- 1Department of Medicine IV - Nephrology and Primary Care, Faculty of Medicine and Medical Center, University of Freiburg, Freiburg, Germany
- 2Interdisciplinary Medical Intensive Care, Faculty of Medicine and Medical Center, University of Freiburg, Freiburg, Germany
- 3Department of Cardiology, Pneumology, Angiology, and Intensive Care, Ortenau Clinical Center Offenburg-Kehl, Freiburg, Germany
Objectives: Deep sedation on the ICU is linked to poor outcome. This study investigated the link between Richmond Agitation-Sedation Scale (RASS) and outcome in venovenous extracorporeal membrane oxygenation (V-V ECMO).
Methods: We performed a secondary analysis of a single-center V-V ECMO cohort. RASS was used as a surrogate measure of sedation depth, patients with a score ≥ −1 were considered awake. V-V ECMO durations below 24 h were excluded. Primary endpoint was 30-day survival. Secondary endpoints were hospital survival and weaning from both ventilator and ECMO therapy.
Results: A total of 343 patients were reanalyzed. The median age was 55 years and 52.2% (179/343) survived for 30 days after ECMO cannulation. Median duration of ECMO was 7.9 (4.7–15.0) days and the median duration of mechanical ventilation after ECMO cannulation was 11.8 (6.7–23.8) days.
In the whole cohort, median RASS on day one and seven after ECMO were − 4 (−4 to −1) and − 3 (−4 to 0), respectively. ECMO survivors consistently had significantly higher RASS scores during the first 7 days of ECMO compared to non-surviving patients (p < 0.01). On day two after ECMO, survival of awake patients (i.e., RASS ≥-1) was significantly better compared to sedated [i.e., RASS −4 to −2; OR 2.20 (1.28–3.71), p < 0.01] or unresponsive patients [i.e., RASS -5; OR 2.27 (1.15–4.64), p = 0.02]. The survival benefit of awake ECMO was consistent from day two to seven. Patients awake at least once during ECMO showed higher 30-day survival rates [64.4% vs. 39.6%, OR 2.75 (1.77–4.24), p < 0.01].
Conclusion: In this retrospective study, awake patients on V-V ECMO showed higher 30-day survival rates compared to sedated or unresponsive patients. These data should encourage further research on awake V-V ECMO.
Introduction
Deep sedation hampers mobilization (1). In our 10-year data on venovenous extracorporeal membrane oxygenation (V-V ECMO) support (2), mobilization was connected to improved outcome.
In V-V ECMO, mobilization seems safe and feasible (3–5). Mobilization however is only one of multiple ways how sedation impacts outcome. There are abundant data that light sedation on intensive care unit (ICU) is liked to outcome including reduction of delirium, distress, and enabling spontaneous breathing (6–9). In critically ill patients on ICU, including ECMO patients, data show that complications including bacterial pneumonia may be reduced in awake patients (10–13). Additionally, deep sedation related muscle loss is another typical complication, developing within days on the ICU (14–16).
We therefore hypothesized that awake V-V ECMO independently improves outcome. Here, we investigated the association of the Richmond Agitation-Sedation Scale (RASS) and 30-day survival.
Methods
In this retrospective cohort study, we reanalyzed data on mobilization during V-V ECMO support (2). Primary endpoint was 30-day survival. Secondary endpoints were hospital survival and weaning from both ventilator and ECMO therapy. Inclusion criteria were an age of at least 18 years at cannulation, primary venovenous support (excluding veno-venoarterial and veno-arterial ECMO), and a duration of V-V ECMO support of at least 24 h. The ethics committee of the University of Freiburg (file number 21–1683) approved this registry.
Daily decisions on sedation and mobilization are made individually at the bedside. For this analysis, we grouped patients into those with 30-day survival and non-survival. As for statistics, in Table 1, Mann–Whitney-U test was used on continuous data and Fishers Exact test on categorial data. In Figure 1A and Supplementary Figures 1A–3A, 2-way ANOVA was used to analyze the relation of RASS and survival. In Figure 1B and Supplementary Figures 1B–3B, Chi-square test was used to compare groups. Odds ratios were calculated using Fisher’s exact test. In Supplementary Table S1, univariate and multivariate logistic regression analysis was used based on predefined confounders of the primary endpoint. In Supplementary Table S2, 2-way ANOVA was used including only patients still on ECMO. Kaplan–Meier survival analysis was used for Supplementary Figure S4. A p-value of <0.05 was considered statistically significant. Data are given as median (interquartile range) or as number of patients (percentage of group).
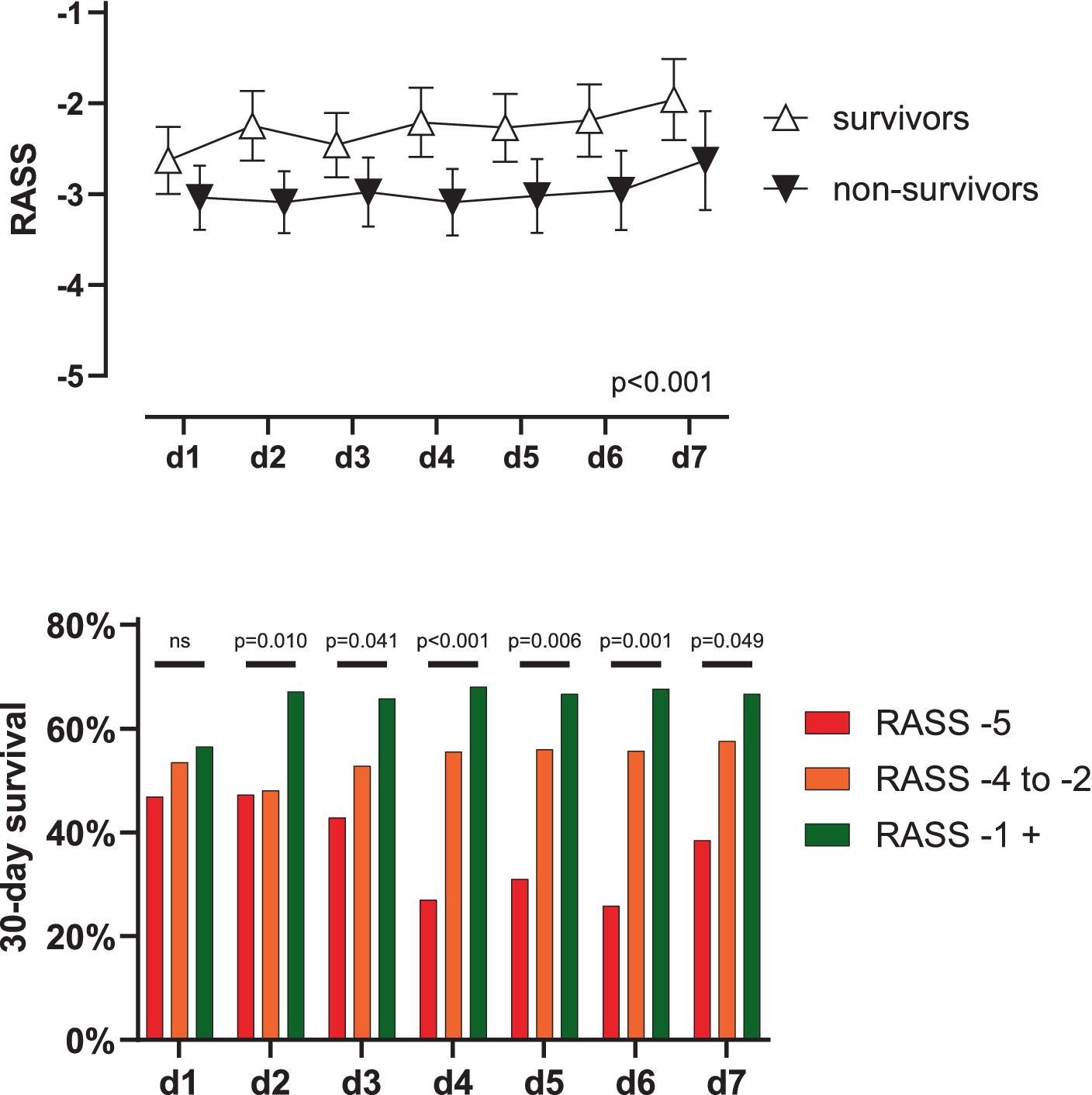
Figure 1. Outcome in V-V ECMO according to RASS-score. (A) Patients surviving 30 days showed significantly higher RASS-scores during day 1 to 7 (p < 0.001) while RASS-scores did not change over time and factors did not interact (p = 0.304 and 0.859). Data shown as mean with 95% CI. (B) On day two after ECMO-implantation, survival of awake patients (i.e., RASS-score ≥ −1) was significantly better compared to unresponsive patients [i.e., RASS-score − 5, 67.1 vs. 47.3%, OR 2.271 (1.145–4.638), p = 0.023] or sedated patients [i.e., RASS-score − 4 to −2, 67.1 vs. 48.1%, OR 2.200 (1.276–3.712), p = 0.004]. This trend was consistent to day seven. RASS, Richmond Agitation-Sedation Scale; d, day; ns, not significant; CI, confidence interval.
RASS was evaluated at least three times a day for each ECMO patient. For this analysis, the highest documented RASS score each day was considered. Patients were categorized as awake (RASS ≥-1), sedated (RASS −4, −3 or − 2) or unresponsive (RASS -5) according to highest RASS-score.
Results
Patient cohort
As previously reported (2), 343 patients were treated with V-V ECMO for ≥24 h between October 2010 and May 2021 (canulation window). 30-day survival was reached in 179/343 (52.2%) patients. Non-surviving patients suffered from significantly more preconditions, i.e., immunodeficiency, lung and liver disease and oncological disorders (all p < 0.05). There were no relevant differences in the respiratory status before ECMO cannulation, see Table 1. Surviving patients stayed significantly longer on the ICU after ECMO cannulation (21.9 (12.7–37.8) compared to 9.5 (5.0–14.9) days, p < 0.001) and were on mechanical ventilation significantly longer (16.8 (8.8–34.9) compared to 9.4 (5.0–14.8) days, p < 0.001). ECMO runtime was 7.8 (4.3–14.8) days not differing between the groups (p = 0.052).
RASS on ECMO
Median RASS on the day of ECMO implantation was −4 (−4 to −1) and − 3 (−4 to 0) on day 7. A 2-way ANOVA showed significantly higher RASS-scores over the first 7 days in surviving patients (p < 0.001) while RASS-scores did not change over time (p = 0.304) without interaction (p = 0.859), see Table 1 and Figure 1A.
Awake ECMO
When grouping patients’ consciousness, 30-day survival did not differ statistically on day one after ECMO (56.5 vs. 53.5 vs. 46.9%. p = 0.445) in awake, sedated and unresponsive patients, respectively. Starting on day two however, survival of awake patients was significantly higher compared to sedated (67.1 vs. 48.1%, OR 2.200 (1.276–3.712), p = 0.004) or unresponsive patients (67.1 vs. 47.3%, OR 2.271 (1.145–4.638), p = 0.023). This trend was consistent until day seven, see Figure 1B. Patients who were awake at least once during ECMO had a significantly higher survival rate (64.4% vs. 39.6%, OR 2.750 (1.773–4.240) p < 0.001). The outcome of awake patients on ECMO was also significantly better when evaluating the outcomes ‘hospital survival’, ‘weaning from ECMO’, or ‘weaning from the ventilator’, see supplementary Figures S1–S3. A Kaplan–Meier survival analysis also confirmed these findings, see Supplementary Figure S4.
Bias
This retrospective registry study of awake ECMO faces a significant risk of bias. We performed a multivariate logistic regression analysis including potential reasons for cerebral damage including CPR before ECMO, which did not significantly correlate with our primary endpoint. Of note being awake during the first 7 days of ECMO was an independent predictor of the primary endpoint while mobilization was not, see Supplementary Table S1.
The 2-way ANOVA on RASS on ECMO showed similar results when only analyzing patients still on ECMO, see Supplementary Table S2.
Discussion
The analysis showed significantly better survival in more awake patients, especially if patients survived until day two after ECMO cannulation.
There are many data showing that deep sedation correlates with poor outcome (17–23). Lighter sedation and daily interruption of sedation might influence outcome by various means one being better mobilization (13). Data from the ELSO registry showed better survival in patients with early mobilization and better mobilization among others in patients avoiding mechanical ventilation and thus sedation (11). These plausible results match smaller previous studies (24–27). We also showed this correlation of mobilization and outcome in this patient cohort (2). Mobilization however is not the only mechanism by which awake ECMO might influence survival (13) and mobilization was not an independent predictor of outcome in the logistic regression. The improved outcome of awake ECMO patients however was consistent over all investigates secondary endpoints. A potential confounder of these results might be that surviving patients have a higher chance of being awake at least once during ECMO compared to early deceased patients. This was addressed by excluding patients not surviving at least 24 h from the analysis and by focusing on the first 7 days of ECMO, only. When evaluating only patients still on ECMO (excluding patient weaned early from ECMO) our results could be confirmed.
Another important potential confounder is that sicker patients might have needed higher sedation depth. Low RASS therefore would be a marker of illness rather than independently influencing risk of death. This bias cannot be excluded and has to be considered when drawing clinical decisions from retrospective data.
We saw a significantly better survival in awake patients only on day two after ECMO implantation. Without stretching the data, this fact might suggest that the vulnerable first 24 h after ECMO do not have to be complicated by too ambitious sedation reduction.
Lastly, not all trials on awake patients on the ICU were positive (17). Reasons might be found in the heterogeneous patient cohort and the potential increased risk of accidental extubation (28) or ECMO decannulation (29–31) in awake patients.
Conclusion
In this retrospective study, awake patients on V-V ECMO showed higher 30-day survival rates compared to sedated or unresponsive patients. There are many confounders and biases to be considered when interpreting retrospective data. Pending robust data, deep sedation strategies in V-V ECMO might be advisable only for specific indications.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics committee of the University of Freiburg (file number 21–1683). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
FR: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. VZ: Conceptualization, Methodology, Validation, Writing – review & editing. AS: Conceptualization, Methodology, Resources, Supervision, Validation, Writing – review & editing. CN: Data curation, Investigation, Writing – review & editing. TW: Conceptualization, Methodology, Resources, Supervision, Validation, Writing – review & editing. DS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. We acknowledge support by the Open Access Publication Fund of the University of Freiburg.
Conflict of interest
AS declares a research grant and lecture honoraria by the CytoSorbents. TW received lecture honoraria or travel support from Abbot Medical, AstraZeneca and Boston Scientific. DS received lecture honoraria or travel support from the Abiomed, AstraZeneca, Dahlhausen, Getinge, Medtronic, Orion Pharma, and was part of a dual lumen advisory board by the Medtronic.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1394698/full#supplementary-material
References
1. Bright, L, Van Der Lee, L, Hince, D, Wood, FM, and Edgar, DW. Quantification of the negative impact of sedation and inotropic support on achieving early mobility in burn patients in ICU: a single center observational study. Burns. (2021) 47:1756–65. doi: 10.1016/j.burns.2021.09.015
2. Rottmann, FA, Noe, C, Bemtgen, X, Maier, S, Supady, A, Wengenmayer, T, et al. Survival outcomes and mobilization during venovenous extracorporeal membrane oxygenation: a retrospective cohort study. Front Med. (2023) 10. doi: 10.3389/fmed.2023.1271540
3. Hodgson, CL, Hayes, K, Linnane, M, Tronstad, O, Reddy, N, Young, M, et al. Early mobilisation during extracorporeal membrane oxygenation was safe and feasible: a pilot randomised controlled trial. Intensive Care Med. (2020) 46:1057–9. doi: 10.1007/s00134-020-05994-8
4. Braune, S, Bojes, P, Mecklenburg, A, Angriman, F, Soeffker, G, Warnke, K, et al. Feasibility, safety, and resource utilisation of active mobilisation of patients on extracorporeal life support: a prospective observational study. Ann Intensive Care. (2020) 10:161. doi: 10.1186/s13613-020-00776-3
5. Marhong, JD, DeBacker, J, Viau-Lapointe, J, Munshi, L, Del Sorbo, L, Burry, L, et al. Sedation and mobilization during Venovenous extracorporeal membrane oxygenation for acute respiratory failure: an international survey. Crit Care Med. (2017) 45:1893–9. doi: 10.1097/CCM.0000000000002702
6. DeBiasi, EM, Akgün, KM, and Pisani, M. Awake or sedated: trends in the evaluation and Management of Agitation in the intensive care unit. Semin Respir Crit Care Med. (2015) 36:899–913. doi: 10.1055/s-0035-1564875
7. Jean, R, Shah, P, Yudelevich, E, Genese, F, Gershner, K, Levendowski, D, et al. Effects of deep sedation on sleep in critically ill medical patients on mechanical ventilation. J Sleep Res. (2020) 29:e12894. doi: 10.1111/jsr.12894
8. Shehabi, Y, Chan, L, Kadiman, S, Alias, A, Ismail, WN, Tan, MATI, et al. The sedation practice in intensive care evaluation (SPICE) study group investigators. Sedation depth and long-term mortality in mechanically ventilated critically ill adults: a prospective longitudinal multicentre cohort study. Intensive Care Med. (2013) 39:910–8. doi: 10.1007/s00134-013-2830-2
9. Hyun, D, Ahn, JH, Gil, HY, Nam, CM, Yun, C, and Lim, CM. Longitudinal trajectories of sedation level and clinical outcomes in patients who are mechanically ventilated based on a group-based trajectory model: a prospective, multicentre, longitudinal and observational study in Korea. BMJ Open. (2023) 13:e072628. doi: 10.1136/bmjopen-2023-072628
10. Zangrillo, A, Landoni, G, Biondi-Zoccai, G, Greco, M, Greco, T, Frati, G, et al. A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Crit Care Resusc. (2013) 15:172–8. doi: 10.1016/S1441-2772(23)01792-1
11. Tonna, JE, Bailey, M, Abrams, D, Brodie, D, and Hodgson, CL. Predictors of early mobilization in patients requiring VV ECMO for greater than 7 days: an international cohort study. Heart Lung. (2023) 1:57–63. doi: 10.1016/j.hrtlng.2023.05.022
12. Yu, X, Gu, S, Li, M, and Zhan, Q. Awake extracorporeal membrane oxygenation for acute respiratory distress syndrome: which clinical issues should be taken into consideration. Front Med. (2021) 8:682526. doi: 10.3389/fmed.2021.682526
13. Langer, T, Santini, A, Bottino, N, Crotti, S, Batchinsky, AI, Pesenti, A, et al. “Awake” extracorporeal membrane oxygenation (ECMO): pathophysiology, technical considerations, and clinical pioneering. Crit Care. (2016) 20:150. doi: 10.1186/s13054-016-1329-y
14. Ramsay, DA, Zochodne, DW, Robertson, DM, Nag, S, and Ludwin, SK. A syndrome of acute severe muscle necrosis in intensive care unit patients. J Neuropathol Exp Neurol. (1993) 52:387–98. doi: 10.1097/00005072-199307000-00006
15. Berg, HE, Larsson, L, and Tesch, PA. Lower limb skeletal muscle function after 6 wk of bed rest. J Appl Physiol. (1997) 82:182–8. doi: 10.1152/jappl.1997.82.1.182
16. Mendes, JN d S, Rodrigues, IG, Arcoverde, GMPF, Floro, CCP, Fortunato, WSL, Lima, RM d S, et al. Evaluation of muscle loss by ultrasonography in critically ill patients. Nutr Clin Pract. (2023) 38:664–71. doi: 10.1002/ncp.10945
17. Olsen, HT, Nedergaard, HK, Strøm, T, Oxlund, J, Wian, KA, Ytrebø, LM, et al. Nonsedation or Light sedation in critically ill, mechanically ventilated patients. N Engl J Med. (2020) 382:1103–11. doi: 10.1056/NEJMoa1906759
18. Devlin, JW, Skrobik, Y, Gélinas, C, Needham, DM, Slooter, AJC, Pandharipande, PP, et al. Clinical practice guidelines for the prevention and Management of Pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. (2018) 46:e825:–e873. doi: 10.1097/CCM.0000000000003299
19. Girard, TD, Kress, JP, Fuchs, BD, Thomason, JW, Schweickert, WD, Pun, BT, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (awakening and breathing controlled trial): a randomised controlled trial. Lancet. (2008) 371:126–34. doi: 10.1016/S0140-6736(08)60105-1
20. Kress, JP, Pohlman, AS, O’Connor, MF, and Hall, JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. (2000) 342:1471–7. doi: 10.1056/NEJM200005183422002
21. Aragón, RE, Proaño, A, Mongilardi, N, de Ferrari, A, Herrera, P, Roldan, R, et al. Sedation practices and clinical outcomes in mechanically ventilated patients in a prospective multicenter cohort. Crit Care. (2019) 23:130. doi: 10.1186/s13054-019-2394-9
22. Strøm, T, Martinussen, T, and Toft, P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomised trial. Lancet. (2010) 375:475–80. doi: 10.1016/S0140-6736(09)62072-9
23. Augustes, R, and Ho, KM. Meta-analysis of randomised controlled trials on daily sedation interruption for critically ill adult patients. Anaesth Intensive Care. (2011) 39:401–9. doi: 10.1177/0310057X1103900310
24. Wells, CL, Forrester, J, Vogel, J, Rector, R, Tabatabai, A, and Herr, D. Safety and feasibility of early physical therapy for patients on extracorporeal membrane oxygenator: University of Maryland Medical Center experience*. Crit Care Med. (2018) 46:53–9. doi: 10.1097/CCM.0000000000002770
25. Munshi, L, Kobayashi, T, DeBacker, J, Doobay, R, Telesnicki, T, Lo, V, et al. Intensive care physiotherapy during extracorporeal membrane oxygenation for acute respiratory distress syndrome. Annals ATS. (2017) 14:246–53. doi: 10.1513/AnnalsATS.201606-484OC
26. Hayes, K, Hodgson, CL, Pellegrino, VA, Snell, G, Tarrant, B, Fuller, LM, et al. Physical function in subjects requiring extracorporeal membrane oxygenation before or after lung transplantation. Respir Care. (2018) 63:194–202. doi: 10.4187/respcare.05334
27. Wildschut, ED, Hanekamp, MN, Vet, NJ, Houmes, RJ, Ahsman, MJ, Mathot, RAA, et al. Feasibility of sedation and analgesia interruption following cannulation in neonates on extracorporeal membrane oxygenation. Intensive Care Med. (2010) 36:1587–91. doi: 10.1007/s00134-010-1931-4
28. Li, P, Sun, Z, and Xu, J. Unplanned extubation among critically ill adults: a systematic review and meta-analysis. Intens Crit Care Nurs. (2022) 70:103219. doi: 10.1016/j.iccn.2022.103219
29. Hadano, H, Kamio, T, Fukaguchi, K, Sato, M, Tsunano, Y, and Koyama, H. Analysis of adverse events related to extracorporeal membrane oxygenation from a nationwide database of patient-safety accidents in Japan. J Artif Organs. (2023) 27:15–22. doi: 10.1007/s10047-023-01386-z
30. Kim, DH, Cho, WH, Son, J, Lee, SK, and Yeo, HJ. Catastrophic mechanical complications of extracorporeal membrane oxygenation. ASAIO J. (2021) 67:1000–5. doi: 10.1097/MAT.0000000000001354
Keywords: extracorporeal membrane oxygenation, mobilization, acute respiratory distress syndrome, intensive & critical care, RASS, awake
Citation: Rottmann FA, Zotzmann V, Supady A, Noe C, Wengenmayer T and Staudacher DL (2024) Awake venovenous extracorporeal membrane oxygenation and survival. Front. Med. 11:1394698. doi: 10.3389/fmed.2024.1394698
Edited by:
Bryan D. Kraft, Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, School of Medicine, Duke University, United StatesReviewed by:
John Grotberg, Washington University in St. Louis, United StatesCraig Rackley, Duke University Medical Center, Duke University, United States
Copyright © 2024 Rottmann, Zotzmann, Supady, Noe, Wengenmayer and Staudacher. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dawid L. Staudacher, ZGF3aWQuc3RhdWRhY2hlckB1bmlrbGluaWstZnJlaWJ1cmcuZGU=
†ORCID: Felix A. Rottmann, orcid.org/0000-0002-7458-6521
Dawid L. Staudacher, orcid.org/0000-0002-9423-9682
Alexander Supady, orcid.org/0000-0003-4056-3652
 Felix A. Rottmann
Felix A. Rottmann Viviane Zotzmann
Viviane Zotzmann Alexander Supady
Alexander Supady Christian Noe
Christian Noe Tobias Wengenmayer2
Tobias Wengenmayer2 Dawid L. Staudacher
Dawid L. Staudacher