
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med. , 03 May 2024
Sec. Gastroenterology
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1399429
 Danilo Menichelli1,2†
Danilo Menichelli1,2† Gianluca Gazzaniga3†
Gianluca Gazzaniga3† Francesco Del Sole1
Francesco Del Sole1 Arianna Pani4
Arianna Pani4 Pasquale Pignatelli1
Pasquale Pignatelli1 Daniele Pastori1*
Daniele Pastori1*Acute upper and lower gastrointestinal (GI) bleeding may be a potentially life-threatening event that requires prompt recognition and an early effective management, being responsible for a considerable number of hospital admissions. Methods. We perform a clinical review to summarize the recent international guidelines, helping the physician in clinical practice. Older people are a vulnerable subgroup of patients more prone to developing GI bleeding because of several comorbidities and polypharmacy, especially related to an increased use of antiplatelet and anticoagulant drugs. In addition, older patients may have higher peri-procedural risk that should be evaluated. The recent introduction of reversal strategies may help the management of GI bleeding in this subgroup of patients. In this review, we aimed to (1) summarize the epidemiology and risk factors for upper and lower GI bleeding, (2) describe treatment options with a focus on pharmacodynamics and pharmacokinetics of different proton pump inhibitors, and (3) provide an overview of the clinical management with flowcharts for risk stratification and treatment. In conclusion, GI is common in older patients and an early effective management may be helpful in the reduction of several complications.
Gastrointestinal (GI) bleeding is one of the most frequent gastroenterological conditions that require medical attention. Its incidence varies with age, with older patients being more frequently affected. The severity of gastrointestinal bleeding can vary from a mild form to a potentially threatening life condition. The estimated prevalence for overall GI bleeding is approximately 3.1% (1). The origin of the bleeding defines its clinical presentation and definition, with hemorrhages originating before the Treitz ligament being classified as upper GI bleeding and those originating after this landmark as lower GI bleeding.
Upper GI bleeding in the older population is frequently associated with gastric and duodenal ulcer or esophagitis, being responsible for the episode in 80% of the cases (2). The incidence of upper GI bleeding can also vary depending on the geographical region. In Northern Europe, the annual incidence ranges from 213 to 570 per 100,000 patients (3); in a UK cohort of older patients affected with acute upper GI bleeding, the rate was 63% in those above 60 years of age (4); and in a North American cohort, this rate ranged between 35 and 45% (5). Although the incidence of upper GI bleeding in older patients is high, there is an even higher incidence of lower GI bleeding (6).
The incidence of hospitalizations for upper GI bleeding increased with age, being 197.4 per 100,000 population between 66 and 75 years of age and rising to 425.2 per 100,000 in people older than 75 years in a North American Cohort (7). In addition, the risk of readmittance after a first hospitalization remains higher than that of the general population.
As the risk of complications increases, so does the risk of mortality in a patient aged over 60 years experiencing an upper GI bleeding episode, estimated to be between 12 and 25%, compared to the 10% of the general population (2). In a study based on the Welsh population, the case fatality for an upper GI bleeding ranged from 11.2 to 21.5% in men above 65 years of age and from 9.1 to 20.7% in women, increasing with age (8).
Lower GI bleeding is a condition that most frequently appears in older patients, with an incidence that also depends on geographic and socio-economic factors and comorbidities (3). The most common etiologies are diverticulosis, ischemic colitis, colitis, hemorrhoids, and colorectal cancer (3). In Northern Europe, the incidence rate for lower GI bleeding ranges from 2.41 to 3.64 in male patients and from 1.72 to 3.10 in female patients, increasing with age (9). In a Spanish study, the incidence of new lower GI bleeding was between 100 and 150 per 100,000 patients in 2005 (6).
With the higher frequency of comorbidities, this cohort of patients also has an increased risk of hospitalization and longer in-hospital stay (10), with an estimated rate between 127.7 and 380.1 per 100,000 population, increasing with age (7). A multicentric study in a European cohort shows that this cohort of patients also suffers from an increased mortality rate, where the hospital mortality for lower GI bleeding was 2.5 and 1.17% in the following 3 months (1).
Medication history should be carefully reviewed upon admission, as many drugs may be associated with gastrointestinal bleeding.
First, anticoagulants and antiplatelets increase bleeding risk (11, 12). Their prescription is very common, as it has been reported that about half of the patients presenting for an upper gastrointestinal bleeding (UGIB) were treated using antithrombotic drugs (13). Clinicians should assess the risk–benefit ratio of antithrombotic administration in this setting. Indeed, as shown by the ASPREE trial enrolling 19,114 healthy older people (>65 years) patients without previous cardiovascular disease, the administration of low-dose aspirin increased the risk of major bleeding (hazard ratio [HR] 1.38, 95% confidence interval [95%CI] 1.18–1.62) and UGIB (HR 1.87, 95%CI 1.32–2.66) without an improvement of cardiovascular disease prevention (14). Furthermore, recent European guidelines advise that the use of aspirin for primary cardiovascular prophylaxis should be discontinued in patients who have a confirmed UGIB, although it should be continued for secondary prevention (15). Similarly, people taking warfarin should have the medication stopped along with anticoagulant reversal in situations of severe UGIB, while continued anticoagulation must be evaluated in cases of less severe UGIB (15).
Second, non-steroidal anti-inflammatory drugs (NSAIDs) are commonly prescribed in adult people; they represent a common risk factor for gastric hemorrhage. Their primary mechanism is the inhibition of cyclooxygenase 1 (COX-1); as a consequence, there is a great reduction of prostaglandin production, which leads to poorer protection of gastric mucosa. Coadministration of aspirin and other NSAIDs have been associated with greater gastric damage because of combined inhibition of both COX-1 and COX-2 mucosae-protective pathways (16).
Beyond these medications, other drugs have been associated with a potentially increased risk of GI bleeding, such as serotonin-selective reuptake inhibitors and calcium channel blockers; therefore, patients should be questioned whether they have been prescribed these drugs as well (17, 18).
Moreover, drugs per se may induce upper bleeding by causing pill esophagitis. A large number of medications have been associated with this phenomenon, with antibiotics, NSAIDS, and bisphosphonates being the most common (19). This occurrence is more frequent in older adults with a reduced esophageal transit; therefore, it should always be excluded in case of retrosternal pain, odynophagia, and drug assumption before sleeping up to 3 days before and whether the real cause of bleeding has not been identified yet (19).
Finally, the patient should be asked about recent consumption of products that may change stool appearance and make it look similar to real bleeding (i.e., iron, bismuth, liquorice, and charcoal), in order to avoid a wrong diagnosis in the initial stages (20, 21).
Pharmacological suppression of gastric acid production is routinely performed in patients with UGIB upon admission. This effective strategy is based on the reason that the coagulation process benefits from a higher than normal gastric pH, which, therefore, leads to a better control of hemorrhage (22).
However, despite this physio-pathological mechanism, not all acid-suppressive strategies have proved to be equal; a meta-analysis of 11 studies highlighted that H2 receptor antagonists are less efficient at stopping recurrent or persistent hemorrhage than proton pump inhibitors (PPIs), in particular for Forrest Ia, Ib, or IIa ulcers (23). For this reason, although there is evidence for a potential role of this drug class in ulcer prevention (24), it is not routinely prescribed in this setting.
There is no clear evidence on the best schedule of PPI use in terms of time, type of administration, and type of PPI. Intravenous (IV) formulation is usually preferred; however, if not available, the oral formulation may be used as well, as it has been suggested to have a similar effectiveness (25).
A recent Cochrane meta-analysis of RCTs has investigated the pre-endoscopic PPI role. There are insufficient data to determine high-certainty evidence; however, it seems that PPI administered before endoscopic procedures may not reduce mortality and need for surgery (26). On the contrary, they may reduce rebleeding and the need for hemostatic treatment performed at index endoscopic procedures with low and moderate certainty of evidence, respectively (26).
Several PPIs are available and widely used (Table 1); however, there are no recommendations on the best one to administer in this setting. As reported, they have similar pharmacokinetic parameters such as high bioavailability, volume of distribution, and protein binding. However, some differences may induce a preference according to specific situations.
Half-lives range from 0.5 to 3 h; given this short period and the fact that not all protonic pump inhibitors are targetable at the same time, a three-day period has been estimated to reach a steady-state inhibition of gastric acid (29). Despite the slightly different half-lives of PPIs, the drug of choice should not be determined on this feature as they all cause irreversible and durable inhibition of protonic pumps. Considering that preclinical models have shown that H+, K+-ATPase has a half-life of approximately 54 h (29), it must be considered that the effects of PPIs do not cease right after the last dose administration.
All the PPIs undergo hepatic metabolism; in particular, CYP2C19 plays a relevant role, followed by CYP3A4. This aspect should be taken into account as CYP2C19 genetic polymorphisms may give an extensive or poor-metabolizer phenotype, which reflects in a lower or higher PPI exposure, respectively (29); in case of genetic variants, esomeprazole should be preferred to omeprazole as its metabolism is less influenced by CYP2C19 polymorphisms (30).
PPIs are eliminated by the kidney; this is less true for lansoprazole, as the kidney accounts only for 14–23% of its elimination. In any case, no dosage adjustment for kidney function is required, regardless of PPI type and severity of the renal disease.
PPI treatment is usually chronic after the acute bleeding episode; older people commonly have concomitant diseases, which require other treatments as well. It has been estimated that 55–98% of people over 65 years old have at least two comorbidities (31). A higher disease burden implies a higher number of medications prescribed, thus leading to a higher risk of interactions among drugs. PPIs may interact with other medications in several ways.
First, PPI-induced modulation of gastric pH may result in reduced bioavailability of certain drugs administered orally; as an example, it has been reported that coadministration of omeprazole may lower bioavailability of methotrexate, ketoconazole, mycophenolate mofetil, and protease inhibitors by affecting their solubility; for this reason, their pharmacokinetic profile may be altered (32).
Second, a certain potential of PPIs to interact with intestinal P-glycoprotein (P-gp) cannot be excluded; this may be an issue, as many P-gp substrates, such as digoxin, nifedipine, amitriptyline, and tacrolimus, are widely administered in people over 65 years old; for this reason, coadministration must be carefully monitored (33).
As previously stated, PPIs are mainly metabolized by CYP2C19; this may lead to drug–drug interactions (DDI) with pharmacological agents that are substrates of the same enzymes. Among the PPIs, omeprazole has a higher DDI potential given its strong affinity for CYP2C19 and CYP3A4; it has been reported to have interaction with diazepam, moclobemide, phenytoin, and warfarin (32). In case of concomitant drugs, which may have an interaction, there is some evidence that pantoprazole, rabeprazole, and lansoprazole may carry a weaker risk of interactions (32).
Interaction between omeprazole/esomeprazole and clopidogrel is of high clinical relevance in this setting and should be monitored: It has been demonstrated that concomitant administration of omeprazole is associated with a lower exposure to active clopidogrel metabolite, regardless of a possible double dose of clopidogrel or a 12-h time period between the administrations of the two drugs (32, 34). In this case, pantoprazole should be preferred given its lower influence on clopidogrel metabolism.
Beyond pharmacological interactions, numerous studies have shown that PPIs are linked to an increased risk of a variety of negative effects, including Clostridium difficile infection, osteoporotic-related fractures, renal impairment, community-acquired pneumonia, vitamin B12 deficiency, and dementia (35). Given the range of potential side effects linked to long-term use in older people, an assessment for the need to continue PPI therapy should be routinely conducted.
This is the reason why guidelines for PPI deprescribing have been developed. A periodic assessment of PPI indications should be conducted regularly, to lower exposure; however, discontinuation is not indicated for patients with severe gastro-esophageal diseases (36).
Pharmacological agents acting on vasoconstriction (e.g., terlipressin, octreotide, and somatostatin) are recommended in addition to endoscopy in patients with UGIB from varices (or at risk for varices) (37); therapy should last from presentation to 3–5 days after bleeding cessation.
As a class, they are associated with a better hemostasis, lower need of blood transfusions, and a lower risk of 1-week mortality (38).
Among the others, terlipressin (2 mg IV q4h and then 1 mg IV q4h) has proved a 34% relative risk reduction in mortality (39). From a pharmacological point of view, terlipressin is a vasopressin analog, which acts by constricting mesenteric artery; this leads to a lower portal venous flow and, therefore, to a lower portal pressure.
Octreotide is a synthetic analog of somatostatin, a hormone which reduces release of vasodilators, thus causing a reduced portal inflow. It is administered by bolus 50 mcg IV, followed by continuous infusion (CI) 50 mcg IV each hour, and is the most widespread choice in USA in these cases, as terlipressin is not available in this country. Compared to somatostatin, octreotide has a longer half-life, but causes a similar prompt reduction of variceal pressure; however, despite adding continuous infusion, these effects only last some minutes, probably due to a pharmacodynamic desensitization (40). Nevertheless, a longer term effect mediated by other pathways may not be excluded (41).
When compared with octreotide, in a randomized controlled trial (RCT) of cirrhotic patients, terlipressin has shown a longer effect in reducing portal pressure (42); therefore, it should be preferred if available.
No vasoactive treatments should be used in place of endoscopic variceal ligation.
Prokinetic drugs may be administered as they help in cleaning the stomach from blood clots and other residues, thus allowing endoscopist have a better visualization of active bleeding sources.
Erythromycin has been studied in this setting due to its role as motilin receptor agonists. A meta-analysis of RCT has proved that it may improve visualization of gastric mucosa (43), while another showed that it is statistically associated with a lower rate of second-look endoscopies and a shorter length of stay in hospital (44). There is no clear evidence on whether adding erythromycin has a further benefit compared to nasogastric tube lavage only (43).
Notably, erythromycin is a strong CYP3A4 inhibitor: In older patients, this may represent an issue as concomitant drugs are often administered and their metabolism may be altered (45). Similarly, it has been associated with QTc prolongation and a higher risk of torsades de pointes, which should be taken into account in this population (46). Obviously, the shorter the exposure to this drug, the lower the risk of clinically significant DDI, which, however, may not be excluded.
It may be argued that, given the similar mechanism, metoclopramide may have a role as well. However, previous evidence already discussed metoclopramide role and found no effect. For this reason, despite a similar function, erythromycin is currently preferred to metoclopramide in UGIB (47).
Patients with cirrhosis and GI bleeding are frequently diagnosed with bacterial infections; approximately 22% of patients develop an infection in the first 2 days of hospital stay, while this incidence peaks up to 66% considering the first 2 weeks (48). For this reason, an antibiotic prophylaxis is usually administered in cirrhotic patients with gastrointestinal hemorrhage. A large spectrum antibiotic prophylaxis has been associated with a lower mortality, rate of bacterial infections, rebleeding rate, and length of hospitalization (49). Broad-spectrum antibiotics should be started before endoscopy and administered for up to 7 days (37).
A usual choice may be ceftriaxone 1 g once daily (OD) IV; If the patient is discharged before a week, change to ciprofloxacin 500 mg bis in die (BID) may be an alternative, although ceftriaxone has proven to be superior (37). However, drug should be selected considering patients characteristics, such as comorbidities and previous exposure to antibiotics, as people above the age of 65 years may have hepatic or renal impairments, which may affect drug metabolism and elimination; for instance, a pharmacokinetic study on older patients with moderate-to-severe impairment in renal function has highlighted a greater ceftriaxone exposure with a 48 h dosing schedule (50). Similarly, bacterial characteristics should be considered as well, as a local pattern of ceftriaxone and quinolone resistance in cirrhotic patients have been reported (51, 52).
However, despite antibiotic administration, bacterial infections still occur in approximately one-fifth of cirrhotic patients admitted for variceal bleeding; therefore, it still remains a crucial issue that should be carefully taken into account (53).
Upper gastrointestinal bleeding (UGIB) is defined as hemorrhage proximal to the Treitz ligament involving the esophagus, stomach, and duodenum (54). The most common symptoms of UGIB are melena, hematemesis, and coffee ground vomiting (54). Hematochezia, instead, is a rare manifestation of UGIB and is commonly a presentation of lower gastrointestinal bleeding (LGIB) (55, 56). Systemic manifestations, in major and life-threatening gastrointestinal (GI) bleeding (both UGIB and LGIB), include hemodynamic instability, hypotension, abdominal pain associated with lethargy, fatigue, syncope, and angina (56, 57).
The most common cause of UGIB is peptic ulcer disease, involving approximately 32–36% of all hospitalized patients; then esophagitis, duodenitis, and gastritis (until 24% of hospitalization); and finally variceal bleeding (approximately 11% of hospitalization, but 90% of UGIB in patients with liver cirrhosis) (4, 56, 58, 59).
The incidence of UGIB is widely different among countries ranging between 67 and 172/100.00 person with similar rates between Europe and the United States (59–61). Although hospitalizations for UGIB have declined due to H. pylori eradication, the use of proton pump inhibitors, and increased access to endoscopy (59, 62, 63), the mortality rate of UGIB is approximately 2–10% (54).
Antiplatelet and anticoagulant use, non-steroidal inflammatory drugs, corticosteroids, liver cirrhosis, the presence of multiple comorbidities, and older age are common risk factors for gastrointestinal bleeding, especially of UGIB (1, 6, 56, 62).
Several guidelines suggest general recommendations for initial management of UGIB (a flowchart of UGIB management is proposed in Figure 1). First, patients with UGIB should be guaranteed an IV access by cannula (≤18 G) in each antecubital fossa and an early fluid resuscitation (37) should be started, reducing the risk of mortality and myocardial infarction (54), achieving 90–100 mmHg systolic blood pressure as target (64, 65). In particular, a first approach with 500 mL of crystalloids infused in less than 15 min are suggested as first choice in hemodynamically unstable patients (66), although studies showed no difference between colloids and crystalloids in fluid resuscitation during UGIB (54).
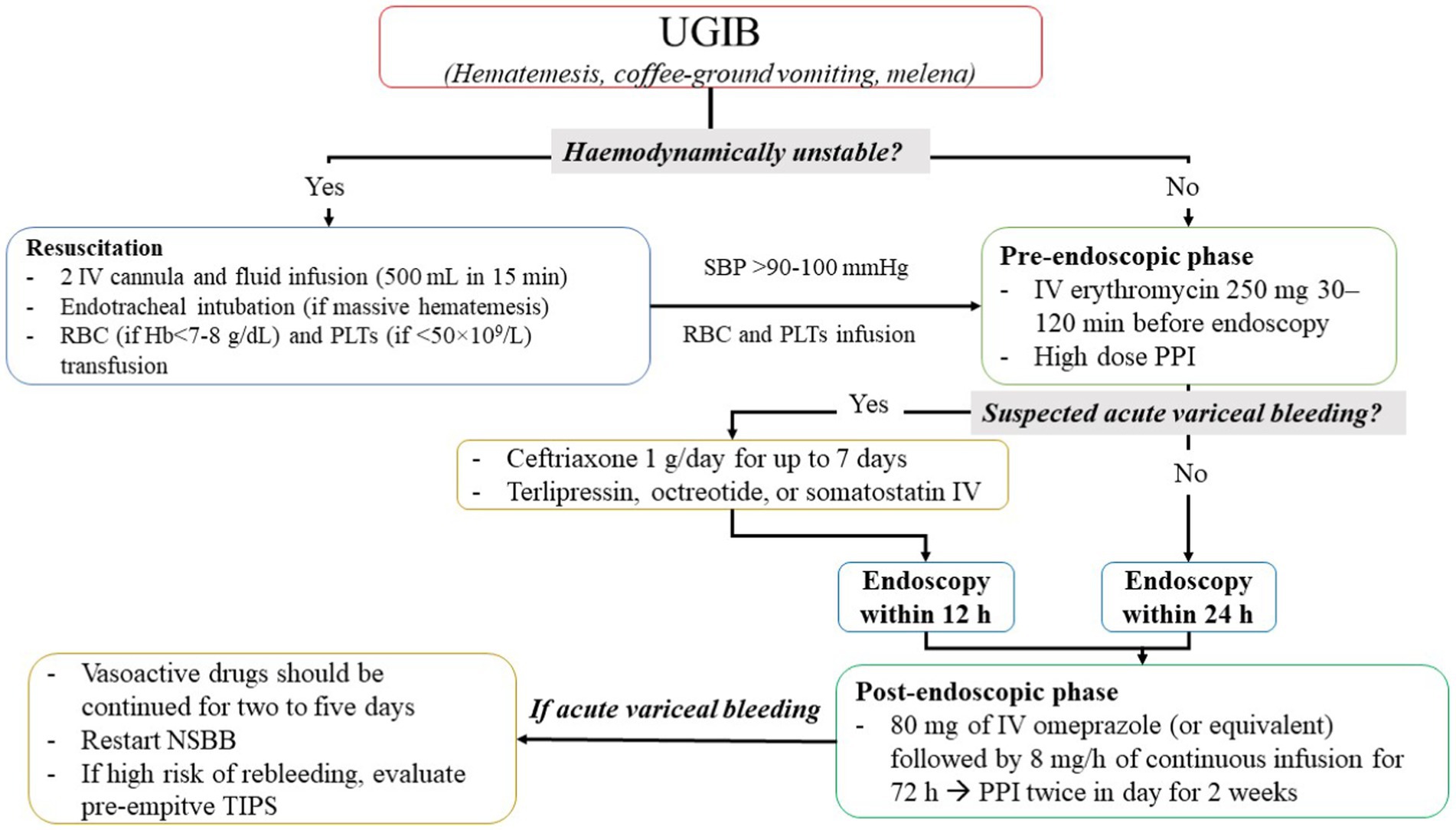
Figure 1. Flowchart of upper gastrointestinal bleeding management. IV, intravenous; NSBB, non-selective beta-blockers; PLTs, platelets; PPI, proton pump inhibitors; RBC, red blood cells; SBC, systolic blood pressure; TIPS, transhepatic intrajugular portosystemic shunt; UGIB, upper gastrointestinal bleeding.
During resuscitation, transfusion of packed red cells should be performed with a restrictive approach using a threshold of 7 g/dL (<8 g/dL in patients with cardiovascular disease) for hemoglobin (Hb) (37, 54) and transfusion of platelets should be performed using a threshold of 50 × 109/L (67, 68). After resuscitation, treatment of UGIB is divided into three phases: pre-endoscopic, endoscopic, and post-endoscopic phases.
In this phase (Figure 1), if patients are hemodynamically stable, erythromycin (250 mg IV infusion approximately 30–120 min before endoscopic procedures) was administered: Indeed, erythromycin, as a prokinetic agent, improves visualization during the endoscopy procedure resulting in a lower length of hospital stay, a lower rate of re-intervention, and less need for blood transfusions (37, 54). Furthermore, a large meta-analysis conducted by the Cochrane Institute showed that PPI may be useful (26) and performed on 2,223 patients included in six RCTs. Indeed, there is moderate-certainty evidence that PPI started before endoscopy for UGIB likely reduces the requirement for endoscopic hemostatic treatment. However, there is insufficient evidence to conclude whether PPI had a role on mortality, rebleeding, and need for surgery.
On the other hand, all guidelines recommended against the use of tranexamic acid in UGIB due to high risk of venous thromboembolism without an improvement on mortality (37, 54, 69).
Patients with compensated advanced chronic liver disease and clinically significant portal hypertension defined as hepatic venous pressure gradient>10 mmHg and/or liver stiffness by transient elastography >25 kPa should be treated for nonselective beta blocker (NSBB) as carvedilol to prevent the development of variceal bleeding (37). For patients unsuitable for NSBB and with high-risk esophageal varices, endoscopic band ligation is the endoscopic prophylactic treatment of choice (37). Of note, in patients with advanced chronic liver disease and portal vein thrombosis, an anticoagulation treatment, if not contraindicated, may be helpful to prevent variceal bleeding: indeed, in a large meta-analysis (70), there were no differences in major or minor bleeding in patients treated or not treated with anticoagulants (11% for both groups), but a lower rate of variceal bleeding was observed in patients taking anticoagulants, maybe due to thrombus resolution in portal vein (70).
In patients with suspected variceal bleeding (37, 54), the use of vasoactive agents such as terlipressin, octreotide, or somatostatin at hospital admission is recommended and continued for a duration of up to 5 days. Furthermore, antibiotic prophylaxis is also recommended in patients with UGIB by suspected esophageal varices (37, 54). In particular, the European Society of Gastrointestinal Endoscopy (ESGE) suggests the use of ceftriaxone 1 g/day for up to 7 days for all patients with suspected variceal bleeding (or in accordance with local antibiotic resistance and patient allergies) (37). In addition, all patients should be stratified according to CHILD-PUGH and MELD scores and the endoscopic evaluation should take place within 12 h from the time of patient presentation/fluid resuscitation.
Predictive pre- and post-endoscopic scores were developed during several years. In particular, pre-endoscopic score may help the physician to choose the optimal management of patients with UGIB evaluating an outpatient approach and estimating the risk of complications and death. In Table 2, we summarize the items of pre-endoscopic risk assessment scores recommended and validated in clinical practice (pre-endoscopic Rockall score, AIMS-65, and Glasgow Blatchford score) (54, 71–73). Recently, a simple ABC score (74) was proposed (Table 2), but not sufficient data are available to recommend it in clinical practice (54).
In particular, a recent multicenter study involving 3,012 consecutive patients with UGIB showed that the Glasgow-Blatchford score has high accuracy at predicting need of hospitalization or death. Furthermore, a score of ≤1 is the optimum threshold for choose an outpatient management (75). For this reason, international guidelines recommend this score as first choice (37, 69, 76, 77).
In patients with UGIB candidate to endoscopy in emergency setting, this should be performed within 24 h of presentation (within 12 h if variceal bleeding is suspected) (37) and hemostatic endoscopic treatment is recommended only for ulcers with active spurting, active oozing, and non-bleeding visible vessels (37), while it is unclear whether endoscopic hemostatic treatment is useful for ulcers with adherent clot resistant to vigorous irrigation (37). No endoscopic treatment indicated whether only flat pigmented spots or clean base is found during endoscopy (69).
Finally, in patients with recurrent bleeding, after previous successfully endoscopic procedure, a new endoscopic treatment with clips is recommended, although with low quality of evidence (37).
In the post-endoscopic phase, medical therapy should be administered to reduce the risk of rebleeding and death. All guidelines recommend the use of high dose of PPIs (54) without the difference between continuous and intermittent regimen (37) (Figure 1).
The American College of Gastroenterology (ACG) guidelines recently suggest a medical therapy for UGIB based on endoscopic features (69). While the treatment of active ulcers or adherent clot findings is coherent with other guidelines (a high-intensity PPIs: for continuous regimen, 80 mg bolus followed by 8 mg/h infusion for 3 days and for intermittent regimens, 40 mg 2–4 times daily for 3 days, orally if feasible, after an initial bolus of 80 mg) (69), ACG guidelines for flat pigmented spot or clean base suggest standard dose-regimen PPI (69).
After high-dose PPIs, in patients undergoing hemostatic treatment, a further 2-week treatment with twice-daily PPIs is recommended to reduce rebleeding risk (37).
In addition, in case of proven variceal bleeding, vasoactive drugs should be continued for 2 to 5 days (54) (Figure 1).
In patients with variceal bleeding at high risk of recurrent bleeding following successful endoscopic hemostasis, pre-emptive transjugular intrahepatic portosystemic shunt (TIPS) within 72 h (preferably within 24 h) must be considered (37). NSBBs (propranolol or carvedilol) in combination with endoscopic therapy for secondary prophylaxis should be continued in patients with advanced chronic liver disease and/or and previous esophageal variceal bleeding.
Lower gastrointestinal bleeding (LGIB) represents the 3% of emergency surgical referrals (78), and its incidence is estimated to be 33–87 for 100.000 patients (79). The mortality is 3.4% rising 18–20% in patients with LGIB during hospitalization (79). The most common cause of LGIB is diverticular bleeding, followed by benign anorectal conditions such as hemorrhoids, fissures, and rectal ulcers (79). Other common causes are telangiectasia in multiple sites of GI tract, colitis, and colorectal cancer. Of note, 23% of hospitalized patients with LGIB in the UK are discharged without a diagnosis (80). Patients with LGIB should be clinically evaluated to establish the hemodynamic stability. Clinical history (bleeding history and comorbidities), clinical evaluation (including digital-rectal exploration), laboratory test, and concomitant therapy are needed to establish the hemodynamic status of patients (81). In particular, shock index (heart rate [HR] and systolic blood pressure [SBP] ratio) is recommended by current guidelines (79). A shock index >1 defined the patient as hemodynamically unstable (79).
Similar to UGIB, a resuscitation strategy (previous described) should be performed in unstable patients (79). In these patients, a computed tomography (CT) scan with angiography should be performed to evaluate the focus of bleeding; then, patients should undergo to interventional radiology (preferably <60 min from hospital admission) or endoscopy (79). Although endoscopy treatment represents the first line of treatment from international guidelines (79, 81, 82), only 2.1% of cases of LGIB undergo endoscopic treatment and the most common intervention is red blood cell transfusions (79). If a treatment failure occurred during endoscopy or radiological intervention, surgery should be evaluated in selected cases. If no focus of bleeding is identified during CT scan with angiography, patients should be considered stable with major bleeding.
If shock index is <1, LGIB should be considered stable and Oakland score should be performed to establish whether major or minor bleeding occurred and whether hospitalization is required (Oakland score ≤ 8 suggests a possible outpatient management) (79).
Fluid resuscitation is needed, and a restricted red blood cell transfusion regimen should be preferred with a threshold of Hb <70 g/L with a target of 70–90 g/L after transfusion, except for patients with previous history of cardiovascular disease with a threshold of Hb <80 g/L with a target of 10.0 g/L (79), and platelets transfusion should be performed using a threshold of 50 × 109/L (82). Figure 2 summarizes the management of LGIB.
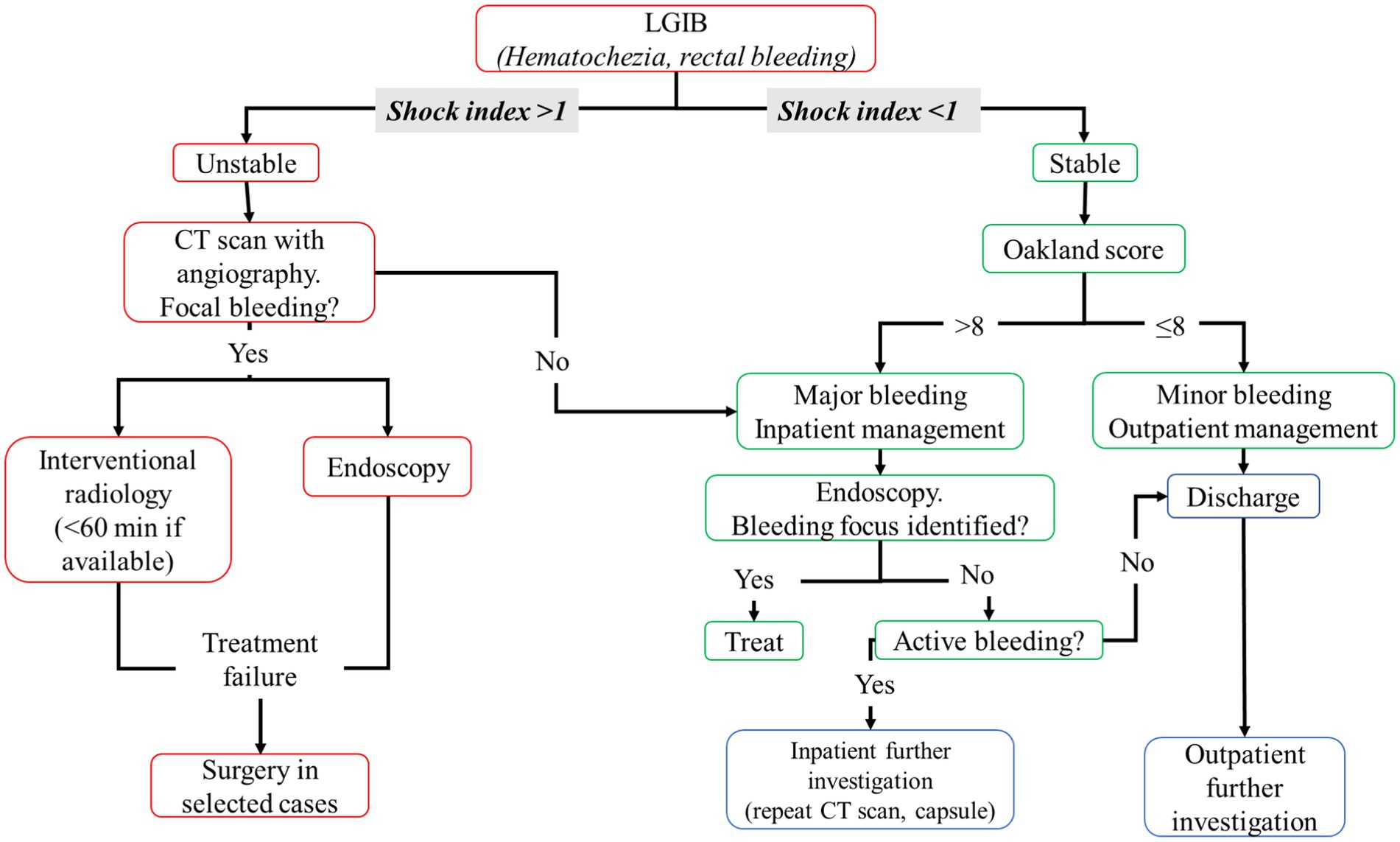
Figure 2. Flowchart of lower gastrointestinal bleeding management. CT, computed tomography; HR, heart rate; LGIB, lower gastrointestinal bleeding; SBP, systolic blood pressure.
In major bleeding, if patient is hemodynamically stable, colonoscopy should be performed after adequate colon cleansing (a nasogastric tube may help colon preparation in patients with a low risk of aspiration and ongoing bleeding) (82). In total, 4–6 liters of a polyethylene glycol (PEG)-based solution or the equivalent should be administered over 3–4 h until the rectal effluent is clear of blood and stool (82).
In patients with high-risk clinical features or ongoing bleeding, endoscopy should be performed within 24 h of patient’s admission to the emergency department, after an adequate colon preparation; otherwise, a colonoscopy should be performed next available after a colon purge (82). Further investigations are needed if no bleeding focus was found, such as CT scan with angiography repetition or use of video capsules (79).
In older people, the severity and prognosis of GI bleeding are influenced by medical comorbidities (1) and therapies as well as the use of antiplatelet and anticoagulants medication (83). Indeed, 70% of UGIB occurred in patients >60 years old and its incidence and mortality risk rise according to age (83). Similarly, patients with LGIB are more common in older patients, with a mean onset age between 63 and 77 years, with higher mortality risk (83). In addition, GI bleeding incidence seems to be reduced only in patients <70 years old (61). For these reasons, GI hemorrhage management is a backbone in the older care and there are some peculiarities of old age that should be addressed.
First, endoscopy, the first line of diagnosis and treatment for GI hemorrhage, has similar mortality risk in older patients compared to general population and old age is not a contraindication to endoscopy (83). Of note, older patients had an increased risk to developing adverse events and oxygen desaturation, especially if benzodiazepines (BDZ) are administered during endoscopy for sedation (83, 84); for this reason, a lower dose of BDZ with careful titration is suggested (83).
In addition, older patients are more likely to be treated with antiplatelets, anticoagulants, especially with complex antithrombotic therapy (CAT) resulting in an increased risk of hospitalization and transfusion as shown in a large cohort study of 78,133 old veterans aged >60 years treated with antiplatelets and/or anticoagulants, with the highest risk of hospitalization and transfusion in patients treated with dual antiplatelet agents and anticoagulant (85).
In patients treated with antiplatelets, a GI bleeding incidence rate of 0.7–1.3% for aspirin and 1.2–2% for aspirin and clopidogrel combination during a follow-up of 1–2 years was observed in Western countries (86–88). In older patients, the GI bleeding incidence rate rise to 2.7% as shown by an observational study on 1852 patients undergone the implantation of drug-eluted stent (DES) with a mean age of 70.9 years (89).
Antiplatelet management had a fundamental role in older patients. If antiplatelet is administered in primary prophylaxis should be permanently discontinued, while antiplatelet in secondary prevention should not be stopped, but if suspension is needed, it should be restarted when hemostasis is guaranteed (79, 82).
In patients treated with dual antiplatelet therapy with aspirin and a P2Y12 inhibitor, P2Y12 should be stopped only in unstable hemorrhage and restarted within 5 days, especially if recent coronary stenting is performed (54, 79, 81).
In older patients, comorbidities such as atrial fibrillation (AF) that require indefinite anticoagulation are common. The most common oral anticoagulant prescribed in older patients is vitamin K antagonists (VKAs) and direct oral anticoagulants (DOACs).
In older patients (≥75 years), the incidence rate of GIB was 2.19% per year for dabigatran 110 mg and 2.80% per year for dabigatran 150 mg (90). Instead, an incidence GIB rate of 1.51 and 0.83% per year was observed for edoxaban 60 mg and 30 mg, respectively (90). The incidence of GIB rate of 2.0% for rivaroxaban and 0.76% for apixaban, but no data according to dose were found (90). The incidence of GIB for warfarin ranged between 0.86 and 1.59% per year in phase III clinical trials (90). A large cohort meta-analysis performed on 129.357 patients by Miller et al. summarized the risk of GIB in patients treated with DOACs and VKAs (pooled rate: 1.5% versus 1.3%, respectively; odds ratio [OR]: 0.98; 95%CI: 0.80–1.21) (91).
Recently, a large network meta-analysis on 605.771 AF patients showed a reduced risk of GIB in patients treated with apixaban compared to ones treated with dabigatran or rivaroxaban (92).
VKAs, as warfarin, should be discontinued during hemorrhage and should be restarted at 7 days after GI bleeding if low thrombotic risk (54, 79, 81). In patients with high thrombotic risk as well as patients with mechanical prosthetic heart valve, AF with prosthetic heart valve or mitral stenosis, <3 months after venous thromboembolism, low molecular weight heparin treatment should be started at 48 h after hemorrhage (54, 79). In patients on warfarin, although the optimal international normalized ratio (INR) to perform endoscopy is <1.3, endoscopy may be also considered when INR is <2.5 without a significantly increased risk of rebleeding (54).
On the other hand, DOAC should be stopped at presentation of GI bleeding and restarted within 7 days after GI bleeding (54, 79, 81).
In life-threatening GI bleeding and hemodynamically unstable patients, the interruption of oral anticoagulants is not enough, and a reversal agent is needed (Figure 3) (12, 54, 79, 81).
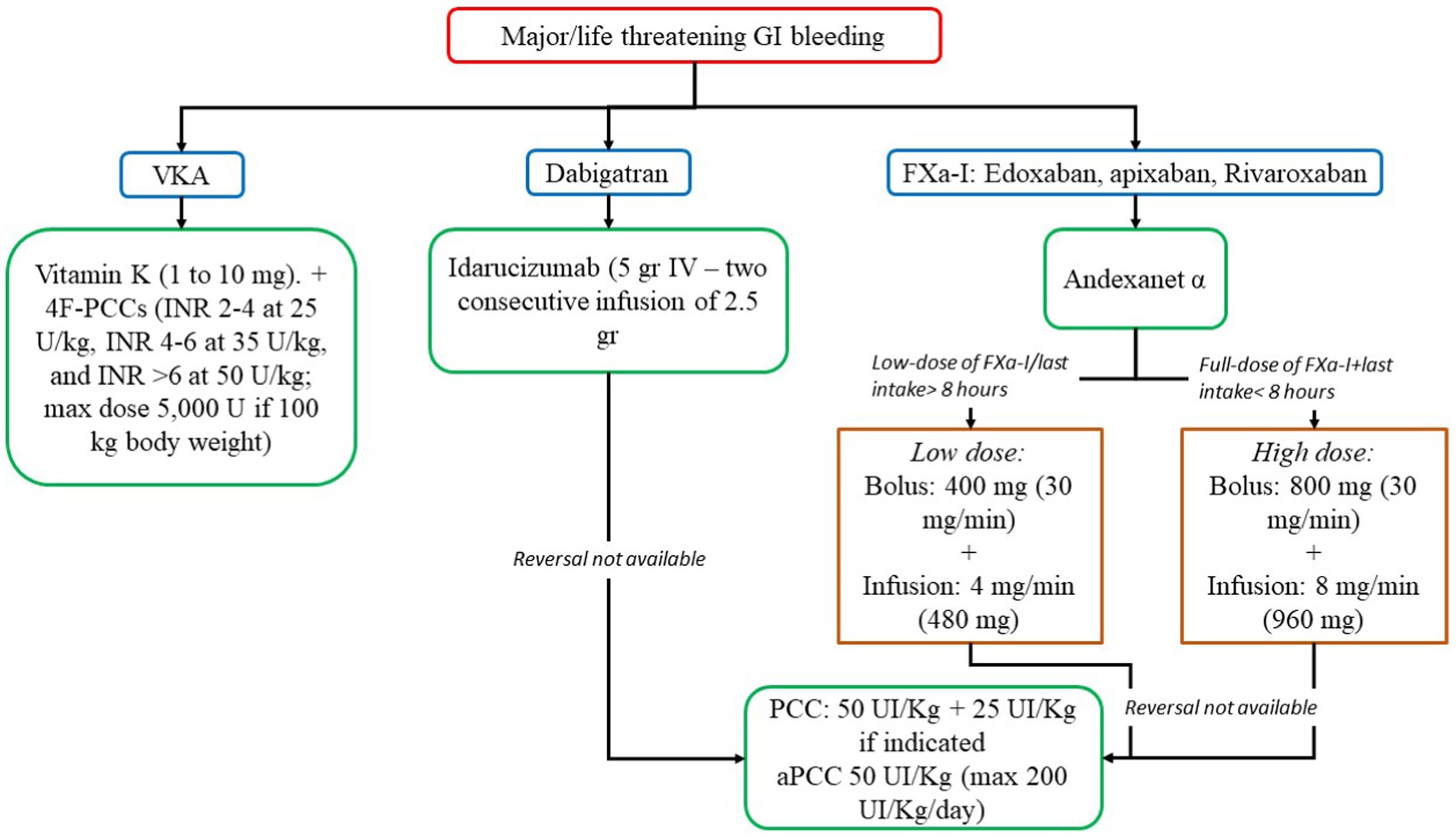
Figure 3. Reversal strategies for anticoagulants in major/life threatening gastrointestinal bleeding. 4F-PCC, 4-factor prothrombin complex concentrate; aPCC, activated prothrombin complex concentrate; FXa-I, inhibitors of FXa; GI, gastrointestinal; INR, international normalized ratio; PCC, prothrombin complex concentrate; VKA, vitamin K antagonist.
In VKAs, Vitamin K is a specific reversal agent in a dose-dependent manner (1 to 10 mg). Slow intravenous administration (in 25 to 50 mL normal saline over 15 to 30 min) causes a rapid reduction in the INR (4–6 h) (93). However, the administration of vitamin K does not result in immediate correction of coagulopathy, and in life threatening bleeding, vitamin K administration must be accompanied by the administration of 4-factor prothrombin complex concentrate (4F-PCCs), or, if not available, plasma (93). 4F-PCCs should be administered according to INR range and body weight (INR 2–4 at 25 U/kg, INR 4–6 at 35 U/kg, and INR >6 at 50 U/kg; max dose 5,000 U if 100 kg body weight) (93).
In DOACs, in dabigatran users, a reversal agent, idarucizumab is suitable (5gr + 2.5gr, IV) (93). However, in patients taking apixaban, rivaroxaban, and edoxaban, andexanet alfa may be useful. If these drugs are not available, 4F-PCC or activated PCC (aPCC) may be an alternative (50 U/kg) (12, 93).
Furthermore, a large cohort study of 3,166 patients treated with antiplatelet and without routine PPI use, due to previous myocardial infarction or cerebrovascular event, showed that the long-term risk of bleeding is higher in older patients than in younger patients with a substantial risk of disabling or fatal UGIB, suggesting that a co-prescription of PPI should be encouraged (94).
On the other hand, the use of PPI is associated with several adverse effects, as well as increased risk of fractures, osteoporosis, higher risk of Clostridium difficile (CD) infection and community-acquired pneumonia (CAP), especially in older patients (95).
In particular, a large meta-analysis including 2,181,546 individuals taking or not taking PPI showed that patients not taking PPI, those taking PPI, had an increased risk of developing any-site fractures, hip fractures, spine fracture, and osteoporosis (96), this evidence is confirmed independently from dose and duration of therapy, as suggested by a large meta-analysis on 2,103,800 patients showing a high risk of hip fracture in patients with long- and short-term therapy and in low, medium, and high dosage of PPI (97).
In addition, PPI is also associated with an increased risk of developing CD infection as shown in a large meta-analysis of 56 studies (40 case control and 16 cohort) involving 356,683 patients (98). This risk is estimated approximately 64% compared to ones not taking PPI (99) and probably is related to PPI-gut dysbiosis (100, 101) that increases also all-cause mortality (101).
Finally, several studies showed an increased risk of developing CAP in patients taking PPI compared to ones who are not taking these drugs, particularly within 30 days (102–104). A pathogenic mechanism has been proposed to explain the association between PPI use and the incidence of CAP: PPIs may increase the gastric pH altering also normal oropharyngeal flora, which could increase susceptibility to respiratory infections by permitting survival of pathogens that lead to CAP (104).
Osteoporosis, bone fractures, CD infection, and CAP may be deadly for older patients, and these complications should be avoided. For this reason, PPI in older patients should be used only according to clinical indications, with long-term treatments only for selected cases (36).
In conclusion, UGIB and LGIB represent a severe common complication, especially in older patients with comorbidities and on treatment with antiplatelet and/or anticoagulant drugs. Several drugs are available to reduce bleeding complications, especially for UGIB. Current evidence and guidelines suggest a clinical approach based on hemodynamic status with endoscopy as the first line for diagnosis and treatment.
DM: Conceptualization, Writing – original draft. GG: Writing – original draft. FS: Writing – original draft. AP: Writing – review & editing. PP: Supervision, Writing – review & editing. DP: Conceptualization, Supervision, Visualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Lenti, MV, Pasina, L, Cococcia, S, Cortesi, L, Miceli, E, Caccia Dominioni, C, et al. Mortality rate and risk factors for gastrointestinal bleeding in elderly patients. Eur J Intern Med. (2019) 61:54–61. doi: 10.1016/j.ejim.2018.11.003
2. Lingenfelser, T, and Ell, C. Gastrointestinal bleeding in the elderly. Best Pract Res Clin Gastroenterol. (2001) 15:963–82. doi: 10.1053/bega.2001.0252
3. Hreinsson, JP, Kalaitzakis, E, Gudmundsson, S, and Bjornsson, ES. Upper gastrointestinal bleeding: incidence, etiology and outcomes in a population-based setting. Scand J Gastroenterol. (2013) 48:439–47. doi: 10.3109/00365521.2012.763174
4. Hearnshaw, SA, Logan, RF, Lowe, D, Travis, SP, Murphy, MF, and Palmer, KR. Acute upper gastrointestinal bleeding in the UK: patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut. (2011) 60:1327–35. doi: 10.1136/gut.2010.228437
5. Farrell, JJ, and Friedman, LS. Gastrointestinal bleeding in the elderly. Gastroenterol Clin N Am. (2001) 30:377–407. viii. doi: 10.1016/s0889-8553(05)70187-4
6. Lanas, A, Garcia-Rodriguez, LA, Polo-Tomas, M, Ponce, M, Alonso-Abreu, I, Perez-Aisa, MA, et al. Time trends and impact of upper and lower gastrointestinal bleeding and perforation in clinical practice. Am J Gastroenterol. (2009) 104:1633–41. doi: 10.1038/ajg.2009.164
7. Laine, L, Yang, H, Chang, SC, and Datto, C. Trends for incidence of hospitalization and death due to GI complications in the United States from 2001 to 2009. Am J Gastroenterol. (2012) 107:1190–5; quiz 1196. doi: 10.1038/ajg.2012.168
8. Button, LA, Roberts, SE, Evans, PA, Goldacre, MJ, Akbari, A, Dsilva, R, et al. Hospitalized incidence and case fatality for upper gastrointestinal bleeding from 1999 to 2007: a record linkage study. Aliment Pharmacol Ther. (2011) 33:64–76. doi: 10.1111/j.1365-2036.2010.04495.x
9. Vora, P, Pietila, A, Peltonen, M, Brobert, G, and Salomaa, V. Thirty-year incidence and mortality trends in upper and lower gastrointestinal bleeding in Finland. JAMA Netw Open. (2020) 3:e2020172. doi: 10.1001/jamanetworkopen.2020.20172
10. Comay, D, and Marshall, JK. Resource utilization for acute lower gastrointestinal hemorrhage: the Ontario GI bleed study. Can J Gastroenterol. (2002) 16:677–82. doi: 10.1155/2002/156592
11. Guo, CG, Cheung, KS, Zhang, F, Chan, EW, Chen, L, Wong, IC, et al. Incidences, temporal trends and risks of hospitalisation for gastrointestinal bleeding in new or chronic low-dose aspirin users after treatment for Helicobacter pylori: a territory-wide cohort study. Gut. (2020) 69:445–52. doi: 10.1136/gutjnl-2019-318352
12. Pastori, D, Menichelli, D, Gingis, R, Pignatelli, P, and Violi, F. Tailored practical management of patients with atrial fibrillation: a risk factor-based approach. Front Cardiovasc Med. (2019) 6:17. doi: 10.3389/fcvm.2019.00017
13. Dunne, PDJ, Laursen, SB, Laine, L, Dalton, HR, Ngu, JH, Schultz, M, et al. Previous use of antithrombotic agents reduces mortality and length of hospital stay in patients with high-risk upper gastrointestinal bleeding. Clin Gastroenterol Hepatol. (2019) 17:e442:440–447.e2. doi: 10.1016/j.cgh.2018.04.046
14. Mcneil, JJ, Wolfe, R, Woods, RL, Tonkin, AM, Donnan, GA, Nelson, MR, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. (2018) 379:1509–18. doi: 10.1056/NEJMoa1805819
15. Veitch, AM, Radaelli, F, Alikhan, R, Dumonceau, JM, Eaton, D, Jerrome, J, et al. Endoscopy in patients on antiplatelet or anticoagulant therapy: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guideline update. Gut. (2021) 70:1611–28. doi: 10.1136/gutjnl-2021-325184
16. Fiorucci, S, De Lima, OM, Mencarelli, A, Palazzetti, B, Distrutti, E, Mcknight, W, et al. Cyclooxygenase-2-derived lipoxin A4 increases gastric resistance to aspirin-induced damage. Gastroenterology. (2002) 123:1598–606. doi: 10.1053/gast.2002.36558
17. Alam, SM, Qasswal, M, Ahsan, MJ, Walters, RW, and Chandra, S. Selective serotonin reuptake inhibitors increase risk of upper gastrointestinal bleeding when used with NSAIDs: a systemic review and meta-analysis. Sci Rep. (2022) 12:14452. doi: 10.1038/s41598-022-18654-2
18. He, Y, Chan, EW, Leung, WK, Anand, S, and Wong, IC. Systematic review with meta-analysis: the association between the use of calcium channel blockers and gastrointestinal bleeding. Aliment Pharmacol Ther. (2015) 41:1246–55. doi: 10.1111/apt.13211
19. Kikendall, JW. Pill esophagitis. J Clin Gastroenterol. (1999) 28:298–305. doi: 10.1097/00004836-199906000-00004
20. Liu, JF, Srivatsa, A, and Kaul, V. Black licorice ingestion: yet another confounding agent in patients with melena. World J Gastrointest Surg. (2010) 2:30–1. doi: 10.4240/wjgs.v2.i1.30
21. Srygley, FD, Gerardo, CJ, Tran, T, and Fisher, DA. Does this patient have a severe upper gastrointestinal bleed? JAMA. (2012) 307:1072–9. doi: 10.1001/jama.2012.253
22. Green, FW Jr, Kaplan, MM, Curtis, LE, and Levine, PH. Effect of acid and pepsin on blood coagulation and platelet aggregation. A possible contributor prolonged gastroduodenal mucosal hemorrhage. Gastroenterology. (1978) 74:38–43. doi: 10.1016/0016-5085(78)90352-9
23. Gisbert, JP, Gonzalez, L, Calvet, X, Roque, M, Gabriel, R, and Pajares, JM. Proton pump inhibitors versus H2-antagonists: a meta-analysis of their efficacy in treating bleeding peptic ulcer. Aliment Pharmacol Ther. (2001) 15:917–26. doi: 10.1046/j.1365-2036.2001.01012.x
24. Taha, AS, Mccloskey, C, Prasad, R, and Bezlyak, V. Famotidine for the prevention of peptic ulcers and oesophagitis in patients taking low-dose aspirin (FAMOUS): a phase III, randomised, double-blind, placebo-controlled trial. Lancet. (2009) 374:119–25. doi: 10.1016/S0140-6736(09)61246-0
25. Tsoi, KK, Hirai, HW, and Sung, JJ. Meta-analysis: comparison of oral vs. intravenous proton pump inhibitors in patients with peptic ulcer bleeding. Aliment Pharmacol Ther. (2013) 38:721–8. doi: 10.1111/apt.12441
26. Kanno, T, Yuan, Y, Tse, F, Howden, CW, Moayyedi, P, and Leontiadis, GI. Proton pump inhibitor treatment initiated prior to endoscopic diagnosis in upper gastrointestinal bleeding. Cochrane Database Syst Rev. (2022) 1:CD005415. doi: 10.1002/14651858.CD005415.pub4
27. Markar, SR, Mavroveli, S, Petrides, KV, Scarpa, M, Christophe, V, Castoro, C, et al. Applied investigation of person-specific and context-specific factors on postoperative recovery and clinical outcomes of patients undergoing gastrointestinal cancer surgery: multicentre European study. BMJ Open. (2016) 6:e012236. doi: 10.1136/bmjopen-2016-012236
28. Drugbank. Available at: https://www.drugbank.com/ (Accessed 2023).
29. Shin, JM, and Kim, N. Pharmacokinetics and pharmacodynamics of the proton pump inhibitors. J Neurogastroenterol Motil. (2013) 19:25–35. doi: 10.5056/jnm.2013.19.1.25
30. Klotz, U. Clinical impact of CYP2C19 polymorphism on the action of proton pump inhibitors: a review of a special problem. Int J Clin Pharmacol Ther. (2006) 44:297–302. doi: 10.5414/CPP44297
31. Marengoni, A, Angleman, S, Melis, R, Mangialasche, F, Karp, A, Garmen, A, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. (2011) 10:430–9. doi: 10.1016/j.arr.2011.03.003
32. Wedemeyer, RS, and Blume, H. Pharmacokinetic drug interaction profiles of proton pump inhibitors: an update. Drug Saf. (2014) 37:201–11. doi: 10.1007/s40264-014-0144-0
33. Blume, H, Donath, F, Warnke, A, and Schug, BS. Pharmacokinetic drug interaction profiles of proton pump inhibitors. Drug Saf. (2006) 29:769–84. doi: 10.2165/00002018-200629090-00002
34. Angiolillo, DJ, Gibson, CM, Cheng, S, Ollier, C, Nicolas, O, Bergougnan, L, et al. Differential effects of omeprazole and pantoprazole on the pharmacodynamics and pharmacokinetics of clopidogrel in healthy subjects: randomized, placebo-controlled, crossover comparison studies. Clin Pharmacol Ther. (2011) 89:65–74. doi: 10.1038/clpt.2010.219
35. Maes, ML, Fixen, DR, and Linnebur, SA. Adverse effects of proton-pump inhibitor use in older adults: a review of the evidence. Ther Adv Drug Saf. (2017) 8:273–97. doi: 10.1177/2042098617715381
36. Targownik, LE, Fisher, DA, and Saini, SD. AGA Clinical practice update on De-prescribing of proton pump inhibitors: expert review. Gastroenterology. (2022) 162:1334–42. doi: 10.1053/j.gastro.2021.12.247
37. Gralnek, IM, Camus Duboc, M, Garcia-Pagan, JC, Fuccio, L, Karstensen, JG, Hucl, T, et al. Endoscopic diagnosis and management of esophagogastric variceal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. (2022) 54:1094–120. doi: 10.1055/a-1939-4887
38. Wells, M, Chande, N, Adams, P, Beaton, M, Levstik, M, Boyce, E, et al. Meta-analysis: vasoactive medications for the management of acute variceal bleeds. Aliment Pharmacol Ther. (2012) 35:1267–78. doi: 10.1111/j.1365-2036.2012.05088.x
39. Ioannou, G, Doust, J, and Rockey, DC. Terlipressin for acute esophageal variceal hemorrhage. Cochrane Database Syst Rev. (2001) 1:CD002147. doi: 10.1002/14651858.CD002147
40. Escorsell, A, Bandi, JC, Andreu, V, Moitinho, E, Garcia-Pagan, JC, Bosch, J, et al. Desensitization to the effects of intravenous octreotide in cirrhotic patients with portal hypertension. Gastroenterology. (2001) 120:161–9. doi: 10.1053/gast.2001.20892
41. Ludwig, D, Schadel, S, Bruning, A, Schiefer, B, and Stange, EF. 48-hour hemodynamic effects of octreotide on postprandial splanchnic hyperemia in patients with liver cirrhosis and portal hypertension: double-blind, placebo-controlled study. Dig Dis Sci. (2000) 45:1019–27. doi: 10.1023/A:1005553914878
42. Baik, SK, Jeong, PH, Ji, SW, Yoo, BS, Kim, HS, Lee, DK, et al. Acute hemodynamic effects of octreotide and terlipressin in patients with cirrhosis: a randomized comparison. Am J Gastroenterol. (2005) 100:631–5. doi: 10.1111/j.1572-0241.2005.41381.x
43. Adao, D, Gois, AF, Pacheco, RL, Pimentel, CF, and Riera, R. Erythromycin prior to endoscopy for acute upper gastrointestinal haemorrhage. Cochrane Database Syst Rev. (2023) 2:CD013176. doi: 10.1002/14651858.CD013176.pub2
44. Rahman, R, Nguyen, DL, Sohail, U, Almashhrawi, AA, Ashraf, I, Puli, SR, et al. Pre-endoscopic erythromycin administration in upper gastrointestinal bleeding: an updated meta-analysis and systematic review. Ann Gastroenterol. (2016) 29:312–7. doi: 10.20524/aog.2016.0045
45. Periti, P, Mazzei, T, Mini, E, and Novelli, A. Pharmacokinetic drug interactions of macrolides. Clin Pharmacokinet. (1992) 23:106–31. doi: 10.2165/00003088-199223020-00004
46. Fiets, RB, Bos, JM, Donders, A, Bruns, M, Lamfers, E, Schouten, JA, et al. QTc prolongation during erythromycin used as prokinetic agent in ICU patients. Eur J Hosp Pharm. (2018) 25:118–22. doi: 10.1136/ejhpharm-2016-001077
47. Daram, SR, and Garretson, R. Erythromycin is preferable to metoclopramide as a prokinetic in acute upper GI bleeding. Gastrointest Endosc. (2011) 74:234; author reply 234-235. doi: 10.1016/j.gie.2011.01.059
48. Bernard, B, Grange, JD, Khac, EN, Amiot, X, Opolon, P, and Poynard, T. Antibiotic prophylaxis for the prevention of bacterial infections in cirrhotic patients with gastrointestinal bleeding: a meta-analysis. Hepatology. (1999) 29:1655–61. doi: 10.1002/hep.510290608
49. Chavez-Tapia, NC, Barrientos-Gutierrez, T, Tellez-Avila, F, Soares-Weiser, K, Mendez-Sanchez, N, Gluud, C, et al. Meta-analysis: antibiotic prophylaxis for cirrhotic patients with upper gastrointestinal bleeding - an updated Cochrane review. Aliment Pharmacol Ther. (2011) 34:509–18. doi: 10.1111/j.1365-2036.2011.04746.x
50. Tan, SJ, Cockcroft, M, Page-Sharp, M, Arendts, G, Davis, TME, Moore, BR, et al. Population pharmacokinetic study of ceftriaxone in elderly patients, using cystatin C-based estimates of renal function to account for frailty. Antimicrob Agents Chemother. (2020) 64:e00874–20. doi: 10.1128/AAC.00874-20
51. Ghweil, AA, Bazeed, SES, Al Rawy, MH, Khodeary, A, and El-Amir, MI. Fluoroquinolone-resistant strains in cirrhotic patients with spontaneous bacterial peritonitis: microbiological and molecular aspects. Eur J Gastroenterol Hepatol. (2022) 34:64–8. doi: 10.1097/MEG.0000000000001908
52. Hillert, A, Schultalbers, M, Tergast, TL, Vonberg, RP, Rademacher, J, Wedemeyer, H, et al. Antimicrobial resistance in patients with decompensated liver cirrhosis and bacterial infections in a tertiary center in northern Germany. BMC Gastroenterol. (2021) 21:296. doi: 10.1186/s12876-021-01871-w
53. Martinez, J, Hernandez-Gea, V, Rodriguez-De-Santiago, E, Tellez, L, Procopet, B, Giraldez, A, et al. Bacterial infections in patients with acute variceal bleeding in the era of antibiotic prophylaxis. J Hepatol. (2021) 75:342–50. doi: 10.1016/j.jhep.2021.03.026
54. Orpen-Palmer, J, and Stanley, AJ. Update on the management of upper gastrointestinal bleeding. BMJ Med. (2022) 1:e000202. doi: 10.1136/bmjmed-2022-000202
55. British Society of Gastroenterology Endoscopy Committee. Non-variceal upper gastrointestinal haemorrhage: guidelines. Gut. (2002) 51:iv1-6. doi: 10.1136/gut.51.suppl_4.iv1
56. Kim, BS, Li, BT, Engel, A, Samra, JS, Clarke, S, Norton, ID, et al. Diagnosis of gastrointestinal bleeding: a practical guide for clinicians. World J Gastrointest Pathophysiol. (2014) 5:467–78. doi: 10.4291/wjgp.v5.i4.467
57. Peura, DA, Lanza, FL, Gostout, CJ, and Foutch, PG. The American College of Gastroenterology bleeding registry: preliminary findings. Am J Gastroenterol. (1997) 92:924–8.
58. Enestvedt, BK, Gralnek, IM, Mattek, N, Lieberman, DA, and Eisen, G. An evaluation of endoscopic indications and findings related to nonvariceal upper-GI hemorrhage in a large multicenter consortium. Gastrointest Endosc. (2008) 67:422–9. doi: 10.1016/j.gie.2007.09.024
59. Oakland, K. Changing epidemiology and etiology of upper and lower gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. (2019) 42-43:101610. doi: 10.1016/j.bpg.2019.04.003
60. Abougergi, MS, Travis, AC, and Saltzman, JR. The in-hospital mortality rate for upper GI hemorrhage has decreased over 2 decades in the United States: a nationwide analysis. Gastrointest Endosc. (2015) 81:e881:882–888.e1. doi: 10.1016/j.gie.2014.09.027
61. Loperfido, S, Baldo, V, Piovesana, E, Bellina, L, Rossi, K, Groppo, M, et al. Changing trends in acute upper-GI bleeding: a population-based study. Gastrointest Endosc. (2009) 70:212–24. doi: 10.1016/j.gie.2008.10.051
62. Pilotto, A, Leandro, G, Di Mario, F, Franceschi, M, Bozzola, L, and Valerio, G. Role of Helicobacter pylori infection on upper gastrointestinal bleeding in the elderly: a case-control study. Dig Dis Sci. (1997) 42:586–91. doi: 10.1023/A:1018807412030
63. Post, PN, Kuipers, EJ, and Meijer, GA. Declining incidence of peptic ulcer but not of its complications: a nation-wide study in the Netherlands. Aliment Pharmacol Ther. (2006) 23:1587–93. doi: 10.1111/j.1365-2036.2006.02918.x
64. Stanley, AJ, and Laine, L. Management of acute upper gastrointestinal bleeding. BMJ. (2019) 364:l536. doi: 10.1136/bmj.l536
65. Tripathi, D, Stanley, AJ, Hayes, PC, Patch, D, Millson, C, Mehrzad, H, et al. U.K. guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut. (2015) 64:1680–704. doi: 10.1136/gutjnl-2015-309262
66. Siau, K, Hearnshaw, S, Stanley, AJ, Estcourt, L, Rasheed, A, Walden, A, et al. British Society of Gastroenterology (BSG)-led multisociety consensus care bundle for the early clinical management of acute upper gastrointestinal bleeding. Frontline Gastroenterol. (2020) 11:311–23. doi: 10.1136/flgastro-2019-101395
67. Hunt, BJ, Allard, S, Keeling, D, Norfolk, D, Stanworth, SJ, Pendry, K, et al. A practical guideline for the haematological management of major haemorrhage. Br J Haematol. (2015) 170:788–803. doi: 10.1111/bjh.13580
68. Mcdonald, SW, Ginez, HK, Vinturache, AE, and Tough, SC. Maternal perceptions of underweight and overweight for 6-8 years olds from a Canadian cohort: reporting weights, concerns and conversations with healthcare providers. BMJ Open. (2016) 6:e012094. doi: 10.1136/bmjopen-2016-012094
69. Laine, L, Barkun, AN, Saltzman, JR, Martel, M, and Leontiadis, GI. ACG clinical guideline: upper gastrointestinal and ulcer bleeding. Am J Gastroenterol. (2021) 116:899–917. doi: 10.14309/ajg.0000000000001245
70. Loffredo, L, Pastori, D, Farcomeni, A, and Violi, F. Effects of anticoagulants in patients with cirrhosis and portal vein thrombosis: a systematic review and Meta-analysis. Gastroenterology. (2017) 153:e481:480–487.e1. doi: 10.1053/j.gastro.2017.04.042
71. Rockall, TA, Logan, RF, Devlin, HB, and Northfield, TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. (1996) 38:316–21. doi: 10.1136/gut.38.3.316
72. Saltzman, JR, Tabak, YP, Hyett, BH, Sun, X, Travis, AC, and Johannes, RS. A simple risk score accurately predicts in-hospital mortality, length of stay, and cost in acute upper GI bleeding. Gastrointest Endosc. (2011) 74:1215–24. doi: 10.1016/j.gie.2011.06.024
73. Blatchford, O, Murray, WR, and Blatchford, M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet. (2000) 356:1318–21. doi: 10.1016/S0140-6736(00)02816-6
74. Laursen, SB, Oakland, K, Laine, L, Bieber, V, Marmo, R, Redondo-Cerezo, E, et al. ABC score: a new risk score that accurately predicts mortality in acute upper and lower gastrointestinal bleeding: an international multicentre study. Gut. (2021) 70:707–16. doi: 10.1136/gutjnl-2019-320002
75. Stanley, AJ, Laine, L, Dalton, HR, Ngu, JH, Schultz, M, Abazi, R, et al. Comparison of risk scoring systems for patients presenting with upper gastrointestinal bleeding: international multicentre prospective study. BMJ. (2017) 356:i6432. doi: 10.1136/bmj.i6432
76. Barkun, AN, Almadi, M, Kuipers, EJ, Laine, L, Sung, J, Tse, F, et al. Management of Nonvariceal Upper Gastrointestinal Bleeding: guideline recommendations from the international consensus group. Ann Intern Med. (2019) 171:805–22. doi: 10.7326/M19-1795
77. Sung, JJ, Chiu, PW, Chan, FKL, Lau, JY, Goh, KL, Ho, LH, et al. Asia-Pacific working group consensus on non-variceal upper gastrointestinal bleeding: an update 2018. Gut. (2018) 67:1757–68. doi: 10.1136/gutjnl-2018-316276
78. Newman, J, Fitzgerald, JE, Gupta, S, Von Roon, AC, Sigurdsson, HH, and Allen-Mersh, TG. Outcome predictors in acute surgical admissions for lower gastrointestinal bleeding. Color Dis. (2012) 14:1020–6. doi: 10.1111/j.1463-1318.2011.02824.x
79. Oakland, K, Chadwick, G, East, JE, Guy, R, Humphries, A, Jairath, V, et al. Diagnosis and management of acute lower gastrointestinal bleeding: guidelines from the British Society of Gastroenterology. Gut. (2019) 68:776–89. doi: 10.1136/gutjnl-2018-317807
80. Oakland, K, Guy, R, Uberoi, R, Hogg, R, Mortensen, N, Murphy, MF, et al. Acute lower GI bleeding in the UK: patient characteristics, interventions and outcomes in the first nationwide audit. Gut. (2018) 67:654–62. doi: 10.1136/gutjnl-2016-313428
81. Triantafyllou, K, Gkolfakis, P, Gralnek, IM, Oakland, K, Manes, G, Radaelli, F, et al. Diagnosis and management of acute lower gastrointestinal bleeding: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. (2021) 53:850–68. doi: 10.1055/a-1496-8969
82. Strate, LL, and Gralnek, IM. ACG clinical guideline: management of patients with acute lower gastrointestinal bleeding. Am J Gastroenterol. (2016) 111:459–74. doi: 10.1038/ajg.2016.41
83. Yachimski, PS, and Friedman, LS. Gastrointestinal bleeding in the elderly. Nat Clin Pract Gastroenterol Hepatol. (2008) 5:80–93. doi: 10.1038/ncpgasthep1034
84. Christe, C, Janssens, JP, Armenian, B, Herrmann, F, and Vogt, N. Midazolam sedation for upper gastrointestinal endoscopy in older persons: a randomized, double-blind, placebo-controlled study. J Am Geriatr Soc. (2000) 48:1398–403. doi: 10.1111/j.1532-5415.2000.tb02628.x
85. Abraham, NS, Hartman, C, Richardson, P, Castillo, D, Street, RL Jr, and Naik, AD. Risk of lower and upper gastrointestinal bleeding, transfusions, and hospitalizations with complex antithrombotic therapy in elderly patients. Circulation. (2013) 128:1869–77. doi: 10.1161/CIRCULATIONAHA.113.004747
86. Pipilis, A, Makrygiannis, S, Chrisanthopoulou, E, Sourlas, N, Kaliambakos, S, and Ntailianas, P. Gastrointestinal bleeding in patients receiving antiplatelet and anticoagulant therapy: practical guidance for restarting therapy and avoiding recurrences. Hell J Cardiol. (2014) 55:499–509.
87. Ray, G. Incidence and outcome of gastrointestinal bleeding in patients receiving aspirin with or without clopidogrel over 10 years- An observational study. J Family Med Prim Care. (2022) 11:7750–5. doi: 10.4103/jfmpc.jfmpc_1298_22
88. Yasuda, H, Matsuo, Y, Sato, Y, Ozawa, S, Ishigooka, S, Yamashita, M, et al. Treatment and prevention of gastrointestinal bleeding in patients receiving antiplatelet therapy. World J Crit Care Med. (2015) 4:40–6. doi: 10.5492/wjccm.v4.i1.40
89. Alli, O, Smith, C, Hoffman, M, Amanullah, S, Katz, P, and Amanullah, AM. Incidence, predictors, and outcomes of gastrointestinal bleeding in patients on dual antiplatelet therapy with aspirin and clopidogrel. J Clin Gastroenterol. (2011) 45:410–4. doi: 10.1097/MCG.0b013e3181faec3c
90. Benamouzig, R, Guenoun, M, Deutsch, D, and Fauchier, L. Review article: gastrointestinal bleeding risk with direct oral anticoagulants. Cardiovasc Drugs Ther. (2022) 36:973–89. doi: 10.1007/s10557-021-07211-0
91. Miller, CS, Dorreen, A, Martel, M, Huynh, T, and Barkun, AN. Risk of gastrointestinal bleeding in patients taking non-vitamin K antagonist oral anticoagulants: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. (2017) 15:e1673:1674–1683.e3. doi: 10.1016/j.cgh.2017.04.031
92. Menichelli, D, Del Sole, F, Di Rocco, A, Farcomeni, A, Vestri, A, Violi, F, et al. Real-world safety and efficacy of direct oral anticoagulants in atrial fibrillation: a systematic review and meta-analysis of 605 771 patients. Eur Heart J Cardiovasc Pharmacother. (2021) 7:f11–f19. doi: 10.1093/ehjcvp/pvab002
93. Tomaselli, GF, Mahaffey, KW, Cuker, A, Dobesh, PP, Doherty, JU, Eikelboom, JW, et al. 2017 ACC expert consensus decision pathway on management of bleeding in patients on oral anticoagulants: a report of the American College of Cardiology Task Force on expert consensus decision pathways. J Am Coll Cardiol. (2017) 70:3042–67. doi: 10.1016/j.jacc.2017.09.1085
94. Li, L, Geraghty, OC, Mehta, Z, Rothwell, PM, and Oxford Vascular, S. Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: a population-based cohort study. Lancet. (2017) 390:490–9. doi: 10.1016/S0140-6736(17)30770-5
95. Devitt, J, Lyon, C, Swanson, SB, and Desanto, K. What are the risks of long-term PPI use for GERD symptoms in patients > 65 years? J Fam Pract. (2019) 68:E18–9.
96. Liu, J, Li, X, Fan, L, Yang, J, Wang, J, Sun, J, et al. Proton pump inhibitors therapy and risk of bone diseases: An update meta-analysis. Life Sci. (2019) 218:213–23. doi: 10.1016/j.lfs.2018.12.058
97. Poly, TN, Islam, MM, Yang, HC, Wu, CC, and Li, YJ. Proton pump inhibitors and risk of hip fracture: a meta-analysis of observational studies. Osteoporos Int. (2019) 30:103–14. doi: 10.1007/s00198-018-4788-y
98. Trifan, A, Stanciu, C, Girleanu, I, Stoica, OC, Singeap, AM, Maxim, R, et al. Proton pump inhibitors therapy and risk of Clostridium difficile infection: systematic review and meta-analysis. World J Gastroenterol. (2017) 23:6500–15. doi: 10.3748/wjg.v23.i35.6500
99. Mehta, P, Nahass, RG, and Brunetti, L. Acid suppression medications during hospitalization as a risk factor for recurrence of Clostridioides difficile infection: systematic review and meta-analysis. Clin Infect Dis. (2021) 73:e62–8. doi: 10.1093/cid/ciaa545
100. Kiecka, A, and Szczepanik, M. Proton pump inhibitor-induced gut dysbiosis and immunomodulation: current knowledge and potential restoration by probiotics. Pharmacol Rep. (2023) 75:791–804. doi: 10.1007/s43440-023-00489-x
101. Lin, CY, Cheng, HT, Kuo, CJ, Lee, YS, Sung, CM, Keidan, M, et al. Proton pump inhibitor-induced gut Dysbiosis increases mortality rates for patients with Clostridioides difficile infection. Microbiol Spectr. (2022) 10:e0048622. doi: 10.1128/spectrum.00486-22
102. Lambert, AA, Lam, JO, Paik, JJ, Ugarte-Gil, C, Drummond, MB, and Crowell, TA. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS One. (2015) 10:e0128004. doi: 10.1371/journal.pone.0128004
103. Nguyen, PA, Islam, M, Galvin, CJ, Chang, CC, An, SY, Yang, HC, et al. Meta-analysis of proton pump inhibitors induced risk of community-acquired pneumonia. Int J Qual Health Care. (2020) 32:292–9. doi: 10.1093/intqhc/mzaa041
Keywords: endoscopy, older population, anticoagulants, gastrointestinal bleeding, proton pump inhibitors
Citation: Menichelli D, Gazzaniga G, Del Sole F, Pani A, Pignatelli P and Pastori D (2024) Acute upper and lower gastrointestinal bleeding management in older people taking or not taking anticoagulants: a literature review. Front. Med. 11:1399429. doi: 10.3389/fmed.2024.1399429
Received: 11 March 2024; Accepted: 11 April 2024;
Published: 03 May 2024.
Edited by:
Antonietta G. Gravina, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Jonathan Soldera, University of Caxias do Sul, BrazilCopyright © 2024 Menichelli, Gazzaniga, Del Sole, Pani, Pignatelli and Pastori. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniele Pastori, ZGFuaWVsZS5wYXN0b3JpQHVuaXJvbWExLml0
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.