study. Nutrition. (2016) 32:1206–10. doi: 10.1016/j.nut.2016.04.001
16. Lai R-M, Lin S, Wang M-M, Li N, Zhou J-H, Lin X-Y, et al. Tenofovir alafenamide significantly increased serum lipid levels compared with entecavir therapy in chronic hepatitis B virus patients. World J Hepatol. (2023) 15:964–72. doi: 10.4254/wjh.v15.i8.964
17. Surial B, Mugglin C, Calmy A, Cavassini M, Günthard HF, Stöckle M. Weight and metabolic changes after switching from tenofovir disoproxil fumarate to Tenofovir Alafenamide in people living with HIV : a cohort study. Ann Internal Med. (2021) 174:758–67. doi: 10.7326/M20-4853
18. Tao X, Lu Y, Zhou Y, Zhang L, Chen Y. Efficacy and safety of the regimens containing tenofovir alafenamide versus tenofovir disoproxil fumarate in fixed-dose single-tablet regimens for initial treatment of HIV-1 infection: a meta-analysis of randomized controlled trials. Int J Infectious Dis. (2020) 93:108–17. doi: 10.1016/j.ijid.2020.01.035
19. Agarwal K, Brunetto M, Seto WK, Lim Y-S, Fung S, Marcellin P. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection. J Hepatol. (2018) 68:672–81. doi: 10.1016/j.jhep.2017.11.039
20. Li L, Zhou J, Li Y, Wang F, Zhang D, Wang M, et al. Effectiveness and safety of tenofovir amibufenamide and its comparison with tenofovir alafenamide in patients with chronic hepatitis B: results from a retrospective real-world study. Front Pharmacol. (2023) 14:1165990. doi: 10.3389/fphar.2023.1165990
21. Huhn GD, Shamblaw DJ, Baril J-G, Hsue PY, Mills BL, Nguyen-Cleary T, et al. Atherosclerotic cardiovascular disease risk profile of Tenofovir Alafenamide versus Tenofovir Disoproxil Fumarate. Open Forum Infectious Dis. (2020) 7:ofz472. doi: 10.1093/ofid/ofz472
22. Chan HLY, Fung S, Seto WK, Chuang W-L, Chen C-Y, Kim HJ, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. (2016) 1:185–95. doi: 10.1016/S2468-1253(16)30024-3
23. Suzuki K, Suda G, Yamamoto Y, Abiko S, Kinoshita K, Miyamoto S. Effect of switching from tenofovir disoproxil fumarate to tenofovir alafenamide on lipid profiles in patients with hepatitis B. PloS One. (2022) 17:e0261760. doi: 10.1371/journal.pone.0261760
24. Taramasso L, Di Biagio A, Riccardi N, Briano F, Di Filippo E, Comi L. Lipid profile changings after switching from rilpivirine/tenofovir disoproxil fumarate/emtricitabine to rilpivirine/tenofovir alafenamide/emtricitabine: different effects in patients with or without baseline hypercholesterolemia. PloS One (2019) 14:e0223181. doi: 10.1371/journal.pone.0223181
25. Martini S, Maggi P, Gervasoni C, Onorato L, Ferrara S, Alessio L. Dynamics of lipid profile in antiretroviral-naïve HIV-infected patients, treated with TAF-based regimens: a multicenter observational study. Biomedicines (2022) 10:3164. doi: 10.3390/biomedicines10123164
26. Wen J, Huang Y, Lu Y, Yuan H. Associations of non-high-density lipoprotein cholesterol, triglycerides and the total cholesterol/HDL-c ratio with arterial stiffness independent of low-density lipoprotein cholesterol in a Chinese population. Hypertension Res. (2019) 42:1223–30. doi: 10.1038/s41440-019-0251-5
27. Quispe R, Elshazly MB, Zhao D, Toth PP, Puri R, Virani SS. Total cholesterol/HDL-cholesterol ratio discordance with LDL-cholesterol and non-HDL-cholesterol and incidence of atherosclerotic cardiovascular disease in primary prevention: the ARIC study. Eur J Preventive Cardiol. (2020) 27:1597–605. doi: 10.1177/2047487319862401
28. Fung SK, Pan CQ, Wong GL-H, Seto W-K, Ahn SH, Chen C-Y. Atherosclerotic cardiovascular disease risk profile of patients with chronic hepatitis B treated with tenofovir alafenamide or tenofovir disoproxil fumarate for 96 weeks. Alimentary Pharmacol Therapeutics (2024) 59:217–29. doi: 10.1111/apt.17764
29. Hong H, Choi W-M, Lee D, Shim JH, Kim KM, Lim Y-S. Cardiovascular risk in chronic hepatitis B patients treated with tenofovir disoproxil fumarate or tenofovir alafenamide. Clin Mol Hepatol. (2024) 30:49–63. doi: 10.3350/cmh.2023.0328
30. Fabris C, Federico E, Soardo G, Falleti E, Pirisi M. Blood lipids of patients with chronic hepatitis: differences related to viral etiology. Clin Chim Acta Int J Clin Chem. (1997) 261:159–65. doi: 10.1016/S0009-8981(97)06532-7
31. Trépo C, Chan HLY, Lok A. Hepatitis B virus infection. Lancet. (2014) 384:2053–63. doi: 10.1016/S0140-6736(14)60220-8
32. Yi S, Ren G, Zhu Y, Cong Q. Correlation analysis of hepatic steatosis and hepatitis B virus: a cross-sectional study. Virol J. (2024) 21:22. doi: 10.1186/s12985-023-02277-8
33. Tong X, Song Y, Yin S, Wang J, Huang R, Wu C, et al. Clinical impact and mechanisms of hepatitis B virus infection concurrent with non-alcoholic fatty liver disease. Chin Med J. (2022) 135:1653–63. doi: 10.1097/CM9.0000000000002310
34. Price JC, Seaberg EC, Latanich R, Budoff MJ, Kingsley LA, Palella FJ, et al. Risk factors for fatty liver in the multicenter AIDS cohort study. Am J Gastroenterol. (2014) 109:695–704. doi: 10.1038/ajg.2014.32
35. Riebensahm C, Berzigotti A, Surial B, Günthard HF, Tarr PE, Furrer H, et al. Factors associated with liver steatosis in people with human immunodeficiency virus on contemporary antiretroviral therapy. Open Forum Infectious Dis. (2022) 9:ofac538. doi: 10.1093/ofid/ofac538
Keywords: tenofovir alafenamide, lipid safety, chronic hepatitis B, 96-week, treatment
Citation: Zhao W, Liu Y, Zhang M, Cui Z, Qu Z, Li Y, Wan M, Wang W, Chen Y, Shi L, Li J and Ye F (2024) Lipid safety of tenofovir alafenamide during 96-week treatment in treatment-naive chronic hepatitis B patients. Front. Med. 11:1399665. doi: 10.3389/fmed.2024.1399665
Received: 12 March 2024; Accepted: 15 May 2024;
Published: 04 June 2024.
Edited by:
Krzysztof Tomasiewicz, Medical University of Lublin, PolandReviewed by:
Simona Alexandra Iacob, Carol Davila University of Medicine and Pharmacy, RomaniaCopyright © 2024 Zhao, Liu, Zhang, Cui, Qu, Li, Wan, Wang, Chen, Shi, Li and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Ye, eWVmZW5nLmppYW90b25nQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.